Abstract
Objective
Reports regarding the associations between childhood maltreatment (CM) and body fat composition remain heterogeneous in humans although it is indicated in preclinical studies. Moreover, the effects of CM subtypes on different types of body fat are unclear. Thus this study is to determine the associations between CM and its subtypes with body fat and to explore the potential pathways.
Methods
Participants were assessed for a history of CM by the Childhood Trauma Questionnaire, and were divided into the CM group (with CM exposures) and non-CM group (without CM exposures). Body composition was measured by dual-energy X-ray absorptiometry. Subjects provided salivary and blood samples.
Results
Compared with the non-CM group, CM subjects had greater visceral fat mass (1136±160g vs. 836±116g, p<0.05) but not total body fat, android fat, body mass index or waist-to-hip ratio. Moreover, CM subjects had a blunted cortisol awakening response and elevated inflammatory factors. Correlation analysis indicated that CM subtypes had differential effects on visceral adiposity and cortisol awakening response.
Conclusions
Our results suggest that CM exposure is linked with increased visceral fat deposition, and the perturbation of the HPA axis activity and activation of the immune system may be 2 potential pathways that explain this relationship.
Keywords: Childhood maltreatment, Obesity, Visceral fat mass, HPA axis, Inflammatory factors
Introduction
Obesity is a prevalent global health problem. It affects 17% of children and adolescents, and 36% of adults in the United States (1,2). Current epidemiological studies have demonstrated that obesity-related health risk in humans is strongly correlated with an increased ratio of visceral fat mass, indicating that health is associated with maladaptive body fat distribution (3,4). Visceral obesity is defined as excess intra-abdominal adipose tissue accumulation and is estimated as the difference between abdominal fat mass and subcutaneous fat mass (3,4). Accumulating evidence has indicated that a high proportion of abdominal fat, visceral fat in particular, is independently associated with impaired glucose homeostasis and insulin resistance, indicative of the importance of visceral adiposity as a greater and more specific risk for metabolic diseases (5,6).
Preclinical studies suggested that stressful psychosocial experience during the early period of life is associated with metabolic changes that may have a programming effect on the development of overweight and obesity in adults although the mechanisms are not understood (7). There is association between obesity and childhood maltreatment (CM) in humans, however, the data are heterogeneous and it remains unknown whether CM is linked with visceral obesity (8). In addition, studies suggest that different CM subtypes may affect physical health to varying degrees (9). Due to the increasing prevalence and severity of obesity, and limited effective treatments for obesity (1,2,10), it is critical for advancing current knowledge of obesity prevention by identifying potentially modifiable risk factors such as CM and its subtypes to develop novel and specific prevention targets and intervention opportunities.
Studies suggest that stress experienced during the early developmental phases can induce persistent changes in the ability of the hypothalamic-pituitary-adrenal (HPA) axis to respond to stress in adulthood, thus increasing the susceptibility to various stress-related disorders (11–13). A normal HPA axis is characterized by pronounced circadian variations with distinct morning peaks in circulating cortisol levels (14). In contrast, a chronically stressed HPA axis is characterized by a decreased diurnal variability (11–13,15,16). Chronically stressed individuals can show either a blunted cortisol response to a stressor or prolonged elevation with little to no recovery to baseline (17). Cortisol increases appetite and shifts food preferences to so-called “comfort foods”, i.e. foods high in fat and added sugars that may reduce feelings of stress via dampening of the HPA axis activity (18,19). Repeated stressors and associated cortisol release may lead to excessive consumption of “comfort foods” and ultimately excessive weight gain. Therefore, following exposure to repeated stressors, elevated body fat and changes in body fat distribution may be observed.
Evidence from animal models and humans indicates that early life adverse events program monocytes and macrophages toward a pro-inflammatory state (20–22). Cross-sectional studies have shown the elevated levels of cytokines, including interleukin (IL)-6, tumor necrosis factor (TNF)-α and C-reactive protein, in individuals exposed to early life traumatic events (20,21). Preclinical and human studies have indicated that activated immune system may play a central role in the pathogenesis of abdominal obesity and specifically visceral obesity (23). Inflammation-related factors were found to increase in individuals with obesity and to reduce in individuals with weight loss (24,25). Since both early traumatic events and obesity are associated with an inflammatory state, it is possible that the increased systemic inflammation associated with early life adversity may actually be related with obesity, particularly with increased visceral fat deposition.
The objective of this study is to determine the associations between CM and its subtypes with different types of body fat mass, including total body fat and visceral fat mass. A secondary aim is to explore if the dysregulated HPA axis activity and activated inflammation are the potential pathways for these links.
