Abstract
Purpose
Tumor size and stage are important prognostic parameters in renal cell carcinoma. While pathological stage T1 and T2 are defined by size alone, the presence of certain intrinsic features can up stage a tumor to pathological stage T3a regardless of size. We investigate the effect of pathological tumor stage on the relationship between tumor size and risk of disease recurrence.
Materials and Methods
Data were reviewed on patients who underwent nephrectomy at our institution between 2006 and 2013 to identify all those with pathological stage T1, T2 and T3a tumors. A proportional hazards Cox model was built with time to recurrence as outcome, and pathological stage and tumor size as covariates. An interaction term for stage and tumor size was included.
Results
The final cohort included 1,809 patients. On multivariable analysis, when adjusted for tumor size, patients with pT3a tumors had a greater risk of tumor recurrence compared to those with pT1/T2 tumors (HR 3.70; 95% CI 2.31, 5.92; p <0.0001). The risk of disease recurrence increased more rapidly as tumor size increased only with the presence of perinephric fat invasion (p=0.006).
Conclusions
Using the AJCC 2010 staging criteria we validated pathological stage T3a as a poor prognostic factor in renal cell carcinoma regardless of tumor size. Our results also demonstrated an increased rate of risk of recurrence with perinephric fat invasion. Given this increased risk of recurrence, even in tumors less than 4 cm, closer surveillance is warranted in such cases and the role of perinephric involvement necessitates further investigation.
Keywords: carcinoma, renal cell, recurrence, neoplasm staging
Kidney cancer rates in the United States have increased steadily during the last 10 years to more than 65,000 new cases diagnosed in 2013 with 13,680 deaths recorded.1 The management and followup of patients with RCC are largely dependent on accurate pathological tumor staging. According to the AJCC 2010 TNM staging manual, classification of pathological stage T1 and T2 RCC tumors depends solely on size (7 cm or less for pT1 and greater than 7 cm for pT2 lesions limited to the kidney), whereas pT3a tumors are defined by the presence of additional tumor characteristics regardless of tumor size, including renal vein invasion, muscular venous branch invasion, perinephric fat invasion or renal sinus fat invasion.2 Advantages of the latest staging edition include improved ability to differentiate cancer specific survival among stages, recognition of adrenal gland invasion as a more aggressive feature and characterization of MVBI as equivalent in prognosis to RVI.3–5
Small renal masses, defined as tumors 4 cm or less, are effectively treated with NSS with excellent oncologic outcomes. Recent studies have demonstrated that tumors up to 7 cm can also be treated safely and effectively with NSS.6 A majority of these lesions fall in the pT1/2 category. However, with the limitations of current imaging modalities as clinical staging tools, a proportion of SRMs, especially those with a central location, or subtle involvement of PN or SF, are ultimately up staged to pT3a on final pathological review.7
Among pT3a tumors MVBI and RVI have been found to be features associated with decreased CSS compared to pT1/2 tumors, with further venous extension into the inferior vena cava and beyond demonstrating inferior survival.5,8 PN invasion and SF invasion have also been independently associated with death from RCC, with SF invasion portending a poorer outcome than PN invasion.9–11 Furthermore, the combination of fat invasion and RVI has been shown to have a greater impact on survival than each individually.8,12,13 Studies have also implicated the renal sinus as the most common site of extrarenal extension, with SF invasion correlating with higher grade and larger size.14,15 A size dependent difference in CSS has also been noted in pT3a tumors. The 5-year survival rate for cases with tumors 7 cm or less is 70% vs 46% for tumors larger than 7 cm.16 However, controversy remains regarding the prognostic implications of PN invasion and tumor size in pT3a tumors.17,18 Therefore, in this study we investigate the impact of pathological tumor stage on the relationship between tumor size and risk of disease recurrence using the 2010 AJCC TNM staging criteria.
MATERIALS AND METHODS
After receiving institutional review board approval we retrospectively collected data for all patients (2,285) who underwent partial or radical nephrectomy at our institution between 2006 and 2013. Standardized documentation of MVBI began at our institution in 2003 and 2006 was chosen to represent a modern series in which recording by our dedicated uropathologists of MVBI was already standard practice. Patients with benign histology (275), unclear tumor staging (35) or pathological stage greater than pT3aN0M0 (159) were excluded from analysis. In the pT3a group 7 tumors lacked details of the specific type of invasion and were also excluded from final analysis.
