Abstract
Objective(s):
The aim of this study was to evaluate the effects of Malva sylvestris aqueous extract on cutaneous wound healing in BALB/c mice.
Materials and Methods:
Twenty seven male BALB/c mice (2.5 months of age) were used. A cut wound (superficial fascia depth) was made locally. The mice were then divided into three groups: the first, second and third groups received topical administration of M. sylvestris 1% aqueous extract, silver sulfadiazine topical cream and cold cream (positive and negative control groups), respectively. On days 4, 7 and 10 excisional biopsies were performed and wound healing was evaluated histopathologically. The data were analyzed by the ANOVA and Tukey statistical tests.
Results:
On days 4 and 7, the numbers of inflammatory cells in the silver sulfadiazine and M. sylvestris-treated groups were significantly lower than the control group and keratinization at the edges of the wound in both groups was significantly higher than the control group. On the tenth day of the study, the Malva-treated mice showed better healing features and less fibrosis and scar formation, and also fewer hair follicles were damaged in this group. On the tenth day of the study, the numbers of inflammatory cells in M. sylvestris and silver sulfadiazine-treated groups were significantly lower than the control group.
Conclusion:
The present study supports the beneficial effects of M. sylvestris on the wound healing process and suggests a potential clinical application.
Keywords: Malva sylvestris, Mice, Skin, Wound healing
Introduction
The skin is the largest organ of the human body and plays a major role in maintaining homeostasis and protection (1). Maintaining skin integrity is critical for protection against loss of water, bleeding, infection with invasive microorganisms, and to regulate body temperature; as well as an undeniable role in beauty and psychological effects on people (2). Cutaneous wound healing occurs in a really complex process (2, 3). Wound healing takes place in three overlapping phases: inflammatory, proliferative and remodeling. In the inflammatory phase several types of inflammatory cells attend to the injury site and in addition to their phagocytic and anti-microbial activity, they play an important role in helping the wound healing process (2-5). The proliferative phase involves the creation of a permeability barrier (re-epithelization) as well as the establishment of an appropriate blood supply (neovascularization) and reinforcement of the injured tissue (fibroplasia) (2, 3). Remodeling, the third phase of wound healing, consists of deposition of matrix materials and their subsequent change over time (2, 3). Several studies have shown that natural and herbal medicines can affect wound healing (6-13).
Various kinds of topical ointments and creams have been used to treat wounds. However, with the interest in complementary medicine during the recent years, application of various oils and natural products has been proposed for treating burn wounds (14, 15). M. sylvestris, locally known as “Panirak” is an herbaceous, perennial plant. The most commonly used parts of the plant are flowers and leaves without petiole. The active ingredients include mucilage, tannins, malvyn and malvydyn (14, 16, 17). The plant leaves are also the richest in nutraceuticals such as powerful antioxidants (phenols, flavonoids, carotenoids, and tocopherols), unsaturated fatty acids (e.g. α-linolenic acid) and minerals measured in ash content (18). In various herbal references, several medical uses for M. sylvestris such as laxative, diuretic and anti-cough, are listed (14, 16). Anti-inflammatory, antimicrobial and antioxidant activities of this plant have been shown by many studies (19-21). The mixture of M. sylvestris with cetylpyridinium chloride demonstrated antimicrobial activity against 28 strains of Staphylococcus aureus (22). Edema induced by carrageenan and formalin was reduced to 60% in mice taking an aqueous extract of M. sylvestris (23). Moreover, methanolic extracts obtained from the leaves have very strong antioxidant properties and lipid peroxidation in liposomes and brain cell homogenates (18). These reports suggest that M. sylvestris is a good candidate for wound healing. However, there are few and somehow controversial studies concerning the effect of this drug on wound healing. In one study in 2010, Pirbalouti et al showed rat wounds treated with M. sylvestris extract had significant smaller sizes than other groups. Also, collagen fibers in the skin of rats in this treatment group were more organized than the other groups (21). On the other hand, Kovalik et al could not find any beneficial effect for M. sylvestris in the treatment of oral wounds in their study (24).
In the current study, we evaluated the effects of M. sylvestris aqueous extract on cutaneous wound healing in mice.
Materials and Methods
All procedures were approved by the Medical Ethics Committee of Mashhad University of Medical Sciences.
