Abstract
Background
While nicotine is the primary addictive compound in tobacco, other tobacco constituents including minor alkaloids (e.g., nornicotine, anabasine) may also contribute to tobacco addiction by mimicking or enhancing the effects of nicotine. Further evaluating the behavioral effects of minor alkaloids is essential for understanding their impact on tobacco addiction and informing development of tobacco product standards by the FDA.
Methods
This study compared the addiction-related effects of nicotine and the minor alkaloids nornicotine, anabasine, myosmine, anatabine, and cotinine on intracranial self-stimulation (ICSS) thresholds in rats.
Results
Acute injection of nicotine produced reinforcement-enhancing (ICSS threshold-decreasing) effects at low to moderate doses, and reinforcement-attenuating/aversive (ICSS threshold-increasing) effects at high doses. Nornicotine and anabasine produced similar biphasic effects on ICSS thresholds, although with lower potency compared to nicotine. Myosmine only elevated ICSS thresholds at relatively high doses, while anatabine and cotinine did not influence ICSS thresholds at any dose. None of the alkaloids significantly influenced ICSS response latencies, indicating a lack of nonspecific motoric effects.
Conclusions
These findings indicate that some minor tobacco alkaloids can either fully (nornicotine, anabasine) or partially (myosmine) mimic nicotine’s addiction-related effects on ICSS, albeit at reduced potency. These findings emphasize the need for further study of the abuse potential of minor alkaloids, including evaluation of their effects when combined with nicotine and other tobacco constituents to better simulate tobacco exposure in humans. Such work is essential for informing FDA regulation of tobacco products and could also lead to the development of novel pharmacotherapies for tobacco addiction.
Keywords: Nicotine, minor alkaloids, non-nicotine tobacco constituents, intracranial self-stimulation, rat
1. INTRODUCTION
The primary role of nicotine in maintaining tobacco use is well established (Benowitz, 2008; U.S. Department of Health and Human Services, 1999), but non-nicotine tobacco constituents may also contribute to tobacco addiction. For example, minor tobacco alkaloids (e.g., nornicotine, anabasine) can mimic nicotine’s behavioral and neuropharmacological effects, albeit typically at reduced potency compared to nicotine (for review, see Brennan et al., 2014; Hoffman and Evans, 2013). Further evaluation of minor alkaloids is essential for understanding their impact on tobacco addiction and could lead to the development of novel pharmacotherapies for smoking cessation. Given that the Food and Drug Administration (FDA) now has the authority to regulate the content of nicotine and other constituents in tobacco products (Deyton et al., 2010; Hatsukami et al., 2012, 2010), such work could also inform the development of tobacco product standards to reduce the addictiveness of those products (Benowitz and Henningfield, 1994, 2013; Henningfield et al., 2004).
Intracranial self-stimulation (ICSS) has been useful for studying the addiction-related effects of drugs on brain reinforcement systems. Low to moderate doses of nicotine and other drugs reduce the minimal (threshold) stimulation intensity that maintains ICSS, suggesting increased sensitivity to the reinforcing effects of the brain stimulation (Caggiula et al., 2009; Harrison et al., 2002; Huston-Lyons and Kornetsky, 1992). This effect may reflect the more general ability of drugs to enhance the reinforcing effects of environmental stimuli (e.g., sensory stimuli, food; see Caggiula et al., 2009; Chaudhri et al., 2006; Paterson et al., 2008; Wise, 2002), an important behavioral mechanism mediating drug addiction (Caggiula et al., 2009; Chaudhri et al., 2006; Paterson, 2009). The ability of drugs to reduce ICSS thresholds is potentially more predictive of their abuse liability than other measures. For example, some drugs of abuse that are not self-administered (e.g., hallucinogens) nonetheless reduce ICSS thresholds (Wise, 1996, 2002; Wise et al., 1992). High doses of nicotine and other drugs inhibit the function of brain reinforcement systems and elevate ICSS thresholds, a putative measure of aversion (Fowler et al., 2011; Kenny et al., 2003; Spiller et al., 2009). A drug’s aversive effects are an important component of its abuse liability because they limit the amount of drug consumed (for review, see Fowler and Kenny, 2013; Verendeev and Riley, 2013).