The final cohort consisted of 1,809 patients, including 1,483 with pT1 or pT2 tumors (82%) and 326 with pT3a tumors (18%). In the 2010 AJCC TNM staging criteria pT1 tumors are characterized as tumors 7 cm or less (pT1a 4 cm or less and pT1b greater than 4 to 7 cm), pT2 tumors are greater than 7 cm (pT2a greater than 7 to 10 cm and pT2b greater than 10 cm) and pT3a tumors with evidence of PN invasion, SF invasion, MVBI or RVI.2 It is standard practice at our institution to include tumor adjacent perinephric fat in every partial and radical nephrectomy specimen for accurate tumor staging.
We assessed the increased risk of recurrence by tumor size in patients with pT3a disease, and compared that increase to patients with pT1 and pT2 disease. We used locally weighted Kaplan-Meier estimates to plot the 3-year risk of disease recurrence by tumor size separately for pT3a tumors and pT1/T2 tumors. To confirm patients with pT3a disease indeed have a poorer outcome we built a proportional hazards Cox model with time to recurrence as outcome, and pathological stage (pT1/pT2 vs pT3a) and tumor size as covariates.
We also assessed whether the risk of recurrence had a steeper increase by tumor size for pT3a cases compared to pT1/T2 cases. Thus, we included an interaction term for pathological tumor stage and tumor size in the previously mentioned Cox model. To gauge whether the presence of specific types of invasion that define pT3a tumors (renal vein, muscular branch, perinephric or sinus fat) are driving the relationship between recurrence and pT3a tumors, we repeated this analysis, replacing pathological tumor stage for each type of invasion.
Because there is controversy over the aggressive or indolent nature of SRMs that are postoperatively designated T3a, we also compared the risk of disease recurrence for pT1a tumors to those that were pT3a in masses less than 4 cm. All analyses were completed using Stata® 12.0.
RESULTS
Table 1 lists the clinicopathological characteristics of our cohort (1,809) by pathological tumor stage, as pT1/pT2 (1,483) vs pT3a (326). Median age at surgery was 59 years (IQR 50, 67) among patients with pT1/T2 disease and 62 years (IQR 54, 69) for those with pT3a disease (p <0.0001). The majority of our cohort was Caucasian (89% and 87% for the pT1/2 and pT3a groups, respectively) and male (66% and 69% for pT1/2 and pT3a, respectively). The majority of tumors were histologically clear cell (61% in pT1/2 tumors and 74% in pT3a tumors). Median primary tumor size was smaller in the pT1/pT2 group compared to the pT3a group at 3.0 cm (IQR 2, 4.5) vs 5.6 cm (IQR 4, 8.5), respectively. Of patients in the pT1/2 group 89% underwent open or minimally invasive NSS compared to 48% in the pT3a group. In the pT1/T2 group 94% of tumors were classified as pT1 (7 cm or less) and 6% were classified as pT2 (greater than 7 cm). In the pT3a group 65% of tumors were 7 cm or less and 35% were greater than 7 cm.
Table 1.
Clinicopathological features of cohort
| No. pT1/pT2 (%) | No. pT3a (%) | p Value | |
|---|---|---|---|
| Gender: | |||
| F | 505 (34) | 102 (31) | 0.3 |
| M | 978 (66) | 224 (69) | |
| Race: | |||
| White | 1,298 (88) | 276 (85) | 0.023 |
| Black | 89 (6.0) | 12 (3.7) | |
| Asian | 52 (3.5) | 21 (6.4) | |
| Other | 21 (1.4) | 7 (2.1) | |
| Unknown | 23 (1.6) | 10 (3.1) | |
| Surgical approach: | |||
| Open radical nephrectomy | 134 (9.0) | 146 (45) | <0.0001 |
| Open partial nephrectomy | 947 (64) | 120 (37) | |
| Laparoscopic partial nephrectomy | 367 (25) | 35 (11) | |
| Laparoscopic radical nephrectomy | 35 (2.4) | 25 (7.7) | |
| Size of primary lesion (cm): | <0.0001 | ||
| 4 or Less | 1,053 (71) | 91 (28) | |
| Greater than 4 to 7 | 336 (23) | 122 (37) | |
| Greater than 7 to 10 | 59 (4.0) | 69 (21) | |
| Greater than 10 | 35 (2.4) | 44 (13) | |
| Tumor histology: | |||
| Chromophobe | 165 (11) | 35 (11) | <0.0001 |
| Clear cell | 908 (61) | 242 (74) | |
| Other | 169 (11) | 34 (10) | |
| Papillary | 241 (16) | 15 (4.6) | |
| Type of invasion:* | |||
| Renal vein | – | 66 (20) | |
| PN | – | 107 (33) | |
| Muscular branch | – | 94 (29) | |
| Renal SF | – | 137 (42) | |
There were 71 patients with more than 1 type of feature present.
