Abstract
The dopamine D1 receptor is centrally involved in mediating the effects of cocaine and is essential for cocaine-induced locomotor sensitization. Changes in D1 receptor expression has been reported in various models of cocaine addiction; however, the mechanisms that mediate these changes in D1 receptor expression are not well understood. Using preadolescent drd1a-EGFP mice and a binge cocaine treatment protocol we demonstrate that the D1 receptor is post-transcriptionally regulated in the caudate-putamen of cocaine-sensitized animal. While cocaine-sensitized mice express high levels of steady state D1 receptor mRNA, the expression of D1 receptor protein is not elevated. We determined that the post-transcriptional regulation of D1 receptor mRNA is rapidly attenuated and D1 receptor protein levels increase within thirty minutes when the sensitized mice are challenged with cocaine. The rapid increase in D1 receptor protein levels requires de novo protein synthesis and correlates with the cocaine-induced hyperlocomotor activity in the cocaine-sensitized mice. The increase in D1 receptor protein levels in the caudate-putamen inversely correlated to the levels of microRNA 142-3p and 382, both of which regulate D1 receptor protein expression. The levels of these two microRNAs decreased significantly within five minutes of cocaine challenge in sensitized mice. The results provide novel insights into the previously unknown rapid kinetics of D1 receptor protein expression which occurs in a time scale that is comparable to the expression of immediate early genes. Furthermore, the results suggests a potential novel role for inherently labile microRNAs in regulating the rapid expression of D1 receptor protein in cocaine-sensitized animals.
Keywords: behavioral sensitization, gene expression, microRNA, preadolescence, striatum
Introduction
Numerous studies have established a central role for dopamine receptors, in particular, the dopamine D1 receptor in mediating the effects of most classes of drugs of abuse, including cocaine (Unterwald et al., 1994; Tsukada et al., 1996; Hummel and Unterwald, 2002; Sclussman et al., 2003; Karasinska et al., 2005; Karlsson et al., 2008). Pharmacological and genetic studies, using knock-out animals, have shown that D1 receptors are important for mediating cocaine-induced psychomotor sensitization and cocaine-self administration (Xu et al., 2000; Karasinska et al., 2005; Caine et al., 2007; Karlsson et al., 2008). Studies using different cocaine administration paradigms in various animal species have consistently shown increases in D1 receptor mRNA but not D1 receptor protein (Kleven et al., 1990; Henry and White, 1991; Laurier et al., 1994; Unterwald et al., 2001; Hummel and Unterwald, 2002; Brenhouse et al., 2008). Taken together, the overarching conclusion from previous studies suggest a hypothesis that the expression of D1 receptor might be post-transcriptionally regulated in cocaine-treated animals. Here, we directly tested the hypothesis that D1 receptor expression is post-transcriptionally regulated in cocaine-sensitized mice.
The mechanisms that mediate the changes in expression of D1 receptors in cocaine-treated animals are not well understood and it is not clear if the changes in D1 receptor expression in certain brain regions correlates with cocaine-induced alterations in behavior. We have previously shown that the D1 receptor is regulated at the transcriptional and post-transcriptional level in a cell line model, during postnatal mouse brain development, and in rodent models of alcohol addiction; in particular, we have recently shown that the post-transcriptional regulation of D1 receptor expression is directly mediated by microRNAs, miR142-3p and miR382 (Pasuit et al., 2004; Tobón et al., 2012; Li et al 2013). The latter microRNA is important for regulating expression of D1 receptors in models of alcohol addiction (Li et al., 2013). In this report, we assessed the changes in expression of miR142-3p and miR382 in cocaine-sensitized mice and determined if the changes in expression of D1 receptors and the two microRNAs correlated with changes in cocaine-induced hyperlocomotor activity.
The results of this study show that the D1 receptor exhibits post-transcriptional regulation in the caudate-putamen but not the nucleus accumbens of cocaine-sensitized mice. The post-transcriptional regulation is rapidly attenuated upon cocaine administration to sensitized mice resulting in a fast increase in D1 receptor protein levels within 30 minutes. This rapid cocaine-induced increase in D1 receptor protein requires de novo protein synthesis and correlates with rapid decrease in the levels of two microRNAs and increase in locomotor activity.
Materials and Methods
Animals
We have previously described the drd1-EGFP mice (Tg(Drd1-a-EGFP)X60Gsat/Mmmh MMRRC:000297) used in this study (Tobón and Kuzhikandathil, 2014). The drd1-EGFP transgenic mice have a mixed Swiss Webster/FVB genetic background. These mice carry a transgene which expresses the enhanced green fluorescent reporter (EGFP) gene under the control of the D1 receptor promoter. Thus the fluorescent EGFP protein is expressed in cells that endogenously express the D1 receptor mRNA, facilitating single cell-level studies of changes in D1 receptor expression and function. The drd1-EGFP mice were weaned at post natal day 21 (P21) and male mice used for experiments on P23. Animals were housed in individual cages on a 12 hour light/dark cycle (lights on at 0800), and provided food and water ad lib. All procedures were performed during light phase and approved by the IACUC committee at Rutgers-New Jersey Medical School.
Cocaine administration
We have previously described the binge administration protocol used in this paper (Tobón and Kuzhikandathil, 2014). Briefly, beginning on P23, mice received three daily intraperitoneal (i.p.) injections of saline or 15mg/kg of cocaine HCl (Medisca, Plattasburgh, NY), one hour apart, for seven consecutive days in the home cage. Brains were harvested on day 8 for mRNA and protein analysis before and, at various times, after a challenge injection of saline or cocaine (15 mg/kg). Anisomycin (Sigma-Aldrich, St. Louis, MO) was dissolved in HCl and adjusted to pH4.5 with NaOH. Mice were injected with vehicle (saline adjusted to pH4.5) or 150 mg/kg anisomycin s.c., one hour prior to cocaine challenge injections on day 8.
