Abstract
Objective
To assess whether partial mouth protocols (PRPs) result in biased estimates of the associations between smoking, alcohol, obesity, and diabetes with periodontitis.
Methods
Using a sample (n=6,129) of the 1996–1998 Atherosclerosis Risk in Communities study, we used measures of probing pocket depth and clinical attachment level to identify moderate-severe periodontitis. Adjusting for confounders, unconditional binary logistic regression estimated prevalence odds ratios (POR) and 95% confidence limits. Specifically, we compared POR for smoking, alcohol, obesity and diabetes with periodontitis derived from full-mouth to those derived from 4-PRPs [Ramfjörd, National Health and Nutrition Examination survey-III, modified-NHANES-IV and 42-site-Random-site selection-method]. Finally, we conducted a simple sensitivity analysis of periodontitis misclassification by changing the case definition threshold for each PRP.
Results
In comparison to full-mouth PORs, PRP PORs were biased in terms of magnitude and direction. Holding the full-mouth case definition at moderate-severe periodontitis and setting it at mild-moderate-severe for the PRPs did not consistently produce POR estimates that were either biased towards or away from the null in comparison to full-mouth estimates.
Conclusions
PRPs result in misclassification of periodontitis and may bias epidemiologic measures of association. The magnitude and direction of this bias depends on choice of PRP and case-definition threshold used.
Keywords: Periodontitis, Partial mouth protocol, Gold standard, Misclassification, Sensitivity
INTRODUCTION
Full-mouth periodontal examination is the preferred method for assessing periodontitis (Susin et al., 2005, Kingman et al., 2008, Kingman et al., 1988, Beck et al., 2006). It requires an assessment of periodontal pocket depth (PD) and clinical attachment level (CAL) on 6 sites per-tooth for a total of 168 sites in the fully dentate (excluding 3rd molars). Though preferred, full-mouth examination may be inefficient for time and resources (Beck et al., 2006) in epidemiologic surveys thus, several partial mouth protocols (PRPs) have been proposed. For instance, the 1959 periodontal disease index by Ramfjörd (Ramfjörd, 1959), the NIDCR indices used in the NHANES-III and NHANES-IV surveys (Page and Eke, 2007, Albandar et al., 1999) and the random-site-selection method (RSSM) by Beck et al (Beck et al., 2006). Several studies evaluating PRPs have quantified the extent to which prevalence (Kingman et al., 1988, Susin et al., 2005), severity (Kingman et al., 2008) and mean CAL (Beck et al., 2006) estimates are affected.
Periodontitis has been linked to systemic health conditions like diabetes and cardiovascular diseases (Beck et al., 1996, Beck et al., 1998, Beck and Offenbacher, 2001, Demmer et al., 2008). Additionally, modifiable lifestyle factors like smoking and alcohol are also associated with periodontitis. Indeed, smoking is a recognized independent risk factor for periodontitis (Do et al., 2008, Thomson et al., 2007, Tomar and Asma, 2000, Susin et al., 2004), while evidence of an association with alcohol (Tezal et al., 2004, Amaral Cda et al., 2009, Nishida et al., 2005, Nishida et al., 2004, Jansson, 2008, Lages et al., 2012, Kongstad et al., 2008, Pitiphat et al., 2003, Shimazaki et al., 2005, Tezal et al., 2001) and obesity (Genco et al., 2005, Saito and Shimazaki, 2007, Palle et al., 2013, Nishida et al., 2005, Saito et al., 1998, Saito et al., 2001, Wood et al., 2003) is mixed (Amaral Cda et al., 2009). Obesity shows a weak but positive association, while alcohol studies reported both positive and inverse associations. Although, some of these studies evaluated periodontitis from self-report (Pitiphat et al., 2003), others were based on measurements of PD and CAL on 6 sites per tooth (Tezal et al., 2001, Susin et al., 2004, Amaral Cda et al., 2008, Nishida et al., 2004, Nishida et al., 2005, Kongstad et al., 2008, Wood et al., 2003) or measures of either PD and/or CAL derived from PRPs (Tezal et al., 2004, Do et al., 2008, Thomson et al., 2007, Tomar and Asma, 2000, Lages et al., 2012, Shimazaki et al., 2005, Genco et al., 2005) using different case-classification methods and controlling for different confounding factors.
PRPs inherently underestimate prevalence of periodontitis, while severity can either be over- or underestimated (Kingman et al., 1988). This misclassification could lead to biased measures of association and incorrect inferences (Copeland et al., 1977, Thomas et al., 1993, Barron, 1977, Magder and Hughes, 1997). Specifically, if outcome status is misclassified non-differentially according to levels of a binary exposure, the odds ratio will be biased towards the null. However, with differential outcome misclassification, or more than two levels of exposure, the direction of bias becomes hard to predict (Rothman et al., 2008, Copeland et al., 1977).
Assuming there is no measurement error at the tooth-site, it follows that a healthy subject with no diseased sites cannot be misclassified as a case via PRP (no false-positives), because the respective PRP is a subset of the full-mouth (gold standard) examination. In contrast, a subject classified as a case via full mouth-exam can be misclassified as either healthy (false-negatives) if only healthy sites are evaluated for a given PRP or correctly classified as a case (true-positives) if a subset of diseased sites are evaluated. Therefore, with respect to periodontitis prevalence, PRPs have a specificity and positive predictive value of 100% (Kingman et al., 1988). This is necessarily not the case for severity because disease severity of sites evaluated for PRPs may differ from full-mouth severity.
Using the Atherosclerosis Risk in Communities (ARIC) study, we aim to investigate the effect of outcome misclassification from different PRPs on the association between periodontitis and the following factors: smoking, diabetes, alcohol and obesity. We additionally aim to conduct a simple sensitivity analysis by changing the PRP case definition threshold and evaluating the impact on the respective measures of association.
MATERIALS AND METHODS
Data Source/Study population
The Atherosclerosis Risk in Communities study (ARIC) is a prospective cohort study of community dwelling adults residing in 4-U.S. communities – Jackson, Mississippi; Washington county, Maryland; suburban Minneapolis, Minnesota; and Forsyth County, North Carolina. At baseline 15,792 adults aged 45–64 years provided data for the primary study aim, which was to investigate the etiology and natural history of atherosclerosis and clinical cardiovascular diseases. Our analytic dataset consists of a cross-sectional sample of 6,129 participants, eligible for periodontal examination at the 4th follow-up visit from 1996 to 1998 (Beck et al., 2006).
Periodontal assessment
Measures of probing pocket depth (PD) and gingival recession were assessed on 6 sites [mesio-buccal (MB), mid-buccal (B), disto-buccal (DB), mesio-lingual (ML), lingual (L), and disto-lingual (DL)] per tooth for all teeth in the mouth using a UNC 15 manual probe (Beck et al., 2006). Probing depth was defined as the distance from the free gingival margin to the bottom of the sulcus while gingival recession was defined as the distance from the cementoenamel junction (CEJ) to the free gingival margin. Clinical attachment level (CAL) was calculated as the sum of PD and gingival recession. Periodontal examiners had moderate to excellent reliability ranging from 83.2% to 90.2% (Beck et al., 2006).
Outcome definition
Using the Centers for Disease Control and prevention-American Academy for Periodontology (CDC-AAP) case classification, we classified individuals with ≥ 2 interproximal sites with CAL of ≥ 6 mm (not on the same tooth) AND ≥ 1 interproximal sites with PD of ≥5 mm as having severe periodontitis (Eke et al., 2010, Eke et al., 2012, Page and Eke, 2007). Otherwise, individuals with ≥ 2 interproximal sites (not on the same tooth) with CAL of ≥ 4 mm OR ≥ 2 interproximal sites (not on the same tooth) with PD of ≥ 5 mm were classified as having moderate periodontitis. For those individuals not meeting case definitions of moderate or severe periodontitis, those with ≥ 2 interproximal sites (not on the same tooth) with CAL of ≥ 3 mm AND either [≥ 2 interproximal sites (not on the same tooth) with PD of ≥ 4 mm OR ≥ 1 interproximal sites with PD of ≥ 5 mm] were classified as having mild periodontitis (Eke et al., 2010, Eke et al., 2012). Based on this definition, we defined a case as those with moderate or severe periodontitis. Individuals with fewer than 2 eligible interproximal sites were excluded rather than classified as non-cases because if they had more than 2 interproximal sites, could have been classified as cases.
