Abstract
Background
Emerging evidence implicates circadian abnormalities as a component of the pathophysiology of major depressive disorder (MDD). The suprachiasmatic nucleus (SCN) of the hypothalamus coordinates rhythms throughout the brain and body. On a cellular level, rhythms are generated by transcriptional, translational, and post-translational feedback loops of core circadian genes and proteins. In patients with MDD, recent evidence suggests reduced amplitude of molecular rhythms in extra-SCN brain regions. We investigated whether unpredictable chronic mild stress (UCMS), an animal model that induces a depression-like physiological and behavioral phenotype, induces circadian disruptions similar to those seen with MDD.
Methods
Activity and temperature rhythms were recorded in C57BL/6J mice before, during, and after exposure to UCMS, and brain tissue explants were collected from Period2 luciferase (Per2::luc) mice following UCMS to assess cellular rhythmicity.
Results
UCMS significantly decreased circadian amplitude of activity and body temperature in mice, similar to findings in MDD patients and these changes directly correlate with depression-related behavior. While amplitude of molecular rhythms in the SCN was decreased following UCMS, surprisingly, rhythms in the nucleus accumbens were amplified with no changes seen in the prefrontal cortex or amygdala. These molecular rhythm changes in the SCN and the nucleus accumbens (NAc) also directly correlated with mood-related behavior.
Conclusions
These studies find that circadian rhythm abnormalities directly correlate with depression-related behavior following UCMS and suggest a desynchronization of rhythms in the brain with an independent enhancement of rhythms in the NAc.
Keywords: depression, circadian, chronic stress, period2, amplitude, lumicycle
INTRODUCTION
Disruptions in circadian rhythms have been implicated in a variety of psychiatric disorders including major depressive disorder (MDD) (1). Core symptoms of MDD include low mood and anhedonia, with patients experiencing a variety of other symptoms, many of which are thought to stem from disruptions in the circadian system. For instance, MDD subjects display changes in the sleep/wake cycles and altered daily activity patterns (2, 3). In addition they frequently display altered rhythms in body temperature, hormones, cortisol, and certain neurotransmitters (2, 4). Furthermore, treatments modulating the circadian cycle, such as light therapy and Agomelatine (a melatonin receptor agonist) are effective antidepressant treatments (5, 6) and appear to require an intact master circadian pacemaker (7), suggesting that certain MDD symptoms may be related to circadian disruption.
Circadian function is controlled by the master clock in the suprachiasmatic nucleus (SCN) SCN of the anterior hypothalamus (8). Circadian rhythms in the SCN and other brain regions are generated by a cycle of gene expression in individual cells that form transcriptional-translational feedback loops (9). The SCN also coordinates subordinate oscillators throughout the brain and periphery (e.g. rhythms in other brain regions and organ systems). Increasing evidence in rodents suggests that region-specific oscillations in limbic regions are instrumental regulators of emotional and motivational behaviors due to their integration of information from the SCN and homeostatic cues from hormonal states (10). For example, acute and chronic stress, major precipitating factors of MDD, likely affect rhythms in sub-oscillators, without affecting phase and period of the circadian pacemaker (11). In addition, rhythms of the central circadian gene Period 2 (Per2) are selectively altered in emotion-related brain regions (e.g. amygdala) following disruption of circulating corticosterone (12, 13). Further support for a role of molecular clocks in MDD was recently shown in a study reporting significant alterations in the diurnal variation of expression of core circadian genes in extra-SCN brain regions, including the amygdala, prefrontal cortex, hippocampus, and nucleus accumbens (NAc), of human postmortem subjects with MDD (14). In patients with bipolar disorder, skin fibroblasts transfected with Per2 luciferase reporter constructs (Per2::luc) displayed longer circadian periods compared to healthy controls, and lithium (a common treatment for bipolar disorder) resynchronized and enhanced the amplitude of the dampened molecular rhythms in these cells (15). Collectively, these pioneering studies in humans suggest circadian disruptions could precipitate or underlie the psychopathology of mood disorders, and these alterations or disruptions to rhythms may lie in the SCN or other brain regions.
While there is emerging evidence in MDD for disruption of circadian rhythms in the brain, the direct effect of chronic stress on molecular, physiological, and behavioral circadian rhythms is unclear. Furthermore, it is unknown which brain regions are primarily affected by circadian disruptions in MDD, and what specific circadian parameters are altered by chronic stress in mood regulating brain regions. Here, we exposed mice to unpredictable chronic mild stress (UCMS), a well-established rodent model of a depression-like syndrome, to first characterize the effects of chronic stress on physiological and molecular rhythms in mice. We used Per2::luc reporter mice to non-invasively and continuously monitor the effects of UCMS on brain region specific molecular rhythms. By identifying specific brain regions where circadian disruptions are most prominent, we can then target these regions in future studies to fully investigate the role of circadian mechanisms underlying depression.
METHODS AND MATERIALS
Animals
Adult male C57BL/6J (B6) mice were used for the telemetry and gene expression studies (Jackson Laboratory, Bar Harbor, ME). Period2::luciferase mice (Per2::luc) were maintained on a C57BL/6J background. Mice were housed under a standard light-dark (LD) cycle (lights on at 800 h and lights off at 2000 h) during the entire experiment. Experiments were conducted in compliance with the NIH laboratory animal care guidelines and protocols approved by the IACUC at the University of Pittsburgh.
Unpredictable Chronic Mild Stress
Mice (telemetry or lumicycle and gene expression cohorts) were subjected to four weeks of UCMS, as previously described (16) (Supplement 1). Fur ratings were as follows (see 16): 1=Generally well-groomed; 2=Slightly “fluffy” with spiky patches; 3=Most of body fluffy with coat discoloration; eye conjunctivae slightly red; and 4=Fluffy, stained, dirty coat with bald patches; eye conjunctivae red.
Behavioral Assays
Behavioral assays were conducted during the day (Zeitgeber Time (ZT) 4–10 corresponding to ZT0 (or 24) “lights on” and ZT12 “lights off) using the elevated plus maze (EPM), open field (OF), dark/light box (D/L box), novelty suppressed feeding (NSF), and the forced swim test (FST) (Supplement 1).
Telemetry Recording
Telemetry transmitters (TA-F20; Data Sciences International; St. Paul, MN) were implanted in the abdomen of B6 mice (Control: N=7 and UCMS: N=16). Control mice were implanted with non-functioning “dummy” transmitters. Following recovery of two weeks from surgery, locomotor activity and body temperature were recorded for the duration of the experiment (10min bins across 24h per day). Rhythms were recorded before (2 weeks), during (6 weeks), and after (3 weeks) UCMS (Figure 1A).
