Abstract
Objectives
To assess the value of conducting a glaucoma screening randomized controlled trial in the UK.
Methods
Decision model based economic evaluation and value of information analysis. Model derived from a previous health technology assessment. Model updated in terms of structure and parameter estimates with data from surveys, interviews with members of the public and health care providers and routine sources.
Results
On average, across a range of ages of initiating screening (40–60 years), glaucoma prevalence (1–5%), screening uptake (30–100%), and the performance of current case finding, screening was not cost-effective at a £30,000 threshold per quality adjusted life year (QALY) from the perspective of the National Health Service (NHS). The societal value of removing all uncertainty around glaucoma screening is £107 million at a threshold of £20,000 per QALY. For informing policy decisions on glaucoma screening, reducing uncertainty surrounding the NHS and personal social care cost of sight impairment (£74 million) was of most value, followed by reducing uncertainty in test performance (£14 million) and uptake of either screening or current eye care (£8 million each).
Conclusions
A glaucoma screening trial in the UK is unlikely to be the best use of research resources. Further research to quantify the costs of sight impairment falling on the NHS and personal social services is a priority. Further development of glaucoma tests and research into strategies to promote the uptake of screening or current eye care such as through the use of a behavioural intervention would be worthwhile.
Keywords: decision analysis, health policy, public health, ophthalmology
Introduction
Glaucoma, a chronic eye condition, is a leading cause of avoidable blindness.1,2 Open angle glaucoma is the commonest form.3 Sight loss from glaucoma can be avoided as early treatment of the condition reduces the risk of sight loss.4 However, in the UK around 3000 people are newly registered with sight impairment due to glaucoma each year.5 Delayed detection and thus access to early treatment is the main risk factor for sight loss6,7 and may be linked to areal or individual socioeconomic deprivation.8–10 Delayed access to treatment may occur at any stage of the referral pathway. There may be patient delay in terms of attendance for testing, process delay in terms of missed detection, or system delay leading to delayed referral for treatment.11–13 The public health importance of glaucoma could indicate that a screening programme might be warranted. Before a screening policy is adopted evidence is required that the benefits of screening, namely reduced visual impairment, outweigh any harms, for example anxiety and cost.
An earlier evaluation using economic modelling found that screening the UK population, selected on age alone, was unlikely to be cost-effective as the prevalence is too low in all age groups (screening at age 40 or 65 or 75).5,14 A surveillance programme targeted to higher risk groups (a sibling with glaucoma; ethnic minority groups; diabetics; or people with ocular risk factors such as raised intraocular pressure (IOP) and myopia) or those who do not normally use eye care might be worthwhile. The modelling evaluation, hereafter referred to as the Glaucoma Screening Model, used the best data available but still had some uncertainties: how best to screen (tests and location); likely uptake of any screening programme; and the effectiveness and coverage of current eye care services.
The most robust way to evaluate any proposed screening programme is a randomized controlled trial (RCT).15 There are no RCTs evaluating glaucoma screening16 and any trial would need to be large and thus costly. In a recent trial platform study,17 we undertook a multicomponent mixed-methods approach to provide evidence to inform the optimal design for a trial. We followed the Medical Research Council guidance for the development and evaluation of complex interventions.18 Our initial work consisted of addressing the development of the screening test schedule and the factors associated with motivation of the public to attend for screening. We took a systematic, theory-based approach to intervention development (identifying the evidence, modelling process and developing outcomes) and explored the feasibility (for service providers), acceptability (for providers and users), and cost-effectiveness (for health and social services) of trial components. These individual components are published elsewhere.19–21 In this paper, we report the integration of the findings (revised screening test schedule, likely uptake of screening and uptake of usual care) into the glaucoma screening economic model to inform whether a glaucoma screening trial would be worthwhile.
Methods
We used a Markov model to assess how worthwhile a glaucoma screening trial would be. We took the perspective that interventions compared within the model could be delivered (technical feasibility) and were acceptable (likely uptake by providers and the public) in the context of the NHS.
We revised the structure of the existing Glaucoma Screening Model5,14 with the most likely cost-effective glaucoma testing schedules that might be brought to trial based on a prior Delphi survey19 and views of NHS providers.20 We updated parameter estimates for screening attendance based on our survey of the public to identify factors associated with their hypothetical intention (motivation) to attend an eye health test. We also used data collected in the survey on attendance by the public for an eye test within the last three years to estimate uptake of the comparator pathway within the model of opportunistic case finding within current eye care.21 Costs were reported in 2010 prices. We sought estimates of attendance at eye care services for several risk groups (age over 50, black ethnicity, diabetes, myopia, family history of glaucoma, and low socioeconomic status from the British Household Panel Survey data22 to develop and fit the probability distributions around the mean uptake of current eye care for the general population and subgroups using the Excel© add-on Oracle© Crystal Ball. Revised utility data were based on the EQ-5D-3L23 responses from 640 participants with ocular hypertension and glaucoma sampled from a secondary glaucoma service (Prior, personal communication). All other parameters were as detailed in the original Glaucoma Screening Model.5,14 The model allowed movement between health states every year, estimated costs from NHS and personal social services perspectives and used the EQ-5D-3L quality of life weights to calculate quality adjusted life years (QALYs).