Methods
Participants
Eighty one participants were recruited via the Office of Psychiatric Clinical Research (OPCR) at University of Alabama at Birmingham (UAB). The OPCR recruited study subjects from the community, and psychiatric outpatient and inpatient services. Six participants dropped out, thus 75 subjects completed the study (Table. 1). The participants were between 19 and 55 years of age. Participants were excluded if they: (1) had been taking corticosteroids, antibiotics or anti-inflammatory medication; (2) had current infectious diseases or a history of autoimmune, endocrine, inflammatory, or neurological disorders; (3) were pregnant or lactating; or (4) had a history of psychosis, bipolar disorder, and drug or alcohol abuse or dependence within 1 year prior to enrollment. The project was approved by UAB Institutional Review Board. All participants were provided written informed consent prior to any research procedures. The participants were divided into two groups: subjects with- (CM group, n=38) and without a history of CM (non-CM group, n=37). The CM group consisted of participants who reported having been exposed to at least one moderate to severe subtype of CM using the Childhood Trauma Questionnaire (CTQ) manual (26–29). Subjects in the CM groups were further divided into depressed (n=21) and non-depressed samples (n=17). All subjects were evaluated using the Structured Clinical Interview for DSM-IV (SCID), and were diagnosed with depression if they met the criteria for depression (30).
Table 1.
Demographic data and CTQ scores
| Variables | Non-CM (mean±SD) | CM (mean±SD) | P value |
|---|---|---|---|
| N, Sample size | 37 | 38 | |
| Age (years) | 36.1±12.3 | 39.7±10.9 | 0.521 |
| Number of female subjects | 28 | 26 | |
| Number of Caucasians | 21 | 19 | |
| CTQ Physical neglect scale | 5.1±0.9 | 8.8±3.3 | 0.000 |
| CTQ Emotional neglect scale | 6.9±2.5 | 14.2±7.0 | 0.000 |
| CTQ Emotional abuse scale | 6.3±1.8 | 12.6±4.9 | 0.000 |
| CTQ Physical abuse scale | 6.1±1.2 | 10.5±4.6 | 0.000 |
| CTQ Sexual abuse scale | 5.1±0.9 | 8.8±5.2 | 0.005 |
| CTQ Total scale | 29.3±3.9 | 54.7±16.0 | 0.000 |
Note: only African Americans and Caucasians were enrolled in this study.
CM: childhood maltreatment; SD: standard deviation; CTQ: childhood trauma questionnaire.
Assessment of Childhood Maltreatment
All participants completed a modified version of a well-validated measure of child abuse and neglect before age 18, the CTQ-Short Form (CTQ-SF) (26–29). The CTQ-SF has 5 subscales comprised of 5 questions each that assess CM in the areas of physical neglect (PN), emotional neglect (EN), emotional abuse (EA), physical abuse (PA) or sexual abuse (SA). Subjects rated statements about childhood experience on a five-point scale (1=“never true” to 5=“very often true”). Total CTQ score in each participant was calculated by adding the score for each subscale which ranged between 5 and 25.
The CTQ-SF has demonstrated high internal reliability and good convergent validity with both a clinician-rated interview of childhood abuse and therapists’ ratings of abuse (26,28). A cut-off score higher than 8 on the PN, SA and PA, and cut-off score higher than 10 and 15 for EA and EN, respectively, have provided good sensitivity and specificity for confirmed abuse or neglect (26–29). These cut-off scores were applied in this study, which were associated with moderate to severe levels of abuse and neglect (26–29).
Determination of Body Composition
Body weight and height were measured to calculate body mass index (BMI) using the Quetelet index (kg/m2). Waist and hip circumferences were measured for the calculation of waist-to-hip ratio.
Dual-energy X-ray absorptiometry (Lunar iDXA, GE-Healthcare Madison, WI) scan was acquired with a total body scanner for each participant to determine body composition. Participants were required to wear light clothes, remove all metal objects from their body, and lie supine with arms at their sides while undergoing the iDXA scan. iDXA allows the simultaneous measurement of bone mass, fat mass and lean body mass from the ratio of attenuation of two energy beams passing through the body. Android fat is a standard output from the iDXA software and includes the entire abdominal region, including visceral fat and subcutaneous fat. CoreScan software was used to estimate the visceral fat mass based on measurement of abdominal area and subcutaneous adipose tissue.
Saliva Samples Collection and Measurement of Cortisol Levels
All participants were given Salivette saliva collection devices (Sarstedt, NC), and received verbal and written instructions on how to collect saliva samples at home. They were instructed to collect saliva samples at awakening, 15-, 30-, and 60-mins post awakening. Participants kept the samples in their home freezer before they brought samples to the laboratory at UAB, where samples were stored at −20°C until analysis. On the day of the assay, salivettes were centrifuged at 3000g at 4°C for 10 mins. All samples were assayed in duplicate using a high sensitivity salivary cortisol enzyme-linked immunosorbent assay kit (Salimetrics, PA). The inter-assay variability was 8.6%. The intra-assay variation was 6.2% for low values, and 4.6% for samples with high values. The cortisol awakening response (CAR) for each participant was determined by measuring the cortisol values at awakening and 15-, 30-, or 60-mins post awakening. Incremental area under the curve (AUC) for cortisol levels throughout the cortisol awakening response was calculated using the trapezoidal rule. Cortisol AUC is typically used in this manner as an indicator of overall cortisol response from the awakening to final sample (31).
