Abstract
The ability of the body to perceive noxious stimuli lies in a heterogeneous group of primary somatosensory neurons termed nociceptors. The molecular receptors of noxious mechanical, temperature or chemical stimuli are expressed in these neurons and have drawn considerable attention as possible targets for analgesic development to improve treatment for the millions who suffer from chronic pain conditions. A number of thermoTRPs, a subset of the transient receptor potential family of ion channels, are activated by a wide range on noxious stimuli. In this review, we review the function of these channels and examine the evidence that thermoTRPs play a vital role in acute, inflammatory and neuropathic nociception.
Introduction
The capacity to feel pain is normally advantageous, providing powerful motivation to withdraw from noxious stimuli, to guard injured tissue and to avoid dangerous environments in the future. However acute and chronic pain affects hundreds of millions of people and imposes a severe emotional and economic burden on both individuals and society as a whole. Pain is the number one reason for seeking medical care in the United States. It is a major symptom in many illnesses and can be particularly debilitating when it becomes disassociated with initial injury or illness entering a chronic phase in which pain itself become the disease. In such cases, pain is often felt seemingly in the absence of noxious stimuli, or by a lowering of the threshold of stimuli to induce pain such that an innocuous stimulus can trigger pain (allodynia) or that a noxious stimulus evokes a heightened sensation of pain (hyperalgesia).
Pain is a complex phenomenon involving multiple ascending and descending neuronal pathways and complex processing within the brain. Potential targets for therapeutic intervention can occur anywhere throughout the pain system. Many analgesic targets are expressed in central nervous system (CNS) pain circuits. However due to the widespread expression of targets in other neural pathways and tissues, analgesic administration often causes deleterious side effects. For example, analgesics that act on opioid receptors suppress neuronal activity within the pain pathway, but also can evoke euphoria, dependency, sedation, constipation, and suppression of respiration. One avenue for the development of analgesics with the potential for fewer side effects has been to identify targets that are mainly expressed within the pain pathway.
Molecules that regulate the activity of peripheral neurons (nociceptors) that respond to noxious mechanical, thermal and chemical stimuli are strong candidates for therapeutic intervention. Nociceptors (classified as small diameter unmyelinated C-fibers or lightly myelinated small diameter Aδ fibers), have cell bodies located in the dorsal root ganglia (DRG) that innervate the body or in the trigeminal ganglia (TG) that innervate the face. They send afferents to peripheral tissues such as the skin where molecular receptors located on sensory terminals react to noxious stimuli. This information is relayed to the CNS via central afferents, which synapse with second order neurons in the spinal cord. Pathological pain is often associated with elevated nociceptor excitability. This can occur following tissue injury, which prompts an inflammatory response including the release of molecules that act to sensitize nociceptor activity and evoke pain hypersensitivy or hyperalgesia (Fig 1A). Damage to the peripheral nerve itself can lead to ecotopic nociceptor activity in which pain occurs in the absence of noxious stimuli. The receptors for noxious stimuli often have a fairly restricted expression pattern, which could theoretically limit the potential for serious side effects caused by compounds that target their activity.
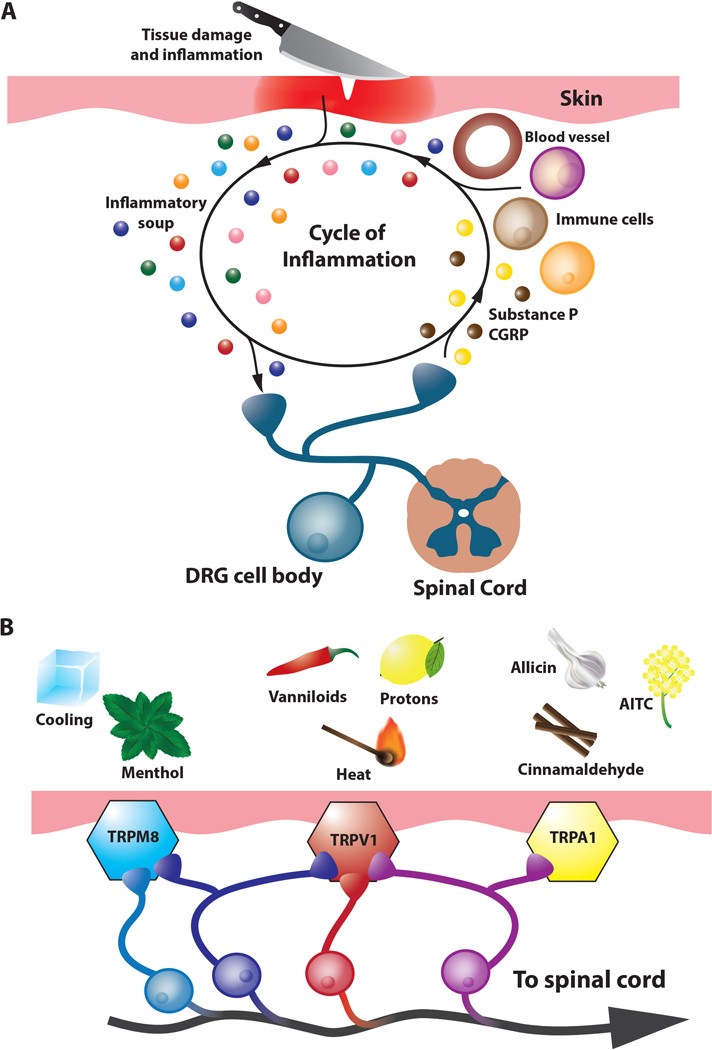
Figure 1.
Pain and inflammation. A. Inflammatory sensitization of nociceptors and the neurogenic response. Primary sensory nociceptors (blue) respond to tissue damage caused by noxious thermal, mechanical or chemical stimuli and contribute to the inflammatory response. In addition to sending painful signals to the spinal cord and then the brain, primary nociceptors release neuropeptides such as CGRP and Substance P which act on peripheral tissues to stimulate vasodialation, vascular leakage and promote the release of inflammatory mediators such as protons, NGF, bradykinin, lipids, prostaglandins, and ATP (also known as the inflammatory soup; colorful spheres) which in turn promote the sensitization of the nociceptor where the threshold of activation of these neurons by physical or chemical stimuli is lowered. B. Pain and coding by thermoTRP receptors. Noxious stimuli and temperatures activate thermoTRP receptors. TRPM8 is activated by cold (< 26°C) and cooling compounds such as menthol. TRPV1 is activated by noxious heat (43°C), vanilloids such as capsaicin, and acidic pH, while TRPA1 is activated by a wide variety of pungent compounds including AITC, cinnamaldehyde (the pungent compound of cinnamon) and allicin, the active ingredient of garlic. Co-expression of TRPV1 and TRPM8 has been reported (violet neurons) as well as co-expression of TRPV1 and TRPA1 (magenta neurons).
Initially identified as temperature sensitive receptors, thermoTRPs, members of the transient receptor potential family of nonselective cation channels, are activated by a wide range of noxious stimuli. TRP channels are tetramers composed of identical subunits, which have six transmembrane domains and cytoplasmic amino and carboxy termini. A role for TRP channels and noxious sensation arose with the discovery of the first identified thermoTRP, TRPV1, activated by noxious stimuli such as capsaicin, the pungent ingredient in chili peppers and noxious heat (Caterina and others 1997). In this review, we will discuss the properties of TRPV1, cool activated TRPM8 and noxious chemical activated TRPA1 as well as three thermoTRPs (TRPM3, TRPV3, and TRPV4) that are expressed in somatosensory neurons or in the case of TRPV3 peripheral targets of these nerves and their function in nociception (Fig 1B).
TRPV1
TRPV1 activation and structure
TRPV1 was first identified by its responsiveness to capsaicin, a vanilloid derived from chili peppers that elicits a burning sensation. Remarkably TRPV1 also responded to noxious heat, with an activation threshold of approximately 43°C and a temperature coefficient of Q10 > 20, as well as extracellular acidification, indicating it might act as a polymodal nociceptor integrating multiple forms of noxious stimuli (Caterina and others 1997). TRPV1 has subsequently been found to respond to other pungent chemicals and endogenous lipid-derived molecules, many of which act cooperatively to stimulate channel activity. For instance capsaicin and acidification both lower the heat activation threshold for the channel activation (Tominaga and others 1998). Immunohistochemical analysis showed that TRPV1 is expressed in approximately 30–50% of all somatosensory neurons in rodent models, predominantly peptidergic C-fiber nociceptors (Tominaga and others 1998). Knockout studies have confirmed a role for TRPV1 in nociception. Recordings from isolated DRG cells of TRPV1 knockout mice as well as behavioral analyses demonstrate significant impairments in the detection of noxious heat and protons along with complete loss of capsaicin sensitivity (Caterina and others 2000; Davis and others 2000).
Recent single particle cryo-EM structural analyses have revealed the structure of TRPV1 at a remarkable 3.4 angstrom resolution. Similar to voltage-gated K+ and Na+ channels (VGCs), TRPV1 is a tetramer that exhibits radial symmetry around a central ion channel that is formed by transmembrane segments 5–6 (S5-S6) and the intervening pore loop and is flanked by S1-S4 domains (Fig. 2) (Liao and others 2013). Unlike VGCs, S1-S4 domains remain stationary during activation, however, major structural rearrangements in the pore helix and selectivity filter, along with the dilation of a lower gate have been reported, suggesting a dual gating mechanism. Furthermore, pharmacological manipulation along with cryo-EM results indicate that these upper and lower gates are allosterically coupled, likely via the pore helix, which may underlie integration of the wide array of noxious signals that TRPV1 processes (Cao, Liao, and others 2013).
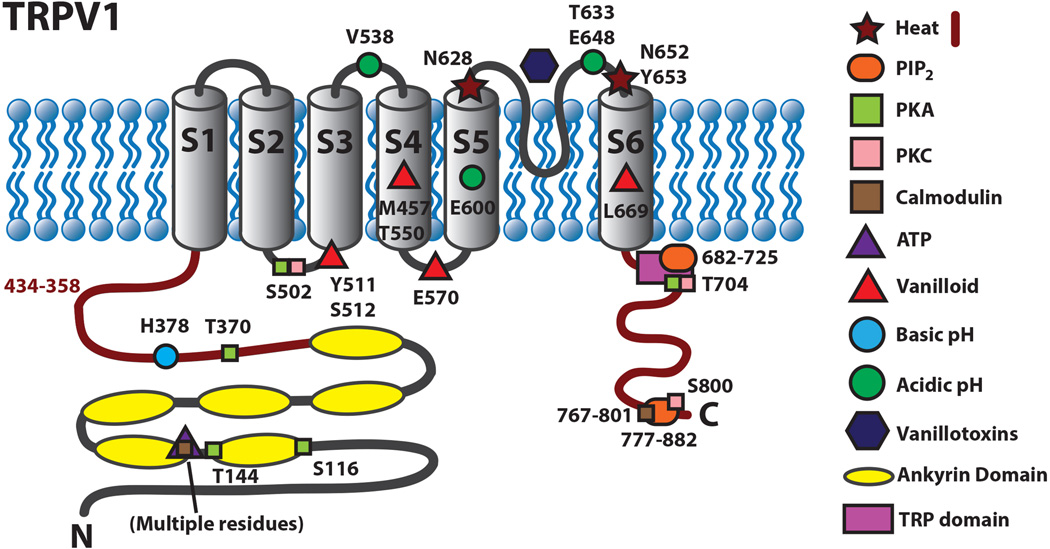
Figure 2.
