Abstract
Angioimmunoblastic T-cell lymphoma (AITL) is the second most common type of peripheral T-cell lymphoma (PTCL) worldwide, and in some countries the most common form. Clinically, AITL usually presents with systemic symptoms, diffuse lymphadenopathy, hepatosplenomegaly and common laboratory abnormalities such as hypergammaglobulinemia. Skin rashes are seen in 50–80% of patients. AITL derives follicular T-helper cells (TFH), that express germinal center markes and produces hyperactivation of B-cell seen in AITL. Although the histologic features of AITL in the skin could be similar to pathologic findings present in lymph node biopsies, we present herein 2 cases of AITL with histologic and immunophenotypic features that were somewhat suggestive of extranodal marginal zone lymphoma (MALT).Caution is urged to exclude the possibility of a systemic T-cell lymphoma such as AITL in cutaneous and lymph node B-cell proliferations.
Background
Angioimmunoblastic T-cell lymphoma (AITL) was initially thought to represent a form of reactive lymphadenopathy with dysproteinemia, as first proposed by Frizzera and Rappaport in 19741–5. Later, Watanabe et al6 hypothesized that AITL was a proliferation of hyperactive B-cells. We now know that AITL is the second most common type of peripheral T-cell lymphoma (PTCL) worldwide, and in some countries the most common form1,7–9. Clinically, AITL usually presents with a constellation of findings that include diffuse lymphadenopathy, hepatosplenomegaly and constitutional symptoms (fever, chills, weight loss, etc). Common laboratory abnormalities include hypergammaglobulinemia, elevated LDH, presence of autoantibodies, and elevated erythrocyte sedimentation rate (ESR)7,8.
Skin rashes are associated with AITL in 50–80% of patients2,10–17. Typically, the rash is morbilliform, and less commonly purpuric, urticarial, nodular or petechial. Pruritus can be seen in up to 84% of cases10. It is now accepted that AITL derives from a population of regulatory T-cells, called follicular T-helper cells (TFH), that express PD1, CD10, BCL6, and CXCL13 and whose normal function is to induce B-cell activation in the germinal center. This explains the hyper activation of B-cell seen in AITL18–20. Although the histologic features of AITL in the skin could be similar to pathologic findings present in lymph node biopsies, we present herein 2 cases of AITL with histologic and immunophenotypic features that were reminiscent of a B-cell lymphoproliferative disorder, such as marginal zone lymphoma (MALT).
Case 1
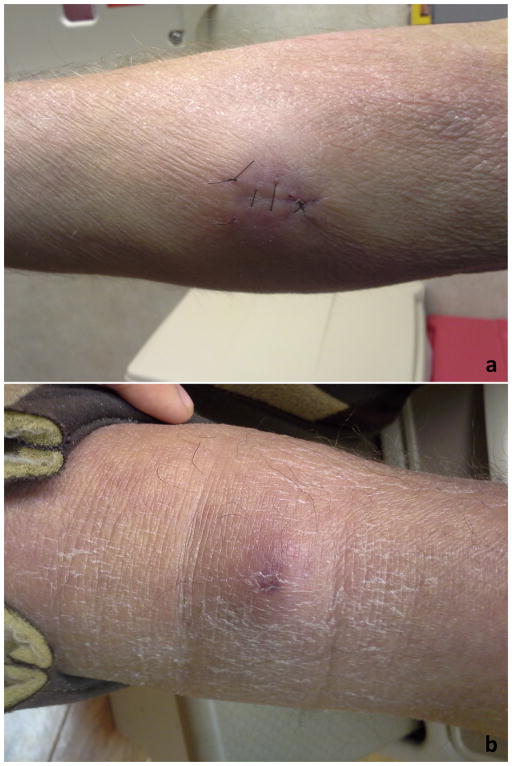
A 59 year-old white male with no previous dermatology history developed generalized erythroderma in September 2012 sparing only his inguinal folds and portions of his thighs (Figure 1a). He was initially seen in January 2013 after several months of symptom control with prednisone tapers. At the time of evaluation, he complained of diffuse and severe itch with prominent dysesthesias. He also had symptoms consistent with Raynaud phenomenon and dilated capillary loops were seen on capillaroscopy. Connective tissue disease was suspected and multiple skin biopsies consistently demonstrated eosinophilic spongiosis. In addition, labs demonstrated a leukocytosis of 20,000 leukocytes/μL with over 1600 eosinophils/μL. Peripheral blood flow cytometry was ordered to better quantitate and characterize his leukocytosis. This demonstrated an abnormal T-cell population with a loss of CD7(46%) and CD26(48%)and a prominently elevated CD4:CD8 ratio(18.3:1).By this time, he had developed prominent epitrochlear and cervical lymphadenopathy and he was referred to the multimodality cutaneous lymphoma clinic. A bone marrow biopsy was performed which revealed a population of T-cells with the same immunophenotypic abnormalities. TCR gene rearrangement analysis showed an oligoclonal population of T-cells. He was suspected to be developing Sezary syndrome versus a primary hypereosinophilic syndrome and was started empirically on bexarotene in May 2013 with substantial improvement of hiserythroderma. JAK2V617 mutation was found to be negative. By July 2013he had developed multiple subcutaneous nodules over his forearms (Figure 1b).
Figure 1.
Figure 1a and 1b. Clinical characteristics of patient #1. 1a shows erythema and scaling of the arm and a biopsy site of a nodule. 1b shows a purple-violaceous nodule on the forearm.
