Abstract
Background
Isoflurane may be protective in pre-clinical models of lung injury but its use in patients with lung injury remains controversial and the mechanism of its protective effects remains unclear. We hypothesized that this protection is mediated at the level of alveolar tight junctions and investigated the possibility in a two-hit model of lung injury that mirrors human acute respiratory distress syndrome.
Methods
Wild-type mice were treated with isoflurane one hour after exposure to nebulized endotoxin (n=8) or saline control (n=9) then allowed to recover for 24 hrs prior to mechanical ventilation (MV, tidal volume 15 mL/kg, 2 hrs) producing ventilator-induced lung injury. Mouse lung epithelial cells were similarly treated with isoflurane one hour after exposure to lipopolysaccharide. Cells were cyclically stretched the following day to mirror the MV protocol used in vivo.
Results
Mice treated with isoflurane following exposure to inhaled endotoxin and prior to MV exhibited significantly less physiologic lung dysfunction. These effects appeared to be mediated by decreased vascular leak, but not altered inflammatory indices. Mouse lung epithelial cells treated with lipopolysaccharide and cyclic stretch and lungs harvested from mice following treatment with lipopolysaccharide and MV had decreased levels of a key tight junction protein (i.e. zona occludens 1) that was rescued by isoflurane treatment.
Conclusions
Isoflurane rescued lung injury induced by a two-hit model of endotoxin exposure followed by MV by maintaining the integrity of the alveolar-capillary barrier possibly by modulating the expression of a key tight junction protein.
Introduction
The acute respiratory distress syndrome (ARDS) affects nearly 200,000 patients per year in the U.S. with mortality rates as high as 45%.1,2 ARDS is characterized by the presence of proteinaceous fluid, inflammatory cells, and hyaline membranes in the alveolar space that cause decreased lung compliance, hypoxemia, and respiratory distress.3 Maintenance of an intact alveolar epithelial barrier requires specialized structures called tight junctions, and data from animal studies suggest that lung injury is mediated, in part, by dysregulation of several key tight junction proteins.4
Supportive care is the mainstay of ARDS treatment and includes mechanical ventilation (MV) when patients develop respiratory failure. While often life-saving, MV can also exacerbate pre-existing lung injury (e.g. from pneumonia or extra-pulmonary sepsis), known as ventilator induced lung injury (VILI).5 Patients undergoing MV, including patients in the intensive care unit (ICU) or operating room, require medications for general anesthesia, sedation, or anxiolysis. Although often necessary to support critically ill patients receiving MV, these medications can also have adverse effects including prolonged MV and increased ICU length of stay.6 Volatile anesthetics are a class of sedatives with favorable pharmacokinetic properties including a rapid onset and rapid recovery upon discontinuation. Volatile anesthetics are routinely used for patients undergoing surgery, but there is debate as to whether they should be used in patients with lung injury that require anesthesia.
Isoflurane is one of the most commonly used volatile anesthetics7 and possesses cytoprotective properties,8 anti-inflammatory properties,9–11 and cardioprotective effects.12 While isoflurane has been shown to confer protection in animal models of lung injury including inhaled endotoxin13 and VILI,14,15 its use in patients with lung injury remains controversial, as some data suggest it may have deleterious effects.16,17 Furthermore, the mechanism of protection with isoflurane following endotoxin induced lung injury and VILI remains unclear. It has been reported that a brief period of preconditioning with isoflurane can confer protection from other types of injury including myocardial ischemia/reperfusion,12 sepsis induced lung injury,13 and ischemic brain injury18 many hours after exposure to the volatile anesthetic and that the mechanism of protection may be due to changes in gene expression.19 Given that isoflurane has been shown to prevent vascular leak in several mouse models of lung injury,20,21 we hypothesized that its protective effects may be due to changes in the expression of key alveolar tight junction proteins, as no studies to date have addressed the role of inhaled anesthetics in epithelial tight junction integrity. To test this hypothesis we used a two-hit model of lung injury that involves MV following lipopolysaccharide exposure. Although the majority of acute lung injury animal studies involve a single injury to the lungs, critically ill patients in the ICU frequently sustain multiple injuries to the lungs (e.g. pneumonia, sepsis, cardiogenic pulmonary edema, transfusion-associated lung injury, etc.) and then require MV and inhalational anesthetics after the initial injury has ensued.22 We set out to use a model of lung injury that mirrored the course of ICU patients with ARDS and to determine whether isoflurane conferred protection in this model.
Materials and Methods
Two hit murine model of lung injury and in vivo isoflurane exposure
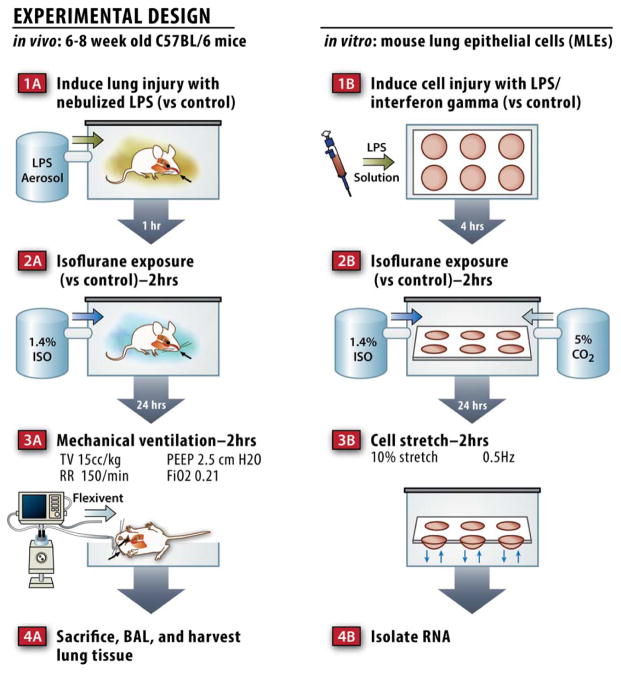
All animal experiments were approved by the Brigham and Women’s Institutional Animal Care and Use Committee (Boston, MA, USA) and were handled according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Wild-type, male, 6–8 week old C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME) were treated with 10mg of nebulized Pseudomonas aeruginosa endotoxin from Sigma-Aldrich (L9143, St Louis, MO) dissolved in phosphate buffered saline (PBS), or PBS alone using an Aeroneb nebulizer (Aerogen, Galway, Ireland) as described previously (Fig 1A).23 One hour later, mice were exposed to 1.4% isoflurane in 100% oxygen for 2 hours as described previously.24 Briefly, mice were randomly assigned to receive 1.4% isoflurane in 100% oxygen or 100% oxygen alone with identical flow rates in identical anesthetizing chambers. This concentration of isoflurane was selected because it represents 1 minimum alveolar concentration in the rodent.24 Animals treated with isoflurane were anesthetized and able to breath spontaneously. The isoflurane concentration was measured continuously with an agent analyzer (Datex, Tewksbury, MA; Ohmeda, Madison, WI). The physiologic status of the mice was monitored in a pilot study using mice with femoral arterial catheters that were treated with similar concentrations of isoflurane (see table, Supplementary Digital Content 1). During isoflurane anesthesia, temperature was maintained at 37°C using heating pads. Anesthesia was terminated by discontinuing isoflurane administration. After anesthesia, the anesthetized and control animals recovered in 100% oxygen for 20 min. Twenty-four hours following isoflurane treatment, mice were anesthetized with 70 mg/kg of pentobarbital (Akorn, Lake Forest, IL) and a tracheostomy was performed prior to pressure limited (30 cm H2O), volume cycled mechanical ventilation with ambient air using a Flexivent™ rodent ventilator (Scireq, Montreal, Canada) with a tidal volume of 15 mL/kg. Other ventilator settings included a respiratory rate of 150 breaths/min and a positive end expiratory pressure of 2.5 cm H2O. Similar minute ventilation in prior mouse studies has been shown to maintain normal PaCO2 and arterial pH levels25 as well as normal oxygenation.26 Measurements of lung physiology including elastance (H) and tissue resistance (G) were measured at baseline and twice hourly thereafter. Standardized recruitment maneuvers were performed prior to each physiologic measurement to prevent atelectasis and standardize volume history.27 When MV was completed, a bronchoalveolar lavage (BAL) was performed by instilling 1 mL of sterile PBS twice. Cells were pelleted from the BAL fluid and assessed for total and differential cell counts using a hemacytometer and a cytospin followed by Diff-Quick staining (Fisher Scientific, Pittsburgh, PA).
