Abstract
The genes encoding Hfq-dependent sRNAs possess a typical Rho-independent transcription terminator. Here, we have studied the molecular events occurring at Rho-independent terminators of sRNA genes, focusing on two well-characterized Hfq-binding sRNAs, SgrS and RyhB. We constructed several hybrid genes in which the DNA sequence corresponding to a strong Rho-independent terminator was placed just downstream from the Rho-independent terminators of sRNA genes. By using this system, we demonstrate that transcripts frequently read through the Rho-independent terminators of sgrS and ryhB in normally growing cells. We show that Hfq does not affect the transcriptional readthrough event itself. We also find that the readthrough products no longer bind to Hfq in vivo. We have developed a competition assay based on a biotin–streptavidin system to analyze the interaction of Hfq and a particular RNA molecule in vitro. By using this method, we verify that the 3′-extended form of SgrS does not bind to Hfq in vitro. Finally, we demonstrate that transcription termination is significantly enhanced under stress conditions where transcription initiation of sRNA genes on the chromosome is induced. We conclude that the production of sRNAs is regulated not only at the step of transcription initiation but also at the step of transcription termination. The mechanism by which transcription termination is enhanced under stress conditions remains to be understood.
Keywords: Hfq, bacterial sRNA, Rho-independent terminator, transcriptional readthrough, termination and stress
INTRODUCTION
Major regulatory small RNAs (sRNAs) in bacteria bind to the RNA chaperone Hfq and regulate the translation and the stability of target mRNAs through base-pairing by the help of Hfq (Waters and Storz 2009; Gottesman and Storz 2010; Vogel and Luisi 2011). The transcription initiation of sRNA genes is induced under specific physiological and/or stress conditions because the promoter of an individual sRNA gene is under the control of a specific transcription factor that is modulated by respective stress conditions. For example, Escherichia coli SgrS is induced in response to glucose-phosphate stress such as accumulation of glucose-6-phosphate (Vanderpool and Gottesman 2004). The induced SgrS sRNA pairs with the target mRNAs to either down- or up-regulate their expression (Vanderpool and Gottesman 2004; Morita et al. 2005; Papenfort et al. 2013). A transcription factor called SgrR is activated, by unknown mechanisms, under the glucose-phosphate stress to stimulate the transcription from the sgrS promoter by RNA polymerase (Vanderpool and Gottesman 2007). Another well-characterized Hfq-binding sRNA, RyhB, is induced in response to Fe2+ depletion to regulate the expression of several mRNAs encoding Fe-binding proteins through base-pairing mechanism (Masse and Gottesman 2002; Masse et al. 2003). In this case, Fur, a repressor protein for the ryhB promoter, is inactivated by the Fe2+ depletion, leading to expression of RyhB (Masse and Gottesman 2002).
The expression of sRNA genes might be regulated also at the step of transcription termination. Genes encoding Hfq-dependent sRNAs are often located in intergenic regions as a single gene (Waters and Storz 2009) while some sRNA genes form operons along with other genes and/or are embedded in the 3′ region of protein-coding genes (Sun and Vanderpool 2011; Chao et al. 2012; Guo et al. 2014). In either case, the sRNA genes possess a typical Rho-independent transcription terminator that is likely to specify the 3′ends of most sRNAs. The hallmark features of Rho-independent terminators, also called factor-independent or intrinsic terminators, are a short GC-rich inverted repeat sequence followed by a run of T residues on the nontemplate strand (d'Aubenton Carafa et al. 1990; Reynolds et al. 1992). When RNA polymerase transcribes the inverted repeat sequence of the terminator, the resulting RNA forms a stem–loop (hairpin) structure that is believed to trigger transcription termination within transcribed U stretches by disrupting the elongation complex due to weak A–U base pairs (Rosenberg and Court 1979; Platt 1986; Yager and von Hippel 1991). We noticed previously that the Rho-independent terminators of sRNA genes possess longer T residue stretches (more than seven) on the nontemplate strand (Otaka et al. 2011) than most other terminators which can vary depending on terminators, but usually less than six (d'Aubenton Carafa et al. 1990). We showed recently that the long polyU tail and hairpin of sRNAs corresponding to their Rho-independent terminator along with a preceding internal U-rich sequence consist of the functional Hfq-binding module of sRNAs (Ishikawa et al. 2012). This implies that the consecutive terminator A residues on the template strand must be efficiently transcribed to generate functional sRNAs. Thus, insights into transcription termination and its regulation will be important to understand how functional sRNAs are generated in cells. To date, however, molecular events at Rho-independent terminators of sRNA genes have been poorly studied. For example, it is largely unknown how frequently transcription termination or readthrough occurs beyond sRNA genes. Because the terminator hairpin and the long polyU tail are essential elements for the Hfq-binding module, another interesting question is whether Hfq affects the transcription termination/readthrough at Rho-independent terminators of sRNA genes. Furthermore, it is not known whether the 3′-extended forms of sRNAs generated by transcriptional readthrough at sRNA terminators still retain the Hfq-binding ability although an early mutational study suggests that the 3′ extension of DsrA sRNA lead to the loss of its regulatory function (Sledjeski and Gottesman 1995).
The aim of this study is to address these questions, focusing on two well-characterized Hfq-binding sRNAs, SgrS and RyhB. By using a “double terminator system” (Abe et al. 1999), we demonstrated that transcriptional readthrough frequently occurs at Rho-independent terminators of sRNA genes and that the readthrough is not affected by Hfq. In addition, we found that the readthrough products, the 3′-extended forms of sRNAs, no longer bind to Hfq both in vivo and in vitro. Finally, we found that transcription termination is markedly enhanced under stress conditions in which the transcription of sRNA genes on the chromosome is induced in cells. The biological relevance of this finding will be discussed.
RESULTS
Double terminator system
The efficiency of termination at Rho-independent terminators is known to vary widely among individual terminators (Reynolds et al. 1992). Thus, elongating RNA polymerases pass through different terminator structures with different frequencies, leading to transcription of the downstream sequences. It is generally difficult to evaluate the efficiency of termination by analyzing RNA transcripts directly by Northern blotting because readthrough products are often heterogeneous in size and/or perhaps unstable. We have used a “double terminator system” (Abe et al. 1999) in which a strong Rho-independent terminator derived from rrnB (rrnBT1) is placed just downstream from a Rho-independent terminator to be tested. The downstream strong terminator efficiently traps the readthrough RNA that is made beyond the test terminator, resulting in stable and distinct longer RNA molecules. To know how frequently transcription termination occurs at Rho-independent terminators of sRNA genes, we constructed hybrid genes on a plasmid in which a strong Rho-independent terminator of rplL (Post et al. 1979) or rrnB (Brosius et al. 1981) was placed just downstream from the Rho-independent terminators of sgrS and ryhB genes encoding SgrS and RyhB sRNAs, respectively. The DNA sequences around the terminators of the genes used along with the predicted RNA secondary structures of transcripts corresponding to the Rho-independent terminators are shown in Figure 1.
FIGURE 1.
(A) DNA sequences around Rho-independent terminators of genes used in this study. The sequences corresponding to Rho-independent terminators of sgrS and ryhB are shown as regular letters, whereas the terminator sequences derived from rplL and rrnB are shown as italic letters. The inverted repeat sequences of Rho-independent terminators are shown by horizontal arrows. (B) The predicted secondary structures of transcripts corresponding to Rho-independent terminators. The free energies of terminator hairpin structures were determined according to the Mfold program (Zuker 2003).