Plant material and extract preparation
To prepare the aqueous extract of M. sylvestris, the plants were collected from the suburb of Birjand city, Iran in the Summer of 2014 and then dried in the shade. A sample of this plant was confirmed by the resident botanist and a documented sample was kept in the Herbarium of Birjand University of Medical Sciences (code 324). The dried plant flowers were milled, powdered and then their aqueous extract was obtained, and dried samples were prepared with a freeze-dryer machine. Topical 1% cream was prepared and the dried aqueous extract was mixed with cold cream.
Experiment animals
In this study, 27 mature (2.5 months of age) male BALB/C mice were used. Mice were purchased from Pasteur Institute, Iran, and were housed in clean, individual cages and had free access to water and standard pellet diet. They were kept in standard environment of 12 hr light/dark cycle, 22±1°C temperature; the air humidity was 60±5%.
Experiment design and wound healing evaluation
First, mice were anesthetized with intraperitoneal injection of ketamine 70 mg/kg. Then, their dorsal skin was shaved. A cut wound with a length of 5 mm was induced locally measuring the depth of the superficial fascia using a surgical blade and toothed forceps (in order to have uniform depth in all wounds). The mice with wounds deeper than superficial fascia and involving the muscles were excluded from the study. The mice were then divided into three groups. To the first group’s wounds, M. sylvestris aqueous extract was applied and the second group as the positive control, received silver sulfadiazine topical cream 1%; the third group received cold cream, as the negative control group. All groups received the treatment twice daily at 8:00 am and 8:00 pm; equal number of mice from each group were selected on days 4, 7 and 10 (10). Excisional biopsies were taken from the back skin and were fixed in 10% formaldehyde solution and then samples were prepared for tissue processing. After preparing the tissue slides, 10 slides from each sample were randomly selected, and were stained with Hematoxylin and Eosin and also with the Trichrome Masson staining protocol. Photos were taken from all slides using a camera-equipped microscope. Finally, two pathologists that were not aware of the slide classifications evaluated the slides and reported the results.
Data analysis
The mean of the values and standard deviations were used to describe the quantitative data, and frequency index was used for qualitative data. One-way ANOVA test was used to compare the variables in groups with each other, and in case of significant results, Tukey test was used. Data analysis was done using SPSS statistical software (ver.16), and data were considered significant at P-values ≤ 0.05.
Results
Sample survey on day 4
The numbers of inflammatory cells, poly morphonuclear (PMN) and mononuclear (MN) cells in the silver sulfadiazine and M. sylvestris treated groups were significantly lower than the control group (P-value<0.0001). However, the types of inflammatory cells did not show significant differences in the three groups (Table 1). Edema was slightly higher in the negative control group than in the other groups. The study of connective tissue evolution showed some granulation tissue formation in the M. sylvestris treated group. Also, this group did not have any fibrous tissue on the 4th day. The amount of granulation tissue in the silver sulfadiazine-treated group was similar to the M. sylvestris group. However, the negative control group did not show signs of granulation tissue formation on this day. Keratinization at the edges of the wound in the M. sylvestris treated group was significantly higher than the control group (P-value=0.01) (Table 1).
Table 1.
Wound healing parameters in three groups on day 4
| P-value ¥ | Control group (cold cream) | Silver sulfadiazine topical cream | Malva sylvestris aqueous extract | ||
|---|---|---|---|---|---|
| <0.0001 | 80±10.5 | 44.8± 8.5 | 40.4± 7* | Number of inflammatory cell (Cell/HPF) | Inflammatory activity |
| - | 93% | 87% | 85% | Type of inflammatory cell (PMN %) | |
| - | 7% | 13% | 15% | Type of inflammatory cell (MN %) | |
| - | +++/++ | ++ | ++ | Edema | |
| - | + | + | + | Fibroblast density | Connective tissue repair |
| - | 100% | 100% | 100% | Fibroblast activity (%) | |
| - | 100% | 100% | 100% | Fibroblast morphology (% of flump or active cells) | |
| - | - | - | - | Collagen synthesis | |
| - | 0 | 0 | 0 | Collagen fibers thickness (micrometer) | |
| - | -/+ | -/+ | -/+ | Granulation tissue formation | |
| - | damaged | damaged | damaged | Basal layer | Epithelial tissue repair |
| - | undetectable | undetectable | undetectable | Scar formation | |
| - | 0 | 0 | 0 | Number of wound surface epidermal layers | |
| 0.01 | 1±0.2 layer | 1±0.3 layer | 2±0.5 layer | Wound edge keratinization | |
| - | Some necrotic follicles | Some necrotic follicles | Some necrotic follicles | Hair follicles in the scar tissue | |
| 0.68 | 19±3.1 | 18±4.5 | 20±2.7* | Hair follicles in the wound edge |
Mean ± SD¥ Anova and Tukey tests; - low, -/+ low to mild, +/- very mild, + mild, ++ mild to moderate, +++ moderate, ++++ severe
Sample survey on day 7
On the seventh day of the study, in all groups epithelial lining met both sides of the wound (Figure 1: D, E and F). The numbers of inflammatory cells (PMN and MN) in the M. sylvestris and silver sulfadiazine-treated groups were significantly lower than the control group (P-value<0.0001), although there was no significant difference between these two groups (P-value=0.13). Edema was mild in the M. sylvestris group compared to a moderate edema in the other groups (Table 2).