The base case analysis conducted from NHS perspective, considered a cohort of 40-year old males with prevalence from 1% to 5%. Sex-specific variables were not available for any of the model parameters except for mortality. We used male mortality in the base-case analysis, consistent with good modelling practice, as they are a conservative assumption for screening. Alternative likelihoods of attending screening (e.g. 30–100%) and estimated costs and QALYs over their estimated lifetime with screening occurring every 10 years were included. All costs and QALYs were discounted at 3.5%. The results are presented as incremental cost-effectiveness ratios (ICERs).
We conducted sensitivity analyses to identify plausible situations where screening might be considered worthwhile by varying screening start age and accuracy of glaucoma detection within current eye care services. We used alternative data for uptake of current eye care (based on the survey of the public21 and British Household Panel Survey (BHPS) data) for the whole population, for cohorts aged 50 and 60 as well as for higher risk subgroups of the adult population: those self-reported as having diabetes, sight problems in the family (excluding using spectacles) as a proxy for family history of glaucoma, and those at low household income (below £10,000 a year). Data were not available within the BHPS to investigate the impact of ethnicity or myopia on the uptake rates of current eye care by these groupings.
The effect of including personal social services costs was explored in the sensitivity analysis by incorporating an annual cost of sight impairment from £1000 to £40,000. The upper value is in line with personal social services expenditure per person with sight impairment for England for 2009–10.24 Variations in cost of sight impairment were combined with variation in both prevalence rates and screening uptake to identify situations where screening might be worthwhile. We explored the impact of increasing screening uptake because of a behavioural intervention and incorporating an additional cost for its provision. Without a behavioural intervention, glaucoma screening uptake was considered to be about 23% (based on our survey where 45% of the sample had strong intention scores (mean intention score of 7 on a 1–7 scale)21 and the health behaviour literature that 50% of strong intenders will perform the intended behaviour.25 We varied screening uptake from 30% to 100% to explore the impact of a behavioural intervention. Reflecting a simple behavioural intervention such as an invitation letter targeted to improve motivation by making it easier to attend a screening appointment to a fully tailored intervention, and thus more costly, to persuade those with low intentions to attend. The mean QALYs were expressed in pounds sterling by multiplying them by the willingness to pay for a QALY threshold (e.g. £30,000, see Figure 1).
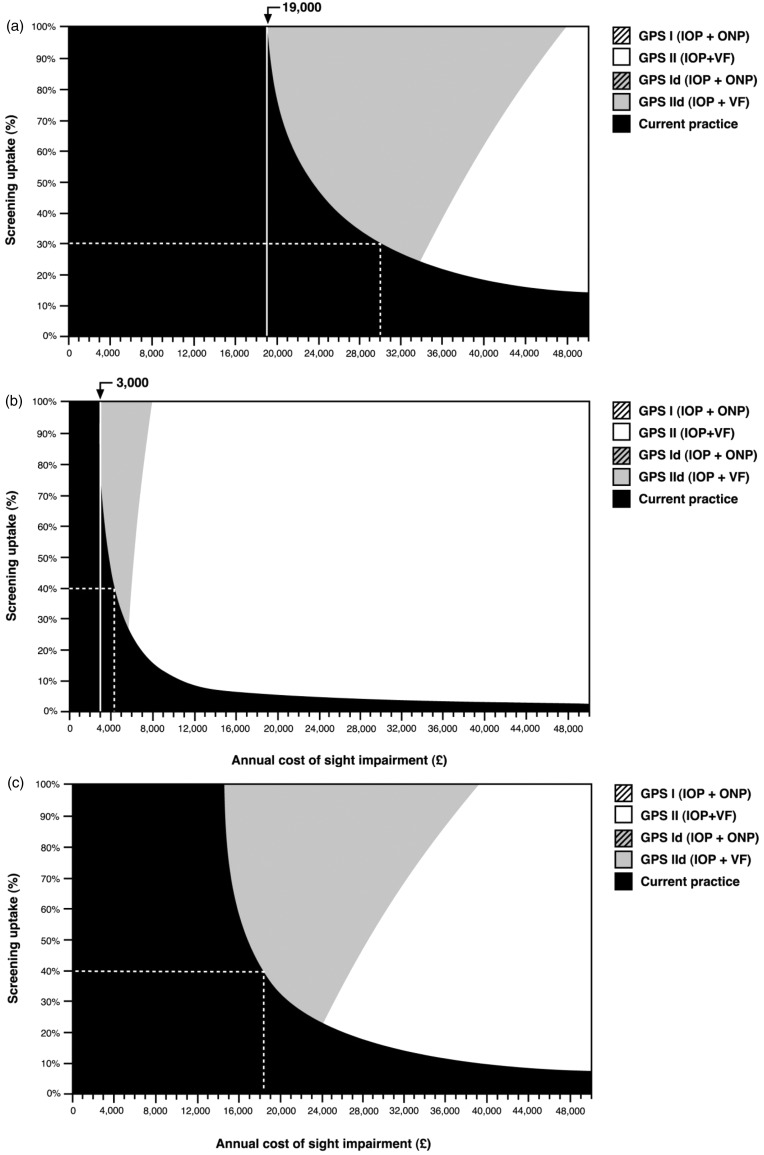
Figure 1.
