Abstract
Objective
Oxidative stress events have been implicated to occur and facilitate multiple failure modes of intracortical microelectrodes. The goal of the present study was to evaluate the ability of a sustained concentration of an anti-oxidant and to reduce oxidative stress-mediated neurodegeneration for the application of intracortical microelectrodes.
Approach
Non-functional microelectrodes were implanted into the cortex of male Sprague Dawley rats for up to sixteen weeks. Half of the animals received a daily intraperitoneal injection of the natural anti-oxidant resveratrol, at 30 mg/kg. The study was designed to investigate the biodistribution of the resveratrol, and the effects on neuroinflammation/neuroprotection following device implantation.
Main Results
Daily maintenance of a sustained range of resveratrol throughout the implantation period resulted in fewer degenerating neurons in comparison to control animals at both two and sixteen weeks post implantation. Initial and chronic improvements in neuronal viability in resveratrol-dosed animals were correlated with significant reductions in local superoxide anion accumulation around the implanted device at two weeks after implantation. Controls, receiving only saline injections, were also found to have reduced amounts of accumulated superoxide anion locally and less neurodegeneration than controls at sixteen weeks post-implantation. Despite observed benefits, thread-like adhesions were found between the liver and diaphragm in resveratrol-dosed animals.
Significance
Overall, our chronic daily anti-oxidant dosing scheme resulted in improvements in neuronal viability surrounding implanted microelectrodes, which could result in improved device performance. However, due to the discovery of thread-like adhesions, further work is still required to optimize a chronic anti-oxidant dosing regime for the application of intracortical microelectrodes.
2. Introduction
Microelectrode arrays capable of recording neuronal signals are emerging as a promising tool in both clinical and research settings (Nicolelis, 2003; Schwartz, 2004; Cogan, 2008). In basic science, chronic neural recordings in animal models can facilitate our understanding of cortical mapping in both normal and disease states (Magnotta et al., 2012; Duffau, 2013; Zhang et al., 2013). In the clinical setting, high-resolution recorded neuronal signals currently provide a way for individuals to control assistive devices, prosthetic limbs, and enable functional movement of the patient’s paralyzed limbs (Donoghue et al., 2007; Kim et al., 2008; Pancrazio and Peckham, 2009; Simeral et al., 2011; Hochberg et al., 2012; Jorfi et al., 2014).
Unfortunately, following implantation of intracortical microelectrodes, multiple failure modes can occur, ultimately resulting in loss of recorded signals weeks to months after surgery (Prasad et al., 2012; Barrese et al., 2013; Prasad et al., 2014). One emerging hypothesis concerning microelectrode failure suggests a leading role for oxidative stress in altering neuronal cell viability and blood brain barrier stability at the device-tissue interface (McConnell et al., 2009; Potter et al., 2013). In addition, it has also been proposed that the same oxidative environment can result in breakdown/corrosion of both the insulator and the metals of the electrode itself (Schmitt et al., 1999; Barrese et al., 2013; Kozai et al., 2014; Prasad et al., 2014; Sankar et al., 2014). Therefore, given this possible role for oxidative stress events, the biological mechanisms that might create and propagate a local oxidative environment around implanted microelectrodes are being investigated.
For example, McConnell et al. demonstrated that following microelectrode implantation, accumulation of hemosiderin-laden macrophages, a cell-type associated with oxidative stress, occurs as early as two weeks (McConnell et al., 2009). In addition, we recently demonstrated that high levels of reactive oxygen species accumulate around implanted microelectrodes at two weeks post device implantation (Potter et al., 2013). Finally, expression of ferritin around the electrode, which can result in increased Fenton (radical) chemistry, has also been associated with failure of microwire and platinum microelectrodes (Prasad et al., 2012; Prasad et al., 2014).
To this end, our group has begun to investigate the use of anti-oxidative approaches to mitigate the buildup of reactive oxygen intermediates around implanted microelectrodes(Potter et al., 2013; Potter-Baker et al., 2014a; Potter et al., 2014). Specifically, we have found that short-term (<48 hours) accumulation/release of anti-oxidants, for example resveratrol or curcumin, around implanted microelectrodes can result in higher densities of neuronal nuclei and more viable neurons at the device interface (Potter et al., 2013; Potter et al., 2014). However, to date, our anti-oxidant delivery systems have yet to sustain neuronal viability around implanted microelectrodes beyond four weeks after device implantation; where it is likely that fast clearance rates and low bioavailability facilitated short-term neuronal protection.
Therefore, building from our previous approaches, we sought to investigate if the use of chronic daily administration of anti-oxidants could provide a sustained anti-oxidative environment around implanted microelectrodes in a rat model. Our results demonstrate that chronic systemic administration of the natural anti-oxidant, resveratrol, can facilitate the presence of sustained concentrations of the anti-oxidant around implanted microelectrodes. In addition, animals receiving daily anti-oxidant treatment had significantly reduced amounts of reactive oxygen species and minimal neuronal degeneration around implanted microelectrodes up to sixteen weeks after implantation. However, we also found that our daily anti-oxidant-dosing regime was correlated with increased hemorrhaging both systemically and around implanted microelectrodes, as well as the presence of thread-like adhesions between the liver and diaphragm. Therefore, taken together, our results suggest that while chronic administration of anti-oxidants, such as resveratrol, can facilitate improvements in neuronal viability around microelectrodes, future studies still need to optimize a dosing regime capable of mitigating unwanted side effects of long-term regular administration.
3. Materials and Methods
3.1 Animals and Surgical Implantation
Sixty-eight adult male Sprague Dawley (201–250 gram) rats, each receiving a chronic non-functional microelectrode in each hemisphere, kept on a 12-hour light cycle, were used for this study. Animals were allowed to survive to time end points of either (1) two or sixteen weeks (chronic implantation (N=8 implants/animals) and biodistribution (N=4 implants/animals)) or (2) one hour, eighteen hours, two, three and seven days (biodistribution (N=4/animals)). For chronically implanted animals, age-matched non-implanted sham control animals (N=4) were also included in the data analysis. Surgical procedures followed our previously established protocols with minor deviations (Potter et al., 2012b; Potter et al., 2012a; Potter et al., 2013). All animal care and procedures were performed in accordance with Case Western Reserve University Institutional Animal Care and Use Committee. To minimize variability, the same surgeon performed all implantation surgeries.
Briefly, rats were anesthetized using an intraperitoneal (IP) injection of a mixture of ketamine (80 mg/kg) and xylazine (10 mg/kg). Anesthesia was maintained throughout the duration of the procedure using isoflurane (0.25 – 2%) by monitoring changes in vitals (heart rate / blood-oxygen content) and toe-pinch reflex. Following initial anesthesia, the surgical area was thoroughly shaved and Marcaine (0.5%) was injected subcutaneously (SQ) to provide local anesthesia. Opthalmic ointment was applied to prevent retinal drying during the procedure. The animal was mounted directly onto a stereotaxic frame. Prior to surgery, animals received meloxicam (1 mg/kg) and cefazolin (16 mg/kg) SQ to manage pain and to prevent infection, respectively. A sterile surgical area was then obtained by painting the surgical field three times with a betadine and then a 70% isopropanol swab. Animal temperature was maintained using a circulating water heating pad that was placed directly below the mounted animal.
For the craniotomy, the skull was first exposed using a one-inch incision down midline and the tissue retracted on both sides. A 3-mm hole in the skull to expose cortical tissue surface was then achieved manually using a 3-mm biopsy punch (PSS Select). For each hemisphere, the 3-mm hole was placed approximately 3 mm lateral to midline and 4 mm caudal to bregma. The dura was then reflected using a 90° dura pick (Fine Science Tools) and visible vasculature avoided. A sterile 2 mm × 123 µm × 15 µm non-functional single shank ‘Michigan-style’ electrode, containing a 1 mm × 1 mm tab was carefully inserted into each hemisphere approximately 2 mm (1 implant per hemisphere). Microelectrodes were sterilized using ethylene-oxide and allowed to out-gas for a minimum of 72 hours prior to implantation (Ravikumar et al., 2013). To prevent cortical tissue dehydration following electrode implantation, Kwik-sil (World Precision Instruments) was applied over the exposed tissue space. Implanted electrodes were then anchored to the skull using a ultra-violet (UV) curing dental acrylic (Fusio/Flow-it ALC, Pentron Dental). The surgical area was closed using 5-0 monofilament polypropylene suture (Henry Schein) and triple antibiotic was applied over the sutures to prevent drying and localized infection.
3.2 Resveratrol Preparation and Administration Schedule
To investigate the effects of daily resveratrol administration in our intracortical microelectrode implantation model, three animal conditions were investigated: (1) no dose (control), (2) dosed with diluent daily (daily diluent) and (3) dosed with resveratrol daily (daily resveratrol). For those receiving injections, animals received the first injection 16 to 24 hours before surgery and a second injection immediately following electrode implantation. Continual injections of either diluent or resveratrol were then administered every 20 to 26 hours until time end points (two and sixteen weeks). The same dosing scheme was also utilized for the acute biodistribution study.
Pharmaceutical-grade resveratrol (MegaResveratrol) was administered i.p. at a dose of 30 mg/kg and was considered sterile as is following our previously reported protocol (Potter et al., 2013). Briefly, resveratrol was first dissolved at 50 mg/mL in 50% ethanol (diluted from 100% in deionized water) at 50° C. Then the appropriate amount of resveratrol was drawn up in a sterile syringe and diluted to a final concentration of 2% ethanol with sterile saline solution. In the case of diluent animals, injections were prepared identically and with the same final volume, albeit without resveratrol. Prior to injection of both diluent and resveratrol, syringes were warmed to increase solubility and maintain animal body temperature. In addition, since it has been reported that resveratrol can lose activity after being dissolved (Delmas et al., 2011), all injections were made fresh daily before use. To ensure accurate dosing, animals were weighed bi-weekly throughout the course of the study and the dosing regime altered accordingly.
3.3 Perfusion, Tissue Extraction and Tissue Allocation
Multiple time points and tissue processing techniques were investigated/utilized in our study. The goal of our analysis was to first determine a resveratrol-dosing scheme that would sustain concentration of the anti-oxidant around the implanted microelectrode for the duration of the implant. Then, we aimed to determine the effects of the dosing regime on the neuroinflammatory response.
First, in order to obtain an initial understanding of the bioavailability of resveratrol in our microelectrode model, following a two-dose resveratrol administration (Potter et al., 2013), blood samples were taken from the tail vein of rats immediately after surgical implantation and at preset time points up to 24 hours after surgery. Blood samples (~200 µL) were immediately placed on ice, frozen and lyophilized for further analysis.
For all quantitative analyses within tissue, animals were perfused transcardially with 1X phosphate-buffered saline (PBS) following our previously outlined methodology(Potter et al., 2012b; Potter et al., 2012a; Potter et al., 2013). Briefly, animals were heavily anesthetized using a mixture of ketamine (80 mg/kg) and xylazine (10 mg/kg) given i.p.. Each animal was then perfused with 1X PBS at a rate of 50 mL/minute until the exudate was clear (~500 mL/animal). For each animal, the brain was removed and the tissue carefully cut down midline with a scalpel blade. Each hemisphere (with implanted electrode) was then assigned into a group at random: (1) fixed tissue staining, (2) non-fixed tissue staining, or (3) biodistribution. Within each subset, for any given time point and animal condition, four to six implants were used for analysis.
