Abstract
Objective
U. urealyticum biovar 2 (UU-2) but not U. parvum (UP) has been associated with non-gonococcal urethritis (NGU), but little is known about species-specific responses to standard therapies. We examined species-specific treatment outcomes and followed men with treatment failure for 9 weeks.
Methods
From May 2007-July 2011, men aged ≥16 attending an STD clinic in Seattle, Washington with NGU (urethral discharge or urethral symptoms plus ≥5 PMNs/HPF) were enrolled in a double-blind, randomized trial. Participants received active azithromycin (1g) + placebo doxycycline or active doxycycline (100mg bid × 7d) + placebo azithromycin. Ureaplasmas were detected in culture followed by species-specific PCR. Outcomes were assessed at 3, 6, and 9 weeks. At 3 weeks, men with persistent Ureaplasmas received “reverse therapy” (e.g., active doxycycline if they first received active azithromycin). At 6 weeks, persistently-positive men received moxifloxacin (400mg × 7d).
Results
Of 490 men, 107 (22%) and 60 (12%) were infected with UU-2 and UP, respectively, and returned at 3 weeks. Persistent infection was similar for UU-2-infected men initially treated with azithromycin or doxycycline (25% vs. 31%, P=0.53), but differed somewhat for men with UP (45% vs. 24%; P=0.11). At 6 weeks, 57% of UU-2-infected and 63% of UP-infected men who received both drugs had persistent infection. Failure after moxifloxacin occurred in 30% and 36%, respectively. Persistent detection of UU-2 or UP was not associated with signs/symptoms of NGU.
Conclusion
Persistent infection after treatment with doxycycline, azithromycin, and moxifloxacin was common for UU and UP, but not associated with persistent urethritis.
Keywords: Ureaplasma, urethritis, treatment
INTRODUCTION
Nongonococcal urethritis (NGU) is a common syndrome among male patients attending sexually transmitted disease (STD) clinics. Several organisms have been implicated in the etiology of NGU; Chlamydia trachomatis and Mycoplasma genitalium account for 15-40% and 15-25% of all NGU cases, respectively; Trichomonas vaginalis, herpes simplex virus and adenovirus are also responsible for a small proportion of cases [1]. Phylogenetic analyses have demonstrated that Ureaplasma urealyticum, a long-suspected causative agent of NGU, is actually two distinct species: U. urealyticum biovar 2 (UU-2) and U. parvum (UP) [2, 3]. Studies differentiating between these two species have found that UU-2 is associated with 16%-26% of NGU cases [4-6], though this is not always the case [7]. UP has not been associated with NGU [4-6], but has been associated with preterm birth [8] and an increased intrauterine inflammatory response [9] suggesting that it may be an important female reproductive tract pathogen.
Standard therapy for men with NGU consists of either seven days of doxycycline (100mg twice daily) or a single 1g dose of azithromycin [1]. These two therapies were similarly efficacious in the treatment of undifferentiated U. urealyticum in a trial of men with NGU conducted in the mid-1990s [10], but no studies have prospectively assessed the efficacy of these therapies separately for differentiated Ureaplasmas. In clinical isolates obtained from women, the two species had different doxycycline resistance profiles [11, 12] suggesting that UU-2 and UP may respond differently to antimicrobial agents. Whether these differential susceptibilities translate to clinical outcomes and eradication of organisms in other settings remains unknown.
In our recent randomized trial of men with NGU [13], microbiologic cure rates (eradication of the organism) were not significantly different for UU-2-infected men treated with azithromycin or doxycycline. However, we did not evaluate the efficacy of these two therapies for UP, nor did we assess treatment outcomes among men who received additional antimicrobials after initial treatment failure. In the current study, we sought to: (1) compare the efficacy of azithromycin versus doxycycline in persistently positive men with UU-2 and UP; and (2) determine if persistence of UU-2 was associated with persisting clinical signs and symptoms of NGU.
METHODS
Study Design and Population
Details of the study design, population, and data collection methods have been previously described [13]. Briefly, from January 2007 to July 2011, men presenting to a Seattle, WA STD clinic were recruited into a double-blind, randomized treatment trial for NGU. Eligible participants had NGU, defined as visible urethral discharge or ≥5 polymorphonuclear leukocytes (PMNs) per high-powered field (HPF), were ≥16 years of age, and reported no antibiotic use in the previous month. Men were randomized 1:1 to receive one of two pre-packaged treatments: (1) doxycycline, 100 mg administered orally twice daily for 7 days and azithromycin placebo, single dose (two or four tablets formulated to look identical to 1g azithromycin), administered orally; or (2) azithromycin, 1g as a single dose (two 500mg or four 250mg tablets), administered orally and doxycycline placebo administered orally twice daily for 7 days (14 capsules formulated to look identical to the active doxycycline capsules). Clinical and sexual history data collected at enrollment were obtained by a single study clinician (M.S.L.). A computer assisted self-interview (CASI) collected additional demographic and behavioral data.