Full-mouth (gold standard)
We identified a case based on a full-mouth examination consisting of measurements of PD and CAL on 6 sites per tooth, for all teeth in the mouth except third molars, for a maximum of 168 sites in the fully dentate.
Partial mouth protocols (PRPs)
Case classification was also performed using four PRPs. As with the gold standard method, only information from interproximal sites contributed to the CDC-AAP case definition.
Ramfjörd periodontitis index
We used measures of PD and CAL on 6 sites per tooth in the following index teeth: right maxillary first molar, left maxillary central incisor, left maxillary first premolar, left mandibular first molar, right mandibular central incisor and right mandibular first premolar (Ramfjörd, 1959), for a maximum of 36 sites per individual and did not substitute any missing Ramfjörd teeth (Ramfjörd, 1959).
NHANES-III
The NHANES-III method was introduced by the NIDCR and used in the 3rd NHANES (Page and Eke, 2007, Albandar et al., 1999). We simulated this method by randomly selecting a maxillary quadrant with its corresponding contralateral mandibular quadrant using a random number generator. The seed was changed 5 times and an arithmetic mean of the prevalence measure derived from each seed was taken. The data used for statistical analysis came from the seed with prevalence closest to the average of the 5 seed specific prevalence measures (within 0.02%). We identified a case from measures of PD and CAL on the MB and B sites on all teeth present in these quadrants except the third molars. This method produces a maximum of 28 sites per individual.
MB, B, DL sites (modified-NHANES-IV)
As in the NHANES-III, we randomly selected a maxillary quadrant with its corresponding contralateral mandibular quadrant using a random number generator and identified cases from measures of PD and CAL on the MB, B and DL sites on all teeth present in these quadrants except the third molars. This method which results in a maximum of 42 sites per individual has been reported to provide better prevalence and severity estimates compared to the NHANES-IV method that assesses the DB in place of the DL site (Kingman et al., 2008). Likewise, we also changed the seed 5 times, taking an arithmetic mean of the seed specific prevalence measures and using data generated from the seed that produced prevalence closest to the average of the 5 seed specific prevalence (within 0.02%).
42-random site selection method (RSSM)
First introduced by Beck et al (Beck et al., 2006). After designating the number of sites (n=42) to be chosen, we used a random number generator to select sites to be evaluated without replacement for each individual. This method allows for a maximum of a randomly selected 42-sites per individual and did not include third molars. The 42-site RSSM was chosen to allow easy comparison of estimates with the modified NHANES-IV method, which also allows for a maximum of 42 sites.
Exposures
Smoking was based on a report of smoking at least 100 cigarettes in a lifetime. Participants were categorized as current, former and never smokers. Alcohol consumption was modeled as continuous (grams/week) and also categorized as never drinkers, drinks <54g of alcohol/week which is equivalent to <5 glasses of wine/week (Sanders et al., 2011), 5–10 glasses of wine/week and >10 glasses of wine/week. Obesity was classified as having BMI ≥30 Kg/m2, overweight (BMI 25–<30 Kg/m2), normal weight (<25 Kg/m2). Presence of diabetes was based on fasting blood glucose ≥126 mg/dl or self-reported doctor’s diagnosis of diabetes (Yes/No).
Covariates
Age in years; gender; study site; race (Black/White); oral hygiene practices in the past day (not at all, 1, 2, ≥3); frequency of dental visits (regularly, when in discomfort, something needs fixing, never and other); educational attainment (<11, 12–17 and >17 years); number of teeth present in each assessment method.
Statistical analysis
From 6,793 eligible participants, 95 were excluded for missing all 6 Ramfjörd teeth. An additional 439 had only one eligible interproximal site and were also excluded. Of the remaining 6,259 participants, complete participant analysis was conducted on 6,129 participants with no missing covariates. Descriptive statistics of socio-demographic characteristics by case status was assessed for the different case identification methods. Chi-square tests assessed differences across categorical variables while unpaired t-tests assessed differences among continuous variables. In stratified analysis, effect measure modification among exposure variables was assessed with multiplicative interaction using a likelihood ratio test, setting threshold for statistical significance at p <0.10. We also assessed higher order polynomial terms for all continuous variables. Unconditional binary logistic regression estimated prevalence odds ratios (POR) and 95% confidence limits (C.L) of the associations between smoking, alcohol, obesity, diabetes and periodontitis adjusted for age, gender, study center, race, diabetes, education level, tooth brushing frequency, dental visits and number of teeth present. To quantify the magnitude of bias we calculated the difference between the natural log of adjusted PRPs POR and full-mouth estimate.
Next, we conducted a simple sensitivity analysis by varying the case definition threshold for each PRP, synonymous to changing their sensitivities. Specifically, we set the case definition threshold at mild-moderate-severe periodontitis and then at severe periodontitis and compared the resulting PORs to those derived from the full-mouth method with case definition held constant at moderate-severe periodontitis. Lastly, multiple linear regression modeled periodontitis as percent of sites with PD ≥4mm or CAL ≥3mm. The results of this analysis are included as an online appendix. Statistical tests were 2-sided and significance was set at p < 0.05. Statistical tests and data analysis were performed using SAS v. 9.4 (SAS Institute, Cary NC).
RESULTS
Table 1 shows the distribution of covariates and missing observations for each variable while Table 2 show distribution of cases and non-cases with no missing covariate information as well as the unadjusted associations. We estimated the prevalence of moderate-severe periodontitis as 57.5%, 23.7%, 17.5%, 33.8% and 48.0% for the full-mouth, Ramfjörd, NHANES-III, modified NHANES-IV and the 42-site RSSM methods respectively. The proportion of individuals with moderate-severe periodontitis appears to increase with increasing severity of smoking, alcohol, BMI and diabetes. However, this trend was not observed for alcohol and BMI for some of the PRPs. For example, the proportion of overweight cases was lower than normal weight (22.0% vs. 23.8%) in the Ramfjörd method. Accordingly, while a greater proportion were reported as cases with increasing levels of alcohol consumption, this trend was not observed for the Ramfjörd and NHANES-III methods (Table 1).
Table 1.
