Abstract
Background
Amphetamine analogues have been demonstrated to have some efficacy in reducing use in cocaine dependent individuals. However, these agents also have potential for abuse. Lisdexamfetamine (LDX), a lysine+dextroamphetamine formulation, has been approved for the treatment of Attention-Deficit/Hyperactivity Disorder (ADHD) and as a prodrug, has less abuse potential.
Objective
This pilot study sought to evaluate the safety, tolerability, and efficacy of LDX as a candidate treatment for cocaine dependence.
Methods
A randomized, double-blind, placebo-controlled parallel group study served to evaluate LDX in 43 cocaine-dependent individuals: (1) Placebo (PBO; 0 mg, n = 21), (2) LDX (70 mg, n = 22). Participants received medication for 14 weeks. Cocaine use was determined based on urine analysis for benzoylecgonine (BE; a cocaine metabolite).
Results
Retention rates were higher though not significantly different in the PBO (71.4%) than the LDX condition (57.1%). Compared to those in the PBO condition, those receiving LDX were more likely to report experiencing (ps < .05) diarrhea (45.5% vs. 14.3%), headaches (45.5% vs. 9.5%), and anxiety (31.8% vs. 4.8%). No differences in medication conditions were observed for blood pressure, heart rate, or body weight. In the randomized sample, no differences in cocaine use were seen. Those receiving LDX reported significantly less craving for cocaine than participants receiving PBO.
Conclusions
LDX did not significantly reduce cocaine use compared to PBO in the randomized sample.
Keywords: cocaine, amphetamine analogues, dextroamphetamine, lisdexamfetamine dimesylate, L-lysine-Dextroamphetamine, agonist-like treatment
1. INTRODUCTION
Numerous candidate pharmacotherapeutics have been evaluated to promote cessation or reduction of cocaine use (Amato et al., 2007, 2011; Castells et al., 2010; Minozzi et al., 2008; Pani et al., 2011). Despite several decades of effort, it has been difficult to identify medications that consistently reduce cocaine use. One approach that has shown promise for the treatment of cocaine dependence involves agonist-like medications (Amato et al., 2011; Grabowski et al., 2004b; Herin et al., 2010; Rush and Stoops, 2012). Agonist-like medications are thought to reduce cocaine use through several mechanisms that support or enhance the dopaminergic system essential in response to cocaine. Mechanisms may involve inhibition of dopamine reuptake or metabolism (e.g., bupropion, disulfiram, methyphenidate, modafinil; Anderson et al., 2009; Poling et al., 2006; Schottenfeld et al., 2014; Winhusen et al., 2006), replenishment of dopamine stores (e.g., levodopa; Schmitz et al., 2008), or indirect enhancement of dopaminergic function via reversal of the dopamine transporter (e.g., dextroamphetamine; Grabowski et al., 2001, 2004b; Levin et al., 2015; Mariani et al., 2012; Mooney et al., 2009; Schmitz et al., 2012; Shearer et al., 2003).
In humans, double-blind clinical studies have demonstrated as much as a 50% reduction in cocaine use following amphetamine analogue treatment in cocaine-dependent individuals (Grabowski et al., 2001, 2004a; Levin et al., 2015; Mooney et al., 2009; Shearer et al., 2003). Evidence of the efficacy of other psychostimulants for the treatment of cocaine dependence has been less clear (Castells et al., 2010). Given the significant reduction in cocaine use achieved with amphetamine analogues, initiation of large scale Phase III trials would not be unreasonable. Several amphetamine mixtures and formulations are approved by the Food and Drug Administration (FDA) for the treatment of Attention-Deficit/Hyperactivity Disorder (ADHD), including Adderall®, Concerta®, and Benzedrine® (Micromedex® Healthcare Series., n.d.). However, a critical barrier has seriously challenged further evaluation of amphetamine analogues or other stimulant-like agents in cocaine-dependent individuals in the community. Specifically, amphetamine analogues have the potential for abuse and diversion (Grinspoon and Hedblom, 1975; Heal et al., 2013b; Kollins, 2007). Indeed high-dose, non-therapeutic use of amphetamines can have dire health effects, and in rare instances, cause death (Barr et al., 2006; Grinspoon and Hedblom, 1975; Lineberry and Bostwick, 2006). Potential risks and benefits along with challenges to evaluation for FDA approval have been very recently reviewed (Negus and Henningfield, 2014).
However, a novel formulation of dextroamphetamine, lisdexamfetamine dimesylate (LXD; Vyvanse®), has been approved by the FDA for the treatment of ADHD. LDX is a prodrug consisting of dextroamphetamine covalently bonded to the amino acid lysine. Following oral administration, unlike other amphetamines, red blood cells convert the pro-drug (LDX) to the active drug (dextroamphetamine) by enzymatic rate-limited hydrolysis of lysine from amphetamine (Pennick, 2010). The kinetics of the rate limiting enzymatic reaction are such that the medication has a slow onset and long-lasting efficacy similar to a sustained-release amphetamine preparation (Biederman et al., 2007; Jasinski and Krishnan, 2009b; Pennick, 2010); this can further minimize abuse potential. In a preclinical study using a cocaine-trained rodent model, LDX did not serve as a reinforcer, although LDX did generalize to dextroamphetamine (Heal et al., 2013a). Most recently, researchers have identified more specifically the hydrolysis pathways determining stability of dose exposure, longer duration of action, and lowered plasma concentrations, along with longer time to peak concentration compared to immediate release dextroamphetamine, notably when delivered by routes other than oral (e.g., IV; Ermer et al., 2010; Jasinski and Krishnan, 2009a, b; Wigal et al., 2010). Studies conducted in rhesus monkeys found that LDX had a slower onset and longer duration of action than amphetamine but retained amphetamine’s efficacy to reduce cocaine choice in a cocaine-vs.-food choice procedure (Banks et al., 2015). These results were interpreted to suggest that LDX may have lower abuse liability than amphetamine but similar therapeutic efficacy for treatment of cocaine abuse (Banks et al., 2015). In combination, these factors are posited to limit abuse liability of LDX (Hutson et al., 2014).
This proof-of-concept study is the first to evaluate the safety, tolerability, and efficacy of LDX as an agonist-like therapy for cocaine dependence. Participants received LDX (70 mg, ≈ 30 mg dextroamphetamine) or Placebo and cognitive-behavioral therapy over the 14-week trial. Compared to placebo, we hypothesized that the greatest reduction in cocaine use would occur in individuals receiving LDX. Safety was determined via monitoring of self-reported side effects and vital signs, and tolerability was operationalized in terms of treatment retention and medication adherence. Efficacy outcomes included cocaine use and cocaine craving.
2. MATERIAL AND METHODS
2.1 Participants
This study and all related materials were reviewed and approved by the University of Minnesota Committee for the Protection of Human Subjects (Study Number: 0812M54801). This was a randomized, placebo-controlled, double-blind parallel groups study in adults with cocaine dependence examining the effects of LDX on cocaine use (ClinicalTrials.gov identifier: NCT00958282). Participants were recruited through advertisements in local media sources (September, 2009 to December, 2012), and underwent a telephone interview to establish initial eligibility. To be included, participants had to be: (a) treatment-seeking; (b) between 18 and 65 years of age; (c) in generally good psychiatric and medical health with a normal electrocardiogram (ECG) and no history of heart disease; and (d) cocaine-dependent at time of intake by Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) criteria (American Psychiatric Association, 1994).
Exclusion criteria included: (a) DSM-IV diagnoses for current psychotic disorders, mood disorders (except substance-induced depression), anxiety disorders, ADHD, and other current substance dependence (except marijuana and nicotine dependence); (b) alcohol dependence (with or without physiological dependence); (c) current use of any prescription medications contraindicated by dextroamphetamine; (d) currently pregnant or nursing; (e) current elevation of liver enzyme levels above twice normal limits; (f) existing cardiovascular disease as determined by physician, and ECG evaluation; (g) history of significant acute or chronic physical illness precluding participation; and (h) history of hyperthyroidism, glaucoma, or seizures. Study enrollment and attrition data are presented in Figure 1.
Figure 1.
