Abstract
Introduction
Mobile phone technology may be a cost-effective and convenient way to deliver proven weight-loss interventions and thereby prevent or delay onset of type 2 diabetes. The purpose of this study was to examine the feasibility and efficacy of a diabetes prevention intervention combined with a mobile app and pedometer in English-speaking overweight adults at risk for type 2 diabetes.
Design
RCT.
Participants
Participants included 61 overweight adults with a mean age (SD) of 55.2 (9.0) years. Seventy-seven percent were women, 48% were racial/ethnic minorities, and baseline BMI was 33.3 (6.0).
Intervention
The curriculum was adapted from the Diabetes Prevention Program, with the frequency of in-person sessions reduced from 16 to six sessions and group exercise sessions replaced by a home-based exercise program. A study-developed mobile phone app and pedometer augmented the intervention and provided self-monitoring tools.
Main outcome measure
Weight loss.
Results
Data were collected in 2012 and 2013 and were analyzed in 2014. In intention-to-treat analyses, the intervention group (n=30) lost an average of 6.2 (5.9) kg (−6.8% [5.7%]) between baseline and 5-month follow-up compared to the control group’s (n=31) gain of 0.3 (3.0) kg (0.3% [5.7%]) (p<0.001). The intervention group’s steps per day increased by 2,551 (4,712) compared to the control group’s decrease of 734 (3,308) steps per day (p<0.001). In comparison, the intervention group had greater reductions in hip circumference (p<0.001); blood pressure (p<0.05); and intake of saturated fat (p=0.007) and sugar-sweetened beverages (p=0.02). The intervention had no significant effect on fasting lipid or glucose levels.
Conclusions
The significant weight loss resulting from this modified combined mobile app and pedometer intervention for overweight adults warrants further investigation in a larger trial.
Introduction
Obesity is an independent and modifiable risk factor for developing type 2 diabetes. Given the worldwide epidemic of obesity and diabetes, prevention of these conditions is a public heath priority. Lifestyle modification programs that result in modest weight loss (5%–10%) by increasing physical activity and reducing caloric and fat intake have been shown to be effective in preventing or delaying the onset of type 2 diabetes.1–3 However, these labor-intensive programs have been expensive to implement and sustain over longer periods of time in clinical and community settings.4
One way to reduce the costs of such programs is to utilize digital technology, such as smartphones and mobile apps, which are popular channels of communication worldwide. Approximately 90% of adults in the U.S. already own a mobile phone, and 58% own a smartphone.5 Use of smartphones and mobile apps has grown exponentially,6 particularly among middle and older age groups and racial/ethnic minorities. These populations are also disproportionately impacted by obesity and type 2 diabetes. Utilizing these communication technologies for the delivery of diabetes prevention interventions has the potential to reduce not only costs but also the time and transportation barriers of traditional programs, thereby reaching a larger segment of the target population.
Despite the potential of smartphones and mobile apps and growing interest in their utilization among the public and researchers, relatively few randomized controlled clinical trials (RCTs) have been published that examine the efficacy of these technologies.7 Thus, the aim of this RCT was to evaluate the feasibility and short-term efficacy of a diabetes prevention intervention enhanced with a mobile phone app among overweight English-speaking adults at risk for developing type 2 diabetes. The primary purpose of the intervention was to assist those adults to achieve moderate weight reduction over the 5-month study period.
Methods
This feasibility RCT with parallel groups was approved by the University of California, San Francisco, Committee on Human Research prior to participant enrollment. All participants provided written informed consent. Participants were recruited from May 2012 to March 2013 at primary care clinics and by posting study flyers in San Francisco and Berkeley, California.
Initial eligibility was assessed by telephone, and final eligibility was confirmed by laboratory testing and in-person screening at the screening/baseline visit. Inclusion criteria were as follows: BMI ≥25 (BMI ≥23 for Asian-Pacific Islanders)8; age ≥35 years; high risk for diabetes (diabetes risk test score ≥5 points,9 fasting plasma glucose [FPG] of 100–125 mg/dL, hemoglobin A1c [HbA1c] of 5.7%–7.0%, or oral glucose tolerance test [OGTT] of 140–200 mg/dL); being physically inactive at work or during leisure time as assessed by the Stanford Brief Activity Survey10; being an English speaker; and willingness to use a mobile phone every day if randomized to the intervention. Exclusion criteria were as follows: self-reported diagnosis of diabetes or other disease associated with disordered glucose metabolism (e.g., suboptimally treated thyroid disease); self-reported medical condition or other physical problem necessitating special attention in an exercise or diet program (e.g., eating disorder, uncontrolled hypertension); current participation in a lifestyle modification program or research study; cognitive impairment (screened by the Mini-Cog test)11,12; planning a trip outside the U.S. during the 5-month study period; planning to have gastric bypass surgery; self-reported use of antibiotics, anti-tuberculosis agents, phenytoin, amphetamines, or prescription weight-loss drugs; known severe hearing or speech problems; or self-report of being currently pregnant. It is important to note that both non-mobile phone users and phone users were recruited if they met all inclusion criteria. If a participant did not have an iPhone, one was provided for the study.
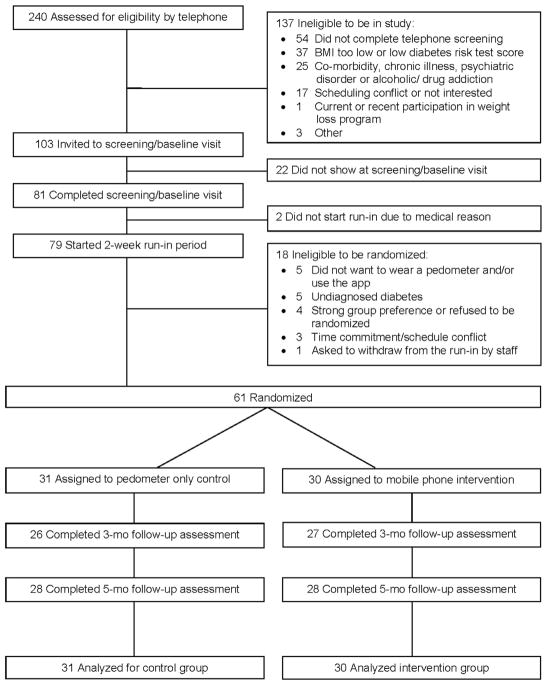
As shown in Figure 1, a total of 240 potential participants were screened for initial eligibility by telephone, and 81 potentially qualified participants attended a screening/baseline visit, two of whom were subsequently excluded owing to uncontrolled hypertension.
Figure 1.
Screening, randomization, and assessments of study participants.
Run-In Period
A total of 79 participants were eligible to start a 2-week run-in period after completing the screening/baseline visit. The run-in period had three purposes: (1) to screen out participants who already had type 2 diabetes according to fasting blood test; (2) to determine if participants were able to comply with the requirements of using the mobile phone app; and (3) to collect baseline average daily steps (physical activity). The research team created a run-in mobile phone app for this phase of the study. This mobile app was designed to mimic the intervention mobile app, but without any specific content to encourage or support behavioral changes (e.g., no goals or goal-oriented messages). During the 2-week run-in period, all participants were asked to use the run-in mobile app daily and wear an Omron Active Style Pro HJA-350IT pedometer (with triaxial accelerometer) on their waist from the time they arose in the morning until the time they retired at night. Step counts were not displayed on the pedometer during the run-in period.