In the case of fixed tissue staining, the brain hemisphere with implanted electrode was then placed directly into 4% paraformaldehyde (PFA) and post fixed for 24 hours at 4° C. After 24 hours, fixed tissue was then placed in fresh 4% PFA and allowed to continue to post-fix for an addition 72 hours. Following fixation, tissue was cryopreserved in a stepwise sucrose gradient (10%-20%-30%) in 1X PBS at 4° C. For non-fixed tissue staining, the explanted brain hemisphere containing the electrode was placed directly into 10% sucrose in 1X PBS for 24 hours at 4° C and then equilibrated in a stepwise sucrose gradient similar to fixed tissue.
For all implanted microelectrodes utilized for biodistribution, a two-step process, following our previously outlined methodology, was utilized. Specifically, using a 3-mm biopsy punch, the tissue surrounding the implanted microelectrode was explanted and assessed for concentration levels of resveratrol spectrophotometrically. Care was taken to ensure that the electrode implantation site was centered in the middle of the biopsied tissue sample. Both the biopsy containing the implantation site and the remaining brain hemisphere were then placed directly into 50% ethanol for biodistribution analysis. Furthermore, immediately after tissue clearing, the following organs were also removed from each animal, weighed and then placed directly into 50% ethanol to be analyzed for biodistribution: liver, lungs, kidney and spleen. Here, 50% ethanol was utilized to enhance the solubility of resveratrol within the solvent.
3.4 Biodistribution of Resveratrol
Both blood and tissue samples were assessed for concentration levels of resveratrol. Specifically, in order to determine the concentration of resveratrol, a fluorescent spectrophotometric analysis was utilized following previously published protocols with minor alterations(Delmas et al., 2011; Potter-Baker et al., 2014a). For all analysis, samples were read in 96-well black plates at an excitation of 315 nm and an emission of 385 nm. For highest accuracy, samples were only read within the center of the plate.
First, a fluorescent standard curve (1 nM to 100 mM) of resveratrol was obtained in neat 50% ethanol. For blood samples, a separate standard curve was run in reconstituted control (non-resveratrol dosed) blood supernatant to account for high levels of background autofluorescence in blood samples. For blood samples, lyophilized blood was reconstituted in 200 µL of 50% ethanol and vortexed for 30 seconds. Following reconstitution, samples were spun quickly in a mini-centrifuge and the supernatant collected for fluorescent analysis.
For all tissue samples, prior to fluorescent assessment for resveratrol, tissue was heavily homogenized (Tissue Tearor) until no visible pieces of tissue were noted. Then, tissue samples were spun for at least 3 minutes at 2,000 rpm on a benchtop centrifuge to pellet cellular debris. The supernatant of each sample was then carefully removed and the final volume was noted.
To determine the amount of resveratrol in each organ, the protein concentration of each supernatant (control and resveratrol administered animals) was first found by UV-vis spectrophotometric measurement at 280 nm. Then, to find the relative fluorescent intensity of the entire sample, Equation 1 was utilized:
Where A denotes the fluorescence of 100 µL protein supernatant and B denotes the total amount of protein (mg) used in fluorescent measurement. The organ volumes for each assessed organ were defined as 0.049 mL (brain implant, defined by the size of the biopsy punch used to extract the tissue) or the weight volume of each respective liver, kidney, spleen, lungs, and remaining brain hemisphere. To account for background tissue and blood auto-fluorescence, the average florescence of control samples was subtracted from resveratrol samples for each time point. Resveratrol samples that displayed a lower fluorescence than control samples were assumed to have a concentration equal to 0. Relative fluorescent intensities of resveratrol-dosed animal supernatants were then inputted into the resveratrol standard curve, and the total concentration was determined. Values are reported as an average concentration ± standard error of the mean (s.e.m.) (n≥4).
3.5 Immunohistochemistry
In addition to biodistribution, we evaluated the neuroinflammatory, neurodegenerative and oxidative stress events surrounding chronically implanted microelectrodes at two and sixteen weeks after implantation. For a complete analysis, fixed tissue was utilized to assess classic neuroinflammatory markers (e.g. microglia, astrocytes, neuronal nuclei, blood brain barrier stability). Then, using non-fixed tissue, we also quantified the level of neurodegeneration and level of reactive oxygen species around the implant.
3.5.1 Tissue Sectioning
Following cryopreservation in the sucrose gradient, both fixed and non-fixed tissue samples were frozen at −80° C in optimal cutting temperature (OCT) compound (Tissue-Tek). Individual brain hemispheres were then sliced axially into 20 µm thick sections and mounted directly onto slides (SuperFrost Plus) and stored at −80° C until immunohistochemical labeling. For fixed tissue, during tissue sectioning, the cryostat was kept at an internal temperature of −22° C, where for non-fixed tissue, an internal temperature of −25° C was utilized.
3.5.2 Antibodies
The following primary antibodies were utilized in this study: mouse anti-neuronal nuclei (NeuN) (1:250, #MAB377, Chemicon), rabbit anti-immunoglobulin G (IgG) (1:250, #618501, AbD Serotec), rabbit anti-IBA-1 (1:250, #019-1974, Wako), and mouse anti-CD68 (1:100, #MAB1435, Chemicon). All antibody tissue labeling was performed on fixed tissue sections.
3.5.3 Neuroinflammatory Markers and Blood-Brain Barrier Permeability
For labeling of microglia/macrophages (IBA-1/CD68), neuronal nuclei (NeuN), and immunoglobulin G (blood-brain barrier stability; IgG), our previously outlined methodology was utilized (Potter et al., 2013). Briefly, fixed tissue sections were removed from the −80° C and allowed to equilibrate to room temperature for a minimum of 30 minutes. Then, remaining OCT was removed using three subsequent washes of 1X PBS and tissue permeated by incubation with 1X PBS containing 0.1% Triton-X 100 (1X PBS-T) for 15 minutes. All slides were then blocked for one hour at room temperature in blocking buffer (4% v/v goat serum (Invitrogen), 0.3% v/v Triton-X 100, 0.1% w/v sodium azide (Sigma)). Following blocking, the primary antibody, diluted in blocking buffer, was added directly to tissue sections and allowed to incubate for 18 hours at 4° C.
Unbound primary antibodies were removed using six subsequent washes in 1X PBS-T. Species-specific secondary antibodies (anti-rabbit or mouse Alexa-Fluor 488 and anti-rabbit or mouse Alexa-Fluor 594, Life Technologies) were diluted 1:1000 in blocking buffer, applied directly to tissue sections and incubated at room temperature for 2 hours. In addition to secondary antibodies, 4’6-diamidino-2-phenylindole (DAPI; Life Technologies) was diluted at 1:32,000 to co-label total cell nuclei. Following incubation in secondaries, tissue sections were thoroughly washed with six subsequent washes of 1X PBS-T. Then, remaining detergent was removed using three addition washes of 1X PBS. To remove hemosiderin-laden macrophage autofluorescence, tissue was then treated for 10 minutes with 0.5 mM copper sulfate buffer (50 mM ammonium acetate, pH 5.0) (Potter et al., 2012a). All slides were then thoroughly rinsed with water and mounted with Fluoromount-G (Southern Biotech). All slides were allowed to dry at room temperature and stored in the dark until imaged. Unless otherwise noted, all wash steps were performed for five minutes.
3.5.4 Neurodegeneration (FJC) Labeling
In addition to total neuronal densities, neuronal viability around implanted devices was investigated for all time points and conditions. Fluorojade-C (FJC), a marker for degenerative neurons, was utilized due to its high resolution, contrast and low background following previously outlined methodology (Gu et al., 2012; Potter et al., 2013). Briefly, non-fixed tissue sections were removed from the −80° C and allowed to equilibrate to room temperature for at least one hour before staining. Slides were then gently washed with 1X PBS to remove remaining OCT and immediately immersed in 0.00005% FJC (Millipore) (diluted in cold (4° C) 1X PBS) for one minute. The FJC solution was then removed gently with 1X PBS and the stained tissue was dried on a slide warmer at 60° C for 5 to 10 minutes. To aide in dehydration, tissue was then immersed in xylenes (Sigma) for one minute and then immediately coverslipped with DPX mounting media (Leica Microsystems).
3.5.5 Intracellular Reactive Oxygen Species
Oxidative stress has been implicated to contribute to multiple failure modes of intracortical microelectrodes. In addition, we and others have previously shown that resveratrol can attenuate the accumulation of reactive oxygen species around injury sites(Ates et al., 2006; Baur and Sinclair, 2006; Constant et al., 2012; Potter et al., 2013). Therefore to investigate the utility of resveratrol in our model, we investigated the amount of intracellular reactive oxygen species around the implanted microelectrode using dihydroethidium (DHE) (Sigma) following our previously used methodology (Potter et al., 2013).
Briefly, DHE stock was prepared to a concentration of 3 mM in dimethyl sulfoxide (DMSO) and stored at −20° C. For tissue staining, non-fixed tissue sections were removed from the −80° C and allowed to equilibrate to room temperature for at least one hour. Next, the DHE staining solution (stock solution diluted 1:100 in 1X PBS (30 µM final concentration)) was added directly onto slides and incubated at room temperature for 30 minutes. DHE was washed off the slides using three brief washes with 1X PBS. All slides were coverslipped with Fluoromount-G and immediately imaged after labeling.
3.5.6 Hematoxylin and Eosin (H&E) Labeling
Liver samples to be assessed for localized hemorrhaging were processed at the Case Western Reserve University Pathology Core. Briefly, tissue samples were embedded in OCT and sliced at 20 µm in a cryostat. Tissue sections were then applied directly to slides and stored at −80° C until labeling.
For staining, slides were removed from the freezer and allowed to equilibrate to RT for approximately 30 minutes. Slides were then incubated in a hemotoxylin stock solution for three minutes. Hemotoxylin was removed using a five minute rinse in deionized water and initially fixed using a dip in acid alcohol. The slides were then thoroughly rinsed with tap water and deionized water each for two minutes. Excess water was then blotted from slides and tissue samples were incubated in eosin stock solution for 30 seconds. Following staining, tissue was thoroughly dried using a serial alcohol wash step (95% and 100%). Tissue was washed two times for five minutes in each alcohol concentration. Finally, tissue was cleared in xylenes and mounted using Permount.
3.6 Imaging and Quantitative Analysis
All fluorescent images for this study were acquired on an AxioObserver Z1 (Zeiss Inc.) and an AxioCam MRm (Zeiss Inc.) using a 10X objective. A large field of view for each acquired image was achieved by stitching together at least nine individual 10X images using the MosaiX software (Zeiss). Exposure times were held constant for each analyzed marker at all time points and conditions. Final acquired images were then exported as 16-bit tagged imaging files (TIFs) using Axiovision LE (Zeiss Inc.) to be quantified in MATLAB.
