Abstract
Objective
To characterize human triglyceride-rich lipoproteins (TRL) with and without apoA-II and to study their metabolism in vivo.
Methods
Plasma from 11 participants on a controlled diet given a bolus infusion of [D5]L-phenylalanine to label apoB was combined into four pools and applied to anti-apoA-II immunoaffinity columns. Fractions with and without apoA-II were separated into VLDL and IDL by ultracentrifugation; lipids and apolipoproteins were measured. For kinetic measurements, apoB was isolated and hydrolyzed to the constituent amino acids. Tracer enrichment was measured by GCMS. Metabolic rates were determined by SAAM-II.
Results
VLDL and IDL with apoA-II comprised 7% and 9% of total VLDL and IDL apoB respectively. VLDL with apoA-II was enriched in apoC-III, apoE, and cholesterol compared to VLDL without apoA-II. Mean apoB FCR of VLDL with apoA-II was significantly lower than for VLDL without apoA-II (2.80±0.96 pools/day v.s. 5.09±1.69 pools/day, P=0.009). A higher percentage of VLDL with apoA-II was converted to IDL than was cleared from circulation, compared to VLDL without apoA-II (96±8% vs. 45±22%; P=0.007). The rate constants for conversion of VLDL to IDL were similar for VLDL with and without apoA-II. Thus, a very low rate constant for clearance accounted for the lower FCR of VLDL with apoA-II.
Conclusion
VLDL with apoA-II represents a small pool of VLDL particles that has a slow FCR and is predominantly converted to IDL rather than cleared from the circulation.
Keywords: Apolipoprotein A-II, Lipid metabolism, VLDL, Triglyceride rich lipoproteins
Introduction
Apolipoprotein A-II (apoA-II) is the second most abundant protein constituent of high-density lipoprotein (HDL), accounting for about 20% of HDL protein.1 The mean plasma total apoA-II concentration is about 30–35mg/dL.2 Although apoA-II is mainly found associated with HDL, a small proportion is associated with the apoB lipoproteins, especially chylomicrons and very-low-density lipoprotein (VLDL).3
Animal models have shown that apoA-II can directly affect VLDL metabolism.4–6 Transgenic mice expressing human apoA-II at two to three times higher than the normal concentration of mouse apoA-II displayed hypertriglyceridemia.4 The lipoprotein lipase mediated lipolysis of VLDL particles containing apoA-II was impaired in these mice.4 Secretion of VLDL was not affected.4 A subsequent study confirmed that human apoA-II transgenic mice had reduced hydrolysis of triglycerides from chylomicrons and VLDL, but also found a 25% increase in triglyceride secretion.5 These findings suggest that the hypertriglyceridemia in human apoA-II overexpressing mice is associated with increased secretion and defective catabolism by lipoprotein lipase of triglyceride-rich lipoproteins.
The role of apoA-II in human VLDL metabolism is unclear. Patients who have severe hypertriglyceridemia (Type V hyperlipoproteinemia) have apoB lipoproteins that are enriched in apoA-II that are poor substrates for lipoprotein lipase. 3 Patients with Tangier disease have VLDL that contain a large amount of apoA-II that is also a poor substrate for lipoprotein lipase, and they have mild hypertriglyceridemia.3,7 Alaupovic et al separated apoB lipoproteins from patients with severe hypertriglyceridemia or Tangier disease into lipoproteins with or without apoA-II.3 Importantly, the apoB lipoproteins from both groups that contained apoA-II had impaired reactivity to lipoprotein lipase compared to lipoproteins that did not contain apoA-II.3 In other respects, the lipid and apolipoprotein composition of the apoB lipoproteins that had apoA-II was similar to VLDL that did not have apoA-II. These studies in mice and humans raise the possibility that apoA-II contributes to hypertriglyceridemia by reducing lipolysis in plasma of triglyceride-rich lipoproteins. It is also possible that apoA-II, as a surface protein, interferes with clearance of VLDL from the circulation by apoE or apoB interacting with hepatic receptors, similar to the action of another VLDL surface protein, apoC-III.8
It is unknown whether apoA-II affects VLDL metabolism in normolipidemia or in common phenotypes of mild hypertriglyceridemia. No studies have directly examined the relationship of apoA-II to VLDL metabolism in vivo in humans. To do this, we studied the metabolism of VLDL and IDL that contain apoA-II compared to those that do not have apoA-II in individuals who have normal or mildly increased fasting triglyceride levels.
Materials and Methods
Subjects
11 participants evaluated were recruited as part of a previous study. 8,9 Exclusion criteria included secondary hyperlipidemia; Apo E2/E2, E4/E4, and E2/E4 genotypes and use of medications that affect lipid metabolism. The study was approved by the Human Subjects Committees at Harvard School of Public Health and Brigham and Women’s Hospital. All participants gave informed consent.
Dietary Period
All study subjects were provided complete diets rich in either monounsaturated fat or carbohydrate for 3 weeks before the kinetic study. The participants as outpatients were required to eat one meal each weekday on-site, either lunch or dinner. The monounsaturated fat diet consisted of 37% fat (8% saturated fat, 24% monounsaturated fat, and 5% polyunsaturated fat), 48% carbohydrate, and 15% protein. The carbohydrate diet consisted of 20% fat (7% saturated fat, 8% monounsaturated fat, and 5% polyunsaturated fat), 65% carbohydrate, and 15% protein. Participants were instructed not to consume alcohol or intake any other source of calories. Energy intake was adjusted to keep body weight constant during the study period.
