Abstract
The suprachiasmatic nucleus (SCN) contains a circadian clock that generates endogenous rhythmicity and entrains that rhythmicity with the day-night cycle. The neurochemical events that transduce photic input within the SCN and mediate entrainment by resetting the molecular clock have yet to be defined. Because GABA is contained in nearly all SCN neurons we tested the hypothesis that GABA serves as this signal in studies employing Syrian hamsters (Mesocricetus auratus). Activation of GABAA receptors was found to be necessary and sufficient for light to induce phase delays of the clock. Remarkably, the sustained activation of GABAA receptors for more than three consecutive hours was necessary to phase delay the clock. The duration of GABAA receptor activation required to induce phase delays would not have been predicted by either the prevalent theory of circadian entrainment or by expectations regarding the duration of ionotropic receptor activation necessary to produce functional responses. Taken together, these data identify a novel neurochemical mechanism essential for phase delaying the “master” circadian clock within the SCN as well as identifying an unprecedented action of an amino acid neurotransmitter involving the sustained activation of ionotropic receptors.
Keywords: entrainment, hamster, photic, neurotransmission, pacemaker
Introduction
The most important property of circadian clocks is their ability to entrain an organism’s physiology and behavior with the day-night cycle. The suprachiasmatic nucleus (SCN) is the location of the mammalian circadian clock that generates endogenous rhythmicity and entrains that rhythmicity with the day-night cycle (Moore and Eichler, 1972;Stephan and Zucker, 1972;Cohen and Albers, 1991). While extraordinary progress has been made in understanding the interacting molecular feedback loops that form the clock (for a review see (Hastings et al., 2014), the signaling pathways within the SCN that mediate light-induced clock resetting are not well understood.
To define the neurochemical signaling pathways responsible for communicating light to the clock it is essential to know the properties of light that are critical for entrainment. Two alternative theories of entrainment have stimulated intensive investigation into how light entrains circadian clocks for over 50 years (for a review see (Roenneberg et al., 2010). The “parametric” theory proposes that clocks entrain to the day-night cycle because light acts in a sustained manner to alter the clock’s speed. Alternatively, the “non-parametric” theory proposes that the phase of the clock is instantaneously shifted by acute changes in light. Because the non-parametric theory has been considered to be the most successful in predicting the properties of entrainment, investigation of the mechanisms mediating light-induced phase shifts within the SCN has focused on acute rather than sustained neurochemical processes.
Although light is communicated to the SCN by the acute activation of glutamate receptors (Colwell and Menaker, 1995;Mintz et al., 1999;Novak and Albers, 2002) recent evidence indicates that brief light exposure can produce sustained changes in neuronal activity within the SCN. Studies employing reduced preparations of the circadian system in transgenic mice (i.e., SCN brain slices) indicate that a brief light pulse triggers increases in neuronal firing and Period (Per) gene expression in SCN neurons that last for more than four hours (Kuhlman et al., 2003;LeSauter et al., 2011). Because GABA is contained in nearly all SCN neurons (Moore and Speh, 1993;van den Pol, 1986) the sustained release of GABA over several hours in response to acute stimulation by light could be responsible for inducing phase shifts in the clock by the sustained activation of GABA receptors. If so, this would represent the first example of a functionally significant response produced by the activation of ionotropic receptors over an interval of hours instead of seconds. In the following experiments, we tested the hypothesis that the sustained activation of GABAA receptors in the SCN over several hours mediates the ability of light to induce phase delays in the fully intact circadian system.
Materials and Methods
Subjects
Adult male Syrian hamsters (Mesocricetus auratus; 110g upon arrival; Charles River Laboratories, Wilmington, MA) were individually housed in polycarbonate cages (20 × 40 × 20 cm) in a 14h:10h light-dark (LD) cycle with ad libitum access to food and water. All procedures were in accordance with the National Institutes of Health Guidelines for the use of animals and were approved by the Institutional Animal Care and Use Committees at Georgia State University or Morehouse School of Medicine.
Stereotaxic Surgery
Seven to 10 days after arrival, hamsters were anesthetized with sodium pentobarbital (90mg/kg) and stereotaxically implanted with guide cannula (26ga, 11mm) aimed at the SCN region (AP=+0.9mm to bregma; ML=1.7mm to bregma; DV=2.2mm to dura; angled medially 10°). As a result, when the needle was inserted into the guide cannula the needle tip was positioned at the lateral border of the SCN. Guide cannulas were affixed to the skull using stainless steel screws and Ortho-Jet dental acrylic (Lang Dental, Wheeling, IL). Buprenorphine (.05mg/kg) was delivered as an analgesic immediately before and 8–12 hours after surgery.
SCN Injections
Hamsters were gently restrained by hand and received microinjections over a 10 second interval under dim red light (<5 lux). The 33ga microinjection needle (PlasticsOne, Roanoke, VA) was attached by polyethylene tubing to a 1µL syringe (Hamilton Co., Reno, NV). The 16mm injection needle extended 5.2mm beyond the guide cannula and a total of 7.4mm ventral to dura. Injection needles were removed 20 seconds after injection and animals immediately returned to their home cage. The GABAA agonist muscimol and the GABAA antagonist bicuculline methbromide were purchased from Sigma-Aldrich (St. Louis, MO) and dissolved into 0.9% saline. 100nL drug injections were delivered at the concentrations indicated in the results section. In all within-subjects experiments the order of drug administration was counterbalanced.
General Data Collection and Analyses
After a 3 to 5 day recovery period cages were fitted with running wheels (16cm diameter) and animals were allowed to entrain to a 14h:10h LD cycle. After verifying entrainment, lights were disabled during the dark phase and remained off for the remainder of the experiment. Injections and light pulses (15 minute duration, 150 lux given at CT13.5) were administered no less than two weeks following release into constant darkness (DD). Behavioral data collection continued for a minimum of 10 days following the final treatment. By convention, circadian time (CT) was used as a marker of circadian phase in DD with CT12 defined as the onset of wheel running activity.
Running wheel data were collected, recorded, and stored in 10-minute bins by ClockLab software (Actimetrics, Wilmette, IL). Daily activity onset was calculated automatically by ClockLab, defined as the beginning of a 6-hour period of high activity following a 6-hour period of inactivity. Circadian phase was estimated by fitting a regression line through daily onsets of activity for the 7 days before and days 4 through 10 after treatment (treatment day = day 0; the first 3 days following treatment were excluded to circumvent unstable onsets). The magnitude of each phase shift was calculated by measuring the difference between the onsets of activity on the day of treatment predicted from the two regression estimates (Daan and Pittendrigh, 1976).
Localization of Injection Site
At the completion of testing animals received an overdose of sodium pentobarbital and 100nL intracranial injection of india ink to mark the drug injection site. 100µm sections were collected through the SCN, stained with cresyl violet, and examined using a light microscope to verify the site of injection. Only data collected from animals receiving drug injections within 500µm of the SCN were included in subsequent data analyses.
Results
Does the sustained activation of GABAA receptors in the SCN mimic the phase-shifting effects of light during early subjective night?