Evaluation of transcriptional readthrough at the sgrS terminator
We first focused on the sgrS terminator. Plasmid pSgrS-S carries the sgrS-S encoding SgrS-S that corresponds to the 3′ portion of SgrS consisting of the minimal base-pairing region and the Hfq-binding module including the Rho-independent terminator sequence (Otaka et al. 2011; Ishikawa et al. 2012). Plasmids pSgrS-S-rrnBT1 and pSgrS-S-rplLT carry the hybrid gene sgrS-S-rrnBT1 and sgrS-S-rplLT, respectively. The sgrS-S gene is under the control of an arabinose-inducible promoter in these plasmids. We first examined transcription termination/readthrough at the sgrS terminator in hfq+ background by Northern analysis. Plasmids described above were introduced into hfq+ cells. As a control, cells were also transformed with the vector plasmid pAraX (Ishikawa et al. 2012). Cells were grown in the presence of arabinose and expression of sRNAs was analyzed by Northern blotting. The sgrS-S gene generates ∼60 nt SgrS-S RNA (Fig. 2A, lane 2). As expected, two hybrid genes, sgrS-S-rrnBT1 and sgrS-S-rplLT, also produce the 60 nt SgrS-S (Fig. 2A, lanes 3,4). The expression levels of SgrS-S were essentially identical among these three genes (sgrS-S, sgrS-S-rrnBT1, and sgrS-S-rplLT). Thus, transcription termination at the sgrS terminator is not affected by the downstream second terminator rrnBT1 or rplLT. We also confirmed that the target ptsG mRNA was dramatically destabilized when SgrS-S was expressed from any of the three genes (Fig. 2A, lower panel), indicating that SgrS-S generated from the hybrid genes is functional to down-regulate the ptsG mRNA. As expected, longer RNA bands corresponding to SgrS-S-rrnBT1 and SgrS-S-rplLT are produced in addition to SgrS-S band in cells harboring pSgrS-S-rrnBT1 or pSgrS-S-rplLT (Fig. 2A, lanes 3,4). These bands can be produced mainly because transcripts that passed through the sgrS terminator terminate at the downstream rrnBT1 or rplLT terminator. It is reported that the termination efficiency of rrnBT1 in vivo is >90% (Brosius et al. 1981). The rplLT is expected to be also strong because of the high stability of its terminator hairpin structure (Fig. 1B). Indeed, the band corresponding to SgrS-S-rplLT is much more distinct than that of SgrS-S-rrnBT1. Hereafter, we have used the rplLT as the downstream terminator to trap the readthrough products.
FIGURE 2.

(A) Properties of transcripts generated from sgrS-S and hybrid genes. TM542 (ΔsgrS hfq+) cells harboring indicated plasmids were grown in LB medium in the presence of 0.2% arabinose. Total RNAs were prepared and 10 μg of RNA samples were subjected to Northern blotting using SgrS-S probe and ptsG probe. (B) Effect of Hfq on expression of SgrS-S and SgrS-S-rplLT. TM542 (ΔsgrS hfq+) and TM772 (ΔsgrS Δhfq) cells harboring indicated plasmids were grown in LB medium in the presence of 0.2% arabinose. Total RNAs were prepared and 10 and 0.25 μg of RNA samples were subjected to Northern blotting using the SgrS-S probe and tmRNA probe, respectively.
The presence of Hfq in cells may affect differentially the accumulation of terminated SgrS-S and elongated SgrS-S-rplLT because SgrS-S is stabilized by Hfq binding (Otaka et al. 2011), while it is not sure at this moment whether the stability of SgrS-S-rplLT is affected by Hfq. We examined the accumulation of terminated and elongated products in both hfq− and hfq+ backgrounds. As expected, the abundance of SgrS-S generated from either pSgrS-S or pSgrS-S-rplLT is markedly reduced in the hfq− background as compared with the hfq+ background (Fig. 2B, lanes 2,4). In contrast, the abundance of SgrS-S-rplLT band generated from pSgrS-S-rplLT is not reduced in the hfq− background (Fig. 2B, lane 4), suggesting that SgrS-S-rplLT is not stabilized by Hfq. Thus, it is appropriate to compare the relative abundance of SgrS-S-rplLT and SgrS-S bands in the hfq− background rather than in the hfq+ background to evaluate the efficiency of termination/readthrough at the sgrS terminator. Interestingly, the abundance of the SgrS-S-rplLT band is almost identical with that of the SgrS-S band (Fig. 2B, lane 4). This implies that ∼50% of elongating RNA polymerase reads through the sgrS terminator if it is assumed that SgrS-S-rplLT and SgrS-S are similar in stability in the hfq− background. In fact, we show that this is the case (Fig. 3B).
FIGURE 3.

Stability of SgrS-S and SgrS-S-rplLT. (A)TM542 (ΔsgrS hfq+) and (B) TM772 (ΔsgrS Δhfq) cells harboring pSgrS-S-rplLT were grown in LB medium in the presence of 0.2% arabinose to A600 = 0.6. Rifampicin (250 μg/mL) was added and the incubation was continued. Total RNAs were prepared at the indicated time after the addition of rifampicin. The RNA samples (10 μg) were subjected to Northern blotting using the SgrS-S probe.
The readthrough products are not stabilized by Hfq
The readthrough product (SgrS-S-rplLT) contains the sequence corresponding to the Hfq-binding module (Ishikawa et al. 2012) although the polyU stretch is located internal in the RNA molecule rather than at the 3′ end. An interesting question is if SgrS-S-rplLT still retains the Hfq-binding ability. The observation that the presence of Hfq does not enhance the abundance of SgrS-S-rplLT (Fig. 2B) suggests that SgrS-S-rplLT is not stabilized much by Hfq whereas SgrS-S is. To test this, we evaluated the stability of terminated and elongated forms of SgrS-S in both hfq− and hfq+ backgrounds. Cells harboring pSgrS-S-rplLT were grown to mid-log phase in the presence of arabinose and rifampicin was added to prevent further initiation of transcription. RNAs were isolated at various times after the addition of rifampicin, and these RNA samples were subjected to Northern blotting. As shown in Figure 3B, the half-lives (t(1/2)) of SgrS-S and SgrS-S-rplLT RNAs are estimated to be 1.3 and 1.1 min, respectively, in hfq− cells. Thus, both SgrS-S and SgrS-S-rplLT are highly unstable in the absence of Hfq. On the other hands, SgrS-S is dramatically stabilized in hfq+ cells (t[1/2] > 30 min) while SgrS-S-rplLT is still unstable in hfq+ cells (Fig. 3A).
Analysis of the ryhB terminator
We also examined transcriptional readthrough at the Rho-independent terminator of the ryhB gene encoding another well-characterized Hfq-binding sRNA, RyhB (Masse and Gottesman 2002) with the double terminator system. Plasmids pRyhB and pRyhB-rplLT carry the ryhB and ryhB-rplLT genes, respectively. RNA transcripts from these plasmids in both hfq− and hfq+ cells were analyzed by Northern blotting. The ryhB gene produces 95 nt RyhB while the hybrid ryhB-rplLT gene produces two RNAs, RyhB plus a longer RNA corresponding to RyhB-rplLT (Fig. 4A). Based on the ratio of the RyhB-rplLT band to the terminated RyhB band, the efficiency of transcription termination at the ryhB terminator is estimated to be ∼80% in the hfq− background (Fig. 4A, lane 4). The abundance of RyhB generated from both pRyhB and pRyhB-rplLT in hfq− cells is significantly reduced compared with the hfq+ background while the abundance of RyhB-rplLT is moderately affected by the hfq− background (20%–30% less in hfq− cells).
