Abstract
Memory formation is thought to occur via enhanced synaptic connectivity between populations of neurons in the brain. However, it has been difficult to localize and identify the neurons that are directly involved in the formation of any specific memory. We have previously used fos-tau-lacZ (FTL) transgenic mice to identify discrete populations of neurons in amygdala and hypothalamus, which were specifically activated by fear conditioning to a context. Here we have examined neuronal activation due to fear conditioning to a more specific auditory cue. Discrete populations of learning-specific neurons were identified in only a small number of locations in the brain, including those previously found to be activated in amygdala and hypothalamus by context fear conditioning. These populations, each containing only a relatively small number of neurons, may be directly involved in fear learning and memory.
Pavlovian or classical conditioning involves the presentation of a neutral stimulus, the conditioned stimulus (CS), paired with a biologically significant stimulus, the unconditioned stimulus (US), such as pain or food. Once learning has occurred, presentation of the CS alone is able to elicit a response, and this learned reaction to the previously neutral CS is known as the conditioned response. Fear conditioning is a robust type of classical conditioning, which uses an aversive US such as a footshock. It is one of the most well characterized and extensively studied models of learning and memory (Paré 2002; Sah et al. 2003; Maren and Quirk 2004; Fanselow and Poulos 2005; Kim and Jung 2006; Ehrlich et al. 2009; Johansen et al. 2011).
A number of major brain regions have been identified which are required for fear conditioning (LeDoux 2000). However, specific neurons within these regions and throughout the brain that are directly involved in fear conditioning have not been identified. The identification of these neurons is required to elucidate the memory trace or engram. Thus, the identification of these neurons is required to determine the neuronal changes that are involved with and encode the fear memory, to define the precise circuits within the brain that are involved in fear learning and memory, and to determine how the neuronal changes affect those neuronal circuits to establish the memory. One method for identifying neurons activated by a certain task is to use the immediate-early gene c-fos as a marker of neuronal activation. A number of studies have examined c-fos expression following fear learning (Smith et al. 1992; Beck and Fibiger 1995; Radulovic et al. 1998), implicating a number of different brain regions including parietal cortex, hippocampus, and amygdala. However, these studies did not determine if the c-fos expression was specifically associated with fear learning or whether it was related to different sensory stimuli the animals received (Radulovic et al. 1998).
More recent studies have utilized c-fos-regulated expression in transgenic mice to identify neuronal populations that are activated by exposure to specific contexts during fear conditioning (Reijmers et al. 2007; Garner et al. 2012; Liu et al. 2012; Ramirez et al. 2013). Subsequent specific inhibition or activation of these neurons directly demonstrated that these c-fos-activated neurons were involved in the memory of specific contexts (Garner et al. 2012; Liu et al. 2012; Ramirez et al. 2013). These experiments not only show that different subpopulations of hippocampal neurons are involved in memory of different contexts, but also validate the approach of using fos-regulated expression as a marker for neurons involved in learning and memory.
Our previous development of the transgenic fos-tau-lacZ (FTL) mouse (Wilson et al. 2002; Murphy et al. 2007) has emerged as an excellent tool for visualizing functionally activated early gene expression in brain subnuclei associated with learning and memory. Previously, we trained FTL mice using context fear conditioning and identified a number of discrete, anatomically defined populations of neurons within amygdala and hypothalamus which were specifically activated by learning (Wilson and Murphy 2009; Trogrlic et al. 2011). While context fear conditioning is a very robust form of fear conditioning, the context CS is complex and relatively undefined, and could include different combinations of visual, olfactory, auditory, and tactile stimuli. It is thus difficult to determine the relative importance of these different stimuli in the fear conditioning process, and the population of activated neurons may be less specific than if a more precise CS is used.
Auditory fear conditioning, using a specific tone as CS, is a more studied and better characterized form of fear learning. We hypothesized that the populations of neurons specifically activated by this form of fear conditioning would represent a more tightly defined subset of the populations activated by context fear conditioning and we have trained FTL mice to determine whether this is the case. In addition, we have undertaken a brain-wide screen of FTL activation, with the exclusion of cortical regions. To maximize the specificity of learning, we modified the traditional auditory fear-conditioning paradigm so that association of the footshock to the context of the training chamber is minimized (Nithianantharajah and Murphy 2008). We find a limited number of discrete, anatomically defined populations of neurons that are activated by auditory-specific fear conditioning, with some overlap in those regions activated by context fear learning.
Results
Behavioral results
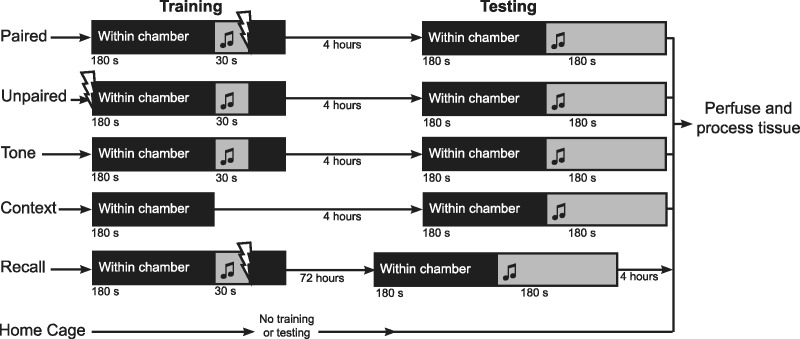
To identify neurons specifically activated by auditory fear learning, we used a protocol in which animals were trained to specifically fear an auditory stimulus, with no fear response to other sensory stimuli that they were exposed to during training (see Nithianantharajah and Murphy 2008). Mice were habituated to the context of the fear-conditioning chamber for 3 min each day for 3 wk prior to training, so that the association of fear to contextual cues would be minimal. Mice were also exposed to an enriched environment during this period as we showed previously that auditory fear learning outcomes are improved by this enrichment protocol (Nithianantharajah and Murphy 2008). Following the period of habituation and environmental enrichment, the Paired group of mice were conditioned to fear an auditory tone by placing them in the conditioning chamber and playing the tone for 30 sec, co-terminating with a footshock (Fig. 1).
Figure 1.
Training and testing paradigm for each group of mice. During training Paired mice were exposed to a paired presentation of the auditory tone and footshock. Four hours after training Paired mice were tested for auditory fear memory by exposing them to the training chamber and the auditory tone. Unpaired mice were exposed to a temporally unpaired tone and shock during training, and tested 4 h later. Tone mice were exposed to the training chamber and the auditory tone, and tested 4 h later. Context mice were exposed to only the context of the training chamber, and tested 4 h later. Paired, Unpaired, Tone, and Context mice were anesthetized immediately following testing, and perfused. Recall mice were trained as Paired mice, but returned to their home cages for 72 h. Recall mice were then tested, anesthetized, and perfused 4 h later. Home Cage mice had no training or testing.
A relatively low shock level (0.2 mA) was used. In previous experiments in context fear conditioning, we used low shock levels because the fear-conditioned mice showed clear, robust freezing whereas control mice showed only very low levels of freezing (Wilson and Murphy 2009; Trogrlic et al. 2011). At higher shock levels, shock-control mice still showed significant levels of freezing (Nithianantharajah and Murphy 2008) indicating some learning had occurred.
We used a number of control groups of mice that enabled us to distinguish between FTL activation due to learning and to nonlearning experiences (Fig. 1). To control for experience of context, the Context group was exposed only to the conditioning chamber during training. Similarly, the Tone group was exposed to both the context of the chamber as well as the auditory tone, but with no footshock. The Unpaired group was exposed to a temporally unpaired tone and shock, thereby providing a control condition in which animals have been exposed to the same set of sensory stimuli as Paired mice, but have not encoded a fear memory. In addition, a Recall group of mice was included, enabling us to determine if neuronal populations that were activated by learning were also activated by the recall and expression of fear. Recall mice were first trained using the paired protocol, and then returned to their home cages for 72 h (giving enough time for FTL transgene expression to return to basal levels [Wilson and Murphy 2009]).
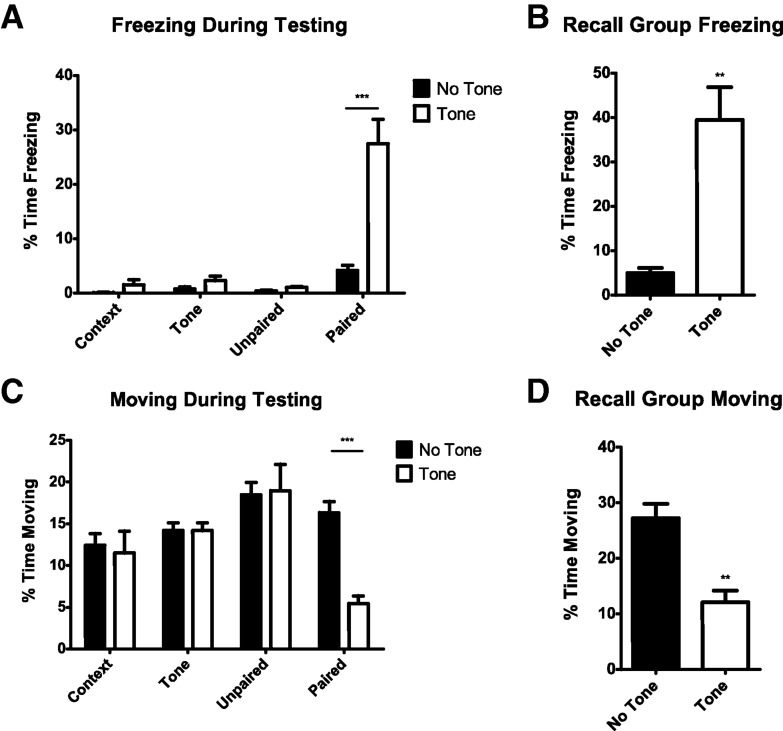
To determine if the mice had acquired a fear response, each mouse was reexposed to the fear conditioning chamber and auditory tone 4 h after training (excluding Recall mice, as described above). The first measure of fear expression was the percent of time the mice spent freezing (see Fig. 2A,B). Context, Tone, and Unpaired mice did not freeze more than base levels to the fear conditioning chamber during testing, either in the presence or absence of the auditory tone, indicating they had not learned to fear either the context of the conditioning chamber or the auditory tone (Fig. 2A). Only Paired mice froze significantly more than base levels in the presence of the auditory tone (t = 8.627, P < 0.001). Thus, only Paired mice learned to fear the auditory tone, and this fear memory was specific to the tone and not to the chamber context (Tone effect F(1,32) = 15.95, P = 0.0004, treatment effect F(3,32) = 20.80, P < 0.0001, interaction F(3,32) = 14.32, P < 0.0001). The time each mouse spent moving was also recorded as a second measure of fear (Fig. 2C,D). Only Paired mice moved significantly less in the presence of the tone (Fig. 2C; t = 7.792, P < 0.001), again indicating that only the Paired group of mice were conditioned to fear the tone (Tone effect F(1,32) = 10.53, P = 0.0028, treatment effect F(3,32) = 5.85, P < 0.0026, interaction F(3,32) = 12.91, P < 0.0001).