3.6.1 Neuroinflammatory Marker Quantification
For all immunohistochemical markers except those for neuronal nuclei (NeuN), our freely available, recently updated MATLAB program, MINUTE v1.5 was utilized (Potter-Baker et al., 2014b). The chronic implant was defined manually within the graphical user interface and undesired regions excluded from image quantification. Then, rings were expanded from the defined region of interest in 2 µm regions and the intensity within the ring measured up to 1500 µm away from the implant interface. Quantified intensity profiles were then normalized to a background region defined as approximately 700 µm away from the interface. Background was defined for each tissue section and held constant for all conditions and time points. For statistical comparisons between conditions, the area under the intensity curve was also calculated in MATLAB at pre-determined binning regions of 0 to 50 µm, 50 to 100 µm, 100 to 200 µm, 200 to 300 µm, and 300 to 400 µm. For presentation purposes, data are reported as the average normalized fluorescent intensity of each analyzed implant ± standard error of the mean (s.e.m.) (n≥4).
3.6.2 Measurement of Neuronal Survival
Neuronal survival around implanted microelectrodes for all conditions and time points was quantified using Adobe Photoshop. Similar to neuroinflammatory markers (see Section 3.6.1), the implantation site was manually defined and then expanding concentric rings at fixed radii were drawn up to 400 µm from the defined region. The area within each ring and the total number of neuronal nuclei were then determined manually. For all conditions and time points, neuronal nuclei densities were normalized to age-matched non-surgery control animals. Neuronal densities for sham animals were defined as 2757.40 ± 274.83 and 1815.34 ± 176.96 for two and sixteen weeks respectively and determined using aforementioned methodology. Thus, all neuronal nuclei densities are represented as a percent to non-surgical sham animal for each respective time point ± s.e.m.
3.7 Statistical Analysis
For this study, a sample was defined as the averaged data within a given implant. For each analyzed marker, a minimum of four implants were used for statistical comparison. A general linearized model three-way analysis of variance (ANOVA) was used to compare the four conditions at each respective time point. Pair-wise comparisons were conducted using a Tukey’s test, where a p<0.05 was considered to be significant.
4. Results
4.1 Bioavailability and Biodistribution of Resveratrol Around Implanted Microelectrodes
Resveratrol has been widely used for therapeutic treatment in many neurodegenerative and inflammatory in vitro and in vivo disease models (Han et al., 2004; Baur and Sinclair, 2006; Han et al., 2012). For in vitro applications, resveratrol has shown the greatest effectiveness in a concentration range of 5 to 25 µM (Sun et al., 2010; Ravikumar et al., 2012a). However, few studies have investigated effective in vivo concentrations of resveratrol in diseased or inflamed tissue. Therefore we sought to determine a dosing regime that would result in a sustained concentration of resveratrol surrounding implanted microelectrodes up to sixteen weeks after implantation.
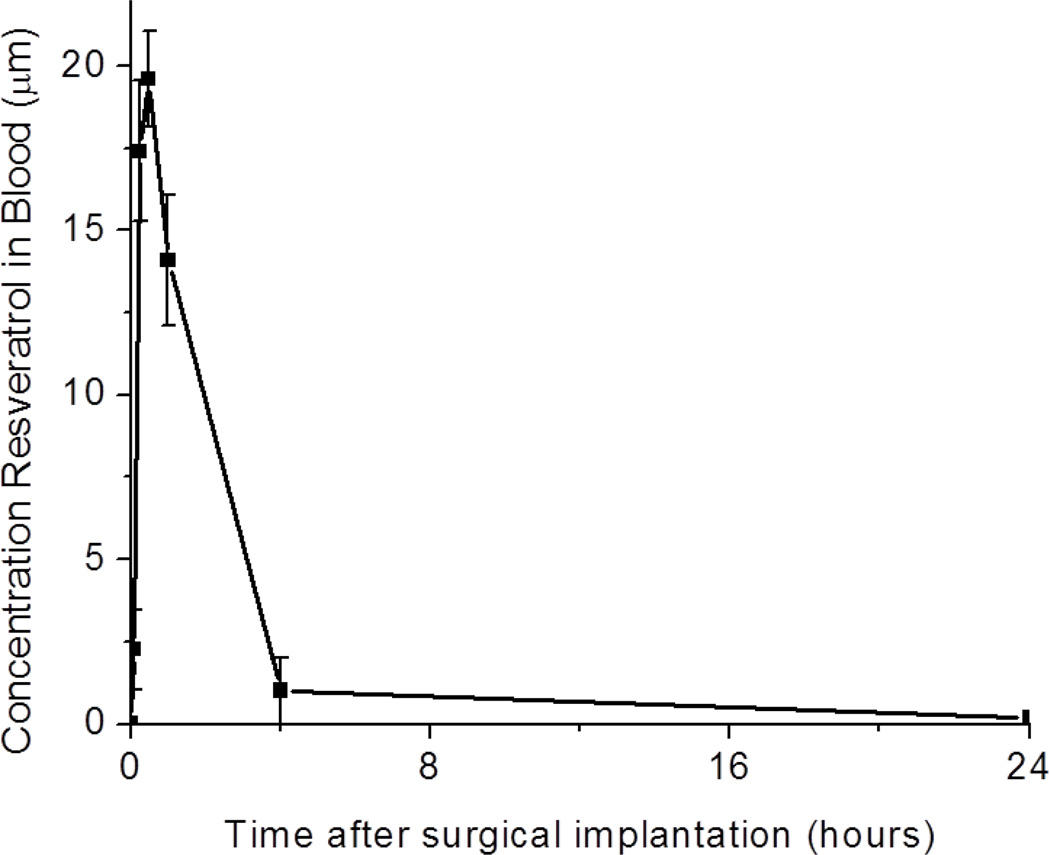
First, to gain a better understanding of the bioavailability of resveratrol in our model, we conducted an initial assessment of the lifespan of the anti-oxidant in the blood stream, following a two-dose administration scheme (the day before and immediately following surgery). Specifically, we found that following a two-dose administration, resveratrol was rapidly cleared from the blood stream within 24 hours post implantation (Figure 1). Peak concentrations of resveratrol (approximately 20 µM) were noted in the blood stream 1 hour after surgery with minimal detection after 4 hours.
Figure 1.
Bioavailability of resveratrol in the blood stream following administration of the anti-oxidant the day before and the day of surgery. N = 4. Data represents average ±
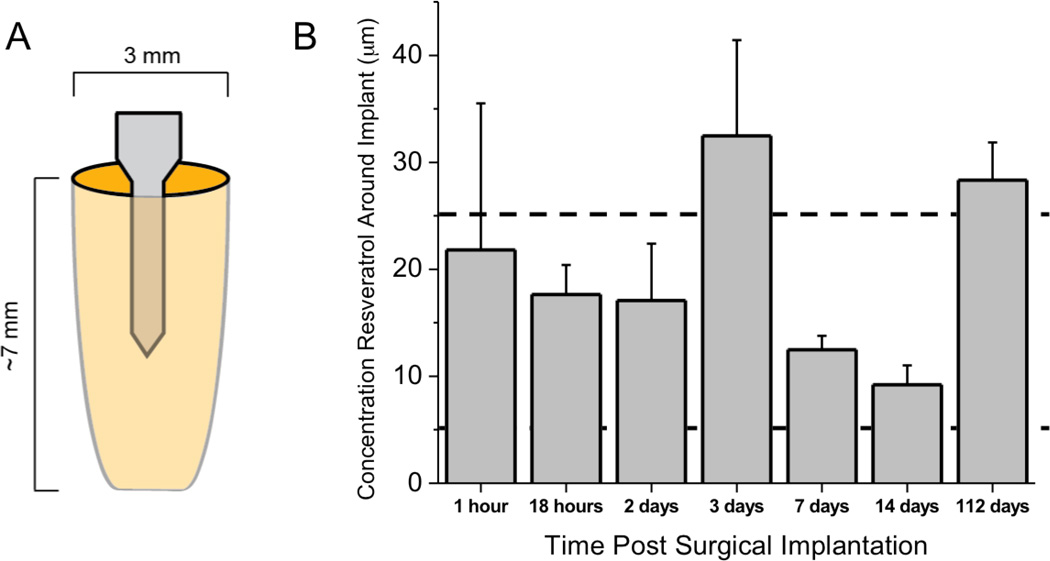
Within cortical tissue immediately adjacent to the implanted microelectrode, we found that a two-dose injection at 30 mg/kg of resveratrol resulted in a sustained range of resveratrol (5 to 25 µM) much longer than what was noted in blood (Figure 2). Specifically, at 18 hours after surgery, the tissue surrounding the implanted microelectrode had a resveratrol concentration of approximately 18 µM. However, by 24 hours after implantation, minimal amounts of resveratrol were detected in brain tissue (data not shown). Therefore, in order to maintain a sustained concentration, we continued to assess the biodistribution of resveratrol in our system following daily injections. Notably, we found that daily injections of the anti-oxidant resveratrol resulted in a range of 5 to approximately 25 µM concentration locally surrounding the implant for sixteen weeks (Figure 2). The most variance in concentrations were noted at early time points (<3 days), likely due to a more instable blood-brain barrier (BBB). In addition, we noted a higher concentration of resveratrol around the implant at 3 days and 16 weeks (112 days). No significant differences were noted between resveratrol concentrations at any of the measured time points. Since 3 days was equally as high as 112 days, we do not anticipate that impaired liver function (see Section 4.6) may have limited resveratrol clearance at chronic time points.
Figure 2.
Biodistribution of resveratrol surrounding implanted microelectrodes up to sixteen weeks after implantation. (A) Implanted microelectrodes were explanted with surrounding tissue and assessed for resveratrol. (B) A dosing regime of daily 30 mg/kg resveratrol resulted in concentrations between 5 to 25 µM around the implanted microelectrode. Data represent average ± s.e.m. N=4.
4.2 Effect of Daily Resveratrol Administration on Neuronal Density and Viability
Neuronal density and viability around implanted microelectrodes have been suggested to directly affect microelectrode device functionality (Buzsáki, 2004; Schwartz, 2004; Jorfi et al., 2014). In addition, we have also previously shown that a two-dose anti-oxidant-dosing regime was capable of providing improved neuronal viability up to four weeks after implantation (Potter et al., 2013). Therefore, we first investigated the effects of chronic daily anti-oxidant administration on neuronal density and viability surrounding implanted microelectrodes.
