Abstract
Objectives
This study investigated the transdentinal cytotoxicity of glutahaldehyde-containing solutions/materials on odontoblast-like cells.
Methods
Dentin discs were adapted to artificial pulp chambers. MDPC-23 cells were seeded on the pulpal side of the discs and the occlusal surface was treated with the following solutions: water, 2%glutaraldehyde (GA), 5%GA, 10%GA, Gluma Comfort Bond+Desensitizer (GCB+De) or Gluma Desensitizer (GDe). Cell viability and morphology were assessed by the Alamar Blue assay and SEM. The eluates were collected and applied on cells seeded in 24-well plates. After 7 or 14 days the total protein (TP) production, alkaline phosphatase activity (ALP) and deposition of mineralized nodules (MN) were evaluated.
Results
Data were analyzed by Kruskal-Wallis and Mann-Whitney tests (p<0.05). GA solutions were not cytotoxic against MDPC-23. GCB+De (85.1%) and GDe (77.2%) reduced cell viability as well as TP production and ALP activity at both periods. After 14 days, GCB+De and GDe groups produced less MN. Affected MDPC-23 presented deformation of the cytoskeleton and reduction of cellular projections.
Conclusions
The treatment with 2.5%, 5% and 10%GA was not harmful to odontoblast-like cells. Conversely, when GA was combined with other components like HEMA, the final material became cytotoxic.
Clinical significance
Glutaraldehyde has been used to decrease dentin hypersensitivity. This substance is also capable of preventing resin-dentin bond degradation by cross-linking collagen and MMPs. This study showed that GA might be safe when applied on acid etched dentin. However, when combined with HEMA the product becomes cytotoxic.
Keywords: glutaraldehyde, cytotoxicity, dentin, odontoblasts, hydroxyethylmetacrylate
INTRODUCTION
Dentin hypersensitivity is a multifactorial disorder that results in an acute response to a non-noxious sensory stimulus.1 Its primary cause appears to be the exposure of dentinal tubules in the oral cavity, which allows movement of dentinal fluid and generates a heightened response to tactile, chemical, thermal and osmotic stimuli that can range from mild discomfort to extreme pain.2 A wide variety of treatments for dentin hypersensitivity is available. Topical application of products able to desensitize the nerve fibers or to occlude the dentinal tubules is the most common form of treatment.3–5
Glutaraldehyde (GA) is a cross-linking fixative and disinfecting agent that reacts with the ε-amino groups to induce the formation of cross-links.6,7 In medical research GA is used in many different ways such as the construction of bioprosthetic heart valves,8,9 modification of gelatins and other materials and tissues.8,10,11 In dental field, it has been used to desensitize sensitive exposed dentin,12 to inhibit MMPs13 and to increase the mechanical properties of demineralized collagen prior to bonding procedures.14
Gluma Desensitizer® and Gluma Comfort® Bond + Desensitizer (De) contain 5% glutaraldehyde, 35% hydroxyethylmethacrylate (HEMA) and 60% water. GA is responsible for precipitating plasma proteins (especially albumin) in dentin to block dentin tubules15 while HEMA reacts with this precipitate to form a mixture of polyHEMA and glutaraldehyde-cross-linked albumin16 that reduces the movement of dentinal fluid and dentin hypersensitivity.
It has been shown that a solution containing only 5% GA applied for 60s on 0.4 mm-thick dentin discs after acid-etching did not exert harmful effects on MDPC-23 cells.17 Conversely, HEMA has been proven able to diffuse through dentinal tubules due to its hydrophylicity and small molecular weight.18 Once this monomer reaches the pulp tissue it inhibits cellular metabolism and pulp tissue inherent defense mechanisms.19–22 Taking into account that Gluma Dessensitizer and Gluma Comfort Bond+De contain both GA and HEMA in their composition the aim of this study was to evaluate the transdentinal cytotoxicity of these products and three different concetrations of GA on odontoblast-like cells.
MATERIALS AND METHODS
Preparation of Dentin Discs
Thirty-six sound third molars were obtained upon approval by the Ethics Committee of the Araraquara School of Dentistry – UNESP, and stored in 0.12% thymol solution at 4°C for up to 3 months. One 0.5 mm-thick dentin disc with no enamel islets or pulp horn projections was obtained from the mid-coronal dentin of each tooth using a precision cutting machine equipped with a water-cooled diamond saw (Isomet 1000, Buehler Ltda., Lake Bluff, IL, USA). The occlusal side of the discs was then manually abraded with wet 320-grit silicon carbide paper to reach a final thickness of 0.4 mm as measured with a digital caliper providing a precision to 0.01 mm (Mitutoyo Sul Americana Ltda, Suzano, Sao Paulo, Brazil).
Permeability Allocations
Dentin permeability was determined to permit a homogeneous distribution of the dentin discs into six groups (n=6). The smear layer on both sides of the discs was removed by 0.5 M EDTA (pH 7.4) applied for 60 s. The discs were rinsed and individually placed in in vitro pulp chambers (IVPCs) modified from Hanks et al.23 The IVPC was connected to a 180 cm column of water for 5 min, and after that the movement of a microbubble introduced through a metallic cannula was recorded during 1 min. The obtained values were transformed into hydraulic conductance values and the discs were allocated into the groups in such a way that the mean hydraulic conductance was statistically similar among them (ANOVA, p>0.05). After measuring the permeability, a fresh smear layer was created on the occlusal side of each disc with a 600-grit silicon carbide paper for 10 s. Then, the discs mounted in the IVPCs were sterilized in ethylene oxide. An area of 0.28 cm2 of exposed dentin was standardized for all discs by o’rings.
Seeding MDPC-23
MDPC-23 is an immortalized cell line from fetal mouse molar dental papillae able to express dentin sialoprotein and other proteins expressed by odontoblasts.24,25 The cells were sub-cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; Sigma Aldrich Corp., St. Louis, MO, USA) containing 10% fetal bovine serum (FBS; Cultilab, Campinas, SP, Brazil), 100 IU/mL penicillin, 100 μg/mL streptomycin and 2 mmol/L glutamine (Gibco, Grand Island, NY, USA) in an humidified incubator with 5% CO2 and 95% air at 37°C (Isotemp Fisher Scientific, Pittsburgh, PA, USA) for 3 days until reaching the number of cells necessary to perform the study. The cells (3×104) were seeded on the pulpal side of the dentin discs (0.28 cm2) in 24-well plates (COSTAR 3595 - Corning Incorporated, Corning, NY, USA) and maintained in an incubator with 5% CO2 and 95% air at 37°C. After 48h the IVPCs were carefully removed from the compartments and returned to the same well with the occlusal side up to receive the treatment solutions/materials.