Figure 2.
Fear expression in mice during testing. (A) Four hours after training, only Paired mice froze in response to the auditory tone, and levels of freezing to the training chamber alone was not significant. (B) Recall mice froze significantly more when exposed to the auditory tone 72 h after training. (C) Four hours after training, only Paired mice show a reduction in movement in response to the auditory tone, and there was no significant decrease in movement without tone. (D) Recall mice showed significantly less movement when exposed to the auditory tone 72 h after training. Numbers are expressed as percent time freezing (A and B) or moving (C and D) ± SEM. (**) P < 0.01 and (***) P < 0.001.
Recall mice were reexposed to the context and tone after 48 h in their home cage, and froze significantly more when presented with the auditory tone than without tone presentation (Fig. 2B; t(8) = 4.622, P = 0.0017). Moving was also significantly reduced in Recall mice in the presence of the tone (Fig. 2B; t8 = 4.487, P = 0.002; Fig. 2D).
Analysis of learning-induced FTL expression
Mice were terminally anesthetized immediately after fear testing and brain sections from each treatment group were qualitatively examined for regions showing differences in FTL expression between the auditory fear learning group and the nonlearning control groups. Regions of interest were described anatomically, and counts of FTL+ neurons were undertaken in defined areas as described below.
Learning-induced activation in the amygdala
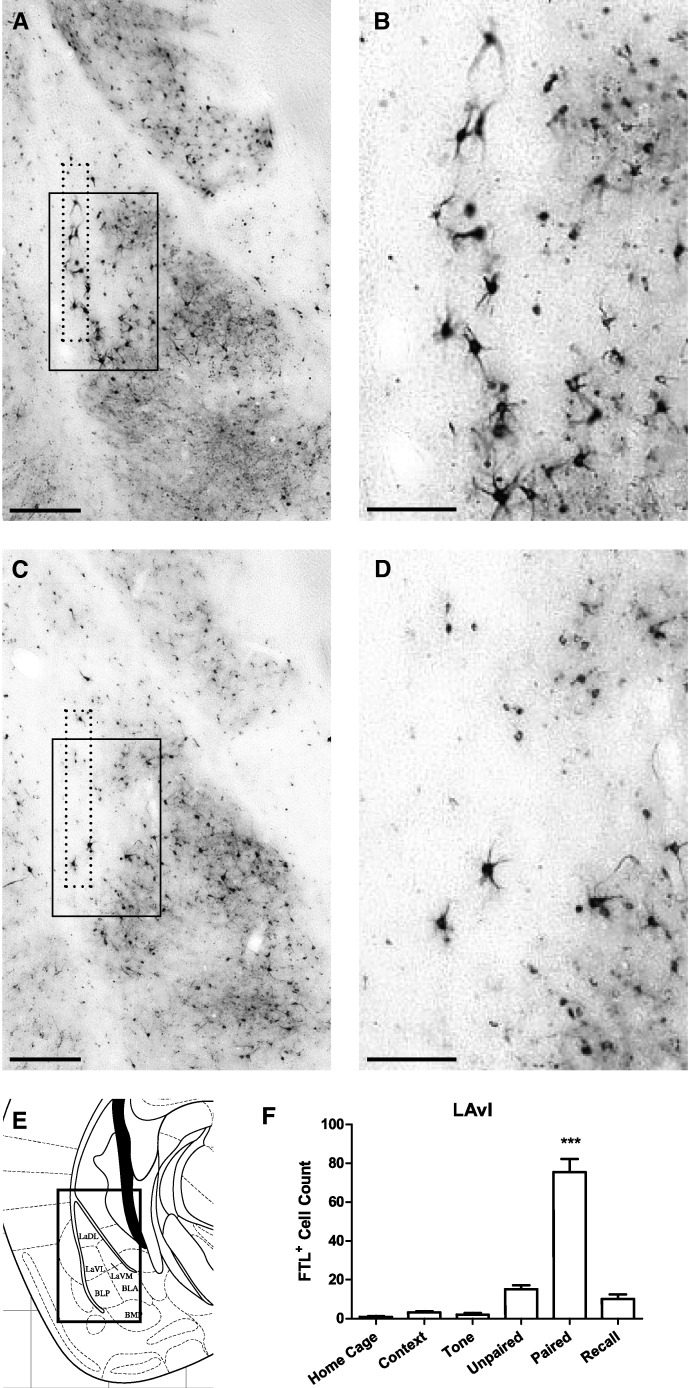
We previously described a population of neurons specifically activated by context fear learning in the ventrolateral subdivision of the lateral amygdala (LAvl) (Wilson and Murphy 2009; Trogrlic et al. 2011). Interestingly, a similar distribution of FTL+ neurons was identified in the current experiment following auditory fear conditioning (Fig. 3). FTL+ neurons were identified in Paired mouse brains, along the lateral border of LAvl, between bregma −1.7 and −2.0 mm. FTL+ neurons were counted within a 100-µm wide rectangle aligned with the lateral border of the LAvl (see Fig. 3A,C), extending the length of the LA in each brain section. There was a four- to sixfold increase in FTL+ neurons in Paired brains compared with Unpaired and Recall, respectively (F(5,43) = 51.93, P < 0.0001; Bonferroni's multiple comparison test: Paired versus Unpaired: P < 0.001; Paired versus Recall: P < 0.001). These FTL activated neurons are a small, discrete population of learning-specific neurons along the border of the LA. We estimate that they represent 5% of neurons in the border region of the LAvl (as described in Materials and Methods).
Figure 3.
Learning-specific FTL+ neurons in ventrolateral Lateral Amygdala (LAvl) following auditory fear conditioning. (A) Bright-field photograph of amygdala at bregma −1.7 mm showing FTL+ neurons in the LAvl in a Paired trained mouse. The solid box encloses the area shown in high power in B. Counted region is encompassed by dotted line. (B) High-power view of FTL+ neurons in Paired LAvl. (C) Bright-field photograph of Unpaired trained mouse at bregma −1.7 mm. The solid box encloses the area shown in high power in D. Counted region is encompassed by dotted line. (D) High-power view of Unpaired LAvl boxed in C. (E) Plate from Mouse Brain Atlas (Franklin and Paxinos 2008) highlighting the region shown in A and C. (F) FTL+ neuron counts of LAvl region in each group of trained mice, shown as mean ± SEM. Significantly more FTL+ neurons were present in Paired mouse LAvl compared with control groups. (***) P < 0.001. Scale bar, 250 µm (A,C), 100 µm (B,D).
Very few FTL+ neurons were identified in this region in Home Cage. Further, exposure to context did not result in any increase in FTL activation (P > 0.05), which may have been due to prior treatment of the mice to EE and habituation to the conditioning chamber. There was also no significant increase in the number of FTL+ neurons following exposure to context in any of the other regions described below (P > 0.05).
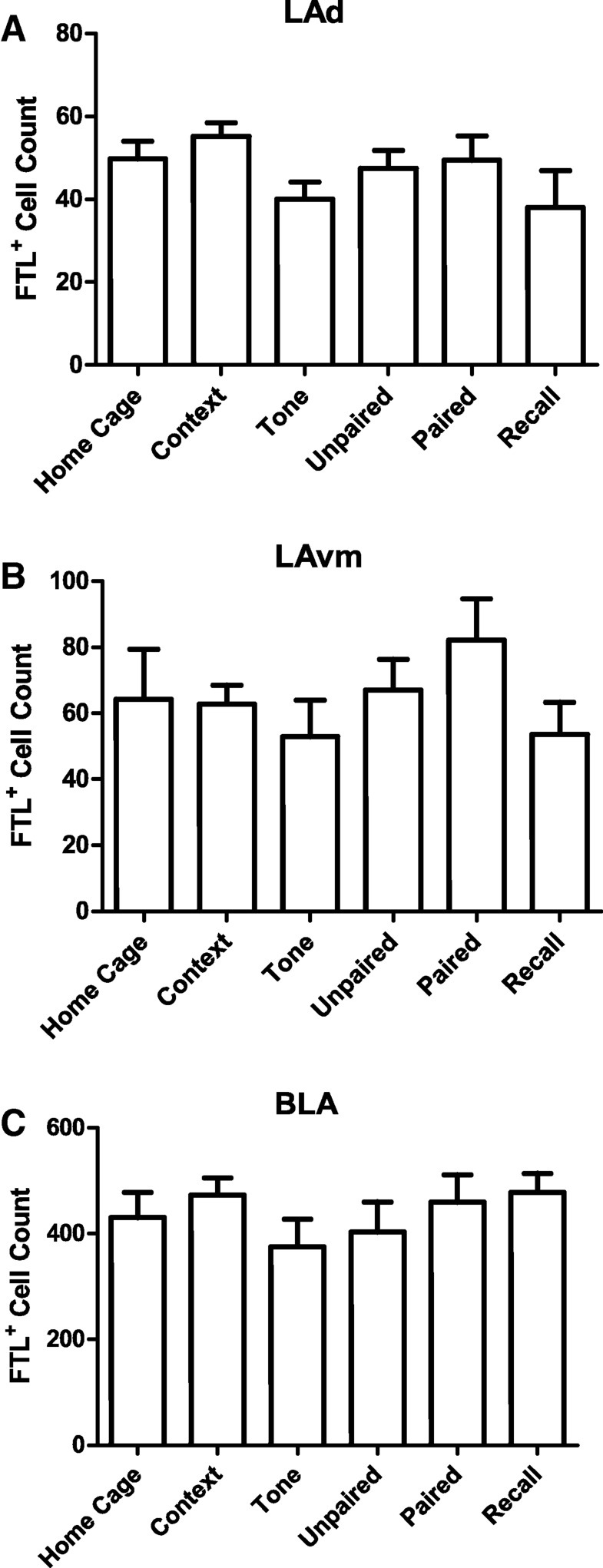
Additionally, there were no effects of auditory fear conditioning on FTL expression in the other divisions of the lateral amygdala, the dorsal (LAd) and ventromedial (LAvm) nuclei (Fig. 4; LAd F(5,39) = 1.099, P = 0.3766; LAvm F(5,39) = 1.016, P = 0.4217). Similarly, we did not find a learning-specific increase in FTL+ neurons in the basolateral amygdala (Fig. 4; F(5,39) = 0.5598, P = 0.730).
Figure 4.
FTL+ neuron numbers in other basolateral amygdala subdivisions. (A) Total numbers of FTL+ neurons in the basolateral amygdala (BLA) in each group of trained mice. (B) Total numbers of FTL+ neurons in the dorsal lateral amygdala (LAd) in each group of trained mice. (C) Total numbers of FTL+ neurons in the medial lateral amygdala (LAm) in each group of trained mice. Numbers are expressed as mean ± SEM. No significant difference in FTL+ neuron number was observed between any of the groups in any of these amygdala regions.