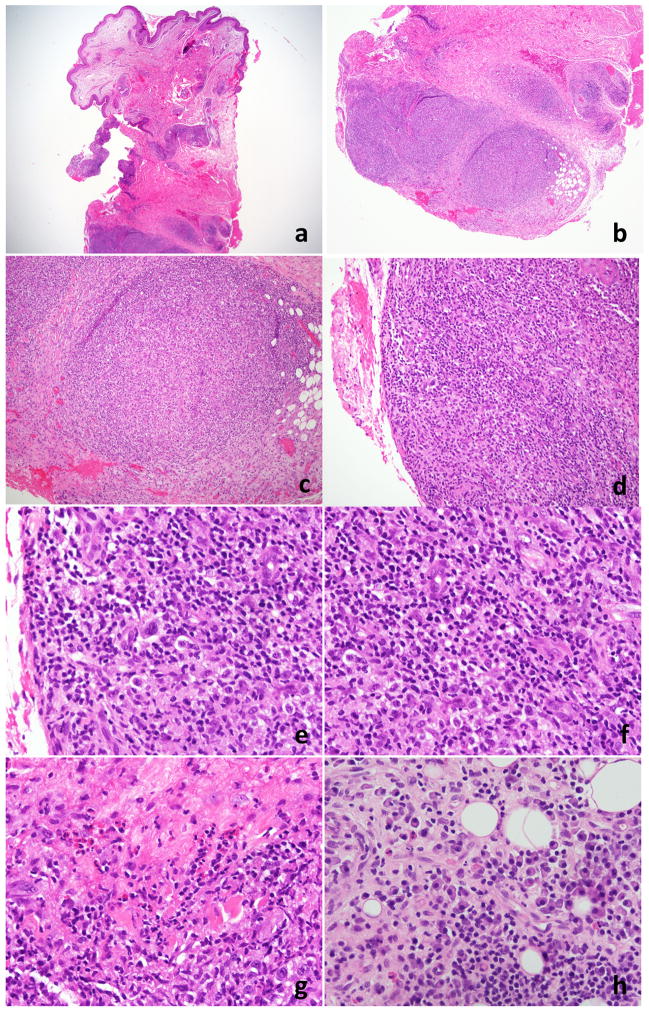
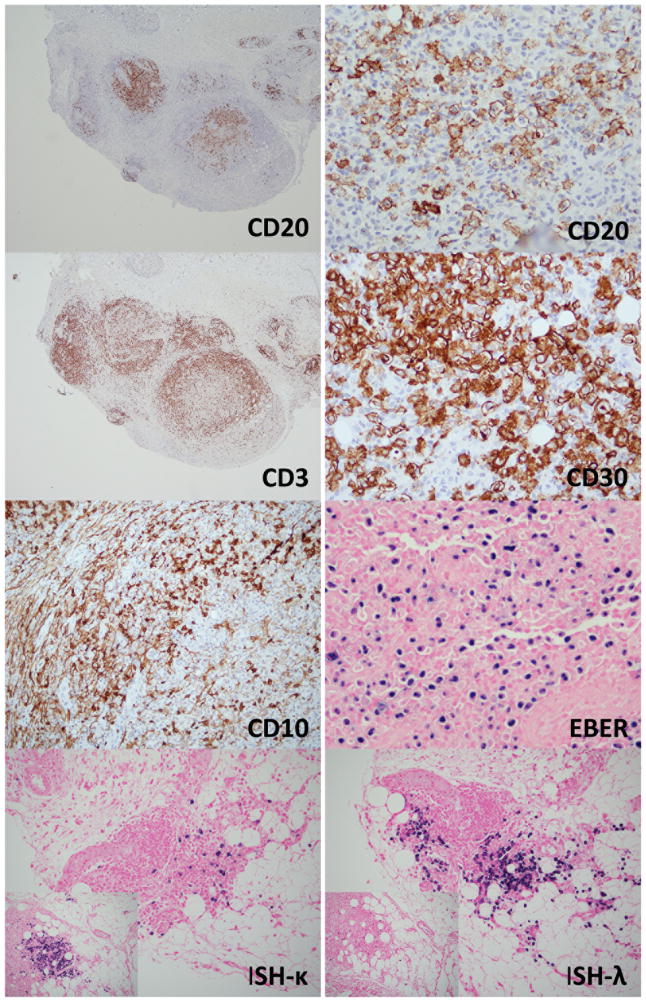
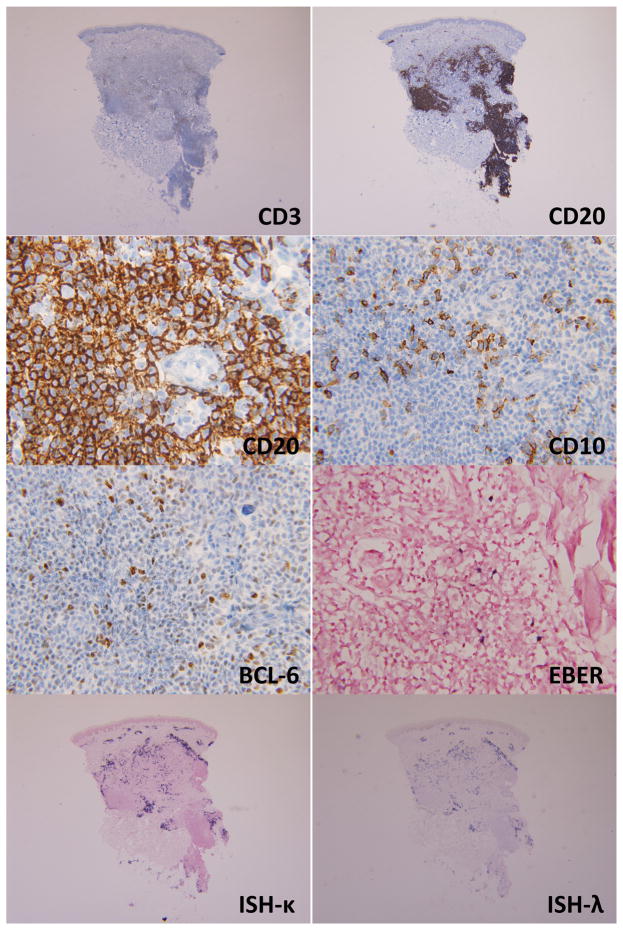
In August 2013, a punch biopsy of a 3 cm tumor on the patient’s right forearm, showed a superficial and deep perivascular and interstitial lymphoid infiltrate with extension into the subcutaneous tissue. A nodular pattern was present and the infiltrate was heavier in the deeper portions of the biopsy (Figure 2). The infiltrate showed a very rich accompanying plasma cell population, composed predominantly of small and mature appearing plasma cells. The lymphoid population was predominantly small to medium in size with some cells showing a plasmacytoid appearance. A larger population of cells with vesicular nuclei and prominent nucleoli was seen. Immunohistochemical stains (Figure 3) showed marked predominance of CD20+B-cells with a smaller component of CD3+ T-cells. CD138 revealed a marked plasmacytosis. In-situ hybridization for kappa and lambda light chains showed an overall polytypic population of plasma cells, with some areas showing kappa light chain predominance and others with the opposite pattern (lambda predominance). The smaller T-cell population showed variable expression of CD10 and BCL6. EBER was positive in scattered lymphoid cells. Molecular studies performed on the skin biopsy showed a clonal population of T-cells and an oligoclonal population of B-cells by IGH gene rearrangement studies. An outside pathologist who reviewed this case entertained a diagnosis of marginal zone lymphoma. A subsequent lymph node biopsy showed effacement of the architecture by a monotonous population of medium sized lymphocytes with scattered intermixed larger cells. A rich arborizing vascular network was seen in the background with very prominent high endothelial venules. Numerous eosinophils and plasma cells in the background were seen. Residual follicles and germinal centers were present focally. Flow cytometric analysis of the inguinal lymph node showed an abnormal population of T-cells with a very high CD4:CD8 ratio(14:2), loss of CD26 (38%), CD7(23%) and aberrant coexpression of CD10. Immunohistochemistry revealed a large population of CD3+ T-cells with coexpression of CD10(16%). The corresponding permanent sections revealed marked disruption of the follicular dendritic networks based on a CD21 immunostain. CD30 was positive in a large number of immunoblasts. A large proportion of T-cells also showed BCL-6 coexpression. Epstein-Barr virus (EBV)-encoded RNAs by in-situ hybridization (EBER-ISH) was positive in numerous lymphoid cells. Based on these findings, the patient was diagnosed with AITL
Figure 2.
Histopathology of case #1. 1a and 1b punch biopsy, low power view (20× and 40×, respectively). There is a predominantly nodular lymphoid infiltrate in the deep dermis and with extension in the subcutis. 2b and 2d – mid power view (100× and 200×). The nodules are composed of a mixture of variable sized lymphocytes and histiocytes that lack germinal centers. 2e and 2f – high power view (400× each). Some of the large cells have morphologic features of immunoblasts, while others show cytologic findings reminiscent of Reed-Sternberg cells and variants. Numerous eosinophils (2g, 400×) and plasma cells (2h, 400×) are present.