Application of the Treatments
Six treatments were tested (n=6) in this study: deionized water (control), 2.5% GA (Sigma-Aldrich Corp) in water, 5% GA in water, 10% GA in water, Gluma Dessensitizer and Gluma Comfort Bond + De (Heraeus Kulzer Inc, Armonk, NY, USA). The occlusal surface of the dentin discs was etched with 35% phosphoric acid (Scotchbond etchant, 3M ESPE. St. Paul, MN, USA) for 15 s, carefully rinsed with deionized water for 10 s and blot dried with sterilized cotton pellets. Then, 20 μL of the GA solutions (2.5%, 5% or 10%) were applied for 60 s, followed by water rinsing and blot drying. Gluma Dessensitizer, Gluma Comfort Bond + D were applied according to the manufacturer instructions (Table 1). All procedures were performed in a vertical laminar flow chamber to prevent contamination and, immediately after, the IVPCs were placed again in a CO2 incubator for additional 24 h.
Table 1.
Main components and instructions for Gluma® Dessensitizer and Gluma Comfort® Bond+Desensitizer use.
| Product (Manufacturer) | Main components | Instructions for use |
|---|---|---|
| Gluma® Desensitizer (Heraeus Kulzer GmbH, Hanau, Germany) | (2-hydroxyethyl-)methacrylate – 25–50% Glutaldedehyde – 5–10% Purified water |
Apply the smallest possible amount of GLUMA Desensitizer required for treatment to the dentinal surface using a microbrush and leave for 30s. Dry the surface carefully by applying a stream of compressed air until the fluid film has disappeared and Ihe surface is no longer shiny. Then rinse thoroughly with water. |
| Gluma Comfort® Bond + Desensitizer (Heraeus Kulzer GmbH, Hanau, Germany) | Ethanol – 25–50% (2-hydroxyethyl-)methacrylate – 10–25% Poly(methacrylic-oligo-acrylic acid) – 5–10% 4-methacryloxyethyltrimellitic acid anhydride – 0–5% Glutaraldehyde 5% |
Afer acid-etching apply a copious amount to the entire cavity surface. Wait for 15s. Use a gentle air blast to evaporate solvent and water. Light cure GLUMA Comfort Bond+De for 20s with a standard lightcuring unit. |
Alamar Blue Assay
Cell viability was analyzed by Alamar Blue® assay (Life Technologies, Carsbad, CA, USA) (n=6). It is based on the reduction of active compound Resazurin by viable cells generating the product Resorufin. After 24 hours of incubation the discs were carefully removed from the IVPCs and placed in a 24-well plate. The cells were rinsed with 1 mL of PBS and then 450 μL of DMEM without FBS and 50 μL of Alamar Blue® solution were added. The discs were incubated at 37°C and 5% CO2 for 4 hours. Three 100 mL aliquots were transferred from the wells to a 96-well plate and the absorbance of each triplicate sample was measured in a plate reader (ELX 800 - Universal Microplate Reader- BIOTEK Instruments, ICC, USA) at wavelengths 570 nm and 600 nm. The absorbance data was transformed into percentage reduction, considering the control group as 100% cell viability.
Total Protein Production
After dentin treatment, the eluate was collected and placed in contact with new-cultured MDPC-23 cells seeded in 24-well plates for 24 hours. Then, the eluate was carefully aspirated and replaced with DMEM. Total protein (TP) production was evaluated at 7 and 14 days laer, according to the Read and Northcote protocol (1981), as previously described by Basso et al.26 The cells were washed three times with 1 mL PBS at 37°C and 1 mL of 0.1% sodium lauryl sulfate (Sigma-Aldrich Corp.) were added to each well for 40 min at room temperature to produce cell lysis. After homogenization an aliquot of 1 mL of each well was transferred to 24 well-plates while the blank received 1 mL of distilled water. Next, the Lowry reagent solution (Sigma-Aldrich Corp, St Louis, MO, USA) was added (1 mL) to all samples, the plates were agitated for 10 s and after 20 min, 500 mL of Folin-Ciocalteau’s phenol reagent solution (Sigma-Aldrich Corp, St Louis, MO, USA) were added to each sample and mixed. Three 100 μL aliquots of each well were transferred to a 96-well plate after 30 min and the absorbance was read at 655 nm in a plate reader (Thermo Plate). The average of the three values was used for statistical analysis. Absorbance values were transformed into percentage as was previously explained above.
Alkaline Phosphatase (ALP) Activity
ALP production was also assessed 7 and 14 days after the 24-hour contact with the eluates, as previously described (Soares et al.27) (n=6). Absorbance was then read at a 590-nm wavelength with an ELISA microplate reader (Tp Reader; Thermoplate, Nanshan District, Shenzhen, China), and converted into IU/L by means of a standard curve with known concentrations of ALP. The amount of total protein (TP) was used for normalization of ALP according to the Read and Northcote protocol, as previously described. The absorbance of the test and blank compartments was measured at a 655-nm wavelength with the microplate reader. The absorbance value obtained was converted into mg/L by a standard protein curve. The final value of ALP was expressed in IU ALP/mg.
Alizarin Red Staining for Mineralized Nodules
To assess the amount of mineralized nodules deposited on the dentin surfaces (n=6) at each time-point (7 and 14 days), cell culture and dentin discs were washed twice with PBS, fixed with 70% ethanol for 1 h, washed 3 times with deionized water and then stained with Alizarin Red dye (40 mM, pH 4.2; Sigma-Aldrich, St Louis, MO, USA) for 20 min, under gentle shaking (VDR Shaker, Biomixer, Ribeirão Preto, SP, Brazil). After aspiration of excess dye, the cells were washed twice with deionized water to remove excess stain, and representative photographs from each group were taken by light microscopy (Olympus BX51, Olympus, Miami, FL, USA). The cells were then incubated with 10% cetylpyridinium chloride (Sigma-Aldrich) for 15 min under agitation to solubilize the nodules. The absorbance of the resulting solution was measured at 570 nm by means of microplate reader. The percentage of mineralized nodules formed for each experimental group was calculated based on the mean value of the control group at 7 days as 100% of staining.