Kinetic Study
Participants received a bolus injection of 1.2 µmol/kg [D5] L-phenylalanine (Tracer Technologies, Cambridge, MA). Blood samples were collected every 30 minutes for the first two hours and hourly thereafter for a total of 14 hours. To maintain a constant postprandial state, small hourly meals were consumed. The daily intake was divided into 12 portions to obtain the hourly intake level. Hourly food intake started 3 hours before the tracer was administered. This technique has been used previously by Zheng et al. in the same kinetic protocol with these participants.9
Separation of Lipoproteins
Because the plasma from these participants in this kinetics protocol was obtained and used in a previous study, quantities were limited. A pooling scheme was therefore devised to create enough sample at baseline and at each time point during the tracer infusion so that the lipoprotein fractionation could yield sufficient quantity for measurements of tracer enrichment and composition of apoA-II containing VLDL which we determined had a very low plasma concentration. The 11 participants were divided into four groups each with 2–3 individuals and their plasma was combined to create 4 pools for analysis of kinetics and composition. One pool came from normolipidemic participants (N=3), and three came from mildly hypertriglyceridemic participants (N=2, 3, 3). Two pools each came from participants on the low-fat diet and the high-monounsaturated fat diets (Table 1). The availability of sufficient plasma governed the selection of the specific participants that were pooled. We emphasize that the aim of this study was to compare the kinetics of TG-rich lipoproteins that contained apoA-II with those that did not apoA-II, and not to explore modulation of the kinetics by diet type for which this protocol is not suited. Another reason for pooling is to reduce the between-subjects variability in kinetic parameters, and to reduce the chance of outliers.
Table 1.
Group characteristics.
| Diet | Group | Weight (kg) |
BMI (kg/m2) |
Total Cholesterol (mg/dL) |
Triglyceride (mg/dL) |
HDL (mg/dL) |
|---|---|---|---|---|---|---|
| Monounsaturated | 1 | 82±28 | 28±5 | 237±26 | 64±31 | 79±36 |
| 2 | 80±14 | 27±2 | 216±25 | 284±10 | 33± 3 | |
| Carbohydrate | 3 | 83±21 | 29±2 | 245±19 | 328± 4 | 39±13 |
| 4 | 105±16 | 40±2 | 215±27 | 219±10 | 32± 8 |
Groups were pools of plasma from 2 or 3 participants as described in methods. Values reflect mean±SD.
An equal amount of plasma from each individual in a group was pooled for each time point. Pooled plasma from each time point was loaded into 20 mL Econo-Pac columns (Bio-Rad Laboratories, Hercules, CA) packed with 2.5mL of anti–apoA-II resin prepared from polyclonal sheep anti–human apoA-II antibody bound to Sepharose 4B Resin at a minimum concentration of 10 mg antibody/mL resin (Academy Biomedical Company Inc, Houston, TX). Samples and resin were incubated for 16 hours at 4 °C with mixi ng. The unbound fraction was eluted from the column by gravity followed by washes with phosphate buffered saline. The bound fraction was then eluted from the columns with 3 mol/L sodium thiocyanate in phosphate-buffered saline. The efficiency of the immunoaffinity separation (percent of apoA-II removed from plasma by the resin) was 93%.The two immunofractions were then fractionated by density ultracentrifugation9. The fractions were centrifuged in an L8–70M instrument (Beckman, Brea, CA) equipped with a Ti25 rotor for 1 hour at 25,000rpm to float light VLDLs [Svedberg units of flotation (Sf) 60–400]. After light VLDL was collected by aspiration, the initial volume was restored with d=1.006 g/mL phosphate buffered saline and dense VLDL (Sf: 20–60) was isolated by spinning for 16 hours at 25,000rpm. Following collection of dense VLDL, the initial volume was restored with potassium bromide (KBr) solution to simultaneously adjust the density to 1.025 g/mL. The samples were then spun at 25,000rpm for 16 hours to isolate IDL (1.006–1.025 g/mL). Finally, after IDL was collected, the sample density was again adjusted with KBr to 1.050 g/mL, the samples were spun for 24 hours at 25,000rpm, and LDL (1.025–1.050) was collected.
We combined light and dense VLDL data because the individual concentrations and tracer enrichments were too low for reliable measurement. Also, in this population, apoA-II concentrations in LDL were near or below the detection limit (0.001mg/dL), not exceeding 0.01mg/dL or 0.5% of total for any individual. Furthermore, the percentage of LDL apoB that contained apoA-II was less than 1% and was not detectable in most samples by GC-MS. Therefore, this study focused on apoB lipoproteins in the VLDL and IDL fractions. In this study, VLDL and IDL refer to the lipoproteins present in the respective density fractions, both apoB48 and apoB100.
Measurement of apoB Tracer Enrichment and Pool Size
ApoB was precipitated from the lipoproteins with isopropanol, a norleucine internal standard was added, and the mixture was converted to volatile heptafluorobutyric acid derivatives. Measurement of tracer enrichment was done as previously described.9 Tracer enrichment was measured on a 6890 gas chromatograph/5973 mass spectrometer (Hewlett-Packard, Palo Alto, CA) using negative chemical ionization and selective ion monitoring. ApoB mass was measured by comparing the ratio between the area under the leucine curve and the area under the norleucine curve with a standard curve of various leucine/norleucine ratios. The ratio calculated in the sample is converted to a concentration of leucine using the equation from the standard curve. The leucine mass is converted to concentration of apoB in the injected sample using the proportion of apoB that is leucine and dividing by the total volume of plasma in the original starting sample. ApoB mass is calculated by multiplying the apoB concentration by the subject’s total plasma volume, which is estimated using their weight in kilograms. Plasma volume (L) was assumed to be 4.4% of body weight (kg) for groups with an average BMI <30 kg/m2. For the obese group (BMI>30 kg/m2) plasma volume was calculated based by an equation described by Nikkila and Kekki.10 To evaluate the possibility of degradation occurring in the samples, we compared the apoB concentration in the samples used in the present study to samples stored at −80C less than 1 year. In our study 0.10% of total apoB was in the VLDL fraction with apoA-II versus 0.08% in the newer samples. We found similar results in the IDL fraction, in our study 0.12% of total apoB was in the IDL fraction with apoA-II versus 0.15% in the newer samples. These results show that with longer storage significant degradation is unlikely.