Figure 3.
Immunohistochemistry of case #1. The nodular aggregates of medium to large cells are CD20+. CD3 shows a background of smaller appearing cells. CD30 is positive in many immunoblasts. CD10 shows positive staining in stromal elements, but is also positive in small lymphocytes. EBER is positive in a large number of immunoblasts. In-situ hybridization reveals an overall polyclonal pattern. While some areas show lambda predominance, others show the opposite (small inlet images).
At the time of diagnosis quantitative real-time (qRT)-PCR for EBV-DNA was performed on the peripheral blood and showed high levels of EBV DNA (36,933 copies/ul; ULN < 2000). He was treated with 6 cycles of EPOCH, achieving a partial response. His qRT-PCR for EBV-DNA in peripheral blood became undetectable. Autologous hematopoietic stem cell transplantation was discussed but the patient’s performance status declined secondary to aortic valve disease with congestive cardiomyopathy, multiple infections, and refractory disease. He died few months thereafter (approximately 1 year after the diagnosis of AITL).
Case 2
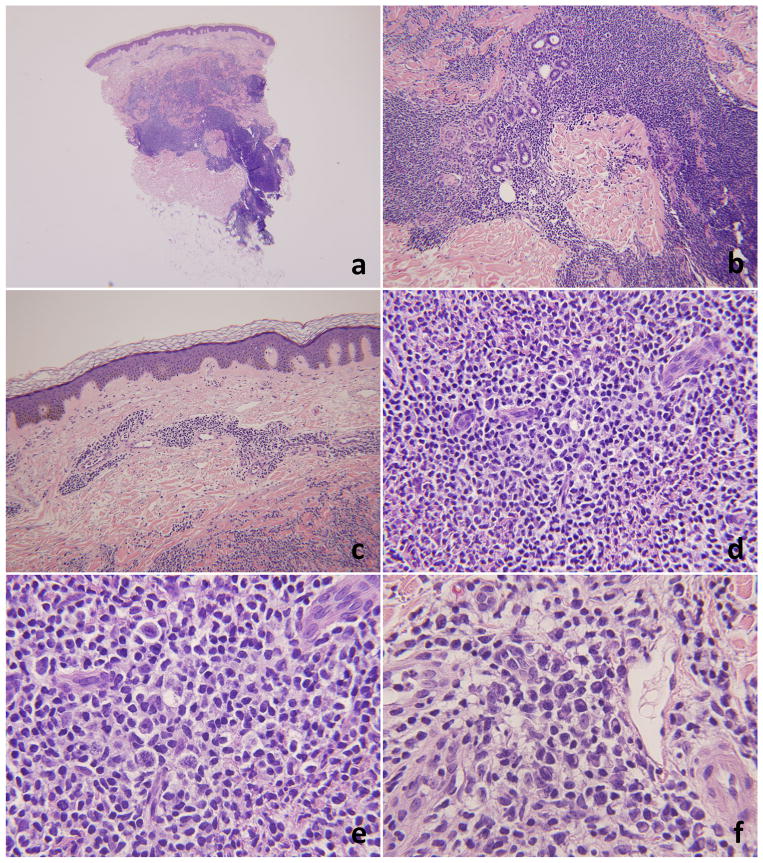
A 68 year-old man presented with a diffuse erythematous rash with accompanying nodules and plaques of varying sizes in the upper and lower extremities. Clinical lymphadenopathy was not evident at presentation. A punch biopsy (Figure 4),obtained from one of the cutaneous nodules, showed a nodular and diffuse lymphoplasmacytic infiltrate centered in the dermis, sparing the epidermis, and extending deeply into the subcutaneous tissue. Perineural, periadnexal and perivascular distribution of the abnormal cells was seen. The lymphoid cells were predominantly small to medium in size, with coarse chromatin and variable nucleoli. A subset of the cells had a plasmacytoid appearance. The plasma cells were markedly increased. The lymphoid infiltrate was composed predominantly of CD20+ B-cells. CD3 was positive in very few scattered small T-cells in the background (Figure 5). A subset of the B-cells showed coexpression of BCL-2, but not CD43, which stained a similar proportion of T-cells. BCL-6 and CD10 was positive in some of the T-cells. CD30 was positive in the larger immunoblasts. In-situ hybridization for kappa and lambda show an abnormally increased kappa to lambda ratio (6–7:1). EBER-ISH was positive in few scattered cells, which appear to be morphologically compatible with immunoblasts. The presumptive diagnosis, based on the in-situ hybridization studies and the plasmacytoid morphology, was marginal zone lymphoma.
Figure 4.
Histopathology of case #2. 4a and 4b low and mid power views (20× and 100×). There is a nodular and diffuse lymphoid infiltrate in the superficial and deep dermis with focal extension into the subcutis. The infiltrate is sparing the epidermis (4c, 100×). 4d and 4e – high power views (200× and 400×, respectively). The infiltrate is heterogenous and includes a large population of immunoblasts. Admixed plasma cells are frequently seen (4f, 400×).
Figure 5.
Immunohistochemistry of case #2. There is a much more prominent infiltrate of CD20+ B-cells, as compared to the CD3+ T-cells. Higher magnification of the CD20 immunostain shows positivity in variable sized lymphoid cells. CD10 is positive in stromal elements and smaller lymphocytes in the background. BCL-6 is positive in numerous lymphocytes, including some of the larger cells. EBER is positive in scattered lymphocytes, which are predominantly small. In-situ hybridization for kappa and lambda shows kappa restriction.
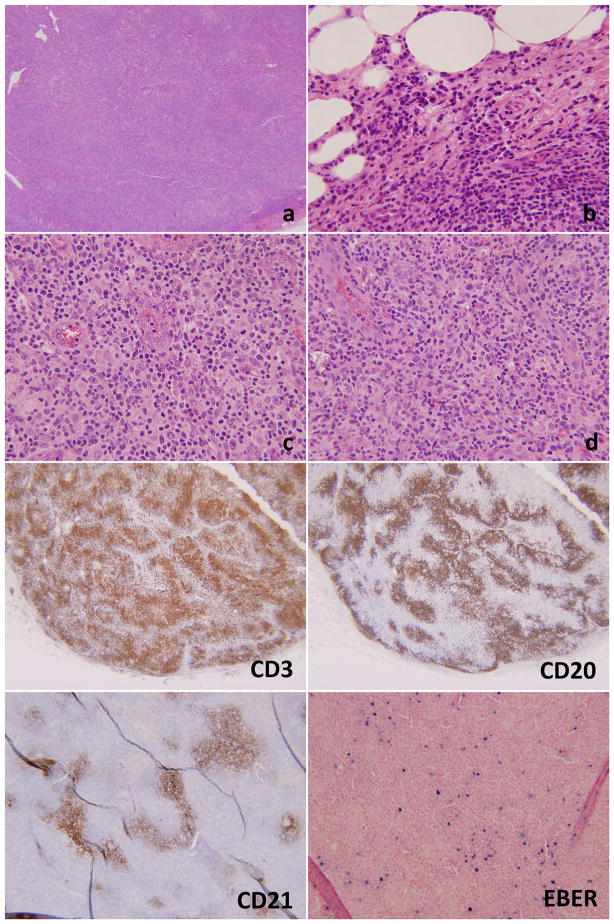
On subsequent clinical follow-up the patient developed inguinal lymphadenopathy. An excisional lymph node biopsy (Figure 6) revealed effacement of the architecture by a population of medium to large cells with an increased number of immunoblasts, eosinophils and plasma cells. Disrupted arborizing follicular dendritic networks were highlighted by CD21 immunostain. There was significant proliferation of high endothelial venules and follicular dendritic cells. The majority of the lymphocytes were CD3+/CD4+ T-cells with aberrant coexpression of CD10. EBER-ISH was also positive. Flow cytometry confirmed the presence of a population of abnormal T-cells with aberrant loss of CD7 and an increased CD4:CD8 ratio. Molecular testing showed a monoclonal TCR gene rearrangement and no evidence of IgH gene rearrangement. A diagnosis of AITL was issued on both the skin and the lymph node biopsies.