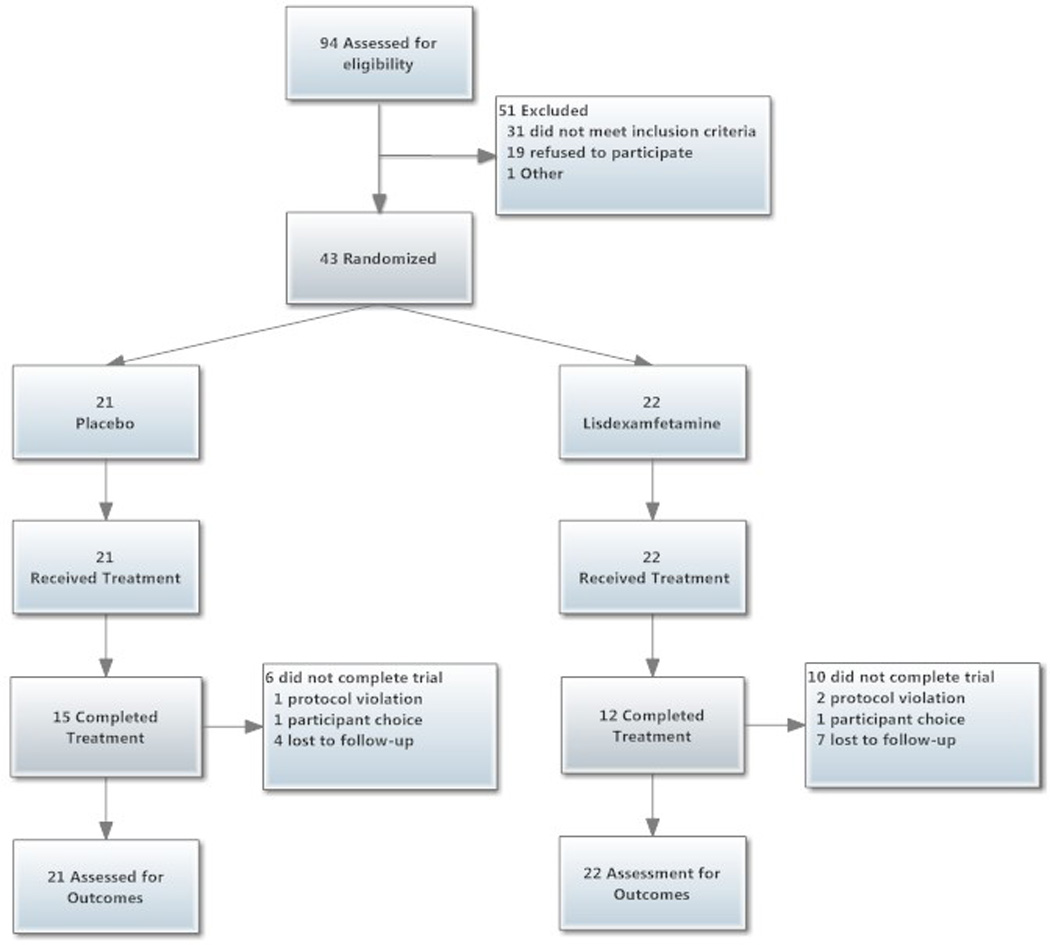
Study participant flow diagram (CONSORT).
2.2 Procedures
The research was conducted at the Ambulatory Research Center, a component of the Department of Psychiatry at the University of Minnesota. The four study phases were: (1) intake (1 week), (2) medication induction (1 week), (3) treatment (14 weeks), and (4) medication reduction (1 week).
2.2.1 Intake
Callers meeting initial telephone screen criteria received an appointment for the consent process and a pre-treatment evaluation, which included a medical history and complete physical examination. Following acquisition of informed consent, a nurse practitioner (under the supervision of S.S.) met with prospective subjects, conducted a physical examination and obtained a medical history. In addition, subjects underwent an ECG and provided urine and blood samples for laboratory tests. Diagnostic interviews were conducted to assess psychiatric history (i.e., Structured Clinical Interview for DSM-IV Axis I Disorders [SCID], First et al., 1995), as well as substance abuse and psychosocial functioning (Addiction Severity Index, [ASI], McLellan et al., 2006). All evaluation data were presented to the study PI (D.H. and later, M.M.) and study physician (S.S.) who determined final eligibility.
2.2.2. Medication Induction
Following intake and randomization to LDX or PBO, subjects underwent a 1-week induction period. Subjects receiving LDX underwent a gradual dose increase until reaching the target dose of 70 mg/day. Subjects randomized to PBO received identical capsules on the same schedule. The medication schedule is based on our previous double-blind cocaine studies (Grabowski et al., 2001, 2004a) using a 1-week run-up to reach 30 mg/day of dextroamphetamine (about equivalent to 70 mg/day of LDX). We used the highest approved dose of LDX, 70 mg; this daily dose contains slightly less than half (≈30 mg) of dextroamphetamine, with the remainder (≈40 mg) composed of the amino acid lysine (Jasinski and Krishnan, 2009b; Krishnan and Zhang, 2008). In this study, the selected dose of LDX was 70 mg, since this was the highest approved dose by the FDA.
2.2.3. Treatment
After daily attendance during medication induction, subjects began the 14-week intervention phase, during which twice weekly attendance was required. Given concerns in the field regarding risks associated with such trials, we applied exceptionally stringent criteria for continuing participation as in previous trials. For any rolling two-week period, participants were required to provide at least 75% of the requested data.
2.2.4. Study end Dose Reduction
The dose reduction sequence reversed the induction phase and was followed by final evaluation.
2.3 Interventions
2.3.1. Medication
Participants were randomly assigned to receive Placebo (0 mg/day, n = 21) or LDX (70 mg/day, n = 22). LDX (purchased from Shire Pharmaceuticals, Inc.) was over-encapsulated in a gel cap by the University of Minnesota Medical Center, Fairview, Investigational Drug Service (IDS) to match identically appearing placebo capsules. Monitoring of medication compliance in urine samples was enhanced by the IDS addition of supradietary levels of riboflavin to each capsule (50 mg) (Del Boca et al., 1996).
2.3.2. Therapy
A manual-based, cognitive-behavioral therapy (CBT) was provided for one hour each week by a master’s-level therapist. The CBT emphasized relapse prevention and coping skills (for a full description see Schmitz et al., 2001)
2.4. Measures
2.4.1. Biological Measures
At each visit, subjects provided urine samples, which were analyzed for benzoylecgonine (BE; a cocaine metabolite) and riboflavin. BE was assessed semi-quantitatively using the PROFILE® -V MEDTOXScan® Drugs of Abuse Test System (MEDTOX, 2009), with cocaine positive tests equaling or exceeding 150 ng/mL. Riboflavin levels range from 0 to 99 fluorescence units, with levels at or below 20 units considered to reflect non-compliance with medication administration (Mooney et al., 2004a).
2.4.2. Subjective Measures
On a weekly basis, patients completed measures of cocaine craving (Halikas et al., 1997). Medication side effects were assessed via a questionnaire previously developed to capture known amphetamine side effects (Grabowski et al., 2001, 2004a; Mooney et al., 2009, 2007; Schmitz et al., 2012). Mood was assessed using the Beck Depression Inventory – II (Beck et al., 1996). The integrity of the study blind was assessed at the end of the treatment phase (i.e., week 14) by having participants and the study physician judge to which medication group the participant had been assigned (Mooney et al., 2004b).
2.5. Statistical Analyses
2.5.1. Assumptions
All analyses were conducted using the Statistical Analysis System, Version 9.4. (SAS Institute Inc., 2014). Except for baseline analyses, all analyses included only data from the 14-week treatment phase. Values of p<.05 were considered statistically significant for main effects and interactions. The Type I error rates in all post-hoc comparisons was controlled using Tukey-Kramer adjustments. Due to participant attrition and frequent missing data, the number of subjects or data points available for statistical analysis varied (see Figure 1 for aggregate attrition figures).
2.5.2. Sample Size
Power analyses were conducted using Monte Carlo simulations for generalized linear mixed models, and using NCSS-PASS for Poisson and logistic regression, with assumptions based on our previous dextroamphetamine studies (Grabowski et al., 2001, 2004). It was determined that a sample of 100 would permit 80% power. However, a total sample of 43 subjects was randomized to treatment, about 40% of that needed for sufficient power. Given the observed cocaine abstinence rates, a total of 102 subjects per treatment (204 total) group would have been needed to have 80% power to detect the observed effect (Rochon, 1998).
2.5.3. Analytic Samples and Treatment of Missing Data
Cocaine use data were approached via two different analytic samples, and two different analytic strategies concerning the treatment of missing data. Two analytic samples were evaluated in this trial: (1) Randomized (N = 43 subjects who were randomized to treatment); and (2) Completers (N = 27 subjects who completed the 14-week treatment phase). Each sample was analyzed in two ways: (1) Intention to treat (ITT; each missing value for a cocaine urine test was imputed to indicate cocaine use); and (2) Missing as missing (MAM; missing values were left as missing).
2.5.4. Techniques
Comparability of study groups across baseline demographic and substance-use variables was evaluated using t-tests for continuous variables and chi-square tests for categorical variables. Kaplan-Meier survival analysis with right censoring was used to test for differences in the duration of treatment as a function of condition. In the case of repeated measures analyses, we employed multilevel models with between-subjects effects of treatment, within-subjects effects of time, and the interaction of treatment and time. Appropriate link functions were employed (e.g., Gaussian, Logit).
2.5.5. Models
In repeated measures models, each model included tests for effects of Medication (i.e., 0 = Placebo, 1 = LDX), Time (i.e., 1 – 14 weeks), and their interaction. The value of the dependent measure during the intake phase was used as a covariate. One exception was cocaine use analyses in which self-reported cocaine use in the 30 days preceding treatment was employed as the covariate (Carroll et al., 2004; McLellan et al., 2006).
3. RESULTS
3.1. Sample Description
Sample characteristics including demographics, substance use variables, and psychosocial functioning are presented in Table 1. No differences were observed across conditions.
Table 1.