Figure 1.
Schematic of the in vivo and in vitro models used.
Histologic assessment of lung injury
Lungs were flushed free of blood by perfusing the right ventricle with 10 mL of sterile PBS. Lungs were inflated to 30 cm H2O and fixed in 10% formalin overnight at 4°C prior to paraffin embedding. Five-micron sections were obtained, and staining with hematoxylin and eosin was performed. Gr-1 (neutrophil) staining was performed and quantitated as described previously.28 Briefly, 3 random 400X images were acquired using an Olympus microscope (Olympus, Center Valley, PA). Images were converted to 8-bit (grayscale) and analyzed using NIH Image J software. Minimum intensity and size thresholds were set and applied to all images. The mean of the 3 random fields was calculated and reported as Gr-1 positive cells per high power field.
Measurement of BAL protein
Protein was measured in BAL supernatants using a standard Bradford assay (Bio-Rad, Hercules, CA) with a standard curve using albumin.
Evans blue assay and wet to dry ratios
Mice were injected intravenously via tail vein with 30 mg/kg of 1% Evans blue dye in PBS (Sigma-Aldrich) 30 minutes prior to MV. Following MV, blood was obtained by right ventricular puncture and plasma was isolated. Lungs were perfused free of blood by injecting the right ventricle with 10 mL of sterile PBS. The left lung was snap frozen in liquid nitrogen and the right lung was weighed (wet weight). The right lung was dried in a 60°C oven and weighed daily for 72 hours or until the weight was unchanged on subsequent days (dry weight). For the Evans blue assay, the left lung was homogenized in 500 μL PBS and 2 volumes of formamide (Sigma-Aldrich) were added. Lung homogenates were incubated overnight at 60°C prior to centrifugation at 13,000 RPM for 30 min. The optical density of the lung homogenate and diluted plasma were obtained at 620 nm and 740 nm and the permeability index was calculated as described previously.29
ELISA
Enzyme linked immunosorbent assay for interleukin-6 was performed using 50 μL of BAL fluid according to the manufacturer’s specifications (Thermo Fisher, Rockford, IL).
Cell culture, in vitro cyclic stretch assay, and isoflurane exposure
Immortalized mouse lung epithelial cells (MLE-15) were the generous gift of Jeffrey Whitsett, M.D. (Professor of Pediatrics, University of Cincinnati College of Medicine, Cincinnati, OH) and were cultured as described previously.30 For cyclic stretch experiments, cells were plated on Pronectin coated Flexcell plates (Flexcell, Hillsborough, NC) and grown to confluence. Cells were then treated with a combination of 0.5 μg/ml Escherichia coli lipopolysaccharide (L2762, Sigma-Aldrich) and 100 units/ml of interferon gamma (315-05, Peprotech, Rocky Hill, NJ) or PBS control for 4 hours (Fig 1B). Cells were subsequently exposed to isoflurane (or control gas containing 23% oxygen, 5% CO2, balance nitrogen) in an airtight chamber for 2 hours as described previously.31 Isoflurane, oxygen, and carbon dioxide concentrations were measured every 30 minutes as described previously.31 After isoflurane exposure, cell were incubated overnight prior to biaxial cyclic stretch (10% stretch, 0.5 Hz) delivered via a Flexcell Strain Unit FX-3000 (Flexcell) for 2 hours prior to isolating RNA or protein. Control cells were placed in the stretching device, but not subjected to stretch.
RNA isolation, reverse transcription, and quantitative PCR
Total RNA was isolated from cells and lung tissue using an RNeasy mini kit (Qiagen, Valencia, CA) according to the manufacturer’s protocol. RNA was treated with recombinant DNAse (Invitrogen, Grand Island, NY) according to the manufacturer’s protocol to remove any genomic DNA contamination. cDNA was synthesized from 1 μg of RNA using the Superscript III reverse transcription kit (Invitrogen). Quantitative real time PCR was performed using a 7300 Real-Time PCR System (Applied Biosystems, Foster City, CA) to determine gene expression for zona occludens 1 (Mm 00493699_m1), occludin (Mm005000912_m1), claudin 4 (Mm00515514_s1), and surfactant protein B (Mm00455681_m1) using validated TaqMan® gene expression assay primer/probe combinations (Applied Biosystems). All qPCR results were normalized to the expression of the endogenous control 18S (Hs99999901_s1). Fold changes in transcripts were determined using the delta delta cycle threshold (i.e. ΔΔCt) method.
Protein isolation, SDS-PAGE, and immunoblotting
Cells were placed on ice and media was aspirated prior to washing twice with ice cold PBS. Cell lysis buffer was prepared by adding a complete mini protease inhibitor tablet (Roche Diagnostics, Indianapolis, IN) and phosphatase inhibitors (Sigma-Aldrich) to protein isolation buffer (40mM HEPES, 120mM NaCl, 1mM EDTA, 3% CHAPS w/v). Ice-cold buffer was added to each well and cells were mechanically disrupted using a rubber scraper. Protein extracts were subjected to a freeze/thaw cycle prior to centrifugation (6,000 RPM × 20 min). Supernatants were collected and protein concentrations were determined using a BCA assay (Thermo Fisher). Protein concentrations were equalized and samples were boiled after adding NuPAGE loading buffer (Life Technologies, Carlsbad, CA) and β-mercaptoethanol. SDS-PAGE was performed using 4–12% Bis/Tris gels (Life Technologies) and proteins were transferred to nitrocellulose membranes. Membranes were blocked with 5% non-fat milk in TBST prior to incubating with anti-zona occludens 1 or anti-occludin (Invitrogen) antibodies at 1:1000 for 2 hours at room temperature. Membranes were washed 3 times in TBST prior to incubating with an HRP-conjugated secondary antibody. Membranes were again washed 3 times prior to being developed with ECL reagent and imaged with a ChemiDoc imaging system (Biorad). Quantitative assessment was performed using Image Lab software (Biorad).
Zona occludens-1 immunostaining and quantitation
Formalin-fixed and paraffin embedded lungs sections were deparaffinized and rehydrated prior to boiling in 10 mM sodium citrate/0.5% Tween 20 (pH 6.0) for 25 minutes for antigen retrieval. Tissue was permeabilized with 0.3% Triton-X (American Bioanalytical, Natick, MA) and blocked with 10% goat serum in PBS (Cellgro, Manassas, VA). Zona occludens (ZO)-1 was identified by immunofluorescence staining using a polyclonal rabbit anti ZO-1 antibody (Zymed, Grand Island, NY) at a concentration of 7.5 μg/ml in 5% goat serum/PBS (or goat serum alone as a control). Alexa fluor 546 (red) goat anti-rabbit-IgG (2 mg/ml) (Invitrogen) at 1:600 dilution was used as the secondary antibody with DAPI counter-staining. Representative images were acquired using a confocal microscope, and 8-bit pictures were used for quantitative analysis. To avoid interference from other channels, only the red channel (ZO-1) was used. Quantitative assessment was performed using Image J software. Briefly, the lung interstitial background was calculated by randomly choosing ten 5.19 μm2 regions. The average of maximum intensity was calculated, and this value was used as the measurement threshold. Next, ten fields were selected at random, and the average of the maximum and mean intensities were calculated correcting for the background threshold. Three images per animal were assessed.