Schematic diagram of TRPV1 illustrating residues that are involved in activation and regulation of the channel.
Many studies have set out to better understand the ability of TRPV1 to respond to noxious stimuli. Chimeric channels created between vanilloid-sensitive and vanilloid-insensitive variants of TRPV1 have demonstrated that residues in transmembrane segments S2-S4 are required for vanilloid sensitivity including a single amino acid substitution I550T that was able to confer capsaicin sensitivity to capsaicin-insensitive rabbit TRPV1 (Jordt and Julius 2002; Gavva and others 2004; Johnson and others 2006). Cryo-EM analysis revealed that vanilloids bind TRPV1 in a hydrophobic pocket comprised of the outer surface of the S3-S4 helices, S4-S5 linker and S6 helix (Cao, Liao, and others 2013). Mutation analysis also revealed amino acid residues required for proton sensitivity. The glutamate residue E648 found between the selectivity filter and S6 mediates agonist effects of protons while E600 was found to mediate the ability of protons to potentiate the activity of other TRPV1 agonists (Jordt and others 2000). Whole-cell recordings of TRPV1 and proton insensitive TRPV2 chimeric channels also revealed residues in the pore helix as well as in the region linking S3-S4 as necessary for responding to acidic pH (Ryu and others 2007).
Studies utilizing reconstituted TRPV1 in artificial membranes have demonstrated that TRPV1 is intrinsically temperature sensitive and large changes in enthalpy accompany temperature-evoked activation, suggesting that conformational changes accompany temperature-evoked gating (Cao, Cordero-Morales, and others 2013). The C-terminal domain, the pore forming region of TRPV1, and the N terminal region, have been implicated in the heat sensitivity of the channel and recently, it was found that PKCβII phosphorylation of T705 is required for TRPV1 thermal sensitivity (Brauchi and others 2006; Grandl and others 2010; Yang and others 2010; Yao and others 2011; Li and others 2014). However, it is not understood how TRPV1 is able to undergo a conformational change in response to heat. Importantly, along with several other TRP channels, TRPV1 has been shown to be weakly voltage dependent such that agonists, including temperature, shift this voltage dependence toward physiological membrane potentials, suggesting that voltage may still be essential for their gating versatility (Nilius and others 2005).
TRPV1 modulation
TRPV1 activity is sensitized via coupling with second messenger signaling cascades by a variety of proalgesic and proinflammatory agents including nerve growth factor (NGF), bradykinin, lipids, prostaglandins, and ATP (Caterina and others 1997; Tominaga and others 1998). Furthermore, TRPV1 activity contributes to neurogenic inflammation in which nociceptors themselves release inflammatory mediators, thus acting cell autonomously to promote inflammation and hyperalgesia (Fig. 1b) (Julius 2013). Analysis of knockout mice has shown that TRPV1 is required for development of inflammatory thermal hyperalgesia, demonstrating an essential role for TRPV1 in pain hypersensitivity associated with chronic pain conditions (Caterina and others 2000; Davis and others 2000).
How do signaling molecules activated by inflammatory mediator signaling cascades modulate TRPV1 activity? Regulation of the phosphorylation state of TRPV1 has been shown to profoundly contribute to its ability to respond to noxious stimuli. De-phosphorylation of TRPV1 has been shown to cause channel desensitization following activation, while protein kinases including PKA and PKC, stimulated downstream of inflammatory mediators, have been shown to phosphorylate TRPV1 and reverse channel desensitization contributing to sustained channel activation (Premkumar and Ahern 2000; Tominaga and others 2001; Bhave and others 2002; Jung and others 2004; Mandadi and others 2006).
Numerous studies have found that the membrane lipid phosphatidylinositol 4,5-bisphosphate (PIP2) can also modulate TRPV1 activity. However, there has been much debate over whether it acts to potentiate or inhibit TRPV1 activity (Prescott and Julius 2003; Yao and Qin 2009; Ufret-Vincenty and others 2011; Cao, Cordero-Morales, and others 2013). A possible explanation for the discrepancies between these findings comes from a recent study which found that PIP2 had opposite effects on TRPV1 function depending on which leaflet of the cell membrane PIP2 is inserted in; insertion into the inner leaflet led to potentiation of capsaicin-activated responses, while insertion into the extracellular leaflet led to inhibition (Senning and others 2014).
TRPV1 in pain conditions
Many studies have shown a strong link between TRPV1 and inflammatory pain. Studies in humans found an increase in the number of TRPV1 immunoreactive fibers in inflamed skin that correlates with inflammatory hyperalgesia (Gopinath and others 2005). Antagonists of TRPV1 have been shown to inhibit thermal and/or mechanical hypersensitivity associated with multiple models of inflammation including UVB-evoked sensitization, complete Freund’s adjuvant (CFA)-induced pain, post-operative pain and bone cancer pain in both rodent and humans (Honore and others 2005; Chizh and others 2007). In multiple rodent models of arthritis, nocifensive behavior was significantly reduced in TRPV1 knockout animals or via treatment with TRPV1 antagonists (Keeble and others 2005; Barton and others 2006; Kelly and others 2015). Furthermore TRPV1 was found mediate nociception induced by activation of the inflammatory mediator, protease-activated receptor-2 (PAR2) in a rodent model of joint inflammation (Helyes and others 2010; Russell and others 2012). Thermal hyperalgesia associated with ischemic pain may also be dependent on TRPV1 (Kwon and others 2014). TRPV1 also appears to play a pivotal role in the pain associated with inflammatory diseases of the gastrointestinal tract. Increased TRPV1 expression has been found in patients suffering from inflammatory bowel disease and functionally correlates with visceral pain hypersensitivity (Akbar and others 2008; van Wanrooij and others 2014). TRPV1 was also observed to have increased expression, promote neurogenic inflammation and mediate nociception in rodent models of pancreatitis (Hutter and others 2005; Xu and others 2007).
Evidence also points to TRPV1 being functionally significant in neuropathic pain conditions. TRPV1 expression increased following nerve injury while blockade of TRPV1 function reduced both thermal and/or mechanical hypersensitivity (Watabiki and others 2011; Urano and others 2012). Chemotherapeutics such as oxaliplatin or paclitaxel increase TRPV1 sensitivity, suggesting an instructive role in chemotherapy-induced peripheral neuropathy (Anand and others 2010;Ta and others 2010; Y. Chen and others 2011). Desensitization of TRPV1, using capsaicin and resiniferatoxin creams, patches or injections, has been shown to provide relief from osteoarthritis, postoperative pain and in a spinal nerve ligation (SNL) model of nerve injury-induced heat sensitivity, further supporting an involvement of TRPV1 in both in inflammatory and neuropathic pain (Forst and others 2002; Backonja and others 2008; Remadevi and Szallisi 2008; King and others 2011).
While TRPV1 antagonists have been essential in better understanding the roles of TRPV1 and have shown promise in treating patients with neurogenic and inflammatory diseases, the development of these drugs has not come without obstacles. Common side effects of first generation TRPV1 antagonists include loss of noxious heat sensation, increased burn risk, and hyperthermia (Gavva and others 2008; Krarup and others 2011; Szolcsányi and Sándor 2012). Fortunately, several compounds have been found that differentially affect the channel. For instance, some block capsaicin, but not heat activation, suggesting that it could be feasible to separate the analgesic effects of TRPV1 antagonists from harmful side effects (Lehto and others 2008) Indeed, second generation antagonists such as AMG8562, APHC1, APHC3 and A-1165442, 52 have already been tested for several inflammatory conditions with success in pain reduction while avoiding hyperthermia (Lehto and others 2008; Reilly and others 2012; Andreev and others 2013; Voight and others 2014).
TRPM8
TRPM8 activation and structure
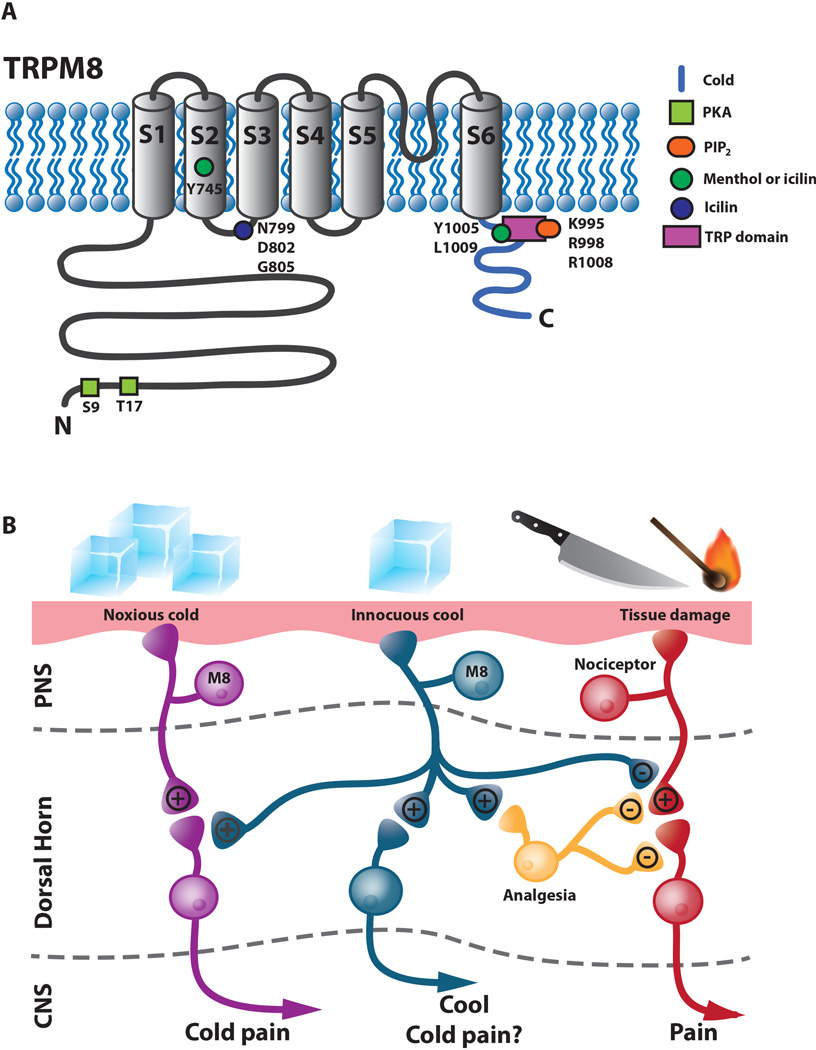
TRPM8 is activated by cool/cold temperatures starting in the innocuous range (≤26°C), menthol and other cooling compounds such as spearmint, eucalyptol and icilin (Fig. 3A) (McKemy and others 2002; Peier, Moqrich, and others 2002; Dhaka and others 2006). TRPM8 is expressed by approximately 10–15% of all somatosensory neurons in the TG and DRG. Consistent with TRPM8 being a cool sensor it is expressed, in a distinct population of small diameter C-fibers and potentially Aδ fibers, the majority of which do not express other known nociceptive or somatosensory markers (McKemy and others 2002; Peier, Moqrich, and others 2002; Takashima and others 2007; Dhaka and others 2008). Similar to TRPV1, studies utilizing artificial membranes have demonstrated that TRPM8 is intrinsically temperature sensitive (Zakharian and others 2010). A clear picture of structural changes that occur during cool activation is still in development. TRPV1-TRPM8 chimeras showed that the C-terminus contains structural determinants necessary for temperature activation of the channel (Brauchi and others 2006). Mutational analyses have identified residues required for ligand activation (Fig. 3a). Mutations in the S2 domain (Y745H) and in the C-terminal TRP domain (L1005R, L1009R) disrupt menthol and icilin sensitivity without affecting cold sensitivity. The S2 domain is most likely involved in ligand binding whereas the TRP domain is most likely required for coupling ligand binding to channel opening (Bandell and others 2006). Residues in the S2-S3 linker and S3 domain are necessary for icilin activation of TRPM8 (Chuang and others 2004). The S3 domain is also required for ligand activation of TRPV1 indicating there could be a partial conservation of mechanism among thermoTRPs. Like TRPV1, TRPM8 exhibits a degree of voltage sensitivity such that thermal or menthol activation affects membrane potentials, and charge-neutralizing mutations have mapped the voltage sensor to the S4 domain and S4-S5 linker (Brauchi and others 2004; Voets and others 2004; Voets and others 2007; Fernández and others 2011).