Activity measurement
We have previously described the methods for measuring the horizontal locomotor activity using the open field photobeam activity system (PAS; SD Instruments, San Diego, CA) (Tobón and Kuzhikandathil, 2014). The animals were placed in the open field arena for 30 minutes prior to injections, for habituation. After the 30 minute period, the recording was paused, the mice were removed from the arena, briefly placed in the home cage while the i.p. injections were administered. Immediately after the injections, the mice were returned to the arena and recording resumed. Photobeam breaks were collected in 5 min bins for half an hour prior to injections and one hour after each of three injections for a total recording time of 3.5 hours. Photo-beam breaks were converted to total distance traveled in cm using the PAS reporter software (version 2). The resting time parameter in the software was set at 4 s.
Brain tissue harvest
Brain tissues was harvested and stored as described previously (Tobón et al., 2012; Tobón and Kuzhikandathil, 2014). Briefly, whole brain was isolated and immersed in ice-cold saline. Brain sections (300μm thick) were obtained using a refrigerated Vibratome® 1500 sectioning system (Vibratome, St. Louis, MO) maintained at 3°C. The nucleus accumbens and caudate-putamen brain regions were micro-punched (2mm) from 300μm coronal sections. The micro-punches for RNA isolation were stored in RNAlater® (Ambion) and those for protein analysis rapidly frozen in a dry ice-ethanol mixture and stored at -80°C.
Real-time reverse transcriptase PCR
RNA isolation and real-time RT-PCR was performed as described previously (Pasuit et al., 2004; Tobón et al., 2012; Tobón and Kuzhikandathil, 2014). PCR was performed using the Roche Light Cycler (Indianapolis, IN, USA) using gene-specific TaqMan® gene expression assays (Applied Biosystems). D1 dopamine receptor cDNA levels were measured using TaqMan® gene expression assay Mm0135211. The internal control GAPDH cDNA was detected using Mm99999915 TaqMan® gene expression assay. To detect and quantitate miR-142-3p (miR-142-3p) and miR-382 (miR-382) levels using real-time RT-PCR we used TaqMan® Small RNA Assays (Invitrogen) for the two microRNAs. MicroRNA levels were normalized to internal control RNU6B. Appropriate negative and positive controls were included in the RT-PCR experiments, as described previously (Pasuit et al., 2004; Tobón et al., 2012; Tobón and Kuzhikandathil, 2014).
Western blotting
Protein lysates were prepared and western blotting performed as described previously (Pasuit et al., 2004; Tobón et al., 2012; Tobón and Kuzhikandathil, 2014). For detecting D1 receptor protein, we used the rat monoclonal anti D1 receptor antibody (Sigma-Aldrich; catalog# D2944) as described previously (Pasuit et al., 2004; Tobón et al., 2012; Tobón and Kuzhikandathil, 2014). Following the detection of D1 receptor protein, the membrane was stripped and reprobed to detect GAPDH. GAPDH protein was detected using a rabbit monoclonal antibody (1:5000 dilution; Cell Signaling Technology; catalog# 5174). The horseradish peroxidase conjugated anti-rat or anti-rabbit secondary antibodies were detected with the SuperSignal® West Dura extended duration substrate chemiluminescence detection kit (Pierce Biochemicals, Rockford, IL). Experiments were repeated at least four independent times with comparable results.
Statistics
One-way, and two-way Analysis of variance (ANOVA) tests and two-tailed Student's t-test were performed with the SigmaPlot® 11 software (SPSS Inc.). Post-hoc multiple comparison was performed using Holm-Sidak test. Data were considered statistically significant when the probability value (P) was less than 0.05.
Results
Changes in D1 receptor expression in preadolescent drd1a-EGFP mice that exhibit cocaine-induced locomotor sensitization
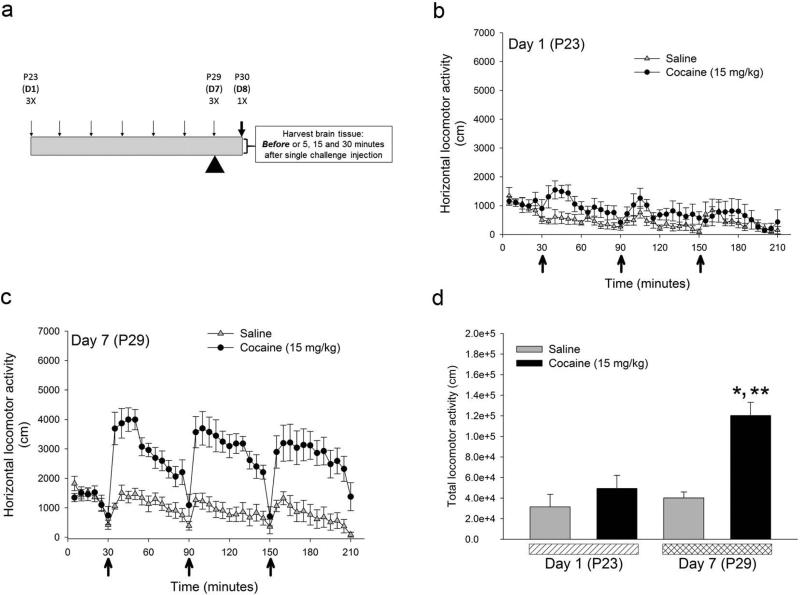
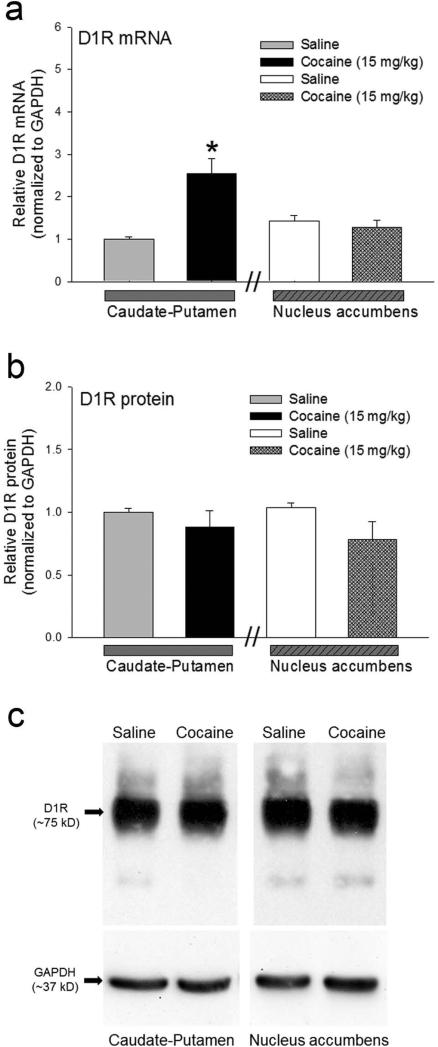