At enrollment, all participants were tested for M. genitalium, C. trachomatis, T. vaginalis, and, beginning in May 2007, for Ureaplasma spp. All microbiologic tests were performed on first-void urine. We used the APTIMA transcription-mediated amplification (TMA) assay to detect C. trachomatis and analyte-specific reagents on the same platform for T. vaginalis (GenProbe, Inc., San Diego, CA). M. genitalium was assessed by in-house polymerase chain reaction (PCR) [14]. Ureaplasmas were detected by a color change in selective broth medium: 0.5 ml fresh urine was inoculated into 4.5ml broth [15] and observed for up to one week [16]. Viable Ureaplasmas could not be recovered after exhaustion of urea from the medium; therefore, Ureaplasma cultures were accomplished by monitoring of growth in serial 10-fold dilutions inoculated from an aliquot of frozen (−80°C) urine. Cultures in the dilution tube that were starting to turn color plus the next higher dilution tube were combined and frozen. After follow-up was completed, species-specific PCR assays were applied to recovered Ureaplasma cultures to identify UU-2 and UP [6] and for the minimum inhibitory concentration (MIC) assays.
Men infected with Ureaplasmas at enrollment returned for up to three additional follow-up visits, each of which included a clinical exam, repeat specimen testing, and completion of a follow-up CASI to obtain sexual behavior data. The first follow-up visit was scheduled three weeks post-enrollment (allowable window=2-5 weeks). At this 3-week follow-up visit, men with recurrent/persistent NGU or a repeat positive Ureaplasma culture received “reverse therapy”: men initially randomized to active azithromycin/placebo doxycycline were given active doxycycline/placebo azithromycin and vice versa. All men were scheduled for an additional 6-week follow-up visit (allowable window=2-5 weeks), at which men with recurrent/persistent NGU or a repeat positive Ureaplasma culture received moxifloxacin (400mg/day for 7 days). Men receiving moxifloxacin were scheduled for a final test-of-cure visit three weeks later (9 weeks post-enrollment; allowable window=2-5 weeks). Any men with persistent detection of Ureaplasmas after the 9 week visit were followed under clinical standard of care.
We defined microbiologic treatment failure as a positive Ureaplasma culture 3 weeks after therapy that was subsequently confirmed via PCR as the same species that was present at enrollment. Clinical treatment failure was defined as self-reported urethral symptoms (dysuria, discharge, itching, tingling) and ≥5 PMNs/HPF or visible urethral discharge at the clinic visit 3 weeks following the receipt of therapy.
Antimicrobial susceptibility testing was performed in triplicate on Ureaplasma culture-positive isolates collected at enrollment from June 2007 to May 2008. The MIC of each antibiotic was determined by the agar dilution method using a Steers replicator to inoculate agar plates with logarithmically growing Ureaplasma cultures (revived from frozen aliquots). MICs were defined as the concentration of antibiotic that inhibited growth by 99%. Two-fold dilutions of antibiotics tested ranged from 0.065μg/ml – 8.0μg/mL for azithromycin, doxycycline and moxifloxacin.
The analytic sample includes men who met a more stringent definition of NGU (self-reported urethral symptoms or clinical signs of visible urethral discharge PLUS ≥5 PMNs/HPF), who tested positive for UU-2 or UP at enrollment, and who returned for at least the 3-week follow-up visit. Men who were positive for both species were included in each species-specific analysis. We excluded men with positive Ureaplasma cultures but negative species-specific PCR tests for both UU-2 and UP.
Statistical Analysis
We summarized demographic, behavioral, and clinical characteristics by infecting organism at enrollment (UU-2 or UP). Statistically significant differences in characteristics among men with UU-2 versus UP were assessed with Pearson's chi-square tests for categorical variables and t-tests for continuous variables.
To compare the efficacy of azithromycin and doxycycline for each species, we used Fisher's exact test to test for significant differences in the proportion of men who experienced microbiologic failure by therapy received, at each follow-up visit. We also evaluated these associations in two sub-populations: men who denied any unprotected sex between follow-up visits and men who had no co-infections at enrollment (i.e., negative tests at enrollment for M. genitalium, C. trachomatis, T. vaginalis and UU-2 or UP – depending on the baseline infection).