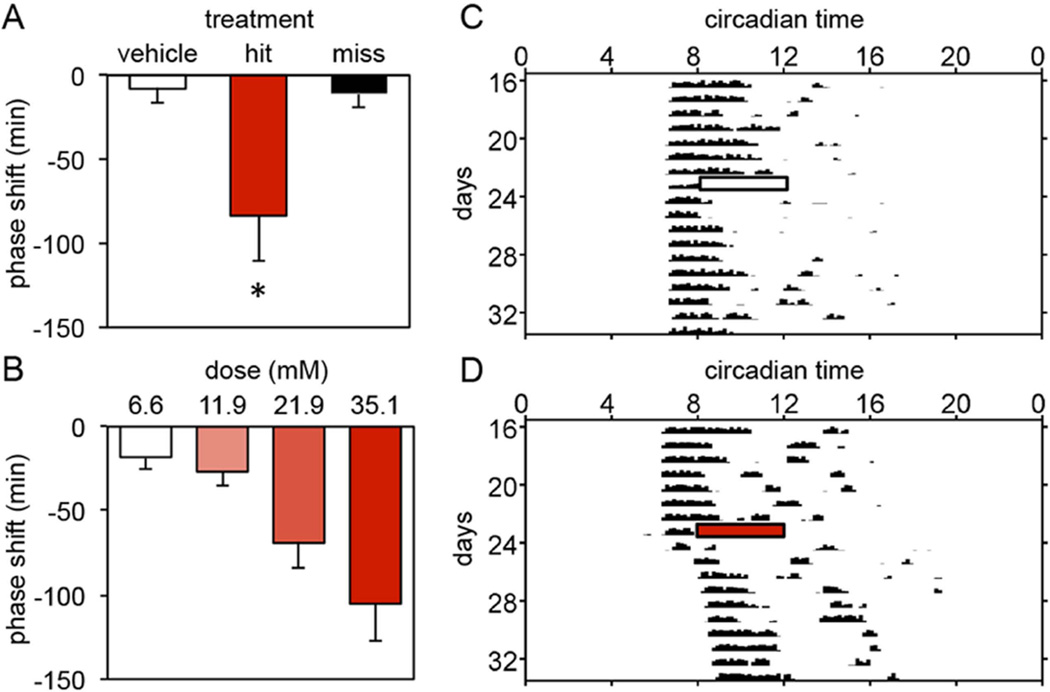
To first examine whether the sustained activation of GABA receptors in the SCN over several hours mediates the ability of light to induce phase delays, hamsters were given 4 consecutive hourly injections of the GABAA agonist muscimol (21.9mM) or vehicle into the SCN region beginning at CT13.5. CT13.5 was chosen because light provided at that phase produces maximal phase delays in Syrian hamsters (Daan and Pittendrigh 1976). The magnitude of drug-induced phase shifts was compared between 3 treatment groups: vehicle (n=7), hits (histologically confirmed injections into the SCN region, n=8), and misses (histologically confirmed injections outside the SCN region, n=5). The group of hamsters injected with muscimol and subsequently confirmed to have injection sites in the SCN area exhibited large phase delays in free-running activity (N=8; −83.5±27.3min) when compared to the group injected with vehicle (N=7; −8.0±8.3min) (One-way ANOVA, F(2,17)=4.910, p=0.021; Dunnett post-hoc test, p=0.023). Phase shifts exhibited by a group injected with muscimol and subsequently confirmed to have injection sites outside the SCN area (N=5; −10.8±8.7min) did not differ from vehicle controls (Figure 1A).
Figure 1.
(A) Mean±SE of phase delays produced by 4 hourly injections of muscimol (21.9mM) into the SCN region between CT13.5 and CT16.5 (hit = animals receiving injections of muscimol within 500µm of the SCN; miss = animals receiving injections of muscimol more than 500µm from the SCN; *, p=0.021, vs. vehicle controls). (B) Mean±SE of phase delays produced by 4 hourly injections of muscimol (doses ranged from 6.6mM to 35.1mM) into the SCN region between CT13.5 and CT16.5. The magnitude of phase delays was dose-dependent (p=0.001). (C, D) Representative activity records demonstrating the effect of 4 hourly injections of vehicle (C) or muscimol (21.9mM) (D) into the SCN region between CT13.5 and CT16.5 on wheel running rhythms in DD. Bars depict the 4-hour injection period (white: saline; shaded: muscimol).
In a second experiment we confirmed that the sustained administration of muscimol induced significant phase delays using a within-subjects design. Hamsters were given 4 consecutive hourly injections of muscimol (21.9mM) or vehicle into the SCN region beginning at CT13.5 in a counterbalanced order (N=5). Muscimol treatment resulted in large phase delays (−129.8±21.9min) in the free-running activity rhythm when compared to vehicle controls (−4.4±13.9min) (paired samples t-test, t(4)=10.581, p<0.001; data not shown).
Are the effects of sustained GABAA receptor activation in the SCN dose-dependent?
To determine whether the sustained effects of muscimol are dose-dependent hamsters were given 4 consecutive hourly injections of muscimol into the SCN region at one of 4 concentrations: 6.6mM (N=6), 11.9mM (N=8), 21.9mM (N=8), or 35.1mM (N=7). The size of the phase shift significantly increased with the injection of increasing doses of muscimol (One-way ANOVA, F(3,25)=7.121, p=0.001). The highest dose of muscimol produced phase delays of nearly two hours (Figure 1B).
What is the duration of GABAA receptor activation in the SCN necessary to induce phase delays?
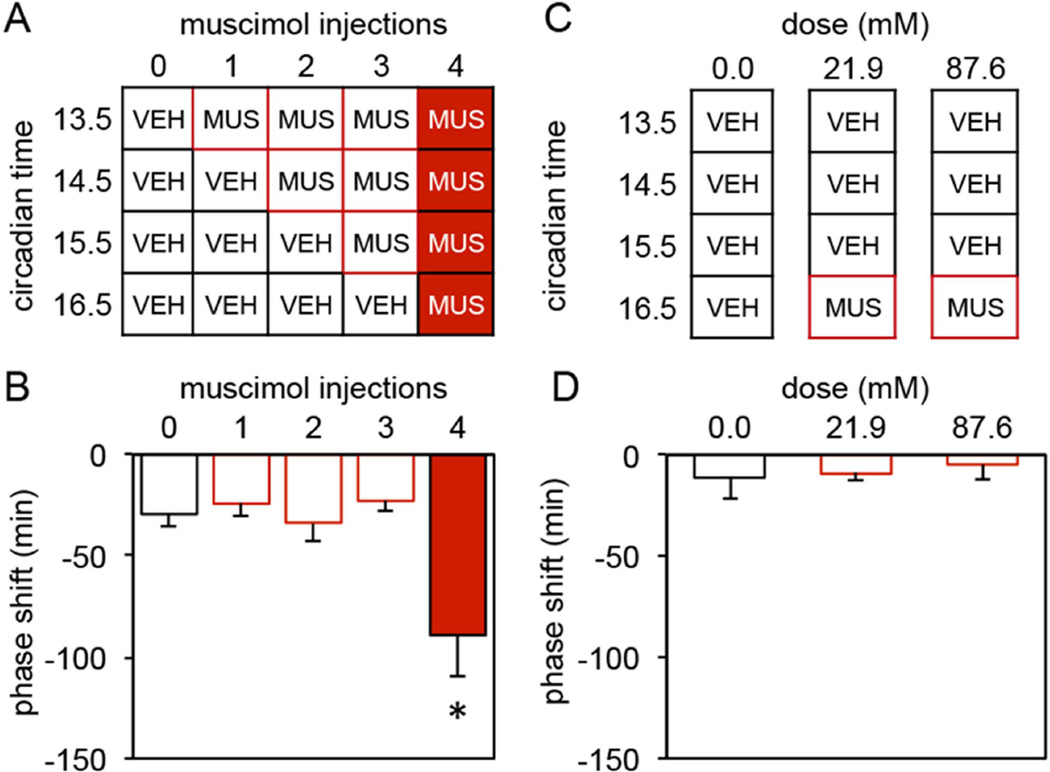
To determine the duration of GABAA receptor activation required to induce a phase delay hamsters were given between 0 to 4 consecutive hourly muscimol (21.9mM) injections into the SCN region beginning at CT13.5 as shown in Figure 2A (N=7–8/group). The size of the subsequent phase shift depended on the number of injections of muscimol administered (One-way ANOVA, F(4,33)=6.350, p=0.001). Only the group receiving 4 hourly injections of muscimol exhibited statistically significant phase delays (−89.1±20.8min) (vs. 0 muscimol injections, −29.4±6.5min, Dunnett post-hoc test, p=0.002, Figure 2B).