Strategies with the highest net-benefit (defined as £30,000 × mean QALYs minus mean costs) for alternative values of annual cost of sight impairment and percentage of screening uptake for a 50-year-old cohort. Willingness to pay is £30,000.
(a) 1% glaucoma prevalence and 17%21 uptake current eye care practice. For the range of values selected for the annual cost of sight impairment and uptake rate, only ‘current practice’, ‘GPS11d (IOP + VF)’ or ‘GPS11 (IOP + VF)’ are potentially cost-effective when society is willing to pay £30,000 per QALY. The dashed line is illustrative. The screening strategy ‘GPS11d (IOP + VF)’ has the highest net-benefit when the screening uptake is 30% and the annual cost of sight impairment is £30,000. The vertical continuous line at £19,000 cost of sight impairment illustrates that screening is not cost-effective below this value, regardless of the screening uptake. (b) 5% glaucoma prevalence and 17%22 uptake of current eye care.
The dashed line is illustrative. The screening strategy ‘GPS11d (IOP + VF)’ has the highest net-benefit for screening attendance of 40% and annual cost of sight impairment just above £4500. The vertical continuous line at £3000 cost of sight impairment illustrates that screening by any pathway is not cost-effective below this value, regardless of screening uptake. (c) 1% glaucoma prevalence rate and 6.5%22 uptake of current eye care. The dashed line is illustrative. The screening strategy ‘GPS11d (IOP + VF)’ having the highest net-benefit for screening attendance of 40% and annual cost of sight impairment above £18,000. IOP: intraocular pressure; GPS: Glaucoma screening Platform Study; QALY: quality adjusted life years; VF: visual field.
We also explored whether enhanced current eye care would be better than enrolling in a screening programme by modifying the model in order to compare two current practice strategies. Two uptake rates of current eye care were compared using BHPS data: 6.5% (corresponding to the uptake rate of low income groups) and 17% (estimated uptake of eye care for people with diabetes).
All analyses incorporated probabilistic sensitivity analyses where the statistical imprecision is allowed for by sampling (e.g. 1000 times) from probability distributions attached to model mean parameter values. The uncertainty in the model parameter values has cost implications as the ‘correct’ strategy might not be chosen. In effect, if the analysis is run with alternative parameter values the choice of strategy might differ from the strategy finally adopted. The sum of the benefits forgone for not being able to make the right decision due to this uncertainty is the expected value of perfect information (EVPI), as perfect information would eliminate the possibility of making wrong decisions.26 The EVPI can be compared with the cost of reducing uncertainty in the model by collecting further information. The expected value of parameter perfect information (EVPPI) is a similar concept but corresponds to a particular parameter or group of parameters in the model. EVPPI was calculated to identify model parameters that contribute the most to the overall model decision uncertainty.26 The total EVPI and EVPPI were obtained by multiplying the EVPI and EVPPI estimated at an individual level by the number of individuals who would benefit from the intervention in a given period of time. We assumed 380,000 individuals with undiagnosed glaucoma in the UK and an annual incidence of 11,000 new cases5 a time horizon of 10 years (representing the lifespan of the technology; in this case a specific screening strategy) and a discount rate of 3.5%.
Results
The revised model, now named the Glaucoma screening Platform Study (GPS) model, considers four possible screening strategies against a current practice comparator (no screening). The pathways modelled are specified in Box 1.
Box 1.