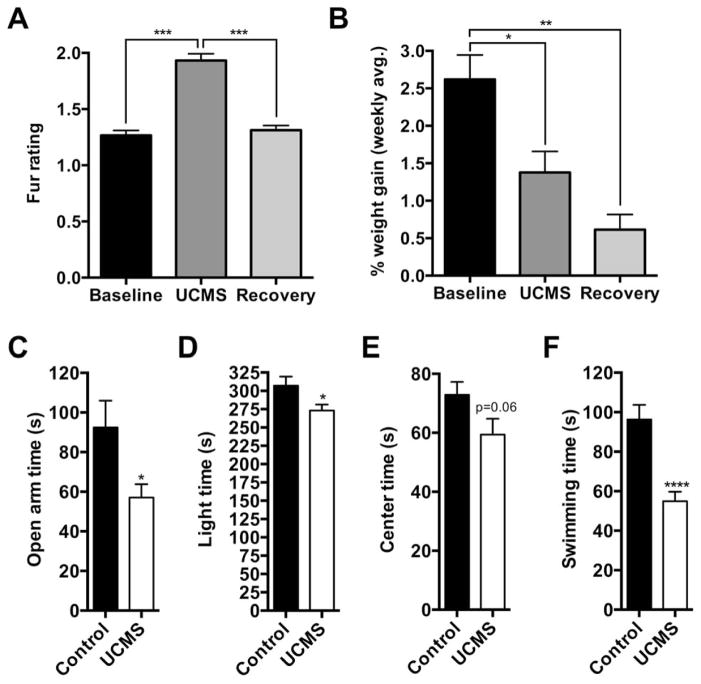
Figure 1.
UCMS induces a physiological and behavioral depression-like phenotype. UCMS worsened coat quality (A) and attenuated weight gain that persisted during the recovery period (B). UCMS increased anxiety-like and depression-like behaviors (C–F), as evident by: reduced time spent on the open arms of the elevated plus maze (C) and in the light side of dark-light box (D); and reduced the amount of time spent swimming during the forced swim test (F). Center time in the open-field approached significance (p=0.06) (E). *, p < 0.05; **, p < 0.01; ****, p < 0.0001; one-way ANOVA followed by Dunnett’s multiple comparisons test or independent sample t test. Data is represented as the mean ± SEM.
Lumicycle Recordings
Following UCMS, Per2::luc mice underwent behavioral testing (Figure 1B), and then were sacrificed to extract explants of brain regions implicated in MDD: medial prefrontal cortex (mPFC), central amygdala (CeA), basolateral amygdala (BLA), nucleus accumbens (NAc), ventral tegmental area (VTA), and suprachiasmatic nucleus (SCN). Individual samples were cultured on a culture membrane in a 35mm dish (PICMORG50, Millipore, Billerica, MA), as previously described (17). Circadian rhythms were assessed for four days by continuous recording of bioluminescence of Per2::luc reporter activity using the LumiCycle 32 (Actimetrics Wilmette, IL).
Quantitative real time RT-PCR
Following UCMS, male B6 mice (Control: N=36 and UCMS: N=36) underwent behavioral testing (Figure 1C) and mice were sacrificed at six ZTs across the day. RNA was isolated and converted to cDNA, followed by gene expression analysis for Per2 normalized to the housekeeping gene Gapdh (Supplement 1).
Data Analysis
Due to the masking effects of light in housing conditions consisting of a standard LD cycle, period and phase are not reported here for locomotor activity and body temperature rhythms because under entrained conditions these become unreliable measures of rhythm. Therefore, we used circadian amplitude as the primary marker of disruption for activity and temperature rhythms. The amplitude of activity and temperature rhythms were calculated as the spectral power at the corresponding peak tau or period of the rhythm as calculated from Chi-square periodogram analyses (ClockLab Software, Actimetrics). Circadian activity and temperature waveforms were constructed for the last week of each experimental period and also over the entire duration of the control or UCMS paradigms. These were analyzed using repeated measures ANOVA (Baseline, UCMS, Recovery) followed by Tukey’s posthoc tests corrected for multiple comparisons. Per2::luc rhythms in brain region explants were de-trended and assessed for period, amplitude, and phase (Supplement 1). Fur rating and body weights were analyzed using one-way ANOVA followed by Dunnett’s multiple comparisons test. Locomotor activity and body temperature rhythms were analyzed using repeated-measures ANOVA followed by Dunnet’s posthoc tests. Behavioral tests were analyzed independent samples t tests. Gene expression was analyzed using CircWave v.1.4 to test for significant rhythmicity using harmonic regression comprised of both sine and cosine waveforms (constrained period of 24 h and α=0.05) and two-way ANOVA with the main factors of group and time and Bonferroni post-hoc tests to investigate significant interactions. The center of gravity for each fitted waveform was used as an estimated acrophase and average amplitude was also extracted from the fitted curve. Amplitude was compared using unpaired t-tests. Correlations between rhythm amplitude and behaviors were assessed using one-tailed Pearson analyses based on a priori hypotheses of the direction of the correlation (R-coefficient), and amplitude correlations with fur ratings were analyzed using Spearman Rank non-parametric analyses (R correlation coefficient). Data is represented as the mean ± SEM. Significance was set at α=0.05.
RESULTS
UCMS alters anxiety-like and depression-like behaviors and reduces the amplitude of physiological circadian rhythms in mice
During the last week of UCMS, mice underwent behavioral testing (behaviors across cohorts were combined for analyses) (Supplemental Figure S1). Fur ratings and body weights were combined also, except for the molecular rhythms cohort (Figure 1B) because the effects of UCMS were more pronounced on weight gain in this cohort (Supplemental figure S2B). As expected, UCMS significantly increased the fur rating, indicating less attention to grooming, which resolved during the recovery phase (F2,30 = 88.36, p<0.001; posthoc, p<0.001) (Figure 1A). In addition, UCMS attenuated weekly body weight gain compared to controls, which persisted during the recovery phase (F2,30 = 15.56, p<0.001; posthoc: baseline vs. UCMS p<0.05, baseline vs. recovery p<0.01) (Figure 1B and supplemental figureS 2A for Per2::luc cohort during and following 4 week UCMS). Mice with or without UCMS displayed similar feeding times during the NSF paradigm (Supplemental figure S2B; t18=−0.03, p=0.84). Other behavioral tests showed that relative to the control group, mice exposed to UCMS spent significantly less time in the open arms of the EPM (Figure 1C; t40=2.56, p=0.014) and in the aversive (light) side of the D/L box (Figure 1D; t70=2.28, p=0.02), with a near significant reduction in the amount of time spent in the center of the OF (Figure 1E; t27=1.93, p=0.06) without changes in total activity (Supplemental figure S1C). UCMS exposed mice also displayed a significant reduction in the amount of time spent swimming during the FST (Figure 1F; t37=4.72, p<0.0001). Thus, the UCMS paradigm successfully induced a physiological and behavioral syndrome that is typically indicative of a depression-like state.
To assess the effect of UCMS on diurnal rhythms, locomotor activity and body temperature rhythms were recorded continuously during baseline, UCMS, and recovery experimental periods (Supplemental figure S1). UCMS significantly reduced the circadian amplitude of both activity and temperature (Activity: F2,30=104.77, p<0.001; posthoc: baseline vs. UCMS, p<0.001. Body temperature: F2,30=139.08, p<0.001; posthoc: baseline vs. UCMS, p<0.001). Decreased activity amplitude persistent into the recovery phase (posthoc: baseline vs. recovery, p<0.001), while body temperature amplitude recovered (posthoc: baseline vs. recovery, p<0.001) (Figures 2A and 2B, respectively). There were no differences in average total activity (p=0.14) or average body temperature (p=0.27) between baseline and UCMS indicating that the decreased amplitude is not simply due to stress-induced changes in locomotor activity (Figures 2C and 2D).