Overall 5-year DFS was 94% (95% CI 92–95). For pT1/2 tumors and pT3a tumors the 5-year DFS was 96% (95% CI 94–97) and 82% (95% CI 74–88), respectively (p <0.005). For pT1a tumors and pT3a tumors less than 4 cm the 5-year DFS was 98% (95% CI 95–99) and 93% (95% CI 79–98), respectively (p <0.005).
SF invasion was the most frequent type of invasion (42%) in the pT3a group. PN invasion, MVBI and RVI were found in 33%, 29% and 20% of patients, respectively. Of the pT3a group 73 (22%) demonstrated more than 1 type of invasion present at pathology.
Median time to followup for patients without disease recurrence was 2.6 years. Overall disease recurred in 72 patients during the study period. Ten patients had recurrence in the prior tumor resection bed, 7 had metachronous tumor formation in the contralateral kidney with the same histology and 55 had distant metastases. Of the distant metastases 85% were clear cell histology, 5.5% were papillary and 9.1% were other histologies.
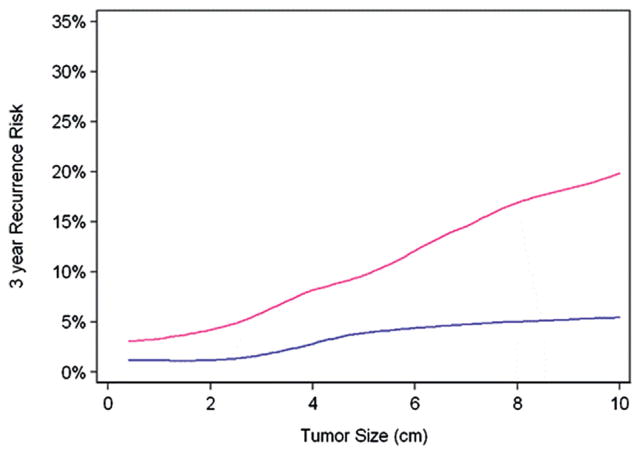
Figure 1 shows the 3-year risk of disease recurrence by tumor size among the pT1/T2 group vs the pT3a group. Patients with increasing tumor size had a greater risk of disease recurrence regardless of tumor stage (p <0.0001). We did not find evidence that the risk of recurrence increased faster by tumor size for pT3a compared to pT1/T2 disease (p=0.3). However, when we tested for the overall effect of pathological tumor stage on disease recurrence using a Cox proportional hazards model adjusted for tumor size, we found that patients in the pT3a group had a greater risk of disease recurrence with a hazard ratio of 3.70 compared to those in the pT1/T2 group (95% CI 2.31, 5.92; p <0.0001).
Figure 1.
Disease recurrence risk (3-year) by primary tumor size for pT1/2 (blue line) vs pT3a (red line) group.
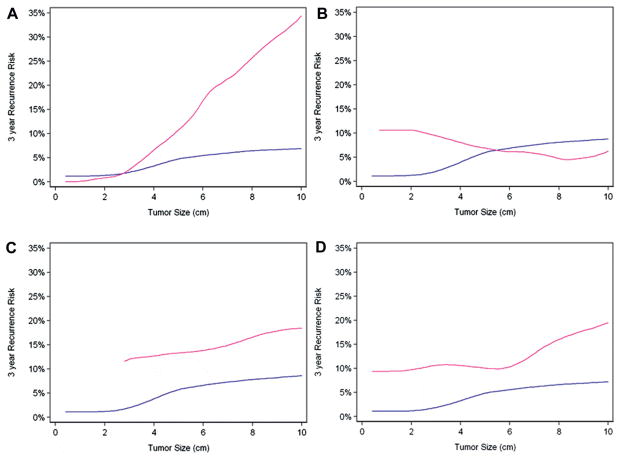
Figure 2 shows disease recurrence risk by tumor size and presence or absence of specific type of invasion. Only PN invasion was statistically significant as an interaction term (p=0.006). This means that the increase in risk for a 1 cm difference in tumor size was greater for patients with PN than for those without PN. We did not find evidence for the same effect with other types of invasion (renal vein invasion p=0.5, muscular branch invasion p=0.2 and renal sinus invasion p=0.4). However, each type of invasion, namely RVI (HR 2.57; 95% CI 1.02, 6.48; p=0.045), MVBI (HR 2.51; 95% CI 1.14, 5.53; p=0.023) and SF invasion (HR 3.91; 95% CI 2.34, 6.54; p <0.0001), had a statistically significant overall effect on disease recurrence on multivariate Cox regression analysis adjusted for tumor size (table 2). Exclusion of contralateral recurrence had no impact on hazard ratios. When limiting our analysis to tumors smaller than 4 cm, we identified 17 cases of recurrence. The hazard ratio for pT3a tumors smaller than 4 cm was 4.38 compared to pT1a tumors (95% CI 1.41, 13.62; p=0.011).