Covariates distribution according to moderate-severe periodontitis in the Atherosclerosis Risk in Communities study; Jackson, MS; Washington county, MD; Suburban Minneapolis, MN; and Forsyth County, NC; 1996–1998
|
|
Full-mouth* (N=6,259)
|
PRP 1† (N=6,259)
|
PRP 2‡ (N=6,259)
|
PRP 3§ (N=6,259)
|
PRP 4|| (N=6,259)
|
|
|---|---|---|---|---|---|---|
| Total N
|
Cases (row %)
|
Cases (row %)
|
Cases (row %)
|
Cases (row %)
|
Cases (row %)
|
|
| Periodontitis | ||||||
| Cases | 3,607 | 3,607 | 1,490 | 1,104 | 2,126 | 3,014 |
| Non-cases | 2,652 | 2,652 | 4,769 | 5,155 | 4,133 | 3,245 |
| Smoking¶ | ||||||
| Non-smoker | 2,989 | 1,440 (48.2) | 524 (17.5) | 348 (11.6) | 769 (25.7) | 1,164 (38.9) |
| Former | 2,533 | 1,622 (64.0) | 674 (26.6) | 511 (20.2) | 964 (38.1) | 1,353 (53.4) |
| Current | 720 | 534 (74.2) | 286 (39.7) | 243 (33.8) | 386 (53.6) | 487 (67.6) |
| missing | 17 | |||||
| Age mean (SD)¶ | 62.3 (5.63) | 62.8 (5.64) | 63.1 (5.62) | 63.4 (5.64) | 63.1 (5.73) | 63.0 (5.66) |
| No. of teeth assessed mean (SD) | 22.4 (5.53) | 22.2 (5.36) | 4.81 (1.27) | 10.8 (2.89) | 10.9 (2.86) | 21.4 (5.67) |
| Gender¶ | ||||||
| Females | 3,417 | 1,664 (48.7) | 600 (17.6) | 424 (12.4) | 888 (26.0) | 1,357 (39.7) |
| Males | 2,842 | 1,943 (68.4) | 890 (31.3) | 680 (23.9) | 1,238 (43.6) | 1,657 (58.3) |
| Race# | ||||||
| White | 5,138 | 2,982 (58.0) | 1,182 (23.0) | 877 (17.1) | 1,703 (33.2) | 2,481 (48.3) |
| Black | 1,077 | 594 (55.2) | 292 (27.1) | 211 (19.6) | 403 (37.4) | 504 (46.8) |
| missing | 44 | |||||
| Center¶ | ||||||
| Forsyth County, NC | 1,623 | 761 (46.9) | 259 (16.0) | 206 (12.7) | 416 (25.6) | 594 (36.6) |
| Jackson, MS | 966 | 515 (53.3) | 254 (26.3) | 173 (17.9) | 348 (36.0) | 430 (44.5) |
| Washington County, MD | 1,536 | 1,034 (67.3) | 476 (31.0) | 335 (21.8) | 634 (41.3) | 896 (58.3) |
| Minneapolis, MN | 2,090 | 1,266 (60.6) | 485 (23.2) | 374 (17.9) | 708 (33.9) | 1,065 (51.0) |
| Missing | 44 | |||||
| Alcohol¶ | ||||||
| Non-drinker | 3,991 | 2,215 (55.5) | 907 (22.7) | 694 (17.4) | 1,300 (32.6) | 1,847 (46.3) |
| <5 drinks/week | 973 | 560 (57.6) | 198 (20.4) | 144 (14.8) | 324 (33.3) | 453 (46.6) |
| 5–10 drinks/week | 615 | 375 (61.0) | 158 (25.7) | 102 (16.6) | 216 (35.1) | 322 (52.4) |
| >10 drinks/week | 663 | 447 (67.4) | 221 (33.3) | 162 (24.4) | 279 (42.1) | 382 (57.6) |
| missing | 17 | |||||
| Times brushed teeth yesterday¶ | ||||||
| Not at all | 89 | 69 (77.5) | 33 (37.1) | 31 (34.8) | 50 (56.2) | 63 (70.8) |
| 1 | 1,734 | 1,098 (63.3) | 497 (28.7) | 365 (21.1) | 677 (39.0) | 926 (53.4) |
| 2 | 3,589 | 2,008 (56.0) | 802 (22.4) | 591 (16.5) | 1,164 (32.4) | 1,671 (46.6) |
| ≥ 3 | 818 | 418 (51.1) | 152 (18.6) | 112 (13.7) | 226 (27.6) | 344 (42.1) |
| missing | 29 | |||||
| Education level (years)¶ | ||||||
| ≤ 11 | 754 | 501 (66.5) | 248 (32.9) | 205 (27.2) | 344 (45.6) | 451 (59.8) |
| 12–16 | 2,683 | 1,568 (58.4) | 628 (23.4) | 476 (17.7) | 926 (34.5) | 1,330 (49.6) |
| ≥ 17 | 2,813 | 1,534 (54.5) | 614 (21.8) | 423 (15.0) | 855 (30.4) | 1,230 (43.7) |
| missing | 9 | |||||
| Dental visit¶ | ||||||
| Regular basis | 4,739 | 2,624 (55.4) | 995 (21.0) | 712 (15.0) | 1,457 (30.7) | 2,132 (45.0) |
| Only when in discomfort | 447 | 300 (67.1) | 149 (33.3) | 127 (28.4) | 211 (47.2) | 268 (60.0) |
| Something needs fixing | 918 | 583 (63.5) | 293 (31.9) | 224 (24.4) | 389 (42.4) | 527 (57.4) |
| Don’t go/Other | 124 | 85 (68.6) | 46 (37.1) | 35 (28.2) | 59 (47.6) | 76 (61.3) |
| missing | 31 | |||||
| BMI (kg/m2)** | ||||||
| Normal (<25) | 1,652 | 896 (54.2) | 393 (23.8) | 277 (16.8) | 513 (31.1) | 735 (44.5) |
| Overweight (25–<30) | 2,592 | 1,492 (57.6) | 570 (22.0) | 445 (17.2) | 886 (34.2) | 1,262 (48.7) |
| Obese (≥30) | 2,004 | 1,212 (60.5) | 523 (26.1) | 381 (19.0) | 723 (36.1) | 1,011 (50.5) |
| missing | 11 | |||||
| Diabetes Mellitus¶ | ||||||
| Yes | 837 | 554 (66.2) | 241 (28.8) | 192 (22.9) | 342 (40.9) | 479 (57.2) |
| No | 5,384 | 3,028 (56.2) | 1,235 (22.9) | 905 (16.8) | 1,765 (32.8) | 2,513 (46.7) |
| missing | 38 | |||||
Full-mouth periodontal examination (6 sites per tooth for all teeth in the mouth except 3rd molars).
Partial mouth protocol based on Ramfjörd teeth (6 teeth and six sites measured per tooth)
Partial mouth protocol based on the NIDCR method which involves selection of a quadrant at random and its contralateral quadrant (examining only the MB and B sites per tooth)
Partial mouth recording protocol based on 2 randomly selected quadrants but examining the MB, B and DL sites.
Partial mouth recording protocol based on random selection of 42 sites per individual. All case definitions for periodontitis were based on the Centers for Disease Control-American Association for periodontology (CDC-AAP) case classification.
p <0.0001 for all assessment methods
p <0.1 for full-mouth, PRP3 and PRP4
p <0.001 for full-mouth.
Final sample size was 6,129 after excluding observations with missing covariates.
Table 2.