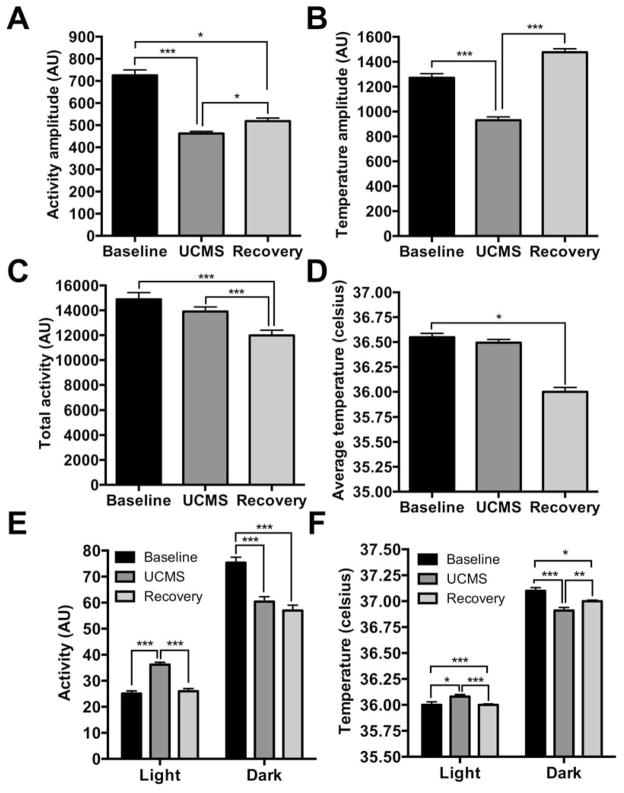
Figure 2.
Effects of UCMS on circadian amplitude of locomotor activity and body temperature. UCMS reduced circadian amplitude of locomotor activity (A) and body temperature (B), which was independent of changes in total activity and average temperature (C,D). Circadian amplitude of locomotor activity remained reduced during the recovery period (A), whereas temperature amplitude recovered to baseline levels (B). UCMS increased locomotor activity specifically during the light phase of light-dark cycle, which returned to baseline levels during the recovery period (E). UCMS induced reductions in activity during the dark phase remained during the recovery period (E). Similarly, body temperature (average) increased during the light phase and decreased during the dark phase of the light-dark cycle (F). *, p < 0.05; **, p < 0.01; ***, p < 0.001; one-way ANOVA followed by Dunnett’s multiple comparisons test or two-way ANOVA followed by Tukey’s post hoc test. Data is represented as the mean ± SEM.
Indeed, mice were more active in the light phase (p<0.001) and less active in the dark phase (p<0.001) during the UCMS period, resembling a “shift” of activity rhythms, rather than an overall change in activity level (Two-way ANOVA: Circadian phase, F1,90=762.8, p<0.0001; Treatment, F2,90=17.29, p<0.0001; Phase x Treatment, F2,90=37.75, p<0.0001). During UCMS, body temperature rhythms were similarly altered across the light (posthoc: baseline vs. UCMS, p<0.05) and dark (posthoc: baseline vs. UCMS, p<0.001) phases (Two-way ANOVA: Circadian phase, F1,90=2601, p<0.0001; Treatment, F2,90=3.363, p=0.039; Phase x Treatment, F2,90=16.94, p<0.0001) (Figure 2F). Activity and body temperature circadian waveforms during the last week of baseline, UCMS, and the recovery period also reflected the reduction in rhythm amplitude compared to baseline, particularly during the night (For activity: time, F48,2160=41.25, treatment, F2,2160=77.99, and interaction, F96,2160=3.71. For temperature: time, F48,2160=464.5, treatment, F2,2160=573.3, and interaction, F96,2160=28.01. All p<0.0001. Posthoc tests: baseline vs. UCMS, time (hr) 8, 9, 9.5, 10, 10.5, 11, 16–17.5, all p<0.05) (Figure 4). Posthoc tests also showed increased activity during the day in UCMS exposed mice (0, 0.5, 1, 7, 7.5, all p<0.05) (Figure 3A). Increased body temperature was observed during the light phase (Posthoc tests: time (hr), 0–7.5 and 20.5–24, all p<0.05) and decreased during the dark phase (Posthoc tests: time (hr), 8–20, all p<0.05) (Figure 3B). There was a decrease in average body temperature and total activity during the recovery phase (Activity, one-way ANOVA: F2,45=10.81, p<0.0001, posthoc: baseline vs. recovery, p<0.001; temperature, one-way ANOVA: F2,45=63.60, p<0.0001, posthoc: baseline vs. recovery, p<0.0001), which could account for post-UCMS changes in rhythm amplitude (Figure 2C and 2D). Circadian waveforms for activity and body temperature averaged across all weeks of each period of the experiment are in supplemental figure S3. These data indicate that chronic stress alters the circadian amplitude of physiological measures.
Figure 4.
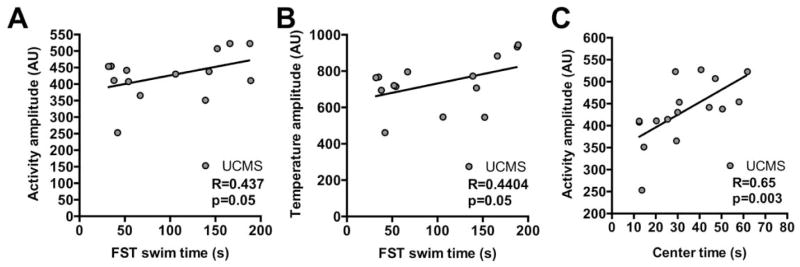
Behavioral and physiological circadian amplitudes positively correlate with depression-like and anxiety-like behaviors. Mice exposed to UCMS tended to have reduced circadian amplitudes of locomotor activity and body temperature. Both activity and temperature circadian amplitudes significantly correlated with time spent swimming during the forced swim test (FST) (A,B), whereas only activity amplitude correlated with time spent in the center of the open-field (C). These correlations strongly suggest that reduced circadian amplitude is associated with increased depression-like and anxiety-like behaviors following UCMS. One-tailed Pearson analyses with R correlation coefficient. Significance was set at α=0.05.
Figure 3.
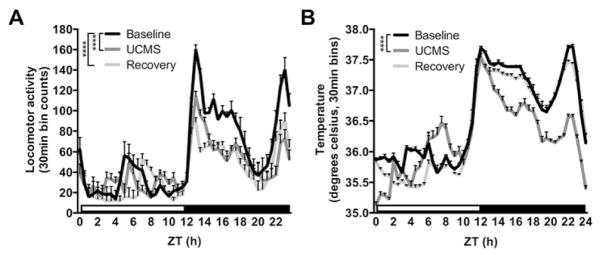
UCMS reduces locomotor activity and body temperature during the dark (or active) phase of the light dark cycle. Circadian waveforms for locomotor activity during the last full week of baseline (week 2), UCMS (week 8), and recovery (week 11) experimental periods show UCMS primarily reduced locomotor activity during the dark (or active) phase, which persisted during the recovery period (A). UCMS also reduced body temperature primarily during the active phase, although this was reversed during the recovery period (B). See results for significant differences between groups at specific time-points. Main effects of groups are depicted. ****, p < 0.0001; repeated-measures two-way ANOVA followed by Tukey’s multiple comparisons tests. Data is represented as the mean ± SEM.