To examine the association between clinical and microbiologic outcomes, we used Fisher's exact test to compare the proportion of men who experienced clinical treatment failure among those who experienced microbiologic treatment failure or cure. We examined this association for each follow-up visit among all men, among men who denied unprotected sex between follow-up visits, among men without co-infections at enrollment, and among men with <10 lifetime vaginal partners, since the association between UU-2 and NGU was previously found to be the strongest in this sub-group [5].
For susceptibility testing, we report the MIC value from the first of the three assays. Isolates from men who were infected with both UU-2 and UP were excluded, since MIC values from positive cultures of these men could not be attributed to one species.
All analyses were performed using Stata statistical software (Version 13.0; StataCorp, College Station, TX). Two-sided tests were performed at a significance level (α) of 0.05. All study procedures and analyses were approved by the University of Washington Institutional Review Board.
RESULTS
A total of 606 men with NGU were enrolled in the parent trial, of whom 567 (94%) were tested for Ureaplasmas. Eighty-six percent (490 of 567) met the revised definition of NGU. Of these, 126 (25.7%) tested positive for UU-2 and 69 (14.1%) tested positive for UP. Twenty-one men were Ureaplasma-positive by culture at the enrollment visit but could not be speciated at either the enrollment or follow-up visit. These men were excluded from species-specific analyses. Eighty-five percent (107 of 126) and 87% (60 of 69) of UU-2 and UP positive men, respectively, returned for the 3-week follow-up visit and were included in this analysis.
The mean age of included cases was 33 years, approximately one-half were white, and most (90%) had visible urethral discharge on exam (Table 1). Consistent with our previous report of this study population at baseline [5], UP-infected men were significantly more likely to report only female sex partners than men with UU-2 (97% vs. 69%, P<0.001). Men with UU-2 reported more sex partners in the past 12 months compared to men with UP (5.4 vs 3.2, P=0.01), and were more likely to report symptoms of urethral discharge and dysuria than men with UP. Roughly similar proportions of men with UU-2 and UP were co-infected with at least one other organism and 4 men (2.5%) were co-infected with both Ureaplasma species. There were no statistically significant differences in demographic, clinical, or behavioral characteristics among men who received azithromycin compared to those who received doxycycline, for men infected with either UU-2 or UP (data not shown).
Table 1.
Baseline characteristics of men infected with U. urealyticum biovar 2 or U. parvum*
| Men with U. urealyticum biovar 2 N = 107 N (%) | Men with U. parvum N = 60 N (%) | P-value | |
|---|---|---|---|
| Treatment arm | |||
| Azithromycin | 52 (48.6) | 31 (51.7) | 0.70 |
| Doxycycline | 55 (51.4) | 29 (48.3) | |
| Age, mean (SD) | 32.6 (±9.3) | 33.8 (±10.2) | 0.37 |
| Race | |||
| White | 53 (50.5) | 29 (49.2) | 0.78 |
| Black | 44 (41.9) | 27 (45.8) | |
| Other | 8 (7.6) | 3 (5.1) | |
| Education | |||
| ≤ High school/GED | 59 (55.1) | 30 (50.0) | 0.52 |
| > High school/GED | 48 (44.9) | 30 (50.0) | |
| Gender of sex partners, past 12 months | <0.001 | ||
| Women | 74 (69.2) | 58 (96.7) | |
| Men | 24 (22.4) | 1 (1.7) | |
| Both | 9 (8.4) | 1 (1.7) | |
| Number of sex partners past 12 months, mean (SD) | 5.4 (±6.1) | 3.2 (±3.6) | 0.01 |
| Self-reported symptoms | |||
| Urethral discharge | 62 (57.9) | 23 (38.3) | 0.02 |
| Dysuria | 63 (58.9) | 24 (40.0) | 0.02 |
| Other urethral symptoms | 29 (27.1) | 12 (20.0) | 0.31 |
| Visible urethral discharge | 96 (89.7) | 55 (91.7) | 0.68 |
| Number of PMNs/HPF | |||
| 5-9 | 24 (22.4) | 14 (23.3) | 0.89 |
| ≥10 | 83 (77.6) | 46 (76.7) | |
| Co-infection | |||
| Mycoplasma genitalium | 19 (17.8) | 7 (11.7) | 0.30 |
| Chlamydia trachomatis | 19 (17.8) | 9 (15.0) | 0.65 |
| Trichomonas vaginalis | 5 (4.7) | 2 (3.3) | 0.68 |
| Ureaplasma urealyticum | -- | 4 (6.7) | -- |
| Ureaplasma parvum | 4 (3.7) | -- | -- |
| Co-infection with at least one other organism | 39 (36.4) | 18 (30.0) | 0.40 |
Abbreviation: SD, standard deviation; GED, general education development
Owing to missing values, not all variables sum to column totals. Denominators for proportions represent those who had data available for that characteristic
Efficacy of therapies
Microbiologic failure for Ureaplasma spp. at the 3-week visit was 34.8% and did not differ for men who received azithromycin or doxycycline (34.7% vs 34.8%, P=1.00).