SEM Cellular Morphology
Two additional discs from each group were prepared for SEM analysis. The cells in contact with the pulp side of the discs were fixed in 2.5% glutaraldehyde (Sigma-Aldrich) in PBS for 1 h at room temperature. Next, the glutaraldehyde was aspirated and the cells were rinsed with PBS, post-fixed with 1% osmium tetroxide (Electron Microscopy Science, Fort Washington, PA, USA) for 1 h and rinsed again with PBS, followed by dehydration with ascending series of water–ethanol solutions (30%, 50%, 70%, 95%, and 100%) two times for 60 min each. The dentin disc and the cells attached to it were immersed for 60 minutes (three 20-minute changes) in 1,1,1,3,3,3-hexamethyldisilazane (HMDS, ACROS Organics, Morris Plains, NY, USA). Finally, the specimens were mounted on metallic stubs and stored in a desiccator for 24 hours, and sputter-coated with a gold layer (SDC 050; Bal-Tec AG, Balzers, Germany), and their morphology was examined with a scanning electron microscope (DSM 960, Carl Zeiss Inc., Oberkochen, Germany). The analysis of the cell morphology was made only descriptively. In this analysis it was considered the cell shape, including the presence of projections from the cell membrane, which is frequently observed in normal MDPC-23 cells. It was also observed membrane disruption and possible presence of cell fragments that remain on the substrate. All these features are compared with the cells from the control group.
Statistical Analysis
Glutaraldehyde-containing treatment agents and period of contact were set as the factors of the study, while the dependent variables were cell viability, total protein production, activity of alkaline phosphatase and deposition of mineralized nodules. As none of the datasets were normally distributed, data were analyzed by the nonparametric Kruskal-Wallis test complemented by Mann-Whitney for pairwise comparisons. Groups were considered statistically different when p-value was smaller than 0.05.
RESULTS
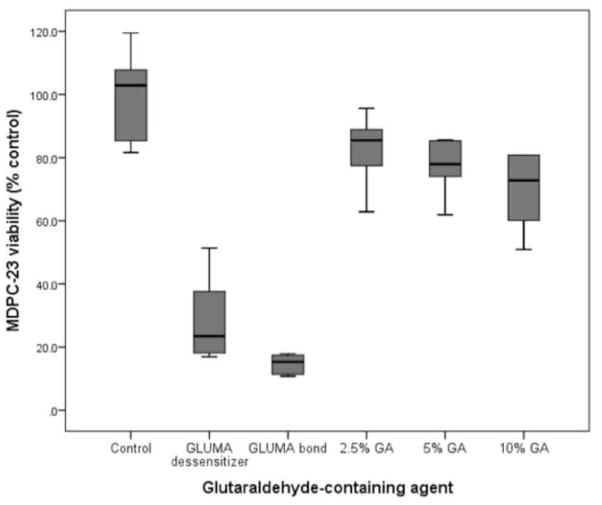
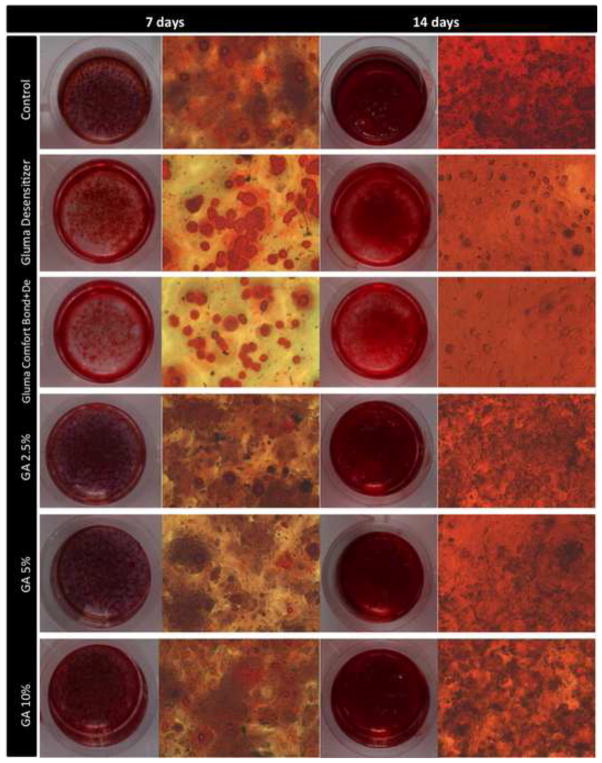
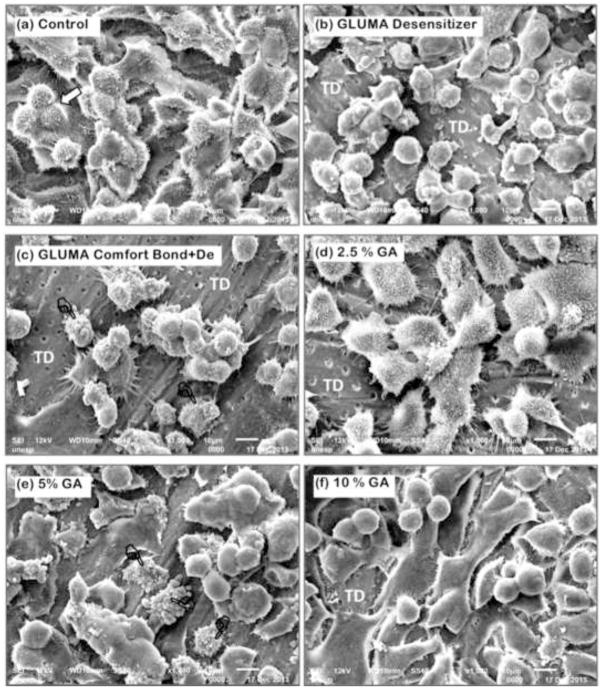
Viability of MDPC-23 cells after 24 hours of contact with glutaraldehyde-containing solutions/materials applied on the occlusal side of 0.4-mm thick dentin discs are given in Table 2 and graphically represented in Figure 1. The highest reduction in cell viability was observed for Gluma Comfort Bond+De (85.1%) followed by Gluma Desensitizer (77.2%) (p<0.05). When GA was applied as a single component solution, reduction in cell viability was much lower and ranged from 16.9% to 29.3% in a concentration-dependent manner and only at 10% it differed from the control (p<0.05). Total protein production (Table 3) was reduced after 7 and 14 days only for the groups treated with Gluma Desensitizer and Gluma Comfort Bond+De (p<0.05). The same was observed when alkaline phosphatase activity was analysed (p<0.05, Table 4). After 7 days from the contact with the eluates, there was no significant difference in deposition of mineralized nodules between control and treatment groups (Table 5 and Figures 2a–f). However, after 14 days, the cells exposed to the eluates obtained from Gluma Desenstizer (Figure 2b′) and Gluma Comfort Bond+De (Figure 2c′) produced significantly fewer mineralized nodules (p<0.05, Table 5). MDPC-23 cells in the control group, assessed by SEM, showed large cytoplasm with numerous projections, which covered most of the dentin surface (Figure 3a). Similar morphology was seen for cells whose dentin was treated with 2% GA (Figure 3d). In contrast, fewer round-shaped cells with little or no-cytoplasmic projections, covering only part of the dentin, were seen in groups treated with Gluma Desensitizer and Gluma Comfort Bond+De (Figures 3b and 3c). Cells exposed to GA 5% and 10% presented a variable morphology with a fewer number of cytoplasmatic projections. Additionally, the cytoplasm of a few cells was disrupted (Figures 3e and 3f).