Randomization
Prior to the trial, the study statistician generated the random allocation sequence, and a staff member who was not involved in the study prepared the sealed envelopes. A permuted block randomization method with randomly selected block sizes of two and four was used, and participants were stratified by fasting plasma glucose levels of ≥100 mg/dL or <100 mg/dL measured at baseline. Using sealed envelopes, research staff randomized 61 participants who completed the 2-week run-in period to either the 5-month diabetes prevention intervention with mobile app and pedometer or to a pedometer-only control (allocation ratio, 1:1), subsequently referred to as the intervention and control groups.
Control
Participants assigned to the control group (n=31) continued using the Omron pedometer, but the settings were changed to display the number of steps. No specific step goals were provided. Research staff removed the run-in mobile app from the participant’s iPhone or collected the iPhone if one had been provided. Each control participant also received a National Institute of Diabetes and Digestive and Kidney Diseases brochure about pre-diabetes.13 Control participants continued to receive standard medical care and attended the outcome assessment visits at 3 and 5 months, but did not receive any intervention.
Intervention
The intervention, called the Mobile Phone–Based Diabetes Prevention Program (mDPP), was modified from the original DPP curriculum1 for overweight adults at risk for type 2 diabetes. The goal of the mDPP intervention was to achieve 10% body weight loss over 5 months, at a pace of 1–2 pounds per week by increasing physical activity, reducing caloric intake, and lowering fat intake. The intervention lasted 5 months and consisted of two delivery modes: in-person and through the mobile app. Two trained, non-medical research staff members delivered a core curriculum consisting of six in-person sessions at randomization and at 0.5-, 1-, 2-, 3-, and 4-month visits (Table 1). The mDPP mobile app, developed by the research team for iOS (iPhone), was used to supplement the in-person sessions and was intended to enhance their effect. The mobile app included electronic diaries for self-monitoring of weight, activity, and caloric intake, with daily reminders to enter this information. To reinforce the domains addressed in the in-person sessions, the mobile app was also used to deliver interactive intervention content through daily messages, video clips, and quizzes. For example, on Day 5 of Study Week 4, participants received the following message: Have you let everyone around you know that you are trying to become more active so they can help you meet your goal? If the participant responded no using the keypad, the next screen displayed: Let others know your physical activity goal. If yes was selected, the screen displayed: Nice work! Responding to the daily message took 1–2 minutes.
Table 1.
Description of Intervention Components
| Format/schedule | Description of intervention components | |
|---|---|---|
| In-person lifestyle intervention sessions adapted from the DPP triala | ||
| Session 1 | 0 month Randomization | Welcome to the Mobile Phone–Based Weight Loss Program and Setting Short- and Long-term Goals Getting Started Being Active Getting Started Losing Weight Being Active: A Way of Life Jump Start Your Activity Plan |
| Session 2 | 0.5 month | Be a Fat Detective Three Ways to Eat Less Fat |
| Session 3 | 1 month | Healthy Eating Tip the Calorie Balance |
| Session 4 | 2 months | Take Charge of What’s Around You The Four Keys to Healthy Eating Out Make Social Cues Work for You |
| Session 5 | 3 months | Problem Solving Talk Back to Negative Thoughts |
| Session 6 | 4 months | The Slippery Slope of Lifestyle Change Ways to Stay Motivated |
| iPhone with mDPP trial app | ||
| Mobile intervention | Daily messages/video clips reinforced the domains addressed in the brief in-person sessions above. A pre-programmed daily message or video clip was automatically sent at 11AM and was accessible on the mobile phone until 7PM. If no reply was made by this time, the daily message disappeared from the phone. An automated reminder was generated at 3PM to alert participants who had not yet responded to the message. Short educational video clips and texts were also available 24 hours per day, 7 days per week (e.g., What is pre-diabetes?) | |
| Mobile weight diary and activity/caloric diary | The weight diary was available 7AM to 7PM every day. Participants were asked to report their weight at least twice (Mondays and Fridays) per week. The activity and caloric diary was accessible 7PM to 11:59PM. If no entry was made by 8:30PM, an automated text message was generated as a reminder for the participant to record the total number of steps per day, the types and duration of physical activities, and total daily caloric intake. Tailored feedback was provided based on their diary entry. |
|
| Physical Activity | ||
| Omron Active Style Pro HJA-350IT pedometer (a triaxial accelerometer) | A home-based physical activity plan was developed for each participant. Daily brisk walking (moderate physical activity) was emphasized and encouraged. Short-term goals were based on 20% increases in step counts each week, with a long-term goal of maintaining 12,000 steps/day. The pedometer facilitated self-monitoring of daily step counts. |
|
The intervention content is based on the original Diabetes Prevention Program (DPP) with 16 sessions over 24 weeks (www.bsc.gwu.edu/dpp/lifestyle/dpp_part.html). Two components of the original DPP (Move Those Muscles and You Can Manage Stress) were included as part of the mobile phone–delivered video clips and daily messages. The original DPP also included group-based exercise programs, but these were replaced with home-based exercise programs in mDPP.
mDPP, Mobile Phone–Based Diabetes Prevention Program.
Intervention participants (n=30) had the run-in mobile app replaced with the intervention (mDPP) mobile app. As with control participants, the intervention group received an Omron pedometer, but only intervention participants received the tailored diabetes prevention program. Diet and physical activity assessments conducted at baseline and during the run-in period were used to tailor the mDPP to each intervention participant by providing individualized short- and long-term goals. Once research staff registered the participant’s average daily steps during the 2-week run-in period in the intervention mobile app, it automatically displayed individualized short-term goals with 20% increases in step counts each week and encouragement for moderate-intensity physical activities (e.g., brisk walking). Each intervention participant’s long-term goal was to increase and maintain their step counts to 12,000 steps per day.14 The intervention also included goals for achieving healthy reductions in total caloric and fat intake based on the DPP recommendations. In addition, intervention participants were advised to reduce consumption of sugar-sweetened beverages by replacing them with non-sweetened alternatives.