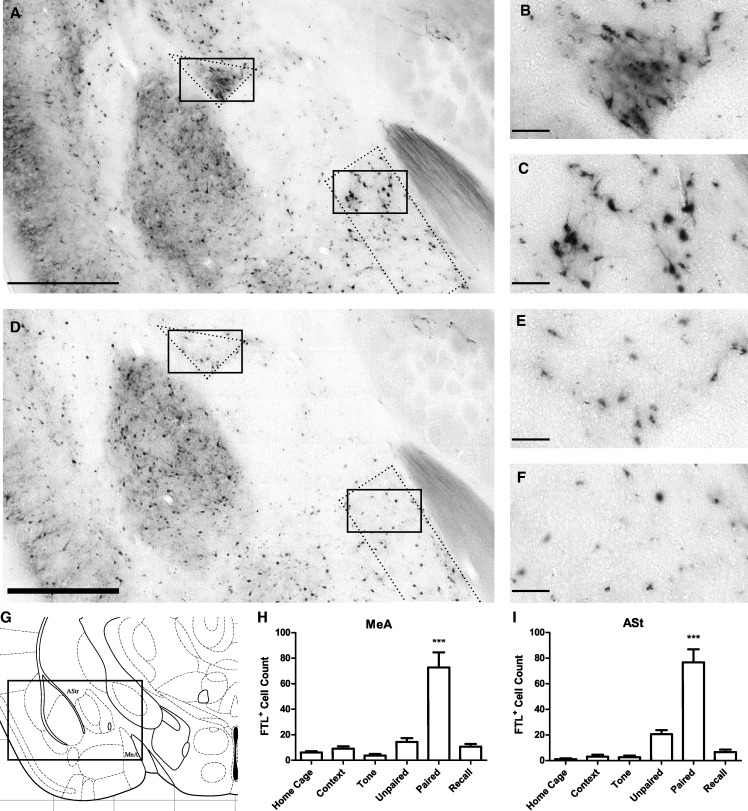
Intense learning-specific FTL expression was also observed within the posterodorsal nucleus of the medial amygdala (MeApd; Fig. 5). The learning-specific expression was most prominent in the dorsal region of the MeApd adjacent to the optic tract and between bregma −1.06 mm and bregma −1.58 mm. FTL+ neurons were counted in each treatment group in the MeApd, within a rectangular region 250 µm wide positioned on the border of the optic tract (Fig. 5A,B), extending the length of the optic tract in each brain section. In this region, there was at least a fivefold increase in the number of FTL+ neurons in the Paired group (F(5,43) = 14.69, P < 0.0001; Bonferroni's multiple comparison test comparing Paired versus Unpaired: P < 0.001; Paired versus Recall: P < 0.001). Additionally, and as described above for the LAvl, the neurons showing FTL activation in the Paired mice are a small population, representing ∼1.2% of neurons within this region of the MeApd.
Figure 5.
Learning-specific FTL expression in the Medial Amygdala and Amygdalostriatal Transition Region following auditory fear conditioning. (A) Bright-field photograph of FTL+ neurons at bregma −1.22 mm in a Paired mouse. Solid boxes enclose areas that are shown in high power in B and C. Regions that were counted are enclosed by dotted lines. (B) High-power view of FTL+ neurons in a Paired mouse AStr. (C) High-power view of FTL+ neurons in MeA from a Paired mouse. (D) Bright-field photograph of section taken at bregma −1.22 mm in an Unpaired trained mouse. Solid boxes encompass areas that are shown in high power in E and F. Regions that were counted are encompassed by dotted lines. (E) High-power view of AStr from an Unpaired mouse. (F) High-power view of Unpaired MeA. (G) Plate from Mouse Brain Atlas (Franklin and Paxinos 2008) highlighting the regions shown in A and D. (H) FTL+ neuron counts of MeA region in each group of mice, shown as mean ± SEM. There were significantly more FTL+ neurons in Paired mouse MeA compared with controls. (I) FTL+ neuron counts of AStr region of each group of mice. There were significantly more FTL+ neurons in Paired AStr compared with controls. (***) P < 0.001. Scale bar, 500 µm (A, D), 50 µm (B, C, E, F).
The amygdalostriatal transition area (AStr) also showed learning-specific expression (Fig. 5A,B). These FTL+ neurons were observed between bregma −1.06 and −1.94 mm in the AStr region, with four times as many FTL+ neurons in Paired brains compared with Unpaired brains. A small increase in FTL+ neurons was also observed in Unpaired brains compared with Context, Tone, and Homecage. There was a significant increase in FTL+ neurons in the Paired AStr (F(5,43) = 23.71, p < 0.0001; Bonferroni's multiple comparison test comparing Paired versus Unpaired: P < 0.001; Paired versus Recall: P < 0.001). The population of FTL+ neurons in Paired mice represented ∼9% of neurons in the AStr.
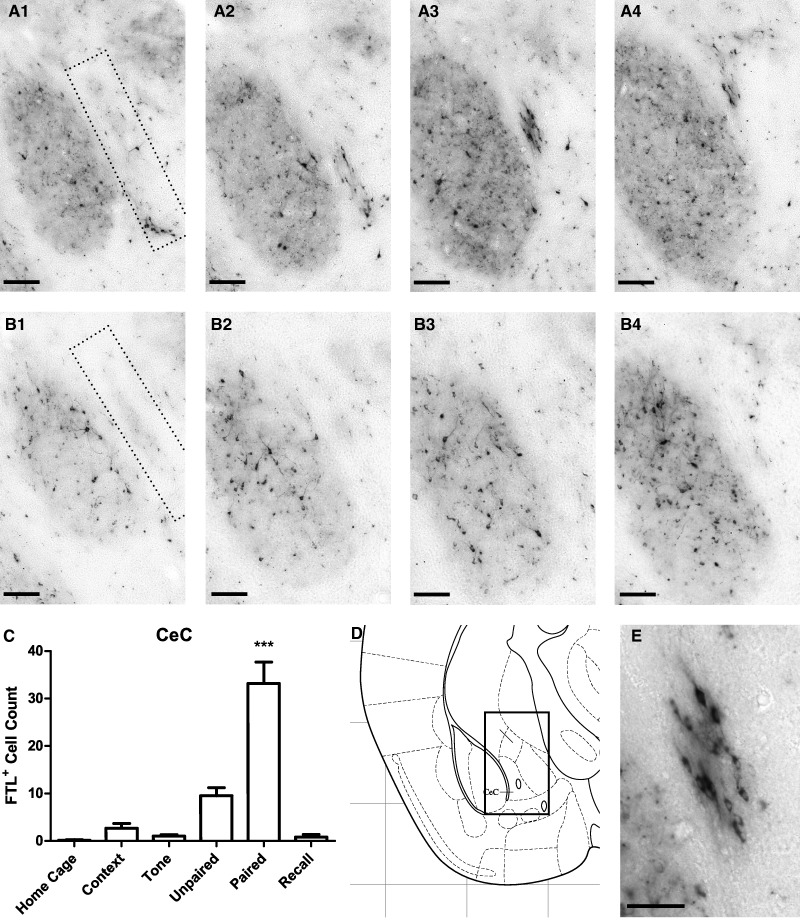
Learning-specific FTL+ neurons were observed within the capsular region of the central amygdala (CeC, Fig. 6). FTL+ neurons were found along the border of the CeC adjacent to the basolateral amygdala, extending rostro-caudally from bregma −0.80–0.95 mm (Fig. 6). In rostral regions of the CeC, these learning-specific neurons were relatively ventral but their location became more dorsal through successive caudal regions of CeC (Fig. 6A1–4). In this region of the CeC, there was a fourfold increase in FTL+ neurons in Paired compared with Unpaired brains (Fig. 6C). As with the AStr population, there was also a small increase in FTL+ neurons in Unpaired brains when compared with the Context, Tone, and Home Cage brains. There was a significant increase in FTL+ neurons in the Paired CeC (F(5,43) = 21.82, P < 0.0001; Bonferroni's multiple comparison test comparing Paired versus Unpaired: P < 0.001; Paired versus Recall: P < 0.001). The population of FTL+ neurons in Paired mice represented ∼3.6% of neurons in this region of the CeC.
Figure 6.
Learning-specific FTL+ expression in CeC following auditory fear conditioning. (A1–4) Images of a series of sections from a Paired brain showing FTL+ neurons in CeC from bregma −0.8–1.0 mm. Region counted is shown encompassed by dotted line. (B1–4) Comparative series of images from an Unpaired brain. (C) FTL+ neuron counts of this region in each group of trained mice, shown as mean ± SEM. There were significantly more FTL+ neurons in Paired mouse brains compared with controls. (D) Plate from Mouse Brain Atlas (Franklin and Paxinos 2008) highlighting the region shown in A1–4 and B1–4. (E) High-power view of Paired FTL+ neurons from A3. (***) P < 0.001. Scale bar, 100 µm (A1–4, B1–4), 50 µm (E).
These results suggest that each of the amygdala populations of neurons described above may be specifically involved in auditory fear conditioning, and the lack of FTL expression in recall brains suggests that FTL activation was not due to fear memory recall or expression of fear.
Learning-induced activation in the hypothalamus
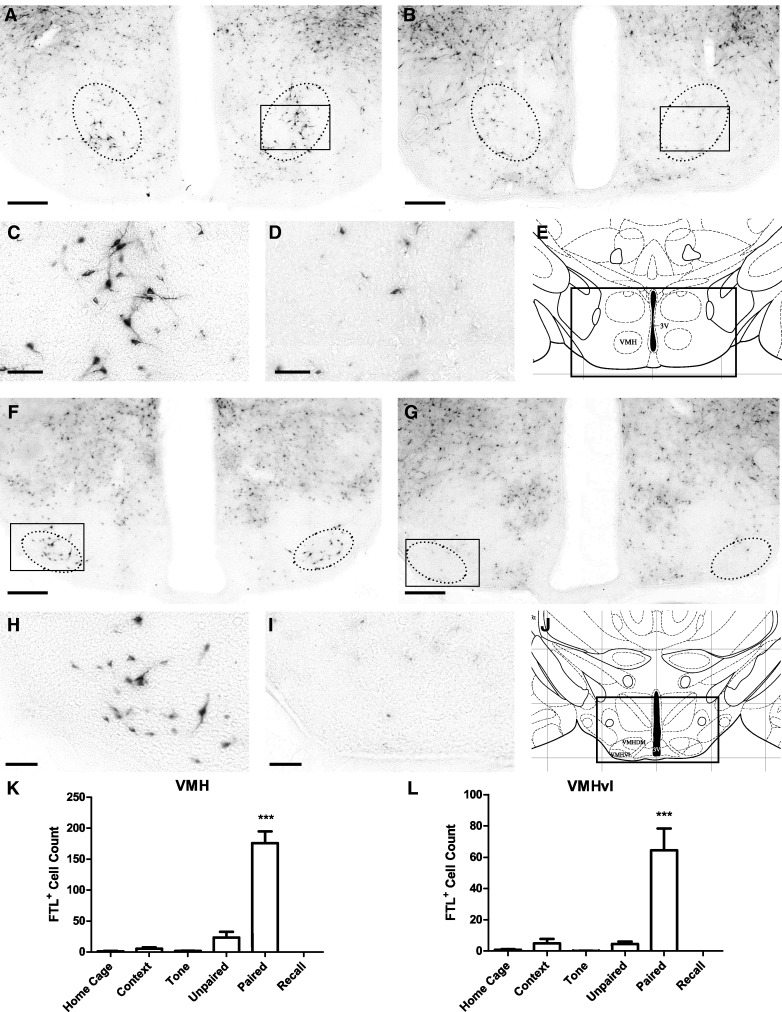
We previously identified a population of neurons within the hypothalamus that was specifically activated following context fear conditioning (Trogrlic et al. 2011). We found a similar population of neurons activated by auditory fear conditioning in the rostral region of the ventromedial hypothalamus (VMH; bregma −1.04–1.44 mm; Fig. 7). There was a ninefold increase in FTL+ neurons in this region in Paired mice compared with Unpaired mice (Fig. 7K). Very few FTL+ neurons were counted in the Home Cage, Context, and Tone control groups. The increase in FTL+ neurons in Paired brains was significant (F(5,43) = 36.42, P < 0.0001, Bonferroni's multiple comparison test comparing Paired versus Unpaired: P < 0.001; Paired versus Recall: P < 0.001). The population of FTL+ neurons in Paired mice represented ∼6% of neurons in this region of the VMH.