Preadolescent (P23) drd1a-EGFP mice were subjected to a binge-treatment regimen in which three i.p. injection of saline or cocaine (15 mg/kg) were administered, 1 hour apart, for seven consecutive days till P29 (Fig. 1a). Locomotor activity was determined on day 1 (P23) and day 7 (P29) in an Open Field test (Fig. 1 b and 1c). Cocaine-induced locomotor activity was significantly enhanced on day 7 in mice administered binge dose of cocaine compared to saline-injected mice (Fig. 1d). Two-way ANOVA showed that there is a statistically significant interaction between cocaine treatment and day of treatment (F1,28 = 7.55, p=0.010) and between saline and cocaine treatment on day 7 (F1,28 = 18.82, p<0.001). These results are consistent with our recent results (Tobón and Kuzhikandathil, 2014). We next determined the levels of D1 receptor mRNA and protein using real-time RT-PCR and western blot analysis, respectively. We assessed expression levels in two brain regions, caudate-putamen and nucleus accumbens, which express high levels of D1 receptor and have been implicated in locomotor and addictive behaviors (Ariano, 1997; Everitt and Robbins, 2005; Zapata et al., 2010; Veeneman et al., 2012). The expression of D1 receptor mRNA and protein was assessed in tissue punches obtained from control and cocaine-sensitized drd1a-EGFP mice on P30, 24 hours after the last binge injection. The results in Figure 2 show that there is a significant increase in steady-state D1 receptor mRNA in the caudate-putamen (p<0.001), but not the nucleus accumbens, in the cocaine-sensitized mice (Fig. 2a). Interestingly, the increase in D1 receptor mRNA levels did not translate into increased D1 receptor protein levels as there was no significant difference in D1 receptor protein in both brain regions between control and cocaine-sensitized mice (Fig. 2b and 2c). These results suggest that the expression of D1 receptor is post-transcriptionally regulated in the caudate-putamen.
Figure 1.
Preadolescent drd1a-EGFP mice exhibit cocaine-induced locomotor sensitization. (a) Twenty three day old (P23) male drd1a-EGFP mice were administered three i.p. doses (3X) of saline or cocaine (15 mg/kg), 1 hour apart, daily for seven consecutive days (D1-D7; downward arrows). On day 8 (P30), twenty four hours after the last injection, brain tissue was harvested before or 5, 15 and 30 minutes after a single challenge i.p. injection of saline or cocaine (15 mg/kg). Total horizontal locomotor activity in an Open Field arena determined on day 1 (P23) (b) and day 7 (P29) (c) for drd1a-EGFP mice administered saline (gray triangles; n=8 mice) or 15 mg/kg cocaine (filled circle; n=12 mice). Arrows indicate the times when the 3 i.p. injections were administered. (d) The total horizontal distance traveled for 3 hours in saline (gray bars) and cocaine (black bars) treated in drd1a-EGFP mice were significantly different between the cocaine treated groups on day 1 and day 7 (*, p < 0.05, n = 8, two-way ANOVA) and between the saline and cocaine treated groups on day 7 (**, p < 0.05, n = 8, two-way ANOVA). Error bars represent ± SEM.
Figure 2.
Post-transcriptional regulation of D1 receptor expression in the caudate-putamen, but not nucleus accumbens, of cocaine-sensitized drd1a-EGFP mice. Relative levels of D1 receptor (D1R) mRNA (a) and protein (b) normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression levels, 24 hours after the last binge injection, in the caudate-putamen and nucleus accumbens of saline (n= 8) and cocaine-sensitized (n=12) drd1a-EGFP mice. Error bars represent ± SEM. *, P<0.001, statistically significant difference in D1R mRNA levels between saline and cocaine-sensitized mice in the caudate-putamen, Student's t-test. (c) Representative western blot showing the expression of D1R and internal control, GAPDH, in tissue punches of caudate-putamen and nucleus accumbens harvested 24 hours after the last injection on day 7 from drd1a-EGFP mice that were binge-treated with saline or 15 mg/kg cocaine for 7 days. The molecular weight (kD) of D1R and GAPDH detected by the antibodies are indicated.