Outcome Measures
The primary outcomes were percentage change in weight and BMI from baseline to 5-month follow-up. Secondary outcomes included other clinical indicators (hip circumference, blood pressure, lipid profile, and glucose levels), as well as objectively measured (via pedometer) physical activity. Self-reported caloric intake and fat intake were assessed with the Block Food Frequency Questionnaire.15 Although the intervention app included a calorie diary, these data were not available for the control group, and thus were not used as a dietary outcome measure. Additional self-report measures included the 7-Day Physical Activity Recall (PAR)16; Self-Efficacy for Physical Activity Survey17; Social Support and Exercise Survey18; Barriers to Being Active Quiz19; and Center for Epidemiologic Studies Depression Scale (CES-D).20
Research staff collected outcome data at baseline and 3-and 5-month follow-ups at the research office. Participants were asked to change into a hospital gown at each outcome assessment visit. Weight was measured with a Tanita WB-110 digital electronic scale, and height was measured at baseline with a standard stadiometer. Hip circumference and blood pressure were measured at each visit using standard protocols. Recorded pedometer data were downloaded to a study computer at each visit. Pedometer data were analyzed as both steps per day and steps per hour to account for possible differences in pedometer wearing time between groups or over time. Data for each week were only included if the pedometer was worn for at least 8 hours per day and at least 4 days per week. Pedometer wearing time was calculated as the number of hours recording at least one step. The Omron Active Style Pro HJA-350IT pedometer measures activity intensity over 60-second epochs and estimates the METs of activity throughout the day. Pedometer measurements were used to calculate minutes of light (1–2 METs); moderate (3–5 METs); and vigorous (6–8 METs) activity. Fasting blood was drawn to obtain lipid and glucose levels during the run-in period and at the 5-month visit. Possible adverse events were assessed at each visit.
Statistical Analysis
A target sample size of 30 participants per group was selected to provide 80% power in two-sided tests to detect between-group differences in change in body weight of 0.25 SD in the planned analysis, adjusting for baseline values and accounting for correlation of baseline and follow-up values, as well as an expected 10% loss to follow-up. Baseline differences between groups were assessed using t tests and chi-square tests, as appropriate. Between-group differences in primary and secondary outcomes were evaluated in intention-to-treat analyses using linear mixed models (LMMs). The fixed effects in each LMM included the baseline value of the outcome of interest, group, time (3 or 5 months), and group-by-time interaction. Random intercepts and slopes were used to account for within-subject correlation of the repeated responses, with robust SEs as needed. Marginal adjusted means with 95% CIs were obtained from the LMMs. Sensitivity analyses were conducted to obtain adjusted estimates of the effect of treatment on primary study outcomes for baseline covariates that differed between groups at p<0.05 and to evaluate the effect of missingness. Mediation analyses21 were conducted to determine the extent to which the effect of the treatment group on weight loss was mediated by factors such as the change in physical activity and dietary intake. Of the 61 participants, 53 (87%) had study-measured weights at 3 months and 56 (92%) at 5 months (Figure 1). The LMM treatment effect estimates are consistent under the assumption that the data are missing at random, given the covariates and outcomes included in each model, provided the fixed and random effects are properly specified. All analyses were conducted in 2014 using Stata, version 13.
Results
Baseline sample characteristics are presented in Table 2. Mean participant age (SD) was 55.2 (9.0) years, 77% were female, and 48% self-identified as belonging to an ethnic minority group. Mean BMI was 33.3 (6.0) kg/m2, mean FPG was 95.9 (7.7) mg/dL, and 30% had an FPG value ≥100 mg/dL. The 61 randomized participants did not differ from the 20 non-randomized participants with respect to age, BMI, past participation in a weight-loss program, or mobile phone use. However, randomized participants were more likely than non-randomized participants to be female (77% vs 50%, p=0.022) and were slightly more likely to own an iPhone (49% vs 25%, p=0.058). No differences in baseline characteristics were observed between participants in the intervention and control groups, except for the family social support for physical activity questionnaire, on which intervention participants had lower mean scores than control participants (p=0.02). Participant retention rate in the intervention group was 90% at 3 months and 93% at 5 months, which did not differ significantly from controls (84% at 3 months and 90% at 5 months) (Figure 1).
Table 2.
Baseline Characteristics
| All participants (n=61) | Control (n=31) | Intervention (n=30) | p-value | |
|---|---|---|---|---|
| Age (years; M±SD [range]) | 55.2±9.0 (36–76) | 53.4±8.7 (36–65) | 57.1±9.1 (36–76) | 0.11 |
| % (n) | % (n) | % (n) | ||
| Race/ethnicitya | 0.14 | |||
| Asian | 22.9 (14) | 12.9 (4) | 33.4 (10) | |
| Black/African American | 4.9 (3) | 9.7 (3) | 0.0 (0) | |
| Hispanic/Latino | 11.5 (7) | 9.7 (3) | 13.3 (4) | |
| White (non-Hispanic) | 52.5 (32) | 61.3 (19) | 43.3 (13) | |
| More than 1 race | 8.2 (5) | 6.4 (2) | 10.0 (3) | |
| Gender | 0.94 | |||
| Male | 23.0 (14) | 22.6 (7) | 23.3 (7) | |
| Female | 77.0 (47) | 77.4 (24) | 76.7 (23) | |
| Annual household income | 0.38 | |||
| ≤$40,000 | 13.1 (8) | 6.4 (2) | 20.0 (6) | |
| $40,001–$75,000 | 16.4 (10) | 12.9 (4) | 20.0 (6) | |
| >75,000 | 54.1 (33) | 64.5 (20) | 43.3 (13) | |
| Decline to state | 11.5 (7) | 12.9 (4) | 10.0 (3) | |
| Don’t know | 4.9 (3) | 3.2 (1) | 6.7 (2) | |
| Marital status | 0.43 | |||
| Never married | 27.9 (17) | 25.8 (8) | 30.0 (9) | |
| Currently married/cohabitating | 57.4 (35) | 64.5 (20) | 50.0 (15) | |
| Divorced/widowed | 14.7 (9) | 9.7 (3) | 20.0 (6) | |
| Fasting blood glucose (mg/dL) | 0.58 | |||
| <100 | 70.5 (43) | 74.2 (23) | 66.7 (20) | |
| ≥100 | 29.5 (18) | 25.8 (8) | 33.3 (10) | |
| HbA1cb | 0.18 | |||
| <5.7% (<39 mmol/mol) | 38.3 (23) | 46.7 (14) | 30.0 (9) | |
| ≥5.7% (>39 mmol/mol) | 61.7 (37) | 53.3 (16) | 70.0 (21) | |
| Family history of diabetes | >0.99 | |||
| No | 13.1 (8) | 12.9 (4) | 13.3 (4) | |
| Yes | 83.6 (51) | 83.9 (26) | 83.3 (25) | |
| Don’t know | 3.3 (2) | 3.2 (1) | 3.4 (1) | |
| Prior use of a commercial weight-loss program | 0.24 | |||
| No | 32.8 (20) | 25.8 (8) | 40.0 (12) | |
| Yes | 67.2 (41) | 74.2 (23) | 60.0 (18) | |
| Phone used for the study | 0.52 | |||
| iPhone provided by the study | 49.2 (30) | 45.2 (14) | 53.3 (16) | |
| Personal iPhone | 50.8 (31) | 54.8 (17) | 46.7 (14) |
Race/ethnicity was self-reported.
n=60 for HbA1c due to one missing value in the control group.
HbA1c, glycosylated hemoglobin.