Despite the utility of ICSS in the study of drug addiction, effects of minor alkaloids have not been examined in this model. To this end, the current study evaluated the acute effects of nicotine and the minor alkaloids nornicotine, anabasine, myosmine, anatabine, and cotinine on ICSS thresholds. Although nornicotine has been studied most extensively (e.g., Bardo et al., 1999; Dwoskin et al., 1999; Green et al., 2000), all of these minor alkaloids can produce behavioral effects under some conditions (Caine et al., 2014; Clemens et al., 2009; Goldberg et al., 1989; Hall et al., 2014; Pratt et al., 1983; Stolerman et al., 1995; Stolerman et al., 1984).
2. METHODS
2.1. Animals
Experimentally-naive male Holtzman Sprague Dawley rats (Harlan, Indianapolis, IN) weighing 250–300 g upon arrival in the colony were housed individually under a reversed 12-hr light/dark cycle and allowed unlimited access to water. All testing occurred during the dark (active) phase. Beginning one week after arrival, rats were food-restricted to ≈18 g/day rat chow to facilitate operant performance and avoid detrimental effects of long-term ad libitum feeding on health. Animals were postnatal day (PND) 144 ± 13.5 (mean ± SEM) at the onset of alkaloid dosing (described below) and, among those rats completing the entire protocol, PND 301.8 ± 23.1 at the completion of the study. Protocols were approved by the Institutional Animal Care and Use Committee of the Minneapolis Medical Research Foundation in accordance with the 2011 NIH Guide for the Care and Use of Laboratory Animals and the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council 2003).
2.2. Drugs
(−)-Nicotine bitartrate, (+/−) nornicotine, (+/−) anabasine, (+/−) myosmine, and (−)-cotinine were obtained from Sigma (St Louis, MO). (+/−) Anatabine was obtained from Toronto Research Chemicals, Inc. (Ontario, Canada). All drugs were prepared in sterile saline, adjusted to a pH of 7.4 using dilute NaOH, and administered s.c. in a volume of 1 ml/kg. All drug doses are expressed as the base.
2.3. Intracranial self-stimulation
Surgery, apparatus, and training procedure used here are described in detail elsewhere (Harris et al., 2013, 2010, 2011; Manbeck et al., 2013). Briefly, animals were anesthetized with i.m. ketamine (75 mg/kg)/xylazine (7.5 mg/kg) and implanted with an electrode in the medial forebrain bundle at the level of the lateral hypothalamus. Rats were later trained to respond for electrical brain stimulation using a modified version of the Kornetsky and Esposito (1979) discrete-trial current-threshold procedure (Markou and Koob, 1992). Each session was approximately 45 minutes and provided two dependent variables: ICSS thresholds (a measure of brain reinforcement function) and response latencies (a measure of non-specific (e.g., motoric) effects).
2.4. Protocol
Animals (N = 15) were tested in daily ICSS sessions conducted Mon-Fri until thresholds were stable (less than 10% coefficient of variation over a 5-day period and no apparent trend). To habituate animals to the injection procedure, saline was administered 10 minutes prior to ICSS testing twice per week (Tuesdays and Fridays) for at least 1 session and until thresholds were stable. Effects of 10-minute pretreatment with nicotine were subsequently determined at nicotine doses of 0, 0.125, 0.25, 0.50, or 1.0 mg/kg. These nicotine doses reduce or increase ICSS thresholds when administered acutely (e.g., Harris et al., 2015; Harrison et al., 2002; Huston-Lyons and Kornetsky, 1992). Injections typically occurred on Tuesdays and Fridays, provided that thresholds were within baseline range on intervening days, and doses were administered in a counterbalanced order.