FIGURE 4.

(A) Transcripts generated from ryhB and ryhB-rplLT genes. TM635 (ΔryhB hfq+) and TM820 (ΔryhB Δhfq) cells harboring indicated plasmids were grown in LB medium in the presence of 1.0% arabinose. Total RNAs were prepared and 4 and 0.25 μg of RNA samples were subjected to Northern blotting using RyhB probe and tmRNA probe, respectively. (B,C) Stability of RyhB and RyhB-rplLT. TM635 (ΔryhB hfq+) (B) and TM820 (ΔryhB Δhfq) (C) cells harboring pRyhB-rplLT were grown in LB medium in the presence of 1.0% arabinose to A600 = 0.6. Rifampicin (250 μg/mL) was added and the incubation was continued. Total RNAs were prepared at the indicated time after the addition of rifampicin. The RNA samples (10 μg) were subjected to Northern blotting using the RyhB probe.
We also evaluated the stability of terminated and elongated forms of RyhB in both hfq− and hfq+ backgrounds. Cells harboring pRyhB-rplLT were grown to mid-log phase in the presence of arabinose and rifampicin was added to prevent further initiation of transcription. RNAs were isolated at various times after the addition of rifampicin, and these RNA samples were subjected to Northern blotting (Fig. 4B,C). The half-lives of RyhB and RyhB-rplLT in the hfq− background were estimated to be 4.6 and 2.3 min, respectively (Fig. 4C). It is interesting to note that RyhB is relatively stable compared with SgrS in the hfq− background. As expected, RyhB is significantly stabilized (t(1/2) = 14.6 min) in hfq+ cells (Fig. 4B) although the effect of Hfq on RyhB stabilization is not as great as on SgrS-S. RyhB-rplLT, like SgrS-S-rplLT, is only moderately stabilized in hfq+ cells.
Hfq does not bind to the 3′-extended forms of SgrS and RyhB
We showed above that the readthrough products, SgrS-S-rplLT and RyhB-rplLT, are only moderately stabilized by Hfq. This strongly suggests that the 3′ extension of SgrS and RyhB impairs the Hfq-binding ability of these extended sRNAs. To test this directly, we performed a pull-down assay using cells expressing SgrS-S-rplLT along with SgrS-S, and Hfq-Flag. Cell extracts were incubated with anti-Flag M2-agarose beads. Proteins bound to the agarose beads were analyzed by Western blotting using anti-Flag antibodies. The affinity-purified Hfq-Flag was treated with phenol and subjected to Northern blotting. As shown in Figure 5A, SgrS but not SgrS-S-rplLT co-purified with Hfq-Flag. We also performed a pull-down assay using cells expressing RyhB-rplLT along with RyhB and Hfq-Flag. Again, the terminated RyhB but not the elongated RyhB-rplLT co-purified with Hfq-Flag (Fig. 5B). These results indicate that the 3′ extention surely impairs the Hfq-binding ability of sRNAs in vivo.
FIGURE 5.

In vivo binding of Hfq to terminated sRNAs and readthrough products. Crude extracts were prepared from TM803 (ΔsgrS hfq-Flag) cells harboring pSgrS-S-rplLT (A) and from TM822 (ΔryhB hfq-Flag) cells harboring pRyhB-rplLT (B). Crude extracts were subjected to the pull-down assay using anti-Flag agarose as described in Materials and Methods. Crude extract (CE; 10 μL) and bound fraction (B; 2 μL) were analyzed by Western blotting using anti-Flag antibodies. For analysis of RNAs, crude extract (CE; 10 μL) and bound fraction (B; 2 μL) were treated with phenol and subjected to Northern blotting using the SgrS-S (A) or RyhB (B) probe.
We also tested the effect of 3′ extension of SgrS on Hfq binding in vitro by using several synthetic RNAs shown in Figure 6A. SgrS50 contains the entire sequence corresponding to the Hfq-binding module of SgrS including eight consecutive uridine residues at the 3′ end (Otaka et al. 2011; Ishikawa et al. 2012). The predicted secondary structure of SgrS50 is shown on the right of Figure 6A. Biotin-SgrS50 is a derivative of SgrS50 in which the 5′ end of SgrS50 is biotinylated. Both SgrS50 and biotin-SgrS50 are expected to retain the ability to bind Hfq. SgrS46 is a derivative of SgrS50 in which the polyU tail is shortened to 4U residues and therefore is expected to lose the Hfq-binding ability (Otaka et al. 2011). SgrS60 is a derivative of SgrS50 in which 10 bases are extended after the polyU stretch. First, Hfq-His6 was incubated with streptavidin-conjugated magnet beads either with or without biotin-SgrS50. Hfq bound and unbound to the magnetic beads was analyzed by SDS-PAGE/silver staining. When Hfq was incubated with the beads in the absence of biotin-SgrS50, Hfq was found only in the unbound fraction (Fig. 6B, lanes 2,8), indicating that Hfq-His6 itself does not bind to the beads. On the other hand, Hfq in the unbound and bound fractions decreased and increased, respectively, with increasing amounts of biotin-SgrS50 (Fig. 6B, lanes 3–6, and lanes 9–12). These results imply that Hfq binds stably to biotin-SgrS50 that tightly interacts with streptavidin-conjugated magnet beads. Then, we examined the effect of excess amounts of SgrS50, SgrS46, and SgrS60 on the interaction between Hfq and biotin-SgrS50. When Hfq was incubated with the beads along with biotin-SgrS50 and excess amounts of SgrS50, Hfq was found predominantly in the unbound fraction (Fig. 6C, lanes 3,8). This implies that Hfq binding to biotin-SgrS50 was inhibited because SgrS50 competes well with biotin-SgrS50 regarding Hfq binding. On the other hand, Hfq was found predominantly in the bound fraction when Hfq was incubated with the beads along with biotin-SgrS50 and excess amounts of SgrS46 (Fig. 6C, lanes 4,9). Thus, SgrS46 affects little the interaction between biotin-SgrS50 and Hfq, indicating that SgrS46 essentially does not bind to Hfq as expected. Then, we performed the same competition assay by using excess amounts of SgrS60. This 3′-extended form of SgrS also did not affect the binding of Hfq to biotin-SgrS50 (Fig. 6C, lanes 5,10), indicating that 3′ extension of SgrS also impairs the Hfq-binding ability of SgrS. These results are consistent with the results of the pull-down assay. Together, we conclude that the Hfq-binding ability of 3′-extended forms of sRNAs is heavily impaired.
FIGURE 6.