Figure 7.
Learning-specific FTL+ neurons in the VMH and VMHvl following auditory fear conditioning. (A) Bright-field photograph of hypothalamic region at bregma −1.2 mm showing FTL+ neurons in the VMH in a Paired mouse. The solid box encloses the area shown in high power in C. Neurons were counted in region encompassed by dotted line. (B) Comparative photograph of Unpaired mouse. (C) High-power view of FTL+ neurons in Paired VMH. (D) High-power view of Unpaired VMH boxed in B. (E) Plate from Mouse Brain Atlas (Franklin and Paxinos 2008) highlighting the region shown in A and B. (F) Bright-field photograph of hypothalamic region at bregma −1.8 mm from a Paired mouse. The solid box encloses the area shown in high power in H. Neurons were counted in region encompassed by dotted line. (G) Comparative image from an Unpaired mouse. (H) High-power view of FTL+ neurons in Paired VMHvl. (I) High-power view of VMHvl from an Unpaired mouse boxed in G. (J) Plate from Mouse Brain Atlas (Franklin and Paxinos 2008) containing the region shown in F and G. (K) FTL+ neuron counts of rostral VMH region in each group of trained mice. Significantly more FTL+ neurons were present in rostral VMH from Paired mice compared with controls. (L) FTL+ neuron counts of caudal VMHvl region in each group of trained mice. There were significantly more FTL+ neurons in Paired mouse VMHvl compared with controls. Numbers are expressed as mean ± SEM. (***) P < 0.001. Scale bar, 250 µm (A, B, F, G), 50 µm (C, D, H, I).
An additional population of learning-specific FTL expression was identified in a more caudal region within the VMH. These neurons correspond to the ventrolateral subdivision of the VMH (VMHvl; Franklin and Paxinos 2008), between bregma −1.7 mm and bregma −1.8 mm (Fig. 7L). There was a 13-fold increase in FTL+ neurons in this region of the VMHvl in Paired compared with Unpaired mice, with very few FTL+ neurons observed in the other control mice. There was a significant increase in FTL+ neurons within the Paired VMHvl (F(5,42) = 9.94, P < 0.0001, Bonferroni's multiple comparison test comparing Paired versus Unpaired: P < 0.001; Paired versus Recall: P < 0.001). The population of FTL+ neurons in Paired mice represented ∼8.7% of neurons in this region of VMHvl.
These results indicate that two specific populations of neurons in the VMH are activated by auditory fear conditioning, and the low numbers of FTL+ neurons in the VMH of recall mice suggests that FTL activation is not associated with the expression of fear.
Learning-induced activation in the lateral septum
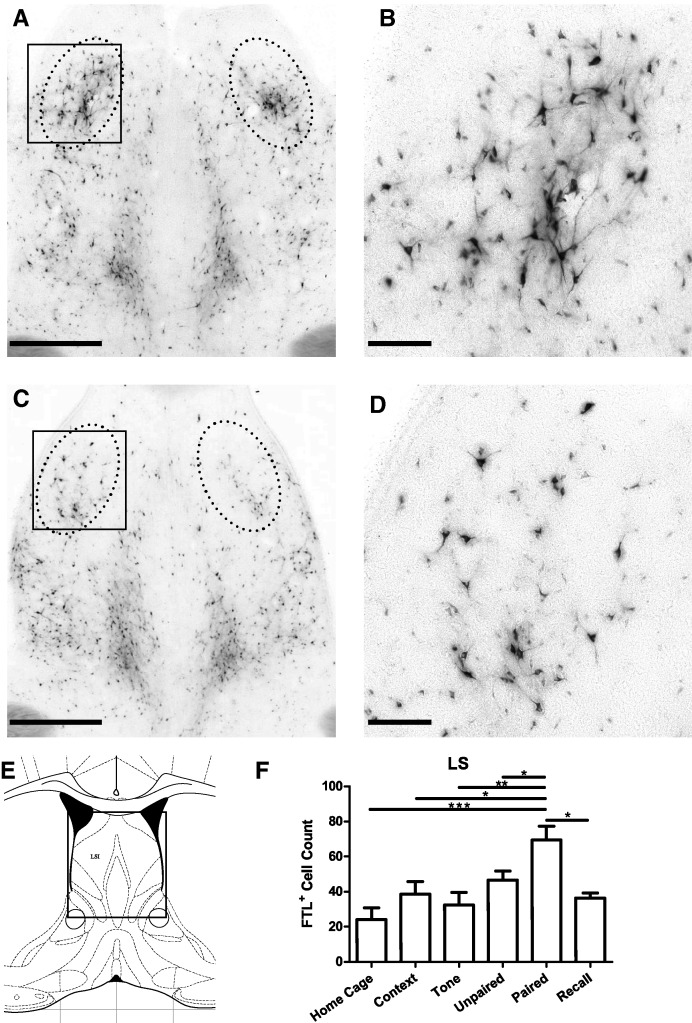
A discrete region within the lateral septum was also found to show learning-associated activation following auditory fear conditioning. At approximately bregma 0.30 mm, a region contained within a 400 × 600-µm oval either side of the midline within the intermediate subdivision of the Lateral septum (LSI) was found to be strongly FTL+ in Paired brains (Fig. 8). Counts of FTL+ neurons within this region of LSI are shown in Figure 8F. There were significant numbers of FTL+ neurons in all conditions, but Paired mice contained at least 60% more FTL+ neurons compared with all other groups. There was a significant effect of treatment on number of FTL-labeled neurons (F(5,42) = 5.7, P = 0.0004, Bonferroni's multiple comparison test comparing Paired versus Unpaired: P < 0.05; Paired versus Recall: P < 0.05). The population of FTL+ neurons in Paired mice represented ∼4.4% of neurons in this region of LSI.
Figure 8.
Learning-specific FTL+ neurons in lateral septum (LSI) following auditory fear conditioning. (A,C) Bright-field images of LSI at bregma 0.3 mm from a Paired and Unpaired mouse, respectively. The solid box encloses the area shown in high power in B. Counted region is encompassed by dotted line. (B,D) High-power views of FTL+ neurons in Paired and Unpaired LSI, respectively. (E) Plate from Mouse Brain Atlas (Franklin and Paxinos 2008) highlighting the region shown in A and C. (F) FTL+ neuron counts of LSI region in each group of trained mice, shown as mean ± SEM. Significantly more FTL+ neurons were present in Paired mouse LSI region compared with controls. (*) P < 0.05, (**) P < 0.01, (***) P < 0.001. Scale bar, 500 µm (A, C), 100 µm (B, D).
Regions with no learning-induced activation
There were some other areas of the brain that may have had learning-related FTL expression when screened qualitatively. These were Ventral Tegmental Area, dorsal lateral Geniculate Nucleus, Pregeniculate Nucleus, and dorso-lateral Periaqueductal Gray. However, upon quantitative analysis of these regions, none were found to have significantly more FTL+ neurons when compared with nonlearning controls (ventral tegmental area; F(5,40) = 4.659, Paired versus Unpaired, P > 0.05; dorsal lateral Geniculate Nucleus, F(5,40) = 1.785, Paired versus Unpaired P > 0.05; Pregeniculate Nucleus, F(5,40) = 4.530, Paired versus Unpaired, P > 0.05; dorso-lateral Periaqueductal Gray, F(5,42) = 1.581, Paired versus Unpaired, P > 0.05). No other brain regions were detected which showed any learning-specific activation.
Discussion
In this study, we aimed to identify discrete populations of neurons in the brain that were specifically activated by auditory fear learning. We trained FTL mice using a model of fear conditioning in which the mice learn to specifically fear an auditory cue with little or no fear of any other contextual stimuli. The use of FTL mice allows a relatively straightforward method for imaging the brain. The advantage over Fos immunohistochemistry or c-fos in situ hybridization results from two principal characteristics of the FTL transgene. First, expression of the transgene is targeted to cell bodies and their processes, and second, transgene activation can be detected either by βgal histochemistry or immunohistochemistry. This allows imaging from the macroscopic level (e.g., whole regions of the brain) to a high-resolution level (e.g., a single neuronal process). In comparison, c-fos expression is restricted to cell nuclei, and thus imaging using conventional c-fos detection techniques does not allow either simple macroscopic imaging or high-resolution imaging and analysis of cell bodies and their processes. In this study, FTL imaging using histochemistry permitted the efficient screening of sections across the brain and the detection of specific patterns of neuronal activation, such as clusters of similarly shaped FTL+ neurons.
We identified a very limited number of regions in the brain that were specifically activated by auditory fear learning, with learning-specific populations found in the amygdala, hypothalamus and lateral septum. We previously showed that some of these populations in amygdala and hypothalamus were activated following the encoding of context fear memories (Trogrlic et al. 2011), while the current findings suggest that either similar or overlapping populations of neurons are directly involved in the process of auditory fear learning.
A number of control groups of animals were used to omit those neurons that were activated due to nonlearning events or stimuli. Mice in the main control group, Unpaired, were exposed to the same sensory stimuli as the Paired group, but did not learn. However, unlike the Paired mice, Unpaired mice do not express fear. We utilized another important control, the Recall group, which enabled us to determine if neuronal populations that showed FTL activation following learning also showed FTL activation following the recall of fear. In our previous studies with context fear conditioning, we found none of the neuronal populations that showed FTL activation following learning also showed FTL activation following recall (Trogrlic et al. 2011). This finding demonstrated that FTL expression in these populations was not due to fear expression. Comparison of the learning group with each of these control groups enabled the identification of neuronal ensembles that were specifically activated by the learning of auditory fear conditioning.
It is important to note that lack of FTL (and c-fos) expression in neurons during recall does not preclude involvement of those neurons in the recall process. These neurons may well fire during recall, but with no subsequent c-fos expression. Induction of c-fos not only requires depolarization and increases in firing rate, but is also associated with strong activation of neurotransmitter receptors and substantial changes in intracellular Ca2+ (Cirelli and Tononi 2000; Kovacs 2008). Thus, c-fos activation is considered to be indicative of strong activation of cell functioning during periods of plasticity or high rates of metabolic activity, and not simply a marker of neuronal firing (Cirelli and Tononi 2000; Kovacs 2008). The learning-specific FTL expression occurring following fear conditioning may thus coincide with plasticity underlying memory formation. We have previously identified different populations of neurons in hypothalamus and other brain regions showing FTL activation after recall of context fear conditioning (Ali et al. 2012).