Figure 6.
Lymph node biopsy of case #2. There is effacement of the architecture at low power magnification (6a, 20×). Numerous plasma cells are seen at the periphery of the lymph node (6b, 200×). 6c and 6d (400×, each) show very prominent vasculature with high endothelial venules and a background very rich in eosinophils. The immunostains show a predominance of CD3+ T-cells as compared to the CD20+ B-cells. CD21 shows arborizing and disrupted follicular dendritic networks. EBER is positive in numerous lymphoid cells.
The patient was treated with chemotherapy (M-CHOP) achieving a CR and then consolidated with autologous bone marrow transplant. Subsequent PET studies showed an excellent nodal response, but the skin rashes continue to recur, some of which showed similar histologic features to the original diagnostic biopsy.
Discussion
The most recent World Health Organization (WHO)classification of hematopoietic neoplasms includes AITL in the category of nodal lymphomas of mature T-cells 1,21. The majority of cases of AITL are associated with EBV, which can be detected with the use of in-situ hybridization studies (EBER) or immunohistochemistry7,9,22–24. Biologically, EBV enters the B-cells and CD21 positive follicular dendritic cells (FDC) and immortalizes them25. Normally the FDCs are confined to the germinal centers. Proliferation of the FDCs retain the ability to rescue B-cells from apoptosis25. It’s been postulated that the infected and immortalized B-cells then produce cytokines that stimulate T-helper cells and particularly T-cells with a regulatory phenotype18,26. The evidence suggesting the role of EBV in AITL includes the presence of EBV viremia and histologic transformation of B-cells. In support of the theory behind the production of neoplastic B-cells in AITL is the development of diffuse large B-cell lymphoma in lymph nodes containing AITL24,27,28. On the other hand, numerous studies have shown that EBV reactivation can be the result of impaired immune surveillance. This pattern of EBV reactivation and viral shedding is the most common form of EBV viremia seen in lymphomas associated with immune-supression, such as Post-transplant lymphoproliferative disorders and lymphomas associated with HIV infection29,30. It is currently believed that the pattern of EBV activation seen in AITL and other types of T-cell lymphomas is likely secondary to shedding of cell-free, tumor associated EBV-DNA fragments from latently infected cells. This is the pattern that also appears to explain the EBV activity seen in immune-competent patients, such as Hodgkin lymphoma cases31. Contrary to previous assumptions, the neoplastic T-cells are not infected by EBV.
The surface marker profile of the neoplastic T-cells in AITL consists of CXCL13, BCL-6 and CD40L22,32,33. Follicular T-helper cells (TFH) that mediate germinal center B-cell differentiation and antibody production normally produce these. Additional markers of TFH include PD-120, SLAM-associated protein (SAP) and c-maf34. Interestingly, overexpression of c-maf has been shown in up to 75% of T-cell lymphomas35,36, and is linked to increased expression of VEGF, or vascular endothelial growth factor, and tumor replication37. Increased vascularity is a well-known feature of AITL in lymph nodes, and neoangiogenesis and increased vascularity has been documented in up to 40% of AITL with cutaneous dissemination2,10,11,14,15,38. Notably, FDC and endothelial cells in AITL show increased expression of FasL, while the tumor cells express Fas (CD95), highlighting the important role played by the interaction of Fas and FasL in the disease pathogenesis, and the close relationship between the environment of tumor cells and vessels10.
Clinically, AITL presents with a very protean appearance and, in most circumstances, is described as morbilliform, and less often as purpuric, urticarial, nodular or petechial10,38. Since the disease is clinically very heterogeneous, the histologic pictures seen with AITL are also variable and often times non-specific, leading to an incorrect diagnosis of benign or reactive conditions. The most typical and recurrent histologic skin findings seen in AITL are: 1) perivascular infiltrate (47% of cases), 2) vascular hyperplasia or proliferations (44% of cases), and 3) vasculitis (27% of cases)10,38. Clonal populations of T-cells are seen in 87% of cases and clonal B-cells can be found in up to 40–80% of cases (with only 5 cases tested from previous reports)10,39. Uncommon histologic presentations include granulomatous dermatitides4,40, an atypical linear IgA dermatosis form40, prurigo-like eruptions41, and rarely an epidermotropic form mimicking mycosis fungoides (MF)42.
In our cases, the cutaneous presentations of the lymphoproliferative disorders preceded in time the development of the clinical adenopathy. The morphology and immunophenotype in both cases were vaguely suggestive of marginal zone lymphoma, as there was a marked predominance of plasma cells, which was associated with an oligoclonal population of B-cells in the first case, and light chains restriction on the second case by in-situ hybridization studies. However, the infiltrates were relatively heavier in deeper portions of the biopsy for the first case, and also reveal a more heterogeneous population of lymphocytes including larger immunoblastic cells, with admixed eosinophils (for both cases). These features are rather unusual in the diagnosis of MALT lymphoma, where the infiltrate tends to be more monotonous, heavier in superficial to mid dermis, and could be (or not) associated with plasmacytic differentiation. Frequent residual germinal centers are seen in MALT lymphomas, with sometimes disrupted (but not arborizing) follicular dendritic netowrks. The typical immunophenotype in MALT shows absence of coexpression of CD5 and CD10, and light chains restriction within the plasma cells (in those cases with plasmacytic differentiation). BCL-2 and, less often, CD43 can be coexpressed in the B-cells. Some cases of MALT lymphoma can show translocation of the MALT1 gene43. Subsequent excisional biopsies from the lymphadenopathies in both of our cases revealed the classic features associated with AITL, including effacement of the architecture, proliferation of arborizing FDCs, prominent vascularity, high endothelial venules, and the increased number of immunoblasts. An immunophenotypically aberrant population of T-cells with coexpression of TFH markers, and T-cell clonality was confirmatory of the diagnosis. The atypical clinical presentations were later accompanied by slight increased hypergammaglobulinemia and EBV viremia.