Figure 2.
(A) Injection regimen used to determine the duration of GABAA receptor activation necessary to induce a phase delay. All groups received a series of 4 hourly injections into the SCN region between CT13.5 and CT16.5. However, the number of consecutive injections containing muscimol (21.9mM) varied from 0 to 4 (VEH = vehicle; MUS = muscimol). (B) Mean±SE of phase delays produced by the 5 treatments outlined in A (* vs. 0 muscimol injections, p=0.002). (C) Injection regimen used to determine whether a single injection of muscimol at CT 16.5 might induce a phase delay or whether a single injection equal to the cumulative dose of 4 individual injections of muscimol might induce a phase delay. Animals in the 0.0 mM group received 4 hourly injections of vehicle into the SCN region between CT13.5 and CT16.5. Animals in the 21.9 and 87.6mM groups received 3 consecutive hourly injections of vehicle followed 1 hour later by a single injection of 21.9mM or 87.6mM muscimol into the SCN region (VEH = vehicle; MUS = muscimol). (D) Mean±SE of phase delays produced by the 3 treatments outlined in C.
The previous experiment demonstrated that 4, but not 1, 2 or 3 hourly injections of muscimol begun at CT13.5 induced large phase delays. While these data support the hypothesis that the duration of GABAA activation necessary for the induction of phase delays is between 3–4 hours, they do not exclude the possibility that either (1) a single injection of muscimol at CT16.5 might induce a phase delay or (2) a single injection equal to the cumulative dose of 4 individual injections of muscimol might induce a phase delay. We investigated each of these alternative possibilities using the same basic protocol as in the previous experiment. In this case, a group receiving 4 consecutive injections of vehicle (CT13.5–16.5, N=10) was compared to groups administered 3 vehicle injections (CT13.5–15.5) followed by injection of muscimol (21.9mM, N=8 or 87.6mM, N=6) at CT16.5 (see Fig 2C). The phase delays resulting from these treatments were small and did not vary by group (One-way ANOVA, F(2,21)=0.179, p=0.837, Figure 2D).
Taken together, these data provide strong support for the hypothesis that activation of GABAA receptors in the SCN for more than 3 hours is sufficient to induce phase delays. The following experiments investigated whether the sustained activation of GABAA receptors is necessary for light to induce phase delays.
Can the sustained inhibition of GABAA receptors in the SCN inhibit the ability of light to induce phase delays?
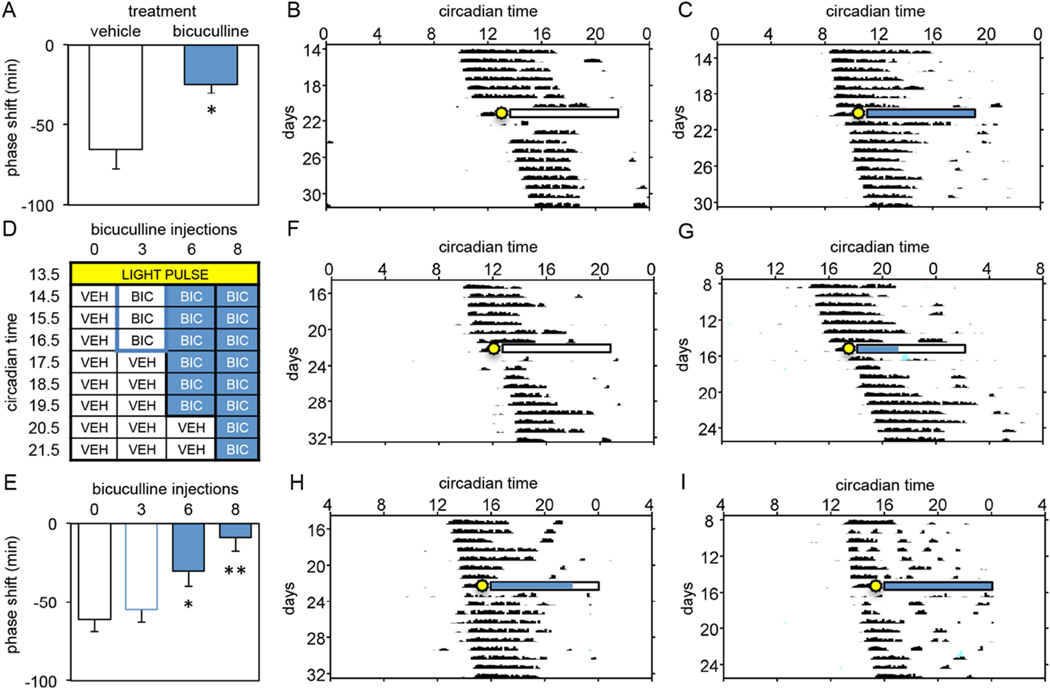
This experiment investigated whether inhibition of GABAA receptors for 8 consecutive hours following a 15-minute light pulse given at CT13.5 would inhibit the production of phase delays. Hamsters were given hourly injections of the GABAA antagonist bicuculline (0.9mM) (N=6) or vehicle (N=8) for 8 consecutive hours beginning one hour after the onset of the light pulse (i.e., CT14.5). Bicuculline-treated hamsters displayed significantly smaller light-induced phase delays (−24.6±5.6min) compared to vehicle-injected controls (−65.4±12.2min) (independent samples t-test, t(12)=−3.043, p=0.013, Figure 3A–C).
Figure 3.
(A) Mean±SE of phase delays produced by a light pulse (15 min, 150 lux) at CT13.5 followed by 8 hourly injections of vehicle or bicuculline (0.9mM) into the SCN region between CT14.5 and CT21.5 (*p=0.013). (B, C) Representative activity records demonstrating the effect of 8 hours of vehicle (B, white bar) or bicuculline (C, shaded bar) administration on light-induced phase delays in DD (sun blast = light pulse). (D) Light pulse and injection regimen used to determine whether the ability of light to induce phase delays requires the sustained activation of GABAA receptors in the SCN. All animals received a light pulse (15 min, 150 lux) at CT13.5 followed by 8 hourly injections into the SCN region between CT14.5 and CT21.5. The number of consecutive injections containing bicuculline (0.9mM) varied from 0 to 8 (VEH = vehicle; BIC = bicuculline). (E) Mean±SE of phase delays produced by each of the treatment regimens outlined in D (*, p=0.035; **, p<0.001, vs. 0 inj controls). (F–I) Representative activity records demonstrating the effect of 0 (F, −96 min), 3 (G, −50 min), 6 (H, 19 min), and 8 hourly injections of bicuculline (I, −14 min) on light-induced phase delays in DD (sun blast = light pulse). White bars and shaded bars indicate times during which vehicle and bicuculline were administered, respectively.
What is the duration of GABAA receptor inhibition in the SCN necessary to inhibit light-induced phase delays?