Sample Characteristics
| Randomized Samplea (N = 43) |
Completers Sampleb (N = 27) |
|||
|---|---|---|---|---|
| Condition | Placebo (n = 21) |
70 mg LDXc (n = 22) |
Placebo (n = 15) |
70 mg LDXc (n = 12) |
| Demographic | ||||
| Aged | 46.4 (10) | 45.1 (5.9) | 46.7 (7.7) | 43.9 (7.7) |
| % Femalee | 18.2 (4) | 21.1 (4) | 20.0 (3) | 16.3 (2) |
| Educationd | 12.9 (1.5) | 12.8 (1.7) | 13 (1.7) | 13.3 (1.7) |
| %Racee | ||||
| Black | 63.2 (12) | 63.6 (14) | 60.0 (9) | 75.0 (9) |
| White | 15.8 (3) | 36.4 (8) | 13.3 (2) | 25.0 (3) |
| Other | 21.1 (4) | 0 (0) | 26.7 (4) | 0 (0) |
| % Marriede | 4.5 (1) | 5.3 (1) | 6.67 (1) | 0 (0) |
| % Employede | 22.7 (5) | 21.1 (4) | 33.3 (5) | 25.0 (3) |
| Drug Use | ||||
| %Intake Cocainee | 68.2 (15) | 89.5 (17) | 86.7 (13) | 84.1 (12) |
| Cocaine Use (30 days) d |
16.1 (9.1) | 12.7 (6.5) | 13.6 (6.4) | 17.9 (6.4) |
| Nicotine Use (30 days) d |
26.5 (7.9) | 28.5 (11.4) | 26.8 (0) | 30.0 (0) |
| Marijuana Use (30 days) d |
9.0 (10.0) | 11.4 (11.9) | 11.3 (12.1) | 12.4 (12.1) |
| Cocaine (Yrs.) d | 7.1 (9.2) | 13.5 (7.7) | 16.5 (9.2) | 6.4 (9.2) |
| Alcohol (Yrs.) d | 5.4 (9.7) | 9.1 (10.3) | 11 (9.2) | 5.7 (9.2) |
| Marijuana (Yrs.) d | 7 (10.2) | 8.9 (10.6) | 7.6 (10.9) | 9 (10.9) |
| ASI Scores | ||||
| Medicald | 0.27 (0.36) | 0.15 (0.22) | 0.17 (0.39) | 0.33 (0.39) |
| Employmentd | 0.72 (0.3) | 0.78 (0.3) | 0.78 (0.37) | 0.65 (0.37) |
| Alcohold | 0.10 (0.15) | 0.19 (0.15) | 0.24 (0.13) | 0.08 (0.13) |
| Drugd | 0.22 (0.08) | 0.21 (0.06) | 0.22 (0.06) | 0.23 (0.06) |
| Legald | 0.06 (0.1) | 0.09 (0.17) | 0.03 (0.12) | 0.07 (0.12) |
| Family/Sociald | 0.18 (0.16) | 0.17 (0.12) | 0.13 (0.16) | 0.17 (0.16) |
| Psychiatricd | 0.11 (0.16) | 0.17 (0.18) | 0.11 (0.12) | 0.09 (0.12) |
Note. Comparability of study groups across baseline demographic and substance-use variables was evaluated using t-tests for continuous variables and chi-square tests for categorical variables. No significant differences were observed among conditions on any variable.
Randomized Sample = 43 subjects who were randomized to treatment.
Completers Sample = 27 subjects finishing the 14-week trial.
LDX = Lisdexamfetamine.
Mean and standard deviation.
Percentage and n.
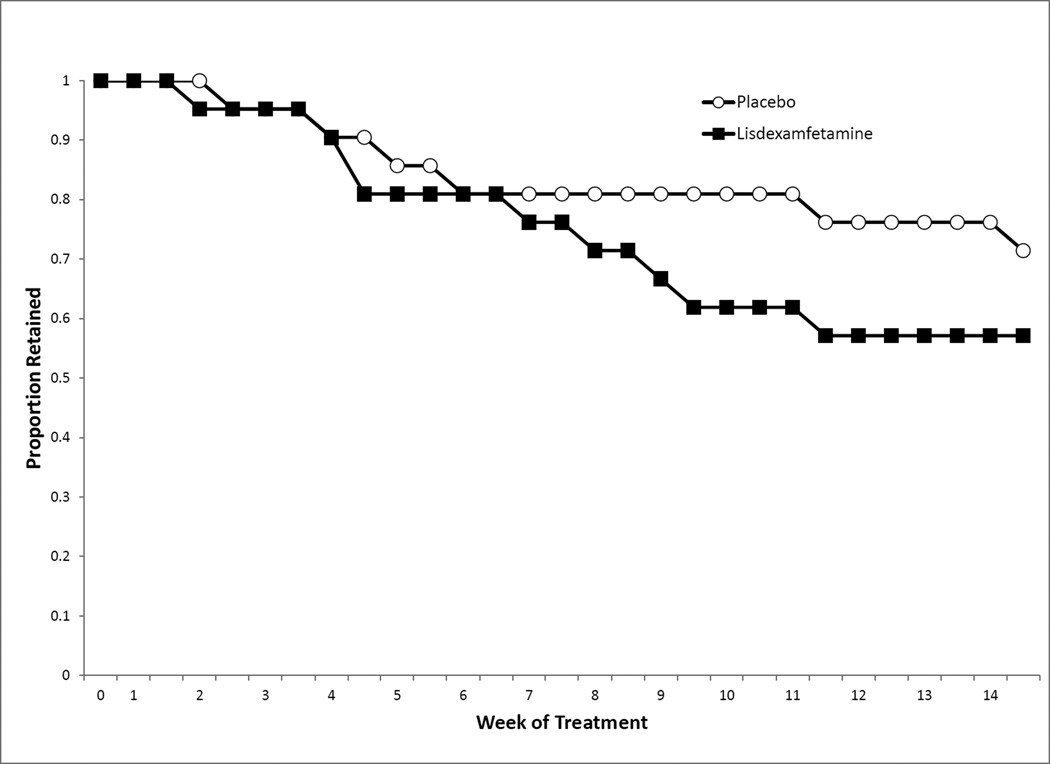
3.2. Retention
Survival analysis indicated no difference in dropout rates (see Figure 2), Log Rank Statistic, χ2(1) = .9318, p = .3344, with 64.3% of participants randomized to treatment completing treatment (Placebo, 71.4%; LDX, 57.1%).
Figure 2.
Subject retention across the treatment phase.
The two groups did not differ in the number of weeks completed (Placebo, M = 11.9, SD = 4.1; LDX, M = 10.6, SD = 4.4). Of the 16 subjects lost from the study (Placebo, N = 6; LDX, N = 10), the majority (n = 11) were lost to follow-up, the others self-excluded from the study (n = 2) or were discontinued from the trial for protocol violations (n = 3, e.g., sporadic attendance).
3.3. Adverse Events
If a subject endorsed an AE one or more times during the 14-week treatment phase, the subject was coded as having the AE (see Table 2). Those in the LDX group reported higher rates of diarrhea, headaches, and anxiety. None of these adverse events required treatment. No subjects discontinued treatment due to intolerance of study medication. There were two serious adverse events in this trial. In the first case, a patient was hospitalized with psychosis (assigned to the placebo condition). She had apparently binged on cocaine prior to her psychotic episode. In addition, she failed to reveal to study staff that she was diagnosed with schizoaffective disorder. She recovered but decided to stop participating in the study. In the second case, a patient was hospitalized for chest pain and diagnosed with angina that was possibly related to the study drug (assigned to LDX condition). He did not continue in the study.
Table 2.
Mean Rates of 15 Self-Reported Medication Adverse Events in the Treatment Phase
| Side Effect |
Placebo N = 21 |
LDX (70 mg) N = 22 |
|---|---|---|
| 1. Nausea | 4.8 | 22.7 |
| 2. Vomiting | 0.0 | 13.6 |
| 3. Diarrhea | 14.3 | 45.5* |
| 4. Abdominal pain | 4.8 | 13.6 |
| 5. Chest pains | 0.0 | 9.1 |
| 6. Changes in appetite | 33.3 | 54.5 |
| 7. Headache | 9.5 | 45.5† |
| 8. Dizziness | 0.0 | 13.6 |
| 9. Fatigue | 14.3 | 27.3 |
| 10. Anxiety | 4.8 | 31.8* |
| 11. Insomnia | 9.5 | 31.8 |
| 12. Somnolence | 0.0 | 4.5 |
| 13. Depression | 14.3 | 13.6 |
| 14. Itching | 4.8 | 13.6 |
| 15. Rash | 0.0 | 9.1 |
Note.
p < 05.
p <01.
Adverse event (AE) rates were monitored during the 14-week treatment phase. If a subject endorsed an AE one or more times during the treatment phase, the subject was coded as having the AE.
3.4. Vital Signs
3.4.1. Weight
Body weight did not differ by medication group, time, or their interaction (See Supplemental Data1; Placebo, M = 192.9 lbs., SE = 8.19; LDX, M = 193.8 lbs., SE = 8.19)
3.4.2. Blood pressure and heart rate
No effects of medication group, time, or their interaction were observed for systolic blood pressure (Placebo, M = 120.9 mmHg, SE = 1.66; LDX, M = 122.6 mmHg, SE = 1.71). Diastolic blood pressure tended to fluctuate slightly over time, F(13, 285) = 2.21, p = 0.0094, (Placebo, M = 74.0 mmHg, SE = 0.96; LDX, M = 74.5 mmHg, SE = 0.99). No effects of medication group, time, or their interaction were observed for heart rate (Placebo, M = 77.4 beats per minute, SE = 1.38; LDX, M = 81.3 beats per minute, SE = 1..43).