Rapid attenuation of D1 receptor post-transcriptional regulation in sensitized mice challenged with cocaine
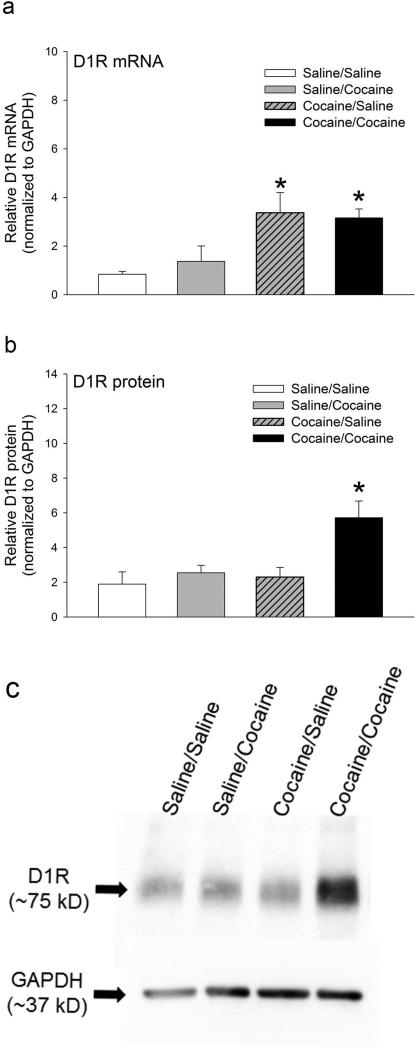
Previous studies, using pharmacological and genetic approaches, have shown that the cocaine-induced hyperlocomotor activity in cocaine-sensitized mice is mediated by D1 receptors. Given this, the paradoxical lack of increase in steady state D1 receptor protein levels in the sensitized mice led us to hypothesize that the D1 receptor protein expression might be dynamically regulated in response to cocaine administration. To test the hypothesis, we administered a single challenge dose of cocaine (15 mg/kg) on day 8 (P30) and harvested brain tissue after 5, 15 and 30 minutes and measured the levels of D1 receptor mRNA and protein using real-time RT-PCR and western blot analysis, respectively. The data were analyzed using two-way ANOVA with binge treatment and challenge treatment as factors. The results in Figure 3a shows that while D1 receptor mRNA levels in the caudate-putamen are different between the non-sensitized and sensitized groups of binge-treated mice (F1,15 = 15.17, p=0.001), they are not significantly different within the challenge treatment group (F1,15 = 0.085, p= 0.775). No significant interaction was found between the binge and challenge treatment factors (F1,15 = 0.44, p= 0.518). These results are consistent with the results shown in Figure 2. In contrast to the effects on D1 receptor mRNA, the levels of D1 receptor protein in the caudate-putamen are different between the non-sensitized and sensitized groups of binge-treated mice (F1,31 = 6.03, p=0.02), and are significantly affected by the challenge treatment (F1,31 = 7.79, p= 0.009). No significant interaction was found between the binge and challenge treatment factors (F1,31 = 3.59, p= 0.07). Post-hoc statistical analysis showed that there was a significant increase in D1 receptor protein levels within 30 minutes only in cocaine-sensitized mice administered a challenge dose of cocaine (t= 3.462, p= 0.002; Fig. 3b and 3c). No significant increase was observed in tissue punches isolated 5 and 15 minutes after the challenge injection (Supplementary Figure 1). These results suggest that the post-transcriptional inhibition of D1 receptor protein expression in the caudate-putamen of cocaine-sensitized mice is rapidly attenuated upon a challenge cocaine administration, leading to an increase in D1 receptor protein levels within 30 minutes of cocaine administration.
Figure 3.
D1 receptor protein levels increases within 30 minutes in cocaine-sensitized drd1a-EGFP mice challenged with cocaine. Relative levels of D1R mRNA (a) and protein (b) normalized to GAPDH expression levels, 30 minutes after a challenge saline or cocaine (15 mg/kg) injection, in the caudate-putamen of saline and cocaine-sensitized drd1a-EGFP mice. N= 4 to 5 mice per treatment group. Error bars represent ± SEM. *, P<0.05, statistically significant difference in D1R mRNA levels between cocaine-sensitized mice, challenged with either saline or cocaine, and all other treatment groups, two-way ANOVA, post-hoc Holm-Sidak test (a). N= 10 to 11 mice per treatment group *, P<0.005, statistically significant difference in D1R protein levels between cocaine challenged mice (black bar) and other treatment groups, two-way ANOVA, post-hoc Holm-Sidak test (b). (c) Representative western blot showing the expression of D1R protein and internal control, GAPDH, in tissue punches of caudate-putamen harvested 30 minute after the challenge injection on day 8 from drd1a-EGFP mice that were binge-treated with saline for 7 days and challenged with saline (saline/saline) or 15 mg/kg cocaine (saline/cocaine) or binge-treated with 15 mg/kg cocaine for 7 days and challenged with saline (cocaine/saline) or 15 mg/kg cocaine (cocaine/cocaine). The molecular weight (kD) of D1R and GAPDH detected by the antibodies are indicated.
The rapid cocaine-induced increase in D1 receptor protein levels requires de novo protein synthesis
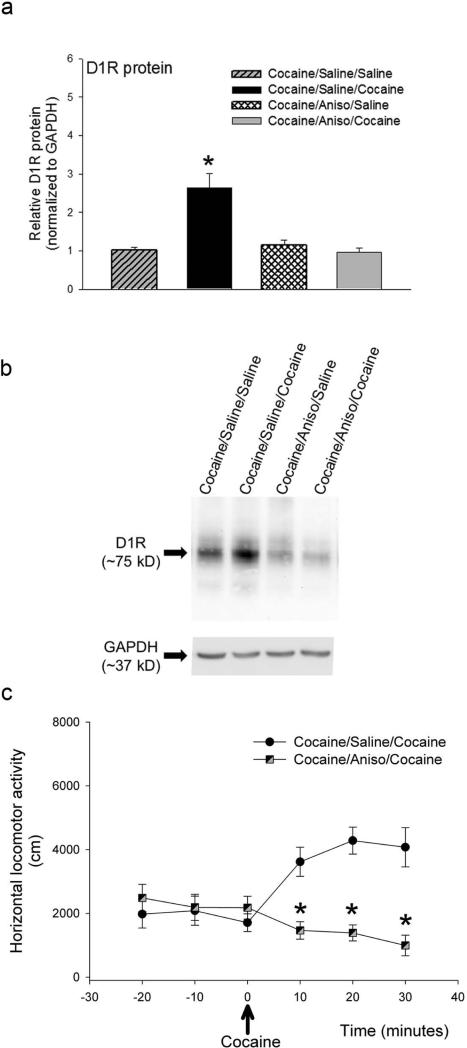
The rapid cocaine-induced increase in D1 receptor protein levels in cocaine-sensitized mice could be due to alterations D1 receptor protein stability or increased D1 receptor protein synthesis. To distinguish these two possibilities, we administered vehicle (saline at pH4.5) or 150 mg/kg anisomycin, a protein synthesis inhibitor (Valjent et al., 2010), to cocaine-sensitized drd1a-EGFP mice 60 minutes before challenging the mice with saline or 15 mg/kg cocaine. The brain tissue was harvested 30 minutes after the challenge injections and the levels of D1 receptor protein levels measured using western blot analysis. The data were analyzed using two-way ANOVA with pre-treatment (saline or anisomycin) and challenge treatment (saline or cocaine) as factors. Significant interaction was found between the pre-treatment and challenge treatment factors (F1,20 = 18.03, p< 0.001). Post-hoc statistical analysis showed that there was a significant increase in D1 receptor protein levels in cocaine-sensitized mice pre-treated with saline and administered a challenge dose of cocaine (t=5.424, p= <0.001) but not in cocaine-sensitized mice pre-treated with anisomycin (t= 0.582, p=0.567). The results in Figure 4a and 4b, shows that pretreatment with anisomycin abolished the cocaine challenge-induced significant increase in D1 receptor protein levels. This suggest that the rapid cocaine-induced increase in D1 receptor protein levels in the caudate-putamen of cocaine-sensitized mice requires de novo protein synthesis.