Statistical analysis
All data are shown as mean ± SEM unless otherwise noted. Data were plotted and analyzed using Prism (GraphPad, La Jolla, CA) software. We did not perform an a priori statistical analysis. Our sample sizes were chosen based on our prior lung injury studies,30 and sample sizes are indicated in each figure legend. Two-tailed hypothesis testing was used for all experiments in our study. A Mann-Whitney test was used for comparisons between two groups when the data were not normally distributed. For comparisons between multiple groups, a one-way ANOVA with Tukey’s multiple comparisons test or a two-way ANOVA with Bonferroni post hoc test was performed depending on the number of experimental variables. A Kruskal-Wallis test with Dunn’s multiple comparisons test was used to compare multiple groups when the data were not normally distributed. A p-value of <0.05 was considered statistically significant.
Results
Isoflurane attenuates lung injury in a two hit murine model of acute lung injury
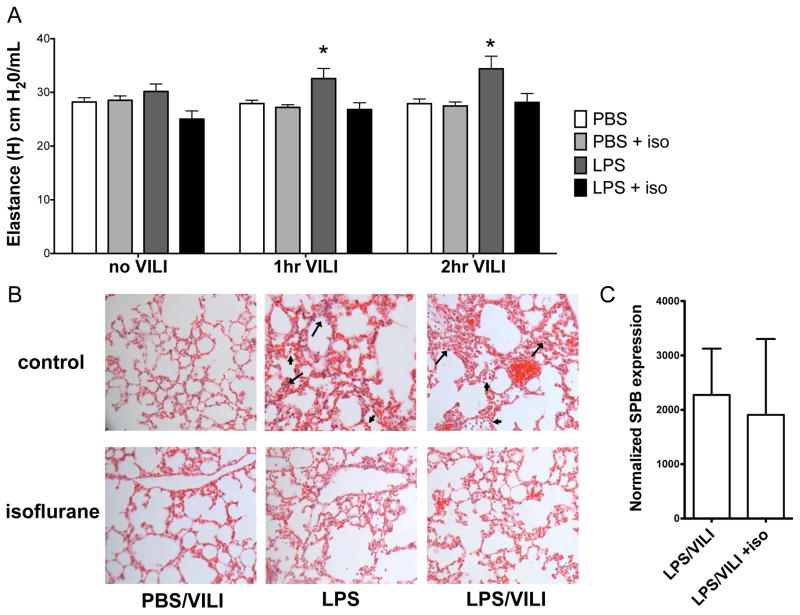
Mice were exposed to nebulized lipopolysaccharide or saline control, with or without MV to induce VILI twenty-four hours later (Fig 1A). Mice exposed to nebulized endotoxin followed by MV developed a significant increase in lung elastance (i.e. stiffness, termed “H”) compared with control mice or VILI alone (Table 1, Fig 2A). Mice were treated with isoflurane or identical control gas for 2 hours after exposure to endotoxin (or saline) and prior to MV (Fig 1A). Mice treated with isoflurane were anesthetized and breathed spontaneously. Hemodynamic monitoring and blood gas analysis in a pilot study demonstrated that mice treated with isoflurane did not have evidence of hypotension, hypercarbia, or hypoxemia (see table, Supplemental Digital Content 1). Mice exposed to inhaled isoflurane either 1 hour after nebulized lipopolysaccharide or the following day (23 hours after lipopolysaccharide) exhibited a significant reduction in lung elastance (H) at 2 hrs following VILI (Fig 2A, Table 1; see figure, Supplementary Digital Content 2). Hematoxylin and eosin staining of lung sections revealed interstitial thickening and infiltration of inflammatory cells after nebulized lipopolysaccharide and MV that appeared more pronounced compared to either injury alone (Fig 2B). Exposure to inhaled isoflurane attenuated histologic lung injury as indicated by pulmonary edema accumulation following lipopolysaccharide and VILI, with the most pronounced effects observed after the combination of both lipopolysaccharide and VILI (Fig 2B), without differences noted in quantity of inflammatory cells (see next section-Results, paragraph 2). We previously reported that physiologic lung dysfunction after injury with endotoxin correlated with surfactant dysfunction, due to decreased levels of the critical protein, surfactant protein B (SPB) at the message and protein levels.30 We therefore measured SPB message levels in lung tissue following the combination of lipopolysaccharide and VILI and found no difference in SPB levels with isoflurane exposure (Fig 2C). Thus, the ameliorated physiologic lung injury parameters with isoflurane are not explained by altered SPB expression in this model.
Table 1.
Summary of detailed lung physiology data presented in Figure 2.
| PBS/VILI | LPS/VILI | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| control | isoflurane | control | isoflurane | |||||
| G | H | G | H | G | H | G | H | |
|
|
||||||||
| baseline | 6.12 ± 0.12 | 28.20 ± 0.80 | 6.02 ± 0.23 | 28.5 ± 0.82 | 6.24 ± 0.28 | 30.2 ± 1.35 | 5.31 ± 0.27 | 25.2 ± 1.48 |
| 30 min | 5.98 ± 0.23 | 27.80 ± 0.67 | 5.78 ± 0.21 | 27.0 ± 0.51 | 6.56 ± 0.25 | 32.0 ± 1.56 | 5.16 ± 0.31 | 26.5 ± 1.35 |
| 60 min | 5.75 ± 0.23 | 27.90 ± 0.63 | 5.67 ± 0.22 | 27.1 ± 0.53 | 6.65 ± 0.31 | 32.6 ± 1.83 | 5.31 ± 0.29 | 27.0 ± 1.22 |
| 90 min | 5.85 ± 0.21 | 28.00 ± 0.70 | 5.42 ± 0.26 | 26.1 ± 1.77 | 6.80 ± 0.40 | 34.3 ± 2.26 | 5.59 ± 0.42 | 28.0 ± 1.44 |
| 120 min | 5.67 ± 0.31 | 27.90 ± 0.94 | 5.68 ± 0.20 | 27.5 ± 0.70 | 6.98 ± 0.37 | 34.4** ± 2.30 | 5.64* ± 0.44 | 28.1* ± 1.69 |
|
|
||||||||
C57BL/6 mice (n=8/group) were treated with nebulized lipopolysaccharide (or saline control) 1 hr prior to exposure to isoflurane (or control gas) 24 hrs prior to mechanical ventilation. Lung elastance (i.e. stiffness, H, cm H2O/mL) and tissue resistance (G, cm H2O/mL) were measured at the onset of mechanical ventilation (i.e. baseline) and every 30 min thereafter. Following 2 hrs of mechanical ventilation, mice injured with lipopolysaccharide (LPS/VILI) had significantly higher lung elastance (H) compared to all other groups
p<0.05 vs PBS, PBS + iso, LPS + iso by 1-way ANOVA with Tukey’s multiple comparisons test.
At 2 hrs, LPS/VILI mice treated with isoflurane had significantly lower elastance (H) and resistance (G) compared to LPS/VILI mice without isoflurane treatment
p<0.05 by 1-way ANOVA with Tukey’s multiple comparisons test.
G = lung tissue resistance; H = lung elastance (stiffness); LPS/VILI = mechanically ventilated mice previously treated with nebulized endotoxin; PBS/VILI = mechanically ventilated mice previously treated with nebulized phosphate buffered saline
Figure 2. Isoflurane attenuates lung injury in a two hit model using nebulized endotoxin and mechanical ventilation.
C57BL/6 mice (n=8/group) were treated with isoflurane (or control gas) 1 hr after exposure to 10 mg nebulized lipopolysaccharide (LPS) and 24 hrs prior to mechanical ventilation (15 mL/kg, 2 hrs). A. The combination of nebulized endotoxin and mechanical ventilation to induce ventilator induced lung injury (LPS/VILI) increased lung elastance (H, stiffness) compared to mice treated with nebulized phosphate buffered saline (PBS, no VILI, n=8) or nebulized PBS followed by VILI (PBS, 2 hr VILI, n=8). Isoflurane prevented the increase in lung stiffness seen following LPS/VILI at both 1 and 2 hrs. (*p<0.05 vs LPS/VILI + isoflurane at same time point by 2-way ANOVA with Bonferroni post test) B. LPS treatment resulted in inflammatory cell infiltration (arrowhead) and interstitial thickening (arrow) consistent with edema that was more pronounced with LPS/VILI and appeared to be ameliorated by isoflurane. C. Isoflurane did not alter lung surfactant protein B (SPB) message levels following LPS/VILI.
