Abstract
Objective(s):
Gardenia jasminoides Ellis (GJ, Cape Jasmine Fruit, Zhi Zi) has been traditionally used for the treatment of infectious hepatitis, aphthous ulcer, and trauma; however, the direct evidence is lacking.
Materials and Methods:
We investigated the effect of the GJ extract (GJ) and gallic acid (GA) on lipopolysaccharide (LPS) induced inflammation of BV-2 microglial cells and acute liver injury in Sprague-Dawley (SD) rats.
Results:
Our results showed that the GJ extract and GA reduced LPS-induced nitric oxide (NO), interleukin (IL)-1, IL-6, reactive oxygen species (ROS), and prostaglandin (PGE2) production in BV-2 cells. The GJ extract and GA significantly decreased serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels in LPS-treated rats. Furthermore, the water extract, but not the ethanol extract, of the GJ dose-dependently inhibited LPS-induced JNK2/1 and slightly p38 mitogen-activated protein kinases (MAPK), and cyclooxygenase-2 (COX-2) expression in BV-2 cells.
Conclusion:
Taken together, these results indicate that the protective mechanism of the GJ extract involves an antioxidant effect and inhibition of JNK2/1 MAP kinase and COX-2 expressions in LPS-induced inflammation of BV-2 cells.
Keywords: BV-2[AGA1] cell, Gardenia jasminoides, MAPKs, NO, ROS
Introduction
Gardenia jasminoides (GJ) is evergreen shrub of Rubiaceae, which is widely used in the treatment of infectious hepatitis, aphthous ulcer, and trauma in Asia. The pharmacological properties of this plant evaluated so far include anti-tumor and anti-inflammatory properties and reducing the risks of gastritis (1-3). The extract of Gardenia jasminoides Ellis, used to treat inflammation, was identified as geniposide. In vivo geniposide was found to plays an anti-inflammatory role via regulating TLR4 and downstream signaling pathways in LPS-induced mastitis in mice (2). It was also observed that geniposide attenuated histopathologic changes of mesenteric lymph node in adjuvant arthritis rats. Collectively, its anti-inflammatory and immune- regulatory effects might be mediated through down-regulating the expression of p-JNK (4). Hepatitis is commonly caused by pathogenic infection (including hepatitis viruses and Gram-negative bacteria), and alcohol- or drug-induced liver toxicity. Its pathology is initiated by a cascade of inflammatory events from viral-, alcohol-, or endotoxin-stimulated inflammatory cells and hepatic Kupffer cells to produce various pro-inflammatory cytokines, including tumor necrosis factor (TNF)-α, interleukin (IL)-1, IL-6, IL-12, and interferon (IFN)-γ (5-7). Lipopolysaccharides (LPS) can induce the Kupffer cells to produce reactive oxygen species (ROS) (8). Inflammatory response to stimuli or injury is often exacerbated by the resultant swelling or edema of tissue, pain (due to increased pressure in tissues or by inflammatory mediators), or even cell damage (9, 10). Therefore, chronic hepatitis leads to cirrhosis and eventually hepatocellular carcinoma. LPS-stimulated microglia, macrophages, and Kupffer cells activate phosphorylation and kinase activities of ERK1/2, c-Jun N-terminal kinase (JNK), p38 mitogen-activated protein kinase (MAPK) and subsequently, cytokine production (11, 12). Evidence indicates that inducible COX may have both pro- and anti-inflammatory properties through the generation of different types of prostaglandins (13). Prostaglandin E2 (PGE2) strongly synergizes with the inflammatory cytokine. Thus, the employment of anti-inflammatory agents may be helpful in the treatment of various inflammatory conditions including hepatitis. It has been reported that Gposidic acid (iridoid glucoside) and genipin (protein) isolated from the GJ extract suppress serum tumor necrosis factor-alpha (TNF-α) and activation of hepatic lipid peroxidation, which was induced by GalN/LPS-induced liver toxicity (14, 15). Gallic acid (GA) is an organic acid found in foods such as blueberries, flaxseeds, tea leaves, and watercress. GA possesses significant anti-inflammatory properties and prevents the expression of inflammatory chemicals including cytokines and histamines (16). However, the mechanism of hepatoprotective effects of GJ extract and GA on LPS-induced liver toxicity has not been reported. Therefore, the aim of present study was to investigate the mechanism of anti-inflammatory effects of the water extract of GJ and GA using in vitro and in vivo models.
Materials and Methods
Reagents
LPS from Escherichia coli serotype 0111:B4 was obtained from Sigma (St. Louis, MO, USA). 2’,7’-Dichlorodihydrofluorescein diacetate (H2DCF-DA) was obtained from Molecular Probe (Eugene, Oregon, USA).