3.5. Medication Adherence
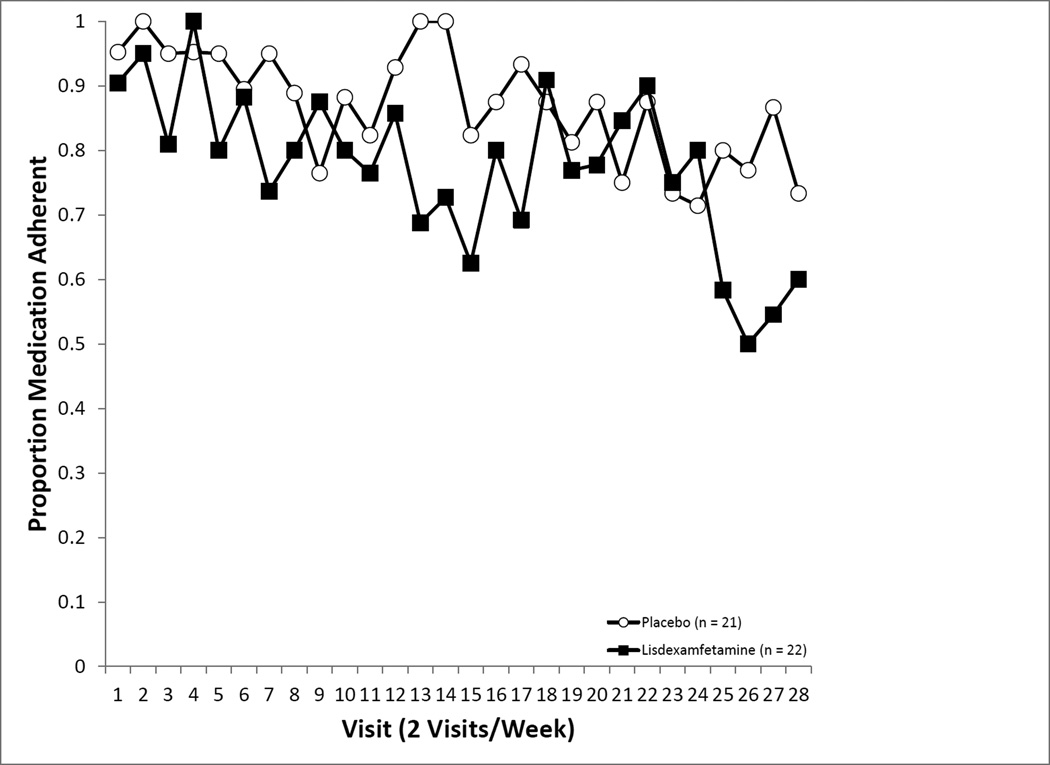
Prior to beginning LDX or PBO capsules that included supradietary levels of riboflavin, the rate of detectable riboflavin was low (2%). Adherence rates based on riboflavin did not differ by medication assignment, F(1, 32.52) = 3.96, p = .0550, but did significantly vary across time, F(1, 27) = 1.94, p = .0030. The interaction term between medication and time could not be estimated due to insufficient variation. Those in the PBO condition tended to show consistently higher rates of adherence (93.4%), while participants in the LDX condition demonstrated lower and somewhat more variable adherence rates (80.3%).
3.6. Cocaine Use (Randomized Sample)
3.6.1. Randomized Sample, ITT
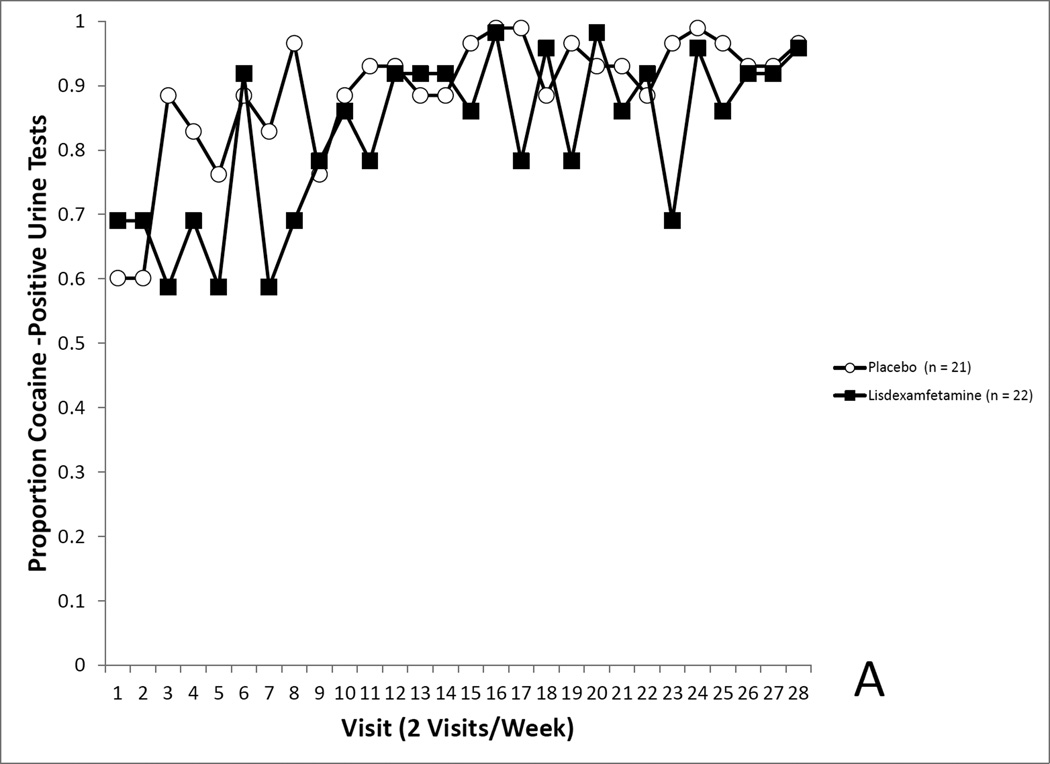
Analysis of cocaine use rates, where missing observations were imputed to indicate cocaine use, revealed no differences by medication assignment on covariate-adjusted cocaine use rates (Placebo, 92.4% positive, LDX, 86.5%), F(1, 32.02) = 0.53, p = 0.4728 (see Figure 4A). Cocaine use rates tended to rise over the course of treatment, F(27, 1063) = 2.21, p<0.0004.
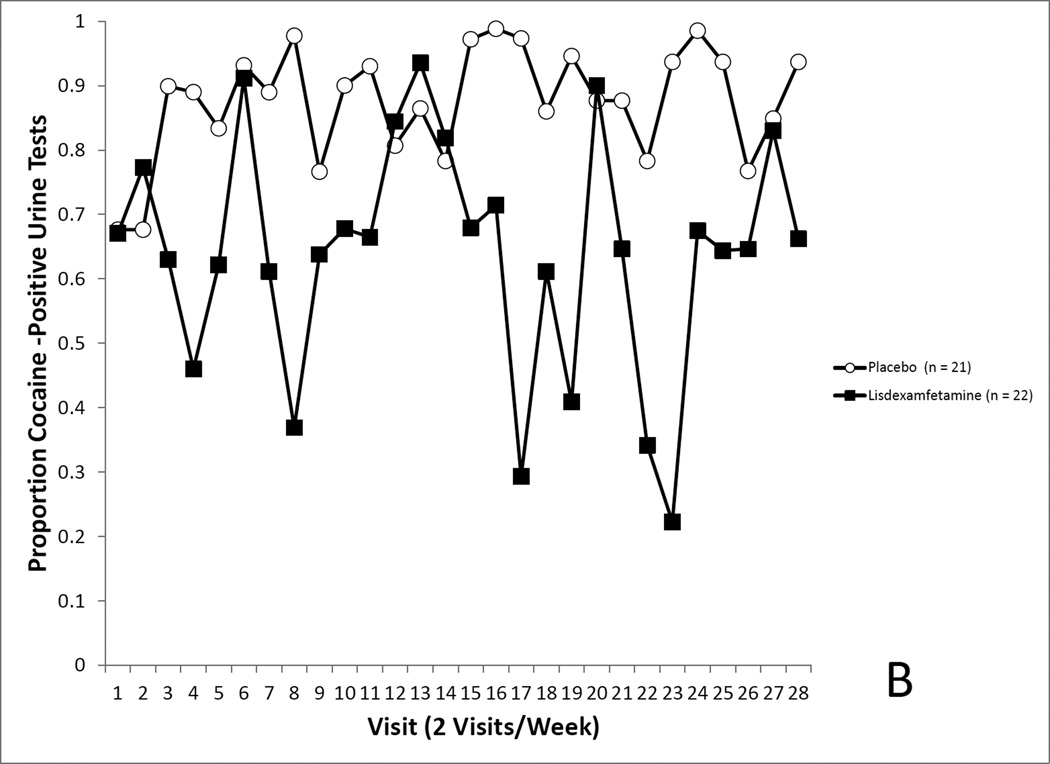
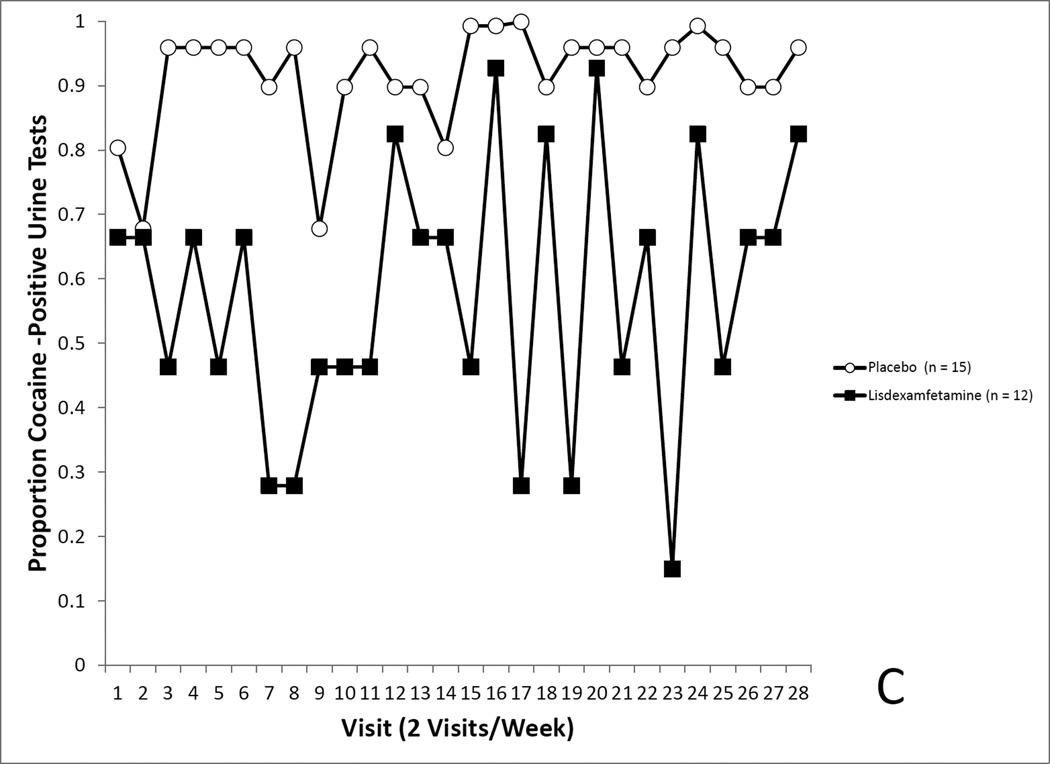
Figure 4.
