Abstract
Background
Mild cognitive impairment (MCI) is the intermediate stage of the cognitive changes between normal aging and dementia. KLOTH is an age-related gene that may contribute to the risk of MCI. The aim of our study was to explore the association between KLOTHO promoter methylation and MCI in Xinjiang Uygur and Han populations.
Methods
DNA methylation assay was performed using the bisulphite pyrosequencing technology among 96 Uygur (48 MCI and 48 controls) and 96 Han (48 MCI and 48 controls) Chinese individuals from Xinjiang province of China.
Results
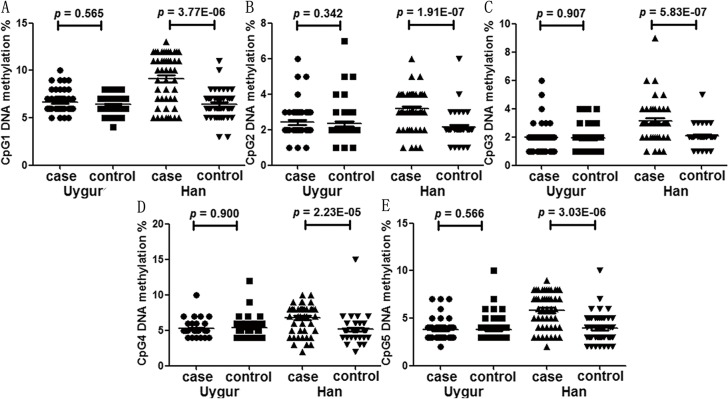
We found significant association between KLOTHO promoter methylation and MCI in the Han Chinese (CpG1: p = 3.77E-06; CpG2: p = 1.91E-07; CpG3: p = 5.83E-07; CpG4: p = 2.23E-05; CpG5: p = 3.03E-06) but not in the Uygur Chinese. Higher KLOTHO promoter methylation levels were found in Han MCI patients than Uygur MCI patients for all the five CpGs (adjusted p values by age < 0.02).
Conclusion
Our results showed that KLOTHO promoter hypermethylation contributed to the MCI risk in Xinjiang Han Chinese but not in Xinjiang Uygur Chinese. The population difference of KLOTHO methylation in the risk of MCI required further investigation in the future.
Introduction
Mild cognitive impairment (MCI) refers to the intermediate stage of the cognitive changes between normal aging and dementia [1]. Individuals with MCI showed cognitive impairment greater than expected for their age and education, but their mental functions are relative complete and do not reach the criteria for dementia. About 10–15% of MCI persons develop into dementia (mostly Alzheimer’s disease, AD) per year, in constrast of 1–2% AD incidence in the general population per year [2]. The incidence of MCI ranges from 1% to 6% per year, while the prevalence ranges from 3% to 22% per year [3, 4] in the Western countries. In China, the prevalences of MCI in different regions vary between 5.4% (Taiyuan city) and 25% (Shaanxi province) [5]. Nowadays, MCI has become a major threat to the eldly, and it should be early detected and intervented in order to delay or prevent the occurrence of dementia.
KLOTHO is a longevity and neuroprotective gene [6], encoding a single-pass transmembrane protein with a long extracellular domain and a short cytoplasmic tail [7]. KLOTHO controls multiple growth factor signaling pathways, including insulin, IGF-1 and Wnt [8]. Klotho is able to protect cells and tissues from oxidative stress by stimulating the expression of antioxidant proteins [9]. Klotho-mutated mice had cognition impairment due to an increased oxidative damage to hippocampus neurons [10], and the similar result was also observed in rhesus monkey [11, 12]. Lower KLOTHO concentration was discovered in the cerebrospinal fluid of older adults, especially in the AD patients [13]. Our previous study indicated lower KLOTHO protein levels in MCI patients than controls [14].
Alteration in promoter methylation is often found to affect gene expression [15] and was shown to be associated with AD [16], and other diseases [17–20]. MCI is a complex disease affected by both genetic and environmental factors [21]. Epigenetics is considered as a link between genetics and environment, and DNA methylation as a major part of epigenetics may play an important role in the development of MCI [22]. Aberrant expression of some important genes, such as APOE [23], presenilin 1 and APP [24], were shown to be associated with the occurrence of MCI. However, the relationship between KLOTHO methylation and MCI is still unclear. Therefore, we examined whether KLOTHO promoter methylation was associated with MCI in the Han and Uygur Chinese in Xinjiang province of China.
Materials and Methods
Samples and clinical data
All the participants over 60 were selected in this study. Among them, there were 96 MCI patients (48 Han and 48 Uygur) and 96 well-matched controls (48 Han and 48 Uygur) collected from epidemiological surveys between 2010 and 2014 in Xinjiang province of China. According to the Diagnostic and Statistical Manual of Mental Disorders 4th edition, the inclusion criteria for MCI comprised the following items: 1) memory complaint; 2) GDS scores were 2–3 grade and MMSE scores for illiterate, primary school and secondary school (and above) were 18–21, 21–24, and 25–27, respectively; 3) decreased daily activities and social participation; 4) HIS score ≤ 4 and no specific causes of cognitive decline; 5) cognitive impairment lasted for over three months; 6) absence of dementia. Subjects were excluded if they had history of mental illness or mental retardation or suffering from severe heart or lung or kidney dysfunction, severe endocrine disease, severe infectious diseases, and toxic encephalopathy. Subjects were also excluded if they had brain dysfunctions in the past 6 months, including stroke, Parkinson's disease, brain tumors, depression, a history of head trauma, or a history of psychotropic drug use, alcohol, or drug addiction. All the collected individuals were Han or Uygur Chinese from Xinjiang in the Western China. All the subjects voluntarily participated in this study. Written informed consent was obtained from each of the subjects following a complete description of the study. The institutional ethics committee of the First Affiliated Hospital at Xinjiang Medical University approved this study.