Description of the pathways compared within the economic model.
| Glaucoma Screening Platform Study (GPS)1. (Tonometry (measurement of intraocular pressure (IOP)) and optic nerve photography (ONP)): The population to be screened are invited to a primary care setting to undergo tonometry and ONP by a technician or nurse who has received some training. Screen positives referred to hospital eye service. |
| GPS11 (IOP and visual field (VF)): As above but screening with tonometry and visual field test (perimetry). Screen positives referred to hospital eye service. |
| GPS1d (IOP + ONP): Screening with tonometry and optic nerve photography and screen positives examined by a specialized optometrist (diagnosis). Diagnostic test positives referred to hospital eye service. |
| GPS11d (IOP + VF): Screening with tonometry and visual field test (perimetry) with further diagnostic refinement and screen positives examined by a specialized optometrist (diagnosis). Diagnostic test positives referred to hospital eye service. |
| Current practice (UK NHS eye care): Opportunistic sight test at community optometrist with referral of suspect glaucoma to the hospital eye service. |
| IOP ≥ 26 mmHg = screen positive. |
| IOP < 26 mmHg + second technology test positive = screen positive. |
| IOP < 26 mmHg + second technology test negative = return to current eye care and re-screen cycle. |
The screening test schedule, based on the views of service providers in terms of practicality and equity, was screening an inception cohort age 40. The screening strategies allow for a technician as first screening contact. However, they differ in the tests performed. Glaucoma tests were either optic nerve photography (ONP) or screening mode perimetry (a measure of visual field (VF) sensitivity) with or without tonometry (a measure of IOP). For those testing positive two pathways were explored, a diagnostic refinement step, using a specialized optometrist to examine screen positives as in the original model, or no referral refinement with screen test positives referred to a hospital based glaucoma service.20 Tables 1 and 2 show the updated data used in the GPS model. All other data in the model remained as for original model.5,14
Table 1.
Prevalence, incidence and progression of glaucoma.
| Probability | Value | Source |
|---|---|---|
| Prevalence of glaucoma | 1–5% | Assumption based on prevalence rates for general population and high prevalence subgroups5,14 |
| Progression to moderate glaucomaa | 0.129 | Progression data from GSM 20075,14 |
| Progression to severe glaucoma | 0.048 | Progression data from GSM 20075,14 |
| Progression to visual impaired | 0.042 | Progression data from GSM 20075,14 |
| Annual probability of having an eye test in current practice (not screening): | ||
| General population (adults over 40 years old) | 0.1728 | Based on survey of the UK public21 |
| General population (adults over 40 years old) | 0.0741 | Based on BHPS data and alternative assumptions22 |
| Individuals with diabetes | 0.1693 | Based on BHPS data22 |
| Individuals with eye problems | 0.1192 | Based on BHPS data22 |
| Individuals within low income households | 0.0653 | Based on BHPS data22 |
| Visual field based glaucoma staging5 | Mean defect score (dB) | |
| Mild glaucoma | −0·01 to −6·00 dB | |
| Moderate glaucoma | −6·01 to −12·00 dB | |
| Severe glaucoma | −12·01 to −20·00 dB | |
| Visual impairment (partial sight/blind) | −20·01 dB or worse | |
GSM: Glaucoma Screening Model; BHPS: British Household Panel Survey.
Visual field based glaucoma staging.
Table 2.
Data on screening tests and test performance.
| Probability | Value | Source |
|---|---|---|
| Current eye care | ||
| Optometry testing, sensitivity | 0.32 | GSM 20075 |
| Optometry testing, specificity | 0.99 | GSM 20075 |
| Proportion of normal (no glaucoma) with IOP < 26 mmHg | 0.96 | GSM 20075 |
| Proportion of glaucoma with IOP ≥ 26 mmHg | 0.35 | GSM 20075 |
| Screening tests | ||
| Optic nerve photography, sensitivity | 0.73 | GSM 20075 |
| Optic nerve photography, specificity | 0.89 | GSM 20075 |
| Perimetry (Frequency Doubling Technology-C-20-1), sensitivity | 0.79 | GSM 20075 |
| Perimetry (Frequency Doubling Technology-C-20-1) specificity | 0.94 | GSM 20075 |
IOP: intraocular pressure; GSM: Glaucoma Screening Model.
Base case analysis (inception cohort aged 40 estimated 1% glaucoma prevalence, screening every 10 years and taking a NHS perspective)
On average, screening the general population selected on age alone was not cost-effective at a £30,000 threshold (Table 3). It should be noted that as this is a population screening model we would expect a relatively small proportion of individuals to be identified with glaucoma and hence, on average for the population, only small gains in benefits.
Table 3.