Figure 2.
Risk of disease recurrence among patients with (pink line) and without (blue line) specific type of invasion by primary tumor size, including perinephric fat invasion (A), renal vein invasion (B), muscular branch invasion (C ) and renal sinus invasion (D).
Table 2.
Multivariable analysis adjusted for tumor size
| HR | 95% CI | p Value | |
|---|---|---|---|
| Pathological stage T3a | 3.70 | 2.31, 5.92 | <0.0001 |
| Pathological stage T3a (size less than 4 cm) | 4.38 | 1.41, 13.62 | 0.011 |
| SF invasion | 3.91 | 2.34, 6.54 | <0.0001 |
| Muscular branch invasion | 2.51 | 1.14, 5.53 | 0.023 |
| RVI | 2.57 | 1.02, 6.48 | 0.045 |
DISCUSSION
In this study we examined the impact of tumor size stratified by pathological stage on the risk of recurrence. Increasing size was associated with increased recurrence in pathological T1/2 tumors as well as T3a tumors. We found that pT3a tumors had an approximately fourfold increased risk of recurrence compared to pT1/2 tumors. We also found that the rate of change of risk of recurrence as tumor size increased was dependent on the presence of perinephric fat invasion but not on other pT3a features. Sinus fat invasion, muscular venous branch invasion and renal vein invasion predicted a higher risk of disease recurrence on multivariable analysis adjusted for tumor size. Due to the large number of pT3a cases with more than 1 type of invasion, we cannot exclude the possibility that the hazard ratios for recurrence may vary from what would occur with only a single invasion type.
The revised AJCC 2010 TNM staging system does not account for size as a criterion when categorizing tumors pT3a and higher, and instead relies solely on extension and invasion into surrounding structures.2 In a multicenter retrospective validation of the 7th edition of the TNM classification, Novara et al confirmed its ability to predict CSS, but were unable to detect a difference in CSS between subgroups pT2b and pT3 and between pT3c and pT4 at a median followup of 42 months.19 Furthermore, they found that pT3a tumors did not encompass a homogenous group. Tumors with PN invasion or RVI had similar outcomes, but those with both types of invasion had inferior CSS. However, this study was limited as pT3a tumors were only divided into PN invasion or RVI without separating SF invasion or including MVBI. Another notable limitation was the lack of central pathological review.
In an evaluation of 120 clear cell RCCs that had renal sinus invasion Bonsib described that 68% of tumors 4 to 7 cm and 97% of tumors larger than 7 cm met the criteria for pT3a disease, making pT2 a rare entity.20 This is consistent with our findings that among the pT1/2 group, 94% of tumors were 7 cm or less. Bonsib et al also reported that the renal sinus is the most frequent site of invasion, which was consistent with our finding that 42% of pT3a tumors in our cohort had SF invasion.14,15
In the present study we demonstrated that the risk of recurrence is similar between RVI and MVBI, which is in agreement with a previous report by Feifer et al, who found that patients with MVBI and RVI have a similarly poor survival compared to those without these types of invasion.5 We also showed that the impact of these forms of invasion remained significant when adjusting for tumor size, which is consistent with a study by Siddiqui et al, who found that PN and SF invasion were associated with worse CSS independent of tumor size.9
On analysis of masses less than 4 cm we found that pT3a tumors were associated with an increased risk of recurrence compared to pT1a tumors (HR 4.38; 95% CI 1.41, 13.62; p=0.011), although our cohort of patients with recurrence in tumors of this size was small (17). In a study of 33 patients with pT1 RCC who died of their disease Thompson et al recognized that 58% had fat or small vein invasion on pathological rereview compared to 21% who did not have recurrence (p <0.001).21 This finding highlights the importance of accurate pathological staging, including review of fat and vascular invasion, as these features are associated with increased recurrence even in SRMs.