Following completion of nicotine dose-response testing, animals were tested for ICSS under drug-free conditions for at least 2 weeks and until ICSS thresholds were stable. Dose-response determinations for a total of five minor alkaloids were conducted. For each rat, three minor alkaloids were randomly chosen to be tested, with each dose-response determination separated by at least a two-week washout period and attainment of stable ICSS thresholds. Test sessions were conducted as described for nicotine, except that rats received 10-minute pretreatment with nornicotine (0, 0.5, 1.0, 3.0, or 6.0 mg/kg), anabasine (0, 0.5, 1.0, 3.0, or 4.0 mg/kg), myosmine (0, 1.0, 6.0, 10.0, or 15.0 mg/kg), anatabine (0, 0.25, 0.5, 1.0, or 3.0 mg/kg), or cotinine (0, 1.0, 6.0, 10.0, or 100.0 mg/kg). These doses were not chosen based on their clinical relevance, as they are considerably higher than those delivered during actual tobacco use (e.g., quantities of nornicotine in the smoke of one cigarette ranged from 27–88 pg, see U.S. Public Health Service, 1988). Rather, they were chosen based on their behavioral effects reported in other animal models of tobacco addiction and to establish the effective dose range in the present model (Caine et al., 2014; Dwoskin et al., 1999; Goldberg et al., 1989; Hall et al., 2014; Stolerman et al., 1995, 1984). Doses and pretreatment time were also based on a pilot study examining acute effects of these minor alkaloids on ICSS (data not shown).
2.5. Statistics
Intracranial self-stimulation thresholds (in µA) and response latencies (in seconds) were expressed as percentage of baseline (i.e., mean during last 5 sessions prior to each dose-response determination). Data for each alkaloid condition were subsequently analyzed using a one-factor, repeated measures ANOVA, followed by a Dunnett post hoc test comparing each alkaloid dose to saline. Degrees of freedom for ANOVAs were adjusted using the method of Geisser and Greenhouse to correct for any violations of sphericity. In the two cases in which rats failed to respond for any ICSS current intensity (both observed following administration of 4.0 mg/kg anabasine), we arbitrarily assigned an ICSS threshold value of 206.0% and a latency value of 132.0%. These threshold and latency values were used because they were slightly larger than those obtained in the animal achieving the highest ICSS threshold throughout the entire experiment. This approach has previously been used to account for missing ICSS data under analogous conditions (see Markou and Koob, 1991).
3. RESULTS
3.1 Attrition and baseline measures
Due to attrition caused by removal of an ICSS headcap or loss of stability of ICSS thresholds, data for some animals were available for only one (n = 3) or two (n = 3) of their three assigned minor alkaloids. Data for the remaining 9 animals were available for all three of their assigned minor alkaloids.
Mean baseline thresholds and response latencies ranged from 96.7 –109.0 µA and 2.3 – 2.5 seconds, respectively, across the different alkaloid dose-response determinations.
3.2. ICSS thresholds
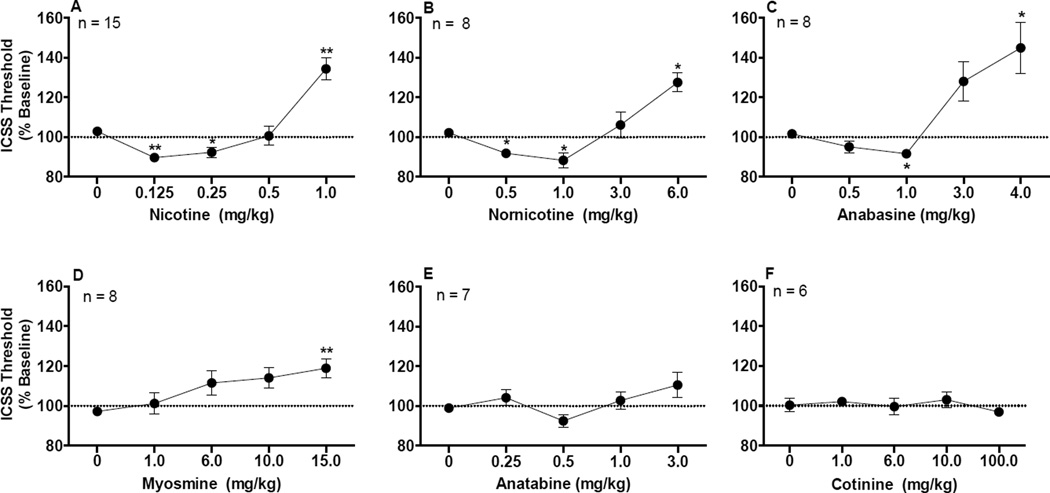
There was a significant effect of dose for the nicotine condition (F(2.6, 36.3)=23.3, p <0.0001), with ICSS thresholds significantly reduced compared to saline at 0.125 mg/kg (q(14) = 5.7, p < 0.01) and 0.25 mg/kg (q(14) = 2.9, p < 0.05) and elevated compared to saline at 1.0 mg/kg (q(14) = 6.0, p < 0.01) (Fig 1A).