Binding of Hfq to synthetic RNAs in vitro. (A) Synthetic RNAs used and their nucleotide sequences. SgrS50 is a 50 nt RNA corresponding to the nucleotides 178–227 of SgrS that is essentially identical with the Hfq-binding module of SgrS (nucleotides 179–227). Biotin-SgrS50 is a derivative of SgrS50 in which the 5′ end was biotinylated. SgrS46 is a derivative of SgrS50 lacking the last 4U of 3′ end. SgrS60 is a derivative of SgrS50 in which the 10 nt sequence corresponding to the readthrough transcript is extended. The predicted secondary structure of SgrS50 is shown on the right. (B) Interaction between biotin-SgrS50 and Hfq. Streptavidin magnetic beads and Hfq-His6 (66.7 nM) were gently shaken with increasing amounts of biotin-SgrS50 in 15 μL of binding buffer for 10 min at 37°C. The following amounts of biotin-SgrS50: lanes 3,9, 8.33 nM; lanes 4,10, 16.7 nM; lanes 5,11, 33.3 nM; lanes 6,12, 66.7 nM. Unbound supernatant and bound precipitate were analyzed by SDS-PAGE and visualized by silver staining. The asterisks (*) indicate the protein (streptavidin) released from the beads during sample heating. (C) Effects of SgrS-S variants on the interaction between biotin-SgrS50 and Hfq. Streptavidin magnetic beads, biotin-SgrS50 (33.3 nM) and Hfq-His6 (66.7 nM) were gently shaken with indicated RNAs (667 nM) in 15 μL of binding buffer for 10 min at 37°C. Unbound supernatant and bound precipitate were analyzed by SDS-PAGE and visualized by silver staining. The asterisks indicate the protein (streptavidin) released from the beads during sample heating.
Transcription termination is enhanced under stress conditions in which sRNAs are induced
So far, we have examined transcription termination at Rho-independent terminators of sRNA genes in normally growing cells. Hfq-binding sRNAs are expressed from their own genes on the chromosome under specific stress and/or physiological conditions. For example, SgrS and RyhB are induced in response to glucose-phosphate stress such as accumulation of glucose-6-phosphate and to depletion of Fe2+, respectively. Because transcription of sRNA genes and their activities are subject to control by Rho-independent termination, we examined the effect of stress on Rho-independent termination of sRNA genes. We first tested the effect of glucose-phosphate stress on transcription termination at the sgrS terminator. Cells harboring pSgrS-S-rplLT were grown in LB medium containing 0.2% arabinose to mid-log phase and exposed to 0.1% nonmetabolizable glucose analog α-methylglucoside (αMG) for 10 min. Then, total RNAs were prepared and subjected to Northern blotting using the sgrS probe. Interestingly, the addition of αMG markedly reduced the amount of the readthrough product SgrS-S-rplLT (Fig. 7A, lanes 1,2). In other words, the glucose-phosphate stress enhanced transcription termination at the sgrS terminator. We also examined the effect of Fe2+ depletion on transcription termination at the sgrS terminator. Cells harboring pSgrS-S-rplLT were grown in LB medium containing 0.2% arabinose to mid-log phase and treated with 2,2′-dipyridyl for 10 min to deplete Fe2+. The addition of 2,2′-dipyridyl clearly enhanced transcription termination at the sgrS terminator (Fig. 7A, lane 3). Next, we examined the effects of glucose-phosphate stress and Fe2+ depletion on transcription termination at ryhB terminator by using cells harboring pRyhB-rplLT. As shown in Figure 7B, termination efficiency at ryhB terminator was also enhanced by both glucose-phosphate stress and Fe2+ depletion. Thus, transcription termination at the Rho-independent terminators of sRNA genes is enhanced not only by the cognate stress but also by noncognate stress.
FIGURE 7.

Transcriptional readthrough at the Rho-independent terminators of sRNA genes under stress conditions. (A) Effects of glucose-phosphate stress or Fe2+ depletion on readthrough at the sgrS terminator. TM772 (ΔsgrS Δhfq) cells harboring pSgrS-S-rplLT were grown in LB medium in the presence of 0.2% arabinose. At A600 = 0.6, 0.1% α-metylglucoside (αMG) or 250 μM 2, 2′-dipyridyl (dip) was added to cultures and incubation was continued for 10 min. Total RNAs were prepared and then the RNA samples were subjected to Northern blotting using SgrS-S probe. Ten μg (lanes 1,2) or 25 μg (lane 3) of RNAs were loaded. (B) Effects of glucose-phosphate stress or Fe2+ depletion on readthrough at the ryhB terminator. TM820 (ΔryhB Δhfq) cells harboring pRyhB-rplLT were grown in LB medium in the presence of 1.0% arabinose. At A600 = 0.6, 250 μM dip or 0.1% αMG was added to cultures and incubation was continued for 10 min. Total RNAs were prepared and then the RNA samples were subjected to Northern blotting using the RyhB probe. Four micrograms (lanes 1,3) or 10 μg (lane 2) of RNAs were loaded. (C,D) Effect of growth phase on the readthrough at sgrS and ryhB terminators. TM772 (ΔsgrS Δhfq) cells harboring pSgrS-S-rplLT (C) and TM820 (ΔryhB Δhfq) cells harboring pRyhB-rplLT (D) were grown in LB medium in the presence of 1.0% arabinose. Total RNAs were prepared from the cultures of A600 = 0.6 (log phase) and A600 = 2.5 (stationary phase). The RNA samples (10 μg) were subjected to Northern blotting using the SgrS-S (C) or RyhB (D) probes. (E) Effects of glucose-phosphate stress and Fe depletion on the readthrough at the crp terminator. TM589 (Δhfq) cells harboring pcrpT-rplLT were grown in LB medium in the presence of 0.2% arabinose. At A600 = 0.6, 0.1% αMG or 250 μM dip was added to cultures and incubation was continued for 10 min. Total RNAs were prepared and then the RNA samples were subjected to Northern blotting using the crp probe. Four micrograms (lanes 1,2) or 10 μg (lane 3) of RNAs were loaded.
It is interesting to test whether other stress conditions that are associated with the induction of sRNAs affect the transcription termination at the Rho-independent terminators. It is known that several sRNAs including RybB are well expressed during stationary phase compared with log phase (Vogel et al. 2003). Therefore, we have examined the effect of growth phase on transcription termination at Rho-independent terminators. Cells harboring pSgrS-S-rplLT or pRyhB-rplLT were grown in LB medium containing 1.0% arabinose. Total RNAs were prepared from cells at mid-log and stationary phases, and subjected to Northern blotting using the sgrS or ryhB probe. As shown in Figure 7, C and D, transcription termination at Rho-independent terminators of these sRNA genes is markedly enhanced during stationary phase. Thus, it is likely that transcription termination at Rho-independent terminators of sRNAs is generally enhanced under stress conditions. Another interesting question is whether the enhanced termination specific of sRNA genes. To address this question, we have constructed the crpT-rplLT hybrid gene and tested the effect of stress on the Rho-independent terminator of crp. The results show that the termination at the crp terminator is markedly enhanced by the stress (Fig. 7E). Thus, the enhanced termination under stress conditions may be a general effect at any Rho-independent transcription terminators throughout the genome.
Analysis of readthrough with endogenous transcripts
We have demonstrated above that transcripts frequently read through the terminators of sRNA genes on the plasmid and transcription termination is enhanced under stress conditions. It is interesting to analyze the transcription termination/readthrough with the endogenous transcripts generated from chromosomal sRNA genes. For this, we have focused on the sgrS-setA operon. The hfq+ and hfq− cells were grown in LB medium to mid-log phase and exposed to either 0.1% glucose or αMG for 10 min. Then, total RNAs were prepared and subjected to Northern blotting. As reported previously (Vanderpool and Gottesman 2004; Kawamoto et al. 2005), SgrS is dramatically induced by αMG (Fig. 8A, lanes 2,4). We also confirmed the previous observation (Kawamoto et al. 2005) that the level of SgrS in the hfq+ cells is much higher than that in the hfq− cells because of the stabilization of SgrS by Hfq. In addition, the readthrough transcripts can be clearly observed as heterogeneous smear bands. Thus, the readthrough occurs with the endogenous transcripts as in the case of plasmid-derived transcripts.