Blood Samples Collection and Measurement
Ten milliliters of blood were drawn from each participant, centrifuged at 3000g for 10 mins, immediately divided into aliquots, and frozen at −80°C until analysis. The analysis for inflammatory factors, including interferon-γ (IFNγ), IL-1β, IL-6, IL-8, IL-10, IL-12 and TNFα, was performed using a Meso Scale Discovery multiplex spot assay and analyzed with MSD Discovery Workbench software (Gaithersburg, MD). Concentrations for cytokines were expressed in pg/ml. Plasma concentrations of leptin and adiponectin (expressed in ng/ml and μg/ml, respectively) were assayed in duplicate using commercially available radioimmunoassay kits according to the procedures supplied by the manufacturer (Millipore Corp, MA).
Statistical analysis
All data are presented as mean ± standard error unless otherwise stated. p values <0.05 were considered significant. All statistical analyses were performed using the Statistical Package for the Social Sciences version 22 (SPSS Inc., IL). All variables were tested for normality of distribution by means of Kolmogorov-Smirnoff tests. Nonparametric tests were applied for data that were not from a normal distribution including IFNγ, IL-β, IL-6, IL-10 and IL-12. Otherwise, independent samples t-tests were carried out to detect the difference between groups. Correlation analyses were used to estimate the levels of association between two variables.
Results
Sample characteristics and CTQ scales
A summary of 75 participants’ characteristics is presented in Table 1. As seen in Table. 1, there were no significant differences in the variables including age, race and sex between participants in the CM-group and non-CM group. However, the CTQ’s subscales and total scores were significantly different between these two groups. Among the subscales of CTQ, emotional neglect was the most common experience with self-reported by 33 (44%) participants on the questionnaire.
Effects of CM on body composition
Mean BMI or waist-to-hip ratio did not differ between the CM group and non-CM group (Figures. 1A and B) although there was a trend of a higher waist-to-hip ratio in the CM group (p=0.10). Visceral fat mass was highly correlated with both total body fat mass and android fat mass in the population studied (Figures. 1C and D). Three different types of body fat mass were compared between the CM- and non-CM subjects. Total body fat mass and android fat mass were similar between the two groups, whereas visceral fat mass was significantly greater in the CM group than in the non-CM group (Figures. 1E–G). The effects of depression on body composition were also examined in the CM group. As shown in Figure 1H, the 3 different types of body fat mass were not significantly affected by the diagnosis of depression although there was a trend for android fat mass and visceral fat mass to be greater in CM subjects with depression (p=0.10 and 0.06, respectively).
Figure 1.
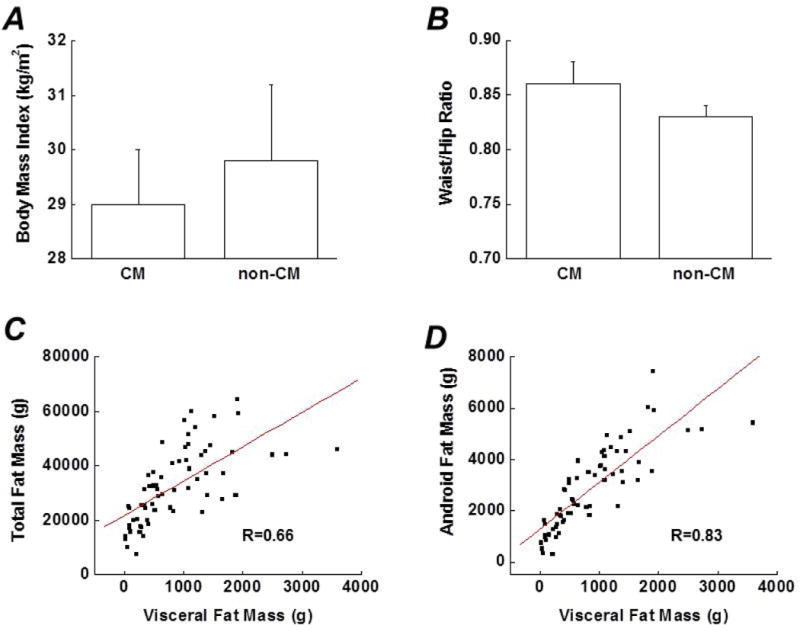
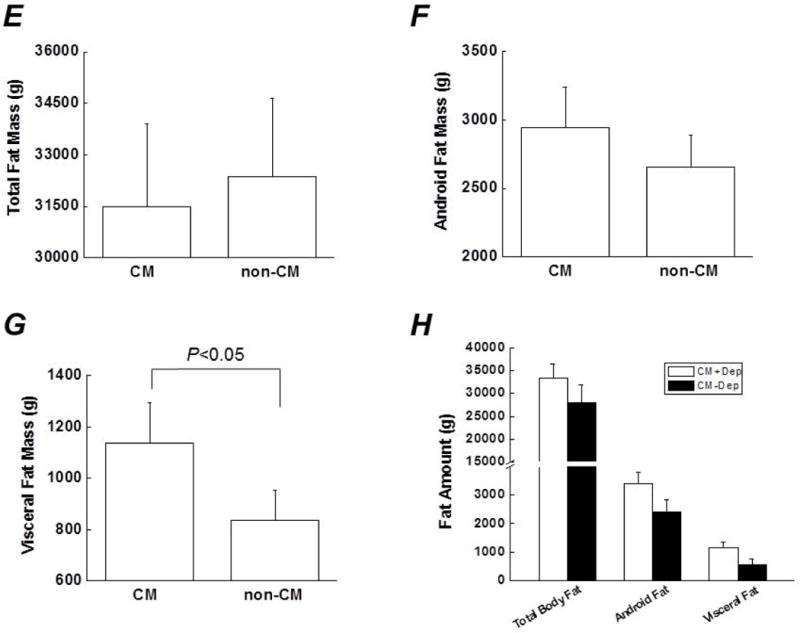
Comparison of several indicators for body composition between the CM group (subjects with a history of CM) and the non-CM group (subjects without a history of CM), including (A) Body mass index (B) Waist-to-hip ratio (E) Total body fat mass (F) Android fat mass and (G) Visceral fat mass. C and D. Associations between visceral fat mass and total body fat mass or android fat mass (correlation coefficients were 0.66 and 0.83, respectively. both p <0.01). H. Effects of depression on 3 types of body fat in subjects with a history of CM. Data are presented as mean ± standard error. CM, childhood maltreatment; Dep, depression.