This experiment investigated whether inhibition of GABAA receptors for 0, 3, 6 or 8 consecutive hours would inhibit light-induced phase delays. Hamsters were exposed to a 15-minute light pulse at CT13.5 and then were given bicuculline as described in Figure 3D (N=10/group). As seen in the previous experiment, 8 hours of GABAA inhibition inhibited light induced phase delays. In addition, 6 but not 3 hourly injections of bicuculline significantly reduced light-induced phase delays when compared to 8 hourly injections of vehicle (One-way ANOVA, F(3,36)=8.026, p=0.001; Dunnett post-hoc tests: 0h vs. 6h, p=0.035; 0h vs. 8h, p<0.001; Figure 3E–I). These data indicate that inhibiting GABAA receptors for 6 but not 3 hours beginning one hour after the onset of the light pulse inhibits the ability of light to induce phase delays. Therefore, the sustained activation of GABAA receptors for 3 hours between CT 14.5 and CT 17.5 is not sufficient for light to induce a phase delay. In contrast, the sustained activation of GABAA receptors for 6 hours between CT 14.5 and CT 20.5 is necessary for light to induce a phase delay.
When must the 6-hour inhibition of GABAA receptor activation in the SCN occur to inhibit light-induced phase delays?
In this experiment, we investigated whether the sustained inhibition of GABAA receptor activation for 6 hours must begin at CT14.5 to inhibit light-induced phase delays, or whether the sustained inhibition of GABAA receptor activation for 6 hours at other phases after the light pulse is sufficient to inhibit light-induced phase delays. Hamsters were given 6 consecutive hourly injections of bicuculline beginning at CT17.5 or CT23.5. Six injections of bicuculline into the SCN region beginning at CT17.5 significantly reduced light-induced phase delays (N=8; −43.4±10.9min) when compared to controls (N=8; −75.5±10.2min)(independent samples t-test, t(14)=−2.161, p=0.049). In contrast, there was no significant difference in the size of light-induced phase delays exhibited by hamsters receiving 6 consecutive injections of bicuculline beginning at CT23.5 (N=9; −43.1±8.4min) and vehicle controls (N=13; −56.6±6.6min)(independent samples t-test, t(20)=−1.281, p=0.215). These data indicate that 6 hours of GABAA receptor activation is necessary for light-induced phase delays either between CT14.5 and CT20.5 or between CT17.5 and CT23.5, suggesting that duration of GABAA receptor activation and not its precise timing within this window is critical for the induction of a light-induced phase delay. Further, these data suggest that a light pulse given at CT13.5 induces sustained activation of GABAA receptors from CT14.5 throughout the remainder of the subjective night or possibly longer and that the inhibition of sustained GABAA receptor activation anytime within this window can inhibit light-induced phase delays. In contrast, GABAA receptor activation from CT23.5 and beyond does not appear to be involved in mediating light-induced phase delays. Taken together, these data indicate that the sustained activation of GABAA receptors necessary for phase delaying the clock occurs during a window beginning around CT14.5 and ending sometime after CT 23.5.
Does the sustained administration of bicuculline in the SCN inhibit light-induced phase delays by inducing phase advances?
In the previous experiment 6 or more hourly injections of bicuculline significantly reduced the magnitude of light-induced phase delays. In this experiment we investigated whether the sustained administration of bicuculline directly inhibits the ability of light to induce phase delays or whether bicuculline counteracts light-induced phase delays indirectly by producing phase advances. No significant differences were observed between the phase shifts exhibited by hamsters given 6 hourly injections of vehicle (N=8; −5.4±4.0min) vs. bicuculline (N=7; −22.5±8.3min) in the absence of a light pulse (independent samples t-test, t(13)= 1.858, p=0.097), thus indicating that bicuculline inhibits the ability of light to induce phase delays rather than counteracting phase delays by inducing phase advances.
Is there a critical phase between CT14.5 and CT21.5 during which activation of GABAA receptors in the SCN is necessary for light to induce phase delays?
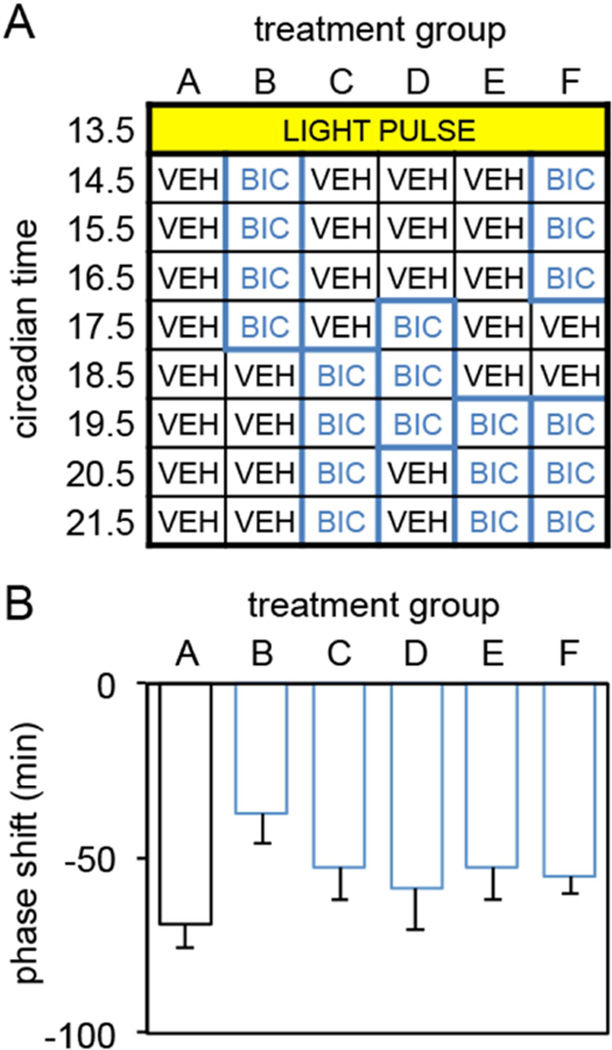
The data from the previous experiments suggest that a light pulse given at CT13.5 induces sustained activation of GABAA receptors from CT14.5 throughout the remainder of the subjective night or possibly longer and that the inhibition of sustained GABAA receptor activation for at least 6 hours anytime within this window can inhibit light-induced phase delays. It remains possible, however, that there is some critical phase within this window where GABAA receptor activation phase delays the clock. In this experiment we tested this possibility by injecting bicuculline for 3, 4 or 6 hours in different temporal patterns between CT14.5 and CT21.5 (see Figure 4A). No significant differences in the size of light-induced phase delays were observed between vehicle controls and any of the temporal patterns of bicuculline administration tested (One-way ANOVA, F(5,63)=1.510, p=0.199; Figure 4B). These data indicate that there is not a critical phase between CT14.5 and CT21.5 during which the sustained activation of GABAA receptors is necessary for light to induce phase delays. Interestingly, administration of bicuculline between CT14.5 – CT16.5 and CT19.5 – CT21.5 did not inhibit light-induced phase delays even though these hamsters received 6 hourly injections of bicuculline (Figure 4B). The reason why 6 consecutive hourly injections of bicuculline consistently inhibited light-induced phase delays but 6 hourly injections interrupted by a two-hour window did not is not known. This finding does suggest, however, that the sustained activation of GABA receptors does not have to occur continuously to induce a phase delay suggesting the possibility that an hourglass type of mechanism might mediate the effects of light on the clock.
Figure 4.
(A) Light pulse and injection regimen used to determine whether the inhibition of GABAA receptors during various phases between CT 14.5 and CT 21.5 would inhibit light-induced phase delays. All groups received a light pulse (15 min, 150 lux) at CT13.5 followed by 8 hourly intracranial injections containing either vehicle or bicuculline (0.9mM) into the SCN region between CT14.5 and CT21.5. Animals received one of 6 injection regimens (VEH = vehicle; BIC = bicuculline) (N’s: A=19, B=7, C=6, D=10, F=17. (B) Mean±SE of phase delays produced by the various injection regimens outlined in A.