FIGURE 8.
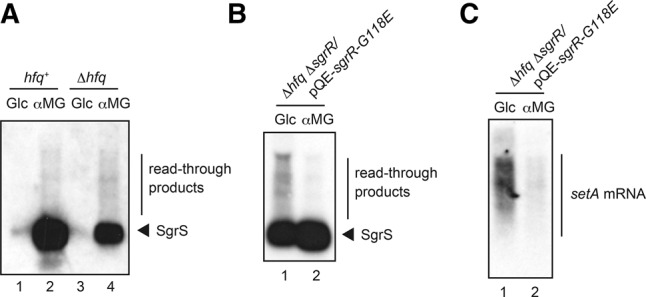
Transcriptional readthrough at the endogenous sgrS terminator and the effect of the stress. (A) IT1568 (hfq+) and TM589 (Δhfq) cells were grown in LB medium. At A600 = 0.6, 0.1% glucose or αMG was added to cultures and incubation was continued for 10 min. Total RNAs were prepared and then the RNA samples (4 μg) were subjected to Northern blotting using SgrS probe. (B,C) TM816 (Δhfq ΔsgrR) cells harboring pQE-sgrR-G118E were grown in LB medium in the presence of 0.1 mM IPTG. At A600 = 0.6, 0.1% glucose or αMG was added to cultures and incubation was continued for 10 min. Total RNAs were prepared and then the RNA samples (20 μg) were subjected to Northern blotting using SgrS probe (B) or setA probe (C), respectively.
It is difficult to examine the effect of stress on the readthrough at the endogenous sgrS terminator because the endogenous SgrS is not expressed under nonstress conditions. It is known that a mutant G118E SgrR can activate the sgrS transcription even without stress (Sun and Vanderpool 2011). Then, we used cells expressing this mutant SgrR to examine how the stress affects the readthrough at the endogenous sgrS terminator. For this, we constructed Δhfq ΔsgrR strain (TM816) in which the C-terminal two-thirds of SgrR coding region on the genome was deleted and plasmid pQE-sgrR-G118E carried the mutant sgrR gene encoding SgrR-G118E. TM816 was transformed with pQE-sgrR-G118E. Cells harboring pQE-sgrR-G118E or pQE80L vector were grown in LB medium containing 0.1 mM Isopropyl β-d-thiogalactopyranoside (IPTG). Total RNAs were prepared from cells at mid-log phase, and subjected to Northern blotting using the sgrS or setA probe. As expected, SgrS is effectively generated in TM816 cells carrying pQE-sgrR-G118E independently from the stress (Fig. 8B) while it is not expressed in TM816 cells harboring the vector plasmid (data not shown). Importantly, the readthrough was markedly reduced under the glucose-phosphate stress (Fig. 8B). The readthrough and its reduction under the stress were also observed in Northern analysis using the setA probe (Fig. 8C). Thus, we conclude that the transcription termination at the endogenous sgrS terminator transcripts is enhanced under stress conditions.
DISCUSSION
Genes encoding Hfq-binding sRNAs end with a Rho-independent transcription terminator. A striking feature of Rho-independent terminators of sRNA genes is the presence of a long consecutive T stretch (more than seven) in the nontemplate strand (Otaka et al. 2011). We showed previously that the RNA sequence corresponding to the Rho-independent transcription terminator makes up a large part of the Hfq-binding module of sRNAs (Ishikawa et al. 2012). Thus, modulation of transcription termination is important for generation of functional Hfq-binding sRNAs in cells. Major questions we have addressed in this study are: (1) how frequently transcriptional readthrough occurs at sRNA genes; (2) whether Hfq affects transcription termination at sRNA genes; (3) whether the readthrough products, the 3′-extended forms of sRNAs, retain Hfq-binding ability; and (4) how stress conditions associated with the induction of sRNAs affect transcription termination. We used a “double terminator system” in which a strong Rho-independent terminator was placed just downstream from the Rho-independent terminators of the plasmid-borne sRNA genes sgrS and ryhB.
We found first that ∼50% and 20% of elongating RNA polymerases read through the Rho-independent terminators of sgrS and ryhB during normal growth (Figs. 2B,4A). Although the stability of the terminator hairpin and the length of polyU tail are important factors that dictate the termination efficiency, there seems to be a little difference between sgrS and ryhB terminators regarding the stability and the length of polyU tail. In fact, the free energy of the terminator hairpin (ΔG) of SgrS and RyhB are calculated to be −11.3 and −10.7, respectively, while the length of the polyU tail of SgrS and RyhB are 8 and 9, respectively (Fig. 1B). It is known that several factors including flanking sequences of a terminator hairpin affect termination efficiency at Rho-independent terminators (Reynolds et al. 1992). The presence of a short hairpin before the terminator hairpin of SgrS (Ishikawa et al. 2012) may limit the formation of terminator hairpin leading to the reduced transcription termination (increased readthrough) at the sgrS terminator.
It is an intriguing question if Hfq plays any roles in transcription termination at the Rho-independent terminators in particular of sRNA genes because Hfq interacts with nascent transcripts of the sRNA genes. However, it has not been tested experimentally whether Hfq affects transcription termination at Rho-independent terminators until the present work. It is reported that Hfq reduces transcription termination by directly inhibiting the activity of termination factor Rho at Rho-dependent terminators (Rabhi et al. 2011). Yet, here we see that the levels of readthrough product are little or only slightly affected by the presence of Hfq at the sgrS and ryhB terminators while the abundance of terminated transcripts is elevated because these RNAs are stabilized against degradation by Hfq binding. The observation that Hfq does not affect readthrough simply implies that Hfq is not involved in modulation of transcription termination at Rho-independent terminators.
The finding that the transcriptional readthrough occurs frequently at the sgrS and ryhB terminators implies that significant amounts of readthrough products are continuously generated in cells. The products generated by readthrough are variants of sRNAs in which their 3′ ends are extended. The 3′-extended forms of SgrS and RyhB retain the entire sequence corresponding to the Hfq-binding module (Ishikawa et al. 2012) though the polyU stretch is no longer located at the 3′ end in these RNA molecules. This raised the question of whether the 3′-extended forms of SgrS and RyhB still retained their regulatory function as Hfq-binding sRNAs. Hfq is known to stabilize sRNAs upon binding in cells (Storz and Gottesman 2006). We demonstrated that the 3′-extended forms of SgrS and RyhB are poorly stabilized by Hfq (Figs. 3, 4), and by pull-down assays that the Hfq-binding ability of the 3′-extended forms of SgrS and RyhB is dramatically decreased (Fig. 5). We also showed by a competition assay using a biotin–streptavidin system that a synthetic RNA corresponding to the 3′-extended form of SgrS does not bind to Hfq in vitro (Fig. 6). We conclude that the 3′-extended forms of SgrS and RyhB produced by transcriptional readthrough no longer retain the Hfq-binding ability and thereby may not function to regulate the target mRNAs. This conclusion is consistent with the early mutational study on DsrA sRNA that showed that mutations in the terminator stem-loop of DsrA led to a significant readthrough resulting in the loss of the regulatory function of DsrA (Sledjeski and Gottesman 1995). In addition, it is reported that the affinity and/or the mode of Hfq binding to a hexauridine (U6) was significantly altered by a single nucleotide substitutions (U5A, U5C, and U5G) at the 3′-terminal position (Sauer and Weichenrieder 2011). This is also consistent with the present finding that the readthrough products of Hfq-binding sRNAs no longer bind to Hfq. Thus, we conclude that the polyU stretch must be located at the 3′ end to act as an element to bind Hfq.