Biochemical analyses
Nucleic acid was extracted from the blood samples using the Genomic DNA Mini Kit (TIANGEN; Beijing, China). The DNA concentration was measured by the ultramicro nucleic acid ultraviolet tester (NANODROP 1000, Wilmington, USA). Plasma levels of biochemical factors (including triglyceride (TG), total cholesterol (TC), high density lipoprotein (HDL), low density lipoprotein (LDL)) were detected by biochemical laboratory (Beckman; Brea, CA, USA). DNA methylation levels of each CpG sites were tested by pyrosequencing technology. Sodium bisulphite (EZ DNA Methylation-Gold Kit; ZYMO RESEARCH, Orange County, California, USA) was used to convert all unmethylated cytosines to uracils, and meanwhile the methylated cytosines unchanged. Polymerase chain reaction (PCR) (Zymo Taq PreMix, ZYMO RESEARCH, Orange County, California, USA) was used to augment target DNA sequences. The primers were all designed by PyroMark Assay Design software. The sequences were 5’-TTGGGTTTTAGAGTGGGAGAAAAGT-3’ (forward primer), 5’-Biotin-AAACCCTCAAATTCATTCTCTTTACCTACC-3’ (reverse primer), and was 5’-AGTGAGAGTAGGTGT-3’ (sequencing primer). All the oligomers were synthesized by the Sangon Biotechnology company (Shanghai, China).
Statistical Program for Social Sciences (SPSS) software 16.0 (SPSS, Inc., Chicago, IL, USA) was used in this study and a p value < 0.05 was considered to be significant. Nonparametric testing was used to compare differences in the each CpG sites of continuous variables between the MCI cases and controls. Spearman rank correlation test was used to analyze the associations between KLOTH methylation and metabolic characteristics of MCI subjects.
Results
A total of five CpGs on the KLOTHO promoter were assessed to explore their associations with the risk of MCI (Fig 1). Significant correlation was found among the methylation levels of the five CpGs (r > 0.524, p < 0.001). As shown in Table 1 and Fig 2, significantly elevated KLOTHO promoter methylation was found in MCI cases than controls in Xinjiang Han Chinese population (CpG1: p = 3.77E-06; CpG2: p = 1.91E-07; CpG3: p = 5.83E-07; CpG4: p = 2.23E-05; CpG5: p = 3.03E-06) but not in Uygur Chinese population. Similar results in the female and male Xinjiang Han Chinese (male: CpG1: p = 0.001; CpG2: p = 2.36E-04; CpG3: p = 0.003; CpG4: p = 0.007; CpG5: p = 0.002, female: CpG1: p = 0.001; CpG2: p = 3.23E-04; CpG3: p = 1.89E-05; CpG4: p = 0.001; CpG5: p = 4.184E-04, Table 2).
Fig 1. The locations of the five KLOTH promoter CpG sites.
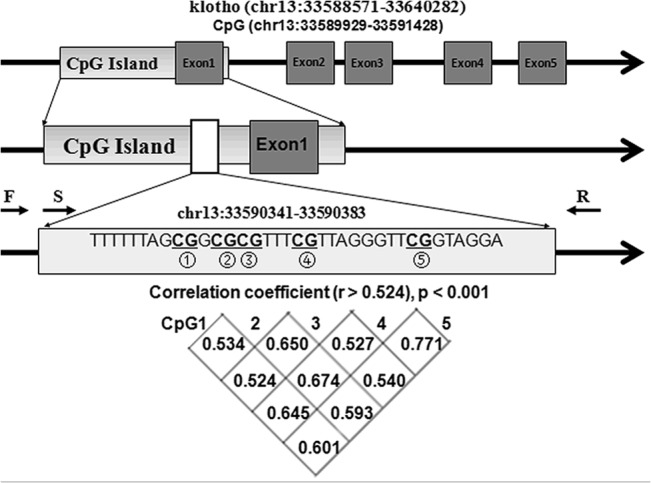
Note: F stand for Forward primer, R stand for Reverse primer, S stand for Sequencing primer.
Table 1. Characteristics of KLOTHO methylation and important parameters in the Xinjiang Uygur and Han Chinese.
| Uygur | Han | |||||
|---|---|---|---|---|---|---|
| Case(n = 48) | Control(n = 48) | p value | Case(n = 48) | Control(n = 48) | p value | |
| BMI | 23.59±3.76 | 23.12±4.14 | 0.57 | 24.49±2.78 | 24.49±3.33 | 0.986 |
| Age(years) | 70.83±4.54 | 71.02±4.42 | 0.838 | 77.41±5.52 | 77.70±5.53 | 0.797 |
| CpG1 | 6(6,7) | 6(6,7) | 0.565 | 10 (7,11.75) | 6 (6,7) | 3.77E-06 |
| CpG2 | 2(2,3) | 2 (2,2) | 0.342 | 3(3.25,4) | 2 (2,2) | 1.91E-07 |
| CpG3 | 2(1,2) | 2(1,2) | 0.907 | 3(2,4) | 2 (2,2) | 5.83E-07 |
| CpG4 | 5(5,5) | 5(5,6) | 0.900 | 8(5,8) | 5 (5,5) | 2.23E-05 |
| CpG5 | 4(3,4) | 3(3,4) | 0.566 | 6(4,8) | 4 (3,5) | 3.03E-06 |
Fig 2. Association between KLOTHO methylation and MCI.