Circadian activity and body temperature amplitudes correlate with mood-related behaviors
We next examined whether circadian activity and body temperature amplitude were correlated with mood-related behaviors. We found that the time spent swimming during FST was strongly correlated with both circadian activity and body temperature amplitude (Figure 4A and 4B, respectively). There was a positive relationship between activity amplitude and the amount of time spent swimming (reduced despair-like behavior) (p=0.05, R=0.437) (Figure 4A). A similar relationship was found for temperature amplitude and FST behavior (p=0.05, R=0.4404) (Figure 4B). In addition, time spent in the center of the OF was significantly correlated with activity amplitude (p=0.003, R=0.65) (Figure 4C). This relationship was unrelated to any change in overall activity (distance traveled in cm) during the OF assay (control: 4759.9 ± 268.1 and UCMS: 4402 ± 106.6; t=1.273, p=0.21). Other behaviors showed no significant correlations with activity amplitude or temperature amplitude (Supplemental figure S4).
UCMS selectively alters circadian amplitude of molecular rhythms in a brain region-specific manner
Per2 is a commonly used marker of molecular rhythm integrity (18). To investigate whether UCMS alters rhythms in specific brain regions associated with depression, Per2::luc mice were exposed to UCMS, tested for anxiety-like and depression-like behaviors, and explants of six brain regions (SCN, NAc, PFC, VTA, BLA, and CEA) were immediately dissected and examined for rhythmicity over a period of four days (Supplemental Figure S1).
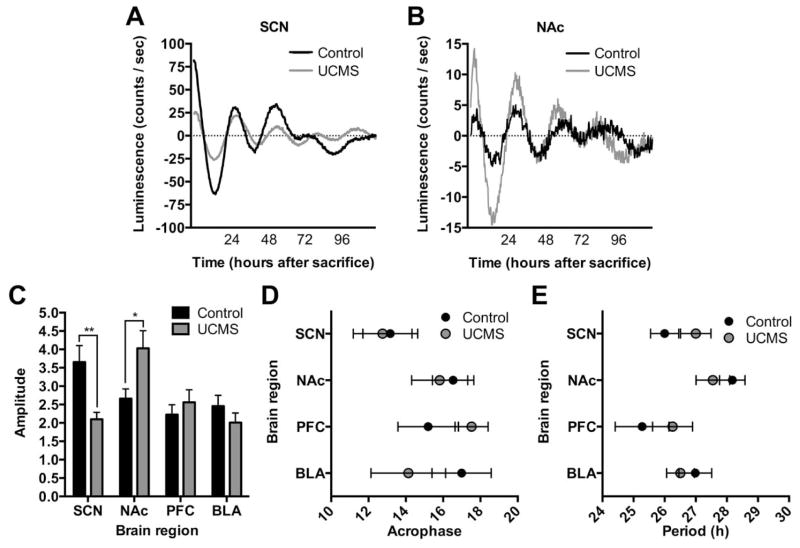
As expected, the SCN displayed the most robust rhythms with only 2 of 44 samples (4.5%) eliminated based on our exclusion criteria (see methods). SCN, NAc, PFC, and BLA showed robust rhythms with a relatively low percentage of sample attrition (4.5%, 13.6%, 6.8%, and 10.3%, respectively). While, it was difficult to obtain consistent rhythms in VTA and CEA samples (Supplemental figure S5), 61% of VTA samples (N=7 control and 11 UCMS) and 66.7% of CEA (N=9 control and 11 UCMS) samples displayed weak rhythms and were able to be used for analysis. Representative plots for each region are shown in Figures 5A,B and supplemental figure S6. Average period, phase, and amplitude of Per2 rhythms were examined across all six regions. Period and phase were unchanged between control and UCMS groups in all regions (Figure 5D,E, respectively). However, UCMS significantly reduced Per2::luc rhythm amplitude in the SCN (p=0.01) (Figure 5A,C), while surprisingly, rhythm amplitude in the NAc was significantly increased (p=0.05) (Figure 5B,C). Luciferase reporter rhythms in other brain regions were unchanged (Figure 5C–E and supplemental figure S5).
Figure 5.
UCMS alters circadian amplitude of molecular rhythms specifically in the SCN and NAc of mice. Representative traces of Per2::luc reporters in the SCN (A) and NAc (B) displaying alterations in molecular rhythm amplitude in UCMS exposed mice. UCMS reduced the Per2::luc reporter circadian amplitude in the SCN (A,C), and increased the amplitude in the NAc (B,C), without affecting amplitude in the PFC or BLA. UCMS had no significant effects on circadian acrophase or period (D,E). *, p < 0.05; two-way ANOVA (main effects: time and treatment) or independent samples t test (control vs. UCMS).
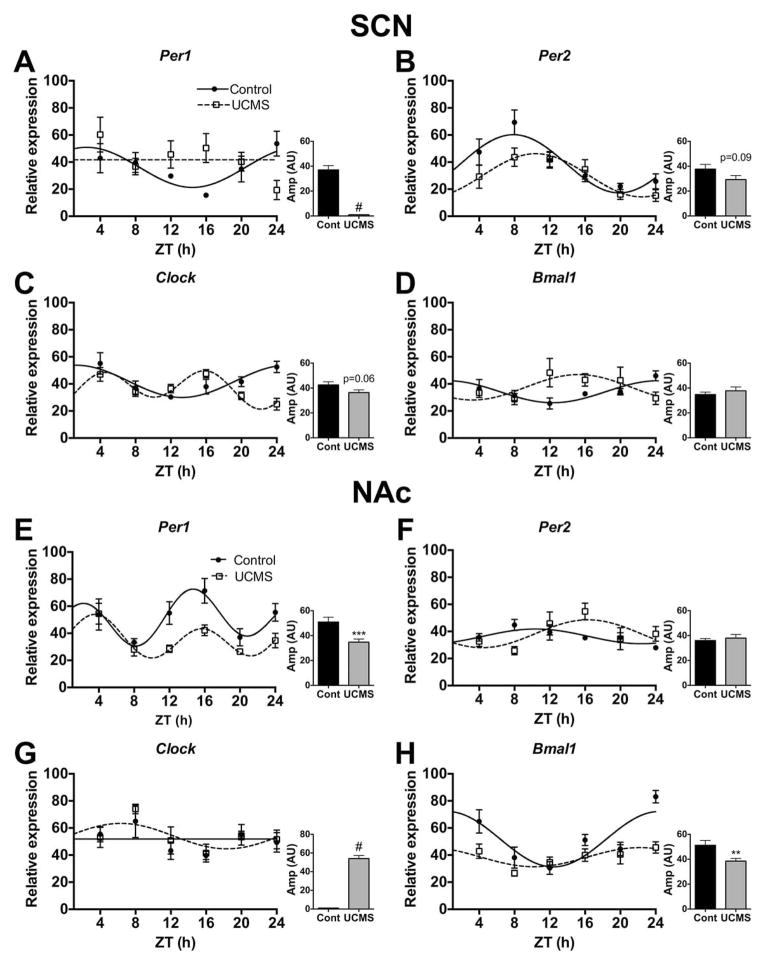
UCMS also altered diurnal rhythms of expression for several of the core circadian genes in the SCN (Figure 6A–D) and NAc (Figure 6E–H; see statistics in supplemental table S1). Rhythms were detected for all genes except for Per1 (UCMS) and Clock (Control) (fitted curves, p<0.05). Chronic stress reduced diurnal rhythms of Per1 in the SCN (n.s. curve; Figure 6A) and NAc (p<0.001; Figure 6E), while significantly altering expression of Per2 in the SCN (p=0.03; Figure 6B) and NAc (p<0.01; Figure 6F). Per1 was also significantly phase advanced (p<0.01; Figure 7B) in the NAc due to stress. Diurnal rhythms of Clock and Bmal1 were also altered by stress. In the SCN, stress significantly delayed the acrophase for both genes (p<0.05; Figure 7A). In the NAc, stress seemed to induce a robust diurnal rhythm of Clock (fitted curve, p<0.05; Figure 6G), while reducing the amplitude of Bmal1 and delaying its acrophase (Figure 6H and 7B, respectively). Thus, UCMS significantly altered the amplitude of Per2::luc rhythms and the diurnal expression rhythms of core circadian genes in the SCN and NAc.