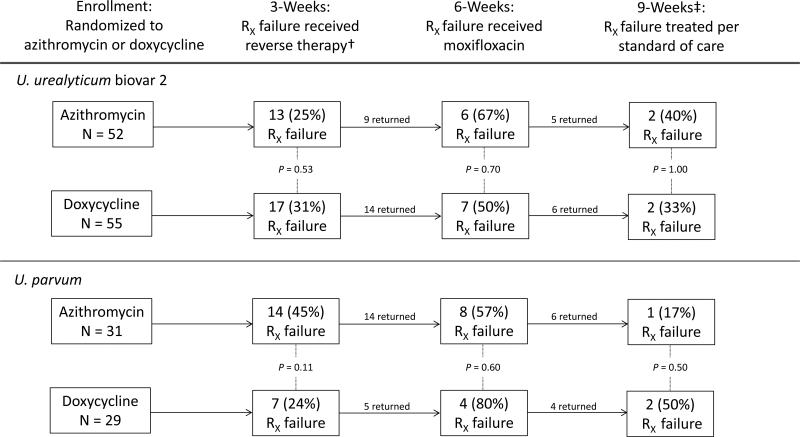
As previously reported, among men with UU-2, microbiologic failure at 3 weeks was similar in men treated with azithromycin and doxycycline (25.0% vs. 30.9%, respectively; P=0.53) [13]. Of those with persistent detection of UU-2 after initial treatment, over one-half had persistent detection of the organism after re-treatment with the alternate therapy (Figure 1). These relationships were similar when analyses were restricted to men who reported no unprotected sex between visits, or to men with no co-infecting organisms (data not shown). Eleven men with persistent UU-2 infection received moxifloxacin and returned for 9-week follow-up; of these, 4 (36%) again experienced microbiologic treatment failure.
Figure 1.
Microbiologic treatment failure at each of three follow-up visits, among all men infected with U. urealyticum biovar 2 or U. parvum participating in a randomized, controlled trial (N=107 for U. urealyticum biovar 2 and N=60 for U. parvum at enrollment)*
Abbreviations: RX, treatment
*P-values derived from Fisher's exact test
†Reverse therapy: Men initially randomized to active azithromycin/placebo doxycycline were given active doxycycline/placebo azithromycin and vice versa
‡Only men who received moxifloxacin were asked to return for a test of cure at 9 weeks
Among UP-infected men, microbiologic treatment failure at 3 weeks occurred somewhat more often after initial azithromycin therapy than after doxycycline therapy (45.2% vs. 24.1%, respectively; P=0.11; Figure 1) and this difference was statistically significant when analyses were restricted to men with no co-infecting organisms (13/23 [56.5%] vs. 4/17 [23.5%], respectively; P=0.05). Microbiologic failure rates after re-treatment with doxycycline and azithromycin at the 6-week visit were high (Figure 1). This finding was not altered when analyses were restricted to men who denied unprotected sex between visits or who had no co-infections. Three of 10 (30%) UP-positive men remained persistently positive after moxifloxacin treatment.
We performed antimicrobial susceptibility testing for 22 UU-2 and 8 UP specimens obtained at enrollment (Table 2). MICs did not exceed 2μg/ml for either species.
Table 2.
In vitro susceptibilities of U. urealyticum biovar 2 and U. parvum to three antibiotics*
| Antibiotic | MIC Values (μg/ml) | ||||
|---|---|---|---|---|---|
| Azithromycin | 0.125 | 0.25 | 0.50 | 1.00 | 2.00 |
| U. urealyticum (N=22) | 0 | 1 | 5 | 13 | 3 |
| U. parvum (N=8) | 2 | 1 | 2 | 2 | 1 |
| Doxycycline | |||||
| U. urealyticum (N=22) | 2 | 4 | 14 | 2 | 0 |
| U. parvum (N=8) | 3 | 4 | 1 | 0 | 0 |
| Moxifloxacin | |||||
| U. urealyticum (N=22) | 0 | 5 | 12 | 5 | 0 |
| U. parvum (N=8) | 1 | 3 | 4 | 0 | 0 |
Cells represent the number of isolates at each MIC value
Association between clinical and microbiologic outcomes
Persistent detection of the organism was not associated with clinical treatment failure at 3-, 6- or 9-weeks for men with UU-2 or men with UP (Table 3). Seven men remained persistently positive for UU-2 or UP after treatment with moxifloxacin, but all experienced resolution of signs/symptoms. There was also no association between clinical and microbiologic failure among men without co-infections, among those with <10 lifetime vaginal sex partners, or among men who had no unprotected sex between visits (data not shown).