An important implication of this study is that pathological stage T3a encompasses tumors that are more aggressive than their T1/2 counterparts, even for tumors less than 4 cm. These tumors are associated with a significantly increased risk of recurrence, suggesting that closer surveillance may be warranted in such patients. Given such information, postoperative surveillance protocols at our institution derive largely from pathological stage rather than size based criteria. As active surveillance for SRMs becomes increasingly used with low rates of disease progression in selected patients, additional studies will be necessary to uncover aggressive tumor characteristics, possibly using molecular or specialized imaging techniques to aid in risk stratification.22,23
There are several limitations of this investigation, and chief among these are the relatively short 2.6-year median followup and small number of events in the study population, particularly for tumors less than 4 cm. This limited our analysis to disease recurrence, since it would not be powered to detect differences in cancer specific or overall survival. Studies with larger numbers of pT3a tumors less than 4 cm will be needed to confirm our findings and help provide insight into the risks associated with each invasion type in SRMs, PN invasion in particular.
CONCLUSIONS
Using the current AJCC 2010 TNM staging criteria we demonstrated that patients with pT3a tumors have an approximate fourfold increased risk of disease recurrence for a given tumor size compared to patients with pT1/T2 disease. Findings are similar for smaller tumors less than 4 cm. Perinephric fat involvement was found to increase the rate of risk of recurrence disproportionately with increasing tumor size. However, understanding the significance of this finding requires further investigation. This study highlights the increased risk of recurrence in pT3a tumors across all sizes, and specifically in tumors less than 4 cm, which are often managed less aggressively.
Acknowledgments
Supported by the Sidney Kimmel Center for Prostate and Urologic Cancers, and the Hanson Family Renal Cancer Research Fund.
Abbreviations and Acronyms
- AJCC
American Joint Committee on Cancer
- CSS
cancer specific survival
- DFS
disease-free survival
- MVBI
muscular venous branch invasion
- NSS
nephron sparing surgery
- PN
perinephric fat
- RCC
renal cell carcinoma
- RVI
renal vein invasion
- SF
sinus fat
- SRM
small renal mass
Footnotes
Study received institutional review board approval.
Nothing to disclose.
References
- 1.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2010. Bethesda: National Cancer Institute; 2013. Based on November 2012 SEER data submission, posted to the SEER web site, April 2013. [Google Scholar]
- 2.Edge S, Byrd DR, Compton CC, et al. AJCC Cancer Staging Manual. 7. New York: Springer; 2010. [Google Scholar]
- 3.Veeratterapillay R, Simren R, El-Sherif A, et al. Accuracy of the revised 2010 TNM classification in predicting the prognosis of patients treated for renal cell cancer in the north east of England. J Clin Pathol. 2012;65:367. doi: 10.1136/jclinpath-2011-200468. [DOI] [PubMed] [Google Scholar]
- 4.Thompson RH, Leibovich BC, Cheville JC, et al. Should direct ipsilateral adrenal invasion from renal cell carcinoma be classified as pT3a? J Urol. 2005;173:918. doi: 10.1097/01.ju.0000153419.98715.24. [DOI] [PubMed] [Google Scholar]
- 5.Feifer A, Savage C, Rayala H, et al. Prognostic impact of muscular venous branch invasion in localized renal cell carcinoma cases. J Urol. 2011;185:37. doi: 10.1016/j.juro.2010.08.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson RH, Siddiqui S, Lohse CM, et al. Partial versus radical nephrectomy for 4 to 7 cm renal cortical tumors. J Urol. 2009;182:2601. doi: 10.1016/j.juro.2009.08.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorin MA, Ball MW, Pierorazio PM, et al. Outcomes and predictors of clinical T1 to pathological T3a tumor up-staging after robotic partial nephrectomy: a multi-institutional analysis. J Urol. 2013;190:1907. doi: 10.1016/j.juro.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 8.Leibovich BC, Cheville JC, Lohse CM, et al. Cancer specific survival for patients with pT3 renal cell carcinoma–can the 2002 primary tumor classification be improved? J Urol. 2005;173:716. doi: 10.1097/01.ju.0000151830.27750.d2. [DOI] [PubMed] [Google Scholar]