Figure 1.
ICSS thresholds (expressed as percent of baseline, mean ± SEM) following injection of nicotine (A), nornicotine (B), anabasine (C), myosmine (D), anatabine (E), or cotinine (F). Number of animals tested with each alkaloid is also shown. *,** Significantly different from saline (0 mg/kg) for that alkaloid, p < 0.05 or 0.01.
For the minor alkaloids, there was a significant effect of dose for the nornicotine condition (F(2.0,14.0)=11.4, p = 0.0012), anabasine condition (F(1.6,11.0)=16.5, p = 0.0008), and myosmine condition (F(2.5,17.5) = 4.4, p = 0.0224) (see Fig 1B–1D). For nornicotine, ICSS thresholds were significantly reduced compared to saline at 0.5 mg/kg (q(7) = 3.9, p < 0.05) and 1.0 mg/kg (q(7) = 3.7, p < 0.05) and elevated compared to saline at 6.0 mg/kg (q(7) = 3.9, p < 0.05) (Fig 1B). For anabasine, ICSS thresholds were significantly reduced compared to saline at 1.0 mg/kg (q(7) = 3.8, p < 0.05) and elevated at 4.0 mg/kg (q(7) = 4.2, p < 0.05) (Fig 1C). Myosmine did not reduce ICSS thresholds compared to saline at any dose, but elevated thresholds at 15.0 mg/kg (q(7) = 4.7, p < 0.01) (Fig 1D). Although anatabine appeared to produce a modest biphasic effect on thresholds (Fig 1E), the effect of anatabine dose was not significant (F(2.5,14.9) = 2.6, p = 0.10) and no dose of anatabine differed significantly from saline (q(6) = 1.0 – 1.8, p = 0.30–0.73). There was also no significant effect of dose for the cotinine condition (F(2.8,13.8) = 0.82, p = 0.50), and no dose of cotinine differed from saline (q(5) = 0.16 – 0.93, p = 0.77–0.99; Fig 1F).
3.3 ICSS latencies
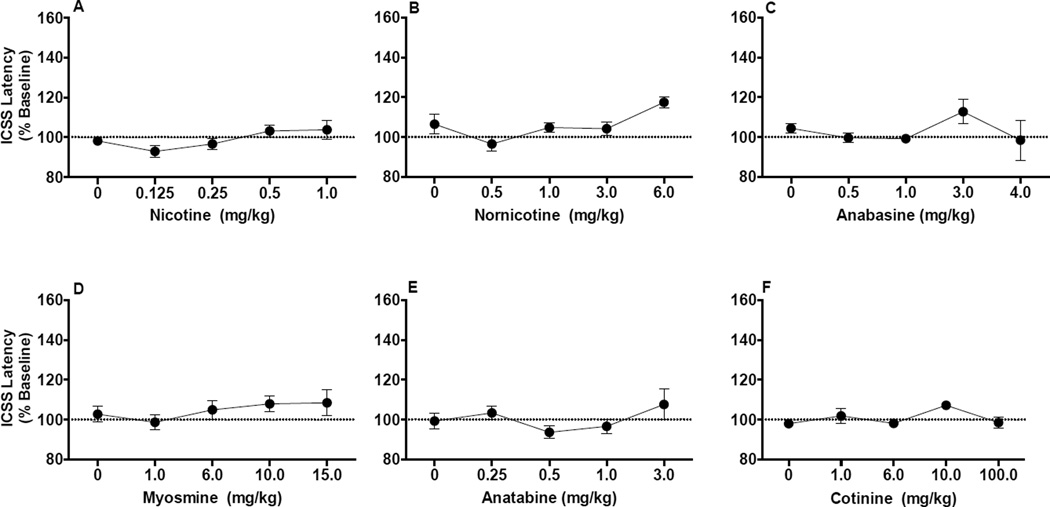
Among all alkaloids studied (Fig 2A–2F), ANOVA indicated a significant effect of dose on response latencies for only nornicotine (F(2.3,16.1)= 4.1, p = 0.032; Fig 2B). However, follow-up pairwise comparisons showed that latencies did not differ significantly from saline at any nornicotine dose (all p-values ≥ 0.063, see Fig 2B).