Unadjusted associations between smoking, alcohol consumption, BMI, and Diabetes with moderate-severe periodontitis in the Atherosclerosis Risk in Communities Study Jackson, MS; Washington county, MD; Suburban Minneapolis, MN; and Forsyth County, NC; 1996–1998 (N=6,129)
| Full-mouth exam* | PRP 1† | PRP 2‡ | PRP 3§ | PRP 4|| | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||
| Cases | Non- cases |
POR (95% C.L) | Cases | Non- cases |
POR (95% C.L) | Cases | Non- cases |
POR (95% C.L) | Cases | Non- cases |
POR (95% C.L) | Cases | Non- cases |
POR (95% C.L) | |
|
| |||||||||||||||
| Smoking | |||||||||||||||
| Non-smoker | 1,410 | 1,522 | 1. | 514 | 2,418 | 1. | 337 | 2,595 | 1. | 752 | 2,180 | 1. | 1,139 | 1,793 | 1. |
| Former | 1,592 | 899 | 1.91 (1.71, 2.13) | 656 | 1,835 | 1.68 (1.48, 1.92) | 499 | 1,992 | 1.93 (1.66, 2.24) | 942 | 1,549 | 1.76 (1.57, 1.98) | 1,327 | 1,164 | 1.80 (1.61, 2.00) |
| Current | 524 | 182 | 3.11 (2.59, 3.73) | 280 | 426 | 3.09 (2.59, 3.70) | 238 | 468 | 3.92 (3.23, 4.75) | 379 | 327 | 3.36 (2.84, 3.98) | 478 | 228 | 3.30 (2.77, 3.93) |
| Alcohol consumption | |||||||||||||||
| Non-drinker | 2,160 | 1,744 | 1. | 882 | 3,022 | 1. | 672 | 3,232 | 1. | 1,264 | 2,640 | 1. | 1,801 | 2,103 | 1. |
| <5 drinks/week | 555 | 409 | 1.10 (0.95, 1.26) | 195 | 769 | 0.87 (0.73, 1.03) | 142 | 822 | 0.83 (0.68, 1.01) | 321 | 643 | 1.04 (0.90, 1.21) | 450 | 514 | 1.02 (0.89, 1.17) |
| 5–10 drinks/week | 371 | 236 | 1.27 (1.07, 1.51) | 156 | 451 | 1.19 (0.97, 1.44) | 102 | 505 | 0.97 (0.77, 1.22) | 213 | 394 | 1.13 (0.94, 1.35) | 317 | 290 | 1.28 (1.08, 1.51) |
| >10 drinks/week | 440 | 214 | 1.66 (1.39, 1.98) | 227 | 437 | 1.70 (1.42, 2.04) | 158 | 496 | 1.53 (1.26, 1.87) | 275 | 379 | 1.52 (1.28, 1.80) | 376 | 278 | 1.58 (1.34, 1.87) |
| BMI (kg/m2) | |||||||||||||||
| Normal (<25) | 880 | 748 | 1. | 383 | 1,245 | 1. | 271 | 1,357 | 1. | 506 | 1,122 | 1. | 724 | 904 | 1. |
| Overweight (25–<30) | 1,459 | 1,084 | 1.14 (1.01, 1.30) | 555 | 1,988 | 0.91 (0.78, 1.05) | 431 | 2,112 | 1.02 (0.87, 1.21) | 862 | 1,681 | 1.14 (1.00, 1.30) | 1,232 | 1,311 | 1.17 (1.04, 1.33) |
| Obese (≥30) | 1,187 | 771 | 1.31 (1.15, 1.50) | 512 | 1,446 | 1.15 (0.99, 1.34) | 372 | 1,586 | 1.17 (0.99, 1.40) | 705 | 1,253 | 1.25 (1.09, 1.44) | 988 | 970 | 1.27 (1.12, 1.45) |
| Diabetes Mellitus | |||||||||||||||
| No | 2,983 | 2,327 | 1. | 1,214 | 4,096 | 1. | 887 | 4,423 | 1. | 1,738 | 3,572 | 1. | 2,476 | 2,834 | 1. |
| Yes | 543 | 276 | 1.54 (1.32, 1.79) | 236 | 583 | 1.37 (1.16, 1.61) | 187 | 632 | 1.48 (1.24, 1.76) | 335 | 484 | 1.42 (1.22, 1.65) | 468 | 351 | 1.53 (1.32, 1.77) |
Full-mouth periodontal examination (6 sites per tooth for all teeth in the mouth except 3rd molars).
Partial mouth protocol based on Ramfjörd teeth (6 teeth and six sites measured per tooth)
Partial mouth protocol based on the NIDCR method which involves selection of a quadrant at random and its contralateral quadrant (examining only the MB and B sites per tooth)
Partial mouth recording protocol based on 2 randomly selected quadrants but examining the MB, B and DL sites.
Partial mouth recording protocol based on random selection of 42 sites per individual.
POR-prevalence odds ratio, C.L-Confidence limits. All case definition for periodontitis were based on the Centers for Disease Control-American Association for periodontology (CDC-AAP) case classification.
In the unadjusted analysis, smoking was significantly associated with greater odds of moderate-severe periodontitis for all the case identification methods with no difference in qualitative conclusion regarding the relationship. Quantitatively, estimates for current smokers derived from the Ramfjörd method was biased towards the null [POR (95% C.L=3.09 (2.59, 3.70)] and away from the null [POR (95% C.L= 3.92 (3.23, 4.75)] for the NHANES-III method as compared to the full-mouth method. Although, also biased away from the null, POR estimates from the modified NHANES-IV and the 42-RSSM methods were quantitatively closer to the full-mouth estimate and to one another [POR (95% C.L=3.36 (2.84, 3.98), 3.30 (2.77, 3.93) and 3.11 (2.59, 3.73), respectively]. Alcohol, BMI and diabetes had a positive unadjusted dose-response trend for the full-mouth, modified-NHANES-IV and the 42-site RSSM methods, but a reversal in direction was observed for some of the individual comparisons obtained via the Ramfjörd and NHANES-III methods (Table 2).
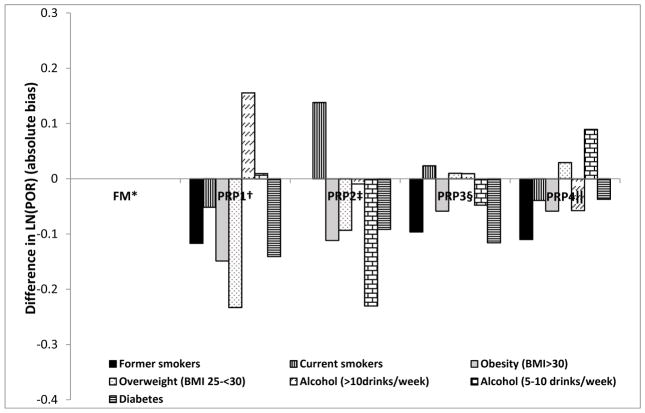
Upon covariate adjustment, the qualitative conclusion regarding the relationship between smoking and periodontitis at a cut-point of moderate-severe was similar across PRPs. Quantitatively, the Ramfjörd and 42-RSSM produced POR estimates that were biased towards the null while the NHANES-III and modified NHANES-IV methods produced POR that were biased away from the null for current vs. non-smokers with the NHANES-III method producing the most biased estimate away from the null relative to the full-mouth method. In contrast, the POR estimate for former vs. non-smokers was biased towards the null for three of the four PRPs (Table 3). Contrary to the results for smoking, the qualitative conclusions about the respective relationship between alcohol, or BMI, and moderate-severe periodontitis had dissimilarities across PRPs. First, the odds of moderate-severe periodontitis based on full-mouth method was slightly greater for drinkers and overweight and significantly higher among obese. While this was also true for the modified NHANES-IV and the 42-RSSM, the alcohol relationships did not have a threshold effect as observed for the full mouth method and the magnitude of associations for some levels of alcohol consumption and/or BMI were also greater. As with the full-mouth method, a joint conclusion of a non-significant relationship with alcohol consumption and a marginally significant relationship with obesity can only be made for the modified NHANES-IV and 42-RSSM (Table 3). Relative to the full-mouth estimate for diabetics, all PRPs produced PORs that were biased towards the null with the 42-RSSM producing estimate closest to the full-mouth estimate [POR (95% C.L= 1.32 (1.12, 1.55), 1.37 (1.16, 1.63)] respectively. The extent of over- or underestimated adjusted PRP POR estimates for smoking, alcohol, obesity and diabetes with moderate-severe periodontitis are presented in Figure 1. This figure shows that the direction of the bias from the PRPs is not always towards-or-away from the null in comparison to full-mouth estimates. Estimated associations were also biased for periodontitis defined as percentage of sites with PD ≥4mm or CAL ≥3mm (Appendix Tables 1, 2, 3).
Table 3.