Figure 1.
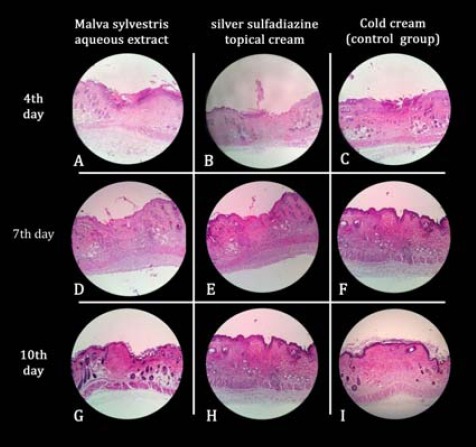
Microscopic pictures of wound healing processes in Malva sylvestris (A, D and G), silver sulfodiazinone (B, E and H) and cold cream (C, F and I) on days 4, 7 and 10 of this study based on hematoxylin and eosin (H& E) stained slides (40x)
Table 2.
Wound healing parameters in three groups on day 7
| P-value ¥ | Control group (cold cream) | Silver sulfadiazine topical cream | Malva sylvestris aqueous extract | ||
|---|---|---|---|---|---|
| <0.0001 | 70.1±9.9 | 41± 10.5 | 32 ± 8* | Number of inflammatory cell (Cell/HPF) | Inflammatory activity |
| - | 40% | 22% | 20% | Type of inflammatory cell (PMN %) | |
| - | 60% | 78% | 80% | Type of inflammatory cell (MN %) | |
| - | ++ | ++ | + | Edema | |
| - | ++ | ++/+++ | +++ | Fibroblast density | Connective tissue repair |
| 0.67 | 97% | 92% | 90% | Fibroblast activity (%) | |
| - | 97% | 92% | 90% | Fibroblast morphology (% of flump or active cells) | |
| - | - | + | + | Collagen synthesis | |
| <0.0001 | 0 | 8.2 ± 1.6 | 10.1±2.6 | Collagen fibers thickness (micrometer) | |
| - | ++ | +++/++ | +++ | Granulation tissue formation | |
| - | Completely formed (with mild edema of the basal layer) | Completely formed (with mild edema of the basal layer) | Completely formed (with mild edema of the basal layer) | Basal layer | Epithelial tissue repair |
| - | undetectable | undetectable | undetectable | Scar formation | |
| 0.007 | 3± 1.6 | 4± 2 | 6± 2.2* | Number of wound surface epidermal layers | |
| 0.008 | 1.9±0.8 layer | 2.1±1 layer | 3.9±1.1* layer | Wound edge keratinization | |
| - | Not seen | Not seen | Not seen | Hair follicles in the scar tissue | |
| 0.54 | 18.9± 7.3 | 20.9±4 | 21.7±5.6* | Hair follicles in the wound edge |
* Mean ± SD¥ ANOVA and Tukey tests; - low, -/+ low to mild, +/- very mild, + mild, ++ mild to moderate, +++ moderate, ++++ severe
Our evaluation showed that the collagen fibers had begun to form in the M. sylvestris and silver sulfadiazine treated groups in this day, but there was no sign of collagen formation in the control group. The thickness of collagen fibers was not significantly different between the M. sylvestris and Silver sulfadiazine treated groups (P-value=0.063). The wound surface of epidermal layers in the M. sylvestris and silver sulfadiazine treated groups was significantly higher than the control group (P-value=0.007), with no significant difference between these two groups (Table 2).