Table 2.
Viability of MDPC-23 (% of control) 24 hours after application of glutaraldehyde-containing agents on 0.4-mm thick dentin discs.
| GA*-containing agents | Cell viability** | Viability reduction |
|---|---|---|
| Control a | 102.9 (85.3–107.8) a | - |
| GLUMA Desensitizera | 23.5 (18.1–37.6) c | 77.2% |
| GLUMA Comfort Bond+De | 15.3 (11.4–17.4) c | 85.1% |
| 2.5% GA a | 85.5 (77.4–88.9) ab | 16.9% |
| 5% GA a | 77.9 (74.0–85.3) ab | 24.3% |
| 10% GA a | 72.8 (60.1–80.8) b | 29.3% |
GA, glutahaldehyde.
Numbers for cell viability are median (percentile 25-percentile 75), n=6.
Medians identified by the same letter are statistically not different (Mann-Whitney, p>0.05).
Figure 1.
Boxplot of the viability of MDPC-23, seven days after application of glutaraldehyde-containing agents on 0.4-mm thick dentin discs. The box contains 50% of the data, and the middle line of the box is the median. The tips of the bars show minimum and maximum values, n=6.
Table 3.
Total protein production by MDPC-23 (% of control) seven and fourteen days after application of glutaraldehyde-containing agents on 0.4-mm thick dentin discs.
| GA*-containing agents | Period after contact
|
||
|---|---|---|---|
| 7 days | 14 days | ||
| Control a | 100.0 (100.0–110.3)** AB | sig | 223.3 (186.6–233.5) a |
| GLUMA Desensitizera | 62.0 (60.5–64.1) C | sig | 111.6 (109.7–117.0) b |
| GLUMA Comfort Bond+De | 57.6 (50.7–62.6) C | sig | 97.6 (87.5–105.4) b |
| 2.5% GA a | 118.8 (106.5–113.8) A | sig | 164.4 (146.7–173.4) ab |
| 5% GA a | 99.8 (97.1–106.2) AB | sig | 145.1 (130.6–181.1) ab |
| 10% GA a | 79.1 (77.9–82.7) BC | sig | 129.1 (114.9–143.8) a |
GA, glutahaldehyde.
Numbers for total protein production are median (percentile 25-percentile 75), n=6.
Within the columns, medians identified by the same letter are statistically not different (Mann-Whitney, p>0.05).
In the rows, sig. indicates statistically significant difference between the periods (Mann-Whitney, p>0.05).
Table 4.
Alkaline phosphatase activity in MDPC-23 (% of control) seven and fourteen days after application of glutaraldehyde-containing agents on 0.4-mm thick dentin discs.
| GA*-containing agents | Period after contact
|
||
|---|---|---|---|
| 7 days | 14 days | ||
| Control a | 96.5 (94.7–109.3)** A | sig | 67.8 (66.5–68.5) a |
| GLUMA Desensitizera | 4.4 (4.3–5.4) B | sig | 0.4 (0.3–0.4) b |
| GLUMA Comfort Bond+Dea | 3.5 (3.2–4.1) B | sig | 0.4 (0.2–0.5) b |
| 2.5% GA a | 117.6 (110.4–118.5) A | sig | 71.7 (67.5–91.7) a |
| 5% GA a | 105.8 (103.4–119.3) A | sig | 69.6 (61.6–81.0) a |
| 10% GA a | 102.9 (94.6–103.8) A | -- | 82.5 (78.8–92.6) a |
GA, glutahaldehyde.
Numbers for alkaline phosphatase activity are median (percentile 25-percentile 75), n=6.
Within the columns, medians identified by the same letter are statistically not different (Mann-Whitney, p>0.05).
In the rows, sig. indicates statistically significant difference between the periods (Mann-Whitney, p>0.05).
Table 5.
Deposition of mineralized nodules (% of control) seven and fourteen days after application of glutaraldehyde-containing agents on 0.4-mm thick dentin discs.
| GA*-containing agents | Period after contact
|
||
|---|---|---|---|
| 7 days | 14 days | ||
| Control a | 99.8 (85.3–109.6)** A | sig | 337.4 (332.8–365.7) a |
| GLUMA Desensitizera | 73.7 (67.5–82.4) A | sig | 167.3 (145.1–189.8) c |
| GLUMA Comfort Bond+Dea | 76.6 (68.3–86.5) A | sig | 116.6 (108.9–133.9) c |
| 2.5% GA a | 80.1 (74.7–85.0) A | sig | 278.7 (272.1–290.7) a |
| 5% GA a | 83.1 (60.2–83.3) A | sig | 277.3 (253.6–340.7) a |
| 10% GA a | 91.8 (71.4–98.8) A | sig | 322.2 (314.5–362.4) a |
GA, glutahaldehyde.