Cost-effectiveness base case analysisa results.
| Screening acceptance (%) | Strategy | Cost | QALYs | ICERb |
|---|---|---|---|---|
| Current practice | £176 | 19.2530 | ||
| 30% | GPS IId (IOP + VF) | £239 | 19.2537 | 88,908 |
| GPS Id (IOP + ONP) | £239 | 19.2536 | (Dominated) | |
| GPS II (IOP + VF) | £266 | 19.2540 | 97,136 | |
| GPS I (IOP + ONP) | £276 | 19.2539 | (Dominated) | |
| Current practice | £176 | 19.2530 | ||
| 50% | GPS IId (IOP + VF) | £261 | 19.2539 | 74,408 |
| GPS 1d (IOP + ONP) | £261 | 19.2538 | (Dominated) | |
| GPS II (IOP + VF) | £304 | 19.2543 | 103,985 | |
| GPS I (IOP + ONP) | £321 | 19.2541 | (Dominated) | |
| Current practice | £176 | 19.2530 | ||
| 70% | GPS IId (IOP + VF) | £282 | 19.2541 | 68,718 |
| GPS 1d (IOP + ONP) | £283 | 19.2540 | (Dominated) | |
| GPS II (IOP + VF) | £342 | 19.2545 | 111,427 | |
| GPS I (IOP + ONP) | £366 | 19.2544 | (Dominated) |
QALY: quality adjusted life years; ICER: incremental cost-effectiveness ratio; IOP: intraocular pressure; GPS: Glaucoma screening Platform Study; VF: visual field.
Forty-year-old inception cohort, 17% uptake of current practice, 1% glaucoma prevalence, lifetime time horizon, NHS costs.
ICERs are related to the, on average, cheapest non-dominated strategy.
Sensitivity analysis
Screening start age and performance of current eye care
For cohorts starting at 50 and 60 years old, ICERs were, regardless of the level of screening uptake, well above the typically accepted threshold value of £30,000 (See Supplementary material online, Tables 4 and 5). Similar results were obtained when the sensitivity and specificity of case detection in current eye care were reduced to plausible minimum levels (See Supplementary material online, Tables 6 and 7).
Varying uptake of screening and current practice
Using alternative assumptions about the estimated uptake of current eye care services, varying from low annual uptake 6.5% (estimated uptake of eye care for low income households) to 17% (estimated uptake of eye care for people with diabetes) based upon BHPS data, and varying the assumed uptake of screening from 50% to 100%, the cost-effectiveness of screening improved, although the ICER remained well above a £30,000 threshold (See Supplementary material online, Tables 8 and 9).
Cost of sight impairment
Figure 1 shows the two-way sensitivity analyses results for the annual cost of sight impairment versus the percentage of screening uptake. That is, for each combination of cost of sight impairment and proportion of screening uptake, the figure which shows the strategy with the highest net-benefit (e.g. willingness to pay multiplied by the mean QALYs minus mean cost), assuming a £30,000 willingness to pay for a QALY.
For the illustrated cohort (inception cohort aged 50, assumed 1% glaucoma prevalence and 17% annual uptake of current practice21) none of the screening pathways would have a higher net-benefit compared with current practice, unless the annual cost of sight impairment is above £19,000. When the annual cost of sight impairment is above £30,000 screening is worthwhile if the uptake of screening is above 30% (Figure 1(a)).
For a higher risk subgroup (assumed glaucoma prevalence of 5%) a targeted screening programme (surveillance) could be worthwhile if the annual cost of sight impairment is above £3000 (Figure 1(b)).
Assuming a low uptake of current eye care (6.5% per year), as might be expected within a low income subgroup screening could be considered cost-effective if the annual cost of visual impairment is above £18,000 per year with screening uptake of 40% for a cohort with an assumed glaucoma prevalence of 1% (Figure 1(c)).
Enhancing current eye care
Increasing the uptake of current eye care for those in higher risk groups (>5% glaucoma prevalence), as opposed to a screening programme, is cost-effective when the annual cost of sight impairment is above £8000 and assuming no cost for a behavioural intervention to improve uptake. Increasing uptake rates would, however, be likely to incur costs. The cost per individual of increasing uptake would need to be <£20 with an annual cost of sight impairment per person of £20,000 for this strategy to approach cost-effectiveness.