A modified auditory fear conditioning paradigm was used to minimize the association of the footshock to more general and less defined contextual cues. This involved extensive habituation to the shock chamber and concurrent exposure to an enriched environment (Nithianantharajah and Murphy 2008). This model of auditory fear conditioning thus enables us to obtain a more specific neural correlate of conditioned fear than that attained using context fear conditioning. Overall levels of FTL expression in the current study appeared less than those observed previously (Trogrlic et al. 2011), which may be due to the pretraining habituation and/or environmental enrichment. Habituation and handling of mice reduces c-fos expression in many areas of the brain (Papa et al. 1993; Asanuma and Ogawa 1994; Ryabinin et al. 1999).
Environmental enrichment results in a series of behavioral and brain changes in the mature animal (Diamond 2001; Nithianantharajah and Hannan 2006). These include improvements in learning and memory, decreased stress, increased cortical thickness and neuronal size, changes in synapse size and number, and increased neurogenesis (Kempermann et al. 1997). Our studies suggest environmental enrichment induces relatively widespread changes in the brain (Nithianantharajah et al. 2004), including effects on FTL expression at the start of enrichment (Ali et al. 2009). Given that environmental enrichment results in decreased stress levels and stress has long been known to increase c-fos expression (Pezzone et al. 1992; Smith et al. 1992), environmental enrichment over a period of weeks may result in generally decreased c-fos expression in the brain. Combined with improved learning and memory, it would thus be expected that environmental enrichment would improve the specificity of the neural correlate of conditioned fear.
C-fos is a widely expressed gene, however its lack of expression in a brain region does not necessarily preclude neuronal activation (Labiner et al. 1993). Thus, we cannot detect neurons that do not utilize c-fos during learning, such as many types of inhibitory neurons. Further, it could not be determined if there was learning-specific expression in some areas of the brain with high levels of baseline FTL expression, such as cortex. Finally, there are some areas of the brain that most likely contribute to auditory fear learning, but are not specifically involved and activated in the formation of an association between the auditory tone and the footshock. For example, in our analysis of context fear conditioning, we did not detect learning-specific activation in hippocampus; this structure was activated by both context exposure and fear learning (M Murphy and Y Wilson, unpubl.). This is consistent with the requirement of the hippocampus in context fear memory through its coding for the context (Garner et al. 2012; Liu et al. 2012).
Learning-induced neuronal activation in the amygdala
The population of FTL+ neurons seen within LAvl is very similar in distribution to that seen following context fear conditioning (Wilson and Murphy 2009; Trogrlic et al. 2011). As previously discussed (see Trogrlic et al. 2011), the LA has been implicated as a site of CS–US association due to the convergence of CS and US sensory signals (Rodrigues et al. 2004; Kim and Jung 2006; Ehrlich et al. 2009). Thus, our hypothesis that the c-fos-related expression within the LAvl neurons following learning may be related to the formation of the CS–US association (Wilson and Murphy 2009; Trogrlic et al. 2011) is further supported by the results of the current study. Whether the same neurons are activated by both context and auditory fear conditioning requires further examination. The activated neurons we identified still only represent a very small percentage of the total neurons within this region of the LA and thus it may be that the auditory and context-specific neurons may be separate or overlapping.
It is also known that phosphorylation of ERK/MAP kinase in the LA is required for auditory fear conditioning in the rat (Schafe et al. 2000). Auditory fear conditioning results in activation of MAP kinase throughout the LA, in particular along the lateral border of the LA, in LAd and LAvl (Schafe et al. 2000; Bergstrom and Johnson 2014). Given that MAP kinase activation partly regulates c-fos expression (Adams and Sweatt 2002), it may be that there is overlap in the FTL+ and MAP kinase activated populations in the LAvl. In any case, these data provide further evidence that discrete, and possibly overlapping, populations of neurons along the lateral border of the LA are activated and involved in auditory- and context fear learning, respectively.
We found no increase in numbers of FTL+ neurons following recall of auditory or context fear memory (Trogrlic et al. 2011) compared with basal levels, indicating that these neurons do not express c-fos following fear memory recall. One interpretation of this finding is that these neurons undergo plasticity following fear learning associated with the formation of the primary fear memory, but there is no further plasticity following fear memory recall. If this were the case, learning-induced plasticity may have altered the firing properties of these neurons and contributed to the conditioned behavior of the mice, but no further changes occurred following recall.
Medial amygdala
The MeA was another site of learning-specific FTL activation following both context- and auditory-fear conditioning (Trogrlic et al. 2011). The learning-specific expression was located in dorsal regions of MeApd. A series of prior studies have examined c-fos expression in MeA using different fear learning models, but none have determined if the c-fos expression was specifically associated with learning (Pezzone et al. 1992; Campeau et al. 1997; Milanovic et al. 1998; Radulovic et al. 1998; Rosen et al. 1998; Trogrlic et al. 2011).
A number of predator odor-induced fear experiments implicate the MeA in fear learning and memory. Predator odor is innately aversive for rodents and can therefore be used as a US in fear conditioning. Ibotenic acid lesions to the rat MeA reduce freezing in response to cat-odor exposure (Li et al. 2004), and temporary inhibition of the rat MeA with GABA agonist, muscimol, completely blocks the expression of fear to fox odor (Müller and Fendt 2006). Thus, the MeA is required for the processing of predator odor-induced fear. In addition, lesions of MeA after predator odor exposure impaired context fear conditioning (Takahashi et al. 2007). These findings suggest that the MeA is required for fear memory of the context in which a predator odor was experienced.
Because of this experimental evidence linking MeA with olfactory stimuli, we previously hypothesized that the activation in MeA following context fear conditioning may be related to undefined olfactory cues within the conditioning chamber (Trogrlic et al. 2011). However, this is not consistent with the current study, given we have specifically conditioned mice to an auditory stimuli. Whereas lesioning or inhibition of MeA does not impair auditory or context fear conditioning (Nader et al. 2001; Walker et al. 2005), another form of fear memory, fear potentiated startle, is affected (Walker et al. 2005). Recent studies also indicate that MeA mediates aversive Pavlovian instrumental transfer (McCue et al. 2014) and neuroendocrine responses induced by context fear conditioning (Yoshida et al. 2014). These experiments thus suggest that MeA contributes to part of the fear memory engram, but may not be required for all aspects of fear learning. Further studies are required to elucidate the precise role of MeA activation following auditory fear conditioning.
Amygdalostriatal transition area
A population of neurons within the AStr showed learning-specific activation both in this study and following context fear conditioning (Trogrlic et al. 2011). In our previous study, there was also some FTL activation in the AStr following exposure to the novel context, immediate shock, and tone (Trogrlic et al. 2011). However in the present study, there was no FTL activation related to context or tone exposure, or recall of fear, and only a small increase in the Unpaired condition. This low-FTL expression in the nonlearning controls is most likely due to the extensive habituation and environmental enrichment prior to training. FTL expression in AStr was therefore predominantly learning specific. In combination with our context fear conditioning study (Trogrlic et al. 2011) this finding further argues that the FTL+ neurons we identified in the AStr have a role in fear learning.
The AStr receives extensive projections from LA as well as the accessory basal nuclei of amygdala, and is likely a major target of both of these regions (Jolkkonen et al. 2001). The projection from the LA is of particular interest because it raises the possibility that the learning-specific neurons we identified in LAvl may project directly to learning-specific neurons in the AStr and form part of the fear learning circuit. Using a rat brain slice preparation with a voltage-sensitive imaging system, the electrophysiological characteristics of the LA pathways were studied (Wang et al. 2002), and the LA signal was shown to propagate specifically to AStr and basolateral nuclei. The LA-AStr signal was characterized as high velocity, high efficiency and the AStr had the property of temporal summation of LA signals (Wang et al. 2002). These characteristics infer strong functional connection between LA and AStr and support the possibility that this connection forms part of the fear learning circuit.
The AStr also receives information, via input from cortex and thalamus, from somatosensory, auditory, and visual modalities (LeDoux et al. 1990). The AStr may thus be a stimulus convergence region for sensory inputs including footshock, auditory, and contextual stimuli, in addition to receiving inputs from LA. The outputs of AStr are predominantly basal ganglia, including striatum, nucleus accumbens, and substantia nigra (Shammah-Lagnado et al. 1999). These structures are involved in movement and higher-order behaviors. The role of AStr in fear learning could thus involve learning-related signaling to control such fear-related behaviors including avoidance and escape.
Central amygdala
In an adjacent region to the AStr, within the CeC, there was a small population of neurons that showed learning-specific activation. These neurons were found only along the lateral border of the CeC within a very narrow rostro-caudal extent of ∼100 µm. They were found at the most rostral extent of the CeC, and along the border where it was directly adjacent to the BLA. A number of other studies have studied c-fos expression in the central amygdala (CeA) following fear conditioning (Pezzone et al. 1992; Beck and Fibiger 1995; Milanovic et al. 1998; Radulovic et al. 1998; Day et al. 2008), but none of these studies determined if any of the c-fos expression was specifically associated with fear learning. No other studies have identified c-fos+ neurons in this very discrete location within the CeC.
The CeA has long been associated as an output site of fearful behavior (Johansen et al. 2011), but has recently been found to be directly involved in the fear-conditioning process (Ciocchi et al. 2010; Haubensak et al. 2010; Li et al. 2013). In particular, inhibitory neurons in the lateral CeA (CeL, which includes CeC) were shown to be directly involved in the fear-conditioning process (Ciocchi et al. 2010; Haubensak et al. 2010). Fear learning was associated with modification of excitatory synapses from LA neurons projecting onto these inhibitory neurons within CeL (Li et al. 2013).
Whether the highly localized neurons we identified in CeC correspond to the learning-specific inhibitory neurons in CeL needs further investigation. One possibility is that during auditory fear conditioning only a small subset of inhibitory neurons in CeL undergoes synaptic modification and correspondingly, only these neurons express c-fos. Although most neurons that express c-fos in amygdala are excitatory (Knapska et al. 2007), it is known that some stimuli can stimulate c-fos in inhibitory neurons (Reznikov et al. 2008). Alternatively, the neurons we identify in CeC may represent a separate population with a different role in the learning process to the inhibitory neurons described above. The CeC receives nociceptive information from spinal cord via the parabrachial nucleus and the BLA, and is a possible site of integration of nociceptive and polymodal information in fear conditioning (Dong et al. 2010). Indeed fear learning specifically induces synaptic potentiation in both parabrachial–CeC and BLA–CeC synapses (Watabe et al. 2013). The learning-specific neurons we identify in CeC may be the post-synaptic neurons of these potentiated synapses and an integration site for fear conditioning.