Sample survey on day 10
The sample survey on the tenth day of the study showed better healing features and less fibrosis and scar formation in the M. sylvestris-treated mice, also less hair follicles were damaged in this group, and PMN cells were rarely seen (Figure 1: G). The silver sulfadiazine-treated group showed moderate scar formation and a slight damage to hair follicles was detectable (Table 3). PMN cells were rarely seen (Figure 1: H).
Table 3.
Wound healing parameters in three groups on day 10
| P-value ¥ | Control group (cold cream) | Silver sulfadiazine topical cream | Malva sylvestris aqueous extract | ||
|---|---|---|---|---|---|
| <0.0001 | 40.1± 9.4 | 8.1± 4.2 | 4.9±2.3* | Number of inflammatory cell (Cell/HPF) | Inflammatory activity |
| - | 13.7% | 1.9% | 1.1% | Type of inflammatory cell (PMN %) | |
| - | 86.3% | 98.1% | 98.9% | Type of inflammatory cell (MN %) | |
| - | + | -/+ | +/- | Edema | |
| - | ++/+++ | ++++/+++ | ++++ | Fibroblast density | Connective tissue repair |
| <0.0001 | 90% | 30% | 5% | Fibroblast activity (%) | |
| 90% | 30.% | 5%* | Fibroblast morphology (% of flump or active cells) | ||
| - | + | +++/++ | +++ | Collagen synthesis | |
| <0.0001 | 9.1±3.7 | 19.8±7 | 19.9±6.1* | Collagen fibers thickness (micrometer) | |
| - | ++ | -/+ | +/- | Granulation tissue formation | |
| - | Completely formed (with mild edema of the basal layer) | Completely formed (without edema of the basal layer) | Completely formed (without edema of the basal layer) | Basal layer | Epithelial tissue repair |
| - | Scar tissue has not yet formed | Moderate scar formation | Mild scar formation | Scar formation | |
| 0.65 | 4±2.3 | 4.1±2.4 | 5±2.3* | Number of wound surface epidermal layers | |
| 0.018 | 3.1±2.6 layer | 4.1±2.4 layer | 6.1±2.6* layer | Wound edge keratinization | |
| - | Not seen | Not seen | Not seen | Hair follicles in the scar tissue | |
| 0.025 | 18.2±5.6 | 23.7±7.5 | 27.3±7.7* | Hair follicles in the wound edge |
Mean ± SD ¥ ANOVA and Tukey tests; - low, -/+ low to mild, +/- very mild, + mild, ++ mild to moderate, +++ moderate, ++++ severe
Finally in the control group which only received cold cream, wound healing process was delayed in comparison with the other two groups. Larger scar formation was seen in this group and mononuclear inflammatory cells were still present in the tissue samples (Tables 3). The numbers of inflammatory cells and the percentage of PMN cells in the M. sylvestris and silver sulfadiazine treated groups on the tenth day of the study were significantly lower than the control group (P-value<0.0001 in both), with no significant difference between these two groups (P-value=0.48). On this day, the majority of fibroblast cells in the M. sylvestris-treated mice were fusiform (inactive), and collagen synthesis in this group was more than the other two groups. The thickness of the collagen fibers in the M. sylvestris and silver sulfadiazine-treated groups was almost similar (P-value=0.9). However, both had a higher thickness than the control group (Figure 2), (P-value<0.0001). On the last day of the study, the number of epidermal layers on the wound surface did not differ significantly in the studied groups (P-value=0.65). However, wound edge keratinization in the M. sylvestris and silver sulfadiazine-treated groups was significantly higher than the control group (P-value= 0.018).
Figure 2.