Isoflurane does not alter the degree of lung inflammation after lipopolysaccharide, VILI, or lipopolysaccharide and VILI combined
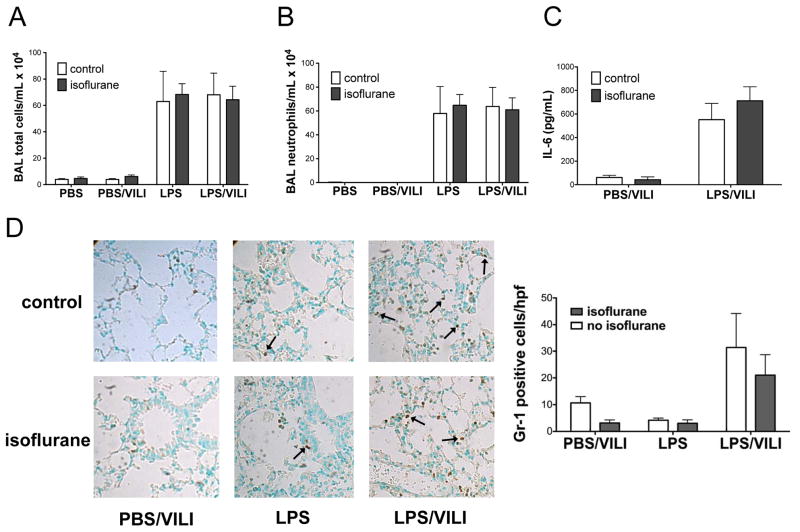
Given that decreased lung inflammation is often observed in the setting of ameliorated lung injury and has been reported in some lung injury studies where isoflurane was protective, we set out to determine whether isoflurane prevented lung injury in our model by modulating markers of lung inflammation. Total BAL inflammatory cells (Fig 3A) and BAL neutrophils (Fig 3B) increased following endotoxin and endotoxin and VILI combined compared to control and VILI animals. However, the number of inflammatory cells in the BAL fluid did not differ with the addition of isoflurane exposure to lipopolysaccharide or the combination of lipopolysaccharide and VILI (Fig 3A, 3B). We next examined BAL levels of interleukin-6, a pro-inflammatory cytokine that is a well-described marker of lung injury.32,33 BAL interleukin-6 levels increased significantly following treatment with lipopolysaccharide and VILI and were not significantly altered in animals that were exposed to isoflurane (Fig 3C). In order to assess for the presence of neutrophils in the interstitial space, we performed immunostaining for the neutrophil marker Gr-1, which revealed increased neutrophils in animals subjected to endotoxin and VILI compared to either injury alone, but there was no significant change in neutrophil infiltration with isoflurane (Fig 3D).
Figure 3. Isoflurane does not impact lung inflammation after nebulized endotoxin, mechanical ventilation, or the combination of both injuries.
Total bronchoalveolar lavage (BAL) inflammatory cells (A) and neutrophils (B) were increased following injury with lipopolysaccharide (LPS, n=3) and LPS followed by mechanical ventilation to induce ventilator induced lung injury (LPS/VILI, n=7) compared to control mice treated with nebulized phosphate buffered saline (PBS, n=2) or PBS treatment followed by mechanical ventilation (PBS/VILI, n=8), but were not affected by isoflurane treatment. Interleukin-6 (IL-6) levels in BAL fluid (C) increased following LPS/VILI (n=7) compared to PBS/VILI (n=7) but were not altered by isoflurane treatment. Staining for Gr-1 revealed increased neutrophils in animals subjected to LPS/VILI compared to either injury alone but there was no significant change in neutrophil infiltration with isoflurane (D).
Isoflurane prevents increased alveolar-capillary barrier permeability following injury with lipopolysaccharide and VILI
To further explore the mechanism of protection of isoflurane in lung injury, we next set out to determine whether isoflurane altered alveolar-capillary membrane permeability during lung injury by assessing BAL protein levels. Mice treated with lipopolysaccharide and VILI exhibited increased BAL protein levels that were significantly reduced with exposure to isoflurane (Fig 4A). To confirm that isoflurane preserves alveolar-capillary barrier integrity, we performed an Evans blue assay and found decreased barrier leak with isoflurane treatment in animals subjected to lipopolysaccharide and VILI when isoflurane was administered either 24 hrs prior to MV or immediately prior to MV (Fig 4B, see figure, Supplemental Digital Content 2). Additionally, mice injured with endotoxin and VILI that were treated with isoflurane had lower wet to dry ratios (Fig 4C), also indicative of decreased barrier leak with isoflurane treatment. These findings were consistent with less edema observed histologically with the addition of isoflurane to mice injured with the combination of lipopolysaccharide and MV (Fig 2B). Thus, the improved lung compliance seen in animals treated with isoflurane correlated with decreased alveolar-capillary membrane permeability assessed by multiple different methods at multiple time points.
Figure 4. Isoflurane prevents increased alveolar-capillary barrier permeability following injury with lipopolysaccharide and mechanical ventilation.
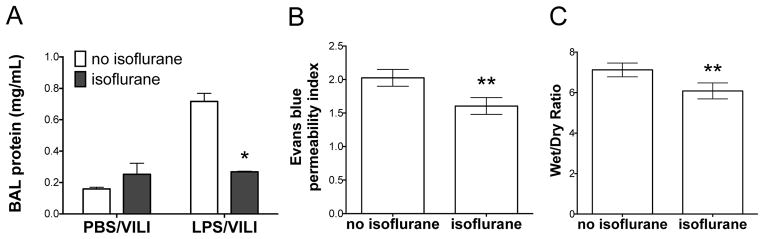
C57BL/6 mice were subjected to injury with nebulized lipopolysaccharide (LPS) and mechanical ventilation to induce ventilator induced lung injury (LPS/VILI) or nebulized phosphate buffered saline followed by mechanical ventilation (PBS/VILI) prior to performing bronchoalveolar lavage (BAL). A. BAL protein increased significantly following LPS/VILI (n=3) compared to PBS/VILI (n=3). Isoflurane treatment following LPS but prior to VILI (n=4) prevented the increase in BAL protein seen with LPS/VILI without isoflurane (n=3). (*p<0.001 vs LPS/VILI no isoflurane by 2-way ANOVA with Bonferroni post-test). In a separate experiment, mice were subjected to LPS/VILI with isoflurane (n=6) or control gas (n=5). Mice treated with isoflurane had significantly less alveolar-capillary permeability as measured by Evan blue assay (B) and wet to dry ratio (C). **p<0.05 vs LPS/VILI no isoflurane by Mann-Whitney test.