Figure 4.
The rapid increase in cocaine-induced D1 receptor protein levels and cocaine-induced locomotor activity in cocaine-sensitized drd1a-EGFP mice requires de novo protein synthesis. (a) Relative levels of D1R protein normalized to GAPDH expression levels, 30 minutes after a challenge saline or cocaine (15 mg/kg) injection, in the caudate-putamen of cocaine-sensitized drd1a-EGFP mice that were pre-injected with saline or 150 mg/kg anisomycin, s.c., 60 minutes prior to the challenge injection. N= 6 mice per treatment group. Error bars represent ± SEM. *, P<0.05, statistically significant difference in D1 receptor protein levels between cocaine-sensitized mice pre-injected with saline and challenged with cocaine and all other treatment groups, two-way ANOVA, post-hoc Holm-Sidak test. (b) Representative western blot showing the expression of D1R and GAPDH, in tissue punches of caudate-putamen harvested 30 minute after challenge injection on day 8. The drd1a-EGFP mice were binge-treated with 15 mg/kg cocaine for 7 days and on day 8 administered either vehicle (saline at pH 4.5) and, 60 minutes later, challenged with saline (cocaine/saline/saline) or 15 mg/kg cocaine (cocaine/saline/cocaine) or administered 150 mg/kg anisomycin and, 60 minutes later, challenged with saline (cocaine/Aniso/saline) or 15 mg/kg cocaine (cocaine/Aniso/cocaine). The molecular weight (kD) of D1R and GAPDH detected by the antibodies are indicated. (c) Total horizontal locomotor activity in an Open Field arena for cocaine-sensitized drd1a-EGFP mice that were pre-injected with saline (n=6) or 150 mg/kg anisomycin, s.c. (n=6), 60 minutes prior to the challenge injection with 15 mg/kg cocaine. *, P<0.05, statistically significant difference in locomotor activity in cocaine-challenged mice that were pretreated with saline (filled circles) and anisomycin (black/gray squares), repeated measures two-way ANOVA, post-hoc Holm-Sidak test.
We next determined the effect of anisomycin on cocaine-induced hyperlocomotor activity in cocaine-sensitized mice. The results in Figure 4c shows that pretreatment of cocaine-sensitized mice with 150 mg/kg anisomycin, 60 minutes before the cocaine challenge, completely blocks the cocaine-induced hyperlocomotor activity (F1,60 = 18.175, p<0.001). These results suggest that the acute cocaine-induced hyperlocomotor activity in cocaine-sensitized mice also requires de novo protein synthesis and correlates with the rapid cocaine-induced increase in D1 receptor protein levels in the caudate-putamen of the cocaine-sensitized mice.
Cocaine-induced rapid downregulation of microRNAs that regulate D1 receptor expression
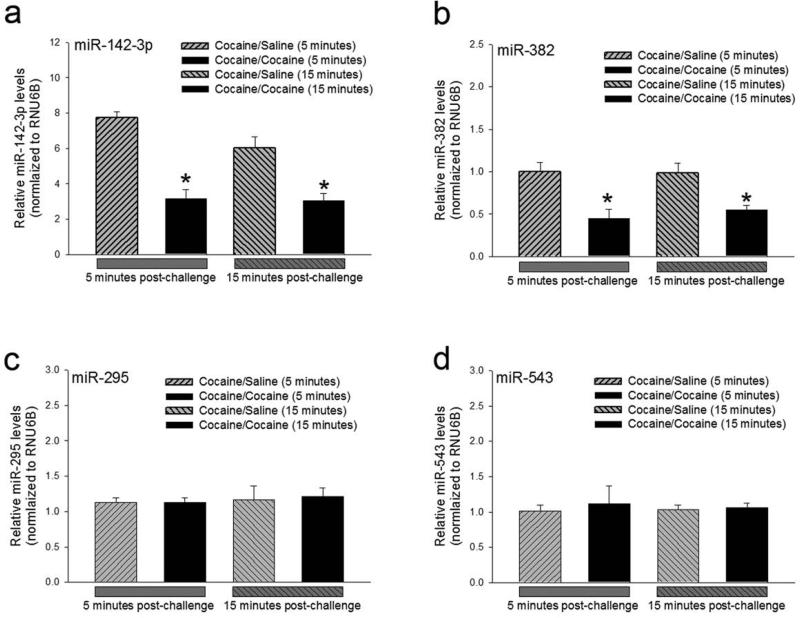
We have recently shown that microRNA miR142-3p and miR382 regulate the expression of D1 receptor (Tobón et al., 2012; Li et al., 2013). Based on the rapid cocaine-induced increase in D1 receptor protein synthesis in sensitized mice, we developed a working hypothesis that post-transcriptional regulation of D1 receptor expression is mediated by microRNA-mediated translational suppression of D1 receptor mRNA. To begin testing temporal feasibility of this model, we measured the levels of miR142-3p and miR382 in the caudate-putamen of cocaine-sensitized mice challenged with saline or 15 mg/kg cocaine. With the expectation that the relief of translational suppression of the D1 receptor mRNA would be preceded by a decrease in levels of these two microRNAs, we measured their levels using real-time RT-PCR at 5 and 15 minutes post challenge injection. The data were analyzed using two-way ANOVA with time (5 minutes or 15 minutes) and challenge treatment (saline or cocaine) as factors. While challenge treatment significantly reduced miR142-3p (F1,16 = 38.256, p<0.001) and miR382 levels (F1,16 = 19.933, p<0.001) at both 5 and 15 minutes, the levels were not significantly different between the two time points (F1, 16 = 2.365, p= 0.144 and F1,16 = 0.161, p= 0.694 for miR142-3p and miR382, respectively). No significant interaction was found between time and challenge treatment factors for either miRNAs (F1,16 = 1.748, p=0.205 and F1,16 = 0.256, p =0.620). Post-hoc analysis of data in Figure 5 showed that miR142-3p (Fig. 5a; t=4.644, p<0.001) and miR382 (Fig. 5b; t= 3.131, p = 0.007) levels are significantly reduced within 5 minutes of the cocaine-challenge in cocaine-sensitized mice. The reduction in microRNA levels are sustained for at least 15 minutes (t=4.130, p<0.001 and t=3.253, p= 0.005 for miR142-3p and miR382, respectively). In contrast to miR142-3p and miR382, two other microRNAs with binding sites in the 3’ untranslated region of the D1 receptor mRNA, miR-295 and miR-543, did not exhibit any significant changes in expression level in cocaine sensitized mice that were challenged with cocaine (Fig. 5c and 5d). The results suggest that the two microRNAs, miR142-3p and miR382, that have been previously shown to post-transcriptionally regulate D1 receptor expression (Tobón et al., 2012; Li et al., 2013), might be involved in the dynamic post-transcriptional regulation of D1 receptor expression in the caudate-putamen of cocaine-sensitized mice.