Figure 6.
UCMS alters diurnal rhythms of circadian gene expression in the SCN and NAc of mice. Brains were collected across the day (ZT4-24) and the SCN and NAc were micropunched for gene expression analyses. Chronic stress altered the diurnal rhythms of gene expression for the core circadian genes, including Per1, Per2, Clock, and Bmal1 in the SCN (A–D), and in the NAc (E–H). Sine wave fits using linear harmonic regression with an assumed period of 24 h for control and UCMS mice (solid and dotted lines, respectively) are superimposed with group means ± SEM for each ZT. Straight line denotes no significant curve fit, and thus a lack of detectable rhythms. All curve fits are significant (p<0.05). **, p<0.01 and ***, p<0.001, unpaired t-tests between control and UCMS amplitude (graph inlets). #, represents no amplitude value due to an undetectable rhythm.
Figure 7.
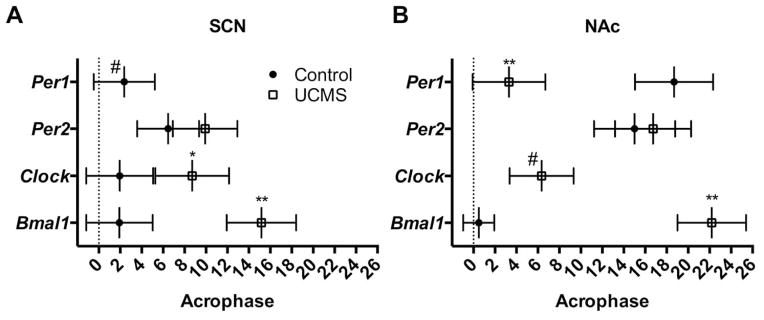
UCMS alters the acrophases of diurnal expression rhythms of core circadian genes in the SCN and NAc of mice. Chronic stress delayed the acrophase of Clock and Bmal1 in the SCN (A) and Bmal1 in the NAc (B), while promoting rhythm robustness of Clock in the NAc (B). Acrophases were derived from the center of gravity values of the fitted harmonic curves (+ SEM) and represent estimations of the circadian phase corresponding to the peak of the rhythm. *, p<0.05; **, p<0.01; unpaired t-tests between control and UCMS. #, represents no amplitude value due to an undetectable rhythm.
Circadian molecular rhythm amplitudes correlate with anxiety-like and depression-like behaviors
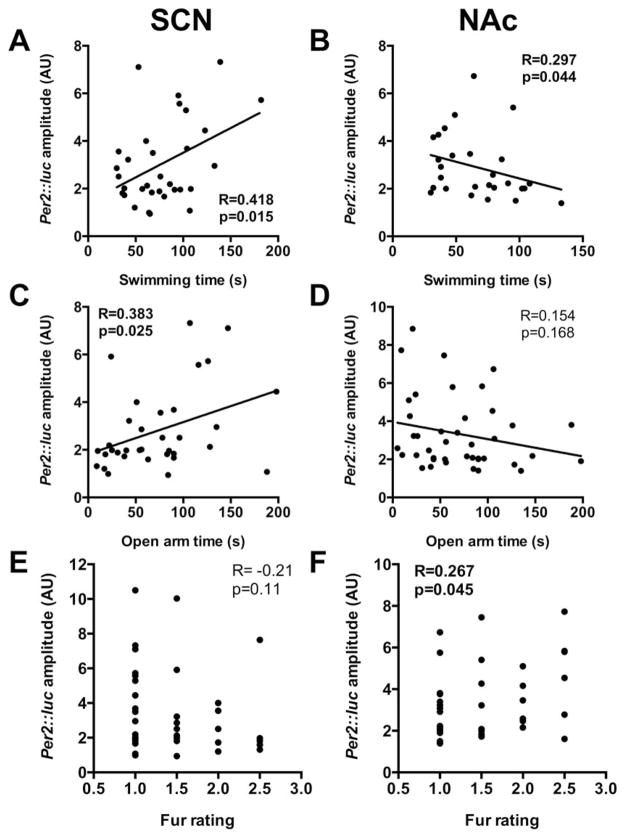
Similar to our previous correlation analysis, we examined whether the amplitude of region-specific molecular rhythms reflected changes in mood-related behaviors. Focusing on the two regions where we saw significant amplitude changes (SCN and NAc), we found strong correlations between circadian amplitude and time spent swimming in the FST and time spent in the open arms in the EPM (Figure 8). Time spent swimming positively correlated with Per2::luc rhythm amplitude in the SCN (p=0.015, R=0.418) (Figure 8A), while rhythm amplitude in the NAc negatively correlated with time spent swimming (p=0.044, R=0.297) (Figure 8B). Time spent in the open arms also positively correlated with Per2::luc rhythm amplitude in the SCN (p=0.025, R=0.383) (Figure 8C), but not with amplitude in the NAc (n.s.) (Figure 8D). These data indicate that increased anxiety-like and depression-like behavior is related to lower rhythm amplitude in the SCN and higher amplitude in the NAc. We also found that Per2::luc rhythm amplitude in the NAc was significantly correlated with fur rating following UCMS, such that increased amplitude was associated with increased fur rating (p=0.045, R=0.267) (Figure 8F). Fur rating failed to significantly correlate with Per2::luc rhythm amplitude in the SCN (p=0.11, R=−0.21) (Figure 8E). Significant correlations were not found for any other brain region (Supplemental figure S6).
Figure 8.
Opposing changes in molecular rhythm amplitude in the SCN and NAc following UCMS are correlated with mood-related behaviors and fur rating. Per2::luc reporter circadian amplitude for the SCN positively correlated while for the NAc amplitude negatively correlated with time spent swimming during the forced swim test (A,B). Similar correlations were found for time spent on the open arms of the elevated plus maze (C,D). Molecular rhythm amplitude in the SCN was also negatively correlated fur rating (E), although this failed to reach significance (p = 0.11), and for the NAc, was positively correlated with fur rating (F). Correlations were analyzed using mice from both control (black dots) and UCMS (red dots) groups. One-tailed Pearson analyses (R correlation coefficient) for amplitude and behavioral measures; Spearman Rank non-parametric analyses (R correlation coefficient) for amplitude and fur ratings. Significance was set at α=0.05.
DISCUSSION
In the present study, we demonstrated that UCMS alters the circadian amplitude of both physiological rhythms and molecular rhythms in the mouse brain. Following UCMS, there were significant reductions in circadian amplitude for both activity and body temperature, which appear to approximate the circadian defects observed in humans suffering from MDD (1, 2). In addition, UCMS induced brain-region specific alterations in circadian amplitude were correlated with behavioral and physiological markers of a depression-like syndrome.