Activating agent
The GJ powder (100 mg) was added to 1 ml of RO water (reverse osmosis), mixed well by vortex for 5 min, and centrifuged at 2000 × g for 10 min. Finally, the GJ extract supernatant was filtered by sterile membrane.
Gallic acid content
GA content was determined by a procedure using 3% Na2CO3 solution and 5% Folin–Ciocalteu’s reagent. The Gardenia jasminoides infusion mixture was reacted for 30 min at room temperature before the absorbance at 760 nm was read. A standard curve was obtained from the gallic acid standard (Sigma-Aldrich Chemie GmbH, Munich, Germany) liquor in five different chroma using the method described by Zhu (17).
Animals
Male Sprague-Dawley rats (300-400 g) obtained from National Laboratory Animal Center (Taipei, Taiwan) and maintained in the Animal Center of Chinese Medical University (Taichung, Taiwan). The animal studies were performed following the guidelines from the Guidebook for the Care and Use of Laboratory Animals (2002) published by the Chinese Society of Animal Science in Taiwan. The rats were divided into six groups and fasted for 12 hr before intraperitoneal (IP) drug administration. One control group was given saline (blank), and the experimental group (L) was given 50 μg/kg of LPS. The GJ extract and GA groups were given 10 or 30 mg/kg of the Gardenia jasminoides extract and 1 or 10 mg/kg of gallic acid by gastric gavage after injection of LPS. Liver tissues were fixed with 10% formaldehyde solution overnight and stained with hematoxylin and eosin (H&E). Hepatic function was assessed by measuring serum alanine aminotranferease (ALT) and aspartate aminotransferase (AST) with an automatic blood analyzer (Hitachi High-Technologies, Tokyo, Japan).
Cell culture
Murine BV-2 cell line obtained from Taichung Veterans General Hospital, Taiwan, was maintained in DMEM (Dulbecco’s Modified Eagle Medium) supplemented with 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37 °C in a humidified incubator under 5% CO2. Confluent cultures were passed by trypsinization. For experiments, cells were washed twice with warm DMEM (without phenol red), and then treated in a serum-free medium. In all experiments, cells were treated with the GJ extract and GA for the indicated times after the addition of 2 μg/ml LPS as activating agent. The GJ extract and GA were dissolved in phosphate-buffered saline (PBS) and filtered through a 0.2 μM filter.
Nitric oxide assay
Nitrite, measured by Griess reaction, was taken as a measure of NO production. Briefly, 100 μl of culture supernatant (12–24 hr) was reacted with an equal volume of Griess reagent (1 part 0.1% naphthylethylene-diamine, 1 part 1% sulfanilamide in 5% H3PO4) in 96-well tissue culture plates for 10 min at room temperature in the dark. The absorbance at 540 nm was determined using a microplate reader (spectraMAX 340, Molecular Devices, Sunnyvale, CA, USA).
Cytokine assay
Cytokines (IL-1β, IL-6) and PGE2 were measured by ELISA kits (R&D, Minnespolis, MN, USA). The absorbance at 450 nm was determined using a microplate reader (spectraMAX 340). The TNF-α, IL-1β and IL-6 levels in the tissue supernatant were determined by ELISA kits (R&D). Protein concentration (pg/μg) in liver tissue was determined using a dye-based protein assay (Bio-Rad Laboratories, Hercules, CA, USA).
Reactive oxygen species generation
Intracellular accumulation of ROS was determined with H2DCF-DA. This nonfluorescent compound accumulates within cells upon deacetylation. H2DCF then reacts with ROS to form fluorescent dichlorofluorescein (DCF). BV-2 cells were plated in 96-well plates and grown for 24 hr before addition of DMEM plus 10 μM H2DCF, incubation for 60 min at 37 °C, and treatment with 1 mM H2O2 60 min. Cells were then washed twice with room temperature Hank’s balanced salt solution (HBSS without phenol red). Cellular fluorescence was monitored on a Fluoroskan Ascent fluorometer (Labsystems Oy, Helsinki, Finland) using an excitation wavelength of 485 nm and emission wavelength of 538 nm.
Serum AST/ALT
Serum levels of liver enzymes such as aspartate aminotransferase (AST) and alanine aminotransferase (ALT) reflect liver function and hepatocyte integrity. Concentrated blood samples were taken from tail veins by venipuncture during the experiment to measure the serum concentrations of AST and ALT in rats of each group. AST and ALT were detected by the automatic blood analyzer (Hitachi High-Technologies, Tokyo, Japan).