Figure 5.
Rapid decrease in miR142-3p and miR382 microRNA levels in cocaine-sensitized drd1a-EGFP mice, challenged with cocaine. Relative miR142-3p (a), miR382 (b), miR295 (c) and miR543 (d) microRNA levels normalized to RNU6B small nuclear RNA levels, 5 or 15 minutes after a challenge saline or cocaine (15 mg/kg) injection, in the caudate-putamen of cocaine-sensitized drd1a-EGFP mice. N= 4 to 7 mice per treatment group. Error bars represent ± SEM. *, P<0.05, statistically significant decrease in miR142-3p and miR382 levels at both 5 and 15 minutes in cocaine-sensitized mice challenged with cocaine when compared to all other treatment groups, two-way ANOVA, post-hoc Holm-Sidak test.
Discussion
Repeated cocaine administration induces long-term changes in behavior, gene expression and protein function (Luscher and Malenka 2011; Jonkman and Kenny, 2013). Cocaine-induced behavior sensitization, characterized by locomotor sensitization is a well-established phenomenon that is blocked by D1 receptor antagonists and absent in D1 receptor null mice (Xu et al., 2000; Karasinska et al., 2005; Caine et al., 2007; Karlsson et al., 2008). While changes in steady-state levels of D1 receptor mRNA and protein have been reported in cocaine-sensitized animals, the relative plasticity of these changes, how rapidly these changes can be induced and the mechanisms underlying them are not well understood. Our results show that D1 receptor mRNA levels are increased in the caudate-putamen but not the nucleus accumbens of preadolescent cocaine-sensitized animals (Fig. 2a). More interestingly, the results in this paper provides novel insights into the degree of plasticity of D1 receptor expression in cocaine-sensitized animals and shows for the first time that cocaine-induced change in D1 receptor protein expression happens in a time-scale that is comparable to changes in expression of immediate-early genes. The cocaine-induced increase in D1 receptor protein level occurs within 30 minutes in the caudate-putamen (Fig. 3), but not in the nucleus accumbens (Supplementary Fig. 2), and is only observed in cocaine-sensitized animals (Fig. 3). The latter result suggests that the 7-day binge cocaine-sensitization causes de novo induction of a post-transcriptional mechanism to regulate D1 receptor protein expression, specifically in the caudate-putamen. This post-transcriptional regulatory mechanism is sensitive to cocaine-treatment as it is rapidly attenuated following a challenge cocaine injection to the sensitized animal. In contrast to the nucleus accumbens, the caudate-putamen is involved in habit formation associated with extended drug administration (Everitt and Robbins, 2005; Zapata et al., 2010; Veeneman et al., 2012). Our results suggest that post-transcriptional regulation of D1 receptor expression in the caudateputamen might be centrally involved in the mechanisms underlying habit formation associated with drug abuse.
Our results using the protein synthesis inhibitor, anisomycin, also showed that the post-transcriptional regulation of D1 receptor expression in the cocaine-sensitized mice is likely mediated by suppression of protein translation as opposed to increased protein turnover (Fig. 4a and b). We also show that the cocaine-induced hyperlocomotor activity in cocaine-sensitized mice correlates with the attenuation of D1 receptor post-transcriptional regulation as anisomycin treatment not only prevented the expression of D1 receptor protein but also the cocaine-induced hyperlocomotor activity (Fig. 4c). Note that the dose and duration of anisomycin treatment in this study did not affect the basal level of D1 receptor expression or basal locomotor activity in the cocaine-sensitized mice. The correlation between increased D1 receptor protein levels in the caudate-putamen and increased locomotor activity is also consistent with the previously described role of D1 receptors in the caudate-putamen in mediating locomotor activity (Xu et al., 2000; Schlussman et al., 2003; Karasinska et al., 2005).
Preadolescent mice were used in this study as, compared to adult mice and in utero treated pups, there is much less known about the effect of cocaine on mice at this developmental stage. Preadolescent and adolescent animals exhibit novelty-seeking and risk-taking behaviors, characteristics which eventually result in acquisitions of addictive habits (Spear 2000). We have recently shown that male preadolescent drd1a-EGFP mice exhibit cocaine-induced locomotor sensitization under the binge cocaine treatment paradigm (Tobón and Kuzhikandathil, 2014). It remains to be determined if the post-transcriptional regulation of D1 receptor expression and changes in microRNA levels observed in preadolescent animals are also observed in adults.