Figure 3.
TRPM8. A. Schematic diagram of TRPM8 illustrating residues that are involved in activation and regulation of the channel. B. Model for TRPM8 function showing possible connections involved in the perception of innocuous cool, noxious cold and analgesia. TRPM8 mediates both noxious cold (magenta neurons) and innocuous cool (blue neurons). Noxious cold perception may involve a pathway separate from innocuous cool sensation or could potentially involve a model in which a single type of TRPM8 expression primary afferent could synapse differentially with second order spinal neurons such that innocuous cool stimuli may be sufficient to activate innocuous cool second order spinal neurons whereas a more intense noxious cold stimulus is necessary to activate a second order nociceptive spinal neuron. Cool analgesia mediated by TRPM8 neurons could be accomplished by direct inhibition of nociceptors (red) or through inhibitory interneurons (yellow) in the spinal cord acting either pre- or post-synaptically in the dorsal horn. Black text indicates strong synapses and grey text indicates weaker synapses.
TRPM8 Regulation
Cold-sensitive neurons are able to acclimate to cold stimuli, which is believed to be important in cold discrimination in changing environments and it has been suggested that these responses could be mediated by modulation of TRPM8. Activation of TRPM8 is followed by extracellular Ca2+ dependent desensitization, presumably via TRPM8 dependent Ca2+ influx (McKemy and others 2002). This process is dependent on Ca2+-mediated activation of phospholipase C (PLC) and subsequent depletion of PIP2. PIP2 can activate TRPM8 and interacts with positive charges in the C-terminal TRP domain. Its depletion leads to reduction in TRPM8 channel activity accompanied by shifting voltage dependence towards more positive potentials (Liu and Qin 2005; Rohacs and others 2005). As PIP2 hydrolysis both negatively regulates TRPM8 and potentially positively regulates TRPV1, it is possible that inflammatory agents acting on PLC-coupled receptors could produce hyperalgesia by sensitizing heat responses, while decreasing any analgesic affects of innocuous cooling.
Other signaling pathways in addition to PLC-PIP2 modulate TRPM8 activity. Lysophospholipids and polyunsaturated fatty acids such as arachidonic acid are created downstream of phospholipase A2 (PLA2) activation, modulate TRPM8 positively or negatively, respectively. Interestingly PLA2 stimulation leads to a net suppression of TRPM8 activity (Vanden Abeele and others 2006; Andersson and others 2007; Bavencoffe and others 2011). PKA and PKC, which act as positive regulators of TRPV1, negatively modulate TRPM8 function (Premkumar and others 2005; Abe and others 2006; De Petrocellis and others 2007). Direct inhibition of TRPM8 by Gαq occurs downstream of the inflammatory mediators bradykinin and histamine (Zhang and others 2012). These findings suggest that inflammatory mediators might potentiate TRPV1 dependent nociception while simultaneously inhibiting potential analgesic effects of TRPM8 dependent cooling.
TRPM8 involvement in pain and analgesia
Knockout studies demonstrate that TRPM8−/− mice exhibit complete loss of acute innocuous cold sensation, impaired responses to noxious cold temperatures and deficits in nocifensive responses to cooling compounds (Bautista and others 2007; Colburn and others 2007; Dhaka and others 2007; Knowlton and others 2010; Knowlton and others 2013; Brenner and others 2014). The membrane protein Pirt has also been shown to regulate TRPM8. Pirt binds directly to TRPM8 and enhances the temperature and menthol threshold for TRPM8, and Pirt knockout mice exhibit impaired behavioral responses to both cold and cooling agents (Tang and others 2013). Taken together these data strongly implicate TRPM8 in cold nociception.
Subpopulations of TRPM8+ neurons respond to different thermal thresholds, suggesting that distinct neurons differentiate innocuous and noxious cold sensations (McKemy 2013). Studies with TRPM8-reporter mouse lines have revealed that ~10–30% of TRPM8 expressing neurons coexpress TRPV1, suggesting that there are at least two populations of TRPM8-expressing neurons: a TRPM8 only population for innocuous cool sensation and a TRPM8+/TRPV1+ population required for noxious cold sensation (Fig. 3b). Consistent with this idea, the number of TRPM8 positive neurons expressing TRPV1 rose 65% following CFA-induced inflammation (Takashima and others 2007; Dhaka and others 2008). However, specific ablation of TRPV1+ neurons in adult mice did not alter aversion to noxious cold temperature or paw withdrawal to noxious cold temperatures, suggesting that TRPM8+/TRPV1+ neurons may be dispensable for TRPM8-mediated noxious cold behavior (Pogorzala and others 2013).
TRPM8 also has roles in inflammatory and neuropathic cold allodynia. In both CFA-induced inflammation and chronic constrictive nerve injury (CCI) models, injury-induced hypersensitivity to the topically administered cooling agent acetone is attenuated in TRPM8−/− mice and in animals treated with TRPM8 antagonist (Colburn and others 2007; Xing and others 2007; Knowlton and others 2011). These results correlate with findings of increased expression of TRPM8 in rat DRG neurons after CCI (Su and others 2011). However, a conflicting study reported reduced TRPM8 expression and no changes in the functional properties of cold/menthol sensitive neurons following CCI in mice (Caspani and others 2009). Cold allodynia is often present in patients following chemotherapy induced peripheral neuropathy. TRPM8 expression was found to be upregulated following oxaliplatin treatment in mice, but oxaliplatin induced cold allodynia was absent in TRPM8−/− mice (Gauchan and others 2009; Knowlton and others 2011). The proalgesic glial derived growth factor artemin has also been shown to potentiate TRPM8-dependent cold hypersensitivity (Lippoldt and others 2013). Cold allodynia follows morphine withdrawal, but is absent in TRPM8−/− mice, and the µ opiate receptor 1 (OPRM1) has been shown to interact with and stimulate TRPM8 internalization following activation (Shapovalov and others 2013). TRPM8 may also potentiate painful bladder syndrome (PBS). TRPM8 expression is elevated in PBS patients compared to controls and the TRPM8 antagonist AMTB attenuated nociceptive reflexes associated with PBS (Mukerji and others 2006; Lashinger and others 2008).
Cold or topical menthol exposure is a common method of pain relief. TRPM8 mediates some aspects of cool-analgesia in addition to having a role in cold nociception. Cool mediated analgesia to an acute noxious stimulus (formalin) was absent in TRPM8−/− mice (Dhaka and others 2007). Activation of TRPM8, either by chemical agonists and/or cooling, caused a significant reduction in nocifensive behavior elicited by CFA- or CCI-induced thermal and/or mechanical hyperalgesia (Proudfoot and others 2006; Su and others 2011). Reversal of CCI induced mechanical hypersensitivity by cooling was also absent in TRPM8−/− animals (Knowlton and others 2013). Menthol evoked analgesia, confirmed to be dependent on TRPM8, also involves endogenous opioid pathways as systemic naloxone treatment reversed TRPM8 dependent analgesia (Liu and others 2013: 8). Taken together, these results demonstrate that depending on context, TRPM8 functions in innocuous cool sensation, nociception or analgesia. How TRPM8 may be able to convey these different sensory modalities is still unclear and awaits further investigation (Fig. 3B).
Finding antagonists specific to TRPM8 may be invaluable in treating conditions such as cold allodynia. Conversely creation of tailored TRPM8 agonists to evoke analgesia could also be useful in the development of novel therapeutics. Blockade of TRPM8 channels with small molecule antagonists reduces acute, inflammatory and neuropathic cold pain responses in mice (Knowlton and others 2011; Calvo and others 2012; Patel and others 2014). However, similar to TRPV1 antagonists, TRPM8 antagonists alter body temperature, but in contrast to TRPV1 antagonists, they cause hypothermia (Knowlton and others 2011; Calvo and others 2012; Patel and others 2014: 8). Therefore it will be necessary to develop drugs that are specific to cold nociception. Recently, a potent TRPM8 antagonist (PF-05105697) was developed that was successfully able to treat pain without causing hypothermia in humans, however patients reported the sensation of excessive warmth (“feeling hot”) as a side effect (Winchester and others 2014).
TRPA1
TRPA1 is activated by a wide array of inflammatory agents
TRPA1, initially termed ANKTM1 due to the presence of multiple N-terminal ankyrin repeats, was originally characterized as a potential noxious cold detector (Fig. 4). In vitro studies found that the channel was activated at an average threshold of 17°C coinciding with the threshold for human noxious cold detection. TRPA1 is expressed in about 30% of TRPV1 expressing peptidergic C-and/or Aδ-fibers providing support for a role for TRPA1 in nociception. These findings however were called into question by studies that found no evidence for cold activation of this channel (Jordt and others 2004). While still controversial, reports utilizing TRPA1−/− mice suggest that at least in mice, TRPA1 does not appear to play a major role in acute noxious cold sensation but may serve to potentiate injury evoked cold hypersensitivity (Obata and others 2005; del Camino and others 2010; Knowlton and others 2010; Obaoa and others 2005). A recent study however has reported that human TRPA1 expressed in artificial membranes is intrinsically cold sensitive and opens up the possibility that TRPA1 has functional role in cold perception in humans (Moparthi and others 2014).