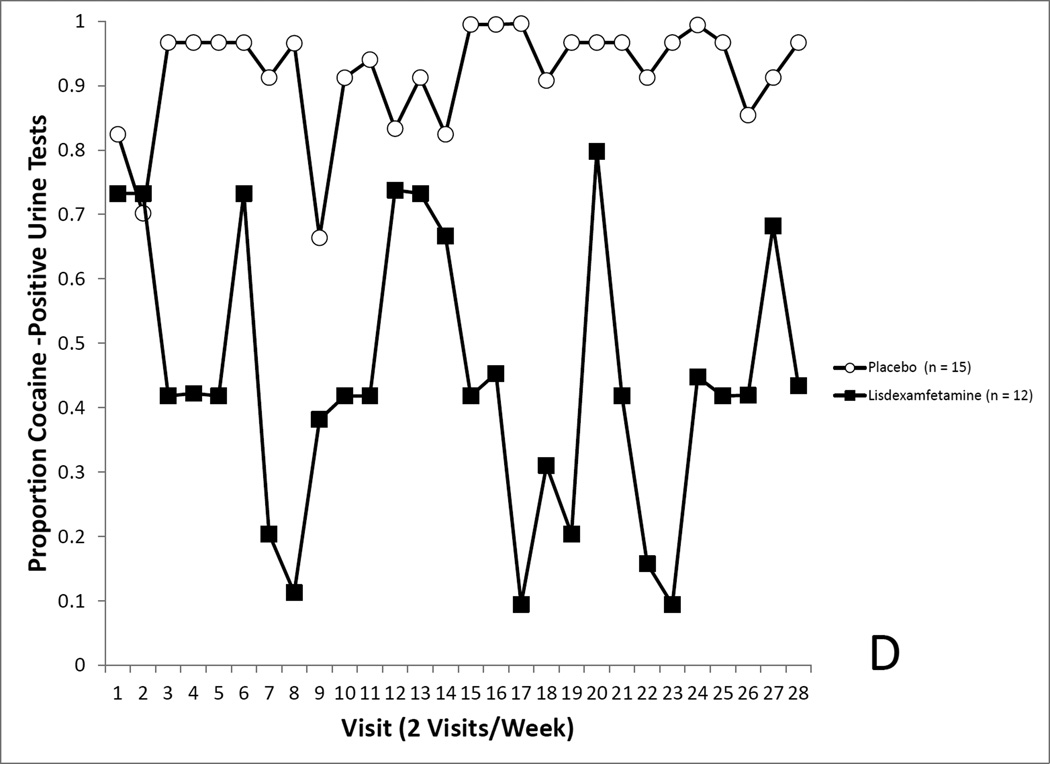
4A. Covariate-adjusted cocaine-use proportions in the randomized sample with missing values imputed as cocaine positive. No differences in cocaine use was seen between the LDX and placebo groups. 4B. Covariate-adjusted cocaine-use proportions in the randomized sample with missing values treated as missing. No differences in cocaine use was seen between the LDX and placebo groups. 4C. Covariate-adjusted cocaine-use proportions in the completers sample with missing values imputed as cocaine positive. Cocaine use proportions reflected a trend for a medication effect. 4D. Covariate-adjusted cocaine-use proportions in the completers sample with missing values treated as missing. Cocaine use proportions were significantly lower in the LDX condition.
3.6.2. Randomized Sample, MAM
The forgoing analysis was repeated leaving missing value as missing, yielding similar results, indicating no effect of medication assignment on covariate-adjusted cocaine use rates (Placebo, 88.0% positive, LDX, 66.3%), F(27, 772) = 2.69, p = 0.1106 (see Figure 4B).
3.6.3. Completer’s Sample, ITT
To evaluate the effects of completed treatment, we conducted an analysis focused on the 27 subjects (PBO, n = 15; LDX, n = 12) finishing the 14-week trial. Prior to analysis, we compared completion status (completer versus non-completer), treatment condition, and their interaction on baseline variables in Table 1. No main effects of completion status or medication assignment were observed, nor their interactions.
This secondary analysis revealed no effect of medication assignment on covariate-adjusted cocaine use rates (Placebo, 94.8% positive, LDX, 59.6%), F(1, 18.06) = 3.95, p = 0.0622 (see Figure 4C).
3.6.4. Completer’s Sample, MAM
Finally, the analysis in section 3.6.3. was conducted while leaving missing values as missing. This secondary analysis did show a significant effect of medication assignment on covariate-adjusted cocaine use rates (Placebo, 95.2% positive, LDX, 42.9%), F(1, 19.11) = 5.18, p = 0.0035 (see Figure 4D).
3.7. Cocaine Craving
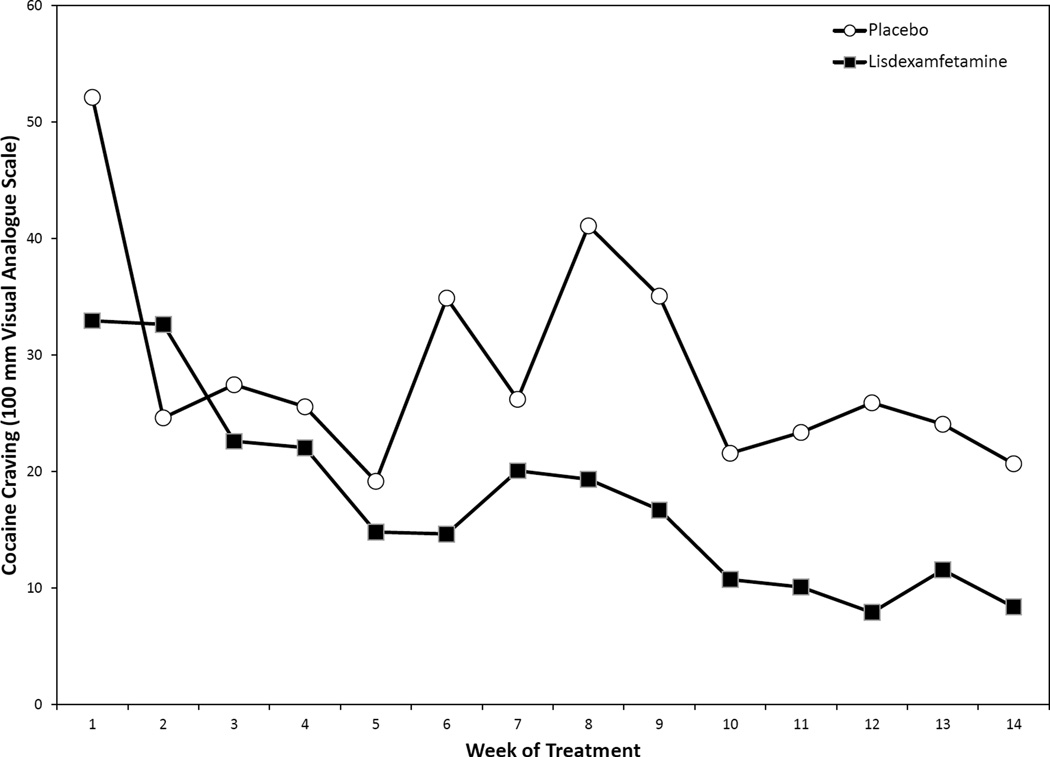
As assessed on a 100-mm visual analogue scale of cocaine craving since last visit, those receiving LDX reported less “Needing cocaine”, F(1, 62.6) = 5.94, p = 0.0176, (Placebo, M = 28.7, SE = 3.21; LDX, M = 17.5, SE = 3.30). “Needing cocaine” tended to decline over the 14 weeks of treatment, F(13, 267) = 1.48, p = 0.0107 (see Figure 5).
Figure 5.
Cocaine craving since last visit, “Needing Cocaine” rated on a 100-mm visual analogue scale. Cocaine craving was significantly lower in those receiving LDX compared to placebo.
3.8. Mood
An effect of time was noted for the BDI-2 with scores tending to decline over the course of the study, F(2, 524) = 3.90, p = 0.0265. At the beginning of medication induction, BDI-2 levels were in the non-depressed range (M = 11.40, SE = 1.33).