Studies to understand how Hfq-binding sRNAs interact with the target mRNAs and Hfq are crucial in understanding how sRNAs act. Various biochemical and biophysical methods have been used to analyze sRNA–mRNA and/or sRNA–Hfq interactions. For example, we previously used an electrophoretic mobility shift assay to analyze the sRNA–mRNA and sRNA–Hfq interactions using RNAs prepared by either in vitro transcription or chemical synthesis (Maki et al. 2010). Another approach for studying RNA–RNA and RNA–protein interactions is the use of biotin-labeled RNAs along with streptavidin beads. Indeed, this approach has proved to be useful to study the sRNA–mRNA interaction (Vincent et al. 2013). In this study, as we have outlined in results, we used the biotin–streptavidin system to study the interaction between sRNAs and Hfq. Our system consists of a 5′-biotinylated synthetic RNA containing a minimal Hfq-binding module, a test RNA, purified Hfq and streptavidin-conjugated magnet beads. The biotinylated RNA binds tightly to the streptavidin beads without losing the Hfq-binding activity. Thus, Hfq can be immobilized on the beads through biotinylated RNA. When excess amounts of test RNAs retaining but not losing Hfq-binding activity can compete with the biotinylated RNA resulting in the inhibition of the Hfq binding to the beads. We showed that this method is quite simple, rapid, and useful to examine the Hfq-binding ability of individual RNA molecules. To our knowledge, this is the first case in which a biotinylated RNA has been successfully used to study the sRNA–Hfq interaction although sRNAs tagged by aptamers such as MS2 and boxB were shown to be useful for the in vivo study on Hfq–sRNA interaction (Said et al. 2009).
The sgrS gene is known to be co-transcribed with the downstream gene setA encoding a putative sugar transporter (Sun and Vanderpool 2011). SetA was shown to contribute to the cellular response to the glucose-phosphate stress although how it acts remains to be determined (Sun and Vanderpool 2011). Similarly, the ryhB seems to be co-transcribed with the downstream gene yhhX (Vassinova and Kozyrev 2000) though this has not been shown experimentally so far. We demonstrated in the present study that transcription termination at the Rho-independent terminators of sgrS and ryhB is markedly enhanced under the stress conditions. The enhanced termination under the stress conditions was also observed in the genomic sgrS-setA transcript (Fig. 8). Thus, the sRNA production is regulated not only at the step of transcription initiation but also at the step of transcription termination. This is biologically significant since increased transcription termination at sRNA genes under stress conditions generate more stable functional sRNAs to deal with the stresses. The enhanced transcription termination at sRNA genes implies that the expression of downstream genes is quite low. In fact, the analysis of the sgrS-setA bi-cistronic transcript showed high SgrS levels relative to setA mRNA levels under glucose-phosphate stress (Sun and Vanderpool 2011). According to our data, the abundance of SgrS-S-rplLT in hfq+ cells is a few % of that of SgrS-S under the stress conditions, indicating most transcripts terminate at the sgrS terminator. It remains to be determined whether this differential expression of the sRNA gene and the downstream gene has biological significance under these stress conditions. We also do not know at this stage the mechanism by which transcription termination is enhanced under stress conditions. Interestingly, the transcription termination at sgrS and ryhB terminators is enhanced not only by a particular cognate stress but also other stress conditions. This suggests that the physiology of cells undergoing stress conditions affects transcription termination at the Rho-independent terminators. One plausible effect of stress might be to reduce nucleoside triphosphates (NTPs) levels in cells, since low NTP concentrations are reported to enhance transcription termination at Rho-independent terminators in vitro (Reynolds et al. 1992). It is interesting to examine if stress conditions such as accumulation of glucose-6-phosphate and depletion of Fe2+ ion lead to the reduction of NTP concentration in cells, and if this is a general effect at Rho-independent transcription terminators throughout the genome.
MATERIALS AND METHODS
Bacterial strains and plasmids
The E. coli K12 strains and plasmids used in this study are listed in Table 1. IT1568 (W3110 mlc−) was used as a parent wild-type strain. To construct TM772 and TM820, the Δhfq::cat allele of TM587 (Morita et al. 2005) was moved to TM542 (Kawamoto et al. 2005) and TM635 (Morita et al. 2006), respectively, by P1 transduction. To construct TM821, the hfq-Flag-cat allele of TM615 (Morita et al. 2005) was moved to TM635 (Morita et al. 2006) by P1 transduction. TM803 and TM822 were constructed by removing the cat gene flanked by two FRT sequences from TM771 (Otaka et al. 2011) and TM821, respectively. The ΔsgrR::kan allele was constructed from BW25113/pKD46 according to Datsenko–Wanner protocol using pKD4 harboring kan gene (Datsenko and Wanner 2000). To construct TM814, the ΔsgrR::kan allele was moved to IT1568 by P1 transduction. To construct TM816, the Δhfq::cat allele of TM587 (Morita et al. 2005) was moved to TM814 by P1 transduction.
TABLE 1.
Bacterial strains and plasmids used in this study

The DNA primers used are listed in Table 2. Plasmids pSgrS-S-rplLT and pSgrS-S-rrnBT1 were constructed as follows: pSgrS-S was used to amplify the DNA fragments containing the sgrS-S sequence and the rplLT or rrnBT1 sequence with primers 1127 and 1415 or 1707, respectively. The amplified DNA fragments were digested with XbaI and HindIII and cloned into pAraX. Plasmid pRyhB-rplLT was constructed as follows: pRyhB was used to amplify the DNA fragment containing the ryhB sequence and the rplL terminator sequences with primers 1144 and 1527. The amplified DNA fragment was digested with XbaI and HindIII and cloned into pAraX. Plasmid pcrpT-rplLT was constructed as follows: pHA7 (Aiba et al. 1982) was used to amplify the DNA fragment containing the crp terminator region and the rplL terminator sequence with primers 1717 and 1718. The amplified DNA fragment was digested with XbaI and HindIII and cloned into pAraX. Plasmid pQE-sgrR-G118E was constructed as follows: chromosomal DNA of W3110mlc was used to amplify the DNA fragment 1 containing the mutated sgrR region (from −19 to +366 relative to ATG start codon) with primers 1596 and 1597. Similarly, DNA fragment 2 containing the mutated sgrR region (+341 to +1656 relative to ATG start codon) was amplified with primers 1595 and 1598. Then, the DNA fragments 1and 2 were used to amplify the mutated sgrR, in which G at +353 is changed to A with 1597 and 1598. The amplified DNA fragment containing sgrS G118E was digested with EcoRI and HindIII and cloned into pQE80L.
TABLE 2.