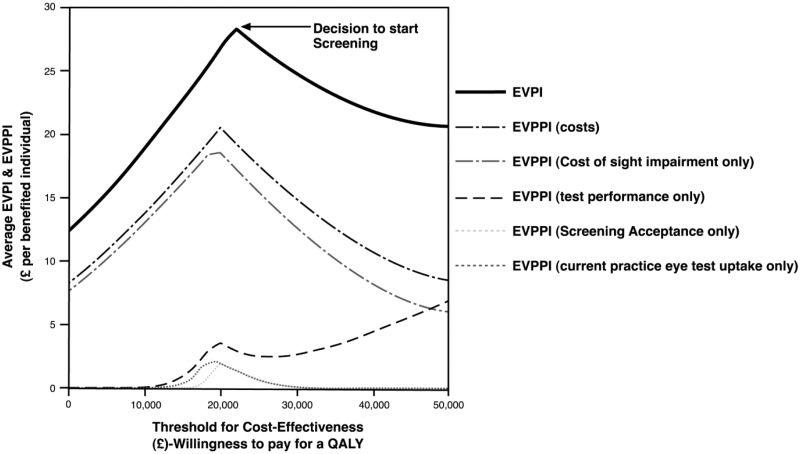
The cost of sight impairment is uncertain. This uncertainty was built into the probabilistic sensitivity analysis by investigating the impact of the annual cost of visual impairment being equally likely to have any value between £1000 and £40,000 (i.e. assuming a uniform distribution) and investigating the value of removing all the imprecision around parameter estimates within the model, i.e. the EVPI as well as the value of removing all uncertainty surrounding a particular (group of) parameter(s), i.e. EVPPI. Figure 2 shows the average EVPI and EVPPI curves for a 50-year-old cohort with a 5% prevalence rate of glaucoma (higher risk subgroup) and when uptake of current practice is 7.4% (described in Table 1). This situation was chosen as, based on the base case results, was the most likely scenario favouring screening. The value of removing the imprecision around the model parameter estimates is illustrated in Figure 2.
Figure 2.
Average expected value of perfect information (EVPI) and expected value of parameter perfect information (EVPPI).
Scenario: model start age (and screening) 50 years old, prevalence rate 5%, screening every 10 years, whole population, current practice annual eye test uptake rate 7.4%, average annual cost of sight impairment £20,500. The upper and lower bounds limits for this distribution were informed by the literature,27,28 assuming that NHS treatment as well as PSS cost were included. Incremental cost-effectiveness ratio for moving to screening (GPS1d (IOP + VF) = £21,720). The peak in EVPI corresponds to the uncertainty in the decision of changing from current practice (opportunistic case finding) to screening with a technician conducting tonometry and visual field test (perimetry) with screen positives examined by a specialized optometrist (GPS11d (IOP + VF)). EVPPI shown for selected parameters that contributed the most to decision uncertainty. IOP: intraocular pressure; GPS: Glaucoma screening Platform Study; PSS: personal social services; VF: visual field.
The peak in the EVPI curve corresponds to the willingness to pay for a QALY value where decision uncertainty is at its highest (at around £20,000 WTP) and is the decision point on whether to screen or not. For a willingness to pay threshold of £20,000 for a QALY, the population EVPI is around £107 million, with the costs of sight impairment contributing the most to this decision uncertainty (EVPPI, £74 million), followed by the uncertainty due to test performance (e.g. £14 million), and screening acceptance or current practice eye uptake rate (e.g. £8 million each).
Discussion
Main findings
Findings from the original Glaucoma Screening Model had suggested that glaucoma screening of a population selected on age is unlikely to be considered cost-effective at values for a QALY that society is typically willing to pay, but screening of high risk groups (prevalence of around 4%) might be. Across all the scenarios, we modelled the conclusion from the original evaluation is unchanged. Results were robust to all changes in the model parameter values except for the annual cost of sight impairment. When taking a wider perspective on the costs of sight impairment, findings from the two way sensitivity analysis, varying screening uptake and costs of sight impairment, suggest that assuming higher estimated costs of sight impairment screening the general population selected on age alone as opposed to surveillance of high risk groups might be worthwhile.
Data on the annual cost of sight impairment are very limited. The health costs of severe sight impairment due to glaucoma have been estimated as £800 per year (updated to 2010–2011 prices).27 For adults with physical disabilities, additional social care costs are estimated at £41,000 per year. These data include estimates from people with sight loss but data were not reported separately.24 The cost of sight impairment in the UK is reported by the Royal National Institute for the Blind as £12,457 per person per year.28 However, these calculations include indirect costs (productivity losses), cost due to lower employment, premature mortality, as well as £8782 corresponding to burden of disease cost – the use of such figures entails some double counting as some of these effects are captured within the QALY estimates. Estimates by Mead and Hyde suggest that the cost of blindness for another chronic eye condition, age related macular degeneration, range around £8000 (updated to 2010–2011 prices) for the first year of blindness but highlighted the limitations in the data to determine the true costs of failing eyesight.29
The purpose of updating the original Glaucoma Screening Model was to determine whether, given the newly defined screening pathways and target population, a large glaucoma screening RCT would be value for money in terms of informing policy decisions. The value of removing all uncertainties in the model was around £107 million, indicating that further research on glaucoma screening might be worthwhile. The uncertainty around cost of sight impairment contributed most followed by the uncertainty around screening test performance, and the uptake of screening or current eye care services.
These findings suggest that before proceeding to a large RCT evaluating a glaucoma screening or surveillance programme, further research to understand and quantify the cost of sight impairment is a priority. These data would be best collected within a prospective cohort study, following individuals through the spectrum of visual impairment and as needs change and adaptation to sight loss occurs.