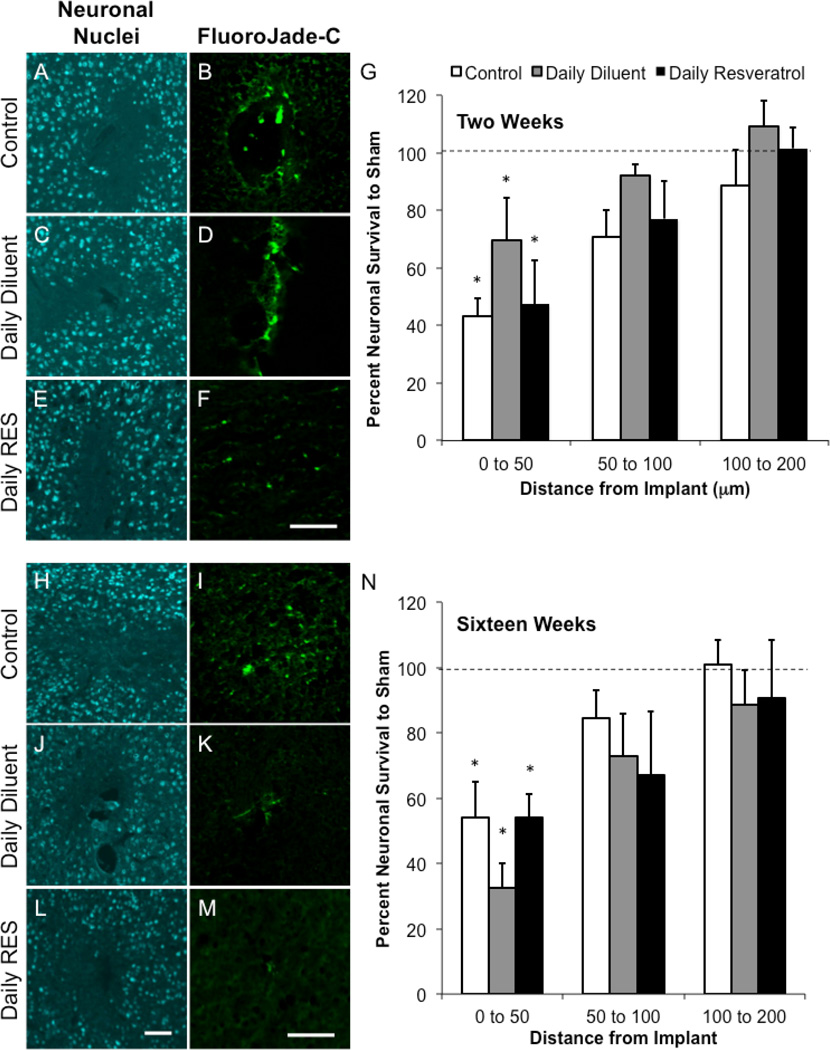
Interestingly, we did not note the same neuronal trends in our daily dosing regime in comparison to our previous study around implanted microelectrodes (Potter et al., 2013). Specifically we found that control and animals receiving daily diluent or resveratrol demonstrated statistically similar neuronal populations at both two and sixteen weeks post-implantation (Figure 3). Furthermore, at two weeks, all studied groups had significantly lower neuronal densities in comparison to sham background densities from 0 to 50 µm (Figure 3G). Similarly, by sixteen weeks, neuronal densities had recovered to background by 50 µm away from the interface of the device (Figure 3N).
Figure 3.
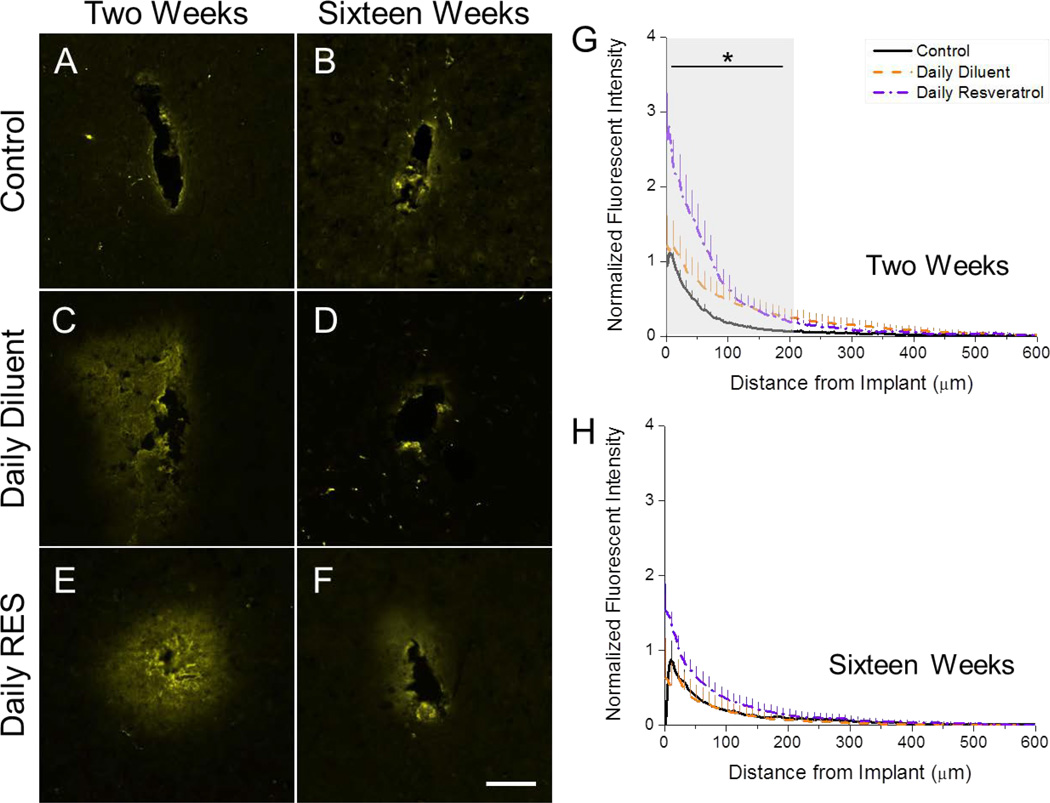
Neuronal nuclei densities and viability around implanted microelectrodes. Neuronal nuclei (NeuN) and fluorojade-C labeling was assessed at two (A-G) and sixteen (H-N) weeks following device implantation in tested animal groups. At two weeks, no statistical differences between animal conditions were noted (G). However, all conditions, from 0 to 50 µm, demonstrated significantly lower densities than background (*p<0.05). By sixteen weeks, only changes were noted in fluorojade-C labeling, where daily diluent and daily resveratrol animals had smaller populations of degenerating neurons (I, K, M). Only the binning region of 0 to 50 µm had significantly lower densities than background (N; *p<0.05). Data represent average ± s.e.m. N≥4. Scale = 100 µm.
While no changes in neuronal densities were noted between animal conditions, we did observe qualitative differences in neuronal viability around implanted microelectrodes between conditions at both two and sixteen weeks after surgery. At two weeks, we found that animals receiving daily resveratrol qualitatively appeared to have fewer degenerating neurons in comparison to controls and animals receiving a daily diluent (Figure 3B, D, F). Similar trends were noted at sixteen weeks; however, at this time point, both animals receiving daily resveratrol and diluent had smaller quantities of degenerating neurons in comparison to control animals (Figure 3I, K, M).
4.3 Accumulation of Reactive Oxygen Species
Secretion of reactive oxygen species from inflammatory cells around implanted microelectrodes can directly affect neuronal health, and has also been implicated in material failure modes of functional electrodes (Balaban et al., 2005; Gandhi and Abramov, 2012; Prasad et al., 2012; Prasad et al., 2014). Administration of resveratrol can directly reduce the amount of reactive oxygen species released by inflammatory cells, such as astrocytes and microglia (Lu et al., 2010). In addition, resveratrol administration has also been shown to instill neuroprotection in both in vitro and in vivo models (Wang et al., 2002; Li et al., 2010; Liu et al., 2011; Ravikumar et al., 2012a; Potter et al., 2013). Therefore, to better understand the role of reactive oxygen species in our model, we next investigated the accumulation of superoxide anion around implanted microelectrodes.
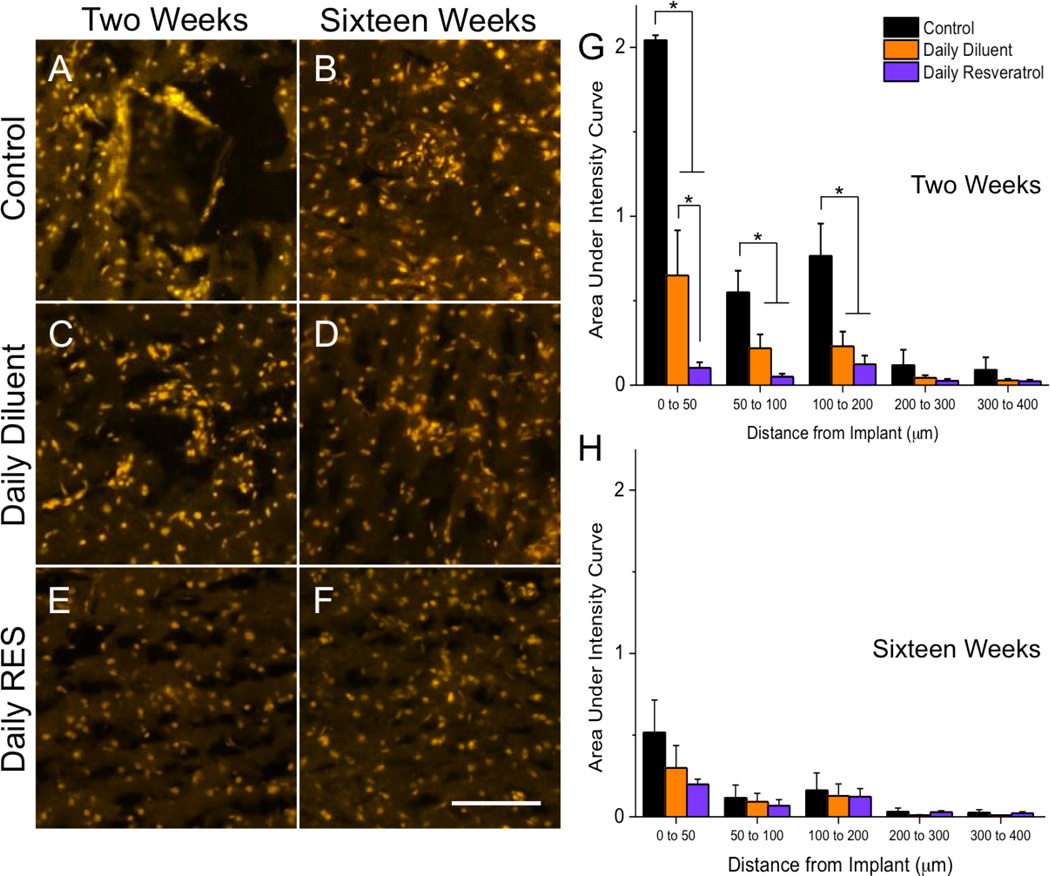
At two weeks after implantation, we found that control animals had high levels of superoxide anion, as noted by dihydroethidium (DHE) labeling, around the implanted device, with the highest amounts accumulating from 0 to 50 µm away from the implant (Figure 4A, G). Animals receiving a daily diluent injection had significantly less DHE staining than control animals up to 200 µm from the implanted device (Figure 4C, G). Similarly, animals that were administered a daily resveratrol injection had significantly lower superoxide anion accumulations in comparison to control animals out to 200 µm from the interface (Figure 4E, G). In addition, however, animals receiving daily resveratrol also had significantly less DHE labeling noted from 0 to 50 µm away from the implant in comparison to daily diluent animals. Our results collectively suggest that both daily injections alone and treatment with resveratrol can significantly alter reactive oxygen species accumulation around the implanted microelectrode at two weeks post-implantation.
Figure 4.