Model Development and Kinetic Analysis
A multi-compartment model was used to describe VLDL and IDL apoB kinetics. The SAAM II software (The Epsilon Group, Charlottesville, VA) was used to model the data. The model allowed for direct secretion into VLDL and IDL fractions, as well as direct clearance from the corresponding compartment. The VLDL and IDL apoB fractional catabolic rates (FCR) were determined from the model parameters giving the best fit. Since VLDL metabolism is a multiple pool process, as part of our model development process, we tested for multiple pools of VLDL and IDL within subfractions that contain apoA-II, as well as conversion pathways among lipoproteins of different apoA-II content.
Statistical analyses
Weight and BMI, as well as serum total cholesterol, triglyceride, and HDL cholesterol were summarized by mean ± standard deviation for each diet group separately. Outcomes of interest included fractional catabolic rate, percent lipolytic conversion from VLDL to IDL, percent clearance of VLDL, and molar ratio of VLDL and IDL constituents per apoB. The difference for each outcome with and without apoA-II was calculated separately for VLDL and IDL, and the mean within-group paired difference tested against the null hypothesis of zero using paired t-test. All tests were two-sided, and deemed statistically significant at P<0.05.
Results
Results of Modeling
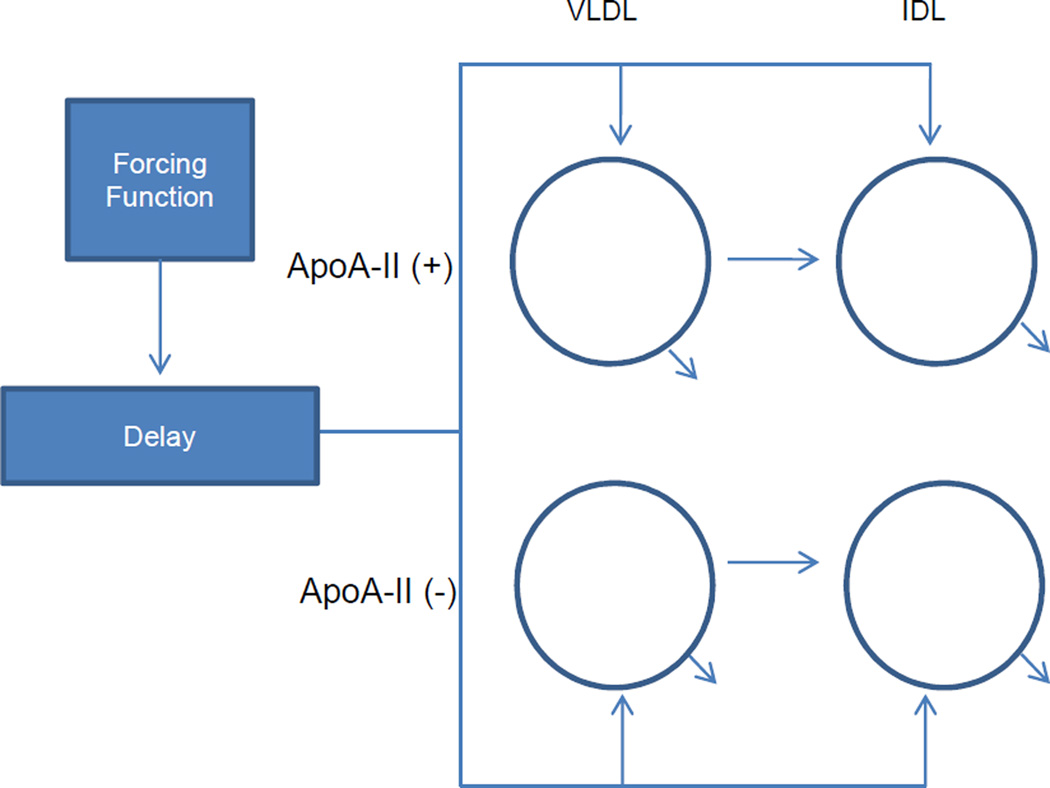
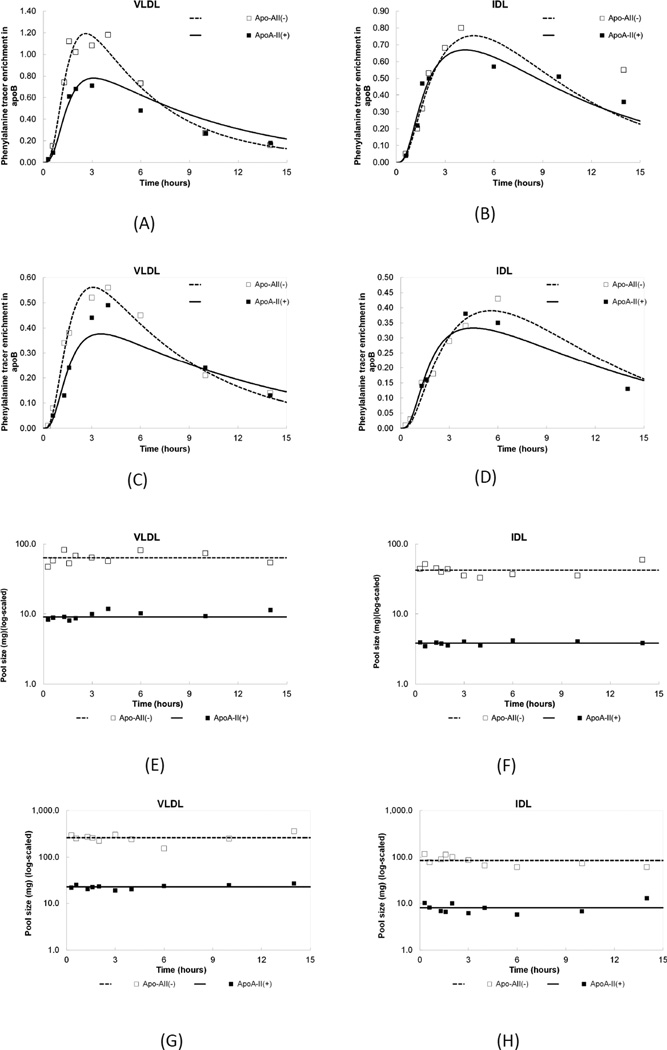
The model utilized a plasma amino acid forcing function followed by a delay compartment to account for the time required for synthesis and secretion of apoB into plasma. We tested for conversion pathways among lipoproteins of different apoA-II composition, specifically, A-II(+) VLDL→A-II(−) IDL as well as A-II(−) VLDL→A-II(+) VLDL. We also tested two models with multiple VLDL pools. The models and summaries of results are included in the supplementary online appendix. The tracer data did not support the presence of such conversion pathways or multiple VLDL pools and this was reflected by poor precision of model parameters. The two multiple VLDL pool models we tested gave similar results to a single VLDL pool model but with higher CVs. One of the models allowed for conversion from the