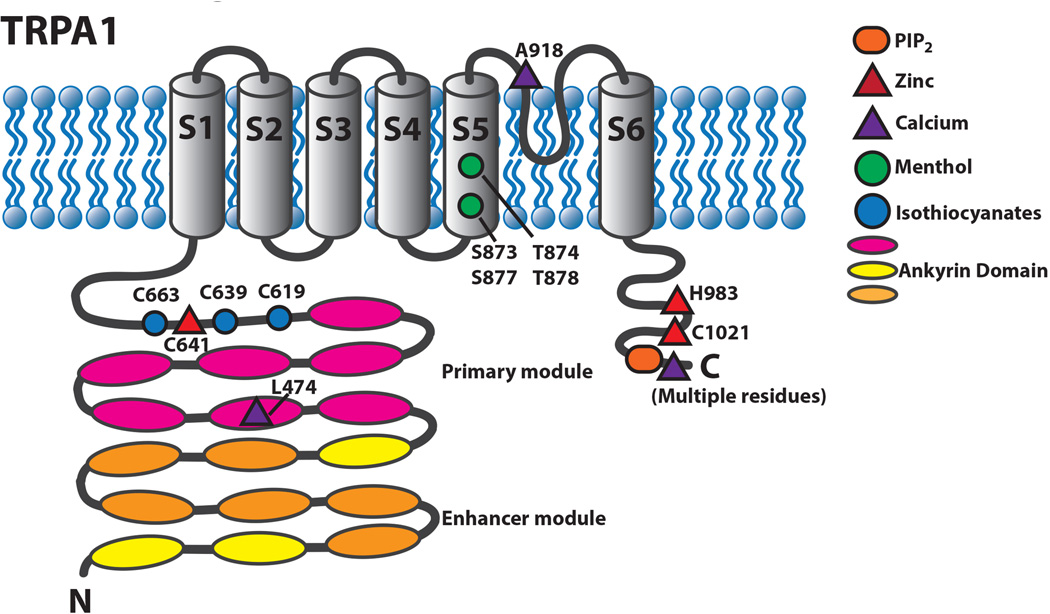
Figure 4.
Schematic diagram of TRPA1 illustrating residues that are involved in activation and regulation of the channel.
There is a clear consensus that TRPA1 is a promiscuous detector of noxious chemical stimuli. TRPA1 is activated by an array of exogenous chemical irritants and endogenous agents that cause pain and inflammation. As for other thermoTRP channels, plant-based compounds function as TRPA1 agonists. Examples of these pungent compounds, which elicit burning and tingling sensations, include allyl isothiocynate (AITC, mustard/wasabi), allicin (garlic), methyl salicylate (wintergreen) and cinnamaldehyde (cinnamon) (Bandell and others 2004; Jordt and others 2004; Macpherson and others 2005). Other toxic agents including environmental irritants such as acrolein, ozone, ammonia, formalin, zinc and therapeutics with known painful side effects such as disulfiram and glibenclamide also serve as agonists for the channel (Bautista and others 2006; Macpherson, Xiao, and others 2007; Maher and others 2008; Dhaka and others 2009; Taylor-Clark and Undem 2010; Babes and others 2013). Endogenous compounds that function as inflammatory mediators and are linked to painful inflammatory syndromes, such as 4-hydroxynonenal (4-HNE), 15-deoxy-δ(12,14)-prostaglandin J2 (15d-PGJ2), hydrogen peroxide, and hydrogen sulfide, also activate TRPA1 (Macpherson, Xiao, and others 2007; Andersson and others 2008; Cruz-Orengo and others 2008; Streng and others 2008; Wang and others 2011). A human TRPA1 gain of function mutation that causes familial episodic pain syndrome suggests a fundamental role for TRPA1 in pain perception, perhaps functioning alongside TRPV1 as critical detectors of painful stimuli (Kremeyer and others 2010).
Many TRPA1 agonists have diverse and on first inspection seemingly unrelated chemical structures. How then could these compounds all activate this channel? The answer for a great many of these agonists lies in their electrophilic properties (Cruz-Orengo et al., 2008; Hinman et al., 2006; Macpherson et al., 2007b). Instead of a shared common binding site as usually required for traditional ligand-channel interaction, it is the shared chemical reactivity of these compounds that facilitate TRPA1 activation. These electrophiles react with three cysteine residues located in the intracellular N-terminal region. Mutation of these three cysteine residues leaves TRPA1 largely insensitive to electrophiles (Hinman and others 2006; Macpherson, Dubin, and others 2007). Additional residues including a N-terminal lysine and cysteines outside the N-terminus might contribute to the regulation or minimally to the activation of the channel (Fig. 4) (Zygmunt and Högestätt 2014).
TRPA1 can also serve as a receptor-operated channel where activation or sensitization occurs through coupling with other receptors via second messenger signaling cascades. For instance inflammatory mediators such as bradykinin or ATP can activate TRPA1 via PLC-dependent pathways downstream of their Gq-protein coupled receptors (Bandell and others 2004; Jordt and others 2004). While it is not known what signaling molecules directly activate TRPA1, calcium, PKA and PIP2 have been shown to modulate channel activity (Akopian and others 2007; Doerner and others 2007; Zurborg and others 2007; Karashima and others 2008; Kim and others 2008). Intriguingly, the toll-like receptor (TLR7) can complex with TRPA1 showing association in co-immunopreciptiation assays. Both receptors are required to elicit single channel activity from exposure to the extracellular microRNA let-7b, which remarkably has been shown to elicit nociceptive responses in mice (Park and others 2014).
TRPA1 and Pain
The creation of TRPA1−/− animals and the development of potent antagonists for TRPA1 have firmly established TRPA1 as a central player in acute and inflammatory nociception. Although electrophiles modify proteins indiscriminately causing cellular/organismal damage, TRPA1−/− mutation drastically reduces or eliminates nociceptive responses to chemicals including AITC, 4-HNE and acrolein in knockout mice (Bautista and others 2006; Kwan 2006). AITC is a known inducer of neurogenic inflammation and mechanical and thermal hyperalgesia (Bánvölgyi and others 2004; Bautista and others 2006). Coupled with the fact that TRPA1 is primarily expressed in peptidergic C-fibers, this suggested that TRPA1 might be required for neurogenic inflammation. Investigations with TRPA1−/− animals showed that not only is TRPA1 required for AITC-induced mechanical and thermal hyperalgesia, but also for the neurogenic inflammatory response and tissue edema associated with AITC-induced inflammation (Bautista and others 2006; Moilanen and others 2012). Other lines of evidence indicate that TRPA1 may be a key mediator in inflammatory pain. TRPA1 is required for neurogenic inflammation induced by carrageenan, a chemical commonly used to induce inflammation (Moilanen and others 2012). Its expression is increased in somatosensory neurons following CFA-induced inflammation, and inflammatory mediators modulate cell surface expression of TRPA1 (Obata and others 2005; da Costa and others 2010). Furthermore pharmacological blockade of TRPA1 by antagonists AP18 and/or HC-030031 inhibits CFA-evoked mechanical and cold hyperalgesia (Petrus and others 2007; del Camino and others 2010).
TRPA1 has been implicated in a number of inflammatory disease models. In rodent models of arthritis, rodents treated with TRPA1 antagonists or TRPA1−/− mice have shown attenuated mechanical hyperalgesia evoked by injection of TNFα, CFA or monoiodoacetate (McGaraughty and others 2010; Fernandes and others 2011). Activation of intestinal TRPA1 can induce acute neurogenic inflammatory pancreatitis and TRPA1 antagonists act synergistically with TRPV1 antagonists to inhibit pancreatic pain, preventing the progression from acute to chronic pain. This suggests these two channels act in concert to promote pain in animal models of this disease (Ceppa and others 2010; Schwartz and others 2011; Schwartz and others 2013). TRPA1 has also been implicated in pain associated with bladder hyperalgesia in a mouse model of cystitis (DeBerry and others 2014) and other models of inflammatory diseases of the gastrointestinal tract. For example, TRPA1 appears to mediate neurogenic inflammation in rodent models of colitis and contributes to visceral mechanical hypersensitivity (Engel and others 2011).
TRPA1 may be a player in neuropathic pain conditions. Following nerve injury, pharmacological blockade of TRPA1 reduced mechanical allodynia evoked by nerve injury (Eid and others 2008). However, studies investigating nerve injury-evoked cold allodynia have provided conflicting data, perhaps due to differences in the methodology of nerve injury. Some studies have found that nerve injury increased TRPA1 expression in the DRG and cold allodynia inhibited following antisense knockdown or pharmacological blockade of TRPA1, while others found decreased TRPA1 expression and function in the DRG following nerve injury (Katsura and others 2006; Caspani and others 2009; J. Chen and others 2011). TRPA1 does seem to play an important role in chemotherapy-induced neuropathic pain. Mechanical and cold allodynia, induced by the chemotherapeutics cisplatin and oxaliplatin, are reduced in TRPA1−/− animals or by treatment with TRPA1 antagonists (Nassini and others 2011; Zhao and others 2012). Disease models of diabetic neuropathy strongly implicate TRPA1 in the mechanical hypersensitivity elicited by streptozotocin-induced diabetes (Wei and others 2009). Methylglyoxal, an electrophilic compound of which production is linked to diabetic neuropathy, directly activates TRPA1 and evokes TRPA1 dependent nociceptive behavior (Andersson and others 2013).
TRPM3
While less extensively studied than other thermoTRPS, TRPM3 has been recently identified as a noxious heat receptor. TRPM3 expressing HEK293T cells exhibited strong responses to heat (40°C). TRPM3 has a fairly moderate temperature coefficient (Q10=7.2) where temperature evoked current increases gradually at temperature >15°C compared TRPV1 (Q10=16.8) where steep activation is observed at temperatures >42°C. Like TRPV1, TRPM3 exhibits voltage sensitivity where thermal activation might shift voltage dependent activation closer to physiological membrane potential (Vriens and others 2011). TRPM3 is expressed in somatosensory neurons with one study showing TRPM3 expression in ~80% of these neurons, although detailed analysis of which populations of somatosensory neurons express TRPM3 remains to be determined (Nealen and others 2003; Staaf and others 2010; Vriens and others 2011).
The neuroactive steroid pregnenolone sulfate (PS) is an agonist for TRPM3 (Wagner and others 2008). Physiological levels of PS were found to dramatically potentiate TRPM3 activity to heat at temperatures as low as 37°C, suggesting that TRPM3 could play a role in heat sensitization. In cultured DRG neurons roughly 60% of neurons responded to PS and responses were limited to small-diameter neurons. About half of these neurons responded to capsaicin, indicating that there is substantial overlapping expression between TRPM3 and TRPV1. A potential role for TRPM3 in nociception was found when nocifensive behaviors elicited by PS were absent in TRPM3−/− animals. Specific deficits in acute and inflammatory noxious heat sensation were also observed in TRPM3−/− animals suggesting that TRPV1 and TRPM3 might act in concert to detect noxious heat (Vriens and others 2011). Corroborating a role for TRPM3 in noxious heat sensation, specific TRPM3 antagonists isosakuranetin and hesperetin significantly inhibited noxious heat and PS-induced nociception (Straub and others 2013).
TRPV3
There is conflicting evidence supporting a significant role for the warm/camphor-activated channel TRPV3 in thermal nociception. TRPV3, which shares 40% homology with TRPV1, has a thermal threshold of 33°C and exhibits increasing responses as temperatures elevate into the noxious range (Peier, Reeve, and others 2002). In rodents, TRPV3 is primarily expressed in skin keratinocytes as well as other epithelia of the tongue, palate, nose, and distal colon. However, TRPV3 is not expressed in rodent sensory neurons, though mRNA and protein analysis in monkey and human have reported that TRPV3 expression in sensory neurons of these species (Smith and others 2002; Xu and others 2002). If accurate, TRPV3 may function to detect temperature differently in rodents and primates. The restricted expression of TRPV3 to epithelial cells suggests that any role the channel could have in thermal sensation and/or nociception would likely be indirect through a relay of information between these cells and sensory neurons.