Accumulation of superoxide anion (dihydroethidium (DHE)) around implanted microelectrodes. At two weeks, high levels of DHE labeling were noted in control animals (A, G). Administration of a daily diluent alone resulted in significant decreases in superoxide anion accumulation in comparison to controls (*p<0.05; C, G) up to 200 µm from the implant interface. However, daily resveratrol administration had significantly lower DHE labeling than both other test groups from 0 to 50 µm from the implant interface at two weeks post implantation (E, G). By sixteen weeks, no significant differences between conditions were noted for all investigated binning intervals (B, D, F, H). Data represent average ± s.e.m. N≥4. Scale = 100 µm.
By sixteen weeks after implantation, no significant differences were noted between animal conditions for accumulated superoxide anion. It is important to note that a significant drop in DHE labeling was noted in control animals from two to sixteen weeks from 0 to 50 µm away from the implant (p<0.05). However, no significant changes in DHE labeling were noted in either daily dosed animal group between two and sixteen weeks.
4.4 Blood-Brain Barrier Stability
Fluctuations in neuronal viability have also been correlated with a leaky blood-brain barrier (BBB) (Winslow et al., 2010). In fact, it was also recently reported that viability of local vasculature could also directly affect neuronal signal recording quality (Saxena et al., 2013). In addition, increased accumulation of reactive oxygen species can directly affect the integrity of localized blood vessels (Abbott et al., 2006). Therefore to investigate BBB stability in our model, we utilized labeling of immunoglobulin-G (IgG), a serum protein non-native to cortical tissue (Aihara et al., 1994).
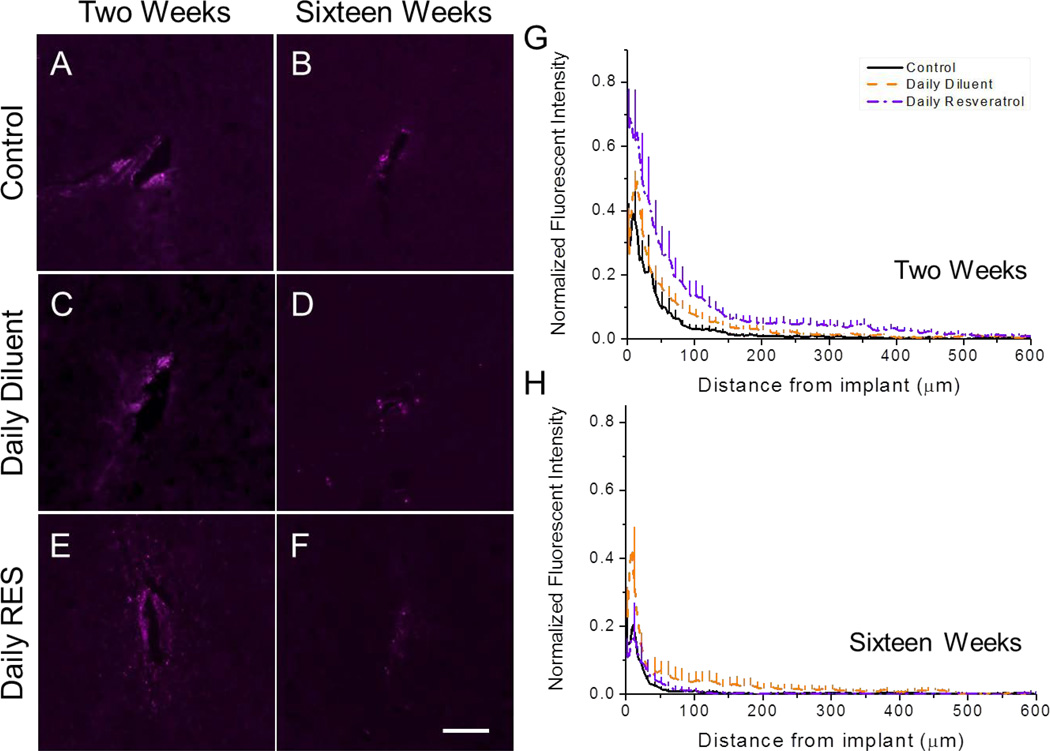
Notably we found that all animal conditions, for all investigated time points, had significantly higher amounts of IgG at the interface of the device than background. At two weeks, significantly higher amounts of IgG were noted in resveratrol-dosed animals in comparison to control animals up to 100 µm from the implant interface (Figure 5; Table 1). Similarly, animals receiving a daily diluent injection had greater IgG accumulation from 100 to 200 µm from the implant interface in comparison to the control (Figure 5G). No significant differences were noted between daily resveratrol and daily diluent injected animals at two weeks after implantation.
Figure 5.
Accumulation of immunoglobulin G (IgG) surrounding intracortical microelectrodes at two and sixteen weeks post implantation. At two weeks, significant differences were noted between the animal conditions up to 200 µm away from the implant interface (A,C,E,G) (*See Table 1). By sixteen weeks, no noted significances were found between animal groups for all investigated binning intervals (B,D,F,H). Data represent average ± s.e.m. N≥4. Scale = 100 µm.
Table 1.
Statistical Comparisons for IgG Accumulation at Two Weeks
| Control | Daily Resveratrol | ||
|---|---|---|---|
| Daily Diluent | 0 to 50 µm | p<0.3231 | p<0.1238 |
| 50 to 100 µm | p<0.1334 | p<0.5073 | |
| 100 to 200µm | p<0.0433 | p<0.8530 | |
| Daily Resveratrol | 0 to 50 µm | p<0.0054 | -- |
| 50 to 100 µm | p<0.0140 | -- | |
| 100 to 200 µm | p<0.1234 | -- |
By sixteen weeks, all animal groups displayed similar IgG accumulation profiles with return to background intensity being noted by 200 µm away from the implant (Figure 5H).
4.5 Effect of Daily Resveratrol Administration on Microglia/Macrophage Activation
Activated microglia and macrophages can directly facilitate localized neurodegeneration and BBB breakdown (Fawcett and Asher, 1999; Guillemin, 2003; Kettenmann et al., 2013). Specifically, it has been shown that following activation, microglia and macrophages can release high concentrations of numerous pro-inflammatory molecules, including reactive oxygen species, which can influence the surrounding environment (Zhang et al., 2011; Aguzzi et al., 2013). Therefore, because of our noted differences in superoxide anion accumulation and BBB permeability, we next quantified the amount of CD68+ cells around microelectrodes to determine the role of activated microglia and macrophages in our system.
Interestingly, at two and sixteen weeks after implantation we did not note any significant differences in CD68+ cell distribution profiles between investigated animal conditions (Figure 6). However, both control and daily diluent animals demonstrated similar CD68+ cell accumulation profiles between two and sixteen weeks, while a reduction in CD68+ labeling was noted in daily resveratrol-dosed animals from two to sixteen weeks post-implantation (Figure 6G–H).
Figure 6.
Accumulation of CD68+ cells around implanted microelectrodes at two and sixteen weeks. The amount of activated microglia and macrophages was quantified using CD68 labeling at the device-tissue interface. At both two (A,C,E,G) and sixteen (B,D,F,H) no significant differences were noted between animal conditions. Data represent average ± s.e.m. N≥4. Scale = 100 µm.
4.6 Anatomical Observations of Peripheral Organs
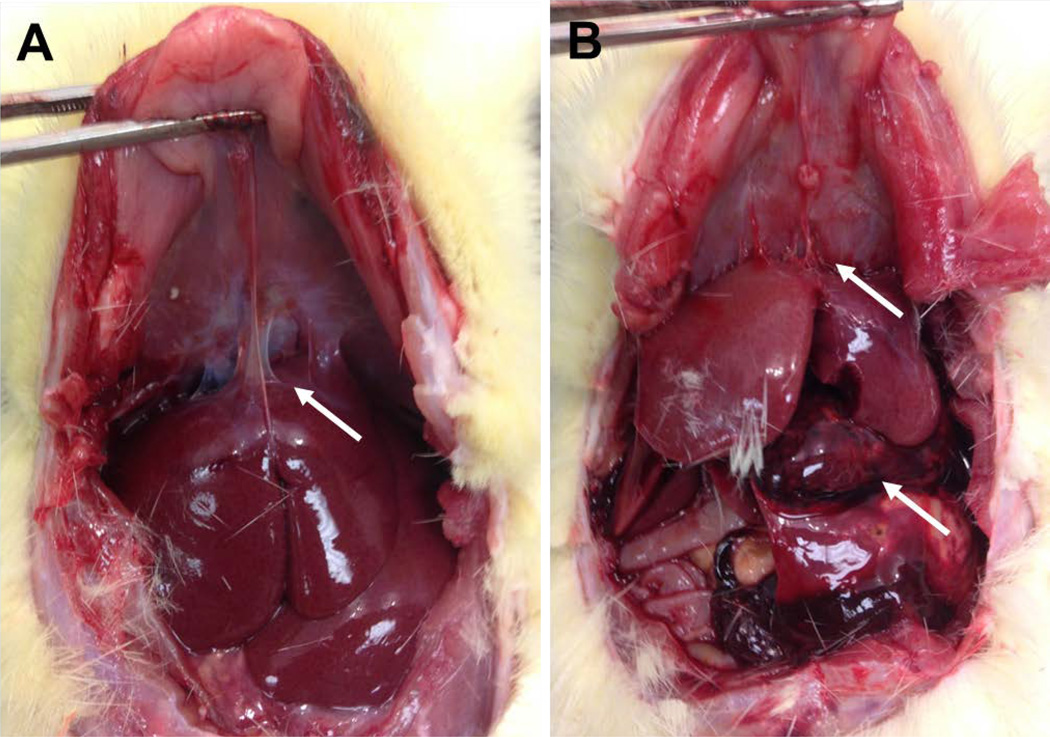
In addition to investigating the neuroinflammatory and neurodegenerative response around implanted microelectrodes, we also assessed any gross changes in peripheral organs at time end points. Of note, we found hemorrhage concentrated at the liver during animal perfusion in 50% of chronically (16-week) administered resveratrol animals (4 of 8) (Figure 7). In addition, 75% of daily resveratrol animals (6 of 8) displayed thread-like adhesions between the liver and diaphragm (Figure 7A). No abdominal hemorrhage was noted in two-week animals.
Figure 7.
Gross anatomical observations in rats receiving daily resveratrol injections for sixteen weeks. (A, B) Violin-string type adhesions were noted between the liver and diaphragm in animals receiving daily resveratrol injections. (B) In addition, approximately 50% (N=4) of animals displayed large hemorrhaging directly on the liver.