DNA primers used for construction of plasmids

Northern blotting
Cells carrying the indicated plasmids were grown at 37°C to mid-log phase unless specified in LB medium supplemented with kanamycin (15 μg/mL) and indicated amounts of arabinose. In Figure 8B,C, cells carrying the indicated plasmids were grown at 37°C to mid-log phase with ampicillin (50 μg/mL) and indicated amounts of IPTG. Total RNAs were isolated as described (Aiba et al. 1981). To detect SgrS-S, RyhB, and crp RNAs, RNA samples were resolved by 12%, 6%, and 8% polyacrylamide gel electrophoresis in the presence of 8 M urea and blotted onto Hybond-N+ membrane (GE Healthcare). To detect ptsG mRNA and setA mRNA, RNA samples were resolved by 1.2% agrarose gel electrophoresis in the presence of formaldehyde and blotted onto Hybond-N+ membrane (GE Healthcare). To detect SgrS and tmRNA, RNA samples were resolved by 1.5% agrarose gel electrophoresis in the presence of formaldehyde and blotted onto Hybond-N+ membrane (GE Healthcare). The RNAs were visualized using digoxigenin (DIG) reagents and kits for nonradioactive nucleic acid labeling and a detection system (Roche Applied Science) according to the procedure specified by the manufacturer. The SgrS-S RNA probe corresponding to antisense of 3′ portion (+168 to +227) of sgrS was prepared by DIG RNA labeling kit (Roche Applied Science). The following DIG-labeled DNA probes were prepared by PCR using DIG-dUTP: a 305-bp fragment corresponding to the 5′ region of ptsG (ptsG probe); 95-bp fragment corresponding to the ryhB (RyhB probe); 227-bp fragment corresponding to the sgrS (SgrS probe); 363-bp fragment corresponding to the tmRNA (tmRNA probe); 90-bp fragment corresponding to the 3′ region of crp (crp probe); 220-bp fragment corresponding to +11 to +230 region relative to ATG start codon of setA (setA probe). Multi Gauge ver. 3.1 software (Fujifilm) was used to quantify RNA bands on the films.
Pull-down assay
Cells were grown in 400 mL of LB medium in the presence of 1.0% arabinose, harvested, and washed with 10 mL STE buffer (100 mM NaCl, 10 mM Tris–HCl at pH 8.0, and 1 mM EDTA). The cell pellets were suspended in ice cold 10 mL of IP buffer (20 mM Tris–HCl at pH 8.0, 0.1 M KCl, 5 mM MgCl2, 10% glycerol, and 0.1% Tween20). The cell suspension was sonicated and centrifuged at 10,000g for 10 min at 4°C. The supernatant (crude extract: CE) was incubated with 50 μL of anti-Flag M2-agarose suspension (Sigma-Aldrich) for 30 min at 4°C. The mixture was filtered by using a mini chromatography column (Bio-Rad). The agarose beads were washed twice by 10 mL of IP buffer. The proteins bound to the beads were eluted with 50 μL of IP buffer containing 0.4 mg/mL Flag peptide (Sigma-Aldrich) and used as bound fraction (B). To analyze proteins, the crude extract (10 μL) and the bound fraction (2 μL) were mixed with SDS-PAGE loading buffer (6.25 mM Tris–HCl at pH 6.8, 2% SDS, 10% glycerol, 5% b-mercaptoethanol, 0.1% Bromophenol blue). The samples were heated for 5 min at 100°C and subjected to a 15% SDS gel electrophoresis, and then transferred to an Immobilon membrane (Milipore). The membrane was treated with an anti-Flag monoclonal antibody (Sigma-Aldrich). Signals were visualized by the Lumi-light Western Blotting Substrate (Roche). To analyze RNAs, the crude extract (10 μL) and the bound fraction (2 μL) were treated with phenol, precipitated, and washed with ethanol. Each precipitate was dissolved in 6 μL of RNA buffer (0.02 M sodium acetate at pH 5.5, 0.5% SDS, and 1 mM EDTA). The RNA samples were subjected to Northern blotting.
Purification of His-tagged Hfq
TM589 harboring pQE80L-Hfq-His (Kawamoto et al. 2006) was cultured in 200 mL of LB medium at 37°C in the presence of 25 μM IPTG and 50 μg/mL ampicillin. At A600 = 0.2, 2 mM of IPTG was added to the culture and incubation was continued for 70 min. Cells were harvested and washed with 20 mL STE, and suspended in Lysis Buffer Native (pH 8.0) (Qiagen). The cell suspension was treated with lysozyme (1 mg/mL) for 10 min at 0°C, sonicated, and centrifuged at 10,000g for 10 min at 4°C. The supernatant was treated with RNase A (1 μg/mL) for 10 min at 0°C and then heated for 10 min at 80°C. The sample was centrifuged at 10,000g for 10 min at 4°C. The supernatant was incubated with 500 μL of Ni2+-NTA agarose resin (Qiagen) for 20 min at 4°C and Hfq-His6 protein was purified according to the manufacturer's instruction. Purified Hfq-His6 was concentrated by Amicon Ultra (0.5 mL 3K) Centrifugal Filters (Milipore). The Hfq-His6 concentration was estimated by 15% SDS-PAGE and SimplyBlue SageStain (Invitrogen). Purified Hfq-His6 was stored with storage buffer (20 mM Tris–HCl at pH 8.0, 0.1 M KCl, 5 mM MgCl2, 50% glycerol, and 0.1% Tween20, 1 mM DTT) at −30°C.
In vitro competition assay
Synthetic RNAs including 5′- biotinylated RNA were obtained from GeneDesign Inc. Two microliters of Dynabeads MyOne Streptavidin C1 magnetic beads (Lifetechnologies), purified Hfq-His6 (66.7 nM), indicated amounts of biotinylated RNA, and an excess amount (667 nM) of different competitor RNAs were mixed together in 15 μL of binding buffer (20 mM Tris–HCl at pH 8.0, 1 mM DTT, 1 mM MgCl2, 20 mM KCl, 10 mM Na2HPO4–NaH2PO4 at pH 8.0) containing 1 mg of yeast tRNA (Invitrogen Japan). The mixtures were gently shaken for 10 min at 37°C. Streptavidin magnetic beads were collected by magnet, and 10 μL of supernatant was used as unbound fraction (UB). Streptavidin magnetic beads were washed with 100 μL of wash buffer (20 mM Tris–HCl at pH 8.0, 0.2 M KCl). Ten microliters of H2O was added to streptavidin magnetic beads, and the suspension was used as bound fraction (B). The UB and B samples were mixed with SDS-PAGE loading buffer. The sample was heated for 5 min at 100°C and subjected to a 15% SDS gel electrophoresis. Hfq-His6 was visualized by SilverQuest silver staining kit (Life Technologies).
ACKNOWLEDGMENTS
We thank Susan Gottesman and Donald Court for critical reading of the manuscript and helpful comments. This work was supported by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to H.A. and T.M.) and Takeda Science Foundation (T.M.).
Footnotes
Article published online ahead of print. Article and publication date are at http://www.rnajournal.org/cgi/doi/10.1261/rna.051870.115.
Freely available online through the RNA Open Access option.