Figure 2.
ICSS response latencies (expressed as percent of baseline, mean ± SEM) following injection of nicotine (A), nornicotine (B), anabasine (C), myosmine (D), anatabine (E), or cotinine (F).
4. DISCUSSION
This study provides new information on the addiction-related effects of the minor tobacco alkaloids nornicotine, anabasine, myosmine, anatabine, and cotinine on ICSS in rats. As expected, nicotine produced reinforcement-enhancing (ICSS threshold-decreasing) effects at low to moderate doses, and reinforcement-attenuating/aversive (ICSS threshold-increasing) effects at high doses. Nornicotine and anabasine produced similar biphasic effects on ICSS thresholds, although with lower potency compared to nicotine. Myosmine only elevated thresholds at high doses, while anatabine and cotinine did not affect ICSS thresholds. None of the alkaloids significantly affected ICSS response latencies, indicating a lack of non-specific motoric effects.
Our findings extend previous reports that nornicotine and anabasine can mimic nicotine’s effects in other behavioral models (e.g., nicotine discrimination; Dwoskin et al., 1999; Goldberg et al., 1989; Green et al., 2000; Pratt et al., 1983). The ≈ 5–10 fold lower potency of nornicotine and anabasine compared to nicotine was similar to that reported in some of these studies (e.g., Dwoskin et al., 1999). Together, these findings support nornicotine and anabasine as potential targets for smoking cessation medication development and establishment of tobacco product standards by the FDA. To the extent that the present findings are indicative of the abuse potential of nornicotine and anabasine, they support the inclusion of these compounds on the FDA’s list of harmful and potentially harmful constituents in tobacco products and tobacco smoke (Food and Drug Administration, 2012).
Limited data are available on the behavioral effects of myosmine, anatabine, and cotinine. A cocktail containing these three minor alkaloids, in addition to nornicotine and anabasine, did not itself maintain i.v. self-administration or influence locomotor activity, but potentiated nicotine’s reinforcing and locomotor stimulant effects (Clemens et al., 2009). Myosmine, anatabine, or cotinine alone also potentiated nicotine’s locomotor stimulant effects in Clemens et al. (2009). Other studies have reported that myosmine, anatabine, and cotinine can produce nicotine-like behavioral effects (Caine et al., 2014; Goldberg et al., 1989; Hall et al., 2014; Wiley et al., 2015). However, some of these effects were partial, not related to dose, and/or only observed at very high doses that may have produced toxicity or contained nicotine as an impurity. Taken together with the current data, these findings suggest that the abuse liability of myosmine, anatabine, and cotinine may be limited. However, further behavioral and neurobiological characterization of these minor alkaloids is clearly warranted. For example, the non-significant trend for anatabine to produce a biphasic effect on ICSS thresholds (see Fig 5E) could be further explored using larger sample sizes to confirm this pattern of effects.
We recently reported that nicotine alone and nicotine dose-equivalent concentrations of smokeless tobacco extracts produced similar acute effects on ICSS thresholds (Harris et al., 2015). Those extracts contained levels of nornicotine and anabasine more representative of exposure levels in smokeless tobacco users and that were at least 33- and 125-fold lower, respectively, than those shown to have behavioral effects in the current studies (see Table 2 in Harris et al., 2015). The lack of differences between nicotine alone and extracts in this study may therefore not be particularly surprising. Nonetheless, nicotine and non-nicotine constituents can produce additive or synergistic effects when administered in combination as occurs during actual tobacco use (Arnold et al., 2014; Belluzzi et al., 2005; Clemens et al., 2009). As such, regardless of their effects when studied in isolation, all of the current minor alkaloids could influence ICSS through interactions with nicotine, other minor alkaloids, and/or other behaviorally relevant tobacco constituents (e.g., acetaldehyde). Examining the effects of variations in tobacco constituent cocktails is needed to better understand the potential role of minor alkaloids in the abuse liability of tobacco products.