first VLDL pool to a second VLDL pool or IDL. A second model allowed conversion from the first VLDL pool to a second VLDL pool and then from the second VLDL pool to IDL. Consequently, we had abided by the principle of parsimony, which was to select the model with the least numbers of parameters that fit the data best.11 The precision of our final model parameters were relatively high with CVs averaged over the four density fractions of <25%. The final model is shown in Figure 1. The tracer-to-tracee ratios and pool sizes in VLDL and IDL fractions are provided in Figure 2.
Figure 1.
Structure of the model. Plasma apoB was separated into 4 different lipoprotein fractions (circles) by anti-apoA-II immunoaffinity chromatography and then ultracentrifugation. Plasma tracer forcing function is followed by a delay compartment. The delay compartment accounts for the time required for the synthesis and secretion of apoB into plasma. ApoA-II(+) and apoA-II(−): denote lipoproteins that do or do not contain apoA-II, respectively.
Figure 2.
(A–D) Tracer enrichment of [D5]L-phenylalanine in apoB of VLDL and IDL. Lines represent model derived curves fit to the data points. (A,B) Monounsaturated fat diet and (C,D) Carbohydrate diet. ApoA-II(+) and apoA-II(−): denote lipoproteins that do or do not contain apoA-II, respectively.
(E–H) Measured apoB pool sizes and model-derived fits in VLDL and IDL. Subfractions on a monounsaturated fat (E,F) diet and on a carbohydrate diet. (G,H). ApoA-II(+) and apoA-II(−): denote lipoproteins that do or do not contain apoA-II, respectively.
Subject Characteristics
Eleven participants (5 men and 6 women) had mean body mass index of 30 ± 5 kg/m2, total cholesterol of 208 ± 35 mg/dL, fasting triglyceride (TG) of 174 ± 103mg/dL, and HDL cholesterol of 50 ± 21 mg/dL (Table 1).
A small fraction of total VLDL and IDL apoB contains apoA-II
Mean concentration of apoB in VLDL with apoA-II was 0.42 ± 0.10 mg/dL, compared to 5.27 ± 3.16 mg/dL for VLDL without apoA-II,(mean paired difference = 4.84 ± 3.07, P=0.05). Thus, VLDL with apoA-II comprised 7% of total VLDL apoB. Mean concentration of apoB in IDL with apoA-II was 0.23 ± 0.16 mg/dL compared to 2.39 ± 2.07 mg/dL for IDL without apoA-II, representing 9% of total IDL apoB.