UCMS as a Model of Circadian Amplitude Disruptions in MDD
While a rodent model that fully recapitulates human depression is unattainable, UCMS is becoming a well-validated paradigm to induce a depression-like syndrome that has high face validity to human depression, including response to antidepressant treatment (16). UCMS was used to investigate the biological links between circadian rhythms and depression, in part, because mice are exposed to stressors randomly across the circadian cycle, which prevents entrainment to daily stressors. Our data indicates that UCMS accurately recapitulates physiological disruptions of circadian rhythms reported in humans with MDD (1, 19)—namely decreased circadian amplitude in both activity and body temperature. In our study, mice exposed to UCMS displayed decreased amplitude of physiological and behavioral rhythms that is not due to a change in average activity level or body temperature; however, changes in average activity level and body temperature could account for altered amplitude in the recovery phase and could indicate long-term consequences of UCMS (20). Rats exposed to a more intensive chronic stress procedure were also shown to have decreased amplitude of circadian activity (21) and mice susceptible to chronic social defeat stress have decreased body temperature amplitude (22). Moreover, chronic restraint stress in mice and chronic unpredictable stress in rats both alter PER2 expression in the SCN (23, 24). Thus, these changes in rhythms are common among different depression-related paradigms, suggesting that they are important in the development of a similar depression-like syndrome.
A recent study from human postmortem brains reported reduced rhythm amplitude of circadian genes, which were accompanied by shifts in acrophase and disrupted phase relationships between individual circadian genes (14). We also found that overall Per2 expression in the SCN was dampened. In the NAc, we found a robust increase in Per2::luc rhythm amplitude when measured within individual animals, but did not see a similar increase in amplitude in Per2 gene expression when measured from separate cohorts over the light/dark cycle. Continuous monitoring of ex-vivo Per2::luc reporter rhythms poses distinct advantages over reconstructing circadian gene rhythms by cross-sectional sampling, such as enhanced measurement sensitivity of gene rhythms over multiple days within the same animal. In humans with MDD, the primary circadian abnormality most often reported is dampened circadian amplitude (19, 25). Importantly, it has been postulated that the therapeutic effects of some first line medications for mood disorders, including lithium, valproic acid, and fluoxetine, are partially due to their strong ability to increase circadian rhythm amplitude (14, 15, 26–29). Future experiments will determine whether UCMS leads to an enhancement of circadian “coupling” between individual neurons in the NAc or “decoupling” between pacemaker neurons in the SCN.
In both UCMS and human depression the negative feedback of the HPA stress axis, which enhances glucocorticoid (GC) release in response to stress, is impaired (30–34). Interestingly, UCMS increased and decreased the amplitude Per2::luc rhythms in the NAc and SCN, respectively, while these rhythms were relatively unaffected in other regions commonly associated with anxiety and depression. Differential effects of chronic stress on rhythm amplitude between the NAc and SCN may be due to differences in glucocorticoid receptor (GR) distribution. GRs are highly expressed in the NAc, and other areas of the mesocorticolimbic system, which regulate the activity of dopaminoceptive neurons in response to glucocorticoid (GC) release (35–37). Similar to peripheral tissues, GR may bind to glucocorticoid response promoter elements to enhance the expression of Per1 and Per2 (38–40). Previously, we implicated the Per genes in response to stress and anxiety behavior specifically in the NAc (41). It is possible that chronic stress enhances the amplitude of local molecular clocks in the NAc independently of SCN-derived inputs, possibly through GC signaling. GC signaling may entrain, or induce other alterations, in the NAc, which has been shown in peripheral tissues (42). However, the effects of UCMS on SCN rhythm amplitude are likely independent of GC signaling. The SCN is among the few brain regions that completely lack GR expression (38) and GR agonists fail to alter the circadian amplitude of Per2 (43). Future studies will examine the effects of UCMS on the activity of SCN afferents, including the paraventricular nucleus, medial raphe, and the dorsomedial nucleus of the hypothalamus, on SCN neuronal activity and rhythm amplitude (44, 45).
It is also possible that the SCN may send indirect projections to the NAc through the VTA, which suggests UCMS may “uncouple” these two brain regions. We were consistently faced with weak or immeasurable rhythms in the CEA and VTA, which is likely due to the rapid desynchronization of individual neurons in these regions upon dissociation from the SCN (46–48). The SCN sends multisynaptic connections to the VTA primarily through the medial preoptic area (48–50) and lesions to the SCN tend to produce an “antidepressant” effect, strongly suggesting the master circadian pacemaker regulates mood and affect through modulation of limbic circuits (51, 52). Also, the SCN strongly entrains the activity and firing of certain neuronal populations in the VTA (53), and following SCN lesions the rhythms of both the dopamine transporter (DAT) and tyrosine hydroxylase (TH) expression are disrupted—leading to constitutively higher DAT and higher TH levels across the diurnal cycle (54). Therefore, the SCN may inhibit neuronal activity in the NAc at certain times of day, which potentially has a direct role in the circadian regulation of emotion and mood.
Circadian Amplitude Correlates with Mood-related Behaviors
Chronic stress induced alterations in molecular rhythm amplitude may affect neuronal activity and firing, cellular synchrony within a brain region, and/or neurocircuitry communication and coherence. We found that circadian amplitude of locomotor activity, body temperature, and molecular rhythms in the SCN and the NAc were significantly correlated with behaviors related to anxiety and depression. Both circadian locomotor activity and temperature were positively associated with swimming time during the FST, a model of behavioral despair (55–58). Molecular rhythm amplitude was significantly correlated with swimming time during the FST, such that lower SCN amplitude and higher NAc amplitude were associated with increased depression-like behavior. A similar direction of association was found for open arm time during the EPM, a behavioral measure of anxiety-like behavior. In addition, lower SCN and higher NAc amplitude was associated with poorer fur quality. These results strongly suggest that variations in circadian behavioral, physiological, and molecular rhythms reflect variations in mood-related behaviors, in particular depression-like behavior in mice may be more related to altered circadian rhythms than anxiety-like behaviors.
Conclusions and Future Directions
Circadian disruption of specific brain regions may underlie certain mood-related behaviors and these results have implications for future investigations targeting region specific circadian mechanisms related to MDD. Combining Per2::luc rhythm assays with powerful tools, such as other molecular assays, in vitro and in vivo electrophysiology, and optogenetics, we will be able to specifically test the functional and physiological relevance of UCMS induced changes in circadian rhythm amplitude. Neurotherapeutics, behavioral modifications, and other treatments that alter circadian amplitude may contribute to alleviating depression-related symptoms.
Supplementary Material
Acknowledgments
We would like to thank Mary Harrington, David Welsh and members of their laboratories for help with data analysis and useful discussions. We would also like to thank Heather Buresch for assistance with mouse breeding and Emily Webster for assistance with genotyping. These studies were supported by MH082876 to CAM and MH098613 to NE.