Table 3.
Association between clinical failure (presence of signs/symptoms) and microbiologic failure (persistence of the organism) at each of three follow-up visits among men infected with UU or UP at time of initial diagnosis of urethritis
| U. urealyticum biovar 2 | U. parvum | |||||
|---|---|---|---|---|---|---|
| Persistence of organism | Persistence of organism | |||||
| Clinical failure at follow-up | Yes n/N (%)* | No n/N (%)* | P-value† | Yes n/N (%)* | No n/N (%)* | P-value† |
| 3-weeks | 5/30 (16.7) | 19/77 (24.7) | 0.45 | 5/21 (23.8) | 6/39 (15.4) | 0.49 |
| 6-weeks | 3/13 (23.1) | 2/10 (20.0) | 1.00 | 4/12 (33.3) | 3/7 (42.9) | 1.00 |
| 9-weeks | 0/4 (0.0) | 0/7 (0.0) | -- | 0/3 (0.0) | 2/7 (28.6) | 1.00 |
n/N = number of men with clinical failure / number of men with or without persistence of the organism
Derived from Fisher's exact test
DISCUSSION
In this population of men with NGU, infection with UU-2 was more common than infection with UP (25% vs. 14%, respectively). Approximately 24-45% of men had persistent detection of Ureaplasmas. after initial treatment with azithromycin or doxycycline, and re-treatment with the alternative therapy was unsuccessful in eradicating UU-2 and UP in many cases, irrespective of the regimen used. These high rates of treatment failure did not appear to be explained by antimicrobial resistance, as all clinical isolates demonstrated relatively low MICs to the three therapies. Somewhat surprisingly, there was no association between persistence of UU-2 and persistent signs/symptoms of NGU.
The rates of microbiologic treatment failure among men with UU-2 and UP treated with azithromycin (25% and 45%, respectively) or doxycycline (31% and 24%, respectively) were lower than that observed by Stamm and colleagues [10] among men with NGU with undifferentiated U. urealyticum infection (55% vs. 53%, for azithromycin and doxycycline, respectively). However, our findings are similar to a more recent trial of men with prostatitis attributed to undifferentiated U. urealyticum, with microbiologic failure rates of 22% and 26%, respectively [17]. Consistent with both previous studies, we found little difference in the efficacy of the two therapies for UU-2, suggesting that either treatment is an appropriate first-line regimen for men with UU-2-associated NGU. However, among men with UP, treatment failure was somewhat more common among men who received azithromycin compared to those that received doxycycline.
Although the difference in failure rates by therapy among men with UP was not statistically significant, this may have been due to low statistical power, and doxycycline may be slightly more effective at eradicating UP from the male genital tract. Given the consistent evidence that UP does not cause NGU [4-6, 18], this does not have direct implications for men, but may have relevance for women. Infection with undifferentiated Ureaplasma spp. has been associated with adverse pregnancy outcomes in studies conducted prior to species differentiation [19-22] and UP, but not UU-2, has been associated with preterm birth or late abortion [8] as well as premature rupture of the membranes [9]. In animal model studies of Ureaplasma pathogenesis, amniotic inoculation with UP stimulates preterm labor in non-human primates [23] and is associated with lung inflammation and altered development of the fetus in sheep [24]. Further recent findings from murine and sheep models indicate that host genetic background and immune response to UP may be the primary determinants of pathogenesis [25, 26]. This suggests that UP likely plays a role in reproductive health but the extent of its importance and the need for treatment requires further study.
Re-treatment of men infected with either UU-2 or UP after initial treatment failure was relatively unsuccessful, irrespective of the antibiotic used. These high rates of treatment failure contrast with the apparent in vitro susceptibility of UU-2 and UP isolates that we observed. Consistent with our findings, studies examining Ureaplasma susceptibility to azithromycin [27] and moxifloxacin [27-30] have generally observed isolates in the susceptible range and have not noted differences in susceptibility patterns by species. However, the universal in vitro susceptibility to doxycycline in this study is in contrast to studies conducted in Europe and West Africa, where 18-37% of Ureaplasma isolates demonstrated resistance to doxycycline [11, 12, 29]. Given the high failure rates that we observed in the presence of low MICs, other factors likely influence persistent infection with Ureaplasmas. For example, the bacteriostatic nature of doxycycline may allow their persistence during treatment and subsequent regrowth. Alternatively, the penetrance and activity of these antibiotics in the urogenital tract may affect their treatment efficacy.