Isoflurane attenuates the reduction of the tight junction protein zona occludens 1 following lipopolysaccharide and cyclic stretch in vitro
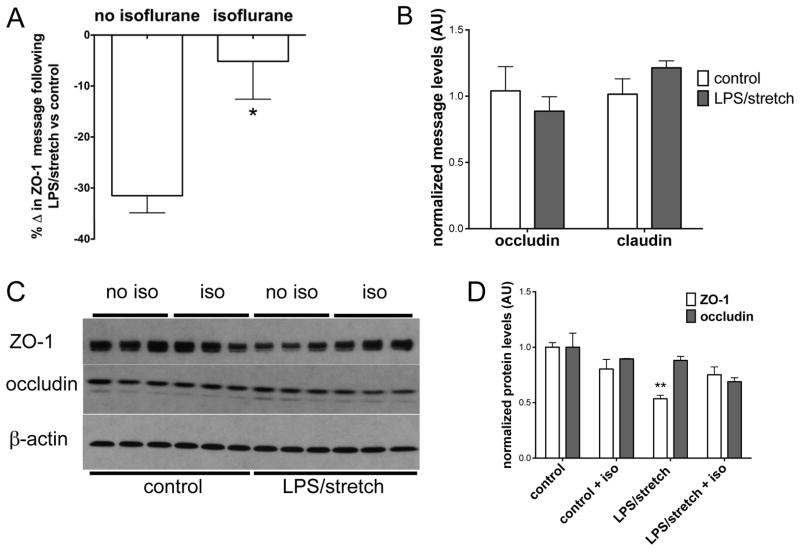
In order to dissect the mechanism by which isoflurane protected against increased alveolar-capillary membrane permeability after injury with lipopolysaccharide and MV, we treated mouse lung epithelial cells with a combination of lipopolysaccharide and interferon-γ (Fig 1B) which has been shown to mimic the downstream effects of lipopolysaccharide in vivo in mice.30 Cells were then exposed to isoflurane after endotoxin but prior to cyclic stretch in order to mirror our in vivo lung injury model. We hypothesized that expression of tight junction protein family members known to be critical for maintenance of barrier function4,34 might be dysregulated in our two hit model and that isoflurane might modulate expression of these proteins. We focused on zona occludens (ZO)-1, given our recent study demonstrating the importance of this protein in maintaining barrier function in the gut in sepsis.35 The combination of endotoxin and interferon-γ followed by cyclic stretch significantly decreased ZO-1 message levels (Fig 5A, see figure, Supplementary Digital Content 3) compared to control cells. Treatment with isoflurane after lipopolysaccharide exposure but prior to cyclic stretch significantly attenuated the decrease in ZO-1 message levels (Fig 5A). Interestingly, the effect of isoflurane appeared to be specific to ZO-1, as message levels of other tight junction proteins, including occluding and claudin-4, were not significantly altered by lipopolysaccharide/interferon-γ and stretch (Fig 5B). ZO-1 protein levels decreased significantly after cells were subjected to lipopolysaccharide/interferon-γ and stretch, but this decrease was prevented with addition of isoflurane (Fig 5C, 5D). Occludin protein levels did not change significantly following lipopolysaccharide/interferon-γ and stretch and were not affected by isoflurane treatment (Fig 5C, 5D).
Figure 5. Isoflurane attenuates the reduction of zona occludens 1 following lipopolysaccharide and cyclic stretch in vitro.
Mouse lung epithelial cells were plated in 6-well dishes on flexible silico-elastic membranes and grown to confluence prior to treatment with lipopolysaccharide (LPS) and interferon gamma (IFN-γ). Following LPS/IFN-γ treatment, cells were exposed to isoflurane (or control gas) 1 day prior to cyclic stretch (10% stretch, 2 hrs). cDNA was synthesized, and message levels were analyzed by qPCR after normalizing for the housekeeping gene 18S. In separate experiments protein was isolated and tight junction protein levels were probed by immunoblotting. A. The combination of LPS/IFN-γ and cyclic stretch (denoted LPS/stretch, n=13 wells) decreased zona occludens 1 (ZO-1) message levels compared to control cells (no LPS/IFN-γ and no stretch, n=8 wells). The decrease in ZO-1 message was significantly attenuated by isoflurane treatment (n=15 wells, *p<0.05 vs no isoflurane by Mann-Whitney test.) B. Occludin and claudin 4 message levels did not change significantly following the combination of LPS/IFN-γ + stretch compared to control cells (no LPS/IFN-γ, no stretch) C. ZO-1 protein levels, but not occludin protein levels, decreased following LPS/IFN-γ + stretch. D. Image quantitation revealed that ZO-1 expression (panel C) decreased significantly with LPS/IFN-γ + stretch, but ZO-1 expression did not differ significantly from control (no LPS/IFN-γ, no stretch) levels when cells were treated with isoflurane. (**p<0.05 vs control/no iso by Kruskal-Wallis test with Dunn’s multiple comparisons test). All data n=3 wells/group unless otherwise noted.
Isoflurane exposure attenuates the reduction in lung epithelial ZO-1 levels in vivo in a two hit model of acute lung injury
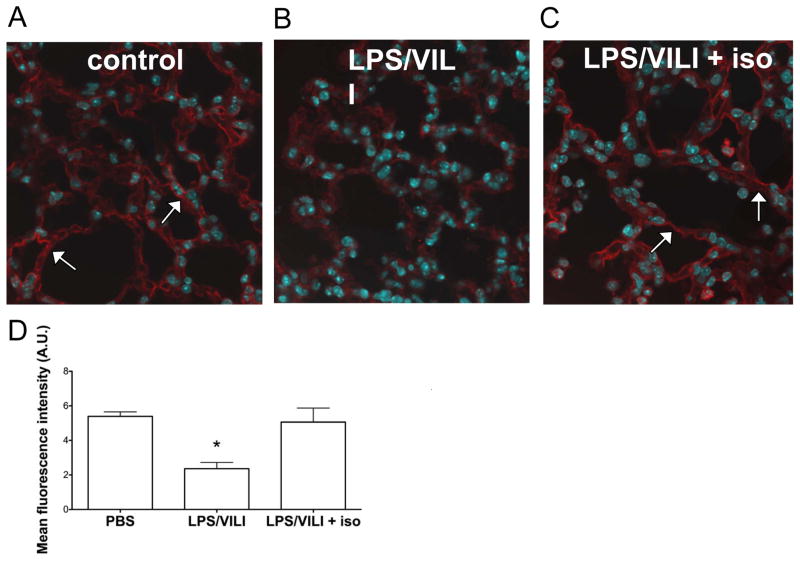
To determine whether ZO-1 levels were similarly altered following lung injury with the combination of lipopolysaccharide and MV in vivo, we performed immunostaining of lung sections harvested from control mice or mice subjected to endotoxin and MV with or without isoflurane exposure. Lung sections from control animals revealed smooth linear ZO-1 staining along the alveolar epithelium consistent with intact tight junctions4 (Fig 6A). Lung sections from mice injured with lipopolysaccharide and MV mice revealed decreased ZO-1 expression marked by staining that was irregular and interrupted in nature (Fig 6B). Sections from mice exposed to lipopolysaccharide and MV with isoflurane revealed restoration of smooth, linear ZO-1 staining along the alveolar epithelium (Fig 6C), consistent with intact tight junctions. Quantitation of ZO-1 staining revealed that levels of ZO-1 in mice injured with endotoxin and MV and treated with isoflurane had returned nearly to baseline prior to lung injury (Fig 6D). Thus the amelioration of physiologic lung dysfunction by isoflurane correlates with reduced alveolar-capillary membrane dysfunction and dysregulation of the tight junction protein, ZO-1.
Figure 6. Isoflurane exposure attenuates the reduction in lung epithelial zona occludens 1 after injury with lipopolysaccharide and mechanical ventilation in vivo.
Wild-type C57BL/6 mice (n=3/group) were subjected to lung injury with the combination of lipopolysaccharide followed by mechanical ventilation to induce ventilator induced lung injury (LPS/VILI) with or without isoflurane treatment and compared to control mice subjected to treatment with nebulized phosphate buffered saline (PBS) without VILI. Zona occludens 1 (ZO-1) immunostaining revealed decreased ZO-1 expression in LPS/VILI (B) that was discontinuous in nature compared to control animals (A). The addition of isoflurane treatment after LPS but prior to mechanical ventilation (C) restored ZO-1 levels back to those seen in control mice, and staining was smooth and continuous in nature similar to that seen in control mice. Quantitation of ZO-1 staining (D, 3 images per mouse) from lung sections confirmed that ZO-1 levels significantly decreased with LPS/VILI and that isoflurane treatment prevented this decrease. *p<0.05 vs all other groups by 1-way ANOVA with Tukey’s multiple comparisons test.