Highlights.
We tested effects of tobacco alkaloids on intracranial self-stimulation (ICSS).
Moderate nicotine doses reduced while high nicotine doses increased ICSS thresholds.
Nornicotine and anabasine produced similar effects, although with lower potency.
High-dose myosmine elevated thresholds, while anatabine and cotinine had no effect.
Some minor alkaloids can either fully or partially mimic nicotine’s effects on ICSS.
Acknowledgements
The authors would like to thank Danielle Burroughs, Theresa Harmon, and Christine Egan for technical assistance.
Role of Funding Source. This work was supported by NCI U19-CA157345 (Hatsukami, DK and Shields, PG, Co-PI; LeSage, PL) and the Minneapolis Medical Research Foundation (MMRF) Translational Addiction Research Program (Harris, PI). The NIH and MMRF had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors. Mark LeSage and Andrew Harris supervised the conduct of the study and were responsible for the conception and design of the study. Laura Tally, Peter Muelken, Andrew Banal, and Clare Schmidt assisted with developing specific protocols, daily conduct of the experiment, and compiling data. Qing Cao contributed to the statistical analysis. Andrew Harris wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of Interest. All authors declare that they have no conflict of interest.
REFERENCES
- Arnold MM, Loughlin SE, Belluzzi JD, Leslie FM. Reinforcing and neural activating effects of norharmane, a non-nicotine tobacco constituent, alone and in combination with nicotine. Neuropharmacology. 2014;85:293–304. doi: 10.1016/j.neuropharm.2014.05.035. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Green TA, Crooks PA, Dwoskin LP. Nornicotine is self-administered intravenously by rats. Psychopharmacology. 1999;146:290–296. doi: 10.1007/s002130051119. [DOI] [PubMed] [Google Scholar]
- Belluzzi JD, Wang R, Leslie FM. Acetaldehyde enhances acquisition of nicotine selfadministration in adolescent rats. Neuropsychopharmacology. 2005;30:705–712. doi: 10.1038/sj.npp.1300586. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Henningfield JE. Establishing a nicotine threshold for addiction. The implications for tobacco regulation. N. Engl. J. Med. 1994;331:123–125. doi: 10.1056/NEJM199407143310212. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Henningfield JE. Reducing the nicotine content to make cigarettes less addictive. Tob. Control. 2013;22(Suppl. 1):i14–i17. doi: 10.1136/tobaccocontrol-2012-050860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan KA, Laugesen M, Truman P. Whole tobacco smoke extracts to model tobacco dependence in animals. Neurosci. Biobehav. Rev. 2014;47C:53–69. doi: 10.1016/j.neubiorev.2014.07.014. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Palmatier MI, Liu X, Chaudhri N, Sved AF. The role of nicotine in smoking: a dual-reinforcement model. Nebr. Symp. Motiv. 2009;55:91–109. doi: 10.1007/978-0-387-78748-0_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine SB, Collins GT, Thomsen M, Wright C, Lanier RK, Mello NK. Nicotine-like behavioral effects of the minor tobacco alkaloids nornicotine, anabasine, and anatabine in male rodents. Exp. Clin. Psychopharmacol. 2014;22:9–22. doi: 10.1037/a0035749. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF. Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology. 2006;184:353–366. doi: 10.1007/s00213-005-0178-1. [DOI] [PubMed] [Google Scholar]
- Clemens KJ, Caille S, Stinus L, Cador M. The addition of five minor tobacco alkaloids increases nicotine-induced hyperactivity, sensitization and intravenous self-administration in rats. Int. J. Neuropsychopharmacol. 2009:1–12. doi: 10.1017/S1461145709000273. [DOI] [PubMed] [Google Scholar]