Ventromedial hypothalamus
Following auditory fear conditioning, there was strong learning-specific FTL activation in two discrete regions within the VMH. The largest number of FTL+ neurons was found in the rostral VMH, the same region identified in our context fear conditioning experiments (Trogrlic et al. 2011). Additionally, we identified a population of neurons showing learning-specific FTL activation within a caudal region of the VMHvl. We did not detect FTL activation within VMHvl in the context fear conditioning experiments. As discussed above, this may have been due to the generally higher levels of FTL expression in the brains of the mice in the context fear conditioning experiments, which is reduced in the current experiments following habituation and environmental enrichment. FTL expression in both of these regions was very highly learning specific with very little expression in the nonlearning control mice or following fear memory recall.
The VMH, along with other hypothalamic nuclei, is known to be involved in regulation of aggression (Kruk et al. 1983; Lin et al. 2011) and fearful behavior (Swanson 2000; Martinez et al. 2008; Silva et al. 2013). However, the lack of FTL expression in VMH and VMHvl following fear memory recall indicates the VMH is not merely activated by the expression of fear. Experiments using predator odor as a US also support involvement of VMH in fear learning, whereby c-fos expression was more closely correlated with fear learning compared with fear expression (Staples et al. 2005). Additionally, there is direct evidence that the VMH is involved in conditioned fear, in experiments using the fear potentiated startle response. Injections of the GABA agonist, muscimol, into the VMH prior to testing for fear potentiated startle, produced a reduction in conditioned fear (Santos et al. 2008). While this does not establish that VMH neurons are involved in fear learning and memory, it distinguishes this region from other hypothalamic nuclei, such as dorsomedial hypothalamus and premamillary nuclei, which have a clear role in fear behaviors but no identifiable involvement in conditioned fear (Santos et al. 2008).
The major inputs of the VMH are from basolateral and medial amygdala (Risold et al. 1994; Canteras et al. 2001; Gross and Canteras 2012); where basolateral amygdala provides a link between LA and VMH, medial amygdala shows learning-specific activation in our experiments. Experiments using antagonists to the neuropeptide Substance P injected into each of these three areas result in inhibition of fear-potentiated startle (Zhao et al. 2009). Based on this and other data it was proposed that a critical pathway for conditioned fear runs from MeA to VMH, and is regulated by Substance P. This is consistent with our findings and supports the idea that the fear-learning circuit includes a pathway from MeA to VMH.
Lateral septum
Auditory fear conditioning also stimulated FTL expression in dorsal LS, an area known to be implicated in processing aversive stimuli. In this brain region, there was significant FTL activation in Home Cage mice as well as the other nonlearning controls and the recall group of mice. The degree of learning-specific activation was thus lower than that observed in the other brain regions we identified (∼60% greater than Unpaired or any other group). We did not examine this region in our previous studies (Trogrlic et al. 2011), which were restricted to amygdala and hypothalamus, and thus we did not determine if there was also learning-specific neuronal activation following context fear conditioning.
Expression of c-fos has previously been found in LS in a number of different models of fear learning and memory, including fear conditioning (Pezzone et al. 1992; Beck and Fibiger 1995) and fear potentiated startle (Campeau et al. 1991; Veening et al. 2009), however these studies did not analyse if c-fos expression correlated specifically with learning. However, Calandreau et al. (2007) found an increase in the number of c-fos+ neurons in dorsal LS following paired auditory fear conditioning compared with unpaired training, very similar to our results. These findings indicate that a subpopulation of neurons in dorsal LS express c-fos specifically associated with auditory fear learning.
There is significant evidence that LS is involved in different emotional states, and in particular in fear and anxiety (Sheehan et al. 2004). There are also a number of studies that have examined the role of LS in fear learning and memory. Electrolytic lesions to the LS result in potentiated freezing following context fear conditioning (Sparks and LeDoux 1995), suggesting an involvement of LS in the conditioning process. However the complete lesioning of LS in these experiments may have simply affected fear expression without affecting fear learning or memory. Experiments involving reversible inhibition of LS with lidocaine prior to fear-conditioning training resulted in complete disruption of auditory fear conditioning, indicating that LS is required for auditory fear learning (Calandreau et al. 2007). In related experiments with context fear conditioning, reversible inhibition of LS with cobalt chloride prior to fear conditioning had no effect (Reis et al. 2010). These results suggest a specificity of involvement of LS in auditory, or possibly different forms of cued conditioning, over context conditioning. Further experiments involving glutamate agonists and antagonists injected into LS prior to training further support this idea (Calandreau et al. 2010). In these experiments, injection of glutamate agonists into LS promoted auditory fear conditioning and disrupted context fear conditioning, whereas the glutamate antagonists had the opposite effect (Calandreau et al. 2010). These findings thus suggest that the LS plays a key role in the specificity of fear conditioning, via selection of the relevant stimuli.
The LS is therefore implicated as contributing to the selection of environmental stimuli best able to predict the unconditioned stimulus, and the FTL activation seen in the LS in the current study may be due to this role. Further studies need to be undertaken to determine if the learning-related c-fos expression is related to some form of cellular or synaptic plasticity or whether it is associated with some metabolic response in these neurons.
Conclusions
The regions we identified were all very highly localized and were comprised of small numbers of activated neurons. A comparison of the current study on auditory fear conditioning with our previous study with context fear conditioning (Trogrlic et al. 2011) suggests that most of these regions are shared. We identified additional populations in the current study, but this was due to a more extensive brain screen as well as prior EE and habituation of the mice, as discussed above. If these areas are directly involved in the memory process, this suggests that at least a part of auditory and context fear learning and memory are contained within small numbers of neurons in the same very specific regions of the brain. This implies that the general circuits for auditory and context fear conditioning are shared, at least at the subcortical level. The LS is a possible exception and may not be involved in context fear learning, as discussed above. Additionally, there may be auditory and context specificity within these circuits as the auditory-specific and context-specific neurons in these brain regions may be different, or overlapping, populations.
Each of these populations of neurons may form part of a larger fear memory engram, and may be fitted into current hodological studies. The connections within the amygdala with regard to fear pathways have been extensively described, reciprocally connecting both the LA and MeA to the VMH (Canteras et al. 1994; LeDoux 2000; Usunoff et al. 2009). AStr is also known to connect reciprocally with the LA (Jolkkonen et al. 2001), while the connections between the VMH and LS have been described in detail (Risold and Swanson 1996). Each of the brain areas identified and described in the current study are therefore interconnected, and these nuclei may exist as nodes in the larger, auditory-fear memory circuit. What is required next is to determine exactly the inputs and output projections of the learning-specific neurons we have identified. Do the FTL+ neurons we identified project directly or indirectly to each other? This information is required to form an accurate description of the fear-conditioning circuit.
Materials and Methods
Animals
Male FTL+ mice aged 8–12 wk were obtained from the Biomedical Sciences Animal Facility, University of Melbourne. Mice were housed in standard 15 cm × 30 cm × 12 cm cages on a 12-h light–dark cycle, with food and water supplied ad libitum. All experiments were conducted with approval of the Animal Ethics Committee of the University of Melbourne and in accordance with the guidelines of the National Health and Medical Research Council (Australia). At least 2 d prior to the commencement of the experiment, mice were singly housed in cages as described, and relocated to a dedicated behavioral laboratory. This facility consisted of quiet (60 dB) and low light (15–20 Lux) conditions on a 12-h light–dark cycle.
Habituation and enrichment
Mice were subjected to a slightly modified version of the habituation and environmental enrichment paradigm outlined by Nithianantharajah and Murphy 2008. Habituation to the conditioning chamber was performed to minimize any association of the footshock to context, and consisted of placing the mice into the conditioning chamber for 3 min each day. As well as habituating the mice to the conditioning chamber they also underwent environmental enrichment. This has been previously shown to improve the specificity of learning outcomes in auditory fear conditioning (Nithianantharajah and Murphy 2008). Environmental enrichment consisted of placing the mice into separate large (53 cm × 58 cm × 31 cm) plastic containers containing an assortment of objects of varying size, color, and texture (including cardboard tubes, plastic containers, wooden pet toys, and foam packing material) for 60 min per day. Mice were habituated to the conditioning chamber and underwent environmental enrichment once per day for 21 d prior to auditory fear conditioning. The order in which the mice were exposed to either the conditioning chamber or the enriched environment was varied each day. Mice then remained in their home cages for 2 d without any handling prior to conditioning, to ensure that FTL expression induced by exposure to the enriched environment and to the conditioning chamber returned to basal levels of FTL expression.
Auditory fear conditioning
Fear conditioning was conducted using a computerized system (Med Associates, VT, USA). The conditioning chamber was contained within a PVC sound-attenuating compartment (16 cm × 14 cm × 13 cm). The floor of the chamber consisted of stainless steel rods (diameter 3.2 mm, spaced 5 mm apart), connected to a shocker–scrambler unit capable of delivering electric shocks of defined duration and intensity. The chamber was cleaned with 70% ethanol between each habituation, training, and testing sessions. For auditory fear conditioning, mice were assigned to separate groups (Context, Tone, Unpaired, Paired, and Recall) and trained accordingly (see Fig. 1). This ensured that there was a cohort of mice exposed to each of the different experiential aspects of the auditory fear conditioning procedure. On the day of training, Paired mice (the learning group) were exposed to a paired presentation of the tone and footshock. These animals would undergo associative learning, and thus learn to associate the tone and footshock. Paired mice were placed into the chamber for 3 min, followed by exposure to the tone (75 ± 5 dB, 10 kHz) for 30 sec, which coterminated with a 0.2 mA, 2-sec footshock. The mice then remained within the chamber for a further 30 sec before being returned to their home cages. There were a series of control groups in which the mice did not undergo associative learning: (1) unpaired mice were immediately shocked (0.2 mA, 2 sec) upon entry to the chamber. They then remained in the chamber for 3 min, followed by exposure to the tone (75 ± 5 dB, 10 kHz) for 30 sec. After a further 30 sec in the conditioning chamber, Unpaired mice were returned to their home cage. (2) Tone mice were exposed to the chamber for 3 min, followed by exposure to an auditory tone (75 ± 5 dB, 10 kHz) for 30 sec. After a further 30 sec in the conditioning chamber, Tone mice were returned to their home cages. (3) Context mice were exposed to the conditioning chamber for 3 min and 30 sec and then returned to their home cages. (4) Recall mice were included to determine the pattern of FTL induced by the expression of tone-induced fear (freezing). Recall mice had training as for Paired mice but were treated differently for testing (see below).
Paired, Unpaired, Tone, and Context groups of mice remained in their home cages for 4 h after training, which allows for the detection of FTL activity that has been up-regulated by the training conditions. Four hours after exposure to the conditioning chamber, all mice were tested for context- and tone-associated fear (Testing), by placing the mice back in the same training chamber for 3 min in the absence of the tone, followed by 3 min with the tone. Freezing behavior was recorded for each 3-min period, and was defined as the absence of any visible movement of the body except for breathing. Moving data were also recorded as the time each mouse spent moving forward laterally to the floor of the training chamber. Immediately following training mice were terminally anesthetized with 100 µL Lethabarb (Virbac) injected intraperitoneally. Previous work has shown that the minimum time needed to see a significant change in FTL expression due to mice experiencing a stimulus such as training or testing for fear memory is ∼60 min. As the mice are killed <10 min after being tested for context- and tone-associated fear, all FTL expression we see in these mice is only due to the training conditions the mice experienced 4 h previously.