Discussion
Isoflurane has been shown to confer protection in various models of acute lung injury including zymosan induced lung injury,36,37 systemic endotoxemia,21 inhaled endotoxin,7,13 hemorrhagic shock,20 and VILI.14,15 Although isoflurane has been studied in models using a single lung injury, our study is, to the best of our knowledge, the first to examine the effects of isoflurane in a clinically relevant two-hit model of lung injury where isoflurane was administered after lung injury was established. We chose this model given debate about the choice of anesthetic for patients with lung injury who require MV. One important group of patients felt to be particularly susceptible to VILI is patients with established lung injury that do not require MV but do require invasive procedures or surgical intervention. Examples of this scenario include a patient with lung injury in the setting of necrotizing pancreatitis who requires pancreatic fluid drainage, debridement, or necrosectomy, or a patient with burns that requires debridement/escharotomy. The use of volatile anesthetics in these types of scenarios remains controversial.17
Although prior studies have shown that isoflurane is protective in each of the injuries that comprise our model,13,14 the mechanism of its protective effects remains incompletely understood. In a mouse model of endotoxin induced lung injury, isoflurane decreased BAL inflammatory cells in mice that were either pre-treated or treated 1 hour after nebulized lipopolysaccharide.13 Surprisingly, we did not see evidence of decreased BAL inflammatory cells, neutrophils, or altered BAL interleukin-6 levels in mice injured with the combination of lipopolysaccharide and MV that were treated with isoflurane (Fig 3). This difference may be due to the use of different serotypes of endotoxin, due to the fact that we assessed lung inflammation at a later time point compared to most studies, and/or due to the fact that our model produces overwhelming lung inflammation that might not be easily modifiable. Our findings are consistent with Giraud et al. who showed that another inhaled anesthetic (halothane) attenuated influx of inflammatory cells into rat lungs following injury with lipopolysaccharide and MV at 4 hours but not 24 hours (as was used in our study), stressing the importance of later time point assessments in these models.38 In addition to the effect of isoflurane on lung inflammation, the study by Reutershan also showed that isoflurane attenuated the alveolar-capillary barrier leak that occurred following injury with lipopolysaccharide.13 Barrier leak and inflammatory cell infiltration often occur simultaneously in lung injury suggesting a common pathophysiologic mechanism. However, in some instances, the mechanisms of each process are distinct. Recruitment of neutrophils and other inflammatory cells to the alveolar space is an active process involving cell migration in response to chemokines and non-cardiogenic pulmonary edema results from dysfunction of the pulmonary capillary and epithelial barriers. The protective effect of isoflurane and other volatile anesthetics has been primarily attributed to dampened inflammation, and the fact that multiple lung injury studies have demonstrated that volatile anesthetics abrogate vascular leak has led investigators to suggest that dampened inflammation might be responsible for this effect.7,20,37,39 Our study raises a novel concept that isoflurane directly affects the alveolar-capillary membrane independent of effects on lung inflammation.
In recent years it has become clear that tight junctions play a key role in the pathogenesis of non-cardiogenic pulmonary edema that is characteristic of ARDS.40–42 Tight junctions are complex sub-cellular structures comprised of both trans-membrane and cytosolic proteins that create a semi-permeable barrier between adjacent epithelial cells.43 In order to determine how isoflurane prevented increased lung edema following injury with lipopolysaccharide and MV (Fig 4), we developed an in vitro model using mouse lung epithelial cells subjected to lipopolysaccharide and interferon-γ prior to cyclic stretch. To our knowledge, we are the first to use this type of in vitro stretch model to study the molecular effects of volatile anesthetics. Given that brief exposure to isoflurane provided durable protection against further lung injury 24 hours after exposure, we hypothesized that the protective effect may be due to alterations in gene expression, specifically the expression of key tight junction proteins. We chose to focus on the role of ZO-1 given prior data showing the importance of this tight junction protein in lung injury following endotoxemia4 and hyperoxia42 induced lung injury. Using our model, we determined that the combination of lipopolysaccharide and interferon-γ followed by cyclic stretch significantly reduced the expression of ZO-1 (Fig 5) and then observed that ZO-1 expression was similarly reduced in vivo (Fig 6). Although others have described the role of another tight junction protein, claudin-441 in VILI, this is the first report of the role of ZO-1 in a model of VILI.
There are several unique features of our study. Our study is the first to describe the protective role of isoflurane in a clinically relevant lung injury model where MV was instituted after lung injury was established. Decreased lung compliance is a key clinical variable that is important in management of ARDS, and our study is the only one that we are aware of that has shown improved physiologic lung function with isoflurane. Interestingly, isoflurane did not improve measures of lung function in a mouse model of VILI alone14 even though it did reduce other injury parameters, suggesting that volatile anesthetics may possess greater protective effects when used after lung injury is established. We have previously shown that physiologic lung dysfunction following endotoxin treatment was due to decreased SPB levels30 and others have shown that sevoflurane increased SPB levels in a rat model of lung injury that combined lipopolysaccharide and MV.39 However, we did not observe any changes in lung SPB message levels following treatment with isoflurane (Fig 2) which may be due to differential effects of individual volatile anesthetics or due to differences in the model. In addition to our use of a clinically relevant lung injury model and our novel effects of isoflurane on improving lung physiology, we are also the first to use an in vitro system to test the effects of isoflurane on lung epithelial cells prior to cyclic stretch. This model enabled us to perform mechanistic studies which ultimately lead to our observation that isoflurane prevents the downregulation of ZO-1 following injury with the combination of lipopolysaccharide and MV.
There are several potential limitations to our study, including that our model utilized MV with a tidal volume of 15 mL/kg. Although lower tidal volumes (i.e. 6 mL/kg) are used in patients with ARDS, lung injury is heterogeneous with regional variation in lung mechanics that leads to local areas of high levels of stretch.44 In order to model the injury that occurs in these areas, the standard approach is to use MV with higher tidal volumes in preclinical models.22,29,45 Another limitation of the two hit model is that it is difficult to determine the exact mechanism of protection with isoflurane as it may exert its effect on either injury independently or in combination. In our model using a short period of moderate tidal volume ventilation, MV alone does not induce significant injury and was employed to exacerbate pre-existing lung injury, as is often seen when patients with an initial ‘hit’ to the lung acquire a second ‘hit’ with MV (even if employed with protective ventilatory strategies). Although we show here that isoflurane modulates ZO-1 levels and prevents non-cardiogenic pulmonary edema, it remains unclear whether this effect is specific to isoflurane or generalizable to all anesthetics (volatile or intravenous). Finally, our study only examined the effect of isoflurane on epithelial barrier integrity, and it is possible that isoflurane also affects other factors that contribute to non-cardiogenic pulmonary edema in ARDS including changes in endothelial permeability and by active transport of salt and water across the epithelial barrier. Despite these limitations, there are several clinically relevant implications of our findings. Now that there is a growing literature to support the beneficial effects of isoflurane and other volatile anesthetics in lung injury, and several reports of using volatile anesthetics for more prolonged periods of time in the ICU,46 there are potential opportunities to translate our findings to critically ill patients. There remains substantial debate surrounding the choice of anesthetic regimens for patients with ARDS that require surgery, and our data suggest that isoflurane may be advantageous in this situation even when administered at different time points after the initial lung injury, but further studies will be needed to confirm this. Enthusiasm for longer-term use of volatile anesthetics in the ICU should be tempered by preclinical reports of potential adverse cerebral effects of these medications.47,48 These issues will be important to address in models of critical illness, as the aggregate effects may be different in the setting of significant lung disease. In summary, we have shown that isoflurane is protective in a two-hit model of lung injury where isoflurane was administered after lung injury was established. Future studies will be needed to determine whether isoflurane might some day have a role in the management of critically ill patients with ARDS in the ICU or in the operating room.
Supplementary Material
Acknowledgments
Source of funding: This work was supported, in part, by the National Institutes of Health (Bethesda, Maryland) grants R01 HL091957 and R01 HL112747 (to Dr. Baron), R01 GM088817 (to Dr. Crosby), K08 GM083207 (to Dr. Fredenburgh), T32 HL00763327 and K08 GM102695 (to Dr. Englert), and the Milton Fund of Harvard University (Cambridge, Massachusetts) to Dr. Crosby.