The effectiveness of any screening or surveillance programme requires that the target population attend. Our findings suggest that behavioural intervention, such as a carefully worded invitation with a reminder or a more intensive intervention (e.g. tailored message, SMS reminder, buddy system) may improve attendance.21
Strengths and limitations
This study used a transparent iterative approach building on a robust evidence synthesis and sought views of all stakeholders to inform decisions regarding glaucoma screening. We used robust qualitative and quantitative methods to provide an evidence-based recommendation for future research. It was not within the scope of this study to estimate the accuracy of screening tests or to evaluate the accuracy of current eye care services in the detection of glaucoma. We used the same estimates of performance of community optometric detection of glaucoma (current eye care) as used in the original model, based on a survey in the late 1980s which may not represent current practice. To estimate screening test performance we used estimates from an evidence synthesis but these were based on evidence from studies with heterogeneous populations and high risk of bias.
We had limited data on the uptake of current eye care services by higher risk groups, particularly by ethnic minority groups and those who might expect to have a higher uptake of current eye care, those with a family history of glaucoma. We did however use new primary data from our survey on eye testing within the last few years and used data from the BHPS that is considered to be representative.
The value for information analysis can only capture those uncertainties incorporated into the economic model and is dependent on the model structure. The model did not consider the potential harms due to screening (e.g. anxiety for those with false positives) or the side effects of treatment (e.g. cataracts). While it is unlikely that these omissions would have a major impact on the model results, they are a limitation of the analysis. Finally, while the EVPI analysis constitutes a necessary condition in determining whether further research is worthwhile it is not in itself sufficient to determine whether research should be conducted (although it can give a strong indication that further research is not worthwhile, if for example EVPI is less than the costs of conducting further research). A positive expected net-benefit of sampling (ENBS) constitutes a necessary and sufficient condition to conduct further research. ENBS is the difference between the benefits of reducing uncertainty with a particular sample size study and the cost of obtaining that sample size. Unfortunately, ENBS can be obtained only under very restrictive assumptions and is often computationally prohibitive and there have been few examples where it has been used in practice to inform, for example, the sample size of a trial.30 In such circumstances, we believe the EVPI establishes a first step to inform the judgment that further research is potentially worthwhile.
Conclusions
A glaucoma screening trial is currently unlikely to be the best use of research resources to inform policy decisions on screening policy in the UK. Further research to quantify the health and personal social services costs of sight impairment is recommended. Further development of glaucoma tests and an evaluation of a behavioural intervention to improve attendance for those who do not use eye care services would be worthwhile. Our findings are UK specific, but the methods used and the modelling framework can be adapted and populated with country specific parameters.
Supplementary Material
Acknowledgements
We thank John Norrie, Alexandra Green, and Jemaima Che-Hamzah for their contribution to the design of the project; members of the advisory group - Rustom Bativala, David Crabb, David Garway-Heath, Roger Hitchings, Stephen McPherson, Anja Tuulonen, Ananth Viswanathan, Heather Waterman, and Richard Wormald.
Funding
This paper was developed from a strategic grant funded by the Medical Research Council (project reference G0701759). The Health Services Research Unit and the Health Economics Research Unit are both core funded by the Chief Scientist Office of the Scottish Government Health Directorates. The views expressed in this report are those of the authors and not necessarily those of the funders. Data sets and models generated during this study are available on request.