Direct secretion rates of apoB in VLDL and IDL with apoA-II were lower than VLDL and IDL without apoA-II
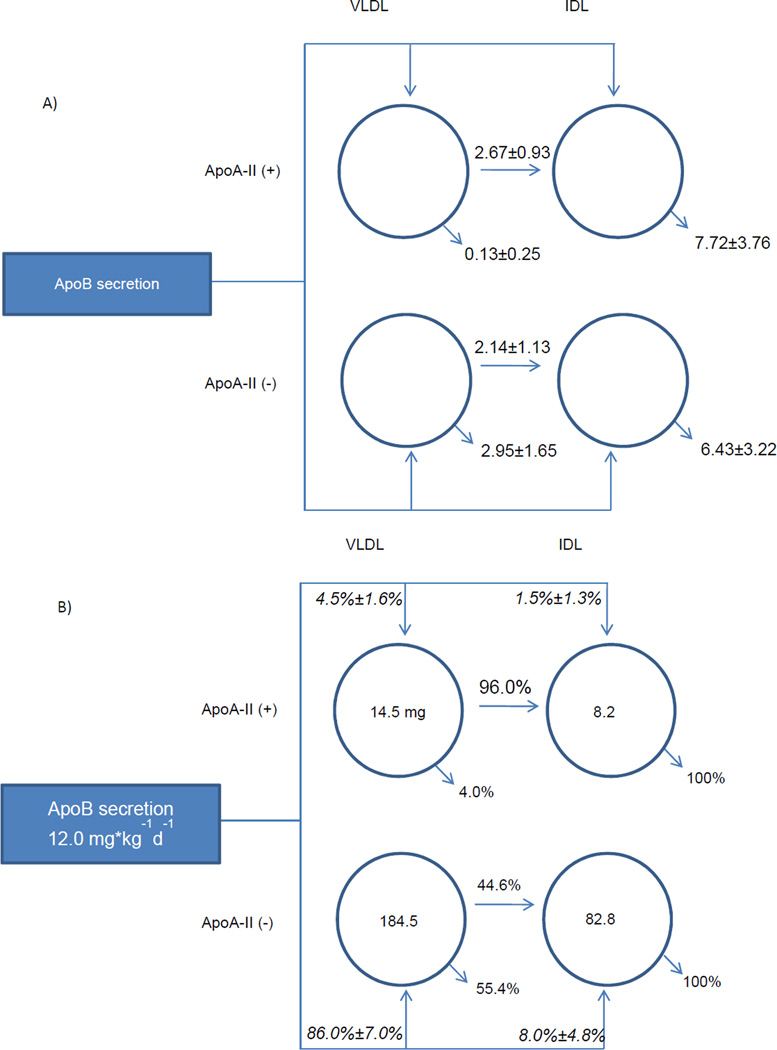
ApoB secretion rates of VLDL with apoA-II were significantly lower than VLDL without apoA-II (Figure 3B). The secretion rate of VLDL with apoA-II was 0.45 mg/kg/day, which represents 4.5% of secreted VLDL apoB, compared to 10.7 mg/kg/day (86%) for VLDL without apoA-II. Secretion of IDL with apoA-II was lower than that of VLDL with apoA-II, 1.5% of total IDL apoB.
Figure 3.
Average rate flux distributions, pool size (mg), and rate constants (pools/day) of apoB lipoproteins. The rectangular box on the left represents the liver, and arrows out of this box represent direct liver secretion into plasma. The circles represent lipoprotein compartments. The numbers inside the circles indicate average measured pool size. Arrows out of the lipoprotein compartments represent conversion to IDL or removal from plasma. (A) Numbers above or next to the arrows represent average rate constants ± SD. (B) Italicized percentages indicate percentage of total liver secretion into each compartment ± SD. Percentages above or below the arrows indicate the relative proportion of flux out of each compartment. ApoA-II(+) and apoA-II(−): denote lipoproteins that do or do not contain apoA-II, respectively. See Figure 4 and text for statistics.
VLDL apoB with apoA-II has a slow fractional catabolic rate
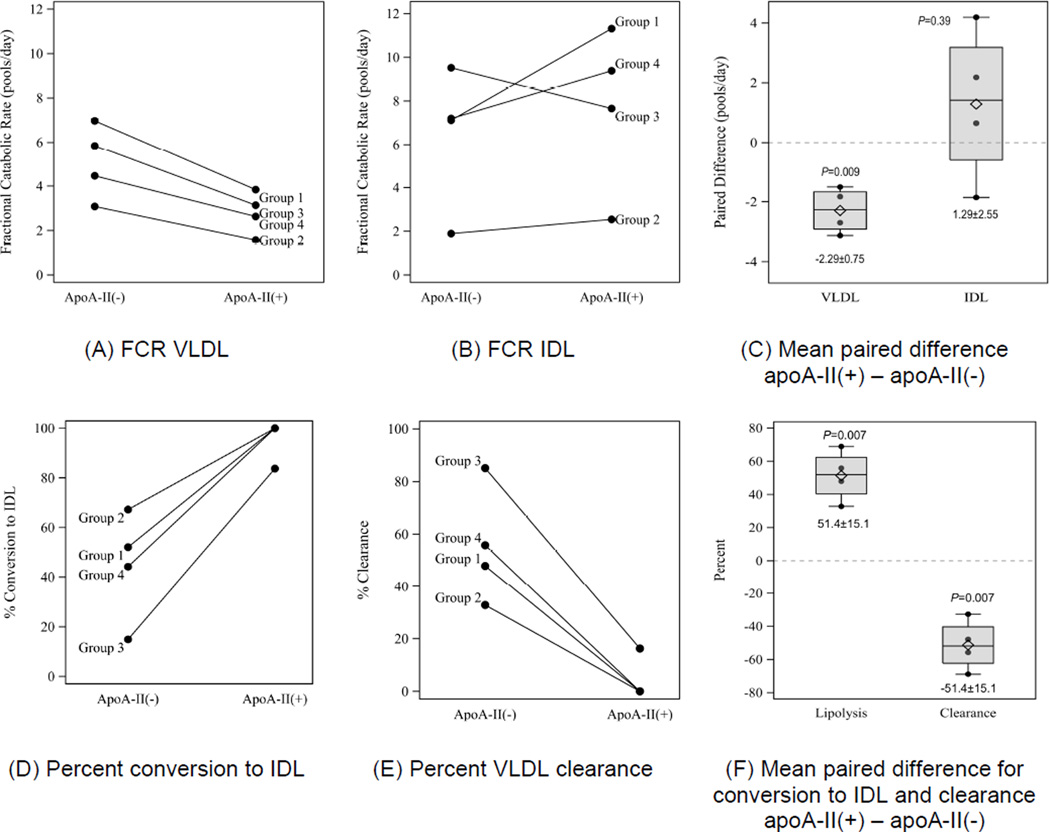
VLDL with apoA-II had a slower apoB fractional catabolic rate (FCR) than VLDL without apoA-II, 2.80 ± 0.96 pools/day vs. 5.09 ± 1.69 pools/day (mean difference −2.29 ± 0.75 pools/day; P=0.009) (Figure 4A,C). In contrast, the mean FCR for IDL without apoA-II was similar to that for IDL with apoA-II, mean difference, 1.29 ± 2.55 (P=0.39) (Figure 4B,C).