Footnotes
The authors declare no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kronfeld-Schor N, Einat H. Circadian rhythms and depression: human psychopathology and animal models. Neuropharmacology. 2012;62:101–114. doi: 10.1016/j.neuropharm.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 2.Monteleone P, Maj M. The circadian basis of mood disorders: recent developments and treatment implications. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2008;18:701–711. doi: 10.1016/j.euroneuro.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Korszun A, Young EA, Engleberg NC, Brucksch CB, Greden JF, Crofford LA. Use of actigraphy for monitoring sleep and activity levels in patients with fibromyalgia and depression. Journal of psychosomatic research. 2002;52:439–443. doi: 10.1016/s0022-3999(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 4.Hickie IB, Naismith SL, Robillard R, Scott EM, Hermens DF. Manipulating the sleep-wake cycle and circadian rhythms to improve clinical management of major depression. BMC medicine. 2013;11:79. doi: 10.1186/1741-7015-11-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Terman M, Terman JS. Light therapy for seasonal and nonseasonal depression: efficacy, protocol, safety, and side effects. CNS spectrums. 2005;10:647–663. doi: 10.1017/s1092852900019611. quiz 672. [DOI] [PubMed] [Google Scholar]
- 6.Carney RM, Shelton RC. Agomelatine for the treatment of major depressive disorder. Expert opinion on pharmacotherapy. 2011;12:2411–2419. doi: 10.1517/14656566.2011.607812. [DOI] [PubMed] [Google Scholar]
- 7.Tuma J, Strubbe JH, Mocaer E, Koolhaas JM. Anxiolytic-like action of the antidepressant agomelatine (S 20098) after a social defeat requires the integrity of the SCN. Eur Neuropsychopharmacol. 2005;15:545–555. doi: 10.1016/j.euroneuro.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Weaver DR. The suprachiasmatic nucleus: a 25-year retrospective. Journal of biological rhythms. 1998;13:100–112. doi: 10.1177/074873098128999952. [DOI] [PubMed] [Google Scholar]
- 9.Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15(Spec No 2):R271–277. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- 10.Amir S, Stewart J. Motivational Modulation of Rhythms of the Expression of the Clock Protein PER2 in the Limbic Forebrain. Biological psychiatry. 2009;65:829–834. doi: 10.1016/j.biopsych.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 11.Meerlo P, van den Hoofdakker RH, Koolhaas JM, Daan S. Stress-induced changes in circadian rhythms of body temperature and activity in rats are not caused by pacemaker changes. Journal of biological rhythms. 1997;12:80–92. doi: 10.1177/074873049701200109. [DOI] [PubMed] [Google Scholar]
- 12.Segall LA, Amir S. Exogenous corticosterone induces the expression of the clock protein, PERIOD2, in the oval nucleus of the bed nucleus of the stria terminalis and the central nucleus of the amygdala of adrenalectomized and intact rats. Journal of molecular neuroscience : MN. 2010;42:176–182. doi: 10.1007/s12031-010-9375-4. [DOI] [PubMed] [Google Scholar]
- 13.Segall LA, Perrin JS, Walker CD, Stewart J, Amir S. Glucocorticoid rhythms control the rhythm of expression of the clock protein, Period2, in oval nucleus of the bed nucleus of the stria terminalis and central nucleus of the amygdala in rats. Neuroscience. 2006;140:753–757. doi: 10.1016/j.neuroscience.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 14.Li JZ, Bunney BG, Meng F, Hagenauer MH, Walsh DM, Vawter MP, et al. Circadian patterns of gene expression in the human brain and disruption in major depressive disorder. Proc Natl Acad Sci U S A. 2013;110:9950–9955. doi: 10.1073/pnas.1305814110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCarthy MJ, Wei H, Marnoy Z, Darvish RM, McPhie DL, Cohen BM, et al. Genetic and clinical factors predict lithium’s effects on PER2 gene expression rhythms in cells from bipolar disorder patients. Translational psychiatry. 2013;3:e318. doi: 10.1038/tp.2013.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mineur YS, Belzung C, Crusio WE. Effects of unpredictable chronic mild stress on anxiety and depression-like behavior in mice. Behav Brain Res. 2006;175:43–50. doi: 10.1016/j.bbr.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 17.Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bae K, Jin X, Maywood ES, Hastings MH, Reppert SM, Weaver DR. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron. 2001;30:525–536. doi: 10.1016/s0896-6273(01)00302-6. [DOI] [PubMed] [Google Scholar]
- 19.Souetre E, Salvati E, Belugou JL, Pringuey D, Candito M, Krebs B, et al. Circadian rhythms in depression and recovery: evidence for blunted amplitude as the main chronobiological abnormality. Psychiatry Res. 1989;28:263–278. doi: 10.1016/0165-1781(89)90207-2. [DOI] [PubMed] [Google Scholar]
- 20.Elizalde N, Gil-Bea FJ, Ramirez MJ, Aisa B, Lasheras B, Del Rio J, et al. Long-lasting behavioral effects and recognition memory deficit induced by chronic mild stress in mice: effect of antidepressant treatment. Psychopharmacology. 2008;199:1–14. doi: 10.1007/s00213-007-1035-1. [DOI] [PubMed] [Google Scholar]
- 21.Gorka Z, Moryl E, Papp M. Effect of chronic mild stress on circadian rhythms in the locomotor activity in rats. Pharmacology, biochemistry, and behavior. 1996;54:229–234. doi: 10.1016/0091-3057(95)02173-6. [DOI] [PubMed] [Google Scholar]
- 22.Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 23.Jiang WG, Li SX, Zhou SJ, Sun Y, Shi J, Lu L. Chronic unpredictable stress induces a reversible change of PER2 rhythm in the suprachiasmatic nucleus. Brain research. 2011;1399:25–32. doi: 10.1016/j.brainres.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Kinoshita C, Miyazaki K, Ishida N. Chronic stress affects PERIOD2 expression through glycogen synthase kinase-3beta phosphorylation in the central clock. Neuroreport. 2012;23:98–102. doi: 10.1097/WNR.0b013e32834e7ec2. [DOI] [PubMed] [Google Scholar]
- 25.Moller-Levet CS, Archer SN, Bucca G, Laing EE, Slak A, Kabiljo R, et al. Effects of insufficient sleep on circadian rhythmicity and expression amplitude of the human blood transcriptome. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E1132–1141. doi: 10.1073/pnas.1217154110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johansson AS, Brask J, Owe-Larsson B, Hetta J, Lundkvist GB. Valproic acid phase shifts the rhythmic expression of Period2::Luciferase. Journal of biological rhythms. 2011;26:541–551. doi: 10.1177/0748730411419775. [DOI] [PubMed] [Google Scholar]
- 27.McCarthy MJ, Welsh DK. Cellular circadian clocks in mood disorders. Journal of biological rhythms. 2012;27:339–352. doi: 10.1177/0748730412456367. [DOI] [PubMed] [Google Scholar]
- 28.McClung CA. Circadian genes, rhythms and the biology of mood disorders. Pharmacol Ther. 2007;114:222–232. doi: 10.1016/j.pharmthera.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uz T, Ahmed R, Akhisaroglu M, Kurtuncu M, Imbesi M, Dirim Arslan A, et al. Effect of fluoxetine and cocaine on the expression of clock genes in the mouse hippocampus and striatum. Neuroscience. 2005;134:1309–1316. doi: 10.1016/j.neuroscience.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 30.Heuser I, Yassouridis A, Holsboer F. The combined dexamethasone/CRH test: a refined laboratory test for psychiatric disorders. Journal of psychiatric research. 1994;28:341–356. doi: 10.1016/0022-3956(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 31.Ising M, Horstmann S, Kloiber S, Lucae S, Binder EB, Kern N, et al. Combined dexamethasone/corticotropin releasing hormone test predicts treatment response in major depression - a potential biomarker? Biological psychiatry. 2007;62:47–54. doi: 10.1016/j.biopsych.2006.07.039. [DOI] [PubMed] [Google Scholar]