Preparation of cell extracts
The test medium was removed from culture dishes and cells were washed twice with ice-cold PBS, scraped off with a rubber policeman, and centrifuged at 200 ×g for 10 min at 4° C. The cell pellets were resuspended in an appropriate volume (approx. 4×107 cells/ml) of lysis buffer (20 Mm Tris–HCl, pH 7.5, 137 mM NaCl, 1 mM phenylmethylsulfonylfluoride (PMSF), 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 5 lg/ml pepstain A), and sonicated. Protein concentration of samples was determined by Bradford assay (Bio-Rad, Hemel, Hempstead, UK), and samples equilibrated to 2 mg/ml with lysis buffer.
Western blotting
BV-2 cells were homogenized in ice-cold lysis buffer (1:10, wt/vol) containing 20 mmol/L 4-(2-hydroxyethyl)-1- piperazineethane sulfonic acid (pH 7.2), 1% Triton X-100, 10% glycerol, 1 mM PMSF, 10 μg/mL leupeptin, and 10 μg/ml aprotinin. This solution was centrifuged at 10,000 × g for 20 min at 4 °C. 50 μg of protein was run on an 8% or 10% sodium dodecyl sulfate-polyacrylamide gel and transferred onto nitrocellulose membranes (NEN Life Science, Boston, MA, USA) at 1.2 A for 3 hr. The membranes were blocked in 5% milk in Tris-buffered saline with Tween-20. The membrane was then incubated with polyclonal rabbit iNOS antibody (BD Biosciences, San Diego, CA, USA), and diluted 1:1000 in blocking buffer. Membranes were incubated with secondary anti-rabbit IgG conjugated to alkaline phosphatase (1:3000; Jackson ImmunoResearch, Philadelphia, PA, USA) and detected with alkaline phosphatase substrate solution (5-bromo-4-chloro-3-indolyl-phosphate/nitroblue tetrazolium) (Kirkegaard & Perry, Baltimore, MD, USA).
Statistical analysis
All data are expressed as mean±SD. For single variable comparisons, Student’s t-test was used. For multiple variable comparisons, data were analyzed by one-way ANOVA using Dunnett’s test. P-values of less than 0.05 or 0.01 were considered significant.
Results
GA contents of Gardenia jasminoides seeds
GA contents obtained from fresh and dry G. jasminoides seeds were 1.62±0.21 mg/g and 7.01±0.54 mg/g, respectively by reported assay method (17).
Effect of Gardenia jasminoides extract and gallic acid on nitrite production in LPS-stimulated BV-2 cells
We first tested whether the GJ extract could inhibit nitrogen free radicals. Murine BV-2 cell line obtained from Taichung Veterans General Hospital, Taiwan, was used as an inflammation model. BV-2 cells were stimulated with LPS (Escherichia coli serotype 0111:B4) and then treated with various concentrations of the GJ extract and GA for 24 hr. Nitrite content was measured using the Griess reaction. The results showed that nitrite production was inhibited dose-dependently by the GJ extract (Figure 1). Therefore, the GJ extract was used in further experiments.
Figure 1.

Effect of the Gardenia jasminoides (GJ) extract on nitric oxide inhibition. BV-2 cells were stimulated with 2 μg/ml LPS and then treated with various concentrations of GJ extract or 1 μM gallic acid (GA) (positive control) for 24 hr. Nitrite production was measured by Griess reagent
Effect of GJ extract and GA on inflammatory cytokines
BV-2 cells were treated with 2 μg/ml LPS in combination with various concentrations (GA or GJ. GA contents that were obtained from fresh and dry G. jasminoides seeds were 1.62±0.21 mg/g and 7.01±0.54 mg/g, respectively) of GJ extract (1, 5, and 10 μg/ml) and 1 μM GA. Cultured supernatants were used to measure IL-1β and IL-6 by ELISA assay. The results showed that the GJ extract dose-dependently decreased IL-1β and IL-6 productions in BV-2 cells (Figure 2), and the effect of GJ on IL-6 production was more prominent than IL-1β.
Figure 2.