Studies utilizing TRPV3−/− mice have reported conflicting findings. Some studies reported nocifensive deficits in response to noxious heat suggesting overlapping function between TRPV1 and TRPV3, while others reported no deficits in noxious heat sensation (Moqrich and others 2005; Huang and others 2011; Miyamoto and others 2011; Marics and others 2014). Differences in mouse strain background or methodology may account for the conflicting findings. The group that reported differences in heat sensation found that TRPV3 activation led to nitric oxide (NO) production in keratinocytes and blockade of NO synthesis inhibited acute thermal nociception via a TRPV3 dependent mechanism. Evidence was also provided suggesting that NO acts upstream of TRPV1 to evoke thermal nocifensive behavior (Miyamoto and others 2011).
While knockout studies did not support a role for TRPV3 in inflammatory thermal hyperalgesia, other studies described a link. When TRPV3 was overexpressed in keratinocytes, these cells released the inflammatory mediator, prostaglandin E(2) (PGE(2)) in a TRPV3 dependent manner. Inhibition of PGE(2) production, with co-inhibition of TRPV1 to mask its contributions to thermal hyperalgesia, decreased inflammatory thermal hyperalgesia in mice overexpressing TRPV3 in keratinocytes (Huang and others 2008). TRPV3 activation in keratinocytes has also been linked to the production and release of the inflammatory mediator ATP, suggesting another TRPV3 dependent route to sensitize nociceptive pathways (Mandadi and others 2009). The endogenous TRPV3 antagonist 17(R)-resolvin D1 was found to reverse inflammatory thermal hyperalgesia, and this effect was blunted upon knockdown of epidermal TRPV3 expression (Bang and others 2012) further support for a role for TRPV3 in inflammatory nociception.
TRPV4
Like TRPV3, TRPV4 is activated by warm temperatures (25–34°C) (Guler and others 2002; Watanabe and others 2002). Unlike TRPV3, which is strongly sensitized by repeated heat activation, TRPV4 is desensitized upon sustained or repeated stimulation at temperatures ≥42°C (Guler and others 2002). PIP2 is required for thermal activation of TRPV4, as mutation of its N-terminal phosphoinositide binding site inhibits activation (Garcia-Elias and others 2013). A tyrosine in its S3 domain has also been shown to be required for thermal sensitivity (Vriens and others 2004). TRPV4 is also activated by osmotic stimuli. Hypotonic solution activates the channel while hypertonic solution inhibits its activity (Liedtke and others 2000; Strotmann and others 2000). Mechanical shear stress and high viscosity solutions have also been reported to activate TRPV4. The channel is broadly expressed in many tissues including keratinocytes and sensory neurons (Everaerts and others 2010). Within sensory neurons there is evidence that TRPV4 is expressed in small/medium diameter neurons with overlap in expression with TRPV1 (Alessandri-Haber and others 2003).
TRPV4−/− mice were reported to have impaired nocifensive responses to a number of stimuli including mechanical stimuli, mild hypertonic solution and hypotonic solution(Alessandri-Haber and others 2003; Liedtke and Friedman 2003; Suzuki and others 2003). There are conflicting reports on the role of TRPV4 in noxious heat sensation. Some studies showed no effects on temperature sensation, normal acute responses to noxious heat but impaired inflammatory thermal hyperalgesia while others showed deficits in acute heat responses yet no differences in behavior after inflammation (Liedtke and Friedman 2003; Suzuki and others 2003; Todaka and others 2004; Lee and others 2005; Huang and others 2011). Additionally, inflammatory mediators such as PGE2 and PAR2 have been shown to sensitize TRPV4 (Alessandri-Haber and others 2005; Grant and others 2007; Zhao and others 2014). These results indicate that TRPV4 could be required for normal nociception and could have a role in abnormal pain conditions. Indeed in a rodent model of chemotherapy-induced neuropathic pain, antisense oligonucleotide knockdown of TRPV4 in sensory neurons inhibited mechanical and hypotonic hyperalgesia (Alessandri-Haber and others 2004). TRPV4 as well as TRPV1 was shown to be required for mechanical hypersensitivity associated with delayed onset muscle soreness (Ota and others 2013: 1). TRPV4 and TRPA1 were both found to contribute to formalin-evoked trigeminal pain (Chen and others 2014). TRPV4 plays a pivotal role in pain associated with a model of temporomandibular joint disorder, as TRPV4−/− animals display reduced masticatory sensitization (Chen and others 2013). While these data implicate TRPV4 as a mediator of nociception, wide spread expression in many tissues might limit its usefulness as a target for analgesic intervention.
Conclusions
Despite massive investment, there has been limited success in the development of effective analgesics for the treatment of chronic pain. It is now clear in the eighteen years since the discovery of TRPV1, that thermoTRP channels play an essential role as primary molecular sensors of noxious stimuli and are integral to pain elicited by inflammatory and neuropathic pain conditions. Whether or not thermoTRPs will be useful targets for analgesic intervention remains to be seen. Antagonists of thermoTRPs, while successfully inhibiting pain caused by inflammatory and chronic pain diseases, have had unforeseen side effects including body temperature dysregulation and blockade of acute thermal sensation used to avoid injury. Promising new studies suggest that these obstacles may be able to be overcome with more specific drugs. It is always difficult to translate research from the bench to the clinical settings but thermoTRPs remain enticing targets for pain intervention.
Acknowledgments
This work was supported by a Scan Design Foundation Innovative Pain Research Grant (AD), and NIH DE023730 (AD).
Footnotes
Conflict of Interest: The authors declare no competing financial interests
References
- Abe J, Hosokawa H, Sawada Y, Matsumura K, Kobayashi S. Ca2+-dependent PKC activation mediates menthol-induced desensitization of transient receptor potential M8. Neurosci. Lett. 2006;397:140–144. doi: 10.1016/j.neulet.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Akbar A, Yiangou Y, Facer P, Walters JRF, Anand P, Ghosh S. Increased capsaicin receptor TRPV1-expressing sensory fibres in irritable bowel syndrome and their correlation with abdominal pain. Gut. 2008;57:923–929. doi: 10.1136/gut.2007.138982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akopian AN, Ruparel NB, Jeske NA, Hargreaves KM. TRPA1 Desensitization in Sensory Neurons is Agonist- Dependent and Regulated by TRPV1-Directed Internalization. J Physiol [Internet] 2007 doi: 10.1113/jphysiol.2007.133231. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17584831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessandri-Haber N, Dina OA, Yeh JJ, Parada CA, Reichling DB, Levine JD. Transient receptor potential vanilloid 4 is essential in chemotherapy-induced neuropathic pain in the rat. J. Neurosci. Off. J. Soc. Neurosci. 2004;24:4444–4452. doi: 10.1523/JNEUROSCI.0242-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessandri-Haber N, Joseph E, Dina OA, Liedtke W, Levine JD. TRPV4 mediates pain-related behavior induced by mild hypertonic stimuli in the presence of inflammatory mediator. Pain. 2005;118:70–79. doi: 10.1016/j.pain.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Alessandri-Haber N, Yeh JJ, Boyd AE, Parada CA, Chen X, Reichling DB, et al. Hypotonicity induces TRPV4-mediated nociception in rat. Neuron. 2003;39:497–511. doi: 10.1016/s0896-6273(03)00462-8. [DOI] [PubMed] [Google Scholar]
- Anand U, Otto WR, Anand P. Sensitization of capsaicin and icilin responses in oxaliplatin treated adult rat DRG neurons. Mol. Pain. 2010;6:82. doi: 10.1186/1744-8069-6-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson DA, Gentry C, Light E, Vastani N, Vallortigara J, Bierhaus A, et al. Methylglyoxal evokes pain by stimulating TRPA1. PloS One. 2013;8:e77986. doi: 10.1371/journal.pone.0077986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson DA, Gentry C, Moss S, Bevan S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J Neurosci. 2008;28:2485–2494. doi: 10.1523/JNEUROSCI.5369-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson DA, Nash M, Bevan S. Modulation of the cold-activated channel TRPM8 by lysophospholipids and polyunsaturated fatty acids. J Neurosci. 2007;27:3347–3355. doi: 10.1523/JNEUROSCI.4846-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreev YA, Kozlov SA, Korolkova YV, Dyachenko IA, Bondarenko DA, Skobtsov DI, et al. Polypeptide modulators of TRPV1 produce analgesia without hyperthermia. Mar. Drugs. 2013;11:5100–5115. doi: 10.3390/md11125100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babes A, Fischer MJM, Filipovic M, Engel MA, Flonta M-L, Reeh PW. The anti-diabetic drug glibenclamide is an agonist of the transient receptor potential Ankyrin 1 (TRPA1) ion channel. Eur. J. Pharmacol. 2013;704:15–22. doi: 10.1016/j.ejphar.2013.02.018. [DOI] [PubMed] [Google Scholar]
- Backonja M, Wallace MS, Blonsky ER, Cutler BJ, Malan P, Rauck R, et al. NGX-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgia: a randomised, double-blind study. Lancet Neurol. 2008;7:1106–1112. doi: 10.1016/S1474-4422(08)70228-X. [DOI] [PubMed] [Google Scholar]
- Bandell M, Dubin AE, Petrus MJ, Orth A, Mathur J, Hwang SW, et al. High-throughput random mutagenesis screen reveals TRPM8 residues specifically required for activation by menthol. Nat Neurosci. 2006;9:493–500. doi: 10.1038/nn1665. [DOI] [PubMed] [Google Scholar]
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, et al. Noxious Cold Ion Channel TRPA1 Is Activated by Pungent Compounds and Bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- Bang S, Yoo S, Yang TJ, Cho H, Hwang SW. 17(R)-resolvin D1 specifically inhibits transient receptor potential ion channel vanilloid 3 leading to peripheral antinociception. Br. J. Pharmacol. 2012;165:683–692. doi: 10.1111/j.1476-5381.2011.01568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bánvölgyi A, Pozsgai G, Brain SD, Helyes ZS, Szolcsányi J, Ghosh M, et al. Mustard oil induces a transient receptor potential vanilloid 1 receptor-independent neurogenic inflammation and a non-neurogenic cellular inflammatory component in mice. Neuroscience. 2004;125:449–459. doi: 10.1016/j.neuroscience.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Barton NJ, McQueen DS, Thomson D, Gauldie SD, Wilson AW, Salter DM, et al. Attenuation of experimental arthritis in TRPV1R knockout mice. Exp Mol Pathol. 2006;81:166–170. doi: 10.1016/j.yexmp.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, et al. TRPA1 Mediates the Inflammatory Actions of Environmental Irritants and Proalgesic Agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, et al. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature [Internet] 2007 doi: 10.1038/nature05910. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17538622. [DOI] [PubMed] [Google Scholar]