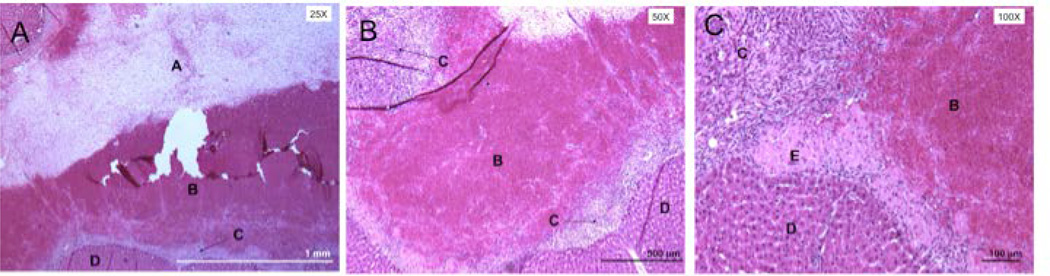
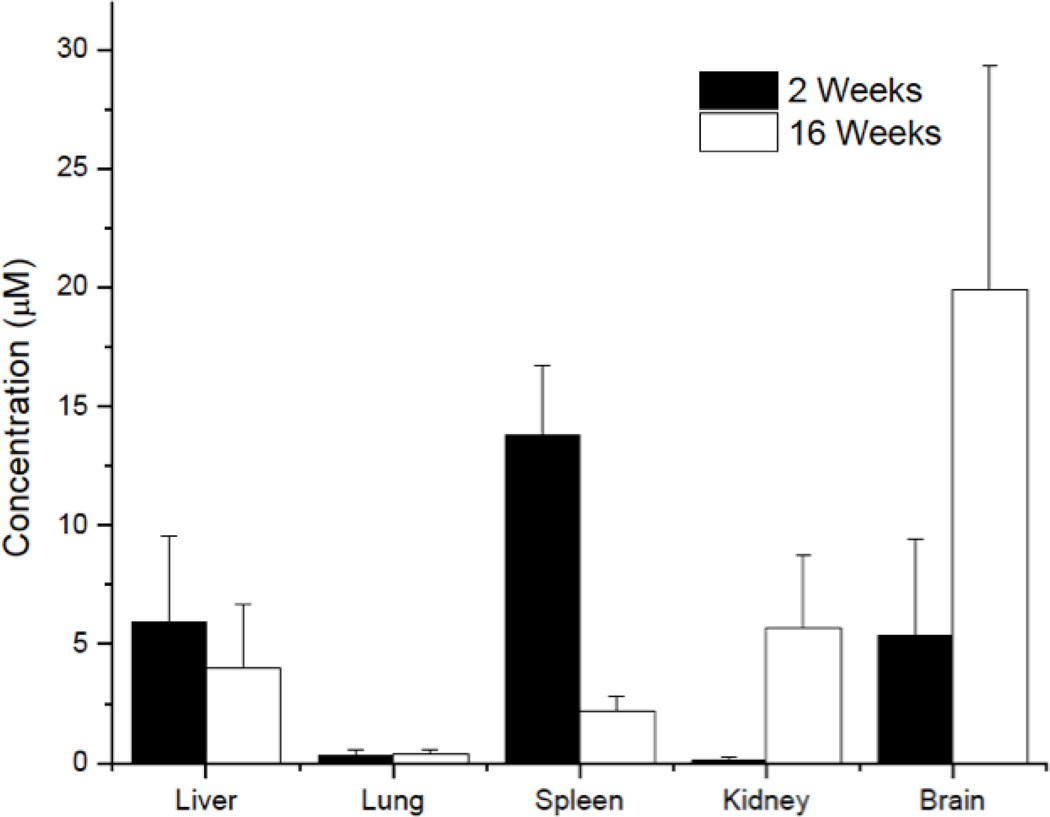
Pathological evaluation further confirmed the presence of increased hemorrhage in the liver (Figure 8). Within the liver, we noted appreciable amounts of granulation tissue, likely in response to the increased hemorrhage (as denoted by clotted blood) in the local tissue space (Figure 8). Administration or accumulation of resveratrol was likely a key factor since no systemic hemorrhaging events were noted in daily diluent animals (data not shown). In addition, we did not find any toxic levels of resveratrol in surrounding intestinal organs, as determined by our biodistribution analysis (Figure 9).
Figure 8.
Hemotoxylin and Eosin (H&E) staining of liver sections in a rat receiving daily resveratrol injections for sixteen weeks. Within our analysis of the affected liver tissue, we found the presence of dissolved clotted blood (A), clotted blood (B), granulation tissue (C), normal liver tissue (D) and dying hepatocytes (E).
Figure 9.
Biodistribution analysis of surrounding organs in animals receiving daily resveratrol injections at both two weeks (black) and sixteen weeks (white). No toxic concentrations (>0.1 mM) of resveratrol were noted at either time point or in any organ sample.
5. Discussion
Chronic reliable use of microelectrode technology could prove critical in understanding the connectivity of the nervous system. Specifically, long-term neuronal recordings have the potential to aide in diagnosing and treating multiple neurodegenerative disease states and conditions (Marg and Adams, 1967; Ward et al., 2009; Duffau, 2013). In addition, chronically recorded single-unit neuronal action potentials can function as command signals to control external devices, such as a computer cursor or robotic limbs, to restore motor deficits in patients with neurological diseases (Kim et al., 2008; Simeral et al., 2011; Hochberg et al., 2012).
However, for any long-term application, implanted microelectrodes all suffer a similar fate – short term and chronic instability and unreliability (Schwartz, 2004). To that end, multiple groups have begun to better understand the mechanisms resulting in microelectrode failure. For example, Barrese et al recently reported on the prevalence of common failure modes in chronically implanted Utah microelectrode arrays and suggested that either (1) mechanical, (2) electrical, (3) material or (4) biological failures were the main reason for final decays/losses in neuronal recording quality (Barrese et al., 2013). Prasad and colleagues have corroborated these results in both microwire and platinum electrodes (Prasad et al., 2012; Prasad et al., 2014), while Kozai et al have also demonstrated them in silicon planar arrays(Kozai et al., 2014).
Within both Barrese and Prasad’s studies, it was hypothesized that oxidative stress may facilitate in and propagate electrical, material and biological failure modes (Barrese et al., 2013; Prasad et al., 2014). This observation is not surprising given that previous groups have suggested the ability of released radicals to directly (1) facilitate the breakdown of insulator/electrical contacts (Schmitt et al., 1999), (2) promote the development and propagation of a glial scar and pro-inflammatory molecules through the nF-κβ pathway (Fawcett and Asher, 1999; Sofroniew, 2009), (3) cause the formation of localized neuronal degeneration (Olanow, 1993; Blumberg, 2004) and (4) lower the integrity of localized vasculature (Abbott, 2000).
Therefore, given the role of reactive oxygen species in microelectrode failure, we hypothesize that a reduced oxidative environment around microelectrodes could not only mitigate neurodegeneration but also prevent the physical breakdown of the electrode itself. In particular we have sought to better understand the biological mechanisms that could facilitate and mitigate the release of radicals around implanted microelectrodes in an attempt to engineer therapeutic approaches.
One promising therapeutic strategy that we have deployed in the application of intracortical microelectrodes has been the use of naturally derived anti-oxidants. Naturally derived anti-oxidants, like resveratrol and curcumin, have gained large popularity in the biosciences due to relatively low side effects and the suggested ability of the body to tolerate long-term administration regimes (Sovak, 2001; Bureau et al., 2008). In the central nervous system, anti-oxidants have had success in alleviating detrimental biological events associated with traumatic brain injury (Ates et al., 2006), spinal cord injury (Lin et al., 2011; Liu et al., 2011), ischemia (Lu et al., 2006; Girbovan et al., 2011; Simao et al., 2012), electrode implantation (Constant et al., 2012; Potter et al., 2013; Potter et al., 2014) and disease states such as Parkinson’s and Alzheimer’s (Olanow, 1993; Sun et al., 2010; Wu et al., 2012).
Chronic studies with anti-oxidants have shown variable results(Baur and Sinclair, 2006; Fonseca-Kelly et al., 2012; Soufi et al., 2012). For example, Fonseca-Kelly and colleagues showed that daily oral gauvage treatment of 100 to 250 mg/kg resveratrol was able to delay the onset of multiple sclerosis up to 30 days. However, Fonseca-Kelly also reported that their resveratrol administration scheme did not alter inflammation or cell phenotypes within the diseased tissue. Similar mixed results have been suggested by, Ghadiri Soufi et al. following a four-month administration regime in a diabetic rat model. For both of these reported studies, it is important to note that no data on the concentration of the anti-oxidant in the target region were provided. Therefore, to ensure that a sustained concentration of our employed anti-oxidant would be apparent around the implanted electrode, we first validated an optimal dosing regime using quantitative biodistribution.
Notably we found that intraperitoneal (i.p) delivery of the anti-oxidant resveratrol resulted in a concentration range of 5 to approximately 25 µM, a range found to be an optimal in vitro (Sovak, 2001; Sun et al., 2010; Ravikumar et al., 2012b), surrounding the implanted microelectrode up to sixteen weeks post implantation (Figure 2). Interestingly, we also noted that oral administration of resveratrol at 200 mg/kg/day was unable to result in any detectable quantities of the anti-oxidant around the implanted microelectrode (data not shown), suggesting that bioavailability of resveratrol around implanted microelectrodes was dictated by the route of administration. This is not surprising since similar studies have suggested that i.p. or intravenous (i.v.) administration of resveratrol results in the greatest bioavailability (Walle et al., 2004; Wenzel and Somoza, 2005; Walle, 2011).
Collectively, we found that a sustained concentration of resveratrol around implanted microelectrodes resulted in marked improvements in neuronal viability in comparison to controls at both two and sixteen weeks after device implantation (Figure 3). Improvements in neuronal viability in resveratrol-dosed animals were correlated with an initial reduction in superoxide anion accumulation (Figure 4). However, since no significant differences between resveratrol-dosed animals and controls were noted for CD68+ cells (Figure 6), this suggests that resveratrol administration did not alter cellular activation. Alternatively, we hypothesize that the anti-oxidant likely altered intracellular pathways of activated cells, including the up-regulation of anti-oxidative enzymes such as superoxide dismutase and catalase, as noted in our previous work (Potter et al., 2013).
As an added control, we also investigated the effects of daily dosing with a saline diluent in implanted rats up to sixteen weeks. Notably, we found that daily dosing with saline alone resulted in significantly less superoxide anion accumulation around implanted microelectrodes, in comparison to controls at two weeks post implantation (Figure 4). Similar to daily resveratrol animals, initial reduction of reactive oxygen species accumulation resulted in qualitative improvements in neuronal viability at sixteen weeks after implantation, in comparison to controls (Figure 3). This result is critical, since no differences in reactive oxygen species accumulation was noted at sixteen weeks between the animal conditions (Figure 4). Therefore taken collectively, our results suggest that an initial suppression of reactive oxygen species can facilitate chronic improvements in neuronal viability around implanted microelectrodes. In addition, given that differences in neuronal viability existed at two weeks between daily diluent and daily resveratrol animals (Figure 3), it is likely that acute reactive oxygen species-mediated neurodegeneration is threshold dependent. Previous work by our group supports a threshold dependent event. Specifically, we have observed other threshold depended cellular responses in cell adhesion, extracellular matrix assemble (Capadona et al., 2005), and more importantly, microglia response to various material-based and endotoxin-mediated neuroinflammatory events (Ravikumar et al., 2012a; Ravikumar et al., 2013).