Differential effects of CM subtypes on visceral fat mass
To determine if CM subtypes have differential effects on visceral adiposity, the associations between the 5 subtypes of CM and visceral fat mass were analyzed. When each subtype was studied individually, we found that the associations between different subtypes and visceral fat mass varied. The correlation analysis revealed that physical abuse was significantly associated with visceral fat mass (Table 2). Other 4 subtypes were also associated with visceral fat mass but not statistically significant. After adjusting for age, sex and race, results remained unchanged.
Table 2.
Correlations between CM subtypes and visceral fat mass
| Subtypes | N | r | P value |
|---|---|---|---|
| PN | 19 | 0.13 | 0.16 |
| EN | 15 | 0.15 | 0.12 |
| EA | 21 | 0.17 | 0.09 |
| PA | 24 | 0.22 | 0.04 |
| SA | 14 | 0.16 | 0.09 |
Note: Among 75 participants, thirty-eight were exposed to childhood maltreatment. N represents sample size exposed to different subtypes of childhood maltreatment.
CM: childhood maltreatment; r: correlation coefficient; PN: physical neglect; EN: emotional neglect; EA: emotional abuse; PA: physical abuse; SA: sexual abuse.
Alterations of the HPA axis functioning in CM group
Baseline cortisol levels at awakening in the CM group were lower than those in the non-CM group (Figure. 2A). Subjects in both the CM- and non-CM groups showed a cortisol response to awakening, however, subjects in the CM group had an attenuated CAR, indicating a blunted cortisol response after awakening (Figure. 2A). A typical CAR is defined by at least a 2.5 nmol/l increase in cortisol during the first 30 mins after awakening (32). About 80% of participants in the non-CM group showed a typical CAR. Instead, less than 30% of participants met this criterion in the CM group. The mean cortisol increase at 15- and 30- mins after awakening in the CM group was greatly lower than that in the non-CM group (Figure. 2A). The AUC, representing the overall levels of cortisol during the CAR, was significantly lower in the CM group than in the non-CM group (Figure. 2B). The cortisol levels and total CTQ scores in the CM group were negatively correlated (R=−0.49; p<0.01) (Figure. 2C). In addition, correlational analyses were performed between the cortisol AUC and CM subtypes. As shown in Table. 3, all CM subtypes were negatively associated with the cortisol AUC, wherein the highest association was observed in sexual abuse, followed by emotional abuse and physical abuse.
Figure 2.
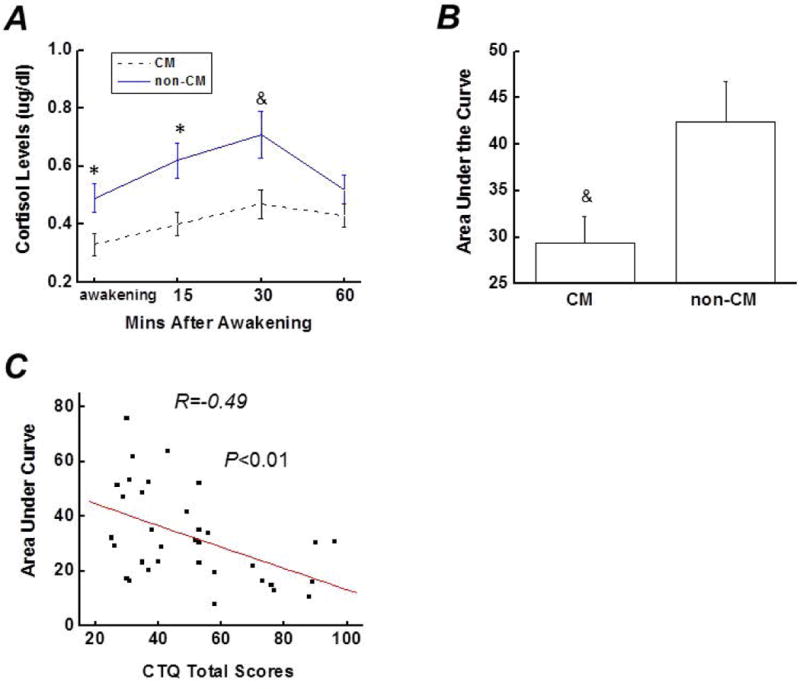
A). Cortisol awakening response at 0, 15-, 30- and 60-mins after awakening in the CM group (subjects with a history of CM) and the non-CM group (subjects without a history of CM). B). Comparison of the area under the curve between the CM group and the non-CM group. C). Scatter plot indicating the relationship between total CTQ score and cortisol area under the curve in the CM group (correlation coefficient was −0.49, p<0.01). Data are presented as mean ± standard error. Compared with the non-CM group at the same time point, &p<0.05; *p<0.01. CTQ, childhood trauma questionnaire; CM, childhood maltreatment.