- Deyton L, Sharfstein J, Hamburg M. Tobacco product regulation--a public health approach. N. Engl. J. Med. 2010;362:1753–1756. doi: 10.1056/NEJMp1004152. [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Crooks PA, Teng L, Green TA, Bardo MT. Acute and chronic effects of nornicotine on locomotor activity in rats: altered response to nicotine. Psychopharmacology. 1999;145:442–451. doi: 10.1007/s002130051079. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration. Harmful and Potentially Harmful Constituents in Tobacco Products and Tobacco Smoke: Established List. Fed. Regist. 2012;77:20034–20037. [Google Scholar]
- Fowler CD, Kenny PJ. Nicotine aversion: Neurobiological mechanisms and relevance to tobacco dependence vulnerability. Neuropharmacology. 2013;76:533–544. doi: 10.1016/j.neuropharm.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ. Habenular alpha5 nicotinic receptor subunit signalling controls nicotine intake. Nature. 2011;471:597–601. doi: 10.1038/nature09797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg SR, Risner ME, Stolerman IP, Reavill C, Garcha HS. Nicotine and some related compounds: effects on schedule-controlled behaviour and discriminative properties in rats. Psychopharmacology. 1989;97:295–302. doi: 10.1007/BF00439441. [DOI] [PubMed] [Google Scholar]
- Green TA, Phillips SB, Crooks PA, Dwoskin LP, Bardo MT. Nornicotine pretreatment decreases intravenous nicotine self-administration in rats. Psychopharmacology. 2000;152:289–294. doi: 10.1007/s002130000524. [DOI] [PubMed] [Google Scholar]
- Hall BJ, Wells C, Allenby C, Lin MY, Hao I, Marshall L, Rose JE, Levin ED. Differential effects of non-nicotine tobacco constituent compounds on nicotine self-administration in rats. Pharmacol. Biochem. Behav. 2014;120:103–108. doi: 10.1016/j.pbb.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AC, Manbeck KE, Schmidt CE, Shelley D. Mecamylamine elicits withdrawal-like signs in rats following a single dose of nicotine. Psychopharmacology. 2013;225:291–302. doi: 10.1007/s00213-012-2814-x. [DOI] [PubMed] [Google Scholar]
- Harris AC, Mattson C, Lesage MG, Keyler DE, Pentel PR. Comparison of the behavioral effects of cigarette smoke and pure nicotine in rats. Pharmacol. Biochem. Behav. 2010;96:217–227. doi: 10.1016/j.pbb.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AC, Pentel PR, Burroughs D, Staley MD, Lesage MG. A lack of association between severity of nicotine withdrawal and individual differences in compensatory nicotine self-administration in rats. Psychopharmacology. 2011;217:153–166. doi: 10.1007/s00213-011-2273-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AC, Tally L, Schmidt CE, Muelken P, Stepanov I, Saha S, Vogel RI, LeSage MG. Animal models to assess the abuse liability of tobacco products: effects of smokeless tobacco extracts on intracranial self-stimulation. Drug Alcohol Depend. 2015;147:60–67. doi: 10.1016/j.drugalcdep.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison AA, Gasparini F, Markou A. Nicotine potentiation of brain stimulation reward reversed by DH beta E and SCH 23390, but not by eticlopride, LY 314582 or MPEP in rats. Psychopharmacology. 2002;160:56–66. doi: 10.1007/s00213-001-0953-6. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Benowitz NL, Donny E, Henningfield J, Zeller M. Nicotine reduction: strategic research plan. Nicotine Tob. Res. 2012;15:2003–2013. doi: 10.1093/ntr/nts214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Perkins KA, Lesage MG, Ashley DL, Henningfield JE, Benowitz NL, Backinger CL, Zeller M. Nicotine reduction revisited: science and future directions. Tob. Control. 2010;19:e1–e10. doi: 10.1136/tc.2009.035584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningfield JE, Benowitz NL, Connolly GN, Davis RM, Gray N, Myers ML, Zeller M. Reducing tobacco addiction through tobacco product regulation. Tob. Control. 2004;13:132–135. doi: 10.1136/tc.2003.006890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AC, Evans SE. Abuse potential of non-nicotine tobacco smoke components: acetaldehyde, nornicotine, cotinine, and anabasine. Nicotine Tob. Res. 2013;15:622–632. doi: 10.1093/ntr/nts192. [DOI] [PubMed] [Google Scholar]