3.9. Study Blind Integrity
Research participants were largely successful at judging medication assignment, χ2(1) = 10.59, p = 0.0011, (accuracy rate, PBO = 81.8%; LDX = 84.6%). The research physician also judged medication assignment, and was also successful in ascertaining actual medication assignments χ2(1) = 721, p = 0.0072, (accuracy rate, PBO = 76.9%; LDX = 85.7%).
4. DISCUSSION
4.1. Key Findings
We conducted a double-blind, randomized, placebo-controlled parallel group design study to investigate the safety, tolerability, and efficacy of LDX in treatment seeking cocaine-dependent subjects. LDX was generally safe and well-tolerated under the restricted conditions of this pilot study. Cocaine use rates did not differ between LDX and placebo-treated subjects. Nonetheless, those receiving LDX reported significantly less craving for cocaine than those receiving placebo treatment.
4.2. Safety and Tolerability
No differences in treatment retention were observed between groups. Those in the LDX group reported higher rates of diarrhea, headaches, and anxiety compared to the placebo group. These side effects have been observed to be elevated in LDX-treated adults compared to placebo (Adler et al., 2008; Childress and Sallee, 2012; Weisler et al., 2009). No differences in heart rate, weight, or blood pressure were observed. As in our previous reports with dextroamphetamine (Grabowski et al. 2001, 2004) and methamphetamine (Mooney et al. 2009), average blood pressures and heart rates fell in the normal range. Overall, LDX was generally safe and well tolerated.
A primary concern about the use of amphetamine analogues for the treatment of those with extant stimulant dependence is that they may cause more illicit stimulant use (Jasinski and Krishnan, 2009a; Kollins, 2008). However, the rationale for using agonist-like medications, including amphetamines for the treatment of cocaine dependence, is homologous conceptually, if not mechanistically, with opioid-replacement therapy for those dependent on opioids (Grabowski et al., 2004a, 2004b; Rush and Stoops, 2012). Thus, Charnaud and Griffiths (1998) reported “substitute prescribing” of methadone for opiate users and dextroamphetamine for amphetamine users in a community treatment sample, noting 67% of the former and 70% of the latter had ceased illicit use at discharge. These findings are consistent with preclinical and human laboratory research (Heal et al., 2013a; Jasinski and Krishnan, 2009a; Rowley et al., 2012). Again, and importantly, the dosage of LDX (70 mg) was modest, equivalent to 30 mg dextroamphetamine (Jasinski and Krishnan, 2009a; Krishnan and Zhang, 2008).
4.3. Efficacy
The quest for cocaine-dependence pharmacotherapies that reduce cocaine use more than a placebo treatment has been protracted (Amato et al., 2007, 2011; Castells et al., 2010; Minozzi et al., 2008; Pani et al., 2011). The current study failed to find a statistically significant difference between LDX and placebo in the randomized sample. While this result might discourage further evaluation of LDX as a candidate medication for cocaine dependence, other observations in this trial suggest LDX may warrant additional study. Compared to placebo-treated subjects, those receiving LDX reported significantly lower craving for cocaine over the 14-week treatment phase. Cocaine cravings can be pervasive and intense and are related to cocaine relapse (Pahwal et al., 2008; Preston et al., 2009). In a secondary data analysis of subjects completing the 14-week course of treatment, LDX-treated subjects had significantly lower cocaine use rates than placebo-treated subjects. Importantly, this relationship cannot be interpreted as causal since the full randomized sample was not analyzed. Yet, the possible efficacy of LDX is suggested and further supported by Banks et al. (2015) preclinical results. Finally, as noted below, FDA dosing constraints existed for this preliminary proof of concept study.
4.4. Future Directions
In this study, per the FDA agreed upon IND, this proof of concept trial was limited to a maximum dosage of LDX, 70 mg/day allowed for ADHD, yielding about 30 mg of dextroamphetamine. Previous studies using 30 mg of a generic dosage of dextroamphetamine (Grabowski et al., 2001; Schmitz et al., 2012) produced a meaningful reduction in cocaine use. However, more favorable findings have been found at higher dosages of dextroamphetamine (Grabowski et al., 2004a). Future research should explore the effects of higher dosages of LDX in cocaine users. Human laboratory models may be useful in elucidating the dose-response function between LDX and cocaine use behavior and subjective responses. Single doses of LDX of 100,150, 200, and 250 mg have been safely tolerated in human subjects (Ermer et al., 2010).
Amphetamine analogues are posited to reduce cocaine use indirectly, enhancing dopaminergic function via reversal of the dopamine transporter (e.g., amphetamines Grabowski et al., 2001, 2004a; Mooney et al., 2009; Shearer et al., 2003). Arguably optimum treatment may require medication combinations targeting additional mechanisms, for example inhibition of dopamine reuptake or metabolism (e.g., bupropion, disfulfiram, methyphenidate, modafinil, Anderson et al., 2009; Poling et al., 2006; Schottenfeld et al., 2014; Winhusen et al., 2006) and replenishment of dopamine stores (e.g., levodopa, Schmitz et al., 2008). However, a recent report of a low dose generic sustained release dextroamphetamine preparation and modafinil (Schmitz et al., 2012) demonstrated no benefit. Still, successful pharmacotherapeutic interventions may require targeting, serotonergic (5-HT) noradrenergic (NE) and cholinergic (Ach) as well as dopaminergic (DA) systems (McDougle et al., 1994; Rothman et al., 2002, 2006). Support for such combination pharmacotherapy comes from a trial combining dextroamphetamine and topiramate, an agent that enhances gamma-Amino butyric acid (GABA; Mariani et al., 2012). Compared to a placebo control condition, the combination of dextroamphetamine and topiramate significantly increased continuous abstinence from cocaine. Interestingly, Mooney et al. (2009), using the broader spectrum analogue, methamphetamine, demonstrated the most substantial beneficial effects of this strategy to date. Still, most recently, Levin et al. (2015) reported dose related concurrent reduction in cocaine use and ADHD in a population with comorbid conditions. In sum, amphetamine analogues are the only agents for which significant benefits have been observed and the “prodrug” strategy represented by LDX may be the current optimal approach (e.g., Banks et al. 2015) while pursuing development of medications with enhanced risk-benefit profiles for cocaine dependence.
4.5. Limitations
The primary limitation of this study was that the proposed sample size was not achieved. In fact, given the observed effect size, a sample of more than 200 would have been required to have 80% power. While cocaine levels were only assessed semi-quantitatively, our other reports using quantitative and semi-quantitative approaches demonstrated no difference in outcome between the methods. Most problematic, though common to studies of stimulant dependent populations, overall rates of treatment completion were low, with fewer than two-thirds of subjects receiving the full course of treatment, yet it was among those completing the full course of treatment with LDX that a significant benefit compared to PBO was detected. This was also the case in an early study by Grabowski et al. (2001). In this study, participants attended twice each week. Most cocaine pharmacotherapy trials involve thrice weekly visits. Accordingly, opportunities to observe cocaine use behavior were limited. The study blind in this trial was not perfectly maintained, but contrary to expectations there were no resultant differences in retention. While weekly manualized CBT was provided, we did not include measures of patient comprehension, treatment engagement, or skills acquisition. While we observed only one serious AE of a cardiovascular nature, more sensitive safety assessments including continuous measurement of vital signs might have revealed more cardiovascular AEs. Cardiovascular AEs are not uncommon in studies of cocaine dependent individuals receiving amphetamine agonist treatment (Castells et al., 2010). Increased usage of energy drinks, which contain variable amounts of vitamin B2 (i.e., riboflavin) may have led to false positive classifications for medication adherence. Finally, though substantial effort was made to have equal numbers of males and females in this study, the current disparity limits the opportunity to explore potential gender differences in response to treatment.
4.6. Conclusions
In summary, this current proof-of-concept trial demonstrated that a novel formulation of dextroamphetamine, LDX, was safe and well tolerated. In a restricted analysis, some evidence was observed for the superior efficacy of LDX in promoting reduced cocaine use. Evaluation of the higher doses of LDX may provide clearer evidence of its efficacy in treating cocaine dependence. Whether LDX, or any agonist-like strategy may realistically be considered for development and receive regulatory approval for stimulant use disorders remains to be determined.
Supplementary Material
Figure 3.
Medication adherence across the treatment phase.
Highlights.
Lisdexamfetamine (LDX) 70 mg QD vs. placebo for 14 weeks in 43 cocaine-dependent treatment seekers.
Twice weekly urine tests for benzoylecgonine analysis (BE; a cocaine metabolite).
No significant differences between treatment groups in cocaine use rates.
LDX-treated subjects reported significantly less craving for cocaine.
Acknowledgements
We thank the participants for taking part in this study.
David Vincent Herin, Ph.D. was the recipient of the K99R00 that funded the work described. Dr. Herin was a stellar young scientist who attended Baylor University as an undergraduate and received his pre doctoral training with Dr. Kathryn Cunningham at the University of Texas Medical Branch-Galveston TX. He received post-doctoral training with Dr. Grabowski at the University of Texas Health Science Center-Houston where he submitted and was awarded an NIH K99R00 grant. He then joined his colleagues Drs. Mooney, Specker and Grabowski as a faculty member in the Department of Psychiatry, Medical School, University of Minnesota in 2008. Dr. Herin received a diagnosis of renal cancer in late 2009. During his illness, Dr. Herin requested, and Dr. Volkow (NIDA Director) concurred, that Dr. Mooney should assume the R00 portion of the award and that his colleagues would complete the work. David died in February of 2011.