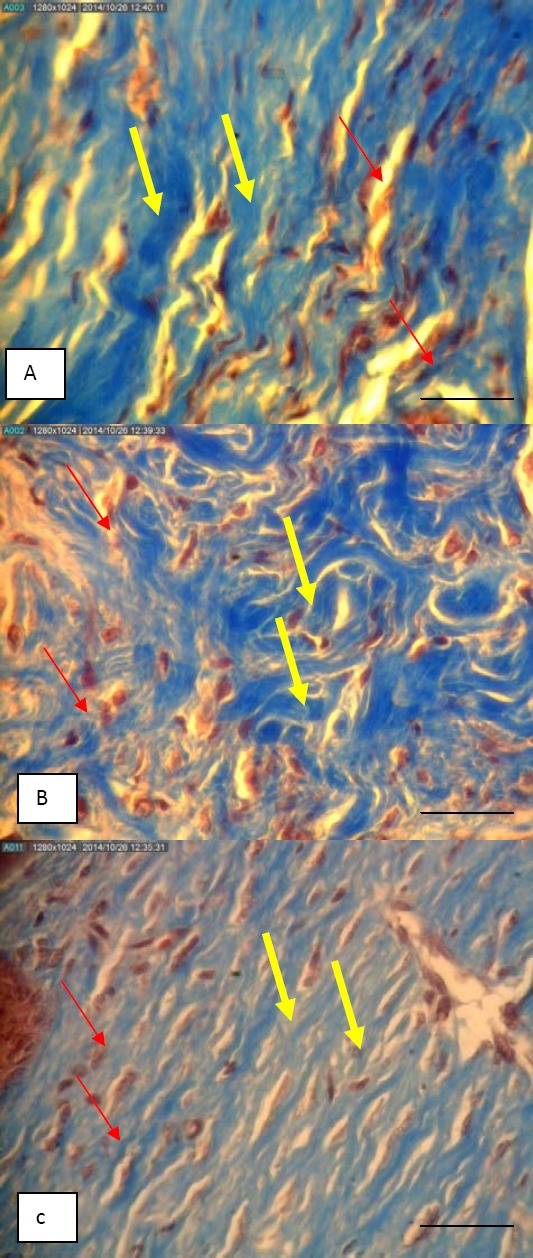
Microscopic pictures of derm skin of mice that showed thickness of collagen fiber and color density in the Malva sylvestris group (A), silver sulfadiazine group (B) and cold cream group (C) on day 10 (Trichrome masson staining, X400); yellow arrow: collagen fibers, red arrow: fibroblast cells; bar = 50 µm
Discussion
From the beginning of the study (on day 4) there was less inflammation in the M. sylvestris-treated mice than other groups. Although later in the study (on days 7 and 10) this group had statistically the same amount of inflammation as the silver sulfadiazine-treated group. Several studies have shown the anti-inflammatory effects of M. sylvestris extract (20, 22, 23, 25, 26). In the studies of Pirbalouti et al, the amount of inflammatory cell infiltration in the M. sylvestris-treated group was lower than the other groups which is similar to our results (15, 20, 21). There is also another study supporting the anti-inflammatory effects of M. sylvestris extract which indicates a strong anti-inflammatory activity of this plant (23). In this study, the effect of M. sylvestris was tested in mice taking a 100 mg/kg oral dose of the aqueous extract. Edema induced by carrageenan and formalin was decreased to 60% in both the chronic and acute inflammation models. Chiclanda et al showed that Malva cream significantly inhibited the edema that was induced by carrageenan. This effect was higher than what was acquired with a cream containing 2% indomethacin (as a positive control), a potent nonselective inhibitor of cyclooxygenase-2(27). These data have confirmed the topical anti-inflammatory effects of this plant.
Using the M. sylvestris aqueous extract in our study caused improvement of the wound healing process, connective tissue formation and re-epithelization. On days 4 and 7, the amount of granulation tissue in the group treated with this plant was more than the other two groups. In fact, the granulation tissue formation had started earlier in the M. sylvestris treated group, thus, there was less of this tissue available on the tenth day and more than 95% of it changed to fibrous tissue. Also, collagen synthesis in this group was more than the control group but did not differ significantly with the silver sulfadiazine-treated group. However, the studies done on the effects of this plant on the wound healing process have had conflicting results, but most of them support the effectiveness of this plant (15, 18, 20, 23). In Pirbalouti et al study, histopathological survey on days 9 and 18 of wound healing, showed more organized collagen fibers and fibroblasts and less inflammation in the M. sylvestris treated group (20).