REFERENCES
- Abe H, Abo T, Aiba H. 1999. Regulation of intrinsic terminator by translation in Escherichia coli: transcription termination at a distance downstream. Genes Cells 4: 87–97. [DOI] [PubMed] [Google Scholar]
- Aiba H, Adhya S, de Crombrugghe B. 1981. Evidence for two functional gal promoters in intact Escherichia coli cells. J Biol Chem 256: 11905–11910. [PubMed] [Google Scholar]
- Aiba H, Fujimoto S, Ozaki N. 1982. Molecular cloning and nucleotide sequencing of the gene for E. coli cAMP receptor protein. Nucleic Acids Res 10: 1345–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius J, Dull TJ, Sleeter DD, Noller HF. 1981. Gene organization and primary structure of a ribosomal RNA operon from Escherichia coli. J Mol Biol 148: 107–127. [DOI] [PubMed] [Google Scholar]
- Chao Y, Papenfort K, Reinhardt R, Sharma CM, Vogel J. 2012. An atlas of Hfq-bound transcripts reveals 3′ UTRs as a genomic reservoir of regulatory small RNAs. EMBO J 31: 4005–4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci 97: 6640–6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Aubenton Carafa Y, Brody E, Thermes C. 1990. Prediction of rho-independent Escherichia coli transcription terminators. A statistical analysis of their RNA stem-loop structures. J Mol Biol 216: 835–858. [DOI] [PubMed] [Google Scholar]
- Gottesman S, Storz G. 2010. Bacterial small RNA regulators: versatile roles and rapidly evolving variations. Cold Spring Harb Perspect Biol 1: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo MS, Updegrove TB, Gogol EB, Shabalina SA, Gross CA, Storz G. 2014. MicL, a new sigmaE-dependent sRNA, combats envelope stress by repressing synthesis of Lpp, the major outer membrane lipoprotein. Genes Dev 28: 1620–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, Otaka H, Maki K, Morita T, Aiba H. 2012. The functional Hfq-binding module of bacterial sRNAs consists of a double or single hairpin preceded by a U-rich sequence and followed by a 3′ poly(U) tail. RNA 18: 1062–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto H, Morita T, Shimizu A, Inada T, Aiba H. 2005. Implication of membrane localization of target mRNA in the action of a small RNA: mechanism of post-transcriptional regulation of glucose transporter in Escherichia coli. Genes Dev 19: 328–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto H, Koide Y, Morita T, Aiba H. 2006. Base-pairing requirement for RNA silencing by a bacterial small RNA and acceleration of duplex formation by Hfq. Mol Microbiol 61: 1013–1022. [DOI] [PubMed] [Google Scholar]
- Maki K, Morita T, Otaka H, Aiba H. 2010. A minimal base-pairing region of a bacterial small RNA SgrS required for translational repression of ptsG mRNA. Mol Microbiol 76: 782–792. [DOI] [PubMed] [Google Scholar]
- Masse E, Gottesman S. 2002. A small RNA regulates the expression of genes involved in iron metabolism in Escherichia coli. Proc Natl Acad Sci 99: 4620–4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masse E, Escorcia FE, Gottesman S. 2003. Coupled degradation of a small regulatory RNA and its mRNA targets in Escherichia coli. Genes Dev 17: 2374–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita T, Maki K, Aiba H. 2005. RNase E-based ribonucleoprotein complexes: mechanical basis of mRNA destabilization mediated by bacterial noncoding RNAs. Genes Dev 19: 2176–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita T, Mochizuki Y, Aiba H. 2006. Translational repression is sufficient for gene silencing by bacterial small noncoding RNAs in the absence of mRNA destruction. Proc Natl Acad Sci 103: 4858–4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otaka H, Ishikawa H, Morita T, Aiba H. 2011. PolyU tail of rho-independent terminator of bacterial small RNAs is essential for Hfq action. Proc Natl Acad Sci 108: 13059–13064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papenfort K, Sun Y, Miyakoshi M, Vanderpool CK, Vogel J. 2013. Small RNA-mediated activation of sugar phosphatase mRNA regulates glucose homeostasis. Cell 153: 426–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt T. 1986. Transcription termination and the regulation of gene expression. Annu Rev Biochem 55: 339–372. [DOI] [PubMed] [Google Scholar]
- Post LE, Strycharz GD, Nomura M, Lewis H, Dennis PP. 1979. Nucleotide sequence of the ribosomal protein gene cluster adjacent to the gene for RNA polymerase subunit β in Escherichia coli. Proc Natl Acad Sci 76: 1697–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabhi M, Espeli O, Schwartz A, Cayrol B, Rahmouni AR, Arluison V, Boudvillain M. 2011. The Sm-like RNA chaperone Hfq mediates transcription antitermination at Rho-dependent terminators. EMBO J 30: 2805–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds R, Bermudez-Cruz RM, Chamberlin MJ. 1992. Parameters affecting transcription termination by Escherichia coli RNA polymerase. I. Analysis of 13 rho-independent terminators. J Mol Biol 224: 31–51. [DOI] [PubMed] [Google Scholar]
- Rosenberg M, Court D. 1979. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet 13: 319–353. [DOI] [PubMed] [Google Scholar]
- Said N, Rieder R, Hurwitz R, Deckert J, Urlaub H, Vogel J. 2009. In vivo expression and purification of aptamer-tagged small RNA regulators. Nucleic Acids Res 37: e133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer E, Weichenrieder O. 2011. Structural basis for RNA 3′-end recognition by Hfq. Proc Natl Acad Sci 108: 13065–13070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sledjeski D, Gottesman S. 1995. A small RNA acts as an antisilencer of the H-NS-silenced rcsA gene of Escherichia coli. Proc Natl Acad Sci 92: 2003–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz G, Gottesman S. 2006. Versatile roles of small RNA regulators in bacteria. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Vanderpool CK. 2011. Regulation and function of Escherichia coli sugar efflux transporter A (SetA) during glucose-phosphate stress. J Bacteriol 193: 143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderpool CK, Gottesman S. 2004. Involvement of a novel transcriptional activator and small RNA in post-transcriptional regulation of the glucose phosphoenolpyruvate phosphotransferase system. Mol Microbiol 54: 1076–1089. [DOI] [PubMed] [Google Scholar]
- Vanderpool CK, Gottesman S. 2007. The novel transcription factor SgrR coordinates the response to glucose-phosphate stress. J Bacteriol 189: 2238–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassinova N, Kozyrev D. 2000. A method for direct cloning of fur-regulated genes: identification of seven new fur-regulated loci in Escherichia coli. Microbiology 146 Pt 12: 3171–3182. [DOI] [PubMed] [Google Scholar]
- Vincent HA, Phillips JO, Henderson CA, Roberts AJ, Stone CM, Mardle CE, Butt LE, Gowers DM, Pickford AR, Callaghan AJ. 2013. An improved method for surface immobilisation of RNA: application to small non-coding RNA-mRNA pairing. PLoS One 8: e79142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel J, Luisi BF. 2011. Hfq and its constellation of RNA. Nat Rev Microbiol 9: 578–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel J, Bartels V, Tang TH, Churakov G, Slagter-Jager JG, Huttenhofer A, Wagner EG. 2003. RNomics in Escherichia coli detects new sRNA species and indicates parallel transcriptional output in bacteria. Nucleic Acids Res 31: 6435–6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters LS, Storz G. 2009. Regulatory RNAs in bacteria. Cell 136: 615–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager TD, von Hippel PH. 1991. A thermodynamic analysis of RNA transcript elongation and termination in Escherichia coli. Biochemistry 30: 1097–1118. [DOI] [PubMed] [Google Scholar]
- Zuker M. 2003. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res 31: 3406–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]