Table 2. Subgroup characteristics of KLOTHO methylation and important parameters in the Xinjiang Uygur and Han Chinese.
| Uygur | Han | |||||
|---|---|---|---|---|---|---|
| Case(n = 24) | Control(n = 24) | p value | Case(n = 24) | Control(n = 24) | p value | |
| male | ||||||
| BMI | 24.96±4.32 | 23.72±3.53 | 0.283 | 25.34±2.82 | 24.15±2.37 | 0.121 |
| Age(years) | 72.00±5.00 | 72.46±4.46 | 0.739 | 78.33±6.32 | 78.71±6.27 | 0.837 |
| CpG1 | 6(6,7) | 7(6,7) | 0.469 | 10(7,11.75) | 6(6,7) | 0.001 |
| CpG2 | 2(2,3) | 2(2,2) | 0.928 | 3(3,4) | 2(2,2) | 2.36E-04 |
| CpG3 | 2(1,2) | 2(2,2) | 0.217 | 3(3,4) | 2(2,3) | 0.003 |
| CpG4 | 5(5,5) | 5(5,6) | 0.484 | 7.5(5,8) | 5(4,5) | 0.007 |
| CpG5 | 3(3,4) | 3(3,4) | 0.572 | 6(4,7) | 4(3,5) | 0.002 |
| female | ||||||
| BMI | 22.21±2.53 | 22.53±4.69 | 0.774 | 23.64±2.52 | 24.85±4.11 | 0.226 |
| Age(years) | 69.67±3.81 | 69.58±3.98 | 0.941 | 76.50±4.55 | 76.71±4.60 | 0.875 |
| CpG1 | 6.5(6,7) | 6(6,7) | 0.125 | 9.5(6,11.75) | 6(5.25,7) | 0.001 |
| CpG2 | 2(2,3) | 2(2,2) | 0.228 | 3(2,4) | 2(2,2) | 3.23E-04 |
| CpG3 | 2(2,2) | 2(1,2) | 0.123 | 3(2,4) | 2(2,2) | 1.89E-05 |
| CpG4 | 5(5,5) | 5(4,5) | 0.518 | 8(5,8) | 5(5,5) | 0.001 |
| CpG5 | 4(3,4) | 3(3,4) | 0.149 | 7(4,8) | 4(3,4) | 4.184E-04 |
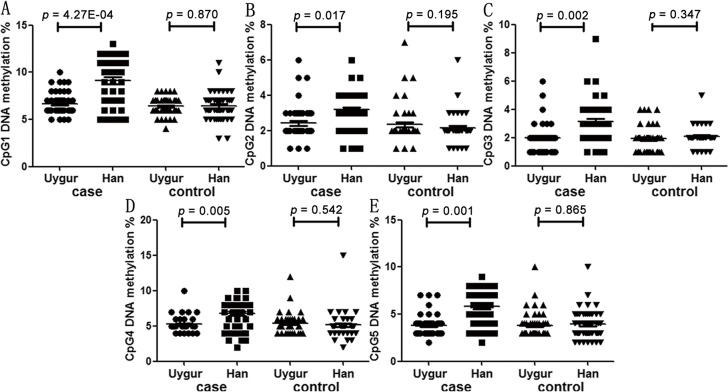
Since there were significant differences in the age between Uygur and Han samples (MCI cases: p = 6.78E-09; controls: p = 3.22E-09; Table 3), thus the population differences of various parameters were adjusted by age. According to the Table 3 and Fig 3, Han MCI patients had significantly higher levels of KLOTHO promoter DNA methylation than Uygur MCI patients (CpG1: adjusted p = 4.27E-04; CpG2: adjusted p = 0.017; CpG3: adjusted p = 0.002; CpG4: adjusted p = 0.005; CpG5: adjusted p = 0.001).
Table 3. Tests for population differences of KLOTHO methylation and important parameters.
| case | control | |||||||
|---|---|---|---|---|---|---|---|---|
| Han | Uygur | p value | adjusted p value (by age) | Han | Uygur | p value | adjusted p value (by age) | |
| BMI | 24.49±2.78 | 23.58±3.76 | 0.186 | 24.49±3.34 | 23.12±4.14 | 0.077 | ||
| Age(years) | 77.42±5.52 | 70.83±4.54 | 6.78E-09 | 77.71±5.53 | 71.02±4.42 | 3.22E-09 | ||
| CpG1 | 10(7,11.75) | 6(6,7) | 1.39E-05 | 4.27E-04 | 6(6,7) | 6(6,7) | 0.973 | 0.870 |
| CpG2 | 3(2.25,4) | 2(2,3) | 1.03E-04 | 0.017 | 2(2,2) | 2(2,2) | 0.288 | 0.195 |
| CpG3 | 3(2,4) | 2(1,2) | 1.11E-06 | 0.002 | 2(2,2) | 2(1,2) | 0.126 | 0.347 |
| CpG4 | 8(5,8) | 5(5,5) | 7.97E-05 | 0.005 | 5(5,5) | 5(5,6) | 0.333 | 0.542 |
| CpG5 | 6(4,8) | 4(3,4) | 9.04E-07 | 0.001 | 4(3,5) | 3(3,4) | 0.668 | 0.865 |
Fig 3. Population difference of KLOTHO methylation.