- Bavencoffe A, Kondratskyi A, Gkika D, Mauroy B, Shuba Y, Prevarskaya N, et al. Complex regulation of the TRPM8 cold receptor channel: role of arachidonic acid release following M3 muscarinic receptor stimulation. J. Biol. Chem. 2011;286:9849–9855. doi: 10.1074/jbc.M110.162016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RWth. cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron. 2002;35:721–731. doi: 10.1016/s0896-6273(02)00802-4. [DOI] [PubMed] [Google Scholar]
- Brauchi S, Orio P, Latorre R. Clues to understanding cold sensation: thermodynamics and electrophysiological analysis of the cold receptor TRPM8. Proc Natl Acad Sci U A. 2004;101:15494–15499. doi: 10.1073/pnas.0406773101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauchi S, Orta G, Salazar M, Rosenmann E, Latorre R. A hot-sensing cold receptor: C-terminal domain determines thermosensation in transient receptor potential channels. J Neurosci. 2006;26:4835–4840. doi: 10.1523/JNEUROSCI.5080-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner DS, Vogt SK, Gereau RW. A technique to measure cold adaptation in freely behaving mice. J. Neurosci. Methods. 2014;236:86–91. doi: 10.1016/j.jneumeth.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo RR, Meegalla SK, Parks DJ, Parsons WH, Ballentine SK, Lubin ML, et al. Discovery of vinylcycloalkyl-substituted benzimidazole TRPM8 antagonists effective in the treatment of cold allodynia. Bioorg. Med. Chem. Lett. 2012;22:1903–1907. doi: 10.1016/j.bmcl.2012.01.060. [DOI] [PubMed] [Google Scholar]
- Del Camino D, Murphy S, Heiry M, Barrett LB, Earley TJ, Cook CA, et al. TRPA1 contributes to cold hypersensitivity. J. Neurosci. Off. J. Soc. Neurosci. 2010;30:15165–15174. doi: 10.1523/JNEUROSCI.2580-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao E, Cordero-Morales JF, Liu B, Qin F, Julius D. TRPV1 Channels Are Intrinsically Heat Sensitive and Negatively Regulated by Phosphoinositide Lipids. Neuron. 2013;77:667–679. doi: 10.1016/j.neuron.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao E, Liao M, Cheng Y, Julius D. TRPV1 structures in distinct conformations reveal activation mechanisms. Nature. 2013;504:113–118. doi: 10.1038/nature12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspani O, Zurborg S, Labuz D, Heppenstall PA. The contribution of TRPM8 and TRPA1 channels to cold allodynia and neuropathic pain. PLoS One. 2009;4:e7383. doi: 10.1371/journal.pone.0007383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Ceppa E, Cattaruzza F, Lyo V, Amadesi S, Pelayo J-C, Poole DP, et al. Transient receptor potential ion channels V4 and A1 contribute to pancreatitis pain in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;299:G556–G571. doi: 10.1152/ajpgi.00433.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Joshi SK, DiDomenico S, Perner RJ, Mikusa JP, Gauvin DM, et al. Selective blockade of TRPA1 channel attenuates pathological pain without altering noxious cold sensation or body temperature regulation. Pain. 2011;152:1165–1172. doi: 10.1016/j.pain.2011.01.049. [DOI] [PubMed] [Google Scholar]
- Chen Y, Kanju P, Fang Q, Lee SH, Parekh PK, Lee W, et al. TRPV4 is necessary for trigeminal irritant pain and functions as a cellular formalin receptor. PAIN®. 2014;155:2662–2672. doi: 10.1016/j.pain.2014.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Williams SH, McNulty AL, Hong JH, Lee SH, Rothfusz NE, et al. Temporomandibular joint pain: a critical role for Trpv4 in the trigeminal ganglion. Pain. 2013;154:1295–1304. doi: 10.1016/j.pain.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Yang C, Wang ZJ. Proteinase-activated receptor 2 sensitizes transient receptor potential vanilloid 1, transient receptor potential vanilloid 4, and transient receptor potential ankyrin 1 in paclitaxel-induced neuropathic pain. Neuroscience. 2011;193:440–451. doi: 10.1016/j.neuroscience.2011.06.085. [DOI] [PubMed] [Google Scholar]
- Chizh BA, O'Donnell MB, Napolitano A, Wang J, Brooke AC, Aylott MC, et al. The effects of the TRPV1 antagonist SB-705498 on TRPV1 receptor-mediated activity and inflammatory hyperalgesia in humans. Pain. 2007;132:132–141. doi: 10.1016/j.pain.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Chuang HH, Neuhausser WM, Julius D. The super-cooling agent icilin reveals a mechanism of coincidence detection by a temperature-sensitive TRP channel. Neuron. 2004;43:859–869. doi: 10.1016/j.neuron.2004.08.038. [DOI] [PubMed] [Google Scholar]
- Colburn RW, Lubin ML, Stone DJ, Wang Y, Lawrence D, D'Andrea MR, et al. Attenuated cold sensitivity in TRPM8 null mice. Neuron. 2007;54:379–386. doi: 10.1016/j.neuron.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Da Costa DSM, Meotti FC, Andrade EL, Leal PC, Motta EM, Calixto JB. The involvement of the transient receptor potential A1 (TRPA1) in the maintenance of mechanical and cold hyperalgesia in persistent inflammation. Pain. 2010;148:431–437. doi: 10.1016/j.pain.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Cruz-Orengo L, Dhaka A, Heuermann RJ, Young TJ, Montana MC, Cavanaugh EJ, et al. Cutaneous nociception evoked by 15-delta PGJ2 via activation of ion channel TRPA1. Mol. Pain. 2008;4:30. doi: 10.1186/1744-8069-4-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- DeBerry JJ, Schwartz ES, Davis BM. TRPA1 mediates bladder hyperalgesia in a mouse model of cystitis. Pain. 2014;155:1280–1287. doi: 10.1016/j.pain.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaka A, Earley TJ, Watson J, Patapoutian A. Visualizing cold spots: TRPM8-expressing sensory neurons and their projections. J Neurosci. 2008;28:566–575. doi: 10.1523/JNEUROSCI.3976-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A. TRPM8 is required for cold sensation in mice. Neuron. 2007;54:371–378. doi: 10.1016/j.neuron.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Dhaka A, Uzzell V, Dubin AE, Mathur J, Petrus M, Bandell M, et al. TRPV1 is activated by both acidic and basic pH. J Neurosci. 2009;29:153–158. doi: 10.1523/JNEUROSCI.4901-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaka A, Viswanath V, Patapoutian A. TRP Ion Channels and Temperature Sensation. Annu Rev Neurosci [Internet] 2006 doi: 10.1146/annurev.neuro.29.051605.112958. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16602906. [DOI] [PubMed] [Google Scholar]
- Doerner JF, Gisselmann G, Hatt H, Wetzel CH. Transient receptor potential channel A1 is directly gated by calcium ions. J Biol Chem. 2007;282:13180–13189. doi: 10.1074/jbc.M607849200. [DOI] [PubMed] [Google Scholar]
- Eid SR, Crown ED, Moore EL, Liang HA, Choong K-C, Dima S, et al. HC-030031, a TRPA1 selective antagonist, attenuates inflammatory- and neuropathy-induced mechanical hypersensitivity. Mol. Pain. 2008;4:48. doi: 10.1186/1744-8069-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel MA, Leffler A, Niedermirtl F, Babes A, Zimmermann K, Filipović MR, et al. TRPA1 and substance P mediate colitis in mice. Gastroenterology. 2011;141:1346–1358. doi: 10.1053/j.gastro.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Everaerts W, Nilius B, Owsianik G. The vanilloid transient receptor potential channel TRPV4: from structure to disease. Prog. Biophys. Mol. Biol. 2010;103:2–17. doi: 10.1016/j.pbiomolbio.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Fernandes ES, Russell FA, Spina D, McDougall JJ, Graepel R, Gentry C, et al. A distinct role for transient receptor potential ankyrin 1, in addition to transient receptor potential vanilloid 1, in tumor necrosis factor α-induced inflammatory hyperalgesia and Freunds complete adjuvant-induced monarthritis. Arthritis Rheum. 2011;63:819–829. doi: 10.1002/art.30150. [DOI] [PubMed] [Google Scholar]
- Fernández JA, Skryma R, Bidaux G, Magleby KL, Scholfield CN, McGeown JG, et al. Voltage-and cold-dependent gating of single TRPM8 ion channels. J. Gen. Physiol. 2011;137:173–195. doi: 10.1085/jgp.201010498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forst T, Pohlmann T, Kunt T, Goitom K, Schulz G, Löbig M, et al. The influence of local capsaicin treatment on small nerve fibre function and neurovascular control in symptomatic diabetic neuropathy. Acta Diabetol. 2002;39:1–6. doi: 10.1007/s005920200005. [DOI] [PubMed] [Google Scholar]
- Garcia-Elias A, Mrkonjic S, Pardo-Pastor C, Inada H, Hellmich UA, Rubio-Moscardó F, et al. Phosphatidylinositol-4,5-biphosphate-dependent rearrangement of TRPV4 cytosolic tails enables channel activation by physiological stimuli. Proc. Natl. Acad. Sci. U. S. A. 2013;110:9553–9558. doi: 10.1073/pnas.1220231110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauchan P, Andoh T, Kato A, Kuraishi Y. Involvement of increased expression of transient receptor potential melastatin 8 in oxaliplatin-induced cold allodynia in mice. Neurosci. Lett. 2009;458:93–95. doi: 10.1016/j.neulet.2009.04.029. [DOI] [PubMed] [Google Scholar]
- Gavva NR, Klionsky L, Qu Y, Shi L, Tamir R, Edenson S, et al. Molecular Determinants of Vanilloid Sensitivity in TRPV1. J. Biol. Chem. 2004;279:20283–20295. doi: 10.1074/jbc.M312577200. [DOI] [PubMed] [Google Scholar]
- Gavva NR, Treanor JJ, Garami A, Fang L, Surapaneni S, Akrami A, et al. Pharmacological blockade of the vanilloid receptor TRPV1 elicits marked hyperthermia in humans. Pain. 2008;136:202–210. doi: 10.1016/j.pain.2008.01.024. [DOI] [PubMed] [Google Scholar]