In both cases EBV reactivity was noted on both skin and lymph node biopsies. Although not typical, EBV reactivity has been described in the setting of MALT, particularly in immunosuppressed individuals44,45. Bayer et al39 described a case of an individual diagnosed with MALT lymphoma who went on to developcutaneous EBV positive diffuse large B-cell lymphoma followed by AITL. The authors suggested that development of multiple clonal populations of B-cells at cutaneous sites should warrant further evaluation for a T-cell lymphoma. We could contrarily argue that this could have been interpreted as AITL masquerading as cutaneous proliferations of B-cells. In fact, the development of DLBCL in the setting of AITL has been well-documented27,28, including the isolated case report at a cutaneous site46. It’s been shown the delay of the diagnosis of AITL from the development of cutaneous non-specific lesions can take up to 2 years9,39. La-chenal et al9 has shown that at the time of diagnosis of AITL patients have adenopathy in 99% of cases and cutaneous lesions in nearly half of the individuals. Federico et al7 has shown a prevalence of adenopathy of 76% in a cohort of over 1,300 patients with a diagnosis of AITL. In their study, skin rashes were present in 21% of cases. Other clinical and laboratory findings that can be seen in AITL include hemolytic anemia (13%) and hypergammaglobulinemia (30%). A study of Balague et al47 showed that in 15 cases of peripheral T-cell lymphomas (PCTL) with frequent cutaneous involvement, there were EBV-negative clonal or monotypic B-cell populations ranging from plasma cell proliferations, B-cell lymphomas with plasmacytic differentiation, and plasmablastic. The presence of clonal plasma cells, were observed in 3 PTCL-NOS patients, 2 AITL, 2 cutaneous CD4+ small/medium-sized pleomorphic T-cell lymphoma, and 1 patient with cutaneous PTCL-NOS. Contrary to our cases, all of them were EBV negative. Again, the authors documented the presence of ‘small EBV+ lymphocytes’ in three of the cases, even though the overall interpretation of the EBER in-situ studies was ‘negative’. A study of Mattoch48 et al de-scribed16 cases of non-MF, non-AITL, cutaneous T-cell lymphomas with associated proliferations of B-cells. The B-cell proliferations were more prominent than the T-cells leading to a misdiagnosis of a B-cell lymphoma 48.Three of these cases were EBV positive. Viraben et al49 also reported on a case of a coexistent cutaneous low-grade B-cell lymphoma in the setting of AITL. The infiltrate was rich in plasma cells and small B-cells. While the authors also described it separately as a distinct diagnosis they comment on ‘a few reactive EBV positive small lymphocytes’ in the skin biopsy.
In the more recent years, numerous studies have paid significant attention to the evaluations of PTCL with a follicular T-helper phenotype18,26,50–53, of which AITL represents a classic example. Some examples where B-cell lymphomas are supported by TFH cells include nodular lymphocyte predominant Hodgkin lymphoma54 and follicular lymphoma55. More interestingly the follicular variant of PTCL-NOS and PTCL-NOS with TFH phenotype were found to have similar immunophenotypes to AITL48,50,51,56. Some cases of PTCL-NOS can show areas very difficult to distinguish from AITL56,57 It appears that the prognosis of lymphomas with TFH is better than other cases of PTCL58 In cutaneous T-cell lymphomas, the provisional diagnostic category of ‘primary cutaneous small to medium size CD4+ T-cell lymphoma (PCSMTCL)’ is a TFHT-cell lymphoma59–63. This disease is characterized by dense nodular and diffuse aggregates of T-cells in the dermis, with a helper phenotype, and a striking degree of pleomorphism. Some of these cases can also have a slight increased proliferation index (up to 30% in some cases). As opposed to AITL, the infiltrate is more dense and the number of B-cells less conspicuous with atypical plasma cells and no association with EBV. Clinically PCSMTCL is usually indolent without systemic manifestations when presenting as a solitary lesion. 11 cases of classic MF with a TFH phenotype (BCL-6 and PD1 expression, negative CD10) have been described.52 All of these cases had a clinical presentation typical of MF (patches or plaques) or Sezary(SS) syndrome (diffuse erythroderma). Histologically, epidermotropism was present in all cases with some cases showing classic Pautriermicroabscesses. All cases were CD4+ and EBV negative. As opposed to AITL, these cases show a clinical presentation typical for MF, the infiltrate was epidermotropic (a feature not present in any case of AITL), and there was absence of CD10 and EBV. More recently some authors have described CD4 and CD8 negative cases of MF with TFH phenotype (a pattern also seen by the authors personal experience)64,65.
Another recently described entity, primary cutaneous T follicular helper lymphoma, has been proposed as a form of an aggressive cutaneous lymphoma50. Clinically, these present with papules, plaques and nodules, distributed in the limbs, trunk and head and neck region. Imaging studies find no evidence of systemic dissemination at diagnosis. Histologically, these show diffuse dense and nodular infiltrate of T and B-cells with almost equal proportions. Admixed immunoblasts are often seen. EBV was negative in all cases. Immunohistochemistry reveals expression of CD10 and BCL-6 in the infiltrate, The proliferation index in those tumors was moderately high (30–50%). All cases showed a clonal T-cell population and one also revealed a clonal B-cell population. As opposed to PCSMTCL or MF with TFH, primary cutaneous T-cell lymphoma with TFH, phenotype is a more aggressive disease, and all patients require systemic treatment50,53. Development of adenopathy occurred in 2 of the patients, and one has been treated with a bone marrow transplant53. Many cases were originally diagnosed as primary cutaneous follicle center lymphoma.
In summary, we report two cases of AITL presenting as a skin rash, with histologic and immunophenotypic features suggestive of marginal zone lymphoma. In the setting of a patient presenting with a ‘primary cutaneous B-cell lymphoma’, caution is urged to exclude the possibility of a systemic T-cell lymphoma such as AITL, in particular when the morphology or immunophenotype are not conclusive, the patient has accompanying systemic symptoms (fevers, weight loss, adenopathy), hypergammaglobulinemia or EBV viremia. Correlation with additional serologic markers, imaging studies, and a prompt an early consulation with clinical hematology is of extraordinart importance in cutaneous and lymph node B-cell proliferations to avoid misdiagnoses.
Acknowledgments
BH is supported by the National Cancer Institute of the National Institutes of Health under Award Number T32CA165998 (PI Miguel Villalona and Steven Devine).
Footnotes
Conflict of Interest statement: None of the authors have a relevant conflict of interest to report.