Numbers for mineralized nodules are median (percentile 25-percentile 75), n=6.
Within the columns, medians identified by the same letter are statistically not different (Mann-Whitney, p>0.05).
In the rows, sig. indicates statistically significant difference between the periods (Mann-Whitney, p>0.05).
Figure 2.
The images represent mineralized nodule deposition by MDPC-23 cells 7 (A–F) and 14 (A′–F′) days after the cells were kept in contact with the eluates (DMEM + products that diffused through the 0.4 mm-thick dentin discs) for 24 h. For each period the left column shows digital photographs of the wells stained with Alizarin Red solution, and at the right column are light-microscopic (10x) images from a representative area of each well. Alizarin Red reacts with calcium to form a complex in a chelation process what allows the quantification of mineral deposition. A and A′= control group; B and B′ = GLUMA Desensitizer; C and C′= GLUMA Comfort Bond+De;aD and D′= 2.5% glutaraldehyde (GA); E and E′= 5% GA and F and F′= 10% GA. Mineralized nodules (arrows) indicate the ability of the cells to mineralize the secreted collagen matrix.
Figure 3.
General view of the odontoblast-like MDPC-23 cells seeded on dentin substrate. SEM, x1000. (a) Control: Cells with large cytoplasm covering most of the dentin surface. No dentinal tubules are visible. These cells have a number of processes which seem to attach them to the substrate. Note the occurrence of cell mitosis (white arrow). (b) Gluma desensitizer: A number of cells that remained adhered to dentin have round shapes and less cytoplasm fewer cytoplamatic projection, making them smaller than in the control group. (c) Gluma Bond: Reduced number of round-shaped MDPC-23 cells remained attached to dentin. Note that a large surface of tubular dentin (TD) with no cells is exposed. Cells with disrupted cytoplasm membrane can also be seen (pointers). (d) 2.5% GA: Cells with large cytoplasm, such as observed in control group, are attached to dentin. However, small surface of tubular dentin (TD) with no cell can be seen. (e) 5% GA: A number of MDPC-23 cells with variable morphology are observed. Note that a few cells show cytoplasm disruption (pointers). (f) 10% GA: Such as observed in (e), cells with different morphology are covering the dentin substrate. Most of these cells show only a few thin and short cytoplasm processes.
DISCUSSION
In the present study, the GA-containing solutions/materials were applied on dentin discs with 0.4 mm of thickness, that is less than the required thickness of 0.5 mm, wich is generally accepted as being biologically capable of protecting the pulp tissue against noxious effects caused by dental materials released components.19,23 The dentin thickness was elected to favor cellular aggression and to facilitate the evaluation of the cytotoxic potential of each solution/material in our standard in vitro screening model. Even in this unfavorable condition, the treatment of etched dentin for 60 s with 2.5% and 5% GA solutions did not provoke any toxic effects on odontoblast-like cells, while 10% GA showed a significant reduction of cell viability (29.3%). Since the dentin discs were kept hydrated by the culture medium (DMEM), during GA diffusion through the demineralized dentin and dentinal tubules, some of the glutaraldehyde was probably consumed by reacting with the exposed collagen as it diffused down at dentin tubules14,28,29 and other available proteins30 and diluted by the fluid inside the tubules. Residues of the unreacted cross-linking agent are a potential source of cytotoxicity of a chemically cross-linked material.31 Gelatin sponges saturated with 0.25 mmol of GA were not cytotoxic when in direct contact with human skin fibroblasts in culture, meaning that all available GA reacted with the gelatin and therefore there was no residual chemical to atack the cells. However, when the sponges were soaked with twice this concentration (0.5 mmol), they were considered moderately toxic.31
GA is known as one of the most effective protein crosslinking agents32 and it is commonly used to crosslink collagen-based biomaterials.33 The crosslinking of dentin collagen using GA and others cross-linkers has been investigated as a promising mechanism to delay the degradation of the hybrid layer34,35. For that purpose, GA is applied as a primer on the etched dentin prior to the application of the adhesive system. Clinically acceptable periods of GA application (30 and 60 seconds) were sufficient to increase collagen stiffness and decrease its degradation.14,29 Based on these findings, in the present study GA solutions were applied for 60 s after acid etching the dentin with phosphoric acid. It has also been demonstrated that during this same period of application, 5% GA is able to inhibit dentin MMP activity14 which may also help to prevent resin-dentin bond degradation. The reaction of MMP-inactivation by GA is rapid and proceeds in aqueous buffer solution under conditions close to physiological pH, ionic strength and temperature.32 Additionaly, 30 s of contact with 6.25% GA is sufficient to kill several microorganisms commonly found in dentin after caries removal.36
For decades, GA has been used for pulpotomy treatment in primary teeth, normally at a concentration of 2mass%. Fibroblasts and HeLa cells in contact with 2% GA instantly killed and are totally fixed.36 Since MDPC-23 cells maintained their viability when 2.5% and 5% GA solutions were applied on the etched dentin, it is plausible to conclude that the GA concentration that reached the MDPC-23 cells was lowered to the point that it did not exert any fixative activity on the cells. At 10%, GA slightly reduced MDPC-23 viability; however, no detrimental effect was seen for total protein synthesis, alkaline phosphatase activity and deposition of mineralized nodules.
Secretion of dentin proteins by odontoblast-like cells was not affected by GA solution, irrespective of the concentrations used. Alkaline phosphatase is a membrane-bound ectoenzyme highly expressed in mineralized tissue cells37 and plays a fundamental role in dentin matrix mineralization. GA solutions also did not affect the activity of alkaline phosphatase, indicating that when applied on dentin, GA would probably not hinder the pulp-dentin complex reparative processes (formation of tertiary dentin). The evidence of that is the undisturbed deposition of mineralized nodules in the collagen matrix even when GA solutions were used.