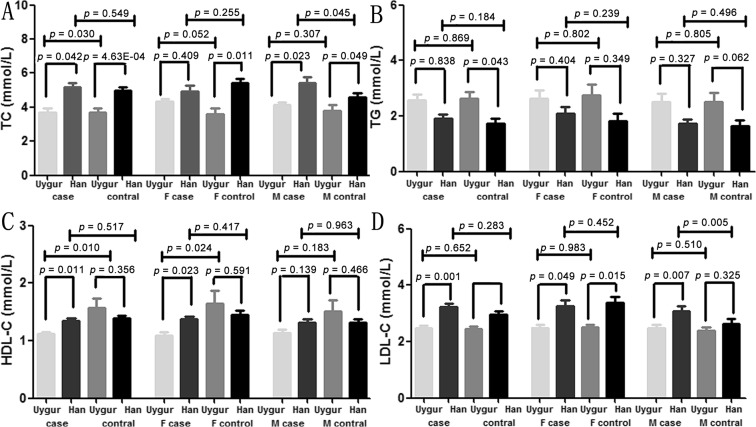
Among the 5 phenotypes, significantly higher TC and lower HDL-C levels were found in the Uygur MCI patients than the Uygur controls (TC: p = 0.030; HDL-C: p = 0.010, Fig 4). Lower levels of HDL-C were found in the Uygur MCI females than the Uygur female controls (p = 0.024, Fig 4). In the Xinjiang Han Chinese, significantly higher levels of TC and LDL-C were found in the male MCI patients compared to the male controls (TC: p = 0.045, LDL-C: p = 0.005, Fig 4).
Fig 4. Differences of important parameters between Xinjiang Uygur and Han populations.
After adjusted by age, significantly higher levels of TC, LDL-C, and HDL-C were found in Han MCI patients than Uygur MCI patients (TC: adjusted p = 0.042; LDL-C: adjusted p = 0.011; HDL-C: adjusted p = 0.011, Fig 4). Significantly lower TG and higher TC and LDL-C levels were found in Han controls than Uygur controls (TG: adjusted p = 0.043; TC: adjusted p = 4.63E-04; LDL-C: adjusted p = 0.008, Fig 4). Besides, significantly higher levels of LDL-C were found in Han patients than Uygur patients (adjusted p = 0.049, Fig 4).
As shown in the Table 4, statistically higher levels of CpG sites were found in Han MCI patients than Uygur MCI patients in the male and female subgroups (male: CpG1: adjusted p = 0.009; CpG2: adjusted p = 0.047; CpG3: adjusted p = 0.009; CpG5: adjusted p = 0.034, Table 4; female: CpG1: adjusted p = 0.012; CpG4: adjusted p = 0.017; CpG5: adjusted p = 0.006, Table 4). In the male subgroup, the levels of TC and LDL-C were all significantly higher in Han patients compared to Uygur patients (TC: adjusted p = 0.023; LDL-C: adjusted p = 0.007, Fig 4). Significantly higher TC levels were also found in the male Han controls compared to the male Uygur controls (adjusted p = 0.049, Fig 4). In the females, significantly higher levels of HDL-C were found in the Han MCI patients than the Uygur MCI patients (adjusted p = 0.023, Fig 4). Statistically higher levels of TC and LDL-C were found in Han female MCI compared to Uygur female controls (TC: adjusted p = 0.011; LDL-C: adjusted p = 0.015, Fig 4).
Table 4. Tests for gender differences of KLOTHO methylation and important parameters.
| case | control | |||||||
|---|---|---|---|---|---|---|---|---|
| Han | Uygur | p value | adjusted p value (by age) | Han | Uygur | p value | adjusted p value (by age) | |
| male | ||||||||
| BMI | 25.34±2.82 | 24.96±4.32 | 0.721 | 24.15±2.37 | 23.72±3.53 | 0.625 | ||
| Age(years) | 78.33±6.31 | 72.00±4.50 | 3.60E-04 | 78.71±6.27 | 72.46±4.46 | 2.44E-04 | ||
| CpG1 | 10(7,11.75) | 6(6,7) | 4.11E-04 | 0.009 | 6(6,7) | 7(6,7) | 0.897 | 0.888 |
| CpG2 | 3(3,4) | 2(2,3) | 0.003 | 0.047 | 2(2,2) | 2(2,2) | 0.291 | 0.533 |
| CpG3 | 3(3,4) | 2(1,2) | 3.73E-04 | 0.009 | 2(2,3) | 2(2,2) | 0.388 | 0.756 |
| CpG4 | 7.5(5,8) | 5(5,5) | 0.012 | 0.121 | 5(4,5) | 5(5,6) | 0.215 | 0.729 |
| CpG5 | 6(4,7) | 3(3,4) | 4.03E-04 | 0.034 | 4(3,5) | 3(3,4) | 0.920 | 0.936 |
| female | ||||||||
| BMI | 23.64±2.52 | 22.21±2.53 | 0.057 | 24.85±4.11 | 22.53±4.69 | 0.075 | ||
| Age(years) | 76.50±4.54 | 69.67±3.81 | 9.88E-07 | 76.71±4.60 | 69.58±3.98 | 7.11E-07 | ||
| CpG1 | 9.5(6,11.75) | 6.5(6,7) | 0.010 | 0.012 | 6(5.25,7) | 6(6,7) | 0.871 | 0.778 |
| CpG2 | 3(2,4) | 2(2,3) | 0.016 | 0.215 | 2(2,2) | 2(2,2) | 0.692 | 0.181 |
| CpG3 | 3(2,4) | 2(2,2) | 0.001 | 0.105 | 2(2,2) | 2(1,2) | 0.128 | 0.39 |
| CpG4 | 8(5,8) | 5(5,5) | 0.003 | 0.017 | 5(5,5) | 5(4,5) | 0.973 | 0.897 |
| CpG5 | 7(4,8) | 4(3,4) | 0.001 | 0.006 | 4(3,4) | 3(3,4) | 0.510 | 0.645 |
Discussion
In the current study, we investigated the association between KLOTHO promoter methylation and MCI in two Xinjiang populations. In the Xinjiang Han, significant results were found in the overall analysis and the gender-based subgroup analyses, although no positive results existed in the Uygur population. Significantly higher level of KLOTHO promoter methylation was found in Han patients compared to Uygur patients. In addition, population differences were also found in multiple clinical phenotypes including the levels of TG, TC, HDL-C and LDL-C. Different dietary cultures in two populations might explain the population differences of the above phenotypes.