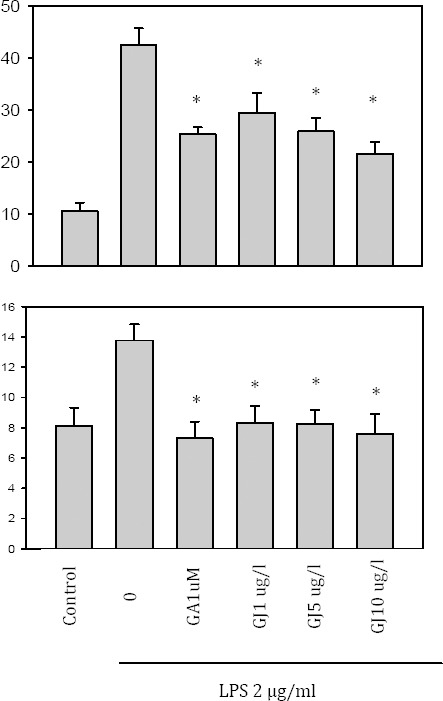
Effect of the Gardenia jasminoides (GJ) extract on cytokine production. BV-2 cells were treated with 2 μg/ml LPS in the presence of 1, 5, and 10 μg/ml of GJ extract or 1 μM GA. GJ dose-dependently decreased IL-1β and IL-6 productions significantly (P<0:05)
Effect of GJ extract and GA on LPS-induced PGE2 release
To investigate the effect of GJ extract and GA on inflammation, PGE2 release was measured in the LPS-induced BV-2 cell culture. The results showed that GJ decreased PGE2 release from BV-2 cells dose-dependently (P<0:05, Figure 3).
Figure 3.
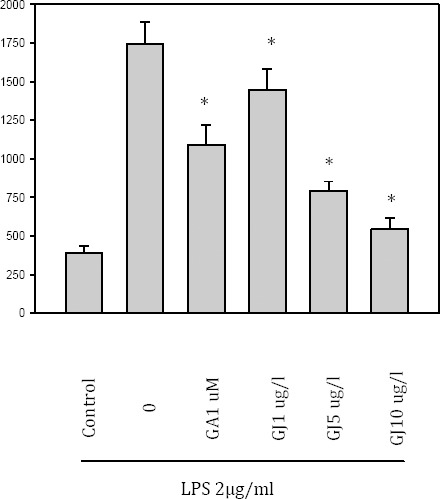
Effect of the Gardenia jasminoides (GJ) extract on prostaglandin E2 production PGE2 released from LPS-induced BV-2 cell culture supernatant, was measured by ELISA. PGE2 was dose-dependently inhibited by the GJ extract (P<0:05)
Effect of GJ extract and GA on ROS generation
To investigate the antioxidant activity of GJ extract and GA on ROS generation, BV-2 cells were treated with H2O2 (1 mM) alone or with the GJ extract and GA. The ROS generation was increased by H2O2, but the generation of ROS was dose-dependently scavenged by the GJ extract (Figure 4).
Figure 4.
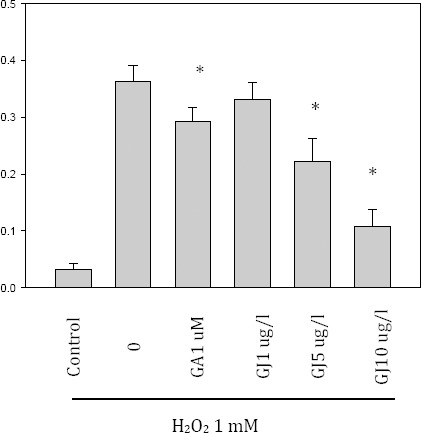
Effect of the Gardenia jasminoides (GJ) extract on reactive oxygen species (ROS) generation. BV-2 cells were treated with H2O2 (1 mM) alone or with the GJ extract and gallic acid (GA) for one hour. The ROS generation was increased by H2O2, but it was dose-dependently scavenged by the GJ extract
Effect of GJ extract and GA on hepatic enzyme activity of SD rats
Anti-inflammatory effect of the GJ extract and GA were further studied in an acute hepatic injury model of SD rats. Rats were pretreated with GA (1 or 10 mg/kg) or the GJ extract (10 or 30 mg/kg) for three days, and then injected with 50 μg/kg LPS to induce liver injury. Both GJ extract and GA supplementation decreased serum AST and ALT levels as compared to the LPS control (Figure 5).
Figure 5.