Adjusted associations between smoking, alcohol consumption, BMI, and Diabetes with moderate-severe periodontitis in the Atherosclerosis Risk in Communities Study; Jackson, MS; Washington county, MD; Suburban Minneapolis, MN; and Forsyth County, NC; 1996–1998 (N=6,129)
| Prevalence (%) | Full-mouth exam*
|
PRP 1†
|
PRP 2‡
|
PRP 3§
|
PRP 4||
|
|---|---|---|---|---|---|
| 57.5% POR (95% C.L) |
23.7% POR (95% C.L) |
17.5% POR (95% C.L) |
33.8% POR (95% C.L) |
48.0% POR (95% C.L) |
|
| Smoking | |||||
| Non-smoker | 1. | 1. | 1. | 1. | 1. |
| Former | 1.63 (1.44, 1.84) | 1.45 (1.26, 1.67) | 1.63 (1.38, 1.91) | 1.48 (1.31, 1.68) | 1.46 (1.30, 1.65) |
| Current | 3.38 (2.77, 4.12) | 3.21 (2.64, 3.91) | 3.88 (3.14, 4.80) | 3.46 (2.87, 4.16) | 3.25 (2.68, 3.93) |
| Alcohol consumption | |||||
| Non-drinker | 1. | 1. | 1. | 1. | 1. |
| <5 drinks/week | 1.02 (0.87, 1.20) | 0.86 (0.71, 1.03) | 0.82 (0.66, 1.01) | 1.05 (0.89, 1.24) | 1.03 (0.88, 1.21) |
| 5–10 drinks/week | 1.07 (0.88, 1.30) | 1.08 (0.87, 1.34) | 0.85 (0.66, 1.09) | 1.02 (0.84, 1.25) | 1.17 (0.97, 1.42) |
| >10 drinks/week | 1.07 (0.88, 1.30) | 1.25 (1.02, 1.53) | 1.06 (0.85, 1.32) | 1.08 (0.89, 1.30) | 1.10 (0.91, 1.33) |
| BMI (kg/m2) | |||||
| Normal (<25) | 1. | 1. | 1. | 1. | 1. |
| Overweight (25–<30) | 1.01 (0.88, 1.15) | 0.80 (0.68, 0.94) | 0.92 (0.77, 1.11) | 1.02 (0.88, 1.18) | 1.04 (0.90, 1.19) |
| Obese (≥30) | 1.23 (1.06, 1.44) | 1.06 (0.89, 1.26) | 1.10 (0.91, 1.34) | 1.16 (0.99, 1.36) | 1.16 (1.00, 1.35) |
| Diabetes Mellitus | |||||
| No | 1. | 1. | 1. | 1. | 1. |
| Yes | 1.37 (1.16, 1.63) | 1.19 (1.00, 1.43) | 1.25 (1.03, 1.52) | 1.22 (1.03, 1.43) | 1.32 (1.12, 1.55) |
Full-mouth periodontal examination (6 sites per tooth for all teeth in the mouth except 3rd molars).
Partial mouth protocol based on Ramfjörd teeth (6 teeth and six sites measured per tooth)
Partial mouth protocol based on the NIDCR method which involves selection of a quadrant at random and its contralateral quadrant (examining only the MB and B sites per tooth)
Partial mouth recording protocol based on 2 randomly selected quadrants but examining the MB, B and DL sites.
Partial mouth recording protocol based on random selection of 42 sites per individual.
All analysis were adjusted for age, study site, race, gender, years of education, tooth-brushing frequency, frequency of dental visits, and number of teeth present in each selection. POR-prevalence odds ratio, C.L-Confidence limits. All case definition for periodontitis were based on the Centers for Disease Control-American Association for periodontology (CDC-AAP) case classification.
Figure 1.
Graphical representation of the absolute bias [LN(POR(full-mouth)) – LN(POR (PRP))] in the adjusted association between smoking, alcohol, obesity and diabetes with moderate-severe periodontitis in the Atherosclerosis risk in communities study, 1996–1998 (n=6,129). The respective bars represent the magnitude of positive (overestimated) or negative (underestimated) POR when the respective PRPs are used as opposed to full-mouth examination in assessing the relationship between each risk factor (smoking, alcohol, obesity, diabetes) and periodontitis.
*Full mouth examination for periodontitis (6 sites per tooth for all teeth in the mouth). †Partial mouth protocol based on Ramfjörd teeth (6 teeth and six sites per tooth) ‡ Parti al mouth protocol based on the NIDCR method which involves selection of a quadrant at random and its contralateral quadrant (examining only the MB and B sites per tooth). §Partial mouth recording protocol based on 2 randomly selected quadrants but examining the MB, B and DL sites. ||Partial mouth recording protocol based on random selection of 42 sites per individual. All case definitions for periodontitis were based on the Centers for Disease Control-American Association for periodontology (CDC-AAP) case classification.
In sensitivity analysis, setting the PRP case definition cut-point at mild-moderate-severe periodontitis produced estimates closer to those derived from the full-mouth method for some of the PRPs (Table 4). Holding the case definition threshold constant at moderate-severe periodontitis, produced prevalence of 57.5% for the full-mouth method. Correspondingly, a threshold of mild-moderate-severe for the PRPs produced prevalence of 47.0% (NHANES-IV) and 59.1% (42-site RSSM). Accordingly, PORs for smoking and diabetes derived with these cut-points were biased towards the null compared to the full-mouth estimate. Conversely, a more stringent case definition of severe periodontitis for the PRPs produced prevalence estimates of 7.41% (NHANES-IV) and 11.6% (42-site RSSM) and correspondingly POR estimates that were biased in terms of magnitude away from the null in comparison to the full-mouth estimates (Table 5).
Table 4.
Sensitivity analysis assessing the independent relationships between smoking, alcohol consumption, BMI, and Diabetes with full-mouth moderate-severe periodontitis in comparison with partial mouth mild-moderate-severe periodontitis, in the Atherosclerosis Risk in Communities Study Jackson, MS; Washington county, MD; Suburban Minneapolis, MN; and Forsyth County, NC; 1996–1998 (N=6,129)
| Moderate-Severe | Mild-Moderate-Severe Periodontitis | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Prevalence (%) | Full-mouth* | PRP 1† | PRP 2‡ | PRP 3§ | PRP 4|| |
|
|
|
||||
| 57.5% POR (95% C.L) |
36.5% POR (95% C.L) |
25.2% POR (95% C.L) |
47.0% POR (95% C.L) |
59.1% POR (95% C.L) |
|
|
|
|
||||
| Smoking | |||||
| Non-smoker | 1. | 1. | 1. | 1. | 1. |
| Former | 1.63 (1.44, 1.84) | 1.37 (1.21, 1.56) | 1.40 (1.22, 1.60) | 1.38 (1.23, 1.56) | 1.41 (1.24, 1.59) |
| Current | 3.38 (2.77, 4.12) | 3.19 (2.65, 3.85) | 3.28 (2.71, 3.98) | 3.26 (2.70, 3.94) | 3.23 (2.63, 4.00) |
| Alcohol consumption | |||||
| Non-drinker | 1. | 1. | 1. | 1. | 1. |
| <5 drinks/week | 1.02 (0.87, 1.20) | 1.00 (0.85, 1.17) | 0.93 (0.78, 1.11) | 1.16 (0.99, 1.36) | 1.08 (0.92, 1.27) |
| 5–10 drinks/week | 1.07 (0.88, 1.30) | 1.05 (0.87, 1.28) | 0.97 (0.79, 1.20) | 1.13 (0.93, 1.36) | 1.12 (0.92, 1.37) |
| >10 drinks/week | 1.07 (0.88, 1.30) | 1.20 (0.99, 1.45) | 1.07 (0.87, 1.30) | 1.07 (0.88, 1.29) | 1.14 (0.93, 1.40) |
| BMI (kg/m2) | |||||
| Normal (<25) | 1. | 1. | 1. | 1. | 1. |
| Overweight (25–<30) | 1.01 (0.88, 1.15) | 0.92 (0.80, 1.06) | 1.00 (0.86, 1.18) | 1.05 (0.92, 1.21) | 1.07 (0.93, 1.23) |
| Obese (≥30) | 1.23 (1.06, 1.44) | 1.25 (1.07, 1.46) | 1.25 (1.06, 1.49) | 1.36 (1.17, 1.58) | 1.22 (1.05, 1.43) |
| Diabetes Mellitus | |||||
| No | 1. | 1. | 1. | 1. | 1. |
| Yes | 1.37 (1.16, 1.63) | 1.14 (0.96, 1.34) | 1.24 (1.04, 1.48) | 1.20 (1.02, 1.41) | 1.34 (1.13, 1.59) |
Full-mouth periodontal examination (6 sites per tooth for all teeth in the mouth except 3rd molars).