Figure 4.
Paired observations for (A) Fractional catabolic rate (FCR) VLDL, (B) FCR IDL, (C) Box plots showing mean paired difference in FCR for VLDL and IDL that do or do not contain apoA-II. (D) Percent conversion of VLDL to IDL, (E) Percent clearance of VLDL, (F) Box plots showing paired differences for conversion of VLDL to IDL and clearance of VLDL. Differences are apoA-II(+) minus apoA-II(−). ApoA-II(+) and apoA-II(−): denote lipoproteins that do or do not contain apoA-II, respectively. Each box-whisker plot represents the sample mean (diamond), median (center line), lower quartile (bottom of the box), upper quartile (top of the box), and extremes (vertical whiskers) outside the interquartile range.
Most VLDL containing apoA-II are converted to IDL before clearance from the circulation
A larger percentage of VLDL with apoA-II compared to VLDL without apoA-II underwent conversion by lipolysis to IDL rather than clearance from the circulation, 96 ± 8% vs. 45 ± 22% respectively (mean difference 51.4 ± 15.1% P=0.007). Only 4% of VLDL with apoA-II left the circulation without becoming IDL, compared to 55% to VLDL without apoA-II. The rate constants for conversion of VLDL to IDL were similar for the VLDL with and without apoA-II (2.67 ± 93 pools/day vs. 2.14 ± 1.14), whereas the rate constants for clearance of VLDL with apoA-II were greatly reduced (Figure 3A). Flux percentages are shown in Figure 3B. Percent conversion to IDL, percent VLDL clearance, and mean differences for the groups is shown in Figures 4 D, E, and F respectively.
Triglyceride, Cholesterol, and Apolipoprotein Content of VLDL and IDL
The mean apoC-III/apoB molar ratio was 27 ± 12 for VLDL without apoA-II versus 86 ± 37 for VLDL with apoA-II (P=0.02); and for IDL 19 ± 12 versus 75 ± 40 (P=0.06) (Table 2). The Spearman rank correlation of FCR and apoC-III content is −0.81 for VLDL and 0.62 for IDL. The mean apoE/apoB molar ratio was 4 ± 1 for VLDL without apoA-II versus 23 ± 4 for VLDL with apoA-II (P=0.004); and for IDL 3 ± 1 versus 7 ± 4 (P=0.05). VLDL with apoA-II tended to have a lower TG/apoB ratio and a higher cholesterol/apoB ratio than VLDL without apoA-II but the differences were not statistically significant (Table 2).
Table 2.
Molar Ratios
| Molar Ratio | apoA-II (−) | apoA-II (+) | Paired Difference | P | |
|---|---|---|---|---|---|
| Apo E/B | VLDL | 4±1 | 23±4 | 19.1±4.8 | 0.0004 |
| IDL | 3±1 | 7±4 | 4±3 | 0.05 | |
| Apo CIII/B | VLDL | 27±12 | 86±37 | 60±28 | 0.02 |
| IDL | 19±12 | 75±40 | 56±37 | 0.06 | |
| Cholesterol/B | VLDL | 6255±3376 | 9406±5567 | 3151±2212 | 0.07 |
| IDL | 4261±945 | 4565±757 | 304±756 | 0.48 | |
| Triglyceride/B | VLDL | 17564±4954 | 14065±1791 | −3499±3294 | 0.12 |
| IDL | 2148±867 | 7102±4958 | 4954±4175 | 0.10 |
Table shows molar ratios (mean ± SD) of apoE, apoCIII, cholesterol, and triglyceride per apoB in VLDL and IDL fractions without and with apoA-II. Mean paired difference apoA-II (+) – apoA-II (−).
Discussion
The main findings are that the plasma apoB pool size of VLDL containing apoA-II is much smaller than that of VLDL without apoA-II, and this was caused by a very low rate of secretion of this VLDL type into plasma. This low rate of secretion was sufficiently low to compensate for a slower FCR of VLDL with apoA-II which would tend to increase the pool size. The slow FCR of VLDL with apoA-II is accounted for by greatly reduced clearance from plasma, and not by slow lipolytic conversion to IDL.