Interestingly, there was a significant negative correlation (Spearman’s, rs=−0.48; p<0.001) between the duration of bicuculline administration and the magnitude of phase shifts produced across all experiments where repeated injections of bicuculline were administered.
Taken together, these data indicate that 3 or more hours of sustained activation of GABAA receptors is sufficient to phase delay the circadian clock and that the sustained activation of GABAA receptors is necessary for light to phase delay the circadian clock.
Histological analysis of the sites of SCN injection
Histological analysis of injection sites was conducted in all animals. Only hamsters with injections sites that surrounded the SCN, but that did not damage the SCN or enter the third ventricle, were included in the data. The administration of 8 hourly injections produced minimal brain damage comparable to that produced by a single injection (see Figure 5).
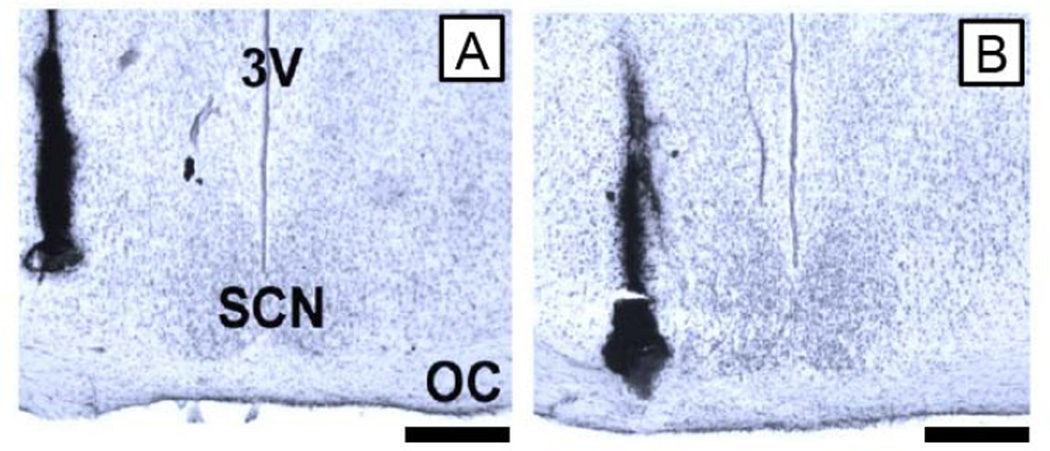
Figure 5.
Illustration of the minimal amount of damage produced by multiple injections into the SCN region as compared to the damage produced by single injections. (A) Photomicrograph from an animal that received a single injection into the SCN region. (B) Photomicrograph from a representative animal that received 8 hourly injections into the SCN region. In each case, a viscous ink was microinjected via the guide cannula to mark the drug injection track; it does not indicate the spread of drugs during experiments. Scale bars are 300µm.
Discussion
These data support the hypothesis that the sustained activation of GABAA receptors in the SCN is sufficient to induce phase delays and that sustained activation of GABAA receptors is necessary for light to phase delay the clock (Figure 6). Three consecutive hourly injections of muscimol was ineffective in producing phase delays while four consecutive hourly injections of muscimol increased phase delays by three-fold. While it is not possible to determine the exact duration of GABAA activation necessary to delay the clock, these data suggest that the duration may be between 3–5 hrs depending on the duration of muscimol’s effects within the SCN. These findings provide the first evidence of a functionally significant response produced by the activation of ionotropic receptors over an interval of hours instead of seconds.
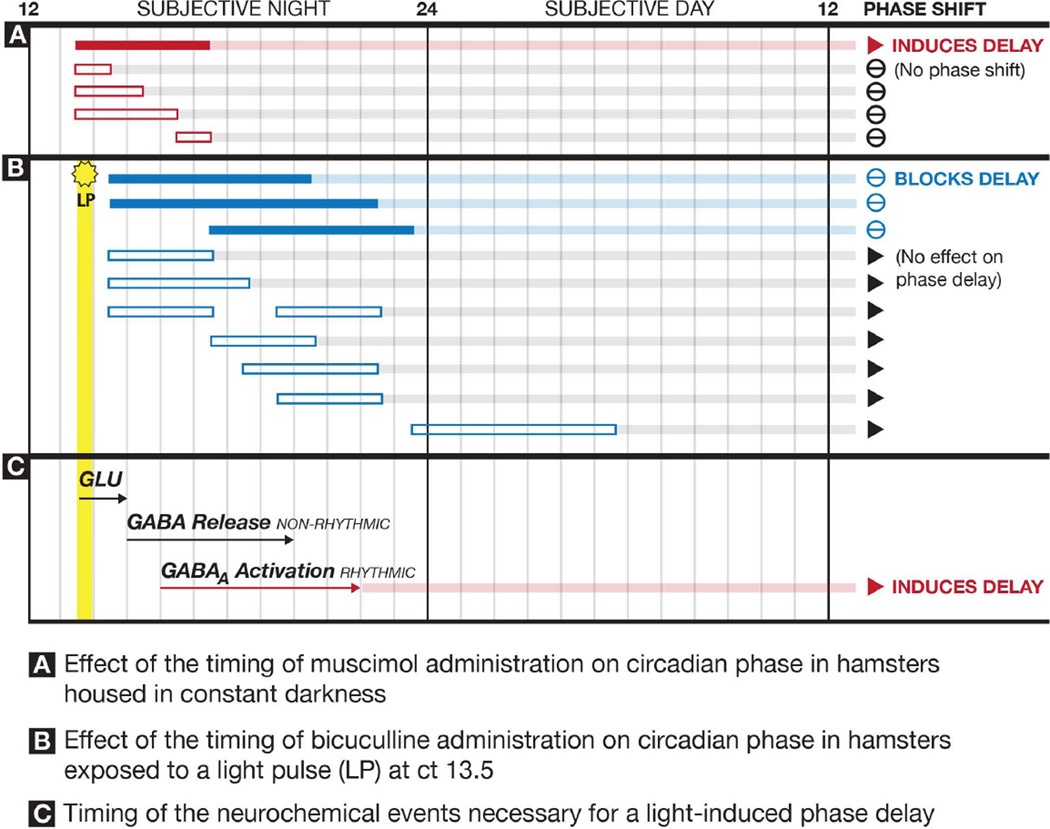
Figure 6.
Summary of the effects of over 1700 injections containing muscimol, bicuculline or vehicle into the SCN region on the phase of circadian rhythms. A. Solid red bar indicates the timing of SCN injections in which muscimol induces a significant phase delay in the locomotor rhythm. Open red bars indicate the timing of SCN injections of muscimol that did not produce significant phase delays. B. Solid blue bars indicate the timing of SCN injections in which bicuculline significantly inhibited light-induced phase delays. Open blue bars indicate the timing of SCN injections of bicuculline that did not significantly inhibit light-induced phase delays. Yellow bar indicates the timing of the 15-minute light pulse. C. Proposed sequence of neurochemical events within the SCN necessary for a light pulse to induce a phase delay. Light induces release of glutamate (GLU) that activates NMDA receptors within the SCN for seconds and possibly minutes (initial transient response). The transient responses to light induce activity in non-rhythmic SCN neurons that begins 30–60 minutes after the light pulse resulting in the sustained release of GABA for 6 or more hours. The sustained GABA release from non-rhythmic neurons results in the sustained activation of GABAA receptors on rhythmic SCN neurons producing a phase delay in the molecular clock mechanisms.