Some of the studies supported the idea of further wound size reduction in the early days when using the M. sylvestris extract (20, 21). However, some other studies such as the Kovalik et al study do not support the wound healing activity of this plant (24). Some of the beneficial effects of this plant on wound healing could be caused by its antimicrobial and antioxidant activities. In Zare et al study, all forms of M. sylvestris extracts had antimicrobial activity against Staphylococcus aureus. aureus and Pseudomonas aeruginosa (28). Watanabe et al showed that mouth washes based on cetylpyridinium chloride (CPC) mixed with M. sylvestris extract displayed stronger antimicrobial activities than those containing only CPC. This mixture showed antimicrobial activity against 28 strains of S. aureus, while mouth washes containing only CPC showed antimicrobial activity against only three strains (22). The antioxidant capacity of M. sylvestris has also been confirmed in different investigations. Methanolic extracts of flowers and leaves were evaluated by several different assays and models, including diphenyl-2-picrylhydrazyl radical absorption neutralization of linoleate free radicals and beta-carotene models. All parts of this plant were shown to have antioxidant activity (13, 29). As mentioned before, therapeutic parts of M. sylvestris (leaves and flowers) contain known powerful antioxidants, antimicrobial and strong anti-inflammatory effects. These effects may be responsible for the plant’s beneficial effects on the wound healing process (15, 20, 22, 23, 26).
Conclusion
The results of the present study support the beneficial effects of this plant on wound healing process and its potential clinical applications.
The authors of this study recommend the evaluation of all effective ingredients of this plant on wound healing process and using the most effective compounds in wound healing topical treatments.
Acknowledgment
This study is part of a medical student thesis and has been funded by a joint research grant (code: 920483) between Mashhad University of Medical Sciences, Mashhad, Iran and Birjand University of Medical Sciences, Birjand, Iran. The authors are thankful to Mr seyyed Amirreza Mohajeri and MR Seyyed Amin Nabavi for their technical support.
References
- 1.Kahan V, Andersen M, Tomimori J, Tufik S. Stress, immunity and skin collagen integrity: evidence from animal models and clinical conditions. Brain Behav Immun. 2009;23:1089–1095. doi: 10.1016/j.bbi.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Bolognia JL, Jorizzo JL, Rapini R. Mosby Elsevier; 2008. Dermatology. [Google Scholar]
- 3.Pakyari M, Farrokhi A, Maharlooei MK, Ghahary A. Critical role of transforming growth factor beta in different phases of wound healing. Adv Wound Care. 2013;2:215–224. doi: 10.1089/wound.2012.0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin P. Wound healing--aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 5.Baum CL, Arpey CJ. Normal Cutaneous Wound Healing: Clinical Correlation with Cellular and Molecular Events. Dermatol Surg. 2005;31:674–686. doi: 10.1111/j.1524-4725.2005.31612. [DOI] [PubMed] [Google Scholar]
- 6.Adikwu M, Attama A, Akah P. Natural products in wound healing. Ethnopharmacology. 2008:87–100. [Google Scholar]
- 7.Fatima Ad Modolo LV, Conegero Sanches AC, Porto RR. Wound healing agents: the role of natural and non-natural products in drug development. Mini Rev Med Chem. 2008;8:879–888. doi: 10.2174/138955708785132738. [DOI] [PubMed] [Google Scholar]
- 8.Kumar B, Vijayakumar M, Govindarajan R, Pushpangadan P. Ethnopharmacological approaches to wound healing-exploring medicinal plants of India. J Ethnopharmacol. 2007;114:103–113. doi: 10.1016/j.jep.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 9.Davis SC, Perez R. Cosmeceuticals and natural products: wound healing. Clin Dermatol. 2009;27:502–506. doi: 10.1016/j.clindermatol.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 10.Ghaderi R, Afshar M, Akhbarie H, Golalipour MJ. Comparison of the efficacy of honey and animal oil in accelerating healing of full thickness wound of mice skin. Int J Morphol. 2010;28:193–198. [Google Scholar]