- 9.Siddiqui SA, Frank I, Leibovich BC, et al. Impact of tumor size on the predictive ability of the pT3a primary tumor classification for renal cell carcinoma. J Urol. 2007;177:59. doi: 10.1016/j.juro.2006.08.069. [DOI] [PubMed] [Google Scholar]
- 10.Bertini R, Roscigno M, Freschi M, et al. Renal sinus fat invasion in pT3a clear cell renal cell carcinoma affects outcomes of patients without nodal involvement or distant metastases. J Urol. 2009;181:2027. doi: 10.1016/j.juro.2009.01.048. [DOI] [PubMed] [Google Scholar]
- 11.Thompson RH, Leibovich BC, Cheville JC, et al. Is renal sinus fat invasion the same as perinephric fat invasion for pT3a renal cell carcinoma? J Urol. 2005;174:1218. doi: 10.1097/01.ju.0000173942.19990.40. [DOI] [PubMed] [Google Scholar]
- 12.Baccos A, Brunocilla E, Schiavina R, et al. Differing risk of cancer death among patients with pathologic T3a renal cell carcinoma: identification of risk categories according to fat infiltration and renal vein thrombosis. Clin Genitourin Cancer. 2013;11:451. doi: 10.1016/j.clgc.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Bedke J, Buse S, Pritsch M, et al. Perinephric and renal sinus fat infiltration in pT3a renal cell carcinoma: possible prognostic differences. BJU Int. 2009;103:1349. doi: 10.1111/j.1464-410X.2008.08236.x. [DOI] [PubMed] [Google Scholar]
- 14.Bonsib SM. The renal sinus is the principal invasive pathway: a prospective study of 100 renal cell carcinomas. Am J Surg Pathol. 2004;28:1594. doi: 10.1097/00000478-200412000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Bonsib SM, Gibson D, Mhoon M, et al. Renal sinus involvement in renal cell carcinomas. Am J Surg Pathol. 2000;24:451. doi: 10.1097/00000478-200003000-00015. [DOI] [PubMed] [Google Scholar]
- 16.Lam JS, Klatte T, Patard JJ, et al. Prognostic relevance of tumour size in T3a renal cell carcinoma: a multicentre experience. Eur Urol. 2007;52:155. doi: 10.1016/j.eururo.2007.01.106. [DOI] [PubMed] [Google Scholar]
- 17.Yoo C, Song C, Hong JH, et al. Prognostic significance of perinephric fat infiltration and tumor size in renal cell carcinoma. J Urol. 2008;180:486. doi: 10.1016/j.juro.2008.04.034. [DOI] [PubMed] [Google Scholar]
- 18.Gofrit ON, Shapiro A, Pizov G, et al. Does stage T3a renal cell carcinoma embrace a homogeneous group of patients? J Urol. 2007;177:1682. doi: 10.1016/j.juro.2007.01.064. [DOI] [PubMed] [Google Scholar]
- 19.Novara G, Ficarra V, Antonelli A, et al. Validation of the 2009 TNM version in a large multi-institutional cohort of patients treated for renal cell carcinoma: are further improvements needed? Eur Urol. 2010;58:588. doi: 10.1016/j.eururo.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 20.Bonsib SM. T2 clear cell renal cell carcinoma is a rare entity: a study of 120 clear cell renal cell carcinomas. J Urol. 2005;174:1199. doi: 10.1097/01.ju.0000173631.01329.1f. [DOI] [PubMed] [Google Scholar]
- 21.Thompson RH, Blute ML, Krambeck AE, et al. Patients with pT1 renal cell carcinoma who die from disease after nephrectomy may have unrecognized renal sinus fat invasion. Am J Surg Pathol. 2007;31:1089. doi: 10.1097/PAS.0b013e31802fb4af. [DOI] [PubMed] [Google Scholar]
- 22.Sun M, Abdollah F, Bianchi M, et al. Treatment management of small renal masses in the 21st century: a paradigm shift. Ann Surg Oncol. 2012;19:2380. doi: 10.1245/s10434-012-2247-0. [DOI] [PubMed] [Google Scholar]
- 23.Crispen PL, Viterbo R, Boorjian SA, et al. Natural history, growth kinetics, and outcomes of untreated clinically localized renal tumors under active surveillance. Cancer. 2009;115:2844. doi: 10.1002/cncr.24338. [DOI] [PMC free article] [PubMed] [Google Scholar]