Despite moderate to high rates of microbiologic failure, the persistence of signs/symptoms of infection was not associated with persistent detection of the organism. There are two key implications of this finding. First, it argues against a casual role for UU-2 in NGU. If UU-2 causes urethritis, we would expect some relationship between presence of the organism and signs and symptoms of disease. Though several studies have demonstrated an association between UU-2 and NGU [4-6], this association was not found in the largest study to date [7] and the topic remains controversial. If UU-2 does cause urethritis, the clinical syndrome may be self-limited and symptoms may resolve over several weeks, with or without effective therapy. Second, the absence of any association between persisting signs and symptoms and persisting organisms calls into question the necessity to re-treat persistently positive men. While there are currently no recommendations for diagnostic testing for Ureaplasmas, some clinics opt to do this. Although few would argue against re-treating symptomatic men, the decision to treat asymptomatic men should balance the benefits of eradicating the organism (e.g., disrupting transmission to female sexual partners) with the drawbacks of side effects and potential expansion of antibiotic resistance.
This study has a number of strengths, including its randomized design, use of sensitive species-specific PCR assays, and pairing of in vitro antimicrobial susceptibility testing with clinical outcomes. This study also has several limitations that merit discussion. First, because this analysis only included persistently positive men, our sample sizes at later follow-up visits were small which limited our ability to detect statistically significant differences in the proportion of treatment failures. Second, we ceased antimicrobial susceptibility testing after the first year of the study and it is possible that isolates from men enrolled later in the trial had a different susceptibility profile then the ones included here. Third, data on sexual exposures between visits were self-reported and are limited by recall bias and social desirability bias. Finally, resistance patterns vary geographically and the extent to which our findings may generalize to other settings is unknown.
In conclusion, azithromycin and doxycycline were similarly efficacious in the treatment of UU-2 among these persistently positive men with NGU, but doxycycline may be more effective against UP. Microbiologic treatment failure among persistently positive men was common, but not associated with persistent clinical urethritis. Several commercial laboratories in the U.S. and the U.K. offer tests for Ureaplasmas. However, this absence of an association between persistent UU-2-infection and clinical signs, coupled with the difficulty in eradicating Ureaplasmas after initial treatment failure suggests that individuals with asymptomatic UU-2 infections do not benefit from nor require on-going antimicrobial therapy.
ACKNOWLEDGEMENTS
The authors would like to thank the men who participated in the trial, as well as the clinicians and staff in the Public Health – Seattle & King County STD Clinic (Yolanda Bantolino, Sylvia Berry, Irene King, Eduardo Muñoz, Victory Murphy, Sally Pendras, Sue Szabo, Michael Verdon, Fred Koch, Roxanne Kerani, Barbara Krekeler); study staff (Sarah McDougal, Noa Kay, Dwyn Dithmer-Schreck); Sabina Astete, Lisa Lowenstein, and Linda Arnesen in the Totten Laboratory; Linda Cles in the UW Chlamydia Laboratory; Gen-Probe, Inc for reagents; Ana-Maria Xet-Mull and William Whittington for Trichomonas testing at the University of Washington; HMC IDS (Jeffrey Purcell, Bao Chau Vo, Asaad Awan, Kelly Nguyen); and the data safety and monitoring board (Edward W. Hook III, David H. Martin, H. Hunter Handsfield, Sarah Holte). We also thank Carolyn Deal, Elizabeth Rogers, and Peter Wolff at the Division of Microbiology and Infectious Diseases at the National Institutes of Health, and Pfizer, Inc, for supplying study drugs.
FUNDING
This work was supported by the University of Washington (UW) Sexually Transmitted Infections and Topical Microbicides Cooperative Research Center (NIH/NIAID U19 AI31448), the Center for AIDS Research (P30 AI027757) and by a grant from the National Institutes of Health (NIH/NIAID R01 AI072728). CWG and CMK were supported by the UW STD/AIDS Research Training Fellowship program (NIH/NIAID T32 AI07140). Pfizer, Inc. provided study drugs (active azithromycin, active doxycycline and placebo azithromycin). Harborview Investigational Drug Service provided placebo doxycycline. This trial is registered at www.ClinicalTrials.gov (NCT00358462).
Footnotes
This trial is registered at www.ClinicalTrials.gov (NCT00358462).
COMPETING INTERESTS
The authors have no competing interests to report.