References
- 1.Jaffe ES. The 2008 WHO classification of lymphomas: implications for clinical practice and translational research. Hematology/the Education Program of the American Society of Hematology. American Society of Hematology. Education Program. 2009:523–531. doi: 10.1182/asheducation-2009.1.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matloff RB, Neiman RS. Angioimmunoblastic lymphadenopathy. A generalized lymphoproliferative disorder with cutaneous manifestations. Archives of dermatology. 1978 Jan;114(1):92–94. doi: 10.1001/archderm.114.1.92. [DOI] [PubMed] [Google Scholar]
- 3.Murakami T, Ohtsuki M, Nakagawa H. Angioimmunoblastic lymphadenopathy-type peripheral T-cell lymphoma with cutaneous infiltration: report of a case and its gene expression profile. The British journal of dermatology. 2001 Apr;144(4):878–884. doi: 10.1046/j.1365-2133.2001.04150.x. [DOI] [PubMed] [Google Scholar]
- 4.Suarez-Vilela D, Izquierdo-Garcia FM. Angioimmunoblastic lymphadenopathy-like T-cell lymphoma: cutaneous clinical onset with prominent granulomatous reaction. The American journal of surgical pathology. 2003 May;27(5):699–700. doi: 10.1097/00000478-200305000-00015. [DOI] [PubMed] [Google Scholar]
- 5.Frizzera G, Moran EM, Rappaport H. Angio-immunoblastic lymphadenopathy with dysproteinaemia. Lancet. 1974 Jun 1;1(7866):1070–1073. doi: 10.1016/s0140-6736(74)90553-4. [DOI] [PubMed] [Google Scholar]
- 6.Watanabe S, Sato Y, Shimoyama M, Minato K, Shimosato Y. Immunoblastic lymphadenopathy, angioimmunoblastic lymphadenopathy, and IBL-like T-cell lymphoma. A spectrum of T-cell neoplasia. Cancer. 1986 Nov 15;58(10):2224–2232. doi: 10.1002/1097-0142(19861115)58:10<2224::aid-cncr2820581011>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 7.Federico M, Rudiger T, Bellei M, et al. Clinicopathologic characteristics of angioimmunoblastic T-cell lymphoma: analysis of the international peripheral T-cell lymphoma project. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013 Jan 10;31(2):240–246. doi: 10.1200/JCO.2011.37.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grogg KL, Attygalle AD, Macon WR, Remstein ED, Kurtin PJ, Dogan A. Angioimmunoblastic T-cell lymphoma: a neoplasm of germinal-center T-helper cells? Blood. 2005 Aug 15;106(4):1501–1502. doi: 10.1182/blood-2005-03-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lachenal F, Berger F, Ghesquieres H, et al. Angioimmunoblastic T-cell lymphoma: clinical and laboratory features at diagnosis in 77 patients. Medicine. 2007 Sep;86(5):282–292. doi: 10.1097/MD.0b013e3181573059. [DOI] [PubMed] [Google Scholar]
- 10.Balaraman B, Conley JA, Sheinbein DM. Evaluation of cutaneous angioimmunoblastic T-cell lymphoma. Journal of the American Academy of Dermatology. 2011 Oct;65(4):855–862. doi: 10.1016/j.jaad.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 11.Brown HA, Macon WR, Kurtin PJ, Gibson LE. Cutaneous involvement by angioimmunoblastic T-cell lymphoma with remarkable heterogeneous Epstein-Barr virus expression. Journal of cutaneous pathology. 2001 Sep;28(8):432–438. doi: 10.1034/j.1600-0560.2001.028008432.x. [DOI] [PubMed] [Google Scholar]
- 12.Ferran M, Gallardo F, Baena V, Ferrer A, Florensa L, Pujol RM. The ‘deck chair sign’ in specific cutaneous involvement by angioimmunoblastic T cell lymphoma. Dermatology. 2006;213(1):50–52. doi: 10.1159/000092841. [DOI] [PubMed] [Google Scholar]
- 13.Huang CT, Chuang SS. Angioimmunoblastic T-cell lymphoma with cutaneous involvement: a case report with subtle histologic changes and clonal T-cell proliferation. Archives of pathology & laboratory medicine. 2004 Oct;128(10):e122–124. doi: 10.5858/2004-128-e122-ATLWCI. [DOI] [PubMed] [Google Scholar]
- 14.Mahendran R, Grant JW, Hoggarth CE, Burrows NP. Angioimmunoblastic T-cell lymphoma with cutaneous involvement. Journal of the European Academy of Dermatology and Venereology : JEADV. 2001 Nov;15(6):589–590. doi: 10.1046/j.1468-3083.2001.00356.x. [DOI] [PubMed] [Google Scholar]
- 15.Martel P, Laroche L, Courville P, et al. Cutaneous involvement in patients with angioimmunoblastic lymphadenopathy with dysproteinemia: a clinical, immunohistological, and molecular analysis. Archives of dermatology. 2000 Jul;136(7):881–886. doi: 10.1001/archderm.136.7.881. [DOI] [PubMed] [Google Scholar]
- 16.Papadavid E, Panayiotides I, Dalamaga M, Katoulis A, Economopoulos T, Stavrianeas N. Cutaneous involvement in angioimmunoblastic T-cell lymphoma. Indian journal of dermatology. 2010 Jul-Sep;55(3):279–280. doi: 10.4103/0019-5154.70704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoon GS, Choi YK, Bak H, et al. Angioimmunoblastic T cell lymphomas: frequent cutaneous skin lesions and absence of human herpes viruses. Annals of dermatology. 2009 Feb;21(1):1–5. doi: 10.5021/ad.2009.21.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahearne MJ, Allchin RL, Fox CP, Wagner SD. Follicular helper T-cells: expanding roles in T-cell lymphoma and targets for treatment. British journal of haematology. 2014 May 12; doi: 10.1111/bjh.12941. [DOI] [PubMed] [Google Scholar]
- 19.Attygalle A, Al-Jehani R, Diss TC, et al. Neoplastic T cells in angioimmunoblastic T-cell lymphoma express CD10. Blood. 2002 Jan 15;99(2):627–633. doi: 10.1182/blood.v99.2.627. [DOI] [PubMed] [Google Scholar]
- 20.Dorfman DM, Brown JA, Shahsafaei A, Freeman GJ. Programmed death-1 (PD-1) is a marker of germinal center-associated T cells and angioimmunoblastic T-cell lymphoma. The American journal of surgical pathology. 2006 Jul;30(7):802–810. doi: 10.1097/01.pas.0000209855.28282.ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swerdlow SH International Agency for Research on Cancer., World Health Organization. WHO classification of tumours of haematopoietic and lymphoid tissues. 4. Lyon, France: International Agency for Research on Cancer; 2008. [Google Scholar]
- 22.Jones D, O’Hara C, Kraus MD, et al. Expression pattern of T-cell-associated chemokine receptors and their chemokines correlates with specific subtypes of T-cell non-Hodgkin lymphoma. Blood. 2000 Jul 15;96(2):685–690. [PubMed] [Google Scholar]
- 23.Weiss LM, Jaffe ES, Liu XF, Chen YY, Shibata D, Medeiros LJ. Detection and localization of Epstein-Barr viral genomes in angioimmunoblastic lymphadenopathy and angioimmunoblastic lymphadenopathy-like lymphoma. Blood. 1992 Apr 1;79(7):1789–1795. [PubMed] [Google Scholar]