KOLOTHO is a longevity gene and has been considered to be involved in MCI which is an age-related disease. KOLOTHO mainly expressed in brain and kidney, secreting into CSF and serum, respectively [25]. KLOTHO as a related factor to oxidative damage has the ability to protect neurons in brain [26]. KLOTHO was considered as an aging suppressor gene [27], and over expression of KLOTHO significantly extended the life span of mice [28, 29].Meanwhile, we found a higher methylation level in KLOTHO promoter in the MCI patients of Xinjiang Han population than matched controls, and the same results were also observed in the subgroup stratified by gender. No difference was found in Uygur population. DNA methylation alteration of promoter was often found to influence gene expression [ 30 ]. In addition, hypermethylation of KLOTHO was shown to low expression in hepatocellular carcinoma tissues [31]. Repressions of KLOTHO migth have an adverse effect for MCI patients according to a previous study [27]. Hence, we hypothesized that hypermethylation of KLOTHO promoter might play a role in the development of MCI of Xingjian Han population via the pathway of down regulation of KLOTHO expression.
Population difference could change gene activity as described previously [32]. Populations are complicated in Xinjiang Province. Uygur population and Han population belong to Caucasian and Mongolian, respectively [33]. Previous study had investigated significant association between IL-4, IFN-gamma promoter methylation and allergic rhinitis patients of Uygur and Han Chinese in Xinjiang [34]. Significantly higher DNA methylation levels of microRNA-375 promoter were found in Han T2D patients than Kazak T2D patients in Xinjiang [35]. In this study, significantly higher level of KLOTHO promoter methylation was discovered in Han MCI patients than Uygur patients, suggesting a population specific mode of KLOTHO methylation in the susceptibility of MCI.
Dyslipidemia is associated with both the occurrence and progression of MCI. HDL-C and TC were belong to vascular risk factors and could increase the risk of MCI and the risk of conversion from MCI to AD [36, 37]. A correlation between HDL-C and dementia was also revealed in the elderly [38]. A cross-sectional study about cognitive impairment suggested that TC and LDL-C were independent risk factors for MCI [39]. An interdisciplinary longitudinal study showed that higher TC levels were associated with an increased risk for cognitive disorders [40]. Higher TC and lower HDL-C levels were found in Chinese T2D patients with MCI compared to simple T2D patients [41]. Consistent with the previous findings, our study showed a significantly higher TC level in MCI compared to controls in the Uygur population.
Population differences were also found in four phenotypes including TG, TC, HDL-C and LDL-C. Higher levels of TC and LDL-C were found in Han population than Uygur population in both MCI and control subgroup. However, elevation of HDL-C level was only found in the Han MCI patients than Uygur MCI patients. In addition, significantly lower levels of TG were found in Han patients and controls than Uygur patients and controls. These phenotypes are shown to be influenced by many factors including genetic factors, eating habit, and lifestyle [42, 43]. The population differences in both the genetic and the environmental factors might explain the differences of above phenotypes in two populations.
Ethnic difference has been observed in the diagnosis, clinical manifestation, disease management, prevalence, and cause of MCI. Race difference was found in the memory performance even after adjusting by the status of education, depression, gender, and memory complaints [44]. As the measurement of cognitive functioning, Hachinski ischemic scale scores were shown to differ by ethnicity [45]. African American MCI patients were found to have faster rate of cognitive decline compared with non-African American ones [46]. Ethnic disparities were also observed in the management of MCI patients [47]. Caucasian MCI patients underwent significant more neuropsychologic testing; while African-Americans had more depression screening tests and were less likely to be prescribed with acetylcholinesterase inhibitors [47]. The prevalence of dementia has been reported to be higher among African Americans and Caribbean Hispanics, lower among Japanese Americans, and similar among Native Americans and Mexican Americans compared with non-Hispanic whites [48]. English usage in Australia was shown to be related to to lower prevalence of MCI and higher rates of reversion from MCI to normal [49]. In the United States, more African American and Hispanic MCI patients were attributable to diabetes than non-Hispanic white MCI patients [50]. Racial disparities in financial capacity were found to exist among patients with amnestic MCI [51]. Predictive risk factors for MCI in non-Hispanic cohorts were not associated with MCI in Mexican Americans [52], who were more likely to be possessed the ApoE ε4 allele less frequently [52]. In addition, ApoE ε4 allele was protective for MCI only in Han Chinese but not in Hui Chinese [53].
In summary, our study identified the contribution of KLOTHO promoter methylation to MCI in Xinjiang Han population. The lack of significant results in the Xinjiang Uygur population might be due to the differences in genetics and environment. Further investigation on the population difference was needed in the future.
Acknowledgments
The research was supported by the grants from the National Natural Science Foundation of China (81471398, 81070873, 31100919 and 81371469), 973 program from the Ministry of Science and Technology of China (2013CB835100), Natural Science Foundation of Zhejiang Province (LR13H020003), Disciplinary Project of Ningbo University (B01350104900), and K. C. Wong Magna Fund in Ningbo University.
Data Availability
All relevant data are within the paper.
Funding Statement
The research was supported by the grants from the National Natural Science Foundation of China (81471398, 81070873, 31100919 and 81371469), the 973 Program from the Ministry of Science and Technology of China (2013CB835100), the Natural Science Foundation of Zhejiang Province (LR13H020003), the Disciplinary Project of Ningbo University (B01350104900), and the3 K. C. Wong Magna Fund in Ningbo University.