REFERENCES
- 1.Centers for Disease Control and Prevention Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12):1–110. [PubMed] [Google Scholar]
- 2.Kong F, James G, Ma Z, et al. Phylogenetic analysis of Ureaplasma urealyticum--support for the establishment of a new species, Ureaplasma parvum. Int J Syst Bacteriol. 1999;49:1879–89. doi: 10.1099/00207713-49-4-1879. [DOI] [PubMed] [Google Scholar]
- 3.Robertson JA, Stemke GW, Davis JW, Jr., et al. Proposal of Ureaplasma parvum sp. nov. and emended description of Ureaplasma urealyticum (Shepard et al. 1974) Robertson et al. 2001. Int J Syst Evol Microbiol. 2002;52(Pt 2):587–97. doi: 10.1099/00207713-52-2-587. [DOI] [PubMed] [Google Scholar]
- 4.Deguchi T, Yoshida T, Miyazawa T, et al. Association of Ureaplasma urealyticum (biovar 2) with nongonococcal urethritis. Sex Transm Dis. 2004;31(3):192–5. doi: 10.1097/01.olq.0000114653.26951.71. [DOI] [PubMed] [Google Scholar]
- 5.Wetmore CM, Manhart LE, Lowens MS, et al. Ureaplasma urealyticum is associated with nongonococcal urethritis among men with fewer lifetime sexual partners: a case-control study. J Infect Dis. 2011;204(8):1274–82. doi: 10.1093/infdis/jir517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ondondo RO, Whittington WL, Astete SG, et al. Differential association of ureaplasma species with non-gonococcal urethritis in heterosexual men. Sex Transm Infect. 2010;86(4):271–5. doi: 10.1136/sti.2009.040394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradshaw CS, Tabrizi SN, Read TR, et al. Etiologies of nongonococcal urethritis: bacteria, viruses, and the association with orogenital exposure. J Infect Dis. 2006;193(3):336–45. doi: 10.1086/499434. [DOI] [PubMed] [Google Scholar]
- 8.Kataoka S, Yamada T, Chou K, et al. Association between preterm birth and vaginal colonization by mycoplasmas in early pregnancy. J Clin Microbiol. 2006;44(1):51–5. doi: 10.1128/JCM.44.1.51-55.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kasper DC, Mechtler TP, Reischer GH, et al. The bacterial load of Ureaplasma parvum in amniotic fluid is correlated with an increased intrauterine inflammatory response. Diagn Microbiol Infect Dis. 2010;67(2):117–21. doi: 10.1016/j.diagmicrobio.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 10.Stamm WE, Hicks CB, Martin DH, et al. Azithromycin for empirical treatment of the nongonococcal urethritis syndrome in men. A randomized double-blind study. JAMA. 1995;274(7):545–9. [PubMed] [Google Scholar]
- 11.Abele-Horn M, Wolff C, Dressel P, et al. Association of Ureaplasma urealyticum biovars with clinical outcome for neonates, obstetric patients, and gynecological patients with pelvic inflammatory disease. J Clin Microbiol. 1997;35(5):1199–202. doi: 10.1128/jcm.35.5.1199-1202.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Domingues D, Tavira LT, Duarte A, et al. Ureaplasma urealyticum biovar determination in women attending a family planning clinic in Guine-Bissau, using polymerase chain reaction of the multiple-banded antigen gene. J Clin Lab Anal. 2002;16(2):71–5. doi: 10.1002/jcla.10023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manhart LE, Gillespie CW, Lowens MS, et al. Standard treatment regimens for nongonococcal urethritis have similar but declining cure rates: a randomized controlled trial. Clin Infect Dis. 2013;56(7):934–42. doi: 10.1093/cid/cis1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dutro SM, Hebb JK, Garin CA, et al. Development and performance of a microwell-plate-based polymerase chain reaction assay for Mycoplasma genitalium. Sex Transm Dis. 2003;30(10):756–63. doi: 10.1097/01.OLQ.0000078821.27933.88. [DOI] [PubMed] [Google Scholar]
- 15.Nash P, Krenz MM. In: Culture media, in Manual of Clinical Microbiology. 5th ed Balows A, et al., editors. American Society for Microbiology; Washington, D.C.: 1991. pp. 1226–1288. [Google Scholar]
- 16.Kenny GE, Cartwright FD. In: Mycoplasmas, in Manual of Clinical Microbiology. 5th ed Balows A, et al., editors. American Society for Microbiology; Washington, D.C.: 1991. pp. 478–482. [Google Scholar]