Supplementary Material
The online tables are available at http://hsr.sagepub.com/supplemental-data
References
- 1.Bunce C, Xing W, Wormald R. Causes of blind and partial sight certifications in England and Wales: April 2007-March 2008. Eye (Lond) 2010; 24: 1692–1699. [DOI] [PubMed] [Google Scholar]
- 2.Pascolini D and Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol 2012; 96(5): 614–618. [DOI] [PubMed]
- 3.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol 2006; 90: 262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maier P, Funk J, Schwarzer G, et al. Treatment of ocular hypertension and open angle glaucoma: meta-analysis of randomised controlled trials. BMJ 2005; 331: 134–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burr J, Mowatt G, Siddiqui MAR, et al. The clinical and cost effectiveness of screening for open angle glaucoma: a systematic review and economic evaluation. Health Technol Assess 2007; 11: iii–170. [DOI] [PubMed] [Google Scholar]
- 6.Grant W, Burke J. Why do some people go blind from glaucoma. Ophthalmology 1982; 89: 991–998. [DOI] [PubMed] [Google Scholar]
- 7.Chen PP. Blindness in patients with treated open-angle glaucoma. Ophthalmology 2003; 110: 726–733. [DOI] [PubMed] [Google Scholar]
- 8.Fraser S, Bunce C, Wormald R, et al. Deprivation and late presentation of glaucoma: case-control study. BMJ 2001; 322: 639–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ng WS, Agarwal PK, Sidiki S, et al. The effect of socio-economic deprivation on severity of glaucoma at presentation. Br J Ophthalmol 2010; 94: 85–87. [DOI] [PubMed] [Google Scholar]
- 10.Sukumar S, Spencer F, Fenerty C, et al. The influence of socioeconomic and clinical factors upon the presenting visual field status of patients with glaucoma. Eye (Lond) 2009; 23: 1038–1044. [DOI] [PubMed] [Google Scholar]
- 11.Safer MA, Tharps QJ, Jackson T, et al. Determinants of three stages of delay in seeking care at a medical clinic. Med Care 1979; 17: 11–29. [DOI] [PubMed] [Google Scholar]
- 12.Andersen BL, Cacioppo JT. Delay in seeking a cancer diagnosis: delay stages and psychophysiological comparison processes. Br J Soc Psychol 1995; 34(Pt 1): 33–52. [DOI] [PubMed] [Google Scholar]
- 13.Evans J, Ziebland S, McPherson A. Minimizing delays in ovarian cancer diagnosis: an expansion of Andersen’s Model of ‘total patient delay’. Fam Pract 2007; 24: 48–55. [DOI] [PubMed] [Google Scholar]
- 14.Hernandez RA, Burr JM, Vale LD. OAG screening project G. Economic evaluation of screening for open-angle glaucoma. Int J Technol Assess Health Care 2008; 24: 203–211. [DOI] [PubMed] [Google Scholar]
- 15.Flanders WD, Longini IM. Estimating benefits of screening from observational cohort studies. Stat Med 1990; 9: 969–980. [DOI] [PubMed] [Google Scholar]
- 16.Hatt S, Wormald R, Burr J. Screening for prevention of optic nerve damage due to chronic open angle glaucoma. Cochrane Database Syst Rev 2006; (4): 006129–006129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burr J, Francis J, Azuara-Blanco A, et al. Developing the intervention and outcome components of a proposed randomised controlled trial (RCT) of a National Screening Programme for Open Angle Glaucoma (OAG), 2008. www.abdn.ac.uk/aura (accessed 1 April 2013).
- 18.Craig P, Dieppe P, Macintyre S, et al. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008; 337: a1655. [DOI] [PMC free article] [PubMed]
- 19.Campbell SE, Azuara-Blanco A, Campbell MK, et al. Developing the specifications of an open angle glaucoma screening intervention in the United Kingdom: a Delphi approach. BMC Health Serv Res 2012; 12: 447, 6963–12-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burr JM, Campbell MK, Campbell SE, et al. Glaucoma screening Platform Study group. Developing the clinical components of a complex intervention for a glaucoma screening trial: a mixed methods study. BMC Med Res Methodol 2011; 11: 54–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prior M, Burr JM, Ramsay CR, et al. Evidence base for an intervention to maximise uptake of glaucoma testing: a theory-based cross-sectional survey. BMJ Open 2012; 2(2): e000710–e000710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.British Household Panel Survey (BHPS). Institute for Social & Economic Research, University of Essex. http://www.iser.essex.ac.uk/bhps (accessed December 2011).
- 23.Euroqol Group. EuroQol - a new facility for the measurement of health related quality of life. Health Policy 1990; 16: 199–208. [DOI] [PubMed] [Google Scholar]
- 24.Personal social services expenditure and unit costs England, 2009-10. http://www.ic.nhs.uk/webfiles/publications/009_Social_Care/pss0910expfinal/pss0910expfinal_update_070311/Personal_Social_Services_Expenditure_Report%202009_10.pdf (accessed December 2011).
- 25.Sheeran P. Intention-behavior relations: a conceptual and empirical review. In: Stroebe W and Hewstone M (eds) European review of social psychology. Oxford: John Wiley & Sons Ltd, 2002, pp.1–36.
- 26.Briggs A, Claxton K and Sculpher M. Decision modelling for health economic evaluation. Oxford University Press, 2006.
- 27.Traverso CE, Walt JG, Kelly SP, et al. Direct costs of glaucoma and severity of the disease: a multinational long term study of resource utilisation in Europe. Br J Ophthalmol 2005; 89: 1245–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Future sight loss UK 1: Economic impact of partial sight and blindness in the UK adult population. http://www.rnib.org.uk/aboutus/Research/reports/prevention/Pages/fsluk1.aspx (accessed December 2011).
- 29.Meads C, Hyde C. What is the cost of blindness? Br J Ophthalmol 2003; 87: 1201–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cook J, Hislop J, Adewuyi T, et al. Assessing methods to specify the target difference for a randomised controlled trial – DELTA (Difference ELicitation in TriAls) review. Health Technol Assess. In press. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.