Partial mouth protocol based on Ramfjörd teeth (6 teeth and six sites measured per tooth)
Partial mouth protocol based on the NIDCR method which involves selection of a quadrant at random and its contralateral quadrant (examining only the MB and B sites per tooth)
Partial mouth recording protocol based on 2 randomly selected quadrants but examining the MB, B and DL sites.
Partial mouth recording protocol based on random selection of 42 sites per individual.
All analysis were adjusted for age, study site, race, gender, years of education, tooth-brushing frequency, frequency of dental visits, and number of teeth present in each selection. POR-prevalence odds ratio, C.L-Confidence limits. All case definition for periodontitis were based on the Centers for Disease Control-American Association for periodontology (CDC-AAP) case classification.
Table 5.
Sensitivity analysis assessing the independent relationships between smoking, alcohol consumption, BMI, and Diabetes with full-mouth moderate-severe periodontitis in comparison with partial mouth severe periodontitis, in the Atherosclerosis Risk in Communities Study Jackson, MS; Washington county, MD; Suburban Minneapolis, MN; and Forsyth County, NC; 1996–1998 (N=6,129)
| Moderate-Severe | Severe Periodontitis | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Prevalence (%) | Full-mouth* | PRP 1† | PRP 2‡ | PRP 3§ | PRP 4|| |
|
|
|
||||
| 57.5% OR (95% C.L) |
4.34% OR (95% C.L) |
2.72% OR (95% C.L) |
7.41% OR (95% C.L) |
11.6% OR (95% C.L) |
|
|
|
|
|
|||
| Smoking | |||||
| Non-smoker | 1. | 1. | 1. | 1. | 1. |
| Former | 1.63 (1.44, 1.84) | 1.15 (0.84, 1.58) | 1.66 (1.10, 2.49) | 1.48 (1.17, 1.88) | 1.76 (1.45, 2.15) |
| Current | 3.38 (2.77, 4.12) | 4.47 (3.16, 6.33) | 4.88 (3.09, 7.72) | 4.15 (3.12, 5.53) | 4.11 (3.21, 5.26) |
| Alcohol consumption | |||||
| Non-drinker | 1. | 1. | 1. | 1. | 1. |
| <5 drinks/week | 1.02 (0.87, 1.20) | 0.98 (0.66, 1.45) | 0.84 (0.50, 1.41) | 0.95 (0.70, 1.29) | 1.03 (0.80 1.32) |
| 5–10 drinks/week | 1.07 (0.88, 1.30) | 0.46 (0.26, 0.84) | 0.71 (0.38, 1.34) | 0.84 (0.58, 1.21) | 0.85 (0.63, 1.16) |
| >10 drinks/week | 1.07 (0.88, 1.30) | 1.43 (0.99, 2.06) | 1.38 (0.88, 2.17) | 1.17 (0.86, 1.59) | 1.25 (0.97, 1.61) |
| BMI (kg/m2) | |||||
| Normal (<25) | 1. | 1. | 1. | 1. | 1. |
| Overweight (25–<30) | 1.01 (0.88, 1.15) | 1.06 (0.76, 1.47) | 0.88 (0.58, 1.33) | 0.78 (0.60, 1.00) | 0.98 (0.79, 1.23) |
| Obese (≥30) | 1.23 (1.06, 1.44) | 1.12 (0.78, 1.60) | 1.25 (0.81, 1.94) | 1.07 (0.82, 1.40) | 1.30 (1.03, 1.64) |
| Diabetes Mellitus | |||||
| No | 1. | 1. | 1. | 1. | 1. |
| Yes | 1.37 (1.16, 1.63) | 1.13 (0.80, 1.60) | 1.04 (0.67, 1.61) | 1.30 (0.99, 1.69) | 1.20 (0.96, 1.51) |
Full-mouth periodontal examination (6 sites per tooth for all teeth in the mouth except 3rd molars).
Partial mouth protocol based on Ramfjörd teeth (6 teeth and six sites measured per tooth)
Partial mouth protocol based on the NIDCR method which involves selection of a quadrant at random and its contralateral quadrant (examining only the MB and B sites per tooth)
Partial mouth recording protocol based on 2 randomly selected quadrants but examining the MB, B and DL sites.
Partial mouth recording protocol based on random selection of 42 sites per individual.
All analysis were adjusted for age, study site, race, gender, years of education, tooth-brushing frequency, frequency of dental visits, and number of teeth present in each selection. POR-prevalence odds ratio, C.L-Confidence limits. All case definition for periodontitis were based on the Centers for Disease Control-American Association for periodontology (CDC-AAP) case classification.
DISCUSSION
The aim of this study was to assess the impact of misclassification of periodontitis on epidemiologic measures of association that characterizes its relationship with smoking, alcohol, obesity and diabetes. For risk indicators like alcohol and BMI, the monotonicity (i.e. dose-response relationship) in proportions for moderate-severe periodontitis in Table 1 and of the POR estimates in Tables 2 and 3 based upon the full-mouth examination was not always preserved with PRPs. While monotonicity was preserved for smoking, it was not the case for alcohol and BMI for some of the PRPs.
While it was usually the case that the unadjusted PRP PORs were biased towards the null, there were exceptions. For instance, the unadjusted and adjusted PORs comparing former to never smokers for the Ramfjörd, modified-NHANES-IV and the 42-site RSSM methods were all biased towards the null relative to full-mouth, the corresponding estimates for NHANES-III was slightly biased away from the null. As has been previously shown, when exposure is measured in more than two levels, non-differential misclassification does not always produce bias of association measures towards the null and there can also be reversals in the direction of the trend (Weinberg et al., 1994).
Explaining the effects of misclassification relies on distinguishing disease classification at the site versus the individual level. According to standard definition, misclassification of periodontitis in individuals is differential when the probability that disease is misclassified varies between exposure groups (e.g., diabetics versus non-diabetics). In PRP designs, it can be shown mathematically that misclassification probabilities at the individual level generally depend in complex ways upon the pattern of disease across sites within the mouth as well as upon site-specific classification probabilities. Although it is possible for diseased sites to be differentially classified between examiners (and by extension, to groups of individuals), for this study we argue it is unlikely. This is because examiners who performed periodontal assessments had no prior knowledge of study aims or subject specific covariate patterns. Therefore, any differences observed at the site-level will be random as opposed to been systematic. On the other hand, by virtue of utilizing PRPs, estimates of disease status for individuals are likely to be differentially classified even if misclassification at the site-level is non-differential.
Indeed, differential misclassification at the subject level occurred in our study, as evidenced by PRP estimates being either biased towards or away from the null in comparison to full-mouth estimates. With a case definition cut-point set at moderate-severe and an assumption of no misclassification for the full-mouth method, we found that PRPs with higher moderate-severe periodontitis prevalence, specifically modified NHANES-IV (33.8%) and the 42-site RSSM (48.0%) tended to produce estimates for smoking, alcohol and BMI that were closer in magnitude to the respective full-mouth estimates.