- 11.Conforti F, Sosa S, Marrelli M, Menichini F, Statti GA, Uzunov D, et al. In vivo anti-inflammatory and in vitro antioxidant activities of Mediterranean dietary plants. J Ethnopharmacol. 2008;116:144–151. doi: 10.1016/j.jep.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 12.Villegas LF, Fernandez ID, Maldonado H, Torres R, Zavaleta A, Vaisberg AJ, et al. Evaluation of the wound-healing activity of selected traditional medicinal plants from Peru. J Ethnopharmacol. 1997;55:193–200. doi: 10.1016/s0378-8741(96)01500-0. [DOI] [PubMed] [Google Scholar]
- 13.Miraldi E, Ferri S, Mostaghimi V. Botanical drugs and preparations in the traditional medicine of West Azerbaijan (Iran) J Ethnopharmacol. 2001;75:77–87. doi: 10.1016/s0378-8741(00)00381-0. [DOI] [PubMed] [Google Scholar]
- 14.Emami AM. 2nd ed. Tehran: Iran University of Medical Sciences; 2010. Handbook of pharmaceutical Herbs. [Google Scholar]
- 15.Pirbalouti AG, Yousefi M, Nazari H, Karimi I, Koohpayeh A. Evaluation of burn healing properties of Arnebia euchroma and Malva sylvestris. Electron J Biol. 2009;5:62–66. [Google Scholar]
- 16.Emami A. 2nd ed. Mashhad: Mashhad University of Medical Sciences; 2011. Botanical Medicine. [Google Scholar]
- 17.Tomoda M, Gonda R, Shimizu N, Yamada H. Plant mucilages. XLII. An anti-complementary mucilage from the leaves of Malva sylvestris var. mauritiana. Chem Pharm Bull. 1989;37:3029–3032. doi: 10.1248/cpb.37.3029. [DOI] [PubMed] [Google Scholar]
- 18.Barros L, Carvalho AM, Ferreira IC. Leaves, flowers, immature fruits and leafy flowered stems of Malva sylvestris: a comparative study of the nutraceutical potential and composition. Food Chem Toxicol. 2010;48:1466–1472. doi: 10.1016/j.fct.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 19.Billeter M, Meier B, Sticher O. 8-hydroxyflavonoid glucuronides from Malva sylvestris. Phytochem. 1991;30:987–990. [Google Scholar]
- 20.Pirbalouti AG, Azizi S, Koohpayeh A, Hamedi B. Wound healing activity of Malva sylvestris and Punica granatum in alloxan-induced diabetic rats. Acta Pol Pharm. 2010;67:511–516. [PubMed] [Google Scholar]
- 21.Pirbalouti AG, Koohpyeh A. Wound healing activity of extracts of Malva sylvestris and Stachys lavandulifolia. Int J Biol. 2010;3:174. [Google Scholar]
- 22.Watanabe E, Tanomaru JM, Nascimento AP, Matoba-Junior F, Tanomaru-Filho M, Yoko Ito I. Determination of the maximum inhibitory dilution of cetylpyridinium chloride-based mouthwashes against Staphylococcus aureus: an in vitro study. J Appl Oral Sci. 2008;16:275–279. doi: 10.1590/S1678-77572008000400009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sleiman N, Daher C. Malva sylvestris water extract: a potential anti-inflammatory and anti-ulcerogenic remedy. Planta Med. 2009;75:PH10. [Google Scholar]
- 24.Kovalik AC, Bisetto P, Pochapski MT, Campagnoli EB, Pilatti GL, Santos FA. Effects of an orabase formulation with ethanolic extract of Malva sylvestris L. in oral wound healing in rats. J Med Food. 2014;17:618–624. doi: 10.1089/jmf.2013.0001. [DOI] [PubMed] [Google Scholar]
- 25.Cheng C, Wang Z. Bacteriostasic activity of anthocyanin of Malva sylvestris. J For Res. 2006;17:83–85. [Google Scholar]
- 26.Yousefi M. Malva Sylvestris in the treatment of hand eczema. Iran J Dermatol. 2010;13:131–134. [Google Scholar]
- 27.Chiclana CF, Enrique A, Consolini A. antiinflammatory activity of Malva sylvestris L (Malvaceae) on carragenin-induced edema in rats. Lat Am J Pharm. 2009;28:275–278. [Google Scholar]
- 28.Zare P, Mahmoudi R, Shadfar S, Ehsani A, Afrazeh Y, Saeedan A, et al. Efficacy of chloroform, ethanol and water extracts of medicinal plants, Malva sylvestris and Malva neglecta on some bacterial and fungal contaminants of wound infections. J Med Plants Res. 2012;6:4550–4552. [Google Scholar]
- 29.Ugurlu E, Secmen O. Medicinal plants popularly used in the villages of Yunt Mountain (Manisa-Turkey) Fitoterapia. 2008;79:126–131. doi: 10.1016/j.fitote.2007.07.016. [DOI] [PubMed] [Google Scholar]