- Huston-Lyons D, Kornetsky C. Effects of nicotine on the threshold for rewarding brain stimulation in rats. Pharmacol. Biochem. Behav. 1992;41:755–759. doi: 10.1016/0091-3057(92)90223-3. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Polis I, Koob GF, Markou A. Low dose cocaine self-administration transiently increases but high dose cocaine persistently decreases brain reward function in rats. Eur. J. Neurosci. 2003;17:191–195. doi: 10.1046/j.1460-9568.2003.02443.x. [DOI] [PubMed] [Google Scholar]
- Kornetsky C, Esposito RU. Euphorigenic drugs: effects on the reward pathways of the brain. Fed. Proc. 1979;38:2473–2476. [PubMed] [Google Scholar]
- Manbeck KE, Shelley D, Schmidt CE, Harris AC. Effects of oxytocin on nicotine withdrawal in rats. Pharmacol. Biochem. Behav. 2013;116C:84–89. doi: 10.1016/j.pbb.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Markou A, Koob GF. Postcocaine anhedonia. An animal model of cocaine withdrawal. Neuropsychopharmacology. 1991;4:17–26. [PubMed] [Google Scholar]
- Markou A, Koob GF. Construct validity of a self-stimulation threshold paradigm: effects of reward and performance manipulations. Physiol. Behav. 1992;51:111–119. doi: 10.1016/0031-9384(92)90211-j. [DOI] [PubMed] [Google Scholar]
- Paterson NE. The neuropharmacological substrates of nicotine reward: reinforcing versus reinforcement-enhancing effects of nicotine. Behav. Pharmacol. 2009;20:211–225. doi: 10.1097/FBP.0b013e32832c7083. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Balfour DJ, Markou A. Chronic bupropion differentially alters the reinforcing, reward-enhancing and conditioned motivational properties of nicotine in rats. Nicotine Tob. Res. 2008;10:995–1008. doi: 10.1080/14622200802097571. [DOI] [PubMed] [Google Scholar]
- Pratt JA, Stolerman IP, Garcha HS, Giardini V, Feyerabend C. Discriminative stimulus properties of nicotine: further evidence for mediation at a cholinergic receptor. Psychopharmacology. 1983;81:54–60. doi: 10.1007/BF00439274. [DOI] [PubMed] [Google Scholar]
- Spiller K, Xi ZX, Li X, Ashby CR, Jr, Callahan PM, Tehim A, Gardner EL. Varenicline attenuates nicotine-enhanced brain-stimulation reward by activation of alpha4beta2 nicotinic receptors in rats. Neuropharmacology. 2009;57:60–66. doi: 10.1016/j.neuropharm.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolerman IP, Garcha HS, Mirza NR. Dissociations between the locomotor stimulant and depressant effects of nicotinic agonists in rats. Psychopharmacology. 1995;117:430–437. doi: 10.1007/BF02246215. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Garcha HS, Pratt JA, Kumar R. Role of training dose in discrimination of nicotine and related compounds by rats. Psychopharmacology. 1984;84:413–419. doi: 10.1007/BF00555223. [DOI] [PubMed] [Google Scholar]
- U.S. Public Health Service. The Health Consequences Of Smoking: Nicotine Addiction: A Report Of The Surgeon General. Washington, DC.: U.S. Department of Health and Human Services; 1988. [Google Scholar]
- Verendeev A, Riley AL. The role of the aversive effects of drugs in self-administration: assessing the balance of reward and aversion in drug-taking behavior. Behav. Pharmacol. 2013;24:363–374. doi: 10.1097/FBP.0b013e32836413d5. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Marusich JA, Thomas BF, Jackson KJ. Determination of behaviorally effective tobacco constituent doses in rats. Nicotine Tob. Res. 2015;17:368–371. doi: 10.1093/ntr/ntu194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Addictive drugs and brain stimulation reward. Annu. Rev. Neurosci. 1996;19:319–340. doi: 10.1146/annurev.ne.19.030196.001535. [DOI] [PubMed] [Google Scholar]
- Wise RA. Brain reward circuitry: insights from unsensed incentives. Neuron. 2002;36:229–240. doi: 10.1016/s0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bauco P, Carlezon WA, Jr, Trojniar W. Self-stimulation and drug reward mechanisms. Ann. N. Y. Acad. Sci. 1992;654:192–198. doi: 10.1111/j.1749-6632.1992.tb25967.x. [DOI] [PubMed] [Google Scholar]