Table 3.
Correlations between CM subtypes and cortisol AUC
| Subtypes | N | r | P value |
|---|---|---|---|
| PN | 19 | −0.33 | 0.10 |
| EN | 15 | −0.02 | 0.48 |
| EA | 21 | −0.49 | 0.03 |
| PA | 24 | −0.38 | 0.06 |
| SA | 14 | −0.51 | 0.04 |
Note: Among 75 participants, thirty-eight were exposed to childhood maltreatment. N represents sample size exposed to different subtypes of childhood maltreatment.
CM: childhood maltreatment; r: correlation coefficient; PN: physical neglect; EN: Emotional neglect; EA: emotional abuse; PA: physical abuse; SA: sexual abuse.
Activation of immune system in CM group
To determine if the immune system is activated in response to the CM and its roles in the development of visceral obesity, the levels for cytokines were measured in subjects. The levels for IFNγ and IL-β in 2 subjects in CM group were 3 standard deviations away from the mean, so their values were not included in data analysis. Pairwise comparison between subjects in two groups revealed that: (1) In contrast to the non-CM group, the levels of inflammatory factors, including IL-1β, IL-12 and TNFα, were elevated in the CM group (Figure. 3A); (2) No differences regarding IFNγ, IL-6, IL-8 and IL-10 levels were observed between the two groups (data not shown); (3) The two groups exhibited similar levels of leptin and adiponectin (Figure. 3B).
Figure 3.
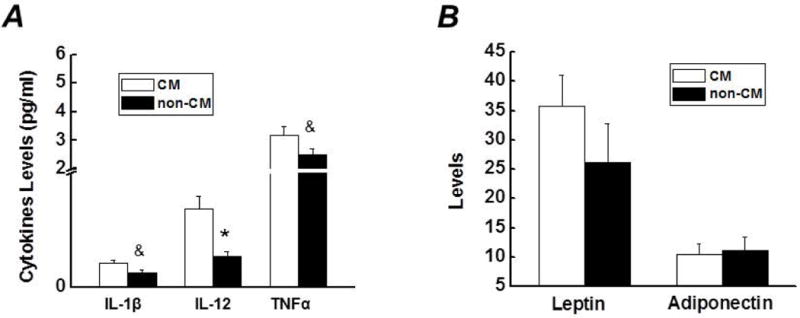
A). Comparison of levels for IL-1β, IL-12, and TNFα between subjects with- and without- a history of childhood maltreatment. B). Comparison of levels for adiponectin (μg/ml) and leptin (ng/ml) between subjects with- and without- a history of CM. Data are presented as mean ± standard error. Compared with the non-CM group, &p<0.05; *p<0.01. IL, interleukin; TNF, tumor necrosis factor; CM, childhood maltreatment.
Discussion
In this study, we investigated the roles of CM and its subtypes on the risk for obesity, particularly visceral obesity, in individuals with early life adverse experience. The major findings are that early life adverse experience is associated with greater visceral fat mass but not total body fat or android fat mass, and statistical analysis reveals that CM subtypes correlate with visceral adiposity differentially. Our data suggest that visceral adiposity is a more specific marker for CM-related obesity. Our data also suggest that the association between CM and visceral fat accumulation is perhaps through, in part, the suppression of the HPA axis functioning and activation of immune system. To our knowledge, this is the first study to document the link between early life adverse experience / its subtypes and visceral adiposity in humans.
Obesity has been commonly assessed using BMI and waist-to-hip ratio. Although BMI is a tool to report the prevalence of obesity, it relies on the assumption that adipose tissue is evenly distributed over the body. Similarly, waist-to-hip ratio is another widely used index for body fat distribution, but the variations in waist do not distinguish subcutaneous adiposity from visceral adiposity. Recent studies have indicated that the proportion of abdominal adipose tissue is a key correlate and perhaps a main factor of obesity-related health problems (3,4). Visceral obesity has now been established as being part of complex phenotype, including adipose tissue storage dysfunction (33). In the current study, the impacts of CM on several indicators for body fat distribution, including BMI, waist-to-hip ratio, total body fat, android fat and visceral fat were investigated. Among these different indicators, only visceral fat mass was found to be associated with CM. Although obesity is a heterogeneous condition, our finding highlights the importance of visceral fat mass as a relatively accurate indicator for body fat composition and a good marker for obesity-related disorders.