Intention-to-treat analyses indicated that intervention participants lost an average of 6.8% (5.7%) of their body weight between baseline and 5-month follow-up, compared to a gain of 0.3% (5.7%) among controls (p<0.001) (Table 3). These changes reflected an absolute decrease of 6.2 (5.9) kg in the intervention group relative to a slight gain of 0.3 (3.0) kg among controls. The net difference in weight change between the groups was 6.9 kg (95% CI=4.6, 9.1 kg). Similarly, mean BMI decreased in the intervention group with almost no change among controls (p<0.001). Sensitivity analyses indicated that missing data and baseline differences in family support had no material effect on these results. The proportion of participants who achieved the goal of losing at least 10% of their baseline body weight was 29% (n=8) in the intervention group versus 0% in the control group (p=0.002). Forty-three percent (n=12) of the intervention participants achieved a clinically significant weight loss of at least 7% reduction in body weight1 versus 0% of the control group participants (p<0.001).
Table 3.
Primary Outcomes and Other Diabetes Risk Factors Over 5 Months
| Variable | Control (M±SD [range or n]) | Intervention (M±SD [range or n]) | p-value | Overall p-valuea |
|---|---|---|---|---|
| Primary outcomes | ||||
| Baseline weight (kg) | 93.1±21.8 (61–131) | 86.2±18.5 (60–143) | 0.18b | |
| Change in weight (kg) | <0.001 | |||
| Baseline to 3-month | 0.4±1.8 (26) | −5.2±4.4 (27) | <0.001c | |
| Baseline to 5-month | 0.3±2.7 (28) | −6.2±5.9 (28) | <0.001c | |
| Percent change in weight (%) | <0.001 | |||
| Baseline to 3-month | 0.3±2.0 (26) | −5.8±4.1 (27) | <0.001c | |
| Baseline to 5-month | 0.3±3.0 (28) | −6.8±5.4 (28) | <0.001c | |
| Baseline BMI (kg/m2) | 33.3±6.0 (26–47) | 32.2±5.6 (24–54) | 0.45b | |
| Change in BMI (kg/m2) | <0.001 | |||
| Baseline to 3-month | 0.2±0.7 (26) | −2.0±1.7 (27) | <0.001c | |
| Baseline to 5-month | 0.1±1.0 (28) | −2.2±2.2 (28) | <0.001c | |
| Percent change in BMI (%) | <0.001 | |||
| Baseline to 3-month | 0.4±2.0 (26) | −5.9±4.2 (27) | <0.001c | |
| Baseline to 5-month | 0.3±3.0 (28) | −6.6±5.7 (28) | <0.001c | |
| Other diabetes risk factors | ||||
| Hip circumference (cm) | <0.001 | |||
| Baseline | 117.1±15.0 (31) | 114.1±12.7 (30) | 0.39d | |
| 3-month | 116.0±14.7 (26) | 110.6±11.9 (27) | 0.04d | |
| 5-month | 115.9±14.4 (28) | 108.8±10.9 (28) | 0.008d | |
| Mean resting blood pressure | ||||
| SBP (mmHg) | 0.01 | |||
| Baseline | 128.3±14.3 (31) | 125.7±13.8 (30) | 0.47d | |
| 3-month | 123.3±13.2 (25) | 124.5±16.1 (27) | 0.50d | |
| 5-month | 129.5±12.1 (28) | 121.1±11.1 (28) | 0.03d | |
| DBP (mmHg) | 0.009 | |||
| Baseline | 81.4±11.6 (31) | 79.6±9.7 (30) | 0.52d | |
| 3-month | 77.4±8.2 (25) | 75.8±12.3 (27) | 0.73d | |
| 5-month | 80.2±8.1 (28) | 73.7±7.9 (28) | 0.003d | |
| Lipid panel* | ||||
| Triglycerides (mg/dL) | 0.40 | |||
| Baseline | 115.2±67.4 (30) | 132.0±63.3 (30) | 0.48d | |
| 5-month | 122.5±73.2 (27) | 118.7±55.5 (28) | 0.84d | |
| Total cholesterol (mg/dL) | 0.29 | |||
| Baseline | 204.4±33.9 (31) | 201.4±30.6 (30) | 0.72d | |
| 5-month | 202.9±38.3 (27) | 190.1±28.2 (28) | 0.26d | |
| LDL (mg/dL) | 0.58 | |||
| Baseline | 128.7±28.1 (30) | 122.0±26.0 (30) | 0.44d | |
| 5-month | 123.7±33.9 (27) | 113.9±26.2 (28) | 0.36d | |
| HDL (mg/dL) | 0.30 | |||
| Baseline | 50.7±15.5 (31) | 52.9±13.4 (30) | 0.57d | |
| 5-month | 55.2±18.8 (27) | 53.5±13.3 (28) | 0.94d | |
| Glucose | ||||
| HbA1c (%) | 0.25 | |||
| Baseline | 5.70±0.27 (30) | 5.83±0.31 (30) | 0.09d | |
| 5-month | 5.66±0.29 (27) | 5.73±0.36 (28) | 0.49d | |
| Fasting plasma glucose (mg/dL) | 0.63 | |||
| Baseline | 95.9±7.7 (31) | 98.9±10.5 (30) | 0.23d | |
| 5-month | 96.2±10.3 (27) | 98.5±10.5 (28) | 0.34d | |
Note: Boldface indicates statistical significance (p<0.05).
Overall p-values based on linear mixed models for changes from baseline to 3 and 5 months, adjusting for baseline.
p-values for baseline group comparisons of weight and BMI are based on simple regression analyses.
p-values for time-specific comparisons of changes in weight and BMI based on linear mixed models for changes from baseline to 3 and 5 months, adjusting for baseline.
p-values for time-specific comparisons of other diabetes risk factors based on linear mixed models for baseline, 3-month, and 5-month outcomes.
DBP, diastolic blood pressure; HbA1c, glycosylated hemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SBP, systolic blood pressure
The intervention also led to reductions in hip circumference (p<0.001) and both systolic (p=0.01) and diastolic (p=0.009) blood pressure, with little change observed among controls (Table 3). Although not statistically significant in this small sample, the intervention group had clinically meaningful22 improvements in triglycerides, total cholesterol, and low-density lipoprotein cholesterol. Mean fasting plasma glucose and HbA1c levels were close to normal at baseline and were not affected by the intervention (Table 3).
Participants wore the pedometer at least 8 hours per day and 4 days per week for an average of 87.3% (18.3%) of the 20 study weeks (range = 2–20 weeks), with no significant difference between the intervention and control groups. Based on the pedometer data, the intervention led to a marked increase in physical activity (Table 4). Intervention participants increased their daily step count by a mean of 2,551 (4,712) steps (a 38% increase) compared with a mean decrease of 734 (3,308) steps (an 11% decrease) among controls (p=0.02). The intervention increased moderate levels of physical activity (p<0.001) but had less impact on light and vigorous levels of physical activity, which were not intervention targets. Sensitivity analyses indicated that missing data and baseline differences in family support had no material effect on these results.
Table 4.