Recall mice remained in their home cages for 72 h after training, so that FTL expression would return to basal levels. Recall mice were then tested as for the other groups, but returned to their home cages for 4 h before being anesthetized. Any changes in FTL expression in these mice is due to the recall of fear and expressing a fear-conditioned response, such as freezing.
Home cage controls were also included, and consisted of mice taken directly from their home cage with no other treatment prior to anesthetization.
Histochemical procedures
Immediately following anesthesia, mice were transcardially perfused with 12 mL 10% sucrose, 2.5 × 10–3 units per microliter Heparin (Sigma-Aldrich, Australia) in analytical grade water, followed by 24 mL of 4% paraformaldehyde (PFA, Merck), and 0.005% gluteraldehyde (EM sciences) in 0.1 M phosphate buffer (pH 7.4). After 30 min, the brains were dissected out and post-fixed in 4% PFA, 0.1 M phosphate buffer for 30 min, rinsed twice with phosphate-buffered saline (PBS), and submerged in cryoprotectant (20% sucrose in 0.1 M PBS) for 48 h. Brains were then snap-frozen in OCT (Sakura Finetek) and kept at −80°C. Coronal sections (50 µm) were cut on a cryostat and transferred into a 24-well tissue culture plate containing PBS, with each well containing 2–3 sections.
The PBS was aspirated from the wells, and replaced with 400 µL per well of assay buffer (10 mM potassium ferricyanide, 10 mM potassium ferrocyanide, 40 mM MgCl2, and 2 mg/mL 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-gal; Roche) in 0.1 M PBS) for 24 h at room temperature, with agitation. Volumes were kept the same for all experiments in order to standardize the conditions of the enzymatic reaction. The sections were then rinsed with PBS and stored in 4% PFA until mounting in 0.5% gelatin solution onto glass slides coated with 0.5% gelatin, 10% potassium chromium alum. Sections were left to dry, dehydrated in histolene and coverslipped (Murphy et al. 2007).
FTL analysis
Stained sections were screened microscopically (Olympus BX61) and brain regions were identified by comparison with a mouse brain atlas (Franklin and Paxinos 2008). Initially, regions of learning-specific FTL expression throughout the brain were identified qualitatively by comparison of sections from Paired mice brains with matching sections from Unpaired brains. Subsequently, slides were scanned using a MIRAX SCAN (Zeiss, Germany) and studied using MIRAX viewer software, enabling side by side comparisons of each section from Paired and Unpaired mouse brains. Sections from all areas of the brain from bregma 3.20 mm to bregma −8.00 mm (excluding cerebellum, which had very little FTL expression) were examined and systematically compared (Ali et al. 2009) this way to identify any additional learning-specific brain regions. Areas that showed a qualitative difference between the Paired and Unpaired conditions in levels of activation were then examined quantitatively in the corresponding sections from all groups. The counting was performed in defined regions of interest (ROI) using a frame generated by the ROI tool of analySIS software (Olympus) of the same shape and size for each brain region (see Results for further details of which areas were counted).
FTL cell counts were done using an Olympus microscope (BX61) at 100× magnification. For all areas where neurons were counted, the area of section was examined in detail by focusing throughout its thickness. In the LAvl only neurons with the following characteristics were counted; neurons had to be within 100 µm of the border of the LaVL with the external capsule, were FTL+ through the entire cell body, and had a stellate morphology with at least two processes. For MeA, AStr, and CeC regions, all cells that were clearly identifiable were counted, even cells with relatively weak FTL staining. In the VMH, only the large stained neurons with visible processes within the defined region (see Results) were included in the count. For the LS region, FTL+ neurons were counted within a 400 µm × 600 µm oval either side of the midline throughout the entire LS (bregma 1.10–0.14 mm), and the three consecutive sections with the highest counts were pooled for each brain. For quantification of each region, the following numbers of mice were used: Home Cage n = 7, Context n = 6, Tone n = 8, Unpaired n = 9, Paired n = 13, Recall n = 5.
To determine the fraction of neurons activated in each region, the density of neurons was estimated by counting the number of Nissl+ cells at least 10 µm in diameter in Nissl-stained sections of known thickness for each counting region from a mouse brain atlas (Franklin and Paxinos 2008). Each density was then multiplied by the volume of the corresponding counting region. Total neuron numbers for each counting region were as follows: LAvl border region 1560; AStr 860; MeApd 5850; CeC 910; VMH 3000; VMHvl 740; LSI 1590. Numbers of activated neurons were represented as a percentage of this cell number.
Statistical significance was determined using a one-way ANOVA and Bonferroni's multiple comparison test comparing Home cage to Context, Paired to Unpaired, and Paired to Recall. Prism software (GraphPad Software) was used for statistical analysis.
Acknowledgments
We thank Rowena Mortimer for technical assistance and Maya Kesar and Sarah Travener for mouse husbandry.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.037663.114.
References
- Adams JP, Sweatt JD. 2002. Molecular psychology: roles for the ERK MAP kinase cascade in memory. Annu Rev Pharmacol Toxicol 42: 135–163. [DOI] [PubMed] [Google Scholar]
- Ali AE, Wilson YM, Murphy M. 2009. A single exposure to an enriched environment stimulates the activation of discrete neuronal populations in the brain of the fos-tau-lacZ mouse. Neurobiol Learn Mem 92: 381–390. [DOI] [PubMed] [Google Scholar]
- Ali AE, Wilson YM, Murphy M. 2012. Identification of neurons specifically activated after recall of context fear conditioning. Neurobiol Learn Mem 98: 139–147. [DOI] [PubMed] [Google Scholar]
- Asanuma M, Ogawa N. 1994. Pitfalls in assessment of c-fos mRNA expression in the brain: effects of animal handling. Rev Neurosci 5: 171–178. [DOI] [PubMed] [Google Scholar]
- Beck CH, Fibiger HC. 1995. Conditioned fear-induced changes in behavior and in the expression of the immediate early gene c-fos: with and without diazepam pretreatment. J Neurosci 15: 709–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrom HC, Johnson LR. 2014. An organization of visual and auditory fear conditioning in the lateral amygdala. Neurobiol Learn Mem 116C: 1–13. [DOI] [PubMed] [Google Scholar]
- Calandreau L, Jaffard R, Desmedt A. 2007. Dissociated roles for the lateral and medial septum in elemental and contextual fear conditioning. Learn Mem 14: 422–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calandreau L, Desgranges B, Jaffard R, Desmedt A. 2010. Switching from contextual to tone fear conditioning and vice versa: the key role of the glutamatergic hippocampal-lateral septal neurotransmission. Learn Mem 17: 440–443. [DOI] [PubMed] [Google Scholar]
- Campeau S, Hayward MD, Hope BT, Rosen JB, Nestler EJ, Davis M. 1991. Induction of the c-fos proto-oncogene in rat amygdala during unconditioned and conditioned fear. Brain Res 565: 349–352. [DOI] [PubMed] [Google Scholar]
- Campeau S, Falls WA, Cullinan WE, Helmreich DL, Davis M, Watson SJ. 1997. Elicitation and reduction of fear: behavioural and neuroendocrine indices and brain induction of the immediate-early gene c-fos. Neuroscience 78: 1087–1104. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW. 1994. Organization of projections from the ventromedial nucleus of the hypothalamus: a Phaseolus vulgaris-leucoagglutinin study in the rat. J Comp Neurol 348: 41–79. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Ribeiro-Barbosa ER, Comoli E. 2001. Tracing from the dorsal premammillary nucleus prosencephalic systems involved in the organization of innate fear responses. Neurosci Biobehav Rev 25: 661–668. [DOI] [PubMed] [Google Scholar]
- Ciocchi S, Herry C, Grenier F, Wolff SBE, Letzkus JJ, Vlachos I, Ehrlich I, Sprengel R, Deisseroth K, Stadler MB, et al. 2010. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature 468: 277–282. [DOI] [PubMed] [Google Scholar]
- Cirelli C, Tononi G. 2000. On the functional significance of c-fos induction during the sleep-waking cycle. Sleep 23: 453–469. [PubMed] [Google Scholar]
- Day HEW, Kryskow EM, Nyhuis TJ, Herlihy L, Campeau S. 2008. Conditioned fear inhibits c-fos mRNA expression in the central extended amygdala. Brain Res 1229: 137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond MC. 2001. Response of the brain to enrichment. An Acad Bras Cienc 73: 211–220. [DOI] [PubMed] [Google Scholar]
- Dong Y-L, Fukazawa Y, Wang W, Kamasawa N, Shigemoto R. 2010. Differential postsynaptic compartments in the laterocapsular division of the central nucleus of amygdala for afferents from the parabrachial nucleus and the basolateral nucleus in the rat. J Comp Neurol 518: 4771–4791. [DOI] [PubMed] [Google Scholar]
- Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Luthi A. 2009. Amygdala inhibitory circuits and the control of fear memory. Neuron 62: 757–771. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Poulos AM. 2005. The neuroscience of mammalian associative learning. Annu Rev Psychol 56: 207–234. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. 2008. The mouse brain in stereotaxic coordinates. Academic Press, San Diego. [Google Scholar]
- Garner AR, Rowland DC, Hwang SY, Baumgaertel K, Roth BL, Kentros C, Mayford M. 2012. Generation of a synthetic memory trace. Science 335: 1513–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross CT, Canteras NS. 2012. The many paths to fear. Nat Rev Neurosci 13: 651–658. [DOI] [PubMed] [Google Scholar]
- Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, Biag J, Dong H-W, Deisseroth K, Callaway EM, et al. 2010. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature 468: 270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JP, Cain CK, Ostroff LE, LeDoux JE. 2011. Molecular mechanisms of fear learning and memory. Cell 147: 509–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolkkonen E, Pikkarainen M, Kemppainen S, Pitkänen A. 2001. Interconnectivity between the amygdaloid complex and the amygdalostriatal transition area: a PHA-L study in rat. J Comp Neurol 431: 39–58. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. 1997. More hippocampal neurons in adult mice living in an enriched environment. Nature 386: 493–495. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Jung MW. 2006. Neural circuits and mechanisms involved in Pavlovian fear conditioning: a critical review. Neurosci Biobehav Rev 30: 188–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapska E, Radwanska K, Werka T, Kaczmarek L. 2007. Functional internal complexity of amygdala: focus on gene activity mapping after behavioral training and drugs of abuse. Physiol Rev 87: 1113–1173. [DOI] [PubMed] [Google Scholar]