Role of funding source: All authors were supported by funding from the National Institute on Drug Abuse of the National Institutes of Health. Initially, Dr. Herin was supported by NIDA K99R00-DA-023548. Grabowski, Mooney and Specker were supported in part by DA RO1 23561. Dr Mooney was supported in part by National, Institute of Drug Abuse (NIDA) grant K01-DA-019446 and NIDA K99-DA-023548.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Author Disclosures
Contributors: All authors were involved in the conduct of the study. Authors Herin and Grabowski designed and wrote the study protocol. Author Mooney served as PI during the majority of the study period and undertook statistical analysis, conducted therapy training and fidelity monitoring. Author Specker served as the study physician. Author Babb was responsible for many aspects of study implementation, data collection and organization, IRB coordination and other study features.
Conflict of interest statement:
Drs. Mooney, Herin and Specker declare no conflicts of interest. Drs. Grabowski and Levin served on a Shire Pharmaceuticals substance use disorder advisory group between 2004–2006. Medication for this study was not provided by Shire Pharmaceuticals but was purchased with NIH granted funds. Shire Pharmaceuticals had no influence, input, or control over this clinical trial or its presentation.
REFERENCES
- Adler LA, Goodman DW, Kollins SH, Weisler RH, Krishnan S, Zhang YX, Biederman J. Double-blind, placebo-controlled study of the efficacy and safety of lisdexamfetamine dimesylate in adults with attention-deficit/hyperactivity disorder. J. Clin. Psychiatry. 2008;69:1364–1373. doi: 10.4088/jcp.v69n0903. [DOI] [PubMed] [Google Scholar]
- Amato L, Minozzi S, Pani PP, Davoli M. Antipsychotic medications for cocaine dependence. Cochrane Database Syst. Rev. 2007:006306. doi: 10.1002/14651858.CD006306.pub2. [DOI] [PubMed] [Google Scholar]
- Amato L, Minozzi S, Pani PP, Solimini R, Vecchi S, Zuccaro P, Davoli M. Dopamine agonists for the treatment of cocaine dependence. Cochrane Database Syst. Rev. 2011:003352. doi: 10.1002/14651858.CD003352.pub3. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic And Statistical Manual Of Mental Disorders: DSM-IV. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Anderson AL, Reid MS, Li SH, Holmes T, Shemanski L, Slee A, Smith EV, Kahn R, Chiang N, Vocci F, Ciraulo D, Dackis C, Roache JD, Salloum IM, Somoza E, Urschel HC, 3rd, Elkashef AM. Modafinil for the treatment of cocaine dependence. Drug Alcohol Depend. 2009;104:133–139. doi: 10.1016/j.drugalcdep.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Hutsell BA, Blough BE, Poklis JL, Negus SS. Preclinical assessment of lisdexamfetamine as an agonist medication candidate for cocaine addiction: effects in rhesus monkeys trained to discriminate cocaine or to self-administer cocaine in a cocaine versus food choice procedure. Int. J. Neuropsychopharmacol. Epub ahead of print. 2015 doi: 10.1093/ijnp/pyv009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr AM, Panenka WJ, MacEwan GW, Thornton AE, Lang DJ, Honer WG, Lecomte T. The need for speed: an update on methamphetamine addiction. J. Psychiatry Neurosci. 2006;31:301–313. [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory®-II (BDI®-II) San Antonio, Texas: Psychological Corporation; 1996. [Google Scholar]
- Biederman J, Krishnan S, Zhang Y, McGough JJ, Findling RL. Efficacy and tolerability of lisdexamfetamine dimesylate (NRP-104) in children with attention-deficit/hyperactivity disorder: a phase III, multicenter, randomized, double-blind, forced-dose, parallel-group study. Clin. Ther. 2007;29:450–463. doi: 10.1016/s0149-2918(07)80083-x. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Fenton LR, Ball SA, Nich C, Frankforter TL, Shi J, Rounsaville BJ. Efficacy of disulfiram and cognitive behavior therapy in cocaine-dependent outpatients: a randomized placebo-controlled trial. Arch. Gen. Psychiatry. 2004;61:264–272. doi: 10.1001/archpsyc.61.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castells X, Casas M, Perez-Mana C, Roncero C, Vidal X, Capella D. Efficacy of psychostimulant drugs for cocaine dependence. Cochrane Database Syst. Rev. 2010:007380. doi: 10.1002/14651858.CD007380.pub3. [DOI] [PubMed] [Google Scholar]
- Charnaud B, Griffiths V. Levels of intravenous drug misuse among clients prescribed oral dexamphetamine or oral methadone: a comparison. Drug Alcohol Depend. 1998;52:79–84. doi: 10.1016/s0376-8716(98)00052-0. [DOI] [PubMed] [Google Scholar]
- Childress AC, Sallee FR. The use of lisdexamfetamine dimesylate for the treatment of ADHD. Exp. Rev. Neurother. 2012;12:13–26. doi: 10.1586/ern.11.175. [DOI] [PubMed] [Google Scholar]
- Del Boca FK, Kranzler HR, Brown J, Korner PF. Assessment of medication compliance in alcoholics through UV light detection of a riboflavin tracer. Alcohol. Clin. Exp. Res. 1996;20:1412–1417. doi: 10.1111/j.1530-0277.1996.tb01142.x. [DOI] [PubMed] [Google Scholar]
- Ermer J, Homolka R, Martin P, Buckwalter M, Purkayastha J, Roesch B. Lisdexamfetamine dimesylate: linear dose-proportionality, low intersubject and intrasubject variability, and safety in an open-label single-dose pharmacokinetic study in healthy adult volunteers. J. Clin. Pharmacol. 2010;50:1001–1010. doi: 10.1177/0091270009357346. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition (SCID -I / P, Version 2.0) NY: Biometric Research Department; 1995. [Google Scholar]
- Grabowski J, Rhoades H, Schmitz J, Stotts A, Daruzska LA, Creson D, Moeller FG. Dextroamphetamine for cocaine-dependence treatment: a double-blind randomized clinical trial. J. Clin. Psychopharmacol. 2001;21:522–526. doi: 10.1097/00004714-200110000-00010. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Stotts A, Cowan K, Kopecky C, Dougherty A, Moeller FG, Hassan S, Schmitz J. Agonist-like or antagonist-like treatment for cocaine dependence with methadone for heroin dependence: two double-blind randomized clinical trials. Neuropsychopharmacology. 2004a;29:969–981. doi: 10.1038/sj.npp.1300392. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Shearer J, Merrill J, Negus SS. Agonist-like, replacement pharmacotherapy for stimulant abuse and dependence. Addict. Behav. 2004b;29:1439–1464. doi: 10.1016/j.addbeh.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Grinspoon L, Hedblom P. Speed Culture: Amphetamine Use and Abuse in America. Cambridge, MA: Harvard University Press; 1975. [Google Scholar]
- Halikas JA, Crosby RD, Pearson VL, Graves NM. A randomized double-blind study of carbamazepine in the treatment of cocaine abuse. Clin. Pharmacol. Ther. 1997;62:89–105. doi: 10.1016/S0009-9236(97)90155-7. [DOI] [PubMed] [Google Scholar]
- Heal DJ, Buckley NW, Gosden J, Slater N, France CP, Hackett D. A preclinical evaluation of the discriminative and reinforcing properties of lisdexamfetamine in comparison to D-amfetamine, methylphenidate and modafinil. Neuropharmacology. 2013a;73:348–358. doi: 10.1016/j.neuropharm.2013.05.021. [DOI] [PubMed] [Google Scholar]
- Heal DJ, Smith SL, Gosden J, Nutt DJ. Amphetamine, past and present - a pharmacological and clinical perspective. J. Psychopharmacol. 2013b;27:479–496. doi: 10.1177/0269881113482532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningfield JE, Negus SS. Agonist medications for the treatment of cocaine use disorder. Neuropsychopharmacology. In press doi: 10.1038/npp.2014.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herin DV, Rush CR, Grabowski J. Agonist-like pharmacotherapy for stimulant dependence: preclinical, human laboratory, and clinical studies. In: Uhl GR, editor. Addiction Reviews 2. 2010. pp. 76–100. [DOI] [PubMed] [Google Scholar]
- Hutson PH, Pennick M, Secker R. Preclinical pharmacokinetics, pharmacology and toxicology of lisdexamfetamine: A novel d-amphetamine pro-drug. Neuropharmacology. 2014;87C:1–50. doi: 10.1016/j.neuropharm.2014.02.014. [DOI] [PubMed] [Google Scholar]
- Jasinski DR, Krishnan S. Abuse liability and safety of oral lisdexamfetamine dimesylate in individuals with a history of stimulant abuse. J. Psychopharmacol. 2009a;23:419–427. doi: 10.1177/0269881109103113. [DOI] [PubMed] [Google Scholar]
- Jasinski DR, Krishnan S. Human pharmacology of intravenous lisdexamfetamine dimesylate: abuse liability in adult stimulant abusers. J. Psychopharmacol. 2009b;23:410–418. doi: 10.1177/0269881108093841. [DOI] [PubMed] [Google Scholar]
- Kollins SH. Abuse liability of medications used to treat attention-deficit/hyperactivity disorder (ADHD) Am. J. Addict. 2007;16:35–44. doi: 10.1080/10550490601082775. [DOI] [PubMed] [Google Scholar]
- Kollins SH. A qualitative review of issues arising in the use of psychostimulant medications in patients with ADHD and co-morbid substance use disorders. Curr. Med. Res. Opin. 2008;24:1345–1357. doi: 10.1185/030079908x280707. [DOI] [PubMed] [Google Scholar]
- Krishnan S, Zhang Y. Relative bioavailability of lisdexamfetamine 70-mg capsules in fasted and fed healthy adult volunteers and in solution: a single-dose, crossover pharmacokinetic study. J. Clin. Pharmacol. 2008;48:293–302. doi: 10.1177/0091270007310381. [DOI] [PubMed] [Google Scholar]
- Levin FR, Mariani JJ, Specker S, Mooney M, Mahony A, Brooks DJ, Babb D, Bai Y, Eberly LE, Nunes EV, Grabowski J. Extended-release mixed amphetamine salts vs placebo for comorbid adult attention-deficit/hyperactivity disorder and cocaine use disorder: a randomized clinical trial. JAMA Psychiatry epub ahead of print. 2015 doi: 10.1001/jamapsychiatry.2015.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lineberry TW, Bostwick JM. Methamphetamine abuse: a perfect storm of complications. Mayo Clin. Proc. 2006;81:77–84. doi: 10.4065/81.1.77. [DOI] [PubMed] [Google Scholar]
- Mariani JJ, Pavlicova M, Bisaga A, Nunes EV, Brooks DJ, Levin FR. Extended-release mixed amphetamine salts and topiramate for cocaine dependence: a randomized controlled trial. Biol. Psychiatry. 2012;72:950–956. doi: 10.1016/j.biopsych.2012.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougle CJ, Black JE, Malison RT, Zimmermann RC, Kosten TR, Heninger GR, Price LH. Noradrenergic dysregulation during discontinuation of cocaine use in addicts. Arch. Gen. Psychiatry. 1994;51:713–719. doi: 10.1001/archpsyc.1994.03950090045007. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Cacciola JC, Alterman AI, Rikoon SH, Carise D. The Addiction Severity Index at 25: origins, contributions and transitions. Am. J. Addict. 2006;15:113–124. doi: 10.1080/10550490500528316. [DOI] [PubMed] [Google Scholar]
- MEDTOX. [accessed on October 21 2014>];PROFILE® -V MEDTOXScan® Drugs of Abuse Test System INSERT. 2009 http://www.medtox.com/Resources/Images/4525.pdf.