Objectively Recorded Physical Activity Using Omron HJ350 Pedometer Over 5 Months
| Variable | Control (n=31) (M±SD) | Intervention (n=30) (M±SD) | p-value | Overall p-value |
|---|---|---|---|---|
| Steps (objectively measured by pedometer) | ||||
| Mean daily steps | ||||
| Baseline | 6,577±4,460 | 6,761±3,572 | ||
| Change in mean daily steps | <0.001 | |||
| Baseline to Month 1 (Week 1–4) | 378±3,507 | 2,564±3,864 | <0.001 | |
| Baseline to Month 2 (Week 5–8) | −288±3,227 | 3,119±4,753 | <0.001 | |
| Baseline to Month 3 (Week 9–12) | −125±3,451 | 2,888±4,998 | <0.001 | |
| Baseline to Month 4 (Week 13–16) | 20±3,847 | 2,604±4,337 | <0.001 | |
| Baseline to Month 5 (Week 17–end) | −734±3,308 | 2,551±4,712 | <0.001 | |
| Mean steps per hour | ||||
| Baseline | 485±320 | 499±275 | ||
| Change in mean steps per hour | <0.001 | |||
| Baseline to Month 1 (Week 1–4) | 46±269 | 131±253 | 0.001 | |
| Baseline to Month 2 (Week 5–8) | 1±229 | 161±292 | <0.001 | |
| Baseline to Month 3 (Week 9–12) | 9±251 | 151±321 | 0.004 | |
| Baseline to Month 4 (Week 13–16) | 31±290 | 145±298 | 0.02 | |
| Baseline to Month 5 (Week 17–end) | −21±240 | 141±327 | 0.001 | |
| Light physical activity (1–2 METs) | ||||
| Mean minutes per day | ||||
| Baseline | 599±144 | 615±160 | ||
| Change in mean minutes per day | 0.06 | |||
| Baseline to Month 1 (Week 1–4) | −18±133 | 36±143 | 0.007 | |
| Baseline to Month 2 (Week 5–8) | −31±146 | 40±153 | 0.004 | |
| Baseline to Month 3 (Week 9–12) | −29±141 | 41±147 | 0.01 | |
| Baseline to Month 4 (Week 13–16) | −31±141 | 10±158 | 0.03 | |
| Baseline to Month 5 (Week 17–end) | −49±151 | 17±167 | 0.05 | |
| Moderate physical activity (3–5 METs) | ||||
| Mean minutes per day | ||||
| Baseline | 51±39 | 57±40 | ||
| Change in mean minutes per day | <0.001 | |||
| Baseline to Month 1 (Week 1–4) | 3.6±31 | 18±36 | <0.001 | |
| Baseline to Month 2 (Week 5–8) | −3.7±27 | 25±44 | <0.001 | |
| Baseline to Month 3 (Week 9–12) | −1.7±30 | 21±46 | <0.001 | |
| Baseline to Month 4 (Week 13–16) | 0.6±33 | 20±40 | 0.003 | |
| Baseline to Month 5 (Week 17–end) | −4.2±29 | 16±46 | <0.001 | |
| Vigorous physical activity (6–8 METs) | ||||
| Mean minutes per day | ||||
| Baseline | 1.6±6.7 | 1.2±4.1 | ||
| Change in mean minutes per day | 0.13 | |||
| Baseline to Month 1 (Week 1–4) | 0.01±3.8 | 1.30±11.0 | 0.09 | |
| Baseline to Month 2 (Week 5–8) | −0.12±3.8 | 0.44±7.7 | 0.03 | |
| Baseline to Month 3 (Week 9–12) | −0.35±4.6 | 0.91±6.3 | 0.14 | |
| Baseline to Month 4 (Week 13–16) | −0.03±3.8 | 1.30±7.4 | 0.20 | |
| Baseline to Month 5 (Week 17–end) | −0.36±5.1 | 2.30±11.0 | 0.01 | |
Note: Boldface indicates statistical significance (p<0.05).
The intervention had no effect on self-reports of total caloric or fat intake, but led to significantly greater reductions than controls in both saturated fat intake (p=0.007) and sugar-sweetened beverage consumption (p=0.02) (Table 5).
Table 5.
Self-reported Dietary Intake Over 5 Months
| Variable (daily values) | Control (M±SD) | Intervention (M±SD) | p-valuea | Overall p-value |
|---|---|---|---|---|
| Caloric intake (kcal) | 0.19b | |||
| Baseline | 1,996±888 (31) | 1,935±851 (30) | 0.88 | |
| 5-month | 1,676±575 (28) | 1,505±588 (28) | 0.19 | |
| Fat intake (g) | 0.18b | |||
| Baseline | 87.4±40.6 (31) | 85.2±39.4 (30) | 0.76 | |
| 5-month | 73.0±28.9 (28) | 64.3±30.0 (28) | 0.14 | |
| Saturated fat intake (g) | 0.007b | |||
| Baseline | 26.3±12.4 (31) | 25.4±13.7 (30) | 0.66 | |
| 5-month | 22.0±9.8 (28) | 16.2±8.3 (28) | 0.007 | |
| Sugar-sweetened beverages (kcal) | 0.02c | |||
| Baseline | 27.8±43.7 (31) | 31.8±68.4 (30) | 0.60 | |
| 5-month | 19.3±36.4 (28) | 7.3±12.3 (28) | 0.02 |
Note: Boldface indicates statistical significance (p<0.05).
p-values for time-specific comparisons based on linear mixed models for baseline and follow-up outcomes.
Overall p-values based on linear mixed models for changes from baseline to follow-up, adjusting for baseline.
Overall p-value based on zero-inflated Poisson model, adjusting for baseline.
Significant group-by-time interactions indicated that the intervention led to increases in self-reported physical activity based on 7-day recall (p=0.03) and reductions in the following barriers to being active: lack of time (p=0.02); social influence (p=0.01); lack of energy (p=0.04); and lack of skill (p=0.01); as well as a marked reduction in lack of willpower (p<0.001) (Table 6). No significant group-by-time interactions were observed on measures of depressive symptoms, self-efficacy for physical activity, social support for physical activity, or fear of injury or lack of resources as barriers to being active.
Table 6.