We are grateful to Thomas Gagliano MA, BS (Lovelace Respiratory Research Institute, Alburqurque, NM) for his assistance with the artwork in Figure 1 and for the technical assistance of Emily Cotran, BA; Megan Mataga, BA; Wini Hwang, BA; and Elysia Heilig, BA (Dept of Anesthesiology, Brigham and Women’s Hospital, Boston, MA). We are also grateful to Lester Kobzik, MD (Harvard School of Public Health, Boston, MA) for his expertise and assistance with review of the pathology specimens.
Footnotes
Competing Interests: The authors declare no competing interests.
A portion of the data from this manuscript were presented at the American Thoracic Society Annual Scientific Meeting, New Orleans, May 14–19, 2010.
References
- 1.ARDS Definition Task Force. Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: The Berlin Definition. JAMA. 2012;307:2526–33. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 2.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–93. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 3.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–49. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 4.Han X, Fink MP, Uchiyama T, Yang R, Delude RL. Increased iNOS activity is essential for pulmonary epithelial tight junction dysfunction in endotoxemic mice. Am J Physiol Lung Cell Mol Physiol. 2004;286:L259–67. doi: 10.1152/ajplung.00187.2003. [DOI] [PubMed] [Google Scholar]
- 5.Tremblay LN, Slutsky AS. Ventilator-induced lung injury: From the bench to the bedside. Intensive Care Med. 2006;32:24–33. doi: 10.1007/s00134-005-2817-8. [DOI] [PubMed] [Google Scholar]
- 6.Ostermann ME, Keenan SP, Seiferling RA, Sibbald WJ. Sedation in the intensive care unit: A systematic review. JAMA. 2000;283:1451–9. doi: 10.1001/jama.283.11.1451. [DOI] [PubMed] [Google Scholar]
- 7.Pang YL, Chen BS, Li SP, Huang CC, Chang SW, Lam CF, Tsai YC. The preconditioning pulmonary protective effect of volatile isoflurane in acute lung injury is mediated by activation of endogenous iNOS. J Anesth. 2012;26:822–8. doi: 10.1007/s00540-012-1456-9. [DOI] [PubMed] [Google Scholar]
- 8.Collange O, Charles AL, Noll E, Bouitbir J, Zoll J, Piquard F, Diemunsch P, Geny B. Isoflurane anesthesia preserves liver and lung mitochondrial oxidative capacity after gut ischemia-reperfusion. Anesth Analg. 2011;113:1438–41. doi: 10.1213/ANE.0b013e3182367a10. [DOI] [PubMed] [Google Scholar]
- 9.Eisenhut M. Role of a reduction of cytokine levels in isoflurane-mediated protection from endotoxin-induced lung injury. Anesthesiology. 2006;105:1280. doi: 10.1097/00000542-200612000-00036. author reply 1280–1. [DOI] [PubMed] [Google Scholar]
- 10.Fujinaga T, Nakamura T, Fukuse T, Chen F, Zhang J, Ueda S, Hamakawa H, Omasa M, Sakai H, Hanaoka N, Wada H, Bando T. Isoflurane inhalation after circulatory arrest protects against warm ischemia reperfusion injury of the lungs. Transplantation. 2006;82:1168–74. doi: 10.1097/01.tp.0000237207.73439.2e. [DOI] [PubMed] [Google Scholar]
- 11.Liu R, Ishibe Y, Ueda M. Isoflurane-sevoflurane adminstration before ischemia attenuates ischemia-reperfusion-induced injury in isolated rat lungs. Anesthesiology. 2000;92:833–40. doi: 10.1097/00000542-200003000-00027. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka K, Ludwig LM, Kersten JR, Pagel PS, Warltier DC. Mechanisms of cardioprotection by volatile anesthetics. Anesthesiology. 2004;100:707–21. doi: 10.1097/00000542-200403000-00035. [DOI] [PubMed] [Google Scholar]
- 13.Reutershan J, Chang D, Hayes JK, Ley K. Protective effects of isoflurane pretreatment in endotoxin-induced lung injury. Anesthesiology. 2006;104:511–7. doi: 10.1097/00000542-200603000-00019. [DOI] [PubMed] [Google Scholar]
- 14.Faller S, Strosing KM, Ryter SW, Buerkle H, Loop T, Schmidt R, Hoetzel A. The volatile anesthetic isoflurane prevents ventilator-induced lung injury via phosphoinositide 3-kinase/Akt signaling in mice. Anesth Analg. 2012;114:747–56. doi: 10.1213/ANE.0b013e31824762f0. [DOI] [PubMed] [Google Scholar]
- 15.Vaneker M, Santosa JP, Heunks LM, Halbertsma FJ, Snijdelaar DG, JVANE, IAVDB, FMVDP, JGVDH, Scheffer GJ. Isoflurane attenuates pulmonary interleukin-1beta and systemic tumor necrosis factor-alpha following mechanical ventilation in healthy mice. Acta Anaesthesiol Scand. 2009;53:742–8. doi: 10.1111/j.1399-6576.2009.01962.x. [DOI] [PubMed] [Google Scholar]
- 16.ChangLai SP, Hung WT, Liao KK. Detecting alveolar epithelial injury following volatile anesthetics by (99m)Tc DTPA radioaerosol inhalation lung scan. Respiration. 1999;66:506–10. doi: 10.1159/000029449. [DOI] [PubMed] [Google Scholar]
- 17.Hung CJ, Liu FY, Shaiu YC, Kao A, Lin CC, Lee CC. Assessing transient pulmonary injury induced by volatile anesthetics by increased lung uptake of technetium-99m hexamethylpropylene amine oxime. Lung. 2003;181:1–7. doi: 10.1007/s00408-002-0109-4. [DOI] [PubMed] [Google Scholar]
- 18.Zhao P, Peng L, Li L, Xu X, Zuo Z. Isoflurane preconditioning improves long-term neurologic outcome after hypoxic-ischemic brain injury in neonatal rats. Anesthesiology. 2007;107:963–70. doi: 10.1097/01.anes.0000291447.21046.4d. [DOI] [PubMed] [Google Scholar]
- 19.Edmands SD, Ladow E, Hall AC. Microarray analyses of genes regulated by isoflurane anesthesia in vivo: A novel approach to identifying potential preconditioning mechanisms. Anesth Analg. 2013;116:589–95. doi: 10.1213/ANE.0b013e31827b27b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harr JN, Moore EE, Stringham J, Wohlauer MV, Fragoso M, Jones WL, Gamboni F, Silliman CC, Banerjee A. Isoflurane prevents acute lung injury through ADP-mediated platelet inhibition. Surgery. 2012;152:270–6. doi: 10.1016/j.surg.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li QF, Zhu YS, Jiang H, Xu H, Sun Y. Isoflurane preconditioning ameliorates endotoxin-induced acute lung injury and mortality in rats. Anesth Analg. 2009;109:1591–7. doi: 10.1213/ANE.0b013e3181baf506. [DOI] [PubMed] [Google Scholar]
- 22.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295:L379–99. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lai PS, Fresco JM, Pinilla MA, Macias AA, Brown RD, Englert JA, Hofmann O, Lederer JA, Hide W, Christiani DC, Cernadas M, Baron RM. Chronic endotoxin exposure produces airflow obstruction and lung dendritic cell expansion. Am J Respir Cell Mol Biol. 2012;47:209–17. doi: 10.1165/rcmb.2011-0447OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palanisamy A, Baxter MG, Keel PK, Xie Z, Crosby G, Culley DJ. Rats exposed to isoflurane in utero during early gestation are behaviorally abnormal as adults. Anesthesiology. 2011;114:521–8. doi: 10.1097/ALN.0b013e318209aa71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frithiof R, Soehnlein O, Eriksson S, Fenhammar J, Hjelmqvist H, Lindbom L, Rundgren M. The effects of isoflurane anesthesia and mechanical ventilation on renal function during endotoxemia. Acta Anaesthesiol Scand. 2011;55:401–10. doi: 10.1111/j.1399-6576.2011.02406.x. [DOI] [PubMed] [Google Scholar]