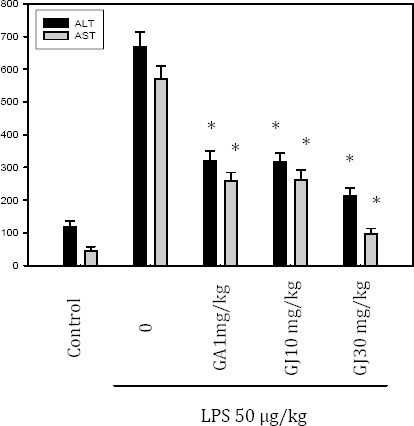
The anti-inflammatory effect of the Gardenia jasminoides (Gj) extract in an acute hepatic injury model. SD rats were pretreated with gallic acid (GA) (1 mg/kg) or the GJ extract (10 or 30 mg/kg) for three days, and then injected with 50 μg/kg LPS to induce liver injury. Both the GJ extract and GA supplementation decreased serum AST and ALT levels as compared to the LPS control
Effect of GJ extract and GA on LPS-induced MAPKs and COX-2 expression
The effect of GJ extract and GA on cell signaling was investigated both in vitro and in vivo. The GJ extract dose-dependently inhibited LPS-induced JNK, and p38 MAPK, and partially inhibited COX-2 in BV-2 cells. Similarly, the GJ extract inhibited LPS-induced p38 MAPK, and partially inhibited COX-2 in the livers of SD rats (Figure 6).
Figure 6.
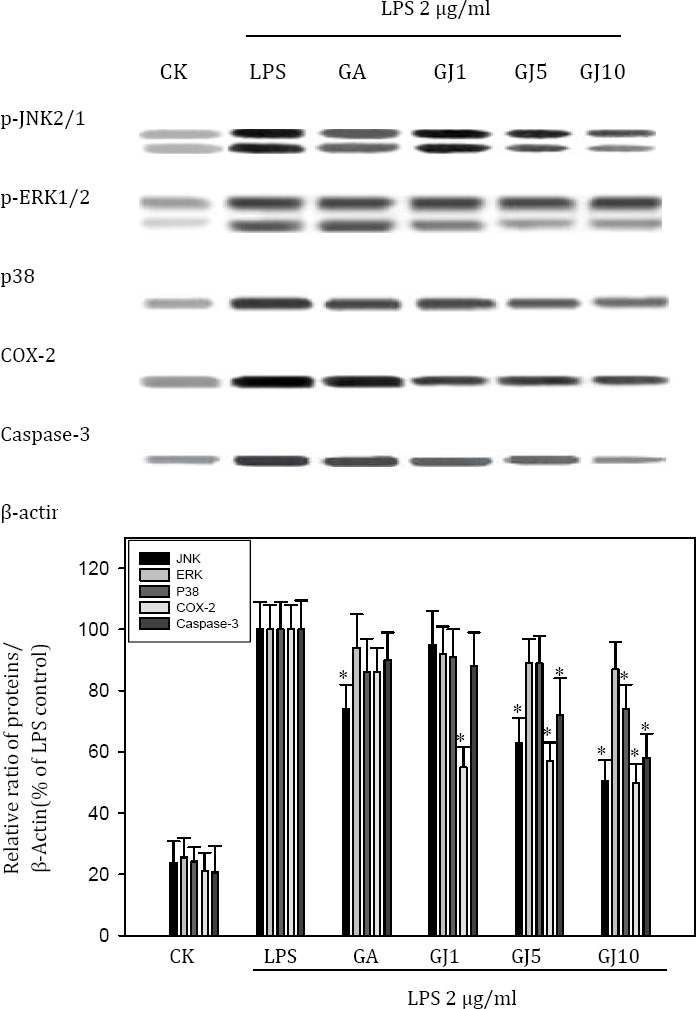
The effect of the Gardenia jasminoides (GJ) extract and gallic acid (GA) on cell signaling was investigated in BV-2 cells. The GJ extract dose-dependently inhibited LPS-induced JNK2/1, partially p38 MAPK, and inhibited COX-2 in BV-2 cells. GA (1 μM) only inhibited LPS-induced JNK2/1 MAPK partially
Effect of GJ extract and GA on LPS-induced hepatic injury of rats
To analyze the effects of GJ extract and GA on LPS-induced hepatic injury of rats, we also examined the liver pathology. Lesions and leukocyte infiltration were prominently induced in LPS-treated rats; however, this pathology was greatly reduced in the GJ extract and GA-treated rats (Figure 7).
Figure 7.

Protective effect of the Gardenia jasminoides (GJ) Extract and gallic acid (GA) on LPS-induced hepatic injury in SD rats. Liver slices from rats with no treatment (A, control) or treated with 50 μg/kg LPS IP (B), and supplemented with 10 mg/kg GA (C) and 30 mg/kg the GJ extract (D) showed the differences in liver pathology. Photographs show the liver slices from rats with 400 × magnification. Partial to extensive vacuolar degeneration of liver cells in LPS stimulated rats was significantly higher than the others, but, this pathology was reduced in the GJ extract and GA-treated rats
Discussion
Our results showed that the GJ extract and GA protected rats from LPS-induced injury. Hepatic lesions and leukocyte infiltration were prominently reduced by the GJ extract and GA treatment. In addition, the levels of AST and ALT were decreased by the GJ extract and GA significantly. This hepatoprotective effect was consistent with the effects of GJ extract on other reports such as concanavalin A, or carbon tetrachloride (CCl4), and hydrophilic bile acids[AGA8] activity (18-20).