These data also provide the first neurobiological evidence that the circadian clock in the SCN is not instantaneously phase shifted by light, but rather requires hours of GABAA activation to induce a phase delay. As such, these findings do not support the theory of non-parametric entrainment. Rather, they indicate that light pulses are transduced into sustained signals that must last hours for the clock to be phase delayed. The relationship between the data of the present study and the predictions of the theory of parametric entrainment are not as clear. On the one hand, no relationship was observed between the duration of muscimol administration and the magnitude of phase shifts. On the other hand, a significant negative relationship was observed between the duration of bicuculline administration and the magnitude of phase shifts. These data raise numerous intriguing questions regarding how such a light transduction mechanism might contribute to the many well known effects of light on circadian timing (e.g., light intensity effects, aftereffects, etc.). Recognition that neither the parametric nor the non-parametric theory can predict all the circadian responses to light has led to new concepts of entrainment that may prove to be more effective for understanding the neurobiological processes underlying entrainment such as those observed here (Roenneberg et al., 2010).
The sustained effects of GABAA agonists and antagonists contrast considerably with their effects when given acutely. Single injections of GABAA agonists into the SCN of hamsters and diurnal grass rats free-running in constant darkness do not mimic the phase shifting effects of light (Smith et al., 1989;Novak and Albers, 2004;Novak et al., 2004;Gillespie et al., 1996;Gillespie et al., 1997). In contrast, single injections of GABAA agonists into the SCN profoundly inhibit the ability of light to induce phase shifts. Injection of a GABAA agonist into the SCN of hamsters significantly inhibits phase delays and the induction of c-Fos when given just prior to a brief light pulse presented at CT13.5. The inhibition of light-induced phase shifts by activation of GABAA receptors is not the result of simply inhibiting the release of glutamate in the SCN in response to light, because GABAA agonists also inhibit the ability of NMDA to induce light-like phase shifts (Mintz et al., 2002). Finally, the acute administration of GABA agonists can significantly inhibit the ability of light to induce Per mRNA within the SCN (Novak et al., 2006;Ehlen et al., 2008). Taken together these data indicate that acute activation of GABAA receptors in the SCN profoundly inhibits the ability of light to induce phase shifts when given just prior to a light pulse and may do so by reducing the levels of Per gene product (Figure 7).
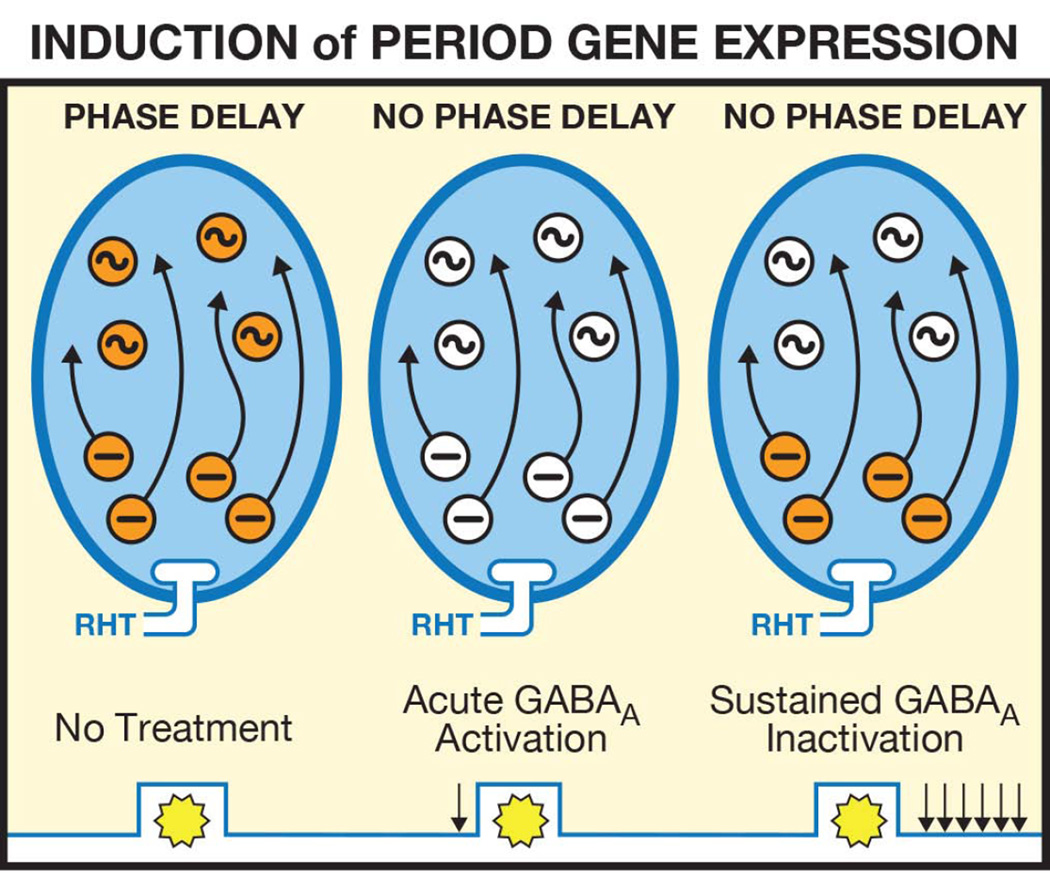
Figure 7.
Proposed regulation of the phase of the circadian clock and Period (Per) gene expression in the SCN by GABAA receptor activation and inactivation. Left Panel: As described in Figure 6, light results in glutamate release from the retinohypothalamic tract (RHT). In response, there is a sustained release of GABA from, as well as a sustained induction of Per in, non-rhythmic neurons. In response, there is a sustained activation of GABAA receptors and a sustained induction of Per in rhythmic neurons resulting in a phase delay of the circadian clock. Middle Panel: Acute activation of GABAA receptors by injection of muscimol prior to a light pulse inhibits light induction of the sustained release of GABA from, as well as an inhibition of Per induction in, non-rhythmic neurons. Acute activation of GABAA receptors inhibits NMDA-induced phase delays suggesting that activation of GABAA receptors does not inhibit light-induced phase delays solely by inhibiting light-induced glutamate release (Mintz et al., 2002). Acute activation of GABAA receptors ultimately blocks light-induced phase delays by preventing Per induction in rhythmic neurons. Right Panel: Sustained inhibition of GABAA receptors by at least six hourly injections of bicuculline following a light pulse blocks light-induced phase delays by inhibiting Per induction in rhythmic neurons.
GABAA receptors are pentameric hetero-oligomers that can be composed of at least 19 distinct subunits (for a review see Farrant and Nusser, 2005). The assembly of GABAA receptors with different combinations of subunits produces receptors with different cellular locations and a complex heterogeneity in their pharmacological properties. The GABAA receptor subtype(s) that mediate the sustained effects of GABA observed in the present study are not known. Perhaps the most fully characterized GABAA receptor subtype contains the γ receptor subunit. Activation of these “GABAA-PHASIC” receptors produce IPSCs that peak and decay within milliseconds and display rapid desensitization. More recently, GABAA receptors that contain the δ subunit have been identified and found to have considerably different properties. These “GABAA-TONIC” receptors respond to low, ‘tonic’ levels of GABA, display low levels of desensitization and are found in extra-synaptic regions. The hamster SCN contains several GABAA receptor subunits including the γ and δ subunits suggesting that both GABAA-PHASIC and GABAA-TONIC receptors are contained within the nucleus (Walton et al., 2014;Naum et al., 2001). Pharmacological studies have found that acute administration of a GABAA-TONIC agonist inhibits light-induced phase shifts during the subjective night, but does not mimic the phase advances produced by muscimol at CT6 (Ehlen and Paul, 2009). In contrast, acute administration of a GABAA-PHASIC agonist phase advances the clock at CT6 but does not reduce phase delays induced by glutamate during the subjective night (McElroy et al., 2009). Thus, both GABAA-PHASIC and GABAA-TONIC receptors appear to be active within the SCN and may mediate different circadian functions. At present, the molecular structure of the GABAA receptors that mediate the sustained effects of GABA reported here is not known.