- Micromedex® Healthcare Series. Greenwood Village, CO: Thompson Healthcare; n.d. Retrieved September 14, 2014 http://www.thomsonhc.com. accessed on. [Google Scholar]
- Minozzi S, Amato L, Davoli M, Farrell M, Lima Reisser AA, Pani PP, Silva de Lima M, Soares B, Vecchi S. Anticonvulsants for cocaine dependence. Cochrane Database Syst. Rev. 2008:006754. doi: 10.1002/14651858.CD006754.pub2. [DOI] [PubMed] [Google Scholar]
- Mooney M, Sayre SL, Green C, Rhoades H, Schmitz J. Comparing measures of medication taking in a pharmacotherapy trial for cocaine dependence. Addict. Disord. Their Treat. 2004a;3:165–173. [Google Scholar]
- Mooney M, White T, Hatsukami D. The blind spot in the nicotine replacement therapy literature: assessment of the double-blind in clinical trials. Addict. Behav. 2004b;29:673–684. doi: 10.1016/j.addbeh.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Mooney ME, Herin DV, Schmitz JM, Moukaddam N, Green CE, Grabowski J. Effects of oral methamphetamine on cocaine use: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2009;101:34–41. doi: 10.1016/j.drugalcdep.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney ME, Schmitz JM, Moeller FG, Grabowski J. Safety, tolerability and efficacy of levodopa-carbidopa treatment for cocaine dependence: two double-blind, randomized, clinical trials. Drug Alcohol Depend. 2007;88:214–223. doi: 10.1016/j.drugalcdep.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Henningfield J. Agonist medications for the treatment of cocaine use disorder. Neuropsychopharmacology epub ahead of print. 2014 doi: 10.1038/npp.2014.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahwal P, Hyman SM, Sinha R. Craving predicts time to cocaine relapse: further validation of the Now and Brief versions of the cocaine craving questionnaire. Drug Alcohol Depend. 2008;93:252–259. doi: 10.1016/j.drugalcdep.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pani PP, Trogu E, Vecchi S, Amato L. Antidepressants for cocaine dependence and problematic cocaine use. Cochrane Database Syst. Rev. 2011:002950. doi: 10.1002/14651858.CD002950.pub3. [DOI] [PubMed] [Google Scholar]
- Pennick M. Absorption of lisdexamfetamine dimesylate and its enzymatic conversion to d-amphetamine. Neuropsychiatr. Dis. Treat. 2010;6:317–327. doi: 10.2147/ndt.s9749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poling J, Oliveto A, Petry N, Sofuoglu M, Gonsai K, Gonzalez G, Martell B, Kosten TR. Six-month trial of bupropion with contingency management for cocaine dependence in a methadone-maintained population. Arch. Gen. Psychiatry. 2006;63:219–228. doi: 10.1001/archpsyc.63.2.219. [DOI] [PubMed] [Google Scholar]
- Preston KL, Vahabzadeh M, Schmittner J, Lin JL, Gorelick DA, Epstein DH. Cocaine craving and use during daily life. Psychopharmacology. 2009;207:291–301. doi: 10.1007/s00213-009-1655-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochon J. Application of GEE procedures for sample size calculations in repeated measures experiments. Stat. Med. 1998;17:1643–1658. doi: 10.1002/(sici)1097-0258(19980730)17:14<1643::aid-sim869>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Blough BE, Baumann MH. Appetite suppressants as agonist substitution therapies for stimulant dependence. Ann. N. Y. Acad. Sci. 2002;965:109–126. doi: 10.1111/j.1749-6632.2002.tb04155.x. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Blough BE, Baumann MH. Dual dopamine-5-HT releasers: potential treatment agents for cocaine addiction. Trends Pharmacol. Sci. 2006;27:612–618. doi: 10.1016/j.tips.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Rowley HL, Kulkarni R, Gosden J, Brammer R, Hackett D, Heal DJ. Lisdexamfetamine and immediate release D-amfetamine - differences in pharmacokinetic/pharmacodynamic relationships revealed by striatal microdialysis in freely-moving rats with simultaneous determination of plasma drug concentrations and locomotor activity. Neuropharmacology. 2012;63:1064–1074. doi: 10.1016/j.neuropharm.2012.07.008. [DOI] [PubMed] [Google Scholar]
- Rush CR, Stoops WW. Agonist replacement therapy for cocaine dependence: a translational review. Future Med. Chem. 2012;4:245–265. doi: 10.4155/fmc.11.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute Inc. The SAS System for Windows. Cary, NC: SAS Institute Inc; 2014. [Google Scholar]
- Schmitz JM, Mooney ME, Moeller FG, Stotts AL, Green C, Grabowski J. Levodopa pharmacotherapy for cocaine dependence: choosing the optimal behavioral therapy platform. Drug Alcohol Depend. 2008;94:142–150. doi: 10.1016/j.drugalcdep.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz JM, Rathnayaka N, Green CE, Moeller FG, Dougherty AE, Grabowski J. Combination of modafinil and d-amphetamine for the treatment of cocaine dependence: a preliminary investigation. Front. Psychiatry. 2012;3:77. doi: 10.3389/fpsyt.2012.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz JM, Stotts AL, Rhoades HM, Grabowski J. Naltrexone and relapse prevention treatment for cocaine-dependent patients. Addict. Behav. 2001;26:167–180. doi: 10.1016/s0306-4603(00)00098-8. [DOI] [PubMed] [Google Scholar]
- Schottenfeld RS, Chawarski MC, Cubells JF, George TP, Lappalainen J, Kosten TR. Randomized clinical trial of disulfiram for cocaine dependence or abuse during buprenorphine treatment. Drug Alcohol Depend. 2014;136:36–42. doi: 10.1016/j.drugalcdep.2013.12.007. [DOI] [PubMed] [Google Scholar]
- Shearer J, Wodak A, van Beek I, Mattick RP, Lewis J. Pilot randomized double blind placebo-controlled study of dexamphetamine for cocaine dependence. Addiction. 2003;98:1137–1141. doi: 10.1046/j.1360-0443.2003.00447.x. [DOI] [PubMed] [Google Scholar]
- Weisler R, Young J, Mattingly G, Gao J, Squires L, Adler L, Study G. Long-term safety and effectiveness of lisdexamfetamine dimesylate in adults with attention-deficit/hyperactivity disorder. CNS Spect. 2009;14:573–585. doi: 10.1017/s1092852900024056. [DOI] [PubMed] [Google Scholar]
- Wigal T, Brams M, Gasior M, Gao J, Squires L, Giblin J. Randomized, double-blind, placebo-controlled, crossover study of the efficacy and safety of lisdexamfetamine dimesylate in adults with attention-deficit/hyperactivity disorder: novel findings using a simulated adult workplace environment design. Behav. Brain Funct. 2010;6:34. doi: 10.1186/1744-9081-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winhusen T, Somoza E, Singal BM, Harrer J, Apparaju S, Mezinskis J, Desai P, Elkashef A, Chiang CN, Horn P. Methylphenidate and cocaine: a placebo-controlled drug interaction study. Pharmacol. Biochem. Behav. 2006;85:29–38. doi: 10.1016/j.pbb.2006.06.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.