Partial to extensive vacuolar degeneration of liver cells were prominently induced in LPS-treated rats. However, this pathology was greatly reduced in the GJ extract and GA-treated rats (Figure 7). The results of this study showed that the PR extract and resveratrol protected rats from LPS-induced injury. Hepatic lesions and leukocyte infiltration were prominently reduced by the GJ extract and GA treatment. In addition, the levels of AST and ALT were decreased by the PR extract and resveratrol significantly. Thus, this hepatoprotective effect is consistent with the effects of resveratrol on mice or rats subjected to CC14, or LPS, D-GaIN, and dimethylnitrosamine [AGA9](21, 22). Therefore, our result confirmed that LPS-induced MAPKs such as ERK (Extracellular signal-regulated kinases and p38 may also contribute to acute liver injury. Nevertheless, other studies had demonstrated that JNK and p38 MAPKs activation following liver ischemia and reperfusion are associated with the induction of apoptosis and necrosis, while inhibitors of p38 and JNK can reduce the liver damage (6, 22).
Our results clearly showed that the mechanism of hepatoprotective effect by the GJ extract was mainly due to the suppression of p38 MAPK and, to a lesser degree, of COX-2. During liver fibrosis formation, JNK and p38 might have opposite effects (23). It has been shown that chronic alcohol induces LPS-induced p38 and ERK1/2 activation, but decreases JNK in murine hepatic macrophages (24). Therefore, LPS-induced MAPKs such as ERK and p38 may also contribute to acute liver injury. The mechanism of the hepatitis virus in causing liver injury also involves the MAPKs pathway. Studies have shown that hepatitis B, C, and E viruses (HBV, HCV and HEV) modulate the MAPK signaling pathway (27). Studies have also shown that deregulated MAPKs are often found to contribute in the development of many cancers, including hepatocarcinoma (HCC). The early studies on the ERK pathway have led to the development of the multikinase inhibitor Sorafenib, the first effective systemic drug for the targeted treatment of human HCC. In addition, the functions and molecular mechanisms of JNK and p38 in HCC development have also been addressed using mouse models (28).
Our findings on the suppression of JNK2/1 MAPK by the GJ extract and GA was consistent with these hepatoprotective mechanisms. Geniposide (Gen), the extract of the GJ has been recently identified as a bioactive iridoid glucoside that has anti-inflammatory effect; in addition, it enhances the intestinal absorption, and protects against hyperlipidemia (29-31). However, other active components of the water extract of the GJ have not been identified. The researchers of the study state that GA may be used to treat inflammatory allergic diseases. GA is one rich compound of the GJ (0.16% fresh seeds, 0.7% dry seeds). Nevertheless, our present data showed that water extract of GJ and GA reduced LPS-induced NO, IL-1β, IL-6, ROS, and PEG2 production in BV-2 cells.
Conclusion
The GJ extract and GA reduced JNK2/1, and p38 MAPKs phosphorylation, and slightly reduced COX-2 expression in BV-2 cells in a dose-dependent manner. Antioxidant effects could also be shown in the LPS-induced liver injury in SD rats, since the JNK2/1 MAPK phosphorylation and COX-2 expression were similarly reduced by GJ extract and GA treatment.
Acknowledgment
We would like to thank Dr Robert H Glew (University of New Mexico, Albuquerque, New Mexico, USA) for his assistance in the preparation of this manuscript.
Footnotes
Conflict of interest
The authors have no financial conflict of interest. The results described in this paper were part of student thesis.