In sensitivity analysis, holding the full-mouth case definition threshold constant at moderate-severe periodontitis, as this was the gold standard but setting it at mild-moderate-severe for the PRPs, produced PRP PORs that were closer or farther from those derived from the full-mouth method. Our sensitivity analysis reduced the degree of underestimation of periodontitis prevalence but not necessarily the bias of the respective measures of association. Likewise, a more stringent case definition cut-point set at severe periodontitis, which equates to decreasing sensitivity, also resulted in PRP estimates that were biased in terms of magnitude and direction for each of the PRPs we investigated. Given changes in specificities tend to have a much larger impact on effect estimates than changes in sensitivities (Edwards et al., 2014, Copeland et al., 1977, Lyles et al., 2011), the magnitude of bias that we observed were not so severe.
These comparisons were possible because data was available on full-mouth examination. However, in the absence of full-mouth data, general approaches for estimation in the presence of disease misclassification such as the maximum likelihood method described by Lyles et al (Lyles et al., 2011) or the multiple imputation method (Edwards et al., 2013) may be useful for PRPs even if misclassification is differential. While these methods may not be directly applicable, future research might extend them using study designs that conduct a full-mouth examination on a fraction of study participants and estimating a misclassification probability of disease status at the subject level that can be used as the basis for statistical adjustments in the estimation of exposure-outcome associations. This study design could lead to development of correction factors to estimate associations with negligible bias. However, given different studies use different criteria in identifying a case, estimating a single correction factor or procedure that cuts across case definitions will be challenging. Thus specifying a range of sensitivities and specificities to assess the robustness of study findings may be preferred.
We have provided a range of estimates resulting from periodontitis misclassification from different PRPs and have reported the magnitude and direction of these biases, providing insight to how PRP case definition threshold can bias measures of association.
STRENGTHS AND LIMITATIONS
PRPs utilized in this study were computer-generated which eliminated examiner errors had data been collected de facto. Our ability to eliminate this error further enhances the robustness of our findings. We had adequate samples that allowed for the adjustment of key confounders without sacrificing precision. Study limitations include case definition criteria that only utilized interproximal sites i.e. 4 sites of 6 (full-mouth), 1 site of 2 (NHANES-III), 2 sites of 3 (modified NHANES-IV) and variable number of interproximal sites (42-site-RSSM). Nevertheless, the CDC-AAP case classification is highly specific, utilizing sites most affected by disease thereby minimizing the false positive rate.
CONCLUSION
This article for the first time demonstrates how underestimation of disease by PRPs leads to bias in epidemiologic measures of association for periodontitis risk factors. The direction of bias in estimates derived from PRPs may not always be in the direction of no association, even if each periodontal measurement is free of any systematic error. This finding is critical as policy and research is governed by the relations between risk factors and disease, which are explicitly quantified by epidemiologic measures of association.
Supplementary Material
CLINICAL RELEVANCE.
Scientific Rationale for the Study
Underestimation of periodontitis prevalence is a well-documented limitation of PRPs. Given disease misclassification biases epidemiologic measures of association (MOA), the corresponding bias from use of PRPs to estimate associations between periodontitis and its risk factors has not been quantified.
Principal Findings
PRPs misclassify periodontitis and biases MOA. The magnitude and direction of bias depends on PRP used and case-definition threshold for classifying periodontitis.
Practical Implications
This finding is critical as clinical standards of care are governed by the relations between risk factors and disease, which are explicitly quantified by epidemiologic measures of association.
Acknowledgments
SOURCES OF FUNDING
A.A. Akinkugbe and V.M. Saraiya were supported by the National Institute of Health NRSA T90 Training Grant NIH/National Institute of Dental and Craniofacial Research (NIDCR) 5T90DE021986-04.
The Atherosclerosis Risk in Communities Study was carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). The authors thank the staff and participants of the ARIC study for their important contributions.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest pertaining to authorship and/or publication of this article.
References Cited
- Albandar JM, Brunelle JA, Kingman A. Destructive periodontal disease in adults 30 years of age and older in the United States, 1988–1994. J Periodontol. 1999;70:13–29. doi: 10.1902/jop.1999.70.1.13. [DOI] [PubMed] [Google Scholar]
- da Amaral CS, Luiz RR, Leao AT. The relationship between alcohol dependence and periodontal disease. J Periodontol. 2008;79:993–998. doi: 10.1902/jop.2008.070525. [DOI] [PubMed] [Google Scholar]
- da Amaral CS, Vettore MV, Leao A. The relationship of alcohol dependence and alcohol consumption with periodontitis: a systematic review. J Dent. 2009;37:643–651. doi: 10.1016/j.jdent.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Barron BA. The effects of misclassification on the estimation of relative risk. Biometrics. 1977;33:414–418. [PubMed] [Google Scholar]
- Beck J, Garcia R, Heiss G, Vokonas PS, Offenbacher S. Periodontal disease and cardiovascular disease. J Periodontol. 1996;67:1123–1137. doi: 10.1902/jop.1996.67.10s.1123. [DOI] [PubMed] [Google Scholar]
- Beck JD, Caplan DJ, Preisser JS, Moss K. Reducing the bias of probing depth and attachment level estimates using random partial-mouth recording. Community Dent Oral Epidemiol. 2006;34:1–10. doi: 10.1111/j.1600-0528.2006.00252.x. COM252 [pii] [DOI] [PubMed] [Google Scholar]
- Beck JD, Offenbacher S. The association between periodontal diseases and cardiovascular diseases: a state-of-the-science review. Ann Periodontol. 2001;6:9–15. doi: 10.1902/annals.2001.6.1.9. [DOI] [PubMed] [Google Scholar]
- Beck JD, Offenbacher S, Williams R, Gibbs P, Garcia R. Periodontitis: a risk factor for coronary heart disease? Ann Periodontol. 1998;3:127–141. doi: 10.1902/annals.1998.3.1.127. [DOI] [PubMed] [Google Scholar]
- Copeland KT, Checkoway H, McMichael AJ, Holbrook RH. Bias due to misclassification in the estimation of relative risk. Am J Epidemiol. 1977;105:488–495. doi: 10.1093/oxfordjournals.aje.a112408. [DOI] [PubMed] [Google Scholar]
- Demmer RT, Jacobs DR, Jr, Desvarieux M. Diabetes Care. United States: 2008. Periodontal disease and incident type 2 diabetes: results from the First National Health and Nutrition Examination Survey and its epidemiologic follow-up study; pp. 1373–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do LG, Slade GD, Roberts-Thomson KF, Sanders AE. Smoking-attributable periodontal disease in the Australian adult population. J Clin Periodontol. 2008;35:398–404. doi: 10.1111/j.1600-051X.2008.01223.x. [DOI] [PubMed] [Google Scholar]