Though our results following daily administration of resveratrol are promising, we cannot discount the observation that both animals injected with daily diluent and daily resveratrol had a significantly more permeable BBB than control animals (Figure 5, Table 1). Collectively our results suggest that both daily injections and incorporation of resveratrol directly or indirectly affected vascular integrity at two weeks after implantation. In the context of pure injection volume, it is likely that changes in blood pressure following i.p. administration contributed to some noted differences in IgG accumulation around implanted microelectrodes (Mayhan, 2001). As a result, future research may need to consider monitoring blood pressure changes both peripherally and within the brain to ensure therapeutic administration does not result in adverse events.
In addition, while our dosing regime resulted in sustained resveratrol concentrations around implanted microelectrodes, it is likely that secondary side effects of the anti-oxidant were contributing to increased hemorrhaging locally and systemically (Figure 7 and Figure 8). In fact it has been suggested that chronic intake of high amounts of some anti-oxidants can result in a reduction in the ability to form blood clots, making patients more susceptible to bleeding (Langseth, 2000). In addition, given the observation of thread-like adhesions in daily resveratrol animals, we suggest that our chronic i.p. administration regime of resveratrol may result in increased abdominal inflammation or possible infection. Further, thread-like adhesions could have occurred from mechanical irritation. Resveratrol has a poor solubility in aqueous solutions. The diluent that we used required both agitation and heating to ‘disperse’ (and not fully dissolve) the resveratrol in solution prior to administration. Therefore, there is no evidence that the resveratrol stayed dispersed once the diluent was diluted with physiological solutions.
In conclusion, despite the limitations of chronic daily anti-oxidant dosing, the results of our study suggest that daily anti-oxidant administration can result in marked improvements in neuronal viability around implanted microelectrodes up to sixteen weeks post-implantation. Further, we have provided evidence that a threshold for reactive oxygen species suppression exists initially and may be a key factor in facilitating chronic neuronal viability to implanted microelectrodes. Therefore given our results, we suggest that future studies further optimize the chronic administration of resveratrol to mitigate undesired systemic side effects that may affect chronic neuronal recording stability. In addition, the anti-oxidant utilized here, resveratrol, was a single approach to investigating the effects of chronic anti-oxidant administration. Given the inherent side effect challenges with resveratrol, we also suggest that future studies consider the use of alternative synthetic or natural anti-oxidants, such as curcumin, vitamin E and melatonin to mitigate neurodegeneration to intracortical microelectrodes.
Acknowledgements
The authors gratefully acknowledge the help of M. Freeberg and J. Nguyen for help in data analysis. The authors also acknowledge the help of S. Siedlak in the histological processing of liver samples. This work was supported by the Department of Biomedical Engineering and Case School of Engineering at Case Western Reserve University through lab start-up funds, the Department of Education Graduate Fellowship in neural engineering, GAANN:P200A100112 (K. Potter) and Medtronic Fellowship (K. Potter). Additional funding on this research was supported in part by the Department of Veterans Affairs Merit Review (J. Capadona, B7122R), Presidential Early Career Award for Scientist and Engineers (J. Capadona, PECASE), and the National Institute of Health (J. Capadona, National Institute of Neurological Disorders and Stroke, 1R01NS082404-01A1). None of the funding sources aided in the collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication.
Footnotes
The authors have no conflicts of interest related to this work to disclose.
References
- Abbott NJ. Inflammatory Mediators and Modulation of Blood-Brain Barrier Permeability. Cellular and molecular neurobiology. 2000;20:131–147. doi: 10.1023/A:1007074420772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nature Reviews Neuroscience. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Aguzzi A, Barres BA, Bennett ML. Microglia: scapegoat, saboteur, or something else? Science. 2013;339:156–161. doi: 10.1126/science.1227901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aihara N, Tanno H, Hall JJ, Pitts LH, Noble LJ. Immunocytochemical localization of immunoglobulins in the rat brain: relationship to the blood-brain barrier. The Journal of Comparative Neurology. 1994;342:481–496. doi: 10.1002/cne.903420402. [DOI] [PubMed] [Google Scholar]
- Ates O, Cayli S, Altinoz E, Gurses I, Yucel N, Sener M, Kocak A, Yologlu S. Neuroprotection by resveratrol against traumatic brain injury in rats. Molecular and Cellular Biochemistry. 2006;294:137–144. doi: 10.1007/s11010-006-9253-0. [DOI] [PubMed] [Google Scholar]
- Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Barrese JC, Rao N, Paroo K, Triebwasser C, Vargas-Irwin C, Franquemont L, Donoghue JP. Failure mode analysis of silicon-based intracortical microelectrode arrays in non-human primates. J Neural Eng. 2013;10:066014. doi: 10.1088/1741-2560/10/6/066014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nature reviews Drug discovery. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- Blumberg J. Free Radicals: The Pros and Cons of Antioxidants. The Journal of Nutrition. 2004;134:3188S–3189S. doi: 10.1093/jn/134.11.3188S. [DOI] [PubMed] [Google Scholar]
- Bureau G, Longpré F, Martinoli MG. Resveratrol and quercetin, two natural polyphenols, reduce apoptotic neuronal cell death induced by neuroinflammation. Journal of Neuroscience Research. 2008;86:403–410. doi: 10.1002/jnr.21503. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Large-scale recording of neuronal ensembles. Nature Neuroscience. 2004;7:446–451. doi: 10.1038/nn1233. [DOI] [PubMed] [Google Scholar]
- Capadona JR, Petrie TA, Fears KP, Latour RA, Collard DM, García AJ. Surface-Nucleated Assembly of Fibrillar Extracellular Matrices. Advanced Materials. 2005;17:2604–2608. [Google Scholar]
- Cogan SF. Neural Stimulation and Recording Electrodes. Annual Review of Biomedical Engineering. 2008;10:275–309. doi: 10.1146/annurev.bioeng.10.061807.160518. [DOI] [PubMed] [Google Scholar]
- Constant JP, Fraley GS, Forbes E, Hallas BH, Leheste JR, Torres G. Resveratrol protects neurons from cannulae implantation injury: Implications for deep brain stimulation. Neuroscience. 2012 doi: 10.1016/j.neuroscience.2012.06.067. [DOI] [PubMed] [Google Scholar]
- Delmas D, Aires V, Limagne E, Dutartre P, Mazué F, Ghiringhelli F, Latruffe N. Transport, stability, and biological activity of resveratrol. Annals of the New York Academy of Sciences. 2011;1215:48–59. doi: 10.1111/j.1749-6632.2010.05871.x. [DOI] [PubMed] [Google Scholar]
- Donoghue JP, Nurmikko A, Black M, Hochberg LR. Assistive technology and robotic control using motor cortex ensemble-based neural interface systems in humans with tetraplegia. The Journal of Physiology. 2007;579:603–611. doi: 10.1113/jphysiol.2006.127209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffau H. Brain mapping in tumors: Intraoperative or extraoperative? Epilepsia. 2013;54:79–83. doi: 10.1111/epi.12449. [DOI] [PubMed] [Google Scholar]
- Fawcett JW, Asher RA. The glial scar and central nervous system repair. Brain Research Bulletin. 1999;49:377–391. doi: 10.1016/s0361-9230(99)00072-6. [DOI] [PubMed] [Google Scholar]
- Fonseca-Kelly Z, Nassrallah M, Uribe J, Khan RS, Dine K, Dutt M, Shindler KS. Resveratrol neuroprotection in a chronic mouse model of multiple sclerosis. Frontiers in Neurology. 2012:00084. doi: 10.3389/fneur.2012.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi S, Abramov AY. Mechanism of oxidative stress in neurodegeneration. Oxidative medicine and cellular longevity. 2012;2012:428010. doi: 10.1155/2012/428010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girbovan C, Morin L, Plamondon H. Repeated resveratrol administration confers lasting protection against neuronal damage but induces dose-related alterations of behavioral impairments after global ischemia. Behavioural Pharmacology. 2011:1. doi: 10.1097/FBP.0b013e32834eafa3. [DOI] [PubMed] [Google Scholar]
- Gu Q, Schmued LC, Sarkar S, Paule MG, Raymick B. One-step labeling of degenerative neurons in unfixed brain tissue samples using Fluoro-Jade C. Journal of neuroscience methods. 2012;208:40–43. doi: 10.1016/j.jneumeth.2012.04.012. [DOI] [PubMed] [Google Scholar]
- Guillemin GJ. Microglia, macrophages, perivascular macrophages, and pericytes: a review of function and identification. Journal of Leukocyte Biology. 2003;75:388–397. doi: 10.1189/jlb.0303114. [DOI] [PubMed] [Google Scholar]
- Han S, Choi JR, Soon Shin K, Kang SJ. Resveratrol upregulated heat shock proteins and extended the survival of G93A–SOD1 mice. Brain research. 2012;1483:112–117. doi: 10.1016/j.brainres.2012.09.022. [DOI] [PubMed] [Google Scholar]
- Han YS, Zheng WH, Bastianetto S, Chabot JG, Quirion R. Neuroprotective effects of resveratrol against beta-amyloid-induced neurotoxicity in rat hippocampal neurons: involvement of protein kinase C. British journal of pharmacology. 2004;141:997–1005. doi: 10.1038/sj.bjp.0705688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg LR, Bacher D, Jarosiewicz B, Masse NY, Simeral JD, Vogel J, Haddadin S, Liu J, Cash SS, van der Smagt P, Donoghue JP. Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature. 2012;485:372–375. doi: 10.1038/nature11076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorfi M, Skousen JL, Weder C, Capadona JR. Progress towards biocompatible intracortical microelectrodes for neural interfacing applications. J Neural Eng. 2014;12:011001. doi: 10.1088/1741-2560/12/1/011001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H, Kirchhoff F, Verkhratsky A. Microglia: new roles for the synaptic stripper. Neuron. 2013;77:10–18. doi: 10.1016/j.neuron.2012.12.023. [DOI] [PubMed] [Google Scholar]
- Kim SP, Simeral JD, Hochberg LR, Donoghue JP, Black MJ. Neural control of computer cursor velocity by decoding motor cortical spiking activity in humans with tetraplegia. J Neural Eng. 2008;5:455–476. doi: 10.1088/1741-2560/5/4/010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozai TD, Catt K, Li X, Gugel ZV, Olafsson VT, Vazquez AL, Cui XT. Mechanical failure modes of chronically implanted planar silicon-based neural probes for laminar recording. Biomaterials. 2014;37C:25–39. doi: 10.1016/j.biomaterials.2014.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langseth L. Antioxidants and their effect on health. 2000 [Google Scholar]