References
- 1. Christa Maree Stephan B, Minett T, Pagett E, Siervo M, Brayne C, McKeith IG. Diagnosing Mild Cognitive Impairment (MCI) in clinical trials: a systematic review. BMJ open. 2013;3(2). Epub 2013/02/07. 10.1136/bmjopen-2012-001909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, et al. Current concepts in mild cognitive impairment. Archives of neurology. 2001;58(12):1985–92. Epub 2001/12/26. . [DOI] [PubMed] [Google Scholar]
- 3. Larrieu S, Letenneur L, Orgogozo JM, Fabrigoule C, Amieva H, Le Carret N, et al. Incidence and outcome of mild cognitive impairment in a population-based prospective cohort. Neurology. 2002;59(10):1594–9. Epub 2002/11/27. . [DOI] [PubMed] [Google Scholar]
- 4. Hanninen T, Hallikainen M, Tuomainen S, Vanhanen M, Soininen H. Prevalence of mild cognitive impairment: a population-based study in elderly subjects. Acta neurologica Scandinavica. 2002;106(3):148–54. Epub 2002/08/14. . [DOI] [PubMed] [Google Scholar]
- 5. Cheng Y, Xiao S. Recent research about mild cognitive impairment in China. Shanghai archives of psychiatry. 2014;26(1):4–14. Epub 2014/08/13. 10.3969/j.issn.1002-0829.2014.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zeldich E, Chen CD, Colvin TA, Bove-Fenderson EA, Liang J, Tucker Zhou TB, et al. The neuroprotective effect of Klotho is mediated via regulation of members of the redox system. The Journal of biological chemistry. 2014;289(35):24700–15. Epub 2014/07/20. 10.1074/jbc.M114.567321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hahn D, Lottaz D, Sterchi EE. C-cytosolic and transmembrane domains of the N-benzoyl-L-tyrosyl-p-aminobenzoic acid hydrolase alpha subunit (human meprin alpha) are essential for its retention in the endoplasmic reticulum and C-terminal processing. European journal of biochemistry / FEBS. 1997;247(3):933–41. Epub 1997/08/01. . [DOI] [PubMed] [Google Scholar]
- 8. Alvarez A, Cacabelos R, Sanpedro C, Garcia-Fantini M, Aleixandre M. Serum TNF-alpha levels are increased and correlate negatively with free IGF-I in Alzheimer disease. Neurobiology of aging. 2007;28(4):533–6. Epub 2006/03/30. 10.1016/j.neurobiolaging.2006.02.012 . [DOI] [PubMed] [Google Scholar]
- 9. Takahashi G, Sakurai M, Abe K, Itoyama Y, Tabayashi K. MCI-186 reduces oxidative cellular damage and increases DNA repair function in the rabbit spinal cord after transient ischemia. The Annals of thoracic surgery. 2004;78(2):602–7. Epub 2004/07/28. 10.1016/j.athoracsur.2004.02.133 . [DOI] [PubMed] [Google Scholar]
- 10. Nagai T, Yamada K, Kim HC, Kim YS, Noda Y, Imura A, et al. Cognition impairment in the genetic model of aging klotho gene mutant mice: a role of oxidative stress. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2003;17(1):50–2. Epub 2002/12/12. 10.1096/fj.02-0448fje . [DOI] [PubMed] [Google Scholar]
- 11. Cherubini A, Spoletini I, Peran P, Luccichenti G, Di Paola M, Sancesario G, et al. A multimodal MRI investigation of the subventricular zone in mild cognitive impairment and Alzheimer's disease patients. Neuroscience letters. 2010;469(2):214–8. Epub 2009/12/08. 10.1016/j.neulet.2009.11.077 . [DOI] [PubMed] [Google Scholar]
- 12. Dohnel K, Sommer M, Ibach B, Rothmayr C, Meinhardt J, Hajak G. Neural correlates of emotional working memory in patients with mild cognitive impairment. Neuropsychologia. 2008;46(1):37–48. Epub 2007/10/05. 10.1016/j.neuropsychologia.2007.08.012 . [DOI] [PubMed] [Google Scholar]
- 13. Semba RD, Moghekar AR, Hu J, Sun K, Turner R, Ferrucci L, et al. Klotho in the cerebrospinal fluid of adults with and without Alzheimer's disease. Neuroscience letters. 2014;558:37–40. Epub 2013/11/12. 10.1016/j.neulet.2013.10.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xiaohui Zhou ZY, Haijun Miao. Relationship of serum Klotho protein content with mild cognitive impairment. Chin J Psychiatry. 2014;47(1):4. Epub February. [Google Scholar]
- 15. Yu Z, Kong Q, Kone BC. Aldosterone reprograms promoter methylation to regulate alphaENaC transcription in the collecting duct. American journal of physiology Renal physiology. 2013;305(7):F1006–13. Epub 2013/08/09. 10.1152/ajprenal.00407.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chang L, Wang Y, Ji H, Dai D, Xu X, Jiang D, et al. Elevation of peripheral BDNF promoter methylation links to the risk of Alzheimer's disease. PloS one. 2014;9(11):e110773 Epub 2014/11/05. 10.1371/journal.pone.0110773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dai D, Cheng J, Zhou K, Lv Y, Zhuang Q, Zheng R, et al. Significant association between DRD3 gene body methylation and schizophrenia. Psychiatry research. 2014;220(3):772–7. Epub 2014/09/30. 10.1016/j.psychres.2014.08.032 . [DOI] [PubMed] [Google Scholar]