One well studied mechanism for mRNA translation suppression is via microRNAs. Recent studies have indicated that microRNAs play an important role in the mechanisms underlying cocaine addiction (reviewed in Jonkman and Kenny, 2013). However, microRNAs that regulate D1 receptor expression in cocaine addiction have not been reported. We have recently identified miR142-3p and miR382 as microRNAs that post-transcriptionally regulate, directly, the D1 receptor expression (Tobón et al., 2012; Li et al., 2013). The microRNA miR382 is particularly interesting as we recently showed that it regulates D1 receptor expression and plays a role in alcohol addiction, raising the possibility that it might be part of a common mechanism for mediating the effects of different drugs of abuse. Our results showing that challenge cocaine injection of cocaine-sensitized mice results in a significant reduction of miR142-3p and miR382 within five minutes (Fig. 5), suggest for the first time that microRNA levels can be rapidly regulated by cocaine; potentially by mechanisms that regulate its stability and levels. While microRNAs, in general, are thought to be stable, interestingly, in vitro studies have shown that miR382 is one of the microRNAs that is inherently unstable with a half-life of ~ 5minutes (Bail et al., 2010). This inherent instability of miR382 makes it ideal for regulating the rapid cocaine-induced translation of D1 receptor mRNA in cocaine-sensitized mice. Mechanisms that regulate microRNA stability, in particular how cocaine rapidly modulates microRNA stability, remains to be determined. With regard to mechanisms that control microRNA levels, a recent study showed rapid depolarization-dependent decrease in microRNA levels in the neurites due to release of microRNA containing exosomes (Goldie et al., 2014). The authors reported significant decrease in miRNA levels following a single 3 minute depolarization pulse. While the authors did not directly measure levels of the proteins targeted by the rapidly downregulated miRNAs, the timescale in which they observe changes in miRNA levels are consistent with our observations of cocaine-induced downregulation of miR142-3p and miR382.
In conclusion, the results in this paper, summarized in Figure 6, provide novel insights into previously unappreciated rapid kinetics of D1 receptor expression in cocaine-sensitized mice challenged with cocaine. It also showed the important role of D1 receptor post-transcriptional regulation in cocaine addiction, revealing a novel mechanism involving translation suppression mediated by highly labile microRNAs whose stability and levels are regulated by cocaine.
Figure 6.
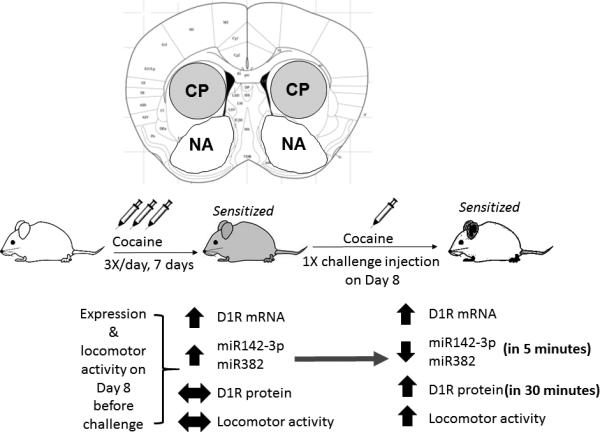
Summary figure showing the changes in expression of D1 receptor mRNA and protein, miRNAs miR142-3p and miR382 at different times in the caudate-putamen (CP) but not nucleus accumbens (NA) of cocaine-sensitized mice. Effects on locomotor activity are also shown. Up and down arrows indicate increase and decrease, respectively. Horizontal arrows indicate no difference between control and sensitized mice.
Supplementary Material
Acknowledgements
This work was supported by NIH grants R03DA026030 and R03DA026030-02S1 and a grant from the F. M. Kirby Foundation to EVK. KET was supported by a PhRMA Foundation pre-doctoral fellowship. SRC was supported by a NIH T32 Predoctoral Training grant (NS 51157-5).
Footnotes
Author's contribution
KET and EVK were responsible for study concept, design and data analysis. KET, JC and SRC contributed to the acquisition of animal data. KET, JC, AS and EVK contributed to RNA, miRNA and protein expression studies and data analysis. KET and SRC performed the behavior studies and analysis. KET and EVK drafted the manuscript. All authors critically reviewed content and approved final version for publication.