- Edwards JK, Cole SR, Chu H, Olshan AF, Richardson DB. Accounting for outcome misclassification in estimates of the effect of occupational asbestos exposure on lung cancer death. Am J Epidemiol. 2014;179:641–647. doi: 10.1093/aje/kwt309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JK, Cole SR, Troester MA, Richardson DB. Accounting for misclassified outcomes in binary regression models using multiple imputation with internal validation data. Am J Epidemiol. 2013;177:904–912. doi: 10.1093/aje/kws340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eke PI, Page RC, Wei L, Thornton-Evans G, Genco RJ. Update of the case definitions for population-based surveillance of periodontitis. J Periodontol. 2012;83:1449–1454. doi: 10.1902/jop.2012.110664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eke PI, Thornton-Evans GO, Wei L, Borgnakke WS, Dye BA. J Dent Res. United States: 2010. Accuracy of NHANES periodontal examination protocols; pp. 1208–1213. [DOI] [PubMed] [Google Scholar]
- Genco RJ, Grossi SG, Ho A, Nishimura F, Murayama Y. A proposed model linking inflammation to obesity, diabetes, and periodontal infections. J Periodontol. 2005;76:2075–2084. doi: 10.1902/jop.2005.76.11-S.2075. [DOI] [PubMed] [Google Scholar]
- Jansson L. Association between alcohol consumption and dental health. J Clin Periodontol. 2008;35:379–384. doi: 10.1111/j.1600-051X.2008.01210.x. [DOI] [PubMed] [Google Scholar]
- Kingman A, Morrison E, Loe H, Smith J. Systematic errors in estimating prevalence and severity of periodontal disease. J Periodontol. 1988;59:707–713. doi: 10.1902/jop.1988.59.11.707. [DOI] [PubMed] [Google Scholar]
- Kingman A, Susin C, Albandar JM. Effect of partial recording protocols on severity estimates of periodontal disease. J Clin Periodontol. 2008;35:659–667. doi: 10.1111/j.1600-051X.2008.01243.x. CPE1243 [pii] [DOI] [PubMed] [Google Scholar]
- Kongstad J, Hvidtfeldt UA, Gronbaek M, Jontell M, Stoltze K, Holmstrup P. Amount and type of alcohol and periodontitis in the Copenhagen City Heart Study. J Clin Periodontol. 2008;35:1032–1039. doi: 10.1111/j.1600-051X.2008.01325.x. [DOI] [PubMed] [Google Scholar]
- Lages EJ, Costa FO, Lages EM, Cota LO, Cortelli SC, Nobre-Franco GC, Cyrino RM, Cortelli JR. Risk variables in the association between frequency of alcohol consumption and periodontitis. J Clin Periodontol. 2012;39:115–122. doi: 10.1111/j.1600-051X.2011.01809.x. [DOI] [PubMed] [Google Scholar]
- Lyles RH, Tang L, Superak HM, King CC, Celentano DD, Lo Y, Sobel JD. Validation data-based adjustments for outcome misclassification in logistic regression: an illustration. Epidemiology. 2011;22:589–597. doi: 10.1097/EDE.0b013e3182117c85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magder LS, Hughes JP. Logistic regression when the outcome is measured with uncertainty. Am J Epidemiol. 1997;146:195–203. doi: 10.1093/oxfordjournals.aje.a009251. [DOI] [PubMed] [Google Scholar]
- Nishida N, Tanaka M, Hayashi N, Nagata H, Takeshita T, Nakayama K, Morimoto K, Shizukuishi S. Association of ALDH(2) genotypes and alcohol consumption with periodontitis. J Dent Res. 2004;83:161–165. doi: 10.1177/154405910408300215. [DOI] [PubMed] [Google Scholar]
- Nishida N, Tanaka M, Hayashi N, Nagata H, Takeshita T, Nakayama K, Morimoto K, Shizukuishi S. Determination of smoking and obesity as periodontitis risks using the classification and regression tree method. J Periodontol. 2005;76:923–928. doi: 10.1902/jop.2005.76.6.923. [DOI] [PubMed] [Google Scholar]
- Page RC, Eke PI. Case definitions for use in population-based surveillance of periodontitis. J Periodontol. 2007;78:1387–1399. doi: 10.1902/jop.2007.060264. [DOI] [PubMed] [Google Scholar]
- Palle AR, Reddy CM, Shankar BS, Gelli V, Sudhakar J, Reddy KK. Association between obesity and chronic periodontitis: a cross-sectional study. J Contemp Dent Pract. 2013;14:168–173. doi: 10.5005/jp-journals-10024-1294. [DOI] [PubMed] [Google Scholar]
- Pitiphat W, Merchant AT, Rimm EB, Joshipura KJ. Alcohol consumption increases periodontitis risk. J Dent Res. 2003;82:509–513. doi: 10.1177/154405910308200704. [DOI] [PubMed] [Google Scholar]
- Ramfjörd SP. Indices for prevalence and incidence of periodontal disease. J Periodontol. 1959;30:51–59. [Google Scholar]
- Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. Lippincott, Williams & Wilkins; Philadelphia, PA: 2008. [Google Scholar]
- Saito T, Shimazaki Y. Metabolic disorders related to obesity and periodontal disease. Periodontol 2000. 2007;43:254–266. doi: 10.1111/j.1600-0757.2006.00186.x. [DOI] [PubMed] [Google Scholar]
- Saito T, Shimazaki Y, Koga T, Tsuzuki M, Ohshima A. Relationship between upper body obesity and periodontitis. J Dent Res. 2001;80:1631–1636. doi: 10.1177/00220345010800070701. [DOI] [PubMed] [Google Scholar]
- Saito T, Shimazaki Y, Sakamoto M. Obesity and periodontitis. N Engl J Med. 1998;339:482–483. doi: 10.1056/nejm199808133390717. [DOI] [PubMed] [Google Scholar]
- Sanders AE, Slade GD, Beck JD, Agustsdottir H. Secondhand Smoke and Periodontal Disease: Atherosclerosis Risk in Communities Study. American Journal of Public Health. 2011;101:S339–S346. doi: 10.2105/AJPH.2010.300069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki Y, Saito T, Kiyohara Y, Kato I, Kubo M, Iida M, Yamashita Y. Relationship between drinking and periodontitis: the Hisayama Study. J Periodontol. 2005;76:1534–1541. doi: 10.1902/jop.2005.76.9.1534. [DOI] [PubMed] [Google Scholar]
- Susin C, Kingman A, Albandar JM. Effect of partial recording protocols on estimates of prevalence of periodontal disease. J Periodontol. 2005;76:262–267. doi: 10.1902/jop.2005.76.2.262. [DOI] [PubMed] [Google Scholar]
- Susin C, Oppermann RV, Haugejorden O, Albandar JM. Periodontal attachment loss attributable to cigarette smoking in an urban Brazilian population. J Clin Periodontol. 2004;31:951–958. doi: 10.1111/j.1600-051x.2004.00588.x. [DOI] [PubMed] [Google Scholar]
- Tezal M, Grossi SG, Ho AW, Genco RJ. The effect of alcohol consumption on periodontal disease. J Periodontol. 2001;72:183–189. doi: 10.1902/jop.2001.72.2.183. [DOI] [PubMed] [Google Scholar]
- Tezal M, Grossi SG, Ho AW, Genco RJ. Alcohol consumption and periodontal disease. The Third National Health and Nutrition Examination Survey. J Clin Periodontol. 2004;31:484–488. doi: 10.1111/j.1600-051X.2004.00503.x. [DOI] [PubMed] [Google Scholar]
- Thomas D, Stram D, Dwyer J. Exposure measurement error: influence on exposure-disease. Relationships and methods of correction. Annu Rev Public Health. 1993;14:69–93. doi: 10.1146/annurev.pu.14.050193.000441. [DOI] [PubMed] [Google Scholar]
- Thomson WM, Broadbent JM, Welch D, Beck JD, Poulton R. Cigarette smoking and periodontal disease among 32-year-olds: a prospective study of a representative birth cohort. J Clin Periodontol. 2007;34:828–834. doi: 10.1111/j.1600-051X.2007.01131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomar SL, Asma S. Smoking-attributable periodontitis in the United States: findings from NHANES III. National Health and Nutrition Examination Survey. J Periodontol. 2000;71:743–751. doi: 10.1902/jop.2000.71.5.743. [DOI] [PubMed] [Google Scholar]
- Weinberg CR, Umbach DM, Greenland S. When will nondifferential misclassification of an exposure preserve the direction of a trend? Am J Epidemiol. 1994;140:565–571. doi: 10.1093/oxfordjournals.aje.a117283. [DOI] [PubMed] [Google Scholar]
- Wood N, Johnson RB, Streckfus CF. Comparison of body composition and periodontal disease using nutritional assessment techniques: Third National Health and Nutrition Examination Survey (NHANES III) J Clin Periodontol. 2003;30:321–327. doi: 10.1034/j.1600-051x.2003.00353.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.