Our mechanistic studies for the association between CM and visceral adiposity revealed the attenuation of morning cortisol response in CM subjects compared with non-CM subjects, consistent with previous reports (34). Heim research group reported that childhood trauma influenced neuroendocrine and autonomic stress reactivity in adulthood although the underlying mechanisms contributing to the reduced HPA axis activity in humans following CM remained unclear (34). Previous studies in rodent models showed that maternal separation induced a reduction in the expression of central glucocorticoid receptors at the epigenetic level or decreased cortical and hippocampal glucocorticoid receptor messenger RNA density (35,36). Childhood trauma, similar to maternal separation in rodents, may also be associated with impaired glucocorticoid-mediated negative feedback inhibition of the pituitary under repeated early life adverse events. Subsequently, stress reactivity becomes suppressed or blunted due to repeated exposures. Thus attenuated morning cortisol response might be a potential pathophysiological mechanism in the development of obesity, visceral adiposity in particular, in individuals with repeated CM exposures. However, at this point, a definite statement can not be made in terms of the causal relationship between attenuated morning cortisol response and visceral adiposity. Future studies should therefore incorporate longitudinal designs to disentangle this complex association and to reveal temporal relationships between CM, suppressed morning cortisol response and visceral obesity.
There could be multiple functional consequences of attenuated morning cortisol response in CM subjects. A previous study suggested that the cortisol awakening response might reflect to some extent the adrenal cortex capacity. Reduced adrenal capacity and subsequent reduced availability of cortisol might allow the increased activation of the immune system, which is known to be regulated by cortisol. Indeed, elevated levels of IL-1β, IL-12 and TNFα were observed in CM subjects in this study. Furthermore, increased inflammatory markers have been previously reported in individuals with exposures of early child abuse (37,38). Given that, it is possible that increased IL-1β, IL-12 and TNFα levels may be the result of early life adversity in CM subjects. Chronic low-grade inflammation is indicated to be present in obesity, and individuals with obesity display altered expression and/or secretion patterns of cytokines including TNFα (23). Taken together, increased immune system activation, in part explained by reduced availability of cortisol, might be one potential pathway leading to increased visceral fat mass in CM subjects.
Our study has several strengths. Among several indicators of body fat distribution, only visceral fat mass was linked to CM. Consistent with previous reports, visceral adiposity is a more specific indicator for obesity in adults, especially in individuals exposed to CM. The second strength of this study is that we observed the differential impacts of CM subtypes on visceral fat mass. The effect of physical abuse on visceral fat mass was the greatest in comparison to the other 4 subtypes. Our findings suggest that more information on subtype differences within group of maltreated individuals is needed because CM subtypes may result in different biological consequences. Furthermore, a better understanding of CM subtypes will provide guidance in developing a more specific interventional strategy. The third strength of this study is that although depression is one of the psychiatric consequences of CM, our data analysis showed that the association between CM and visceral adiposity was independent of the presence of depression. In the other words, CM might be considered as an independent risk factor for visceral adiposity.
Several limitations of our study should be noted. First, the sample size is relatively small, however, all 75 participants were thoroughly investigated and the data set was complete. Statistical analyses were kept simple so that we could attain useful and meaningful results despite the small number of subjects. A second limitation is the reliance on retrospective self-reports of CM using the CTQ, which could be affected by forgetting, non-disclosure and reporting biases. However, a recent meta-analysis study using external corroboration of self-reports suggested that use of validated psychometric instruments and focus on moderate to severe early life trauma could increase the validity of self-reports for CM (39). Consistently, a more strict scale for EN and EA was used in this study. Third, biological consequences in adults induced by CM experience are likely to involve other health-related behaviors such as smoking, physical inactivity and diet, however, these additional variables were not evaluated in the present study. Future studies will include these and other variables for covariate analysis. Lastly, saliva sampling conducted by the subjects at their homes may be prone to measurement error due to a lack of compliance in collecting the sample at the specified time. Less than 10% of study subjects were found to have compliance issues in the present study and were instructed to repeat sample collection. A controlled laboratory environment might be useful to conduct future studies to minimize the confounding factors that influence cortisol levels. In summary, our study addresses one of developmental origins of heterogeneity in risk for visceral adiposity by demonstrating that CM is associated with greater visceral fat mass, and CM could be an independent predictor for visceral adiposity in this particular population. Stressful psychosocial experience in childhood might thus be conceptualized as potentially modifiable risk factor for visceral adiposity. Furthermore, mechanistic studies reveal a blunted HPA axis activity and the activation of immune system in the CM group although prospective studies are required to determine the causality and precise mechanisms. Consideration of the impacts of early adverse experience on visceral adiposity in the future will result in a more precise understanding of the onset of visceral adiposity, and lead to the identification of specific interventional strategies to prevent and treat visceral obesity and its-related disorders.
Supplementary Material
Answer the Study Importance Questions.
-
What is already known about this subject?
Preclinical studies suggest that stressful psychosocial experience during the early period of life is associated with metabolic changes that may have a programming effect on the development of overweight and obesity in adults, however, evidence in humans remains heterogeneous although some studies show that chronic psychosocial stress is associated with obesity in adulthood.
-
What does your study add?