References
- 1.Lim W, Kim O, Jung J, Ko Y, Ha J. Dichloromethane fraction from Gardenia jasminoides : DNA topoisomerase 1 inhibition and oral cancer cell death induction. Pharm Biol. 2010;48:1354–1360. doi: 10.3109/13880209.2010.483246. [DOI] [PubMed] [Google Scholar]
- 2.Song X, Zhang W, Wang T, Jiang H, Zhang Z, Fu Y, Yang Z, Cao Y, Zhang N. Geniposide plays an anti-inflammatory role via regulating TLR4 and downstream signaling pathways in lipopoly-saccharide-induced mastitis in mice. Inflammation. 2014;37:1588–1598. doi: 10.1007/s10753-014-9885-2. [DOI] [PubMed] [Google Scholar]
- 3.Lee JH, Lee DU, Jeong CS. Gardenia jasminoides Ellis ethanol extract and its constituents reduce the risks of gastritis and reverse gastric lesions in rats. Food Chem Toxicol. 2009;47:1127–1131. doi: 10.1016/j.fct.2009.01.037. [DOI] [PubMed] [Google Scholar]
- 4.Dai MM, Wu H, Li H, Chen J, Chen JY, Hu SL, Shen C. Effects and mechanisms of Geniposide on rats with adjuvant arthritis. Int Immunopharmacol. 2014;20:46–53. doi: 10.1016/j.intimp.2014.02.021. [DOI] [PubMed] [Google Scholar]
- 5.Malhi H, Gores GJ. Cellular and molecular mechanisms of liver injury. Gastroenterology. 2008;134:1641–1654. doi: 10.1053/j.gastro.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chao J, Lee MS, Amagaya S, Liao JW, Wu JB, Ho LK, et al. Hepatoprotective effect of shidagonglao on acute liver injury induced by carbon tetrachloride. Am J Chin Med. 2009;37:1085–1097. doi: 10.1142/S0192415X0900751X. [DOI] [PubMed] [Google Scholar]
- 7.Mandrekar P, Szabo G. Signalling pathways in alcohol-induced liver inflammation. J Hepatol. 2009;50:1258–1266. doi: 10.1016/j.jhep.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kono H, Rusyn I, Yin M, Gabele E, Yamashina S, Dikalova A, et al. NADPH oxidase derived free radicals are key oxidants in alcohol-induced liver disease. J Clin Invest. 2000;106:867–872. doi: 10.1172/JCI9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sosa S, Balick MJ, Arvigo R, Esposito RG, Pizza C, Altinier G, et al. Screening of the topical anti-inflammatory activity of some Central American plants. J Ethnopharmacol. 2002;81:211–215. doi: 10.1016/s0378-8741(02)00080-6. [DOI] [PubMed] [Google Scholar]
- 10.Yam MF, Asmawi MZ, Basir R. An investigation of the anti-inflammatory and analgesic effects of orthosiphon stamineus leaf extract. J Med Food. 2008;11:362–368. doi: 10.1089/jmf.2006.065. [DOI] [PubMed] [Google Scholar]
- 11.Geppert TD, Whitehurst CE, Thompson P, Beutler B. Lipopolysaccharide signals activation of tumor necrosis factor biosynthesis through the ras/raf-1/MEK/MAPK pathway. Mol Med. 1994;1:93–103. [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang JX, Zhang Y, Ji SH, Zhu P, Wang ZG. Kinetics of mitogen-activated protein kinase family in lipopolysaccharide-stimulated mouse Kupffer cells and their role in cytokine production. Shock. 2002;18:336–341. doi: 10.1097/00024382-200210000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Poligone B, Baldwin AS. Positive and negative regulation of NF-kappaB by COX-2: roles of different prostaglandins. J Biol Chem. 2001;276:38658–38664. doi: 10.1074/jbc.M106599200. [DOI] [PubMed] [Google Scholar]
- 14.Kim SJ, Kim JK, Lee DU, Kwak JH, Lee SM. Genipin protects lipopolysaccharide-induced apoptotic liver damage in D-galactosamine-sensitized mice. Eur J Pharmacol. 2010;635:188–193. doi: 10.1016/j.ejphar.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Kim SJ, Kim KM, Park J, Kwak JH, Kim YS, Lee SM. Geniposidic acid protects against D-galactosamine and lipopolysaccharide-induced hepatic failure in mice. J Ethnopharmacol. 2013;146:271–277. doi: 10.1016/j.jep.2012.12.042. [DOI] [PubMed] [Google Scholar]
- 16.Kim SH, Jun CD, Suk K, Choi BJ, Lim H, et al. Gallic acid inhibits histamine release and pro-inflammatory cytokine production in mast cells. Toxicol Sci. 2006;91:123–131. doi: 10.1093/toxsci/kfj063. [DOI] [PubMed] [Google Scholar]