Self-Reported Physical Activity, Barriers, Social Support, and Depression Over 5 Months
| Variable | Range of possible scoresa | Control (M±SD) | Intervention (M±SD) | p-valueb | Overall p-valuec |
|---|---|---|---|---|---|
| Physical activity: 7-day recall (kcal/kg/day) | 0.03 | ||||
| Baseline | 34.8±2.4 (31) | 34.4±2.0 (29) | 0.44 | ||
| 3-month | 34.0±1.8 (24) | 35.3±2.5 (26) | 0.06 | ||
| 5-month | 35.0±3.0 (27) | 34.9±2.6 (28) | 0.98 | ||
| Self-efficacy for physical activity | 6–30 | 0.35 | |||
| Baseline | 19.0±5.5 (31) | 19.6±5.5 (30) | 0.69 | ||
| 3-month | 18.1±5.3 (26) | 18.4±6.1 (27) | 0.79 | ||
| 5-month | 18.2±5.7 (28) | 19.8±5.6 (28) | 0.22 | ||
| Barriers to being active | |||||
| Lack of time | 0–9 | 0.02 | |||
| Baseline | 4.26±3.17 (31) | 4.30±2.65 (30) | 0.95 | ||
| 3-month | 4.15±2.89 (26) | 3.31±2.57 (26) | 0.08 | ||
| 5-month | 4.61±2.60 (28) | 3.18±2.48 (28) | 0.03 | ||
| Social influence | 0–9 | 0.01 | |||
| Baseline | 3.58±1.91 (31) | 3.33±2.04 (30) | 0.61 | ||
| 3-month | 4.12±1.97 (26) | 2.58±1.90 (26) | 0.005 | ||
| 5-month | 3.68±2.04 (28) | 2.61±2.10 (28) | 0.03 | ||
| Lack of energy | 0–9 | 0.04 | |||
| Baseline | 4.13±2.39 (30) | 4.00±2.64 (30) | 0.77 | ||
| 3-month | 4.04±2.41 (26) | 2.92±2.28 (26) | 0.02 | ||
| 5-month | 4.21±2.45 (28) | 2.93±2.55 (28) | 0.05 | ||
| Lack of willpower | 0–9 | <0.001 | |||
| Baseline | 6.74±2.13 (31) | 6.23±2.30 (30) | 0.36 | ||
| 3-month | 5.81±2.08 (26) | 3.42±2.64 (26) | <0.001 | ||
| 5-month | 6.04±2.06 (28) | 3.32±3.01 (28) | <0.001 | ||
| Fear of injury | 0–9 | 0.23 | |||
| Baseline | 1.13±1.68 (30) | 1.20±1.37 (30) | 0.95 | ||
| 3-month | 1.36±1.82 (25) | 0.73±1.40 (26) | 0.20 | ||
| 5-month | 1.68±2.31 (28) | 0.89±1.45 (28) | 0.19 | ||
| Lack of skill | 0–9 | 0.01 | |||
| Baseline | 1.77±1.87 (31) | 2.33±2.12 (30) | 0.26 | ||
| 3-month | 2.31±2.36 (26) | 1.08±1.87 (26) | 0.06 | ||
| 5-month | 2.04±2.76 (28) | 1.54±1.97 (28) | 0.31 | ||
| Lack of resources | 0–9 | 0.10 | |||
| Baseline | 1.61±1.52 (31) | 2.23±1.91 (30) | 0.13 | ||
| 3-month | 2.08±1.57 (26) | 1.46±1.73 (26) | 0.16 | ||
| 5-month | 1.89±1.71 (28) | 2.00±2.04 (28) | 0.77 | ||
| Social support for physical activity | |||||
| Family | 13–65 | 0.13 | |||
| Baseline | 36.4±10.3 (31) | 30.6±9.2 (30) | 0.02 | ||
| 3-month | 36.5±11.3 (26) | 36.9±11.9 (27) | 0.87 | ||
| 5-month | 36.2±10.2 (28) | 33.4±11.6 (27) | 0.50 | ||
| Friend | 13–65 | 0.13 | |||
| Baseline | 34.3±8.4 (31) | 34.3±9.4 (30) | 0.98 | ||
| 3-month | 33.9±9.8 (26) | 35.0±9.3 (27) | 0.71 | ||
| 5-month | 31.2±10.2 (28) | 34.6±9.9 (27) | 0.17 | ||
| CES-Dd | 0–60 | 0.41 | |||
| Baseline | 9.7±5.8 (31) | 9.2±5.6 (28) | 0.69 | ||
| 5-month | 13.6±10.4 (28) | 10.7±8.2 (27) | 0.27 | ||
Note: Boldface indicates statistical significance (p<0.05).
For all measures, higher scores indicate more of the construct being measured.
p-values for time-specific comparisons based on linear mixed models for baseline and follow-up outcomes.
Overall p-values based on linear mixed models for changes from baseline to follow-up, adjusting for baseline.
CES-D data were only obtained at baseline and 5 months.
CES-D, Center for Epidemiologic Studies Depression Scale.
Mediation analyses estimated that 26.2% (95% CI=18.1%, 44.8%) of the treatment effect on weight loss was mediated by change in saturated fat intake; 15.9% (95% CI=11.7%, 24.5%) by change in daily step counts; and 0.4% (95% CI=0.3%, 0.7%) by change in consumption of sugar-sweetened beverages.
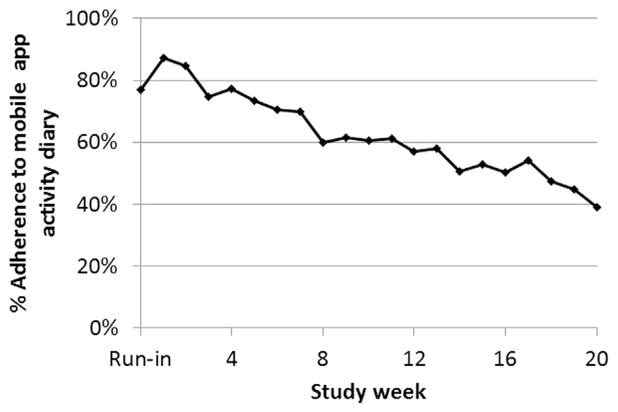
Intervention participants attended an average of 5.1 (1.0) of the six in-person sessions (range = 2–6), and each session had a mean duration of 20.0 (4.7) minutes (range = 12–31 minutes). They wore the pedometer at least 8 hours per day and 4 days per week for an average of 91.2% (16.2%) of the 20 study weeks (range = 9–20 weeks). Intervention participants used the mobile app weight diary at least twice per week (the recommended minimum) during 65.3% (29.2%) (range = 10%–100%) of the study weeks and at least once per week during 80.5% (20.4%) (range = 25%–100%). They used the mobile app diaries to report their daily activity (steps) on 62.0% (27.4%) of the 140 study days (range = 10%–96%) and their daily caloric intake on 46.9% (30.0%) (range = 0%–95%). They responded to 54.3% (23.0%) (range = 11%–85%) of the mobile app daily messages. As illustrated by use of the activity diary in Figure 2, adherence to the mobile app declined over time. Estimates of intervention adherence reflect non-adherence due to participant factors as well as equipment failures (i.e., app glitches, pedometer malfunction). There were no serious adverse events (hospitalization or emergency visits) or deaths associated with the intervention.
Figure 2.
Adherence to the mobile app activity diary over time.
Note that adherence rates reflect equipment failure and glitches as well as participant non-compliance.
Discussion
This pilot study evaluated the combination of a mobile phone app, in-person DPP-based lifestyle intervention sessions, and pedometer use for adults at risk for diabetes. Adherence to the intervention protocol varied across the different components and decreased over the course of the 20-week trial. However, overall, the adherence rates observed in this study were comparable to or better than those reported in similar trials23,24 and suggest that, for the most part, the intervention was feasible for English-speaking adults. Moreover, even with declining adherence, the intervention was efficacious for achieving a mean 6.8% weight loss over the course of the 5-month intervention, with 29% of participants reaching their goal of 10% weight loss, compared with 0% among controls. Increased physical activity and decreased saturated fat intake were mediators of the treatment effect.