Our findings demonstrate that childhood maltreatment is associated with increased visceral adipose tissue but not other indicators for body fat composition, and childhood maltreatment subtypes have differential effects on visceral adiposity. The suppression of the HPA axis activity and activation of inflammatory factors in subjects with a history of childhood maltreatment may be two potential pathways for the association between childhood maltreatment and visceral adiposity.
Acknowledgments
The authors gratefully acknowledge the help of Maryellen Williams of the UAB Metabolism Core Laboratory with laboratory analyses.
Funding: This work was supported by an award, P30DK056336, from the National Institute of Diabetes And Digestive And Kidney Diseases.
Footnotes
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Disclosure: The authors had no conflicts of interest to disclose.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA. 2012;307(5):483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity among adults: United States, 2011–2012. NCHS Data Brief. 2013;(131):1–8. [PubMed] [Google Scholar]
- 3.Kissebah AH, Krakower GR. Regional adiposity and morbidity. Physiol Rev. 1994;74(4):761–811. doi: 10.1152/physrev.1994.74.4.761. [DOI] [PubMed] [Google Scholar]
- 4.Thomas EL, Bell JD. Influence of undersampling on magnetic resonance imaging measurements of intra-abdominal adipose tissue. Int J Obes Relat Metab Disord. 2003;27(2):211–218. doi: 10.1038/sj.ijo.802229. [DOI] [PubMed] [Google Scholar]
- 5.O’Shaughnessy IM, Myers TJ, Stepniakowski K, Nazzaro P, Kelly TM, Hoffmann RG, Egan BM, Kissebah AH. Glucose metabolism in abdominally obese hypertensive and normotensive subjects. Hypertension. 1995;26(1):186–192. doi: 10.1161/01.hyp.26.1.186. [DOI] [PubMed] [Google Scholar]
- 6.Ross R, Aru J, Freeman J, Hudson R, Janssen I. Abdominal adiposity and insulin resistance in obese men. Am J Physiol Endocrinol Metab. 2002;282(3):E657–663. doi: 10.1152/ajpendo.00469.2001. [DOI] [PubMed] [Google Scholar]
- 7.Miki T, Liu JQ, Ohta K, Suzuki S, Kusaka T, Warita K, Yokoyama T, Jamal M, Ueki M, Yakura T, Tamai M, Sumitani K, Hosomi N, Takeuchi Y. Early postnatal maternal separation causes alterations in the expression of beta3-adrenergic receptor in rat adipose tissue suggesting long-term influence on obesity. Biochem Biophys Res Commun. 2013;442(1–2):68–71. doi: 10.1016/j.bbrc.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Danese A, Tan M. Childhood maltreatment and obesity: systematic review and meta-analysis. Mol Psychiatry. 2014;19(5):544–554. doi: 10.1038/mp.2013.54. [DOI] [PubMed] [Google Scholar]
- 9.Petrenko CL, Friend A, Garrido EF, Taussig HN, Culhane SE. Does subtype matter? Assessing the effects of maltreatment on functioning in preadolescent youth in out-of-home care. Child Abuse Negl. 2012;36(9):633–644. doi: 10.1016/j.chiabu.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leblanc ES, O’Connor E, Whitlock EP, Patnode CD, Kapka T. Effectiveness of primary care-relevant treatments for obesity in adults: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155(7):434–447. doi: 10.7326/0003-4819-155-7-201110040-00006. [DOI] [PubMed] [Google Scholar]
- 11.Carpenter LL, Tyrka AR, Ross NS, Khoury L, Anderson GM, Price LH. Effect of childhood emotional abuse and age on cortisol responsivity in adulthood. Biol Psychiatry. 2009;66(1):69–75. doi: 10.1016/j.biopsych.2009.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klaassens ER, Giltay EJ, van Veen T, Veen G, Zitman FG. Trauma exposure in relation to basal salivary cortisol and the hormone response to the dexamethasone/CRH test in male railway employees without lifetime psychopathology. Psychoneuroendocrinology. 2010;35(6):878–886. doi: 10.1016/j.psyneuen.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 13.Tyrka AR, Wier LM, Price LH, Rikhye K, Ross NS, Anderson GM, Wilkinson CW, Carpenter LL. Cortisol and ACTH responses to the Dex/CRH test: influence of temperament. Horm Behav. 2008;53(4):518–525. doi: 10.1016/j.yhbeh.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bao AM, Meynen G, Swaab DF. The stress system in depression and neurodegeneration: focus on the human hypothalamus. Brain Res Rev. 2008;57(2):531–553. doi: 10.1016/j.brainresrev.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Carpenter LL, Carvalho JP, Tyrka AR, Wier LM, Mello AF, Mello MF, Anderson GM, Wilkinson CW, Price LH. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biol Psychiatry. 2007;62(10):1080–1087. doi: 10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33(6):693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Gunnar MR, Frenn K, Wewerka SS, Van Ryzin MJ. Moderate versus severe early life stress: associations with stress reactivity and regulation in 10–12-year-old children. Psychoneuroendocrinology. 2009;34(1):62–75. doi: 10.1016/j.psyneuen.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dallman MF, Akana SF, Laugero KD, Gomez F, Manalo S, Bell ME, Bhatnagar S. A spoonful of sugar: feedback signals of energy stores and corticosterone regulate responses to chronic stress. Physiol Behav. 2003;79(1):3–12. doi: 10.1016/s0031-9384(03)00100-8. [DOI] [PubMed] [Google Scholar]