It is known that GA has a high affinity for free primary amine groups of amino acids38 reacting with demineralized collagen14,29 and other proteins found in dentin. When GA comes in contact with plasma proteins in dentinal fluid it causes their precipitation blocking exposed dentin tubules and reducing dentin permeability.30 Based on that principle, GA-containing dental materials, such as GLUMA, were developed to treat dentin hypersensitivity. This dental product, which contains 35mass% 2-hydroxyhethylmethacrylate (HEMA) in its composition, interacts with tooth structure in different ways.12 While GA clogs proteins within the dentinal tubules,30 HEMA reacts with the precipitated proteins to form a mixture of polyHEMA and glutaraldehyde-cross-linked protein.16 However, in spite of beneficial results regarding dentin desensitizing, Gluma has been reported as toxic to different cell lines.18,39
Gluma desensitizer and Gluma Comfort Bond+De reduced MDPC-23 cells metabolism by 77% and 85.1%, respectively, indicating that both products are considered highly cytotoxic. These finds were confirmed by reductions in total protein synthesis, alkaline phosphatase activity and deposition of mineralized nodules obtained 14 days after the treatments. Additionally, the MDPC-23 cells in contact with components released form both GA-containing dental products showed notable alterations in their morphology, changing from flat, large, cytoplasm conformation to small round-shaped cells. Also, these pulp cells lost most of their thin cytoplasmic processes, which are responsible for cell attachment to the dentin substrate. Fourteen days after the eluates application, cells exposed to Gluma Desensitizer and Gluma Comfort Bond+De presented no signs of recovery.
Gluma desensitizer is a mixture of GA, HEMA and water while Gluma Comfort Bond+De contains these components plus other monomers and adhesive systems components such as a photoinitiator. Since the results of the present study demonstrated that 5% GA did not excert toxic effets on MDPC-23 cells when applied on etched dentin, which is corroborated by Scheffel et al.,17 HEMA may be the main responsible for the deleterious results observed for Gluma products. The high solubility and low molecular mass of HEMA (130.14 g mol−1) favor its diffusion through dentinal tubules to reach the pulp chamber.18 Although this monomer presents less toxic effects compared to BisGMA, UDMA and TEGDMA,40,41 small concentrations of HEMA can cause irreversible inhibitory effect on cultured cells.42 In a recent review, di Giacomo et al.43 reported that HEMA increases the production of reactive oxygen species (ROS) and the oxidative DNA damage, disrupts the intracellular glutathione detoxifying balance, decreases cell proliferation and upregulates COX-2 proteins.
Since dentin thickness plays an important role in dental materials cytotocixity,23,26,39,42 increases in dentin thickness may protect the pulp cells against the toxic effects of Gluma products. In fact, when applied on 1-mm thick dentin specimens, more than twice the thickness used in this study, Gluma Desensitizer was regarded as non cytotoxic.44 Therefore, it could be assumed that Gluma Comfort Bond+De and Gluma Desensitizer are not suitable for very deep cavities as simulated in this study. Despite the results of this in vitro study can not be directly extrapolated to the clinical practice, they raise important questions concerning the use of GA-containing materials currently in the market. Considering that Gluma Desensitizer application is recommended after crown prosthetic preparation, a considerable area of dentin with a thin remaining thickness will be in contact with the material, that may favor pulp tissue damage. A HEMA-free desensitizer might be a safer option to reduce dentin sensitivity. However, further in vivo investigations should be conducted in order to demonstrate that assumption. The clinical efficacy of 5% GA as a dentin desensitizing agent may not be as effective as 5% GA plus 35% HEMA.
CONCLUSION
Glutaraldehyde-containing solutions were not cytotoxic to MDPC-23 cells, while dental products containing a combination of glutaraldehyde plus HEMA and other monomers were highly detrimental to the metabolism of these odontoblast-like pulp cells.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Débora Lopes Salles Scheffel, Department of Orthodontics and Pediatric Dentistry, Araraquara School of Dentistry, UNESP – Univ Estadual Paulista, Araraquara, São Paulo, Brazil. Rua Humaitá, 1680, Araraquara, São Paulo, Brazil, 14801-903.
Diana Gabriela Soares, Department of Dental Materials and Prosthodontics, Araraquara School of Dentistry, UNESP – Univ Estadual Paulista, Araraquara, São Paulo, Brazil. Rua Humaitá, 1680, Araraquara, São Paulo, Brazil, 14801-903.
Fernanda Gonçalves Basso, Department of Physiology and Pathology, Araraquara School of Dentistry, UNESP – Univ Estadual Paulista, Araraquara, São Paulo, Brazil. Rua Humaitá, 1680, Araraquara, São Paulo, Brazil, 14801-903.
Carlos Alberto de Souza Costa, Department of Physiology and Pathology, Araraquara School of Dentistry, UNESP – Univ Estadual Paulista, Araraquara, São Paulo, Brazil. Rua Humaitá, 1680, Araraquara, São Paulo, Brazil, 14801-903.
David Henry Pashley, Department of Oral Biology, College of Dental Medicine, Georgia Regents University, Augusta, Georgia, USA. 1120 15th Street, CL-2112, Augusta, Georgia, USA, 30912-1129.
Josimeri Hebling, Department of Orthodontics and Pediatric Dentistry, Araraquara School of Dentistry, UNESP – Univ Estadual Paulista, Araraquara, São Paulo, Brazil. Rua Humaitá, 1680, Araraquara, São Paulo, Brazil, 14801-903.