ApoA-II overexpression in mice produces VLDL that is a poor substrate for lipoprotein lipase in assays performed in vitro. 4,5 However, hydrolysis of VLDL-TG in vivo in the apoA-II overexpressing mice was similar to control mice.6,12 Alaupovic et al. found that VLDL from patients with Tangier disease had high contents of apoA-II and were less effective as a substrate for human milk lipoprotein lipase than VLDL from normal controls.7 However, other compositional differences of Tangiers VLDL complicates attribution of slow lipolysis specifically to high apoA-II. Alaupovic et al subsequently used apoA-II immunoabsorbers to discover in 3 patients with Tangier disease and in 3 patients with severe hypertriglyceridemia (Type V Hyperlipoproteinemia) a discrete subspecies of VLDL that contained high amounts of apoA-II in combination with apoC-I, apoC-II, apoC-III apoD and apoE. They found in the patients with severe hypertriglyceridemia that the lipolysis rate in vitro of VLDL that contained apoA-II was about 50% slower than VLDL that did not contain apoA-II.3 VLDL with apoA-II from Tangier disease also showed a slow rate of lipolysis, although the researchers did not study VLDL without apoA-II in the Tangier patients. Therefore, we hypothesized that apoA-II reduces the conversion rate of VLDL to IDL, a process that involves lipoprotein lipase. We found to the contrary that VLDL with apoA-II in our normolipidemic and mildly hypertriglyceridemic participants does not have a slower conversion rate to IDL, in vivo, compared to the same participants' VLDL that did not have apoA-II. The rate constants for conversion to IDL are similar for VLDL with and without apoA-II, and should reflect the activity not only of lipoprotein lipase but also of hepatic lipase. Normal conversion of VLDL to IDL in our human study is consistent with normal rate of hydrolysis of VLDL TG in mice overexpressing human apoA-II. 6,12 ApoA-II containing apoB lipoproteins comprise nearly 90% of the apoB lipoproteins in Tangiers disease and about 50% in severe hypertriglyceridemia, much greater amounts than in the normal or mildly hypertriglyceridemic participants in our study.7 It is possible that much higher apoA-II levels in VLDL than our participants had are needed to inhibit lipolysis.
We found that VLDL with apoA-II are not effectively cleared from the circulation. TG content of VLDL with apoA-II is similar to that of VLDL without apoA-II. If VLDL with apoA-II inhibits lipoprotein lipase, the TG content should be much higher than VLDL without apoA-II. In mice overexpressing mouse apoA-II, clearance of chylomicrons, estimated by the retinyl palmitate method, is reduced.5 In mice overexpressing human apoA-II, VLDL clearance rate measured by the cholesteryl oleyl ether method, is not affected.12 These methods are not easily used to interpret in vivo metabolism of VLDL particles in humans which is measured directly by apoB kinetics. Our results suggest that once VLDL enters the circulation, the apoA-II content is a determinant of their flux distribution.
The contents of apoE and apoC-III per VLDL with apoA-II, as measured by their molar ratio to apoB, are higher in VLDL particles with apoA-II compared to VLDL without apoA-II. Previous studies have found that apoA-II can form a complex with apoE on HDL and hypothetically it is possible that it does so on VLDL and reduces the ligand function of apoE.13 It is also possible that apoA-II interacts with apoE in a similar way as apoC-III blocking its interaction with hepatic receptors that remove VLDL from the circulation.8,12,13 This may explain our finding of a slower rate constant for clearance of VLDL particles containing apoA-II from plasma. Alternatively, apoC-III itself may have played a role in reduced clearance of VLDL particles containing apoA-II. Previous human kinetic studies have established apoC-III strongly inhibits hepatic uptake of VLDL. 8 We found that the apoC-III to apoB ratio is associated inversely with VLDL FCR but directly with IDL FCR. In this small sample, it is not possible to determine the association of apoA-II with VLDL and IDL FCR independent of content of apoC-III.
Though we did not specifically isolate the chylomicron fraction, based on previous work in our lab the pool size of VLDL apoB-48 is much smaller than VLDL apoB-100 and once apoB-48 particles are in circulation the metabolic pathways and FCR become similar.14 Thus, apo48 lipoproteins do not contribute materially to the metabolic rates of apoB in VLDL and IDL as prepared in our study.
Limitations of our study include a small sample size as well as pooling of plasma, inability to determine the role of apoA-II in VLDL and IDL that does not have other apolipoproteins such as apoC-III or apoA-I, and participants being on two different diets rather than a single diet. Another limitation was that plasma of participants had to be pooled due to insufficient quantity of plasma to test individuals. However, pooling of samples helps reduce inter-individual variability. Additionally, further studies are needed, specifically with VLDL with apoA-II, but without other apolipoproteins if possible, to understand the unique effect of apoA-II. Diet influences metabolic pathways and secretion of triglyceride-rich lipoprotein particles.9 Nonetheless, the results pertaining to the metabolism of VLDL and IDL according to presence or absence of apoA-II were in the same direction for the two pools that came from participants who ate the low-fat diet and those on the high-monounsaturated fat diet. We found that the concentrations of VLDL and IDL that have both apoA-I and apoA-II are very low (results included in supplementary online appendix); we did not study the metabolism of this type of particle distinct from VLDL and IDL that have apoA-II but not apoA-I.
In summary, a small percentage of VLDL and IDL contains apoA-II. VLDL containing apoA-II have a slower FCR and are less likely to be cleared from plasma and more likely to be converted to IDL. The conversion rate of VLDL to IDL is similar for VLDL that has or does not have apoA-II, suggesting that apoA-II does not inhibit lipolytic conversion of VLDL. Instead VLDL with apoA-II have a slow clearance rate from plasma, thus increasing the percentage that is converted to IDL. Our results have potential implications for understanding the dyslipidemia in familial combined hypercholesterolemia and type V hyperlipoproteinemia, as these phenotypes have significant hypertriglyceridemia. Given the poor clearance of VLDL containing apoA-II, the presence of apoA-II on VLDL may promote atherogenesis.