- Li C, Yan Z, Yang J, Chen H, Li H, Jiang Y, Zhang Z. Neuroprotective effects of resveratrol on ischemic injury mediated by modulating the release of neurotransmitter and neuromodulator in rats. Neurochemistry International. 2010;56:495–500. doi: 10.1016/j.neuint.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Lin MS, Lee YH, Chiu WT, Hung KS. Curcumin provides neuroprotection after spinal cord injury. The Journal of surgical research. 2011;166:280–289. doi: 10.1016/j.jss.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Liu C, Shi Z, Fan L, Zhang C, Wang K, Wang B. Resveratrol improves neuron protection and functional recovery in rat model of spinal cord injury. Brain research. 2011;1374:100–109. doi: 10.1016/j.brainres.2010.11.061. [DOI] [PubMed] [Google Scholar]
- Lu KT, Chiou RYY, Chen LG, Chen MH, Tseng WT, Hsieh HT, Yang YL. Neuroprotective Effects of Resveratrol on Cerebral Ischemia-Induced Neuron Loss Mediated by Free Radical Scavenging and Cerebral Blood Flow Elevation. Journa of Agricultural and Food Chemistry. 2006;54:3126–3131. doi: 10.1021/jf053011q. [DOI] [PubMed] [Google Scholar]
- Lu X, Ma L, Ruan L, Kong Y, Mou H, Zhang Z, Wang Z, Wang J, Le Y. Resveratrol differentially modulates inflammatory responses of microglia and astrocytes. Journal of Neuroinflammation. 2010;7:46. doi: 10.1186/1742-2094-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnotta VA, Heo HY, Dlouhy BJ, Dahdaleh NS, Follmer RL, Thedens DR, Welsh MJ, Wemmie JA. Detecting activity-evoked pH changes in human brain. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:8270–8273. doi: 10.1073/pnas.1205902109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marg E, Adams JE. Indwelling Multiple Microelectrodes in the Brain. Electrocephalography and Clinical Neurophysiology. 1967;23:277–280. doi: 10.1016/0013-4694(67)90126-5. [DOI] [PubMed] [Google Scholar]
- Mayhan WG. Regulation of blood-brain barrier permeability. Microcirculation. 2001:89–104. doi: 10.1111/j.1549-8719.2001.tb00160.x. [DOI] [PubMed] [Google Scholar]
- McConnell GC, Rees HD, Levey AI, Gutekunst C-A, Gross RE, Bellamkonda RV. Implanted neural electrodes cause chronic, local inflammation that is correlated with local neurodegeneration. J Neural Eng. 2009;6:056003. doi: 10.1088/1741-2560/6/5/056003. [DOI] [PubMed] [Google Scholar]
- Nicolelis MAL. Brain-machine interfaces to restore motor function and probe neural circuits. Nature Reviews Neuroscience. 2003;4:417–422. doi: 10.1038/nrn1105. [DOI] [PubMed] [Google Scholar]
- Olanow CW. A radical hypothesis for neurodegeneration. TINS. 1993;16:439–444. doi: 10.1016/0166-2236(93)90070-3. [DOI] [PubMed] [Google Scholar]
- Pancrazio JJ, Peckham PH. Neuroprosthetic devices: how far are we from recovering movement in paralyzed patients? Expert review of neurotherapeutics. 2009;9:427–430. doi: 10.1586/ern.09.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter-Baker KA, Nguyen JK, Kovach KM, Gitomer MM, Srail TW, Stewart WG, Skousen JL, Capadona JR. Development of Superoxide Dismutase Mimetic Surfaces to Reduce Accumulation of Reactive Oxygen Species for Neural Interfacing Applications. Journal of materials chemistry B, Materials for biology and medicine. 2014a;2:2248–2258. doi: 10.1039/C4TB00125G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter-Baker KA, Ravikumar M, Burke AA, Meador WD, Householder KT, Buck AC, Sunil S, Stewart WG, Anna JP, Tomaszewski WH, Capadona JR. An Initial Comparison of Neuroinflammation to Implanted Microelectrodes between a Rat and a Mouse Model. Under Review. 2014b doi: 10.1016/j.biomaterials.2014.03.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter KA, Simon JS, Velagapudi B, Capadona JR. Reduction of autofluorescence at the microelectrode-cortical tissue interface improves antibody detection. Journal of neuroscience methods. 2012a;203:96–105. doi: 10.1016/j.jneumeth.2011.09.024. [DOI] [PubMed] [Google Scholar]
- Potter KA, Buck AC, Self WK, Capadona JR. Stab injury and device implantation within the brain results in inversely multiphasic neuroinflammatory and neurodegenerative responses. J Neural Eng. 2012b;9 doi: 10.1088/1741-2560/9/4/046020. [DOI] [PubMed] [Google Scholar]
- Potter KA, Buck AC, Self WK, Callanan ME, Sunil S, Capadona JR. The effect of resveratrol on neurodegeneration and blood brain barrier stability surrounding intracortical microelectrodes. Biomaterials. 2013;34:7001–7015. doi: 10.1016/j.biomaterials.2013.05.035. [DOI] [PubMed] [Google Scholar]
- Potter KA, Jorfi M, Householder KT, Foster EJ, Weder C, Capadona JR. Curcumin-releasing mechanically adaptive intracortical implants improve the proximal neuronal density and blood-brain barrier stability. Acta Biomater. 2014;10:2209–2222. doi: 10.1016/j.actbio.2014.01.018. [DOI] [PubMed] [Google Scholar]
- Prasad A, Xue QS, Sankar V, Nishida T, Shaw G, Streit WJ, Sanchez JC. Comprehensive characterization and failure modes of tungsten microwire arrays in chronic neural implants. J Neural Eng. 2012;9:056015. doi: 10.1088/1741-2560/9/5/056015. [DOI] [PubMed] [Google Scholar]
- Prasad A, Xue Q-S, Dieme R, Sankar V, Mayrand R, Nishida T, Streit WJ, Sanchez JC. Abiotic-biotic characterization of Pt/Ir microelectrode arrays in chronic implants. Frontiers in neuroscience. 2014 doi: 10.3389/fneng.2014.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravikumar M, Jain S, Miller RH, Capadona JR, Selkirk SM. An organotypic spinal cord slice culture model to quantify neurodegeneration. Journal of neuroscience methods. 2012a;211:280–288. doi: 10.1016/j.jneumeth.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Ravikumar M, Jain S, Miller RH, Capadona JR, Selkirk SM. An organotypic spinal cord slice culture model to quantify neurodegeneration. J Neurosci Methods. 2012b;211:280–288. doi: 10.1016/j.jneumeth.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Ravikumar m, Hageman DJ, Tomaszewski WH, Chandra GM, Skousen JL, Capadona JR. The Effect of Residual Endotoxin Contamination on the Neuroinflammatory Response to Sterilized Intracortical Microelectrodes. Journal of Materials Chemistry B. 2013 doi: 10.1039/C3TB21453B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankar V, Patrick E, Dieme R, Sanchez JC, Prasad A, Nishida T. Electrode impedance analysis of chronic tungsten microwire neural implants: understanding abiotic vs. biotic contributions. Frontiers in neuroengineering. 2014;7:13. doi: 10.3389/fneng.2014.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena T, Karumbaiah L, Gaupp EA, Patkar R, Patil K, Betancur M, Stanley GB, Bellamkonda RV. The impact of chronic blood-brain barrier breach on intracortical electrode function. Biomaterials. 2013;34:4703–4713. doi: 10.1016/j.biomaterials.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Schmitt G, Schultze J-W, Faûbender F, Bub G, Luth H, Schonin MJ. Passivation and corrosion of microelectrode arrays. Electrochimica Acta. 1999;44:3865–3883. [Google Scholar]
- Schwartz AB. Cortical neural prosthetics. Annual Review of Neuroscience. 2004;27:487–507. doi: 10.1146/annurev.neuro.27.070203.144233. [DOI] [PubMed] [Google Scholar]
- Simao F, Matte A, Pagnussat AS, Netto CA, Salbego CG. Resveratrol preconditioning modulates inflammatory response in the rat hippocampus following global cerebral ischemia. Neurochemistry International. 2012 doi: 10.1016/j.neuint.2012.06.009. [DOI] [PubMed] [Google Scholar]
- Simeral JD, Kim SP, Black MJ, Donoghue JP, Hochberg LR. Neural control of cursor trajectory and click by a human with tetraplegia 1000 days after implant of an intracortical microelectrode array. J Neural Eng. 2011;8:025027. doi: 10.1088/1741-2560/8/2/025027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends in Neurosciences. 2009;32:638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soufi FG, Mohammad-nejad D, Ahmadieh H. Resveratrol improves diabetic retinopathy possibly through oxidative stress -nuclear factor kB - apoptosis pathway. Pharmacological Reports. 2012;64:1505–1514. doi: 10.1016/s1734-1140(12)70948-9. [DOI] [PubMed] [Google Scholar]
- Sovak M. Grape extract, resveratrol, and its analogs: a review. Journal of Medicinal Food. 2001;4:193–105. doi: 10.1089/109662001300341752. [DOI] [PubMed] [Google Scholar]
- Sun AY, Wang Q, Simonyi A, Sun GY. Resveratrol as a Therapeutic Agent for Neurodegenerative Diseases. Molecular Neurobiology. 2010;41:375–383. doi: 10.1007/s12035-010-8111-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walle T. Bioavailability of resveratrol. Annuals New York Academy of Science. 2011;1215:9–15. doi: 10.1111/j.1749-6632.2010.05842.x. [DOI] [PubMed] [Google Scholar]
- Walle T, Hsieh F, DeLegge MH, Oatis JE, Walle UK. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metabolism and Disposition. 2004;32:1377–1382. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- Wang Q, Xu J, Rottinghaus GE, Simonyi A, Lubahn D, Sunb GY, Sun AY. Resveratrol protects against global cerebral ischemic injury in gerbils. Brain research. 2002;958:439–447. doi: 10.1016/s0006-8993(02)03543-6. [DOI] [PubMed] [Google Scholar]
- Ward MP, Rajdev P, Ellison C, Irazoqui PP. Toward a comparison of microelectrodes for acute and chronic recordings. Brain research. 2009;1282:183–200. doi: 10.1016/j.brainres.2009.05.052. [DOI] [PubMed] [Google Scholar]
- Wenzel E, Somoza V. Metabolism and bioavailability of trans-resveratrol. Mol Nutr Food Res. 2005;49:472–481. doi: 10.1002/mnfr.200500010. [DOI] [PubMed] [Google Scholar]
- Winslow BD, Christensen MB, Yang W-K, Solzbacher F, Tresco PA. A comparison of the tissue response to chronically implanted Parylene-C-coated and uncoated planar silicon microelectrode arrays in rat cortex. Biomaterials. 2010;31:9163–9172. doi: 10.1016/j.biomaterials.2010.05.050. [DOI] [PubMed] [Google Scholar]
- Wu PF, Xie N, Zhang JJ, Guan XL, Zhou J, Long LH, Li YL, Xiong QJ, Zeng JH, Wang F, Chen JG. Resveratrol preconditioning increases methionine sulfoxide reductases A expression and enhances resistance of human neuroblastoma cells to neurotoxins. The Journal of nutritional biochemistry. 2012 doi: 10.1016/j.jnutbio.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Zhang H, Wang F-W, Yao I-L, Hao A-J. Microglia -- Friend or foe. Frontiers in Bioscience. 2011;S3:869–883. doi: 10.2741/193. [DOI] [PubMed] [Google Scholar]
- Zhang N, Shiramatsu TI, Kanzaki R, Takahashi H. Cortical Mapping of Mismatch Negativity with Deviance Detection Property in Rat. PLoS ONE. 2013;8:e82663. doi: 10.1371/journal.pone.0082663. [DOI] [PMC free article] [PubMed] [Google Scholar]