References
- 1.Curro FA. Tooth hypersensitivity in the spectrum of pain. Dental Clinics of North America. 1990;34:429–37. [PubMed] [Google Scholar]
- 2.Chung G, Jung SJ, Oh SB. Cellular and molecular mechanisms of dental nociception. Journal of Dental Research. 2013;92:948–55. doi: 10.1177/0022034513501877. [DOI] [PubMed] [Google Scholar]
- 3.Cummins D. Recent advances in dentin hypersensitivity: clinically proven treatments for instant and lasting sensitivity relief. American Journal of Dentistry. 2010;23:3A–13A. [PubMed] [Google Scholar]
- 4.Porto IC, Andrade AK, Montes MA. Diagnosis and treatment of dentinal hypersensitivity. Journal of Oral Science. 2009;51:323–32. doi: 10.2334/josnusd.51.323. [DOI] [PubMed] [Google Scholar]
- 5.Schiff T, Delgado E, Zhang YP, Cummins D, Devizio W, Mateo LR. Clinical evaluation of the efficacy of an in-office desensitizing paste containing 8% arginine and calcium carbonate in providing instant and lasting relief of dentin hypersensitivity. American Journal of Dentistry. 2009;22:8A–15A. [PubMed] [Google Scholar]
- 6.Sung HW, Huang DM, Chang WH, Huang RN, Hsu JC. Evaluation of gelatin hydrogel crosslinked with various crosslinking agents as bioadhevises: In vitro study. Journal of Biomedical Material Research. 1999;46:520–30. doi: 10.1002/(sici)1097-4636(19990915)46:4<520::aid-jbm10>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 7.Nimni ME, Cheung D, Startes B, Kodama M, Sheikh . Bio-prosthesis derived from cross-linked and chemically modified collagenous tissues. In: Nimni ME, editor. Collagen. III. Boca Raton, FL: CRC Press; 1988. pp. 1–38. [Google Scholar]
- 8.McDade JK, Brennan-Pierce EP, Ariganello MB, Labow RS, Michael Lee J. Interactions of U937 macrophage-like cells with decellularized pericardial matrix materials: influence of crosslinking treatment. Acta Biomaterialia. 2013;9:7191–9. doi: 10.1016/j.actbio.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 9.Naso F, Gandaglia A, Bottio T, Tarzia V, Nottle MB, d’Apice AJ, et al. First quantification of alpha-Gal epitope in current glutaraldehyde-fixed heart valve bioprostheses. Xenotransplantation. 2013;20:252–61. doi: 10.1111/xen.12044. [DOI] [PubMed] [Google Scholar]
- 10.Lin WH, Tsai WB. In situ UV-crosslinking gelatin electrospun fibers for tissue engineering applications. Biofabrication. 2013;5:035008. doi: 10.1088/1758-5082/5/3/035008. [DOI] [PubMed] [Google Scholar]
- 11.Gomes SR, Rodrigues G, Martins GG, Henriques CM, Silva JC. In vitro evaluation of crosslinked electrospun fish gelatin scaffolds. Materials Science and Engineering C: Materials for Biological Applications. 2013;33:1219–27. doi: 10.1016/j.msec.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 12.Larson TD. Clinical uses of glutaraldehyde/2-hydroxyethylmethacrylate (GLUMA) Northwest Dentistry. 2013;92:27–30. [PubMed] [Google Scholar]
- 13.Scheffel D, Hebling J, Scheffel R, Agee K, Turco G, de Souza Costa C, et al. Inactivation of Matrix-bound Matrix Metalloproteinases by Cross-linking Agents in Acid-etched Dentin. Operative Dentistry. 2014;39:152–8. doi: 10.2341/12-425-L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scheffel DL, Hebling J, Scheffel RH, Agee KA, Cadenaro M, Turco G, et al. Stabilization of dentin matrix after cross-linking treatments, in vitro. Dental Materials. 2014;30:227–33. doi: 10.1016/j.dental.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qin C, Xu J, Zhang Y. Spectroscopic investigation of the function of aqueous 2-hydroxyethylmethacrylate/glutaraldehyde solution as a dentin desensitizer. European Journal of Oral Science. 2006;114:354–9. doi: 10.1111/j.1600-0722.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 16.Munksgaard EC. Amine-induced polymerization of aqueous HEMA/aldehyde during action as a dentin bonding agent. Journal of Dental Research. 1990;69:1236–9. doi: 10.1177/00220345900690060201. [DOI] [PubMed] [Google Scholar]
- 17.Scheffel DL, Bianchi L, Soares DG, Basso FG, Sabatini C, de Souza Costa CA, Pashley DH, Hebling J. Transdentinal cytotoxicity of carbodiimide (EDC) and glutaraldehyde on odontoblast-like cells. Operative Dentistry. 2015;40:44–54. doi: 10.2341/13-338-L. [DOI] [PubMed] [Google Scholar]
- 18.Koulaouzidou EA, Helvatjoglu-Antoniades M, Palaghias G, Karanika-Kouma A, Antoniades D. Cytotoxicity of dental adhesives in vitro. European Journal of Dentistry. 2009;3:3–9. [PMC free article] [PubMed] [Google Scholar]
- 19.Lanza CR, de Souza Costa CA, Furlan M, Alécio A, Hebling J. Transdentinal diffusion and cytotoxicity of self-etching adhesive systems. Cell Biology Toxicology. 2009;25:533–43. doi: 10.1007/s10565-008-9110-x. [DOI] [PubMed] [Google Scholar]
- 20.Bianchi L, Ribeiro AP, de Oliveira Carrilho MR, Pashley DH, de Souza Costa CA, Hebling J. Transdentinal cytotoxicity of experimental adhesive systems of different hydrophilicity applied to ethanol-saturated dentin. Dental Materials. 2013;29:980–90. doi: 10.1016/j.dental.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El-kholany NR1, Abielhassan MH, Elembaby AE, Maria OM. Apoptotic effect of different self-etch dental adhesives on odontoblasts in cell cultures. Archives of Oral Biology. 2012;57:775–83. doi: 10.1016/j.archoralbio.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 22.Gallorini M, Cataldi A, di Giacomo V. HEMA-induced cytotoxicity: oxidative stress, genotoxicity and apoptosis. International Endodontic Journal. 2014;47:813–8. doi: 10.1111/iej.12232. [DOI] [PubMed] [Google Scholar]