- Gopinath P, Wan E, Holdcroft A, Facer P, Davis JB, Smith GD, et al. Increased capsaicin receptor TRPV1 in skin nerve fibres and related vanilloid receptors TRPV3 and TRPV4 in keratinocytes in human breast pain. BMC Womens Health. 2005;5:2. doi: 10.1186/1472-6874-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandl J, Kim SE, Uzzell V, Bursulaya B, Petrus M, Bandell M, et al. Temperature-induced opening of TRPV1 ion channel is stabilized by the pore domain. Nat. Neurosci. 2010;13:708–714. doi: 10.1038/nn.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant AD, Cottrell GS, Amadesi S, Trevisani M, Nicoletti P, Materazzi S, et al. Protease-activated receptor 2 sensitizes the transient receptor potential vanilloid 4 ion channel to cause mechanical hyperalgesia in mice. J Physiol. 2007;578:715–733. doi: 10.1113/jphysiol.2006.121111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guler AD, Lee H, Iida T, Shimizu I, Tominaga M, Caterina M. Heat-evoked activation of the ion channel, TRPV4. J Neurosci. 2002;22:6408–6414. doi: 10.1523/JNEUROSCI.22-15-06408.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helyes Z, Sándor K, Borbély E, Tékus V, Pintér E, Elekes K, et al. Involvement of transient receptor potential vanilloid 1 receptors in protease-activated receptor-2-induced joint inflammation and nociception. Eur. J. Pain Lond. Engl. 2010;14:351–358. doi: 10.1016/j.ejpain.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Hinman A, Chuang HH, Bautista DM, Julius D. TRP channel activation by reversible covalent modification. Proc Natl Acad Sci U A. 2006;103:19564–19568. doi: 10.1073/pnas.0609598103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honore P, Wismer CT, Mikusa J, Zhu CZ, Zhong C, Gauvin DM, et al. A-425619 [1-isoquinolin-5-yl-3-(4-trifluoromethyl-benzyl)-urea], a novel transient receptor potential type V1 receptor antagonist, relieves pathophysiological pain associated with inflammation and tissue injury in rats. J. Pharmacol. Exp. Ther. 2005;314:410–421. doi: 10.1124/jpet.105.083915. [DOI] [PubMed] [Google Scholar]
- Huang SM, Lee H, Chung M-K, Park U, Yu YY, Bradshaw HB, et al. Overexpressed transient receptor potential vanilloid 3 ion channels in skin keratinocytes modulate pain sensitivity via prostaglandin E2. J. Neurosci. Off. J. Soc. Neurosci. 2008;28:13727–13737. doi: 10.1523/JNEUROSCI.5741-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SM, Li X, Yu Y, Wang J, Caterina MJ. TRPV3 and TRPV4 ion channels are not major contributors to mouse heat sensation. Mol. Pain. 2011;7:37. doi: 10.1186/1744-8069-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutter MM, Wick EC, Day AL, Maa J, Zerega EC, Richmond AC, et al. Transient receptor potential vanilloid (TRPV-1) promotes neurogenic inflammation in the pancreas via activation of the neurokinin-1 receptor (NK-1R) Pancreas. 2005;30:260–265. doi: 10.1097/01.mpa.0000153616.63384.24. [DOI] [PubMed] [Google Scholar]
- Johnson DM, Garrett EM, Rutter R, Bonnert TP, Gao Y-D, Middleton RE, et al. Functional mapping of the transient receptor potential vanilloid 1 intracellular binding site. Mol. Pharmacol. 2006;70:1005–1012. doi: 10.1124/mol.106.023945. [DOI] [PubMed] [Google Scholar]
- Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- Jordt SE, Julius D. Molecular basis for species-specific sensitivity to “hot” chili peppers. Cell. 2002;108:421–430. doi: 10.1016/s0092-8674(02)00637-2. [DOI] [PubMed] [Google Scholar]
- Jordt SE, Tominaga M, Julius D. Acid potentiation of the capsaicin receptor determined by a key extracellular site. Proc Natl Acad Sci U A. 2000;97:8134–8139. doi: 10.1073/pnas.100129497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius D. TRP Channels and Pain. Annu. Rev. Cell Dev. Biol. 2013;29:355–384. doi: 10.1146/annurev-cellbio-101011-155833. [DOI] [PubMed] [Google Scholar]
- Jung J, Shin JS, Lee S-Y, Hwang SW, Koo J, Cho H, et al. Phosphorylation of vanilloid receptor 1 by Ca2+/calmodulin-dependent kinase II regulates its vanilloid binding. J. Biol. Chem. 2004;279:7048–7054. doi: 10.1074/jbc.M311448200. [DOI] [PubMed] [Google Scholar]
- Karashima Y, Prenen J, Meseguer V, Owsianik G, Voets T, Nilius B. Modulation of the transient receptor potential channel TRPA1 by phosphatidylinositol 4,5-biphosphate manipulators. Pflüg. Arch. Eur. J. Physiol. 2008;457:77–89. doi: 10.1007/s00424-008-0493-6. [DOI] [PubMed] [Google Scholar]
- Katsura H, Obata K, Mizushima T, Yamanaka H, Kobayashi K, Dai Y, et al. Antisense knock down of TRPA1, but not TRPM8, alleviates cold hyperalgesia after spinal nerve ligation in rats. Exp. Neurol. 2006;200:112–123. doi: 10.1016/j.expneurol.2006.01.031. [DOI] [PubMed] [Google Scholar]
- Keeble J, Russell F, Curtis B, Starr A, Pinter E, Brain SD. Involvement of transient receptor potential vanilloid 1 in the vascular and hyperalgesic components of joint inflammation. Arthritis Rheum. 2005;52:3248–3256. doi: 10.1002/art.21297. [DOI] [PubMed] [Google Scholar]
- Kelly S, Chapman RJ, Woodhams S, Sagar DR, Turner J, Burston JJ, et al. Increased function of pronociceptive TRPV1 at the level of the joint in a rat model of osteoarthritis pain. Ann. Rheum. Dis. 2015;74:252–259. doi: 10.1136/annrheumdis-2013-203413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Cavanaugh EJ, Simkin D. Inhibition of transient receptor potential A1 channel by phosphatidylinositol-4,5-bisphosphate. Am. J. Physiol. Cell Physiol. 2008;295:C92–C99. doi: 10.1152/ajpcell.00023.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King T, Qu C, Okun A, Mercado R, Ren J, Brion T, et al. Contribution of afferent pathways to nerve injury-induced spontaneous pain and evoked hypersensitivity. Pain. 2011;152:1997–2005. doi: 10.1016/j.pain.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton WM, Bifolck-Fisher A, Bautista DM, McKemy DD. TRPM8, but not TRPA1, is required for neural and behavioral responses to acute noxious cold temperatures and cold-mimetics in vivo. Pain. 2010;150:340–350. doi: 10.1016/j.pain.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton WM, Bifolck-Fisher A, Bautista DM, McKemy DD. TRPM8, but not TRPA1, is required for neural and behavioral responses to acute noxious cold temperatures and cold-mimetics in vivo. Pain. 150:340–350. doi: 10.1016/j.pain.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton WM, Daniels RL, Palkar R, McCoy DD, McKemy DD. Pharmacological blockade of TRPM8 ion channels alters cold and cold pain responses in mice. PloS One. 2011;6:e25894. doi: 10.1371/journal.pone.0025894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton WM, Palkar R, Lippoldt EK, McCoy DD, Baluch F, Chen J, et al. A Sensory-Labeled Line for Cold: TRPM8-Expressing Sensory Neurons Define the Cellular Basis for Cold, Cold Pain, and Cooling-Mediated Analgesia. J. Neurosci. 2013;33:2837–2848. doi: 10.1523/JNEUROSCI.1943-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krarup AL, Ny L, Astrand M, Bajor A, Hvid-Jensen F, Hansen MB, et al. Randomised clinical trial: the efficacy of a transient receptor potential vanilloid 1 antagonist AZD1386 in human oesophageal pain. Aliment. Pharmacol. Ther. 2011;33:1113–1122. doi: 10.1111/j.1365-2036.2011.04629.x. [DOI] [PubMed] [Google Scholar]
- Kremeyer B, Lopera F, Cox JJ, Momin A, Rugiero F, Marsh S, et al. A gain-of-function mutation in TRPA1 causes familial episodic pain syndrome. Neuron. 2010;66:671–680. doi: 10.1016/j.neuron.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan KY. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006:50. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Kwon S-G, Roh D-H, Yoon S-Y, Moon J-Y, Choi S-R, Choi H-S, et al. Acid evoked thermal hyperalgesia involves peripheral P2Y1 receptor mediated TRPV1 phosphorylation in a rodent model of thrombus induced ischemic pain. Mol. Pain. 2014;10:2. doi: 10.1186/1744-8069-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashinger ESR, Steiginga MS, Hieble JP, Leon LA, Gardner SD, Nagilla R, et al. AMTB, a TRPM8 channel blocker: evidence in rats for activity in overactive bladder and painful bladder syndrome. Am. J. Physiol. Renal Physiol. 2008;295:F803–F810. doi: 10.1152/ajprenal.90269.2008. [DOI] [PubMed] [Google Scholar]
- Lee H, Iida T, Mizuno A, Suzuki M, Caterina MJ. Altered thermal selection behavior in mice lacking transient receptor potential vanilloid 4. J Neurosci. 2005;25:1304–1310. doi: 10.1523/JNEUROSCI.4745.04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehto SG, Tamir R, Deng H, Klionsky L, Kuang R, Le A, et al. Antihyperalgesic effects of (R,E)-N-(2-hydroxy-2,3-dihydro-1H-inden-4-yl)-3-(2-(piperidin-1-yl)-4-(trifluoromethyl)phenyl)-acrylamide (AMG8562), a novel transient receptor potential vanilloid type 1 modulator that does not cause hyperthermia in rats. J. Pharmacol. Exp. Ther. 2008;326:218–229. doi: 10.1124/jpet.107.132233. [DOI] [PubMed] [Google Scholar]
- Liao M, Cao E, Julius D, Cheng Y. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature. 2013;504:107–112. doi: 10.1038/nature12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke W, Choe Y, Marti-Renom MA, Bell AM, Denis CS, Sali A, et al. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 2000;103:525–535. doi: 10.1016/s0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke W, Friedman JM. Abnormal osmotic regulation in trpv4−/− mice. Proc Natl Acad Sci U A. 2003;100:13698–13703. doi: 10.1073/pnas.1735416100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Hasan R, Zhang X. The basal thermal sensitivity of the TRPV1 ion channel is determined by PKCβII. J. Neurosci. Off. J. Soc. Neurosci. 2014;34:8246–8258. doi: 10.1523/JNEUROSCI.0278-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippoldt EK, Elmes RR, McCoy DD, Knowlton WM, McKemy DD. Artemin, a glial cell line-derived neurotrophic factor family member, induces TRPM8-dependent cold pain. J. Neurosci. Off. J. Soc. Neurosci. 2013;33:12543–12552. doi: 10.1523/JNEUROSCI.5765-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Fan L, Balakrishna S, Sui A, Morris JB, Jordt S-E. TRPM8 is the principal mediator of menthol-induced analgesia of acute and inflammatory pain. Pain. 2013;154:2169–2177. doi: 10.1016/j.pain.2013.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Qin F. Functional control of cold- and menthol-sensitive TRPM8 ion channels by phosphatidylinositol 4,5-bisphosphate. J Neurosci. 2005;25:1674–1681. doi: 10.1523/JNEUROSCI.3632-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, et al. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007;445:541–545. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- Macpherson LJ, Geierstanger BH, Viswanath V, Bandell M, Eid SR, Hwang S, et al. The pungency of garlic: activation of TRPA1 and TRPV1 in response to allicin. Curr Biol. 2005;15:929–934. doi: 10.1016/j.cub.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Macpherson LJ, Xiao B, Kwan KY, Petrus MJ, Dubin AE, Hwang S, et al. An ion channel essential for sensing chemical damage. J Neurosci. 2007;27:11412–11415. doi: 10.1523/JNEUROSCI.3600-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher M, Ao H, Banke T, Nasser N, Wu N-T, Breitenbucher JG, et al. Activation of TRPA1 by farnesyl thiosalicylic acid. Mol. Pharmacol. 2008;73:1225–1234. doi: 10.1124/mol.107.042663. [DOI] [PubMed] [Google Scholar]