- Kovacs KJ. 2008. Measurement of immediate-early gene activation- c-fos and beyond. J Neuroendocrinol 20: 665–672. [DOI] [PubMed] [Google Scholar]
- Kruk MR, Van der Poel AM, Meelis W, Hermans J, Mostert PG, Mos J, Lohman AH. 1983. Discriminant analysis of the localization of aggression-inducing electrode placements in the hypothalamus of male rats. Brain Res 260: 61–79. [DOI] [PubMed] [Google Scholar]
- Labiner DM, Butler LS, Cao Z, Hosford DA, Shin C, McNamara JO. 1993. Induction of c-fos mRNA by kindled seizures: complex relationship with neuronal burst firing. J Neurosci 13: 744–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. 2000. Emotion circuits in the brain. Annu Rev Neurosci 23: 155–184. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Farb C, Ruggiero DA. 1990. Topographic organization of neurons in the acoustic thalamus that project to the amygdala. J Neurosci 10: 1043–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C-I, Maglinao TL, Takahashi LK. 2004. Medial amygdala modulation of predator odor-induced unconditioned fear in the rat. Behav Neurosci 118: 324–332. [DOI] [PubMed] [Google Scholar]
- Li H, Penzo MA, Taniguchi H, Kopec CD, Huang ZJ, Li B. 2013. Experience-dependent modification of a central amygdala fear circuit. Nat Neurosci 16: 332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D, Boyle MP, Dollar P, Lee H, Lein ES, Perona P, Anderson DJ. 2011. Functional identification of an aggression locus in the mouse hypothalamus. Nature 470: 221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Ramirez S, Pang PT, Puryear CB, Govindarajan A, Deisseroth K, Tonegawa S. 2012. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature 484: 381–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. 2004. Neuronal signalling of fear memory. Nat Rev Neurosci 5: 844–852. [DOI] [PubMed] [Google Scholar]
- Martinez RCR, Carvalho-Netto EF, Amaral VCS, Nunes-de-Souza RL, Canteras NS. 2008. Investigation of the hypothalamic defensive system in the mouse. Behav Brain Res 192: 185–190. [DOI] [PubMed] [Google Scholar]
- McCue MG, LeDoux JE, Cain CK. 2014. Medial amygdala lesions selectively block aversive Pavlovian-instrumental transfer in rats. Front Behav Neurosci 8: 329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milanovic S, Radulovic J, Laban O, Stiedl O, Henn F, Spiess J. 1998. Production of the Fos protein after contextual fear conditioning of C57BL/6N mice. Brain Res 784: 37–47. [DOI] [PubMed] [Google Scholar]
- Müller M, Fendt M. 2006. Temporary inactivation of the medial and basolateral amygdala differentially affects TMT-induced fear behavior in rats. Behav Brain Res 167: 57–62. [DOI] [PubMed] [Google Scholar]
- Murphy M, Greferath U, Wilson YM. 2007. A method for detecting functional activity related expression in gross brain regions, specific brain nuclei and individual neuronal cell bodies and their projections. Biol Proc Online 9: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader K, Majidishad P, Amorapanth P, LeDoux JE. 2001. Damage to the lateral and central, but not other, amygdaloid nuclei prevents the acquisition of auditory fear conditioning. Learn Mem 8: 156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nithianantharajah J, Hannan AJ. 2006. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci 7: 697–709. [DOI] [PubMed] [Google Scholar]
- Nithianantharajah J, Murphy M. 2008. Auditory specific fear conditioning results in increased levels of synaptophysin in the basolateral amygdala. Neurobiol Learn Mem 90: 36–43. [DOI] [PubMed] [Google Scholar]
- Nithianantharajah J, Levis H, Murphy M. 2004. Environmental enrichment results in cortical and subcortical changes in levels of synaptophysin and PSD-95 proteins. Neurobiol Learn Mem 81: 200–210. [DOI] [PubMed] [Google Scholar]
- Papa M, Pellicano MP, Welzl H, Sadile AG. 1993. Distributed changes in c-Fos and c-Jun immunoreactivity in the rat brain associated with arousal and habituation to novelty. Brain Res Bull 32: 509–515. [DOI] [PubMed] [Google Scholar]
- Paré D. 2002. Mechanisms of Pavlovian fear conditioning: has the engram been located? Trends Neurosci 25: 436–437. [DOI] [PubMed] [Google Scholar]
- Pezzone MA, Lee WS, Hoffman GE, Rabin BS. 1992. Induction of c-Fos immunoreactivity in the rat forebrain by conditioned and unconditioned aversive stimuli. Brain Res 597: 41–50. [DOI] [PubMed] [Google Scholar]
- Radulovic J, Kammermeier J, Spiess J. 1998. Relationship between fos production and classical fear conditioning: effects of novelty, latent inhibition, and unconditioned stimulus preexposure. J Neurosci 18: 7452–7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez S, Liu X, Lin P-A, Suh J, Pignatelli M, Redondo RL, Ryan TJ, Tonegawa S. 2013. Creating a false memory in the hippocampus. Science 341: 387–391. [DOI] [PubMed] [Google Scholar]
- Reijmers LG, Perkins BL, Matsuo N, Mayford M. 2007. Localization of a stable neural correlate of associative memory. Science 317: 1230–1233. [DOI] [PubMed] [Google Scholar]
- Reis DG, Scopinho AA, Guimarães FS, Corrêa FMA, Resstel LBM. 2010. Involvement of the lateral septal area in the expression of fear conditioning to context. Learn Mem 17: 134–138. [DOI] [PubMed] [Google Scholar]
- Reznikov LR, Reagan LP, Fadel JR. 2008. Activation of phenotypically distinct neuronal subpopulations in the anterior subdivision of the rat basolateral amygdala following acute and repeated stress. J Comp Neurol 508: 458–472. [DOI] [PubMed] [Google Scholar]
- Risold PY, Swanson LW. 1996. Structural evidence for functional domains in the rat hippocampus. Science 272: 1484–1486. [DOI] [PubMed] [Google Scholar]
- Risold PY, Canteras NS, Swanson LW. 1994. Organization of projections from the anterior hypothalamic nucleus: a Phaseolus vulgaris-leucoagglutinin study in the rat. J Comp Neurol 348: 1–40. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, Schafe GE, LeDoux JE. 2004. Molecular mechanisms underlying emotional learning and memory in the lateral amygdala. Neuron 44: 75–91. [DOI] [PubMed] [Google Scholar]
- Rosen JB, Fanselow MS, Young SL, Sitcoske M, Maren S. 1998. Immediate-early gene expression in the amygdala following footshock stress and contextual fear conditioning. Brain Res 796: 132–142. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Wang YM, Finn DA. 1999. Different levels of Fos immunoreactivity after repeated handling and injection stress in two inbred strains of mice. Pharmacol Biochem Behav 63: 143–151. [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ES, Lopez De Armentia M, Power J. 2003. The amygdaloid complex: anatomy and physiology. Physiol Rev 83: 803–834. [DOI] [PubMed] [Google Scholar]
- Santos JM, Macedo CE, Brandão ML. 2008. Gabaergic mechanisms of hypothalamic nuclei in the expression of conditioned fear. Neurobiol Learn Mem 90: 560–568. [DOI] [PubMed] [Google Scholar]
- Schafe GE, Atkins CM, Swank MW, Bauer EP, Sweatt JD, LeDoux JE. 2000. Activation of ERK/MAP kinase in the amygdala is required for memory consolidation of Pavlovian fear conditioning. J Neurosci 20: 8177–8187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shammah-Lagnado S, Alheid G, Heimer L. 1999. Afferent connections of the interstitial nucleus of the posterior limb of the anterior commissure and adjacent amygdalostriatal transition area in the rat. Neuroscience 94: 1097–1123. [DOI] [PubMed] [Google Scholar]
- Sheehan TP, Chambers RA, Russell DS. 2004. Regulation of affect by the lateral septum: implications for neuropsychiatry. Brain Res Brain Res Rev 46: 71–117. [DOI] [PubMed] [Google Scholar]
- Silva BA, Mattucci C, Krzywkowski P, Murana E, Illarionova A, Grinevich V, Canteras NS, Ragozzino D, Gross CT. 2013. Independent hypothalamic circuits for social and predator fear. Nat Neurosci 16: 1731–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Banerjee S, Gold PW, Glowa J. 1992. Induction of c-fos mRNA in rat brain by conditioned and unconditioned stressors. Brain Res 578: 135–141. [DOI] [PubMed] [Google Scholar]
- Sparks PD, LeDoux JE. 1995. Septal lesions potentiate freezing behavior to contextual but not to phasic conditioned stimuli in rats. Behav Neurosci 109: 184–188. [DOI] [PubMed] [Google Scholar]
- Staples LG, Hunt GE, Cornish JL, McGregor IS. 2005. Neural activation during cat odor-induced conditioned fear and ‘trial 2’ fear in rats. Neurosci Biobehav Rev 29: 1265–1277. [DOI] [PubMed] [Google Scholar]
- Swanson LW. 2000. Cerebral hemisphere regulation of motivated behavior. Brain Res 886: 113–164. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Hubbard DT, Lee I, Dar Y, Sipes SM. 2007. Predator odor-induced conditioned fear involves the basolateral and medial amygdala. Behav Neurosci 121: 100–110. [DOI] [PubMed] [Google Scholar]
- Trogrlic L, Wilson YM, Newman AG, Murphy M. 2011. Context fear learning specifically activates distinct populations of neurons in amygdala and hypothalamus. Learn Mem 18: 678–687. [DOI] [PubMed] [Google Scholar]
- Usunoff KG, Schmitt O, Itzev DE, Haas SJ-P, Lazarov NE, Rolfs A, Wree A. 2009. Efferent projections of the anterior and posterodorsal regions of the medial nucleus of the amygdala in the mouse. Cells Tissues Organs 190: 256–285. [DOI] [PubMed] [Google Scholar]
- Veening JG, Böcker KBE, Verdouw PM, Olivier B, De Jongh R, Groenink L. 2009. Activation of the septohippocampal system differentiates anxiety from fear in startle paradigms. Neuroscience 163: 1046–1060. [DOI] [PubMed] [Google Scholar]
- Walker DL, Paschall GY, Davis M. 2005. Glutamate receptor antagonist infusions into the basolateral and medial amygdala reveal differential contributions to olfactory vs. context fear conditioning and expression. Learn Mem 12: 120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Kang-Park M-H, Wilson WA, Moore SD. 2002. Properties of the pathways from the lateral amygdal nucleus to basolateral nucleus and amygdalostriatal transition area. J Neurophysiol 87: 2593–2601. [DOI] [PubMed] [Google Scholar]
- Watabe AM, Ochiai T, Nagase M, Takahashi Y, Sato M, Kato F. 2013. Synaptic potentiation in the nociceptive amygdala following fear learning in mice. Mol Brain 6: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson YM, Murphy M. 2009. A discrete population of neurons in the lateral amygdala is specifically activated by contextual fear conditioning. Learn Mem 16: 357–361. [DOI] [PubMed] [Google Scholar]
- Wilson Y, Nag N, Davern P, Oldfield BJ, McKinley MJ, Greferath U, Murphy M. 2002. Visualization of functionally activated circuitry in the brain. Proc Natl Acad Sci 99: 3252–3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Takayanagi Y, Onaka T. 2014. The medial amygdala-medullary PrRP-synthesizing neuron pathway mediates neuroendocrine responses to contextual conditioned fear in male rodents. Endocrinology 155: 2996–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Yang Y, Walker DL, Davis M. 2009. Effects of substance P in the amygdala, ventromedial hypothalamus, and periaqueductal gray on fear-potentiated startle. Neuropsychopharmacology 34: 331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]