- 17.Zhu QY, Hackman RM, Ensunsa JL, Holt RR, Keen CL. Antioxidative activities of oolong tea. J Agric Food Chem. 2002;50:6929–6934. doi: 10.1021/jf0206163. [DOI] [PubMed] [Google Scholar]
- 18.Mase A, Makino B, Tsuchiya N, Yamamoto M, Kase Y, et al. Active ingredients of traditional Japanese (kampo) medicine, inchinkoto, in murine concanavalin A-induced hepatitis. J Ethnopharmacol. 2010;127:742–749. doi: 10.1016/j.jep.2009.11.029. [DOI] [PubMed] [Google Scholar]
- 19.Lee SJ, Oh PS, Lim KT. Hepatoprotective and hypolipidaemic effects of glycoprotein isolated from Gardenia jasminoides Ellis in mice. Clin Exp Pharmacol Physiol. 2006;33:925–933. doi: 10.1111/j.1440-1681.2006.04466.x. [DOI] [PubMed] [Google Scholar]
- 20.Lee TY, Chang HH, Chen JH, Hsueh ML, Kuo JJ. Herb medicine Yin-Chen-Hao-Tang ameliorates hepatic fibrosis in bile duct ligation rats. J Ethnopharmacol. 2007;109:318–324. doi: 10.1016/j.jep.2006.07.042. [DOI] [PubMed] [Google Scholar]
- 21.Fan G, Tang JJ, Bhadauria M, Nirala SK, Dai F, Zhou B, Li Y, Liu ZL. Resveratrol ameliorates carbon tetrachloride-induced acute liver injury in mice. Environ Toxicol Pharmacol. 2009;28:350–356. doi: 10.1016/j.etap.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 22.Farghali H, Cerný D, Kameníková L, Martínek J, Horínek A, Kmonícková E, Zídek Z. Resveratrol attenuates lipopolysaccharide-induced hepatitis in D-galactosamine sensitized rats: role of nitric oxide synthase 2 and heme oxygenase-1. Nitric Oxid. 2009;21:216–225. doi: 10.1016/j.niox.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Schnabl B, Bradham CA, Bennett BL, Manning AM, Stefanovic B, Brenner DA. TAK1/JNK and p38 have opposite effects on rat hepatic stellate cells. Hepatology. 2001;34:953–963. doi: 10.1053/jhep.2001.28790. [DOI] [PubMed] [Google Scholar]
- 24.Kishore R, Hill JR, Mcmullen MR, Frenkel J, Nagy LE. ERK1/2 and Egr-1 contribute to increased TNF-alpha production in rat Kupffer cells after chronic ethanol feeding. Am J Physiol Gastrointest Liver Physiol. 2002;282:G6–15. doi: 10.1152/ajpgi.00328.2001. [DOI] [PubMed] [Google Scholar]
- 25.King LA, Toledo AH, Rivera-Chavez FA, Toledo-Pereyra LH. Role of p38 and JNK in liver ischemia and reperfusion. J Hepatobiliary Pancreat Surg. 2009;16:763–770. doi: 10.1007/s00534-009-0155-x. [DOI] [PubMed] [Google Scholar]
- 26.Toledo-Pereyra LH, Lopez-Neblina F, Toledo AH. Protein kinases in organ ischemia and reperfusion. J Invest Surg. 2008;21:215–226. doi: 10.1080/08941930802130149. [DOI] [PubMed] [Google Scholar]
- 27.Panteva M, Korkaya H, Jameel S. Hepatitis viruses and the MAPK pathway: is this a survival strategy? Virus Res. 2003;92:131–140. doi: 10.1016/s0168-1702(02)00356-8. [DOI] [PubMed] [Google Scholar]
- 28.Min L, He B, Hui L. Mitogen-activated protein kinases in hepatocellular carcinoma development. Semin Cancer Biol. 2011;21:10–20. doi: 10.1016/j.semcancer.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 29.Fu Y, Liu B, Liu J, Liu Z, Liang D, et al. Geniposide, from Gardenia jasminoides Ellis, inhibits the inflammatory response in the primary mouse macrophages and mouse models. Int Immunopharmacol. 2012;14:792–798. doi: 10.1016/j.intimp.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 30.Chula S, Hang L, Yinying B, Jianning S, Shi R. The effects of notoginsenoside R₁ on the intestinal absorption of geniposide by the everted rat gut sac model. J Ethnopharmacol. 2012;142:136–143. doi: 10.1016/j.jep.2012.04.027. [DOI] [PubMed] [Google Scholar]
- 31.Kojima K, Shimada T, Nagareda Y, Watanabe M, Ishizaki J, Sai Y, et al. Preventive effect of geniposide on metabolic disease status in spontaneously obese type 2 diabetic mice and free fatty acid-treated HepG2 cells. Biol Pharm Bull. 2011;34:1613–1618. doi: 10.1248/bpb.34.1613. [DOI] [PubMed] [Google Scholar]