- 24.Zhou Y, Attygalle AD, Chuang SS, et al. Angioimmunoblastic T-cell lymphoma: histological progression associates with EBV and HHV6B viral load. British journal of haematology. 2007 Jul;138(1):44–53. doi: 10.1111/j.1365-2141.2007.06620.x. [DOI] [PubMed] [Google Scholar]
- 25.Lindhout E, Lakeman A, Mevissen ML, de Groot C. Functionally active Epstein-Barr virus-transformed follicular dendritic cell-like cell lines. The Journal of experimental medicine. 1994 Apr 1;179(4):1173–1184. doi: 10.1084/jem.179.4.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaulard P, de Leval L. Follicular helper T cells: implications in neoplastic hematopathology. Seminars in diagnostic pathology. 2011 Aug;28(3):202–213. doi: 10.1053/j.semdp.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Attygalle AD, Kyriakou C, Dupuis J, et al. Histologic evolution of angioimmunoblastic T-cell lymphoma in consecutive biopsies: clinical correlation and insights into natural history and disease progression. The American journal of surgical pathology. 2007 Jul;31(7):1077–1088. doi: 10.1097/PAS.0b013e31802d68e9. [DOI] [PubMed] [Google Scholar]
- 28.Maegawa RO, Hussain S, Danila DC, Teruya-Feldstein J, Maki RG, O’Connor OA. Angioimmunoblastic T-cell lymphoma with an evolving Epstein Barr virus-positive diffuse large B-cell lymphoma with unusual clinical and pathologic findings. Leukemia & lymphoma. 2007 Oct;48(10):2071–2074. doi: 10.1080/10428190701593628. [DOI] [PubMed] [Google Scholar]
- 29.Dupuis J, Emile JF, Mounier N, et al. Prognostic significance of Epstein-Barr virus in nodal peripheral T-cell lymphoma, unspecified: A Groupe d’Etude des Lymphomes de l’Adulte (GELA) study. Blood. 2006 Dec 15;108(13):4163–4169. doi: 10.1182/blood-2006-04-017632. [DOI] [PubMed] [Google Scholar]
- 30.Rea D, Delecluse HJ, Hamilton-Dutoit SJ, et al. Epstein-Barr virus latent and replicative gene expression in post-transplant lymphoproliferative disorders and AIDS-related non-Hodgkin’s lymphomas. French Study Group of Pathology for HIV-associated Tumors. Annals of oncology : official journal of the European Society for Medical Oncology/ESMO. 1994;5( Suppl 1):113–116. doi: 10.1093/annonc/5.suppl_1.s113. [DOI] [PubMed] [Google Scholar]
- 31.Ambinder RF. Hematology/the Education Program of the American Society of Hematology. American Society of Hematology. Education Program. 2007. Epstein-barr virus and hodgkin lymphoma; pp. 204–209. [DOI] [PubMed] [Google Scholar]
- 32.Grogg KL, Attygalle AD, Macon WR, Remstein ED, Kurtin PJ, Dogan A. Expression of CXCL13, a chemokine highly upregulated in germinal center T-helper cells, distinguishes angioimmunoblastic T-cell lymphoma from peripheral T-cell lymphoma, unspecified. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2006 Aug;19(8):1101–1107. doi: 10.1038/modpathol.3800625. [DOI] [PubMed] [Google Scholar]
- 33.Ortonne N, Dupuis J, Plonquet A, et al. Characterization of CXCL13+ neoplastic t cells in cutaneous lesions of angioimmunoblastic T-cell lymphoma (AITL) The American journal of surgical pathology. 2007 Jul;31(7):1068–1076. doi: 10.1097/PAS.0b013e31802df4ef. [DOI] [PubMed] [Google Scholar]
- 34.Bisig B, Thielen C, Herens C, et al. c-Maf expression in angioimmunoblastic T-cell lymphoma reflects follicular helper T-cell derivation rather than oncogenesis. Histopathology. 2012 Jan;60(2):371–376. doi: 10.1111/j.1365-2559.2011.04022.x. [DOI] [PubMed] [Google Scholar]
- 35.Morito N, Yoh K, Fujioka Y, et al. Overexpression of c-Maf contributes to T-cell lymphoma in both mice and human. Cancer research. 2006 Jan 15;66(2):812–819. doi: 10.1158/0008-5472.CAN-05-2154. [DOI] [PubMed] [Google Scholar]
- 36.Murakami YI, Yatabe Y, Sakaguchi T, et al. c-Maf expression in angioimmunoblastic T-cell lymphoma. The American journal of surgical pathology. 2007 Nov;31(11):1695–1702. doi: 10.1097/PAS.0b013e318054dbcf. [DOI] [PubMed] [Google Scholar]
- 37.Hurt EM, Wiestner A, Rosenwald A, et al. Overexpression of c-maf is a frequent oncogenic event in multiple myeloma that promotes proliferation and pathological interactions with bone marrow stroma. Cancer cell. 2004 Feb;5(2):191–199. doi: 10.1016/s1535-6108(04)00019-4. [DOI] [PubMed] [Google Scholar]
- 38.Jacobson RK, Lee KC, Muglia JJ, Robinson-Bostom L. Cutaneous manifestations of angioimmunoblastic T-cell lymphoma. Journal of the American Academy of Dermatology. 2013 Jul;69(1):e25–26. doi: 10.1016/j.jaad.2012.12.958. [DOI] [PubMed] [Google Scholar]
- 39.Bayerl MG, Hennessy J, Ehmann WC, Bagg A, Rosamilia L, Clarke LE. Multiple cutaneous monoclonal B-cell proliferations as harbingers of systemic angioimmunoblastic T-cell lymphoma. Journal of cutaneous pathology. 2010 Jul;37(7):777–786. doi: 10.1111/j.1600-0560.2009.01408.x. [DOI] [PubMed] [Google Scholar]
- 40.Nassar D, Gabillot-Carre M, Ortonne N, et al. Atypical linear IgA dermatosis revealing angioimmunoblastic T-cell lymphoma. Archives of dermatology. 2009 Mar;145(3):342–343. doi: 10.1001/archdermatol.2008.607. [DOI] [PubMed] [Google Scholar]
- 41.Khaled A, Sfia M, Fazaa B, et al. Chronic prurigo revealing an angioimmunoblastic T cell lymphoma. La Tunisie medicale. 2009 Aug;87(8):534–537. [PubMed] [Google Scholar]
- 42.Ponciano A, de Muret A, Machet L, et al. Epidermotropic secondary cutaneous involvement by relapsed angioimmunoblastic T-cell lymphoma mimicking mycosis fungoides: a case report. Journal of cutaneous pathology. 2012 Dec;39(12):1119–1124. doi: 10.1111/cup.12022. [DOI] [PubMed] [Google Scholar]
- 43.Kempf W, Kazakov DV, Mitteldorf C. Cutaneous lymphomas: an update. Part 2: B-cell lymphomas and related conditions. The American Journal of dermatopathology. 2014 Mar;36(3):197–208. doi: 10.1097/DAD.0b013e318289b20e. quiz 209–110. [DOI] [PubMed] [Google Scholar]
- 44.Gibson SE, Swerdlow SH, Craig FE, et al. EBV-positive extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue in the posttransplant setting: a distinct type of posttransplant lymphoproliferative disorder? The American journal of surgical pathology. 2011 Jun;35(6):807–815. doi: 10.1097/PAS.0b013e3182190999. [DOI] [PubMed] [Google Scholar]