- 18. Xu L, Zheng D, Wang L, Jiang D, Liu H, Liao Q, et al. GCK gene-body hypomethylation is associated with the risk of coronary heart disease. BioMed research international. 2014;2014:151723 Epub 2014/04/04. 10.1155/2014/151723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang LN, Liu PP, Wang L, Yuan F, Xu L, Xin Y, et al. Lower ADD1 gene promoter DNA methylation increases the risk of essential hypertension. PloS one. 2013;8(5):e63455 Epub 2013/05/22. 10.1371/journal.pone.0063455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jiang D, Zheng D, Wang L, Huang Y, Liu H, Xu L, et al. Elevated PLA2G7 gene promoter methylation as a gender-specific marker of aging increases the risk of coronary heart disease in females. PloS one. 2013;8(3):e59752 Epub 2013/04/05. 10.1371/journal.pone.0059752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Young JW, Sharkey J, Finlayson K. Progressive impairment in olfactory working memory in a mouse model of Mild Cognitive Impairment. Neurobiology of aging. 2009;30(9):1430–43. Epub 2008/02/05. 10.1016/j.neurobiolaging.2007.11.018 [DOI] [PubMed] [Google Scholar]
- 22. Coppede F, Migliore L. Evidence linking genetics, environment, and epigenetics to impaired DNA repair in Alzheimer's disease. Journal of Alzheimer's disease: JAD. 2010;20(4):953–66. Epub 2010/02/26. 10.3233/JAD-2010-1415 . [DOI] [PubMed] [Google Scholar]
- 23. Caselli RJ. Phenotypic differences between apolipoprotein E genetic subgroups: research and clinical implications. Alzheimer's research & therapy. 2012;4(3):20 Epub 2012/06/15. 10.1186/alzrt123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lin HC, Hsieh HM, Chen YH, Hu ML. S-Adenosylhomocysteine increases beta-amyloid formation in BV-2 microglial cells by increased expressions of beta-amyloid precursor protein and presenilin 1 and by hypomethylation of these gene promoters. Neurotoxicology. 2009;30(4):622–7. Epub 2009/07/29. 10.1016/j.neuro.2009.03.011 . [DOI] [PubMed] [Google Scholar]
- 25. Chen CD, Tung TY, Liang J, Zeldich E, Tucker Zhou TB, Turk BE, et al. Identification of cleavage sites leading to the shed form of the anti-aging protein klotho. Biochemistry. 2014;53(34):5579–87. Epub 2014/08/12. 10.1021/bi500409n [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brobey R. GD, Gurnani P., Pastor J., Hsieh C.-C., Papaconstantinou J., Kuro-o M., Rosenblatt K. P., editor Klotho activation is neuroprotective against oxidative stress through antioxidant and apoptotic signaling pathways2011; Houston, TX.
- 27. Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, et al. Suppression of aging in mice by the hormone Klotho. Science. 2005;309(5742):1829–33. Epub 2005/08/27. 10.1126/science.1112766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kuro-o M. Klotho as a regulator of oxidative stress and senescence. Biological chemistry. 2008;389(3):233–41. Epub 2008/01/08. 10.1515/BC.2008.028 . [DOI] [PubMed] [Google Scholar]
- 29. Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390(6655):45–51. Epub 1997/11/18. 10.1038/36285 . [DOI] [PubMed] [Google Scholar]
- 30. Razin A, Cedar H. DNA methylation and gene expression. Microbiological reviews. 1991;55(3):451–8. Epub 1991/09/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xie B, Zhou J, Yuan L, Ren F, Liu DC, Li Q, et al. Epigenetic silencing of Klotho expression correlates with poor prognosis of human hepatocellular carcinoma. Human pathology. 2013;44(5):795–801. Epub 2012/11/06. 10.1016/j.humpath.2012.07.023 . [DOI] [PubMed] [Google Scholar]
- 32. Zhang JP, Zhou SF, Chen X, Huang M. Determination of intra-ethnic differences in the polymorphisms of thiopurine S-methyltransferase in Chinese. Clinica chimica acta; international journal of clinical chemistry. 2006;365(1–2):337–41. Epub 2005/10/15. 10.1016/j.cca.2005.09.005 . [DOI] [PubMed] [Google Scholar]
- 33. Shan M, Wang X, Sun G, Ma B, Yao X, Ainy A, et al. A retrospective study of the clinical differences of Uygur breast cancer patients compared to Han breast cancer patients in the Xinjiang region of China. International journal of clinical and experimental medicine. 2014;7(10):3482–90. Epub 2014/11/25. [PMC free article] [PubMed] [Google Scholar]
- 34. Lou Z, Wang H, Yang Q, Jiang X, Zhang Q, Mu NR, et al. [Comparative study of IL-4, IFN-gamma gene methylation for the epigenetics of allergic rhinitis in Xinjiang Uygur, Han people]. Lin chuang er bi yan hou tou jing wai ke za zhi = Journal of clinical otorhinolaryngology, head, and neck surgery. 2012;26(18):795–7. Epub 2012/12/25. . [PubMed] [Google Scholar]
- 35. Chang X, Li S, Li J, Yin L, Zhou T, Zhang C, et al. Ethnic differences in microRNA-375 expression level and DNA methylation status in type 2 diabetes of Han and Kazak populations. Journal of diabetes research. 2014;2014:761938 Epub 2014/04/18. 10.1155/2014/761938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Blasko I, Kemmler G, Jungwirth S, Wichart I, Weissgram S, Jellinger K, et al. Prospective study on association between plasma amyloid beta-42 and atherosclerotic risk factors. J Neural Transm. 2011;118(5):663–72. Epub 2011/02/24. 10.1007/s00702-011-0599-4 . [DOI] [PubMed] [Google Scholar]