- 32.Kunugi H, Ida I, Owashi T, Kimura M, Inoue Y, Nakagawa S, et al. Assessment of the dexamethasone/CRH test as a state-dependent marker for hypothalamic-pituitary-adrenal (HPA) axis abnormalities in major depressive episode: a Multicenter Study. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2006;31:212–220. doi: 10.1038/sj.npp.1300868. [DOI] [PubMed] [Google Scholar]
- 33.Lopez-Duran NL, Kovacs M, George CJ. Hypothalamic-pituitary-adrenal axis dysregulation in depressed children and adolescents: a meta-analysis. Psychoneuroendocrinology. 2009;34:1272–1283. doi: 10.1016/j.psyneuen.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Surget A, Tanti A, Leonardo ED, Laugeray A, Rainer Q, Touma C, et al. Antidepressants recruit new neurons to improve stress response regulation. Molecular psychiatry. 2011;16:1177–1188. doi: 10.1038/mp.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barik J, Parnaudeau S, Saint Amaux AL, Guiard BP, Golib Dzib JF, Bocquet O, et al. Glucocorticoid receptors in dopaminoceptive neurons, key for cocaine, are dispensable for molecular and behavioral morphine responses. Biological psychiatry. 2010;68:231–239. doi: 10.1016/j.biopsych.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 36.Der-Avakian A, Bland ST, Schmid MJ, Watkins LR, Spencer RL, Maier SF. The role of glucocorticoids in the uncontrollable stress-induced potentiation of nucleus accumbens shell dopamine and conditioned place preference responses to morphine. Psychoneuroendocrinology. 2006;31:653–663. doi: 10.1016/j.psyneuen.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 37.Graf EN, Wheeler RA, Baker DA, Ebben AL, Hill JE, McReynolds JR, et al. Corticosterone acts in the nucleus accumbens to enhance dopamine signaling and potentiate reinstatement of cocaine seeking. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:11800–11810. doi: 10.1523/JNEUROSCI.1969-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, et al. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289:2344–2347. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- 39.So AY, Bernal TU, Pillsbury ML, Yamamoto KR, Feldman BJ. Glucocorticoid regulation of the circadian clock modulates glucose homeostasis. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:17582–17587. doi: 10.1073/pnas.0909733106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamamoto T, Nakahata Y, Tanaka M, Yoshida M, Soma H, Shinohara K, et al. Acute physical stress elevates mouse period1 mRNA expression in mouse peripheral tissues via a glucocorticoid-responsive element. The Journal of biological chemistry. 2005;280:42036–42043. doi: 10.1074/jbc.M509600200. [DOI] [PubMed] [Google Scholar]
- 41.Spencer S, Falcon E, Kumar J, Krishnan V, Mukherjee S, Birnbaum SG, et al. Circadian genes Period 1 and Period 2 in the nucleus accumbens regulate anxiety-related behavior. Eur J Neurosci. 2013;37:242–250. doi: 10.1111/ejn.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liston C, Cichon JM, Jeanneteau F, Jia Z, Chao MV, Gan WB. Circadian glucocorticoid oscillations promote learning-dependent synapse formation and maintenance. Nature neuroscience. 2013;16:698–705. doi: 10.1038/nn.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van der Veen DR, Shao J, Xi Y, Li L, Duffield GE. Cardiac atrial circadian rhythms in PERIOD2::LUCIFERASE and per1:luc mice: amplitude and phase responses to glucocorticoid signaling and medium treatment. PloS one. 2012;7:e47692. doi: 10.1371/journal.pone.0047692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kiessling S, Eichele G, Oster H. Adrenal glucocorticoids have a key role in circadian resynchronization in a mouse model of jet lag. The Journal of clinical investigation. 2010;120:2600–2609. doi: 10.1172/JCI41192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Malek ZS, Sage D, Pevet P, Raison S. Daily rhythm of tryptophan hydroxylase-2 messenger ribonucleic acid within raphe neurons is induced by corticoid daily surge and modulated by enhanced locomotor activity. Endocrinology. 2007;148:5165–5172. doi: 10.1210/en.2007-0526. [DOI] [PubMed] [Google Scholar]
- 46.Abe M, Herzog ED, Yamazaki S, Straume M, Tei H, Sakaki Y, et al. Circadian rhythms in isolated brain regions. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:350–356. doi: 10.1523/JNEUROSCI.22-01-00350.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lamont EW, Robinson B, Stewart J, Amir S. The central and basolateral nuclei of the amygdala exhibit opposite diurnal rhythms of expression of the clock protein Period2. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:4180–4184. doi: 10.1073/pnas.0500901102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luo AH, Aston-Jones G. Circuit projection from suprachiasmatic nucleus to ventral tegmental area: a novel circadian output pathway. The European journal of neuroscience. 2009;29:748–760. doi: 10.1111/j.1460-9568.2008.06606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheng MY, Leslie FM, Zhou QY. Expression of prokineticins and their receptors in the adult mouse brain. The Journal of comparative neurology. 2006;498:796–809. doi: 10.1002/cne.21087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron. 2009;61:786–800. doi: 10.1016/j.neuron.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tataroglu O, Aksoy A, Yilmaz A, Canbeyli R. Effect of lesioning the suprachiasmatic nuclei on behavioral despair in rats. Brain Res. 2004;1001:118–124. doi: 10.1016/j.brainres.2003.11.063. [DOI] [PubMed] [Google Scholar]
- 52.Logan RW, Williams WP, 3rd, McClung CA. Circadian rhythms and addiction: mechanistic insights and future directions. Behavioral neuroscience. 2014;128:387–412. doi: 10.1037/a0036268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luo AH, Georges FE, Aston-Jones GS. Novel neurons in ventral tegmental area fire selectively during the active phase of the diurnal cycle. The European journal of neuroscience. 2008;27:408–422. doi: 10.1111/j.1460-9568.2007.05985.x. [DOI] [PubMed] [Google Scholar]
- 54.Sleipness EP, Sorg BA, Jansen HT. Diurnal differences in dopamine transporter and tyrosine hydroxylase levels in rat brain: dependence on the suprachiasmatic nucleus. Brain research. 2007;1129:34–42. doi: 10.1016/j.brainres.2006.10.063. [DOI] [PubMed] [Google Scholar]
- 55.Holmes PV. Rodent models of depression: reexamining validity without anthropomorphic inference. Critical reviews in neurobiology. 2003;15:143–174. doi: 10.1615/critrevneurobiol.v15.i2.30. [DOI] [PubMed] [Google Scholar]
- 56.Petit-Demouliere B, Chenu F, Bourin M. Forced swimming test in mice: a review of antidepressant activity. Psychopharmacology. 2005;177:245–255. doi: 10.1007/s00213-004-2048-7. [DOI] [PubMed] [Google Scholar]
- 57.Porsolt RD. Animal models of depression: utility for transgenic research. Reviews in the neurosciences. 2000;11:53–58. doi: 10.1515/revneuro.2000.11.1.53. [DOI] [PubMed] [Google Scholar]
- 58.Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.