Supplementary Material
Highlights.
We studied the kinetics of VLDL with and without apoA-II in humans.
A small percentage of VLDL and IDL contains apoA-II.
VLDL with apoA-II has a slower FCR than VLDL without apoA-II.
VLDL with apoA-II has greatly reduced clearance from plasma compared to VLDL without apoA-II.
VLDL with apoA-II has similar rate of conversion to IDL as VLDL without apoA-II
Acknowledgements
We express thanks to Sue Wong-Lee for her technical expertise. We also express gratitude to the volunteers who participated in this study.
Funding
This work was supported by grants R01-HL-34980, R01-HL-56210 and RR02635, from the National Institutes of Health, Bethesda, MD
Abbreviations
- TRL
triglyceride rich lipoproteins
- VLDL
Very low density lipoproteins
- apoB
apolipoprotein B
- apoA-II
apolipoprotein A-II
- LPL
lipoprotein lipase
- FCR
fractional catabolic rate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None
References
- 1.Cheung MC, Albers JJ. The measurement of apolipoprotein A-I and A-II levels in men and women by immunoassay. J Clin Invest. 1977;60(1):43–50. doi: 10.1172/JCI108767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bu X, Warden C, Xia Y, Meester C De. Linkage analysis of the genetic dterminants of high density lipoprotein concentrations and composition: evidence for involvement of the apolipoprotein A-II and cholesteryl ester transfer protein loci. Hum Genet. 1994;2:639–648. doi: 10.1007/BF00201563. [DOI] [PubMed] [Google Scholar]
- 3.Alaupovic P, Knight-Gibson C, Wang CS, et al. Isolation and characterization of an apoA-II-containing lipoprotein (LP-A-II:B complex) from plasma very low density lipoproteins of patients with Tangier disease and type V hyperlipoproteinemia. J Lipid Res. 1991;32(1):9–19. [PubMed] [Google Scholar]
- 4.Boisfer E, Lambert G, Atger V, et al. Overexpression of human apolipoprotein A-II in mice induces hypertriglyceridemia due to defective very low density lipoprotein hydrolysis. J Biol Chem. 1999;274(17):11564–11572. doi: 10.1074/jbc.274.17.11564. [DOI] [PubMed] [Google Scholar]
- 5.Castellani LW, Nguyen CN, Charugundla S, et al. Apolipoprotein AII is a regulator of very low density lipoprotein metabolism and insulin resistance. J Biol Chem. 2008;283(17):11633–11644. doi: 10.1074/jbc.M708995200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Escolà-Gil JC, Julve J, Marzal-Casacuberta a, Ordóñez-Llanos J, González-Sastre F, Blanco-Vaca F. Expression of human apolipoprotein A-II in apolipoprotein E-deficient mice induces features of familial combined hyperlipidemia. J Lipid Res. 2000;41(8):1328–1338. [PubMed] [Google Scholar]
- 7.Wang CS, Alaupovic P, Gregg RE, Brewer HB. Studies on the mechanism of hypertriglyceridemia in Tangier disease. Determination of plasma lipolytic activities, k1 values and apolipoprotein composition of the major lipoprotein density classes. Biochim Biophys Acta. 1987;920(1):9–19. doi: 10.1016/0005-2760(87)90305-5. [DOI] [PubMed] [Google Scholar]
- 8.Mendivil CO, Zheng C, Furtado J, Lel J, Sacks FM. Metabolism of very-low-density lipoprotein and low-density lipoprotein containing apolipoprotein C-III and not other small apolipoproteins. Arterioscler Thromb Vasc Biol. 2010;30(2):239–245. doi: 10.1161/ATVBAHA.109.197830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng C, Khoo C, Furtado J, Ikewaki K, Sacks FM. Dietary monounsaturated fat activates metabolic pathways for triglyceride-rich lipoproteins that involve apolipoproteins E and C-III. Am J Clin Nutr. 2008;88(2):272–281. doi: 10.1093/ajcn/88.2.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nikkilä EA, Kekki M. Plasma triglyceride metabolism in thyroid disease. J Clin Invest. 1972;51(8):2103–2114. doi: 10.1172/JCI107017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barrett PHR, Chan DC, Watts GF. Thematic review series: patient-oriented research. Design and analysis of lipoprotein tracer kinetics studies in humans. J Lipid Res. 2006;47(8):1607–1619. doi: 10.1194/jlr.R600017-JLR200. [DOI] [PubMed] [Google Scholar]
- 12.Julve J, Escolà-Gil JC, Marzal-Casacuberta A, Ordóñez-Llanos J, González-Sastre F, Blanco-Vaca F. Increased production of very-low-density lipoproteins in transgenic mice overexpressing human apolipoprotein A-II and fed with a high-fat diet. Biochim Biophys Acta. 2000;1488(3):233–244. doi: 10.1016/s1388-1981(00)00127-x. [DOI] [PubMed] [Google Scholar]
- 13.Weisgraber K, Mahley R. Apoprotein (E--A-II) complex of human plasma lipoproteins. I. Characterization of this mixed disulfide and its identification in a high density lipoprotein. J Biol Chem. 1978;253(17):6281–6288. [PubMed] [Google Scholar]
- 14.Zheng C, Ikewaki K, Walsh BW, Sacks FM. Metabolism of apoB lipoproteins of intestinal and hepatic origin during constant feeding of small amounts of fat. J Lipid Res. 2006;47(8):1771–1779. doi: 10.1194/jlr.M500528-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.