- 45.Kojima M, Nakamura N, Itoh H, et al. Epstein-Barr virus-related atypical lymphoproliferative disorders in Waldeyer’s ring: a clinicopathological study of 9 cases. Pathobiology : journal of immunopathology, molecular and cellular biology. 2010;77(4):218–224. doi: 10.1159/000301154. [DOI] [PubMed] [Google Scholar]
- 46.Yang QX, Pei XJ, Tian XY, Li Y, Li Z. Secondary cutaneous Epstein-Barr virus-associated diffuse large B-cell lymphoma in a patient with angioimmunoblastic T-cell lymphoma: a case report and review of literature. Diagnostic pathology. 2012;7:7. doi: 10.1186/1746-1596-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Balague O, Martinez A, Colomo L, et al. Epstein-Barr virus negative clonal plasma cell proliferations and lymphomas in peripheral T-cell lymphomas: a phenomenon with distinctive clinicopathologic features. The American journal of surgical pathology. 2007 Sep;31(9):1310–1322. doi: 10.1097/PAS.0b013e3180339f18. [DOI] [PubMed] [Google Scholar]
- 48.Mattoch IW, Fulton R, Kim Y, Hoppe R, Warnke RA, Sundram UN. Cutaneous peripheral T-cell lymphoma associated with a proliferation of B cells. American journal of clinical pathology. 2009 Jun;131(6):810–819. doi: 10.1309/AJCP5W0VOCSVOBRA. [DOI] [PubMed] [Google Scholar]
- 49.Viraben R, Brousset P, Lamant L. Cutaneous B-cell lymphoma associated with angioimmunoblastic lymphadenopathy. Journal of the American Academy of Dermatology. 1998 Jun;38(6 Pt 1):992–994. doi: 10.1016/s0190-9622(98)70165-3. [DOI] [PubMed] [Google Scholar]
- 50.Battistella M, Beylot-Barry M, Bachelez H, Rivet J, Vergier B, Bagot M. Primary cutaneous follicular helper T-cell lymphoma: a new subtype of cutaneous T-cell lymphoma reported in a series of 5 cases. Archives of dermatology. 2012 Jul;148(7):832–839. doi: 10.1001/archdermatol.2011.3269. [DOI] [PubMed] [Google Scholar]
- 51.Hu S, Young KH, Konoplev SN, Medeiros LJ. Follicular T-cell lymphoma: a member of an emerging family of follicular helper T-cell derived T-cell lymphomas. Human pathology. 2012 Nov;43(11):1789–1798. doi: 10.1016/j.humpath.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 52.Meyerson HJ, Awadallah A, Pavlidakey P, Cooper K, Honda K, Miedler J. Follicular center helper T-cell (TFH) marker positive mycosis fungoides/Sezary syndrome. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2013 Jan;26(1):32–43. doi: 10.1038/modpathol.2012.124. [DOI] [PubMed] [Google Scholar]
- 53.Ohmatsu H, Sugaya M, Fujita H, Kadono T, Sato S. Primary cutaneous follicular helper T-cell lymphoma treated with allogeneic bone marrow transplantation: immunohistochemical comparison with angioimmunoblastic T-cell lymphoma. Acta dermato-venereologica. 2014 Jan;94(1):54–57. doi: 10.2340/00015555-1626. [DOI] [PubMed] [Google Scholar]
- 54.Nathwani BN, Vornanen M, Winkelmann R, et al. Intranodular clusters of activated cells with T follicular helper phenotype in nodular lymphocyte predominant Hodgkin lymphoma: a pilot study of 32 cases from Finland. Human pathology. 2013 Sep;44(9):1737–1746. doi: 10.1016/j.humpath.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 55.Rawal S, Chu F, Zhang M, et al. Cross talk between follicular Th cells and tumor cells in human follicular lymphoma promotes immune evasion in the tumor microenvironment. Journal of immunology. 2013 Jun 15;190(12):6681–6693. doi: 10.4049/jimmunol.1201363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Attygalle AD, Chuang SS, Diss TC, Du MQ, Isaacson PG, Dogan A. Distinguishing angioimmunoblastic T-cell lymphoma from peripheral T-cell lymphoma, unspecified, using morphology, immunophenotype and molecular genetics. Histopathology. 2007 Mar;50(4):498–508. doi: 10.1111/j.1365-2559.2007.02632.x. [DOI] [PubMed] [Google Scholar]
- 57.Pileri SA, Piccaluga PP. New molecular insights into peripheral T cell lymphomas. The Journal of clinical investigation. 2012 Oct 1;122(10):3448–3455. doi: 10.1172/JCI61205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Iqbal J, Wright G, Wang C, et al. Gene expression signatures delineate biological and prognostic subgroups in peripheral T-cell lymphoma. Blood. 2014 May 8;123(19):2915–2923. doi: 10.1182/blood-2013-11-536359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rodriguez Pinilla SM, Roncador G, Rodriguez-Peralto JL, et al. Primary cutaneous CD4+ small/medium-sized pleomorphic T-cell lymphoma expresses follicular T-cell markers. The American journal of surgical pathology. 2009 Jan;33(1):81–90. doi: 10.1097/PAS.0b013e31818e52fe. [DOI] [PubMed] [Google Scholar]
- 60.Beltraminelli H, Leinweber B, Kerl H, Cerroni L. Primary cutaneous CD4+ small-/medium-sized pleomorphic T-cell lymphoma: a cutaneous nodular proliferation of pleomorphic T lymphocytes of undetermined significance? A study of 136 cases. The American Journal of dermatopathology. 2009 Jun;31(4):317–322. doi: 10.1097/DAD.0b013e31819f19bb. [DOI] [PubMed] [Google Scholar]
- 61.Grogg KL, Jung S, Erickson LA, McClure RF, Dogan A. Primary cutaneous CD4-positive small/medium-sized pleomorphic T-cell lymphoma: a clonal T-cell lymphoproliferative disorder with indolent behavior. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2008 Jun;21(6):708–715. doi: 10.1038/modpathol.2008.40. [DOI] [PubMed] [Google Scholar]
- 62.Toberer F, Hartschuh W, Hadaschik E. Primary cutaneous CD4+ small- to medium-sized pleomorphic T-cell lymphoma: temporary remission by oral doxycycline. JAMA dermatology. 2013 Aug;149(8):956–959. doi: 10.1001/jamadermatol.2013.4162. [DOI] [PubMed] [Google Scholar]
- 63.Volks N, Oschlies I, Cario G, Weichenthal M, Folster-Holst R. Primary cutaneous CD4+ small to medium-size pleomorphic T-cell lymphoma in a 12-year-old girl. Pediatric dermatology. 2013 Sep-Oct;30(5):595–599. doi: 10.1111/pde.12168. [DOI] [PubMed] [Google Scholar]
- 64.Cho-Vega JH, Tschen JA, Vega F. CD4/CD8 double-negative early-stage mycosis fungoides associated with primary cutaneous follicular center lymphoma. Journal of the American Academy of Dermatology. 2011 Oct;65(4):884–886. doi: 10.1016/j.jaad.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 65.Kempf W, Kazakov DV, Cipolat C, Kutzner H, Roncador G, Tomasini D. CD4/CD8 double negative mycosis fungoides with PD-1 (CD279) expression--a disease of follicular helper T-cells? The American Journal of dermatopathology. 2012 Oct;34(7):757–761. doi: 10.1097/DAD.0b013e31825b26d1. [DOI] [PubMed] [Google Scholar]