- 37. Luchsinger JA, Reitz C, Honig LS, Tang MX, Shea S, Mayeux R. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology. 2005;65(4):545–51. Epub 2005/08/24. 10.1212/01.wnl.0000172914.08967.dc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Parnowski T, Kaluza B. [Metabolic syndrome and cognitive dysfunction in the old age]. Psychiatria polska. 2013;47(6):1087–99. Epub 2014/07/11. . [PubMed] [Google Scholar]
- 39. Zou Y, Zhu Q, Deng Y, Duan J, Pan L, Tu Q, et al. Vascular risk factors and mild cognitive impairment in the elderly population in Southwest China. American journal of Alzheimer's disease and other dementias. 2014;29(3):242–7. Epub 2014/01/01. 10.1177/1533317513517042 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Toro P, Degen C, Pierer M, Gustafson D, Schroder J, Schonknecht P. Cholesterol in mild cognitive impairment and Alzheimer's disease in a birth cohort over 14 years. European archives of psychiatry and clinical neuroscience. 2014;264(6):485–92. Epub 2013/11/19. 10.1007/s00406-013-0468-2 . [DOI] [PubMed] [Google Scholar]
- 41. Niu MJ, Yin FZ, Liu LX, Fang Y, Xuan XM, Wu GF. Non-high-density lipoprotein cholesterol and other risk factors of mild cognitive impairment among Chinese type 2 diabetic patients. Journal of diabetes and its complications. 2013;27(5):443–6. Epub 2013/07/13. 10.1016/j.jdiacomp.2013.06.001 . [DOI] [PubMed] [Google Scholar]
- 42. Gupta R, Misra A, Vikram NK, Kondal D, Gupta SS, Agrawal A, et al. Younger age of escalation of cardiovascular risk factors in Asian Indian subjects. BMC cardiovascular disorders. 2009;9:28 Epub 2009/07/07. 10.1186/1471-2261-9-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Merkin SS, Karlamangla A, Crimmins E, Charette SL, Hayward M, Kim JK, et al. Education differentials by race and ethnicity in the diagnosis and management of hypercholesterolemia: a national sample of U.S. adults (NHANES 1999–2002). International journal of public health. 2009;54(3):166–74. Epub 2009/02/17. 10.1007/s00038-008-7030-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McDougall GJ Jr, Vaughan PW, Acee TW, Becker H. Memory performance and mild cognitive impairment in Black and White community elders. Ethnicity & disease. 2007;17(2):381–8. Epub 2007/08/09. . [PMC free article] [PubMed] [Google Scholar]
- 45. Johnson LA, Cushing B, Rohlfing G, Edwards M, Davenloo H, D'Agostino D, et al. The Hachinski ischemic scale and cognition: the influence of ethnicity. Age and ageing. 2014;43(3):364–9. Epub 2013/12/11. 10.1093/ageing/aft189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lee HB, Richardson AK, Black BS, Shore AD, Kasper JD, Rabins PV. Race and cognitive decline among community-dwelling elders with mild cognitive impairment: findings from the Memory and Medical Care Study. Aging & mental health. 2012;16(3):372–7. Epub 2011/10/18. 10.1080/13607863.2011.609533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kalkonde YV, Pinto-Patarroyo GP, Goldman T, Strutt AM, York MK, Kunik ME, et al. Ethnic disparities in the treatment of dementia in veterans. Dementia and geriatric cognitive disorders. 2009;28(2):145–52. Epub 2009/08/20. 10.1159/000235577 . [DOI] [PubMed] [Google Scholar]
- 48. Manly JJ MR. Ethnic differences in dementia and Alzheimer' s disease In: Anderson NB BR, Cohen B, editor. In Critical Perspectives on Racial and Ethnic Differences in Health in Late Life. Washington, DC: The National Academies Press; 2004. p. 95–141. [PubMed] [Google Scholar]
- 49. Low LF, Harrison F, Kochan NA, Draper B, Slavin MJ, Reppermund S, et al. Can mild cognitive impairment be accurately diagnosed in english speakers from linguistic minorities? Results from the Sydney Memory and Ageing study. The American journal of geriatric psychiatry: official journal of the American Association for Geriatric Psychiatry. 2012;20(10):866–77. Epub 2012/01/21. 10.1097/JGP.0b013e31823e31e2 . [DOI] [PubMed] [Google Scholar]
- 50. Luchsinger JA, Reitz C, Patel B, Tang MX, Manly JJ, Mayeux R. Relation of diabetes to mild cognitive impairment. Archives of neurology. 2007;64(4):570–5. Epub 2007/04/11. 10.1001/archneur.64.4.570 . [DOI] [PubMed] [Google Scholar]
- 51. Triebel KL, Okonkwo OC, Martin R, Griffith HR, Crowther M, Marson DC. Financial capacity of older African Americans with amnestic mild cognitive impairment. Alzheimer disease and associated disorders. 2010;24(4):365–71. Epub 2010/07/14. 10.1097/WAD.0b013e3181e7cb05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. O'Bryant SE, Johnson L, Balldin V, Edwards M, Barber R, Williams B, et al. Characterization of Mexican Americans with mild cognitive impairment and Alzheimer's disease. Journal of Alzheimer's disease: JAD. 2013;33(2):373–9. Epub 2012/09/15. 10.3233/JAD-2012-121420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang Z, Ma W, Rong Y, Liu L. The Association between Apolipoprotein E Gene Polymorphism and Mild Cognitive Impairment among Different Ethnic Minority Groups in China. International journal of Alzheimer's disease. 2014;2014:150628 Epub 2014/08/28. 10.1155/2014/150628 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.