The initial response of the SCN to light is an acute activation of glutamate receptors in retino-recipient neurons. These transient responses induce activity in a subset of SCN neurons that begins approximately 30–60 minutes after the light pulse and persists for at least 4–5 hours as indicated by measures of neuronal firing and induction of Per gene expression (Kuhlman et al., 2003;Hamada et al., 2004). Following this initial sustained response Per expression increases in a different subset of SCN neurons beginning approximately 90 minutes after the light pulse and persists for 6 or more hours. The present findings strongly support our hypothesis that the sustained activation of GABAA receptors within the SCN is necessary for light to induce phase delays of the clock and suggests a potential mechanism for the sustained induction of Per expression.
Studies in hamsters have led to the hypothesis that the pacemaker in the SCN is composed of two compartments, one rhythmic and the other non-rhythmic based on the findings that some SCN neurons display endogenous rhythmicity in the expression of specific clock genes, while in other SCN neurons these clock genes are not expressed rhythmically but are induced by light exposure (Hamada et al., 2001). Taken together, the existing data suggest the hypothesis that the sustained firing produced by light results in the sustained release of GABA from photically responsive, but non-rhythmic SCN neurons for six or more hours, and that this sustained GABA release activates GABAA receptors contained on rhythmic SCN neurons for several hours. Further, the sustained activation of GABAA receptors up regulates Per gene expression that ultimately causes a phase delay of the molecular clock. A similar mechanism could underlie entrainment in other species where rhythmic and non-rhythmic SCN neurons exist(Yan and Silver, 2002;Yan and Okamura, 2002;Ramanathan et al., 2009), despite species-specific differences in their anatomical distribution within the SCN(Lee et al., 2003;Morin, 2007).
Several lines of evidence support this hypothesis. In rats, in vitro recordings revealed that subsets of SCN neurons can have a strong phase shifting effect on other SCN neurons (Albus et al., 2005). Incubation of SCN tissue with a GABAA antagonist for 20 hours blocked the communication of phase shifting information between different subsets of SCN neurons. More recently, studies in transgenic mice with reduced GABA neurotransmission as a result of the targeted deletion of the gene that encodes the voltage-gated sodium channel type 1 found light-induced phase delays to be significantly reduced (Han et al., 2012). Chronic treatment of the SCN tissue with GABA transmission-enhancing drugs restored the ability of light to induce phase delays. Taken together, these data support our hypothesis that the phase delaying effects of light are mediated by sustained GABA neurotransmission in the SCN (see Figures 6 and 7).
How the sustained activation of GABAA receptors might mediate changes in Per gene expression are not presently known, although alterations in Ca2+ levels are a likely possibility (for a review see Antle et al., 2009). Critical events mediating phase delays include the activation of ryanodine receptors that amplify the Ca2+ release from internal stores followed by Ca2+/calmodulin-dependent protein kinase (CaMK) II-dependent signaling resulting in the induction of Per gene expression. How the sustained activation of GABAA receptors might influence these events is not known. It is clear that GABAA receptor activation can increase, decrease or have no effect on Ca2+ in SCN neurons depending on circadian phase and their location within the nucleus (Irwin and Allen, 2009). The preponderance of evidence suggests that GABA can have excitatory effects in a subset of SCN neurons during the night (Choi et al., 2008). As such, the sustained excitatory effects of GABA might be responsible for the induction of phase delays. However, the sustained administration of the Na+/K+/Cl− cotransporter 1 inhibitor bumetanide which inhibits GABA-induced membrane depolarization in the SCN does not block light-induced delays, but, significantly increases them (McNeill IV et al., 2014).
In summary, our findings indicate that the sustained activation of GABAA receptors within the SCN is necessary and sufficient to mediate light-induced phase delays of the circadian clock. Whether similar mechanisms regulate light-induced phase advances is not known because phase delays and advances are mediated, at least in part, by different neurochemical pathways (Ding et al., 1998).
Acknowledgements
This work was supported by NIH grant R01-NS078220 to HEA and NSF grant IOS-1022050 to DLH. We thank Dr. James Walton for comments on the manuscript.
Abbreviations
- SCN
suprachiasmatic nucleus
- CT
circadian time
- Per
period
Reference List
- Albus H, Vansteensel MJ, Michel S, Block GD, Meijer JH. A GABAergic mechanism is necessary for coupling dissociable ventral and dorsal regional oscillators within the circadian clock. Curr Biol. 2005;15:886–893. doi: 10.1016/j.cub.2005.03.051. [DOI] [PubMed] [Google Scholar]
- Antle MC, Smith VM, Sterniczuk R, Yamakawa GR, Rakai BD. Physiological responses of the circadian clock to acute light exposure at night. Rev Endocr Metab Disord. 2009;10:279–291. doi: 10.1007/s11154-009-9116-6. [DOI] [PubMed] [Google Scholar]
- Choi HJ, Lee CJ, Schroeder A, Kim YS, Jung SH, Kim JS, Kim dY, Son EJ, Han HC, Hong SK, Colwell CS, Kim YI. Excitatory actions of GABA in the suprachiasmatic nucleus. J Neurosci. 2008;28:5450–5459. doi: 10.1523/JNEUROSCI.5750-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen R, Albers HE. Disruption of human circadian and cognitive regulation following a discrete hypothalamic lesion: A case study. Neurol. 1991;41:726–729. doi: 10.1212/wnl.41.5.726. [DOI] [PubMed] [Google Scholar]
- Colwell CS, Menaker M. Regulation of circadian rhythms by excitatory amino acids. In: Brann DW, Mahesh VB, editors. Excitatory amino acids: Their role in neuroendocrine function. New York: CRC Press; 1995. pp. 223–252. [Google Scholar]
- Daan S, Pittendrigh CS. A functional analysis analysis of circadian pacemakers in nocturnal rodents. II. The variability of phase response curves. J Comp Physiol. 1976;106:253–266. [Google Scholar]
- Ding JM, Buchanan GF, Tischkau SA, Chen D, Kuriashkina L, Faiman LE, Alster JM, McPherson PS, Campbell KP, Gillette MU. A neuronal ryanodine receptor mediates light-induced phase delays of the circadian clock. Nature. 1998;394:381–384. doi: 10.1038/28639. [DOI] [PubMed] [Google Scholar]
- Ehlen JC, Novak CM, Karom MC, Gamble KL, Albers HE. Interactions of GABA A receptor activation and light on period mRNA expression in the suprachiasmatic nucleus. J Biol Rhythms. 2008;23:16–25. doi: 10.1177/0748730407310785. [DOI] [PubMed] [Google Scholar]
- Ehlen JC, Paul KN. Regulation of light's action in the mammalian circadian clock: role of the extrasynaptic GABAA receptor. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1606–R1612. doi: 10.1152/ajpregu.90878.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Gillespie CF, Babagbemi TO, Huhman KL, Albers HE. Bicuculline increases and muscimol reduces the phase-delaying effects of light and VIP/PHI/GRP in the suprachiasmatic region. J Biol Rhythms. 1996;11:137–144. doi: 10.1177/074873049601100206. [DOI] [PubMed] [Google Scholar]