- 19.Vicennati V, Pasqui F, Cavazza C, Garelli S, Casadio E, di Dalmazi G, Pagotto U, Pasquali R. Cortisol, energy intake, and food frequency in overweight/obese women. Nutrition. 2011;27(6):677–680. doi: 10.1016/j.nut.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 20.Carpenter LL, Gawuga CE, Tyrka AR, Lee JK, Anderson GM, Price LH. Association between plasma IL-6 response to acute stress and early-life adversity in healthy adults. Neuropsychopharmacology. 2010;35(13):2617–2623. doi: 10.1038/npp.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coelho R, Viola TW, Walss-Bass C, Brietzke E, Grassi-Oliveira R. Childhood maltreatment and inflammatory markers: a systematic review. Acta Psychiatr Scand. 2014;129(3):180–192. doi: 10.1111/acps.12217. [DOI] [PubMed] [Google Scholar]
- 22.Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5(3):243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- 23.Tchernof A, Despres JP. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013;93(1):359–404. doi: 10.1152/physrev.00033.2011. [DOI] [PubMed] [Google Scholar]
- 24.Ambeba EJ, Styn MA, Kuller LH, Brooks MM, Evans RW, Burke LE. Longitudinal effects of weight loss and regain on cytokine concentration of obese adults. Metabolism. 2013;62(9):1218–1222. doi: 10.1016/j.metabol.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lasselin J, Magne E, Beau C, Ledaguenel P, Dexpert S, Aubert A, Laye S, Capuron L. Adipose inflammation in obesity: relationship with circulating levels of inflammatory markers and association with surgery-induced weight loss. J Clin Endocrinol Metab. 2014;99(1):E53–61. doi: 10.1210/jc.2013-2673. [DOI] [PubMed] [Google Scholar]
- 26.Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, Sapareto E, Ruggiero J. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry. 1994;151(8):1132–1136. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- 27.DiLillo D, Fortier MA, Hayes SA, Trask E, Perry AR, Messman-Moore T, Fauchier A, Nash C. Retrospective assessment of childhood sexual and physical abuse: a comparison of scaled and behaviorally specific approaches. Assessment. 2006;13(3):297–312. doi: 10.1177/1073191106288391. [DOI] [PubMed] [Google Scholar]
- 28.Fink LA, Bernstein D, Handelsman L, Foote J, Lovejoy M. Initial reliability and validity of the childhood trauma interview: a new multidimensional measure of childhood interpersonal trauma. Am J Psychiatry. 1995;152(9):1329–1335. doi: 10.1176/ajp.152.9.1329. [DOI] [PubMed] [Google Scholar]
- 29.Walker EA, Unutzer J, Rutter C, Gelfand A, Saunders K, VonKorff M, Koss MP, Katon W. Costs of health care use by women HMO members with a history of childhood abuse and neglect. Arch Gen Psychiatry. 1999;56(7):609–613. doi: 10.1001/archpsyc.56.7.609. [DOI] [PubMed] [Google Scholar]
- 30.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of clinical psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- 31.Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28(7):916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- 32.Clow A, Thorn L, Evans P, Hucklebridge F. The awakening cortisol response: methodological issues and significance. Stress. 2004;7(1):29–37. doi: 10.1080/10253890410001667205. [DOI] [PubMed] [Google Scholar]
- 33.Despres JP. Excess visceral adipose tissue/ectopic fat the missing link in the obesity paradox? J Am Coll Cardiol. 2011;57(19):1887–1889. doi: 10.1016/j.jacc.2010.10.063. [DOI] [PubMed] [Google Scholar]
- 34.Meinlschmidt G, Heim C. Decreased cortisol awakening response after early loss experience. Psychoneuroendocrinology. 2005;30(6):568–576. doi: 10.1016/j.psyneuen.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 35.Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Plotsky PM. Long-term adaptations in glucocorticoid receptor and mineralocorticoid receptor mRNA and negative feedback on the hypothalamo-pituitary-adrenal axis following neonatal maternal separation. Biol Psychiatry. 2004;55(4):367–375. doi: 10.1016/j.biopsych.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 36.Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7(8):847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 37.Danese A, Moffitt TE, Pariante CM, Ambler A, Poulton R, Caspi A. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Arch Gen Psychiatry. 2008;65(4):409–415. doi: 10.1001/archpsyc.65.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lopes RP, Grassi-Oliveira R, de Almeida LR, Stein LM, Luz C, Teixeira AL, Bauer ME. Neuroimmunoendocrine interactions in patients with recurrent major depression, increased early life stress and long-standing posttraumatic stress disorder symptoms. Neuroimmunomodulation. 2012;19(1):33–42. doi: 10.1159/000327352. [DOI] [PubMed] [Google Scholar]
- 39.Afari N, Ahumada SM, Wright LJ, Mostoufi S, Golnari G, Reis V, Cuneo JG. Psychological trauma and functional somatic syndromes: a systematic review and meta-analysis. Psychosom Med. 2014;76(1):2–11. doi: 10.1097/PSY.0000000000000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