- 26.Dixon DL, De Smet HR, Bersten AD. Lung mechanics are both dose and tidal volume dependant in LPS-induced lung injury. Respir Physiol Neurobiol. 2009;167:333–40. doi: 10.1016/j.resp.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 27.Ingenito EP, Mora R, Cullivan M, Marzan Y, Haley K, Mark L, Sonna LA. Decreased surfactant protein-B expression and surfactant dysfunction in a murine model of acute lung injury. Am J Respir Cell Mol Biol. 2001;25:35–44. doi: 10.1165/ajrcmb.25.1.4021. [DOI] [PubMed] [Google Scholar]
- 28.Baron RM, Lopez-Guzman S, Riascos DF, Macias AA, Layne MD, Cheng G, Harris C, Chung SW, Reeves R, von Andrian UH, Perrella MA. Distamycin A inhibits HMGA1-binding to the P-selectin promoter and attenuates lung and liver inflammation during murine endotoxemia. PLoS One. 2010;5:e10656. doi: 10.1371/journal.pone.0010656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belperio JA, Keane MP, Burdick MD, Londhe V, Xue YY, Li K, Phillips RJ, Strieter RM. Critical role for CXCR2 and CXCR2 ligands during the pathogenesis of ventilator-induced lung injury. J Clin Invest. 2002;110:1703–16. doi: 10.1172/JCI15849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baron RM, Carvajal IM, Fredenburgh LE, Liu X, Porrata Y, Cullivan ML, Haley KJ, Sonna LA, De Sanctis GT, Ingenito EP, Perrella MA. Nitric oxide synthase-2 down-regulates surfactant protein-B expression and enhances endotoxin-induced lung injury in mice. FASEB J. 2004;18:1276–8. doi: 10.1096/fj.04-1518fje. [DOI] [PubMed] [Google Scholar]
- 31.Culley DJ, Boyd JD, Palanisamy A, Xie Z, Kojima K, Vacanti CA, Tanzi RE, Crosby G. Isoflurane decreases self-renewal capacity of rat cultured neural stem cells. Anesthesiology. 2011;115:754–63. doi: 10.1097/ALN.0b013e318223b78b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gurkan OU, He C, Zielinski R, Rabb H, King LS, Dodd-o JM, D’Alessio FR, Aggarwal N, Pearse D, Becker PM. Interleukin-6 mediates pulmonary vascular permeability in a two-hit model of ventilator-associated lung injury. Exp Lung Res. 2011;37:575–84. doi: 10.3109/01902148.2011.620680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolters PJ, Wray C, Sutherland RE, Kim SS, Koff J, Mao Y, Frank JA. Neutrophil-derived IL-6 limits alveolar barrier disruption in experimental ventilator-induced lung injury. J Immunol. 2009;182:8056–62. doi: 10.4049/jimmunol.0801323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yin Q, Nan H, Yan L, Huang X, Wang W, Cui G, Wei J. Alteration of tight junctions in pulmonary microvascular endothelial cells in bleomycin-treated rats. Exp Toxicol Pathol. 2012;64:81–91. doi: 10.1016/j.etp.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 35.Fredenburgh LE, Velandia MM, Ma J, Olszak T, Cernadas M, Englert JA, Chung SW, Liu X, Begay C, Padera RF, Blumberg RS, Walsh SR, Baron RM, Perrella MA. Cyclooxygenase-2 deficiency leads to intestinal barrier dysfunction and increased mortality during polymicrobial sepsis. J Immunol. 2011;187:5255–67. doi: 10.4049/jimmunol.1101186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mu J, Xie K, Hou L, Peng D, Shang L, Ji G, Li J, Lu Y, Xiong L. Subanesthetic dose of isoflurane protects against zymosan-induced generalized inflammation and its associated acute lung injury in mice. Shock. 2010;34:183–9. doi: 10.1097/SHK.0b013e3181cffc3f. [DOI] [PubMed] [Google Scholar]
- 37.Shayevitz JR, Rodriguez JL, Gilligan L, Johnson KJ, Tait AR. Volatile anesthetic modulation of lung injury and outcome in a murine model of multiple organ dysfunction syndrome. Shock. 1995;4:61–7. doi: 10.1097/00024382-199507000-00010. [DOI] [PubMed] [Google Scholar]
- 38.Giraud O, Seince PF, Rolland C, Lecon-Malas V, Desmonts JM, Aubier M, Dehoux M. Halothane reduces the early lipopolysaccharide-induced lung inflammation in mechanically ventilated rats. Am J Respir Crit Care Med. 2000;162:2278–86. doi: 10.1164/ajrccm.162.6.9807113. [DOI] [PubMed] [Google Scholar]
- 39.Voigtsberger S, Lachmann RA, Leutert AC, Schlapfer M, Booy C, Reyes L, Urner M, Schild J, Schimmer RC, Beck-Schimmer B. Sevoflurane ameliorates gas exchange and attenuates lung damage in experimental lipopolysaccharide-induced lung injury. Anesthesiology. 2009;111:1238–48. doi: 10.1097/ALN.0b013e3181bdf857. [DOI] [PubMed] [Google Scholar]
- 40.Ohta H, Chiba S, Ebina M, Furuse M, Nukiwa T. Altered expression of tight junction molecules in alveolar septa in lung injury and fibrosis. Am J Physiol Lung Cell Mol Physiol. 2012;302:L193–205. doi: 10.1152/ajplung.00349.2010. [DOI] [PubMed] [Google Scholar]
- 41.Wray C, Mao Y, Pan J, Chandrasena A, Piasta F, Frank JA. Claudin-4 augments alveolar epithelial barrier function and is induced in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2009;297:L219–27. doi: 10.1152/ajplung.00043.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.You K, Xu X, Fu J, Xu S, Yue X, Yu Z, Xue X. Hyperoxia disrupts pulmonary epithelial barrier in newborn rats via the deterioration of occludin and ZO-1. Respir Res. 2012;13:36. doi: 10.1186/1465-9921-13-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cavanaugh KJ, Jr, Oswari J, Margulies SS. Role of stretch on tight junction structure in alveolar epithelial cells. Am J Respir Cell Mol Biol. 2001;25:584–91. doi: 10.1165/ajrcmb.25.5.4486. [DOI] [PubMed] [Google Scholar]
- 44.Gattinoni L, Caironi P, Cressoni M, Chiumello D, Ranieri VM, Quintel M, Russo S, Patroniti N, Cornejo R, Bugedo G. Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med. 2006;354:1775–86. doi: 10.1056/NEJMoa052052. [DOI] [PubMed] [Google Scholar]
- 45.Peng X, Abdulnour RE, Sammani S, Ma SF, Han EJ, Hasan EJ, Tuder R, Garcia JG, Hassoun PM. Inducible nitric oxide synthase contributes to ventilator-induced lung injury. Am J Respir Crit Care Med. 2005;172:470–9. doi: 10.1164/rccm.200411-1547OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soukup J, Scharff K, Kubosch K, Pohl C, Bomplitz M, Kompardt J. State of the art: Sedation concepts with volatile anesthetics in critically Ill patients. J Crit Care. 2009;24:535–44. doi: 10.1016/j.jcrc.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 47.Lee TA, Wolozin B, Weiss KB, Bednar MM. Assessment of the emergence of Alzheimer’s disease following coronary artery bypass graft surgery or percutaneous transluminal coronary angioplasty. J Alzheimers Dis. 2005;7:319–24. doi: 10.3233/jad-2005-7408. [DOI] [PubMed] [Google Scholar]
- 48.Wu X, Lu Y, Dong Y, Zhang G, Zhang Y, Xu Z, Culley DJ, Crosby G, Marcantonio ER, Tanzi RE, Xie Z. The inhalation anesthetic isoflurane increases levels of proinflammatory TNF-alpha, IL-6, and IL-1beta. Neurobiol Aging. 2012;33:1364–78. doi: 10.1016/j.neurobiolaging.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.