- 23.Hanks CT, Craig RG, Diehl ML, Pashley DH. Cytotoxicity of dental composites and other materials in a new in vitro device. Journal of Oral Pathology. 1988;17:396–403. doi: 10.1111/j.1600-0714.1988.tb01304.x. [DOI] [PubMed] [Google Scholar]
- 24.Hanks CT, Fang D, Sun Z, Edwards CA, Butler WT. Dentin-specific proteins in MDPC-23 cell line. European Journal of Oral Science. 1998;1:260–6. doi: 10.1111/j.1600-0722.1998.tb02185.x. [DOI] [PubMed] [Google Scholar]
- 25.Sun ZL, Fang DN, Wu XY, Ritchie HH, Bègue-Kirn C, Wataha JC, Hanks CT, Butler WT. Expression of dentin sialoprotein (DSP) and other molecular determinants by a new cell line from dental papillae, MDPC-23. Connective Tissue Research. 1998;37:251–61. doi: 10.3109/03008209809002443. [DOI] [PubMed] [Google Scholar]
- 26.Basso FG, Oliveira CF, Kurachi C, Hebling J, Costa CA. Biostimulatory effect of low-level laser therapy on keratinocytes in vitro. Lasers in Medical Science. 2013;28:367–74. [Google Scholar]
- 27.Soares DG, Ribeiro AP, da Silveira Vargas F, Hebling J, de Souza Costa CA. Efficacy and cytotoxicity of a bleaching gel after short application times on dental enamel. Clinical Oral Investigation. 2013;17:1901–9. doi: 10.1007/s00784-012-0883-1. [DOI] [PubMed] [Google Scholar]
- 28.Pashley DH. Consideration of dentine permeability in cytotoxicity testing. International Endodontic Journal. 1988;21:143–54. doi: 10.1111/j.1365-2591.1988.tb00968.x. [DOI] [PubMed] [Google Scholar]
- 29.Bedran-Russo AK, Pashley DH, Agee K, Drummond JL, Miescke KJ. Changes in stiffness of demineralized dentin following application of collagen crosslinkers. Journal of Biomedical Materials Research Part B Applied Biomaterials. 2008;86:330–4. doi: 10.1002/jbm.b.31022. [DOI] [PubMed] [Google Scholar]
- 30.Ishihata H, Finger WJ, Kanehira M, Shimauchi H, Komatsu M. In vitro dentin permeability after application of Gluma® desensitizer as aqueous solution or aqueous fumed silica dispersion. Journal of Applied Oral Science. 2011;19:147–53. doi: 10.1590/S1678-77572011000200011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ulubayram K, Aksu E, Gurhan SID, Serbetci K, Hasirci N. Cytotoxicity evaluation of gelatin sponges prepared with different cross-linking agents. Journal of Biomaterials Science, Plymer Edition. 2002;13:1203–19. doi: 10.1163/156856202320892966. [DOI] [PubMed] [Google Scholar]
- 32.Migneault I, Dartiguenave C, Bertrand MJ, Waldron KC. Glutaraldehyde: behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques. 2004;37:790–6. 798–802. doi: 10.2144/04375RV01. [DOI] [PubMed] [Google Scholar]
- 33.Olde Damink LH, Dijkstra PJ, Van Luyn MJ, Van Wachem PB, Nieuwenhuis P, Feijen J. Changes in the mechanical properties of dermal sheep collagen during in vitro degradation. Journal of Biomedical Materials Research. 1995;29:139–47. doi: 10.1002/jbm.820290202. [DOI] [PubMed] [Google Scholar]
- 34.Bedran-Russo AK, Vidal CM, dos Santos PH, Castellan CS. Long-term effect of carbodiimide on dentin matrix and resin-dentin bonds. Journal of Biomedical Materials Research Part B Applied Biomaterials. 2010;94:250–5. doi: 10.1002/jbm.b.31649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scheffel D, Delgado C, Soares DG, Basso F, de Souza Costa CA, Pashley D, Hebling J. Increased Durability of Resin-Dentin Bonds Following Cross-Linking Treatment. Operative Dentistry. 2015 doi: 10.2341/13-211-L. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hill SD, Berry CW, Sue Seale N, Kaga M. Comparison of antimicrobial and cytotoxic effects of glutaraldehyde and formocresol. Oral Surgery Oral Medicine Oral Pathology. 1991;71:89–95. doi: 10.1016/0030-4220(91)90530-p. [DOI] [PubMed] [Google Scholar]
- 37.Golub EE, Boesze-Battaglia K. The role of alkaline phosphatase in mineralization. Current Opinion in Orthopaedics. 2007;18:444–8. [Google Scholar]
- 38.Cheung DT, Perelman N, Ko EC, Nimni ME. Mechanism of crosslinking of proteins by glutaraldehyde III. Reaction with collagen in tissues. Connective Tissue Research. 1985;13:109–15. doi: 10.3109/03008208509152389. [DOI] [PubMed] [Google Scholar]
- 39.Hanks CT, Wataha JC, Parsell RR, Strawn SE. Delineation of cytotoxic concentrations of two dentin bonding agents in vitro. Journal of Endodontics. 1992;18:589–96. doi: 10.1016/S0099-2399(06)81328-2. [DOI] [PubMed] [Google Scholar]
- 40.Geurtsen W, Lehmann F, Spahl W, Leyhausen G. Cytotoxicity of 35 dental resin composite monomers/additives in permanent 3T3 and three human primary fibroblasts cultures. Journal of Biomedical Materials Research. 1998;41:474–80. doi: 10.1002/(sici)1097-4636(19980905)41:3<474::aid-jbm18>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 41.Reichl FX, Simon S, Esters M, Seiss M, Kehe K, Kleinsasser N, et al. Cytotoxicity of dental composite (co)monomers and the amalgam component Hg2+ in human gingival fibroblasts. Archives of Toxicology. 2006;80:465–72. doi: 10.1007/s00204-006-0073-5. [DOI] [PubMed] [Google Scholar]
- 42.Hanks CT, Straw SE, Wataha JC, Craig RG. Cytotoxic effects of resin components on cultured mammalian fibroblasts. Journal of Dental Research. 1991;70:1450–5. doi: 10.1177/00220345910700111201. [DOI] [PubMed] [Google Scholar]
- 43.di Giacomo V, Pacella S, Rapino M, Di Giulio M, Zara S, Pasquantonio G, et al. pPKC α regulates through integrin β 1 human gingival fibroblasts/Streptococcus mitis adhesion in response to HEMA. International Endodontic Journal. 2013;46:1164–72. doi: 10.1111/iej.12113. [DOI] [PubMed] [Google Scholar]
- 44.Wiegand A, Buchholz K, Werner C, Attin T. In vitro cytotoxicity of different desensitizers under simulated pulpal flow conditions. Journal of Adhesive Dentistry. 2008;10:227–32. [PubMed] [Google Scholar]