- Gillespie CF, Mintz EM, Marvel CL, Huhman KL, Albers HE. GABAA and GABAB agonists and antagonists alter the phase-shifting effects of light when microinjected into the suprachiasmatic region. Brain Res. 1997;759:181–189. doi: 10.1016/s0006-8993(97)00235-7. [DOI] [PubMed] [Google Scholar]
- Hamada T, Antle MC, Silver R. Temporal and spatial expression patterns of canonical clock genes and clock-controlled genes in the suprachiasmatic nucleus. Eur J Neurosci. 2004;19:1741–1748. doi: 10.1111/j.1460-9568.2004.03275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada T, LeSauter J, Venuti JM, Silver R. Expression of Period genes: rhythmic and nonrhythmic compartments of the suprachiasmatic nucleus pacemaker. J Neurosci. 2001;21:7742–7750. doi: 10.1523/JNEUROSCI.21-19-07742.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Yu FH, Schwartz MD, Linton JD, Bosma MM, Hurley JB, Catterall WA, de la Iglesia HO. Na(V)1.1 channels are critical for intercellular communication in the suprachiasmatic nucleus and for normal circadian rhythms. Proc Natl Acad Sci U S A. 2012;109:E368–E377. doi: 10.1073/pnas.1115729109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings MH, Brancaccio M, Maywood ES. Circadian pacemaking in cells and circuits of the suprachiasmatic nucleus. J Neuroendocrinol. 2014;26:2–10. doi: 10.1111/jne.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin RP, Allen CN. GABAergic signaling induces divergent neuronal Ca2+ responses in the suprachiasmatic nucleus network. Eur J Neurosci. 2009;30:1462–1475. doi: 10.1111/j.1460-9568.2009.06944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman SJ, Silver R, Le Sauter J, Bult-Ito A, McMahon DG. Phase resetting light pulses induce Per1 and persistent spike activity in a subpopulation of biological clock neurons. J Neurosci. 2003;23:1441–1450. doi: 10.1523/JNEUROSCI.23-04-01441.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Billings HJ, Lehman MN. The suprachiasmatic nucleus: a clock of multiple components. J Biol Rhythms. 2003;18:435–449. doi: 10.1177/0748730403259106. [DOI] [PubMed] [Google Scholar]
- LeSauter J, Silver R, Cloues R, Witkovsky P. Light exposure induces short- and long-term changes in the excitability of retinorecipient neurons in suprachiasmatic nucleus. J Neurophysiol. 2011;106:576–588. doi: 10.1152/jn.00060.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy B, Zakaria A, Glass JD, Prosser RA. Ethanol modulates mammalian circadian clock phase resetting through extrasynaptic GABA receptor activation. Neuroscience. 2009;164:842–848. doi: 10.1016/j.neuroscience.2009.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill JK, IV, Walton JC, Albers HE. Sustained inhibition of NA+/K+/Cl− co-transporter 1 (NKCC1) enhances the magnitude of light-induced phase delays of the circadian clock. Meeting of the Society for Research on Biological Rhythms. 2014 [Google Scholar]
- Mintz EM, Jasnow AM, Gillespie CF, Huhman KL, Albers HE. GABA interacts with photic signaling in the suprachiasmatic nucleus to regulate circadian phase shifts. Neuroscience. 2002;109:773–778. doi: 10.1016/s0306-4522(01)00519-x. [DOI] [PubMed] [Google Scholar]
- Mintz EM, Marvel CL, Gillespie CF, Price KM, Albers HE. Activation of NMDA receptors in the suprachiasmatic nucleus producers light-like phase shifts of the circadian clock in vivo. J Neurosci. 1999;19:5124–5130. doi: 10.1523/JNEUROSCI.19-12-05124.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic nuclear lesions in the rat. Brain Res. 1972;42:201–206. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- Moore RY, Speh JC. GABA is the principal neurotransmitter of the circadian system. Neurosci Letts. 1993;150:112–116. doi: 10.1016/0304-3940(93)90120-a. [DOI] [PubMed] [Google Scholar]
- Morin LP. SCN organization reconsidered. J Biol Rhythms. 2007;22:3–13. doi: 10.1177/0748730406296749. [DOI] [PubMed] [Google Scholar]
- Naum OG, Fernanda RM, Golombek DA. Rhythmic variation in gamma-aminobutyric acid(A)-receptor subunit composition in the circadian system and median eminence of Syrian hamsters. Neurosci Lett. 2001;310:178–182. doi: 10.1016/s0304-3940(01)02129-2. [DOI] [PubMed] [Google Scholar]
- Novak CM, Albers HE. N-Methyl-D-aspartate microinjected into the suprachiasmatic nucleus mimics the phase-shifting effects of light in the diurnal Nile grass rat (Arvicanthis niloticus) Brain Res. 2002;951:255–263. doi: 10.1016/s0006-8993(02)03168-2. [DOI] [PubMed] [Google Scholar]
- Novak CM, Albers HE. Novel phase-shifting effects of GABAA receptor activation in the suprachiasmatic nucleus of a diurnal rodent. Am J Physiol Regul Integr Comp Physiol. 2004;286:R820–R825. doi: 10.1152/ajpregu.00575.2003. [DOI] [PubMed] [Google Scholar]
- Novak CM, Ehlen JC, Huhman KL, Albers HE. GABA(B) receptor activation in the suprachiasmatic nucleus of diurnal and nocturnal rodents. Brain Res Bull. 2004;63:531–535. doi: 10.1016/j.brainresbull.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Novak CM, Ehlen JC, Paul KN, Fukuhara C, Albers HE. Light and GABA)(A) receptor activation alter period mRNA levels in the SCN of diurnal Nile grass rats. Eur J Neurosci. 2006;24:2843–2852. doi: 10.1111/j.1460-9568.2006.05166.x. [DOI] [PubMed] [Google Scholar]
- Ramanathan C, Campbell A, Tomczak A, Nunez AA, Smale L, Yan L. Compartmentalized expression of light-induced clock genes in the suprachiasmatic nucleus of the diurnal grass rat (Arvicanthis niloticus) Neuroscience. 2009;161:960–969. doi: 10.1016/j.neuroscience.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roenneberg T, Hut R, Daan S, Merrow M. Entrainment concepts revisited. J Biol Rhythms. 2010;25:329–339. doi: 10.1177/0748730410379082. [DOI] [PubMed] [Google Scholar]
- Smith RD, Inouye SIT, Turek FW. Central administration of muscimol phase-shifts the mammalian circadian clock. J Comp Physiol. 1989;164:805–814. doi: 10.1007/BF00616752. [DOI] [PubMed] [Google Scholar]
- Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Science USA. 1972;69:1583–1586. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN. Gamma-aminobutyrate, gastrin releasing peptide, serotinin, somatostatin, and vasopressin: ultrastructural immunocytochemical localization in presynaptic axons in the suprachiasmatic nucleus. Neuroscience. 1986;17:643–659. doi: 10.1016/0306-4522(86)90037-0. [DOI] [PubMed] [Google Scholar]
- Walton JC, McNeill JK, IV, Ross AP, Albers HE. GABA-A receptor ó and ý subunits are expressed in a 24 hour pattern in the suprachiasmatic nucleus of male Syrian hamsters. Society for the Study of Biological Rhythms Meeting. 2014 [Google Scholar]
- Yan L, Okamura H. Gradients in the circadian expression of Per1 and Per2 genes in the rat suprachiasmatic nucleus. Eur J Neurosci. 2002;15:1153–1162. doi: 10.1046/j.1460-9568.2002.01955.x. [DOI] [PubMed] [Google Scholar]
- Yan L, Silver R. Differential induction and localization of mPer1 and mPer2 during advancing and delaying phase shifts. Eur J Neurosci. 2002;16:1531–1540. doi: 10.1046/j.1460-9568.2002.02224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]