- 17.Skerk V, Marekovic I, Markovinovic L, et al. Comparative randomized pilot study of azithromycin and doxycycline efficacy and tolerability in the treatment of prostate infection caused by Ureaplasma urealyticum. Chemotherapy. 2006;52(1):9–11. doi: 10.1159/000090234. [DOI] [PubMed] [Google Scholar]
- 18.Povlsen K, Bjornelius E, Lidbrink P, et al. Relationship of Ureaplasma urealyticum biovar 2 to nongonococcal urethritis. Eur J Clin Microbiol Infect Dis. 2002;21(2):97–101. doi: 10.1007/s10096-001-0665-1. [DOI] [PubMed] [Google Scholar]
- 19.Kundsin RB, Leviton A, Allred EN, et al. Ureaplasma urealyticum infection of the placenta in pregnancies that ended prematurely. Obstet Gynecol. 1996;87(1):122–7. doi: 10.1016/0029-7844(95)00376-2. [DOI] [PubMed] [Google Scholar]
- 20.Yoon BH, Chang JW, Romero R. Isolation of Ureaplasma urealyticum from the amniotic cavity and adverse outcome in preterm labor. Obstet Gynecol. 1998;92(1):77–82. doi: 10.1016/s0029-7844(98)00122-7. [DOI] [PubMed] [Google Scholar]
- 21.Kirchner L, Helmer H, Heinze G, et al. Amnionitis with Ureaplasma urealyticum or other microbes leads to increased morbidity and prolonged hospitalization in very low birth weight infants. Eur J Obstet Gynecol Reprod Biol. 2007;134(1):44–50. doi: 10.1016/j.ejogrb.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 22.Watts DH, Krohn MA, Hillier SL, et al. The association of occult amniotic fluid infection with gestational age and neonatal outcome among women in preterm labor. Obstet Gynecol. 1992;79(3):351–7. doi: 10.1097/00006250-199203000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Novy MJ, Duffy L, Axthelm MK, et al. Ureaplasma parvum or Mycoplasma hominis as sole pathogens cause chorioamnionitis, preterm delivery, and fetal pneumonia in rhesus macaques. Reprod Sci. 2009;16(1):56–70. doi: 10.1177/1933719108325508. [DOI] [PubMed] [Google Scholar]
- 24.Moss TJ, Knox CL, Kallapur SG, et al. Experimental amniotic fluid infection in sheep: effects of Ureaplasma parvum serovars 3 and 6 on preterm or term fetal sheep. Am J Obstet Gynecol. 2008;198(1):122, e1–8. doi: 10.1016/j.ajog.2007.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von Chamier M, Allam A, Brown MB, et al. Host genetic background impacts disease outcome during intrauterine infection with Ureaplasma parvum. PLoS One. 2012;7(8):e44047. doi: 10.1371/journal.pone.0044047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dando SJ, Nitsos I, Kallapur SG, et al. The role of the multiple banded antigen of Ureaplasma parvum in intra-amniotic infection: major virulence factor or decoy? PLoS One. 2012;7(1):e29856. doi: 10.1371/journal.pone.0029856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waites KB, Crabb DM, Duffy LB. Comparative in vitro activities of the investigational fluoroquinolone DC-159a and other antimicrobial agents against human mycoplasmas and ureaplasmas. Antimicrob Agents Chemother. 2008;52(10):3776–8. doi: 10.1128/AAC.00849-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kenny GE, Cartwright FD. Susceptibilities of Mycoplasma hominis, M. pneumoniae, and Ureaplasma urealyticum to GAR-936, dalfopristin, dirithromycin, evernimicin, gatifloxacin, linezolid, moxifloxacin, quinupristin-dalfopristin, and telithromycin compared to their susceptibilities to reference macrolides, tetracyclines, and quinolones. Antimicrob Agents Chemother. 2001;45(9):2604–8. doi: 10.1128/AAC.45.9.2604-2608.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bebear CM, de Barbeyrac B, Pereyre S, et al. Activity of moxifloxacin against the urogenital mycoplasmas Ureaplasma spp., Mycoplasma hominis and Mycoplasma genitalium and Chlamydia trachomatis. Clin Microbiol Infect. 2008;14(8):801–5. doi: 10.1111/j.1469-0691.2008.02027.x. [DOI] [PubMed] [Google Scholar]
- 30.Samra Z, Rosenberg S, Dan M. Susceptibility of Ureaplasma urealyticum to tetracycline, doxycycline, erythromycin, roxithromycin, clarithromycin, azithromycin, levofloxacin and moxifloxacin. J Chemother. 2011;23(2):77–9. doi: 10.1179/joc.2011.23.2.77. [DOI] [PubMed] [Google Scholar]