This pilot trial of the mDPP intervention makes an important contribution to the growing discussion regarding sustainability of diabetes prevention. To our knowledge, this was the first study to successfully modify the original DPP intervention from 16 to six in-person core sessions. Consistent with the current results, recent systematic reviews7,25–28 indicate that text messaging and mobile apps are promising ways of delivering weight-loss interventions and achieving clinically significant weight loss in overweight adults. Although numerous lifestyle modification apps and internet programs are commercially available, 75% of these programs address only one or two behavioral change principles,29 and consumer usage data are not publicly available for data analyses.
The recent advent of smartphones along with wearable sensor devices has taken the potential for diabetes prevention and weight loss to a whole new level. Moreover, data streaming from these smartphone and wearable sensor devices could assist in offering a personalized diabetes prevention program. Incorporation of these technologies for the delivery of weight loss and diabetes prevention interventions also has the potential to significantly reduce costs and improve sustainability and dissemination of such intervention programs. However, almost no evidence is available as to how to optimally apply these types of technologies and utilize continuous real-time data to design a diabetes prevention program. Clearly, there is an urgent need for investigations in this area.
The mDPP intervention included six in-person sessions, in addition to the mobile app and pedometer. Thus, our findings likely reflect a synergistic effect of these intervention components. Although in-person sessions can be labor intensive and inconvenient, past studies have shown them to be helpful for maximizing weight loss.1,30 The six in-person sessions were sufficient when supplemented with the mobile app and pedometer, but it is possible that fewer in-person sessions could be effective. A study employing a factorial or fractional factorial design is therefore needed to test the independent and interactive impacts of the mobile app, pedometer, and in-person intervention components. Moreover, participants may vary with respect to the number of sessions needed to achieve the same weight-loss goal, and developing strategies for identifying the intensity of intervention needed could also help optimize outcomes while managing limited prevention resources.
Several previously published RCTs of weight-loss interventions and diabetes prevention programs delivered via mobile phones have reported mixed results. A large trial in India indicated that a lifestyle intervention delivered by mobile phone messaging reduced the incidence of type 2 diabetes over 2 years among men with impaired glucose tolerance.31 Another trial in China indicated that an intervention combining text messages with group sessions and coaching calls was effective in achieving modest weight loss (2.3% of baseline body weight) over 6 months among overweight adults.32 By contrast, a trial in the U.S. comparing combinations of web-based intervention, in-person groups, phone calls, and text messaging for promoting weight loss among overweight adolescents at risk for type 2 diabetes found no treatment effects for BMI after 12 months.33 Although these studies may be promising, it remains unclear which intervention components are essential for achieving and maintaining clinically meaningful weight loss. Additional studies are also needed to demonstrate that these interventions’ results can be maintained over time, and more importantly, lead to improved glucose tolerance and reduced incidence of type 2 diabetes.
Limitations
These findings need to be considered in light of the study limitations. Our relatively small sample included only English-speaking overweight adults willing to use the mobile phone app and wear a pedometer for 5 months; thus, our findings may not generalize to other populations. The relatively high incomes and large proportion of women in the sample may also limit the generalizability of the findings. As in other studies, it is challenging to obtain reliable and valid estimates of dietary intake, particularly among diverse samples. Finally, although the intervention showed its efficacy for weight loss over the 5-month period, its timing, frequency, and content may benefit from additional adjustment. Furthermore, we do not know whether weight loss is sustained beyond the 5-month intervention period. It would be worth testing different maintenance strategies across a longer-follow-up period, and possibly tailoring them to individuals’ specific needs.
Conclusions
Our study suggests that a modified DPP lifestyle intervention with reduced in-person sessions and the combination of a mobile app and pedometer was feasible for English-speaking overweight adults. The intervention led to clinically and statistically significant weight loss and lowered blood pressure over 5 months by increasing physical activity and reducing intake of saturated fat, thereby reducing risk for both type 2 diabetes and cardiovascular disease. The information learned from this pilot study will guide the design of a larger RCT of the mDPP intervention, including a longer-term weight-loss maintenance phase in the near future, and can also be used to guide the development of other intervention studies.
Acknowledgments
We wish to thank William Haskell, PhD for his consultation on the data analysis and critical review of the manuscript, and Michael B. Potter, MD for his effort to facilitate participant enrollment at the University of California, San Francisco (UCSF) Family Medicine Center at Lakeshore.
This research was supported by the UCSF Diabetes Family Fund for Innovative Patient Care-Education and Scientific Discovery Award, K23 Award (NR011454), and the UCSF Clinical and Translational Science Institute (CTSI) as part of the Clinical and Translational Science Award program funded by the National Center for Advancing Translational Sciences (UL1 TR000004) at the NIH.
Footnotes
No other potential conflicts of interest relevant to this article were reported.
References
- 1.Knowler WC, Barrett-Connor E, Fowler SE, et al. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. http://dx.doi.org/10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20(4):537–544. doi: 10.2337/diacare.20.4.537. http://dx.doi.org/10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 3.Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343–1350. doi: 10.1056/NEJM200105033441801. http://dx.doi.org/10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 4.Hernan WH, Brandle M, Zhang P, et al. Costs associated with the primary prevention of type 2 diabetes mellitus in the diabetes prevention program. Diabetes Care. 2003;26(1):36–47. doi: 10.2337/diacare.26.1.36. http://dx.doi.org/10.2337/diacare.26.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pew Research Institute. Mobile Technology Fact Sheet. 2014 www.pewinternet.org/fact-sheets/mobile-technology-fact-sheet/
- 6.Khalaf S. Apps solidify leadership six years into the mobile revolution. 2014 http://flurrymobile.tumblr.com/post/115191864580/apps-solidify-leadership-six-years-into-the-mobile.