Conflict of Interest
The authors declare no conflict of interest
References
- 1.Ariano MA. Distribution of dopamine receptors. In: Neve KA, Neve RL, editors. The Dopamine Receptors. Humana; New Jersey: 1997. pp. 77–103. [Google Scholar]
- 2.Bail S, Swerdel M, Liu H, Jiao X, Goff LA, Hart RP, Kiledjian M. Differential regulation of microRNA stability. RNA. 2010;16(5):1032–1039. doi: 10.1261/rna.1851510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brenhouse HC, Sonntag KC, Andersen SL. Transient D1 dopamine receptor expression on prefrontal cortex projection neurons: relationship to enhanced motivational salience of drug cues in adolescence. J Neurosci. 2008;28(10):2375–2382. doi: 10.1523/JNEUROSCI.5064-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caine SB, Thomsen M, Gabriel KI, Berkowitz JS, Gold LH, Koob GF, Tonegawa S, Zhang J, Xu M. Lack of self-administration of cocaine in dopamine D1 receptor knock-out mice. J Neurosci. 2007;27(48):13140–13150. doi: 10.1523/JNEUROSCI.2284-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8(11):1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 6.Goldie BJ, Dun MD, Lin M, Smith ND, Verrills NM, Dayas CV, Cairns MJ. Activity-associated miRNA are packaged in Map1b-enriched exosomes released from depolarized neurons. Nucleic Acids Res. 2014;42(14):9195–9208. doi: 10.1093/nar/gku594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henry DJ, White FJ. Repeated cocaine administration causes persistent enhancement of D1 dopamine receptor sensitivity within the rat nucleus accumbens. J Pharmacol Exp Ther. 1991;258(3):882–890. [PubMed] [Google Scholar]
- 8.Hummel M, Unterwald EM. D1 dopamine receptor: a putative neurochemical and behavioral link to cocaine action. J Cell Physiol. 2002;191(1):17–27. doi: 10.1002/jcp.10078. [DOI] [PubMed] [Google Scholar]
- 9.Jonkman S, Kenny PJ. Molecular, cellular, and structural mechanisms of cocaine addiction: a key role for microRNAs. Neuropsychopharmacol. 2013;38(1):198–211. doi: 10.1038/npp.2012.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karasinska JM, George SR, Cheng R, O'Dowd BF. Deletion of dopamine D1 and D3 receptors differentially affects spontaneous behaviour and cocaine-induced locomotor activity, reward and CREB phosphorylation. Eur J Neurosci. 2005;22(7):1741–1750. doi: 10.1111/j.1460-9568.2005.04353.x. [DOI] [PubMed] [Google Scholar]
- 11.Karlsson RM, Hefner KR, Sibley DR, Holmes A. Comparison of dopamine D1 and D5 receptor knockout mice for cocaine locomotor sensitization. Psychopharmacology (Berl) 2008;200(1):117–127. doi: 10.1007/s00213-008-1165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kleven MS, Perry BD, Woolverton WL, Seiden LS. Effects of repeated injections of cocaine on D1 and D2 dopamine receptors in rat brain. Brain Res. 1990;532(1-2):265–270. doi: 10.1016/0006-8993(90)91768-c. [DOI] [PubMed] [Google Scholar]
- 13.Laurier LG, Corrigall WA, George SR. Dopamine receptor density, sensitivity and mRNA levels are altered following self-administration of cocaine in the rat. Brain Res. 1994;634(1):31–40. doi: 10.1016/0006-8993(94)90255-0. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Li J, Liu X, Qin S, Guan Y, Liu Y, Cheng Y, Chen X, Li W, Wang S, Xiong M, Kuzhikandathil EV, Ye JH, Zhang C. MicroRNA expression profile and functional analysis reveal that miR-382 is a critical novel gene of alcohol addiction. EMBO Mol. Med. 2013;5(9):1402–1414. doi: 10.1002/emmm.201201900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lüscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69(4):650–663. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pasuit JB, Li Z, Kuzhikandathil EV. Multi-modal regulation of endogenous D1 dopamine receptor expression and function in the CAD catecholaminergic cell line. J. Neurochem. 2004;89(6):1508–1519. doi: 10.1111/j.1471-4159.2004.02450.x. [DOI] [PubMed] [Google Scholar]
- 17.Schlussman SD, Zhang Y, Kane S, Stewart CL, Ho A, Kreek MJ. Locomotion, stereotypy, and dopamine D1 receptors after chronic “binge” cocaine in C57BL/6J and 129/J mice. Pharmacol Biochem Behav. 2003;75:123–131. doi: 10.1016/s0091-3057(03)00067-4. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt-Mutter C, Muller C, Zwiller J, Gobaille S, Maitre M. Gamma-hydroxybutyrate and cocaine administration increases mRNA expression of dopamine D1 and D2 receptors in rat brain. Neuropsychopharmacol. 1999;21(5):662–669. doi: 10.1016/S0893-133X(99)00066-4. [DOI] [PubMed] [Google Scholar]
- 19.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 20.Tobón KE, Chang D, Kuzhikandathil EV. MicroRNA 142-3p mediates post-transcriptional regulation of D1 dopamine receptor expression. PLoS ONE. 2012;7(11):e49288. doi: 10.1371/journal.pone.0049288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tobón KE, Kuzhikandathil EV. Preadolescent drd1-EGFP mice exhibit cocaine-induced behavioral sensitization. Neurosci. Lett. 2014;558:20–25. doi: 10.1016/j.neulet.2013.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsukada H, Kreuter J, Maggos CE, Unterwald EM, Kakiuchi T, Nishiyama S, Futatsubashi M, Kreek MJ. Effects of binge pattern cocaine administration on dopamine D1 and D2 receptors in the rat brain: an in vivo study using positron emission tomography. J Neurosci. 1996;16(23):7670–7677. doi: 10.1523/JNEUROSCI.16-23-07670.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Unterwald EM, Ho A, Rubenfeld JM, Kreek MJ. Time course of the development of behavioral sensitization and dopamine receptor up-regulation during binge cocaine administration. J Pharmacol Exp Ther. 1994;270(3):1387–1396. [PubMed] [Google Scholar]
- 24.Unterwald EM, Kreek MJ, Cuntapay M. The frequency of cocaine administration impacts cocaine-induced receptor alterations. Brain Res. 2001;900(1):103–109. doi: 10.1016/s0006-8993(01)02269-7. [DOI] [PubMed] [Google Scholar]
- 25.Valjent E, Bertran-Gonzalez J, Aubier B, Greengard P, Hervé D, Girault JA. Mechanisms of locomotor sensitization to drugs of abuse in a two-injection protocol. Neuropsychopharmacol. 2010;35(2):401–415. doi: 10.1038/npp.2009.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veeneman MM, Broekhoven MH, Damsteegt R, Vanderschuren LJ. Distinct contributions of dopamine in the dorsolateral striatum and nucleus accumbens shell to the reinforcing properties of cocaine. Neuropsychopharmacol. 2012;37(2):487–498. doi: 10.1038/npp.2011.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu M, Guo Y, Vorhees CV, Zhang J. Behavioral responses to cocaine and amphetamine administration in mice lacking the dopamine D1 receptor. Brain Res. 2000;852(1):198–207. doi: 10.1016/s0006-8993(99)02258-1. [DOI] [PubMed] [Google Scholar]
- 28.Zapata A, Minney VL, Shippenberg TS. Shift from goal-directed to habitual cocaine seeking after prolonged experience in rats. J Neurosci. 2010;30(46):15457–15463. doi: 10.1523/JNEUROSCI.4072-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.