- 7.Bacigalupo R, Cudd P, Littlewood C, Bissell P, Hawley MS, Buckley Woods H. Interventions employing mobile technology for overweight and obesity: an early systematic review of randomized controlled trials. Obes Rev. 2013;14(4):279–291. doi: 10.1111/obr.12006. http://dx.doi.org/10.1111/obr.12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO. Physical Status: The Use and Interpretation of Anthropometry. Geneva: WHO; 1995. [Google Scholar]
- 9.American Diabetes Association. Are you at risk for type 2 diabetes? Diabetes Risk Test. 2014 www.diabetes.org/are-you-at-risk/diabetes-risk-test/
- 10.Taylor-Piliae RE, Norton LC, Haskell WL, et al. Validation of a new brief physical activity survey among men and women aged 60–69 years. Am J Epidemiol. 2006;164(6):598–606. doi: 10.1093/aje/kwj248. http://dx.doi.org/10.1093/aje/kwj248. [DOI] [PubMed] [Google Scholar]
- 11.Borson S, Scanlan JM, Chen P, Ganguli M. The Mini-Cog as a screen for dementia: validation in a population-based sample. J Am Geriatr Soc. 2003;51(10):1451–1454. doi: 10.1046/j.1532-5415.2003.51465.x. http://dx.doi.org/10.1046/j.1532-5415.2003.51465.x. [DOI] [PubMed] [Google Scholar]
- 12.Borson S, Scanlan J, Brush M, Vitaliano P, Dokmak A. The Mini-Cog: a cognitive “vital signs” measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry. 2000;15(11):1021–1027. doi: 10.1002/1099-1166(200011)15:11<1021::aid-gps234>3.0.co;2-6. http://dx.doi.org/10.1002/1099-1166(200011)15:11<1021::AID-GPS234>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 13.USDHHS. Prediabetes: What You Need to Know. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases; 2007. [Google Scholar]
- 14.Tudor-Locke C, Bassett DR, Jr, Rutherford WJ, et al. BMI-referenced cut points for pedometer-determined steps per day in adults. J Phys Act Health. 2008;5(suppl 1):S126–S139. doi: 10.1123/jpah.5.s1.s126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Block G, Woods M, Potosky A, Clifford C. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol. 1990;43(12):1327–1335. doi: 10.1016/0895-4356(90)90099-b. http://dx.doi.org/10.1016/0895-4356(90)90099-B. [DOI] [PubMed] [Google Scholar]
- 16.Blair SN, Haskell WL, Ho P, et al. Assessment of habitual physical activity by a seven-day recall in a community survey and controlled experiments. Am J Epidemiol. 1985;122(5):794–804. doi: 10.1093/oxfordjournals.aje.a114163. [DOI] [PubMed] [Google Scholar]
- 17.Marcus BH, Selby VC, Niaura RS, Rossi JS. Self-efficacy and the stages of exercise behavior change. Res Q Exerc Sport. 1992;63(1):60–66. doi: 10.1080/02701367.1992.10607557. http://dx.doi.org/10.1080/02701367.1992.10607557. [DOI] [PubMed] [Google Scholar]
- 18.Sallis JF, Grossman RM, Pinski RB, Patterson TL, Nader PR. The development of scales to measure social support for diet and exercise behaviors. Prev Med. 1987;16(6):825–836. doi: 10.1016/0091-7435(87)90022-3. http://dx.doi.org/10.1016/0091-7435(87)90022-3. [DOI] [PubMed] [Google Scholar]
- 19.CDC. Barriers to Being Active Quiz. www.cdc.gov/diabetes/ndep/pdfs/8-road-to-health-barriers-quiz-508.pdf.
- 20.Weissman MM, Sholomskas D, Pottenger M, Prusoff BA, Locke BZ. Assessing depressive symptoms in five psychiatric populations: a validation study. Am J Epidemiol. 1977;106(3):203–214. doi: 10.1093/oxfordjournals.aje.a112455. [DOI] [PubMed] [Google Scholar]
- 21.Imai K, Keele L, Yamamoto T. Identification, inference and sensitivity analysis for causal mediation effects. Stat Sci. 2010;25(1):51–71. http://dx.doi.org/10.1214/10-STS321. [Google Scholar]
- 22.National Cholesterol Education Program. Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Bethesda, MD: National Heart, Lung, and Blood Institute; 2002. [Google Scholar]
- 23.Turner-McGrievy GM, Beets MW, Moore JB, Kaczynski AT, Barr-Anderson DJ, Tate DF. Comparison of traditional versus mobile app self-monitoring of physical activity and dietary intake among overweight adults participating in an mHealth weight loss program. J Am Med Inform Assoc. 2013;20(3):513–518. doi: 10.1136/amiajnl-2012-001510. http://dx.doi.org/10.1136/amiajnl-2012-001510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carter MC, Burley VJ, Nykjaer C, Cade JE. Adherence to a smartphone application for weight loss compared to website and paper diary: pilot randomized controlled trial. J Med Internet Res. 2013;15(4):e32. doi: 10.2196/jmir.2283. http://dx.doi.org/10.2196/jmir.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaw R, Bosworth H. Short message service (SMS) text messaging as an intervention medium for weight loss: a literature review. Health Inform J. 2012;18(4):235–250. doi: 10.1177/1460458212442422. http://dx.doi.org/10.1177/1460458212442422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aguilar-Martinez A, Sole-Sedeno JM, Mancebo-Moreno G, Medina FX, Carreras-Collado R, Saigi-Rubio F. Use of mobile phones as a tool for weight loss: a systematic review. J Telemed Telecare. 2014;20(6):339–349. doi: 10.1177/1357633X14537777. http://dx.doi.org/10.1177/1357633X14537777. [DOI] [PubMed] [Google Scholar]
- 27.Free C, Phillips G, Galli L, et al. The effectiveness of mobile-health technology-based health behaviour change or disease management interventions for health care consumers: a systematic review. PLoS Med. 2013;10(1):e1001362. doi: 10.1371/journal.pmed.1001362. http://dx.doi.org/10.1371/journal.pmed.1001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lyzwinski LN. A systematic review and meta-analysis of mobile devices and weight loss with an intervention content analysis. J Pers Med. 2014;4(3):311–385. doi: 10.3390/jpm4030311. http://dx.doi.org/10.3390/jpm4030311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pagoto S, Schneider K, Jojic M, DeBiasse M, Mann D. Evidence-based strategies in weight-loss mobile apps. Am J Prev Med. 2013;45(5):576–582. doi: 10.1016/j.amepre.2013.04.025. http://dx.doi.org/10.1016/j.amepre.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 30.Wadden TA, Webb VL, Moran CH, Bailer BA. Lifestyle modification for obesity: new developments in diet, physical activity, and behavior therapy. Circulation. 2012;125(9):1157–1170. doi: 10.1161/CIRCULATIONAHA.111.039453. http://dx.doi.org/10.1161/CIRCULATIONAHA.111.039453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramachandran A, Snehalatha C, Ram J, et al. Effectiveness of mobile phone messaging in prevention of type 2 diabetes by lifestyle modification in men in India: a prospective, parallel-group, randomised controlled trial. Lancet Diabetes Endocrinol. 2013;1(3):191–198. doi: 10.1016/S2213-8587(13)70067-6. http://dx.doi.org/10.1016/S2213-8587(13)70067-6. [DOI] [PubMed] [Google Scholar]
- 32.Lin PH, Wang Y, Levine E, et al. A text messaging-assisted randomized lifestyle weight loss clinical trial among overweight adults in Beijing. Obesity. 2014;22(5):E29–E37. doi: 10.1002/oby.20686. http://dx.doi.org/10.1002/oby.20686. [DOI] [PubMed] [Google Scholar]
- 33.Patrick K, Norman GJ, Davila EP, et al. Outcomes of a 12-month technology-based intervention to promote weight loss in adolescents at risk for type 2 diabetes. J Diabetes Sci Technol. 2013;7(3):759–770. doi: 10.1177/193229681300700322. http://dx.doi.org/10.1177/193229681300700322. [DOI] [PMC free article] [PubMed] [Google Scholar]