- Mandadi S, Sokabe T, Shibasaki K, Katanosaka K, Mizuno A, Moqrich A, et al. TRPV3 in keratinocytes transmits temperature information to sensory neurons via ATP. Pflüg. Arch. Eur. J. Physiol. 2009;458:1093–1102. doi: 10.1007/s00424-009-0703-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandadi S, Tominaga T, Numazaki M, Murayama N, Saito N, Armati PJ, et al. Increased sensitivity of desensitized TRPV1 by PMA occurs through PKCepsilon-mediated phosphorylation at S800. Pain. 2006;123:106–116. doi: 10.1016/j.pain.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Marics I, Malapert P, Reynders A, Gaillard S, Moqrich A. Acute heat-evoked temperature sensation is impaired but not abolished in mice lacking TRPV1 and TRPV3 channels. PloS One. 2014;9:e99828. doi: 10.1371/journal.pone.0099828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaraughty S, Chu KL, Perner RJ, Didomenico S, Kort ME, Kym PR. TRPA1 modulation of spontaneous and mechanically evoked firing of spinal neurons in uninjured, osteoarthritic, and inflamed rats. Mol. Pain. 2010;6:14. doi: 10.1186/1744-8069-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKemy DD. The molecular and cellular basis of cold sensation. ACS Chem. Neurosci. 2013;4:238–247. doi: 10.1021/cn300193h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- Miyamoto T, Petrus MJ, Dubin AE, Patapoutian A. TRPV3 regulates nitric oxide synthase-independent nitric oxide synthesis in the skin. Nat. Commun. 2011;2:369. doi: 10.1038/ncomms1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moilanen LJ, Laavola M, Kukkonen M, Korhonen R, Leppänen T, Högestätt ED, et al. TRPA1 Contributes to the Acute Inflammatory Response and Mediates Carrageenan-Induced Paw Edema in the Mouse. Sci. Rep. [Internet] 2. 2012 doi: 10.1038/srep00380. Available from: http://www.nature.com/srep/2012/120424/srep00380/full/srep00380.html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moparthi L, Survery S, Kreir M, Simonsen C, Kjellbom P, Högestätt ED, et al. Human TRPA1 is intrinsically cold- and chemosensitive with and without its N-terminal ankyrin repeat domain. Proc. Natl. Acad. Sci. U. S. A. 2014 doi: 10.1073/pnas.1412689111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moqrich A, Hwang SW, Earley TJ, Petrus MJ, Murray AN, Spencer KS, et al. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science. 2005;307:1468–1472. doi: 10.1126/science.1108609. [DOI] [PubMed] [Google Scholar]
- Mukerji G, Yiangou Y, Corcoran SL, Selmer IS, Smith GD, Benham CD, et al. Cool and menthol receptor TRPM8 in human urinary bladder disorders and clinical correlations. BMC Urol. 2006;6:6. doi: 10.1186/1471-2490-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassini R, Gees M, Harrison S, De Siena G, Materazzi S, Moretto N, et al. Oxaliplatin elicits mechanical and cold allodynia in rodents via TRPA1 receptor stimulation. Pain. 2011;152:1621–1631. doi: 10.1016/j.pain.2011.02.051. [DOI] [PubMed] [Google Scholar]
- Nealen ML, Gold MS, Thut PD, Caterina MJ. TRPM8 mRNA is expressed in a subset of cold-responsive trigeminal neurons from rat. J Neurophysiol. 2003;12:12. doi: 10.1152/jn.00843.2002. [DOI] [PubMed] [Google Scholar]
- Nilius B, Talavera K, Owsianik G, Prenen J, Droogmans G, Voets T. Gating of TRP channels: a voltage connection? J Physiol. 2005;567:35–44. doi: 10.1113/jphysiol.2005.088377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata K, Katsura H, Mizushima T, Yamanaka H, Kobayashi K, Dai Y, et al. TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury. J Clin Invest. 2005;115:2393–2401. doi: 10.1172/JCI25437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota H, Katanosaka K, Murase S, Kashio M, Tominaga M, Mizumura K. TRPV1 and TRPV4 play pivotal roles in delayed onset muscle soreness. PloS One. 2013;8:e65751. doi: 10.1371/journal.pone.0065751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C-K, Xu Z-Z, Berta T, Han Q, Chen G, Liu X-J, et al. Extracellular microRNAs activate nociceptor neurons to elicit pain via TLR7 and TRPA1. Neuron. 2014;82:47–54. doi: 10.1016/j.neuron.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R, Gonçalves L, Newman R, Jiang FL, Goldby A, Reeve J, et al. Novel TRPM8 antagonist attenuates cold hypersensitivity after peripheral nerve injury in rats. J. Pharmacol. Exp. Ther. 2014;349:47–55. doi: 10.1124/jpet.113.211243. [DOI] [PubMed] [Google Scholar]
- Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- Peier AM, Reeve AJ, Andersson DA, Moqrich A, Earley TJ, Hergarden AC, et al. A heat-sensitive TRP channel expressed in keratinocytes. Science. 2002;296:2046–2049. doi: 10.1126/science.1073140. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Starowicz K, Moriello AS, Vivese M, Orlando P, Di Marzo V. Regulation of transient receptor potential channels of melastatin type 8 (TRPM8): effect of cAMP, cannabinoid CB(1) receptors and endovanilloids. Exp. Cell Res. 2007;313:1911–1920. doi: 10.1016/j.yexcr.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Petrus M, Peier AM, Bandell M, Hwang SW, Huynh T, Olney N, et al. A role of TRPA1 in mechanical hyperalgesia is revealed by pharmacological inhibition. Mol Pain. 2007;3:40. doi: 10.1186/1744-8069-3-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogorzala LA, Mishra SK, Hoon MA. The cellular code for mammalian thermosensation. J. Neurosci. Off. J. Soc. Neurosci. 2013;33:5533–5541. doi: 10.1523/JNEUROSCI.5788-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premkumar LS, Ahern GP. Induction of vanilloid receptor channel activity by protein kinase C. Nature. 2000;408:985–990. doi: 10.1038/35050121. [DOI] [PubMed] [Google Scholar]
- Premkumar LS, Raisinghani M, Pingle SC, Long C, Pimentel F. Downregulation of transient receptor potential melastatin 8 by protein kinase C-mediated dephosphorylation. J Neurosci. 2005;25:11322–11329. doi: 10.1523/JNEUROSCI.3006-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott ED, Julius D. A modular PIP2 binding site as a determinant of capsaicin receptor sensitivity. Science. 2003;300:1284–1288. doi: 10.1126/science.1083646. [DOI] [PubMed] [Google Scholar]
- Proudfoot CJ, Garry EM, Cottrell DF, Rosie R, Anderson H, Robertson DC, et al. Analgesia mediated by the TRPM8 cold receptor in chronic neuropathic pain. Curr Biol. 2006;16:1591–1605. doi: 10.1016/j.cub.2006.07.061. [DOI] [PubMed] [Google Scholar]
- Reilly RM, McDonald HA, Puttfarcken PS, Joshi SK, Lewis L, Pai M, et al. Pharmacology of modality-specific transient receptor potential vanilloid-1 antagonists that do not alter body temperature. J. Pharmacol. Exp. Ther. 2012;342:416–428. doi: 10.1124/jpet.111.190314. [DOI] [PubMed] [Google Scholar]
- Remadevi R, Szallisi A. Adlea (ALGRX-4975), an injectable capsaicin (TRPV1 receptor agonist) formulation for longlasting pain relief. IDrugs Investig. Drugs J. 2008;11:120–132. [PubMed] [Google Scholar]
- Rohacs T, Lopes CM, Michailidis I, Logothetis DE. PI(4,5)P2 regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nat Neurosci. 2005;8:626–634. doi: 10.1038/nn1451. [DOI] [PubMed] [Google Scholar]
- Russell FA, Schuelert N, Veldhoen VE, Hollenberg MD, McDougall JJ. Activation of PAR(2) receptors sensitizes primary afferents and causes leukocyte rolling and adherence in the rat knee joint. Br. J. Pharmacol. 2012;167:1665–1678. doi: 10.1111/j.1476-5381.2012.02120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu S, Liu B, Yao J, Fu Q, Qin F. Uncoupling proton activation of vanilloid receptor TRPV1. J. Neurosci. Off. J. Soc. Neurosci. 2007;27:12797–12807. doi: 10.1523/JNEUROSCI.2324-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz ES, Christianson JA, Chen X, La J-H, Davis BM, Albers KM, et al. Synergistic role of TRPV1 and TRPA1 in pancreatic pain and inflammation. Gastroenterology. 2011;140:1283–1291. e1–e2. doi: 10.1053/j.gastro.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz ES, La J-H, Scheff NN, Davis BM, Albers KM, Gebhart GF. TRPV1 and TRPA1 antagonists prevent the transition of acute to chronic inflammation and pain in chronic pancreatitis. J. Neurosci. Off. J. Soc. Neurosci. 2013;33:5603–5611. doi: 10.1523/JNEUROSCI.1806-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senning EN, Collins MD, Stratiievska A, Ufret-Vincenty CA, Gordon SE. Regulation of TRPV1 ion channel by phosphoinositide (4,5)-bisphosphate: the role of membrane asymmetry. J. Biol. Chem. 2014;289:10999–11006. doi: 10.1074/jbc.M114.553180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapovalov G, Gkika D, Devilliers M, Kondratskyi A, Gordienko D, Busserolles J, et al. Opiates modulate thermosensation by internalizing cold receptor TRPM8. Cell Rep. 2013;4:504–515. doi: 10.1016/j.celrep.2013.07.002. [DOI] [PubMed] [Google Scholar]
- Smith GD, Gunthorpe MJ, Kelsell RE, Hayes PD, Reilly P, Facer P, et al. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature. 2002;418:186–190. doi: 10.1038/nature00894. [DOI] [PubMed] [Google Scholar]
- Staaf S, Franck MCM, Marmigère F, Mattsson JP, Ernfors P. Dynamic expression of the TRPM subgroup of ion channels in developing mouse sensory neurons. Gene Expr. Patterns. 2010;10:65–74. doi: 10.1016/j.gep.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Straub I, Krügel U, Mohr F, Teichert J, Rizun O, Konrad M, et al. Flavanones that selectively inhibit TRPM3 attenuate thermal nociception in vivo. Mol. Pharmacol. 2013;84:736–750. doi: 10.1124/mol.113.086843. [DOI] [PubMed] [Google Scholar]
- Streng T, Axelsson HE, Hedlund P, Andersson DA, Jordt S-E, Bevan S, et al. Distribution and function of the hydrogen sulfide-sensitive TRPA1 ion channel in rat urinary bladder. Eur. Urol. 2008;53:391–399. doi: 10.1016/j.eururo.2007.10.024. [DOI] [PubMed] [Google Scholar]
- Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol. 2000;2:695–702. doi: 10.1038/35036318. [DOI] [PubMed] [Google Scholar]
- Su L, Wang C, Yu YH, Ren YY, Xie KL, Wang GL. Role of TRPM8 in dorsal root ganglion in nerve injury-induced chronic pain. BMC Neurosci. 12:120. doi: 10.1186/1471-2202-12-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Mizuno A, Kodaira K, Imai M. Impaired pressure sensation with mice lacking TRPV4. J Biol Chem. 2003;13:13. doi: 10.1074/jbc.M302561200. [DOI] [PubMed] [Google Scholar]
- Szolcsányi J, Sándor Z. Multisteric TRPV1 nocisensor: a target for analgesics. Trends Pharmacol. Sci. 2012;33:646–655. doi: 10.1016/j.tips.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Takashima Y, Daniels RL, Knowlton W, Teng J, Liman ER, McKemy DD. Diversity in the neural circuitry of cold sensing revealed by genetic axonal labeling of transient receptor potential melastatin 8 neurons. J Neurosci. 2007;27:14147–14157. doi: 10.1523/JNEUROSCI.4578-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ta LE, Bieber AJ, Carlton SM, Loprinzi CL, Low PA, Windebank AJ. Transient Receptor Potential Vanilloid 1 is essential for cisplatin-induced heat hyperalgesia in mice. Mol. Pain. 2010;6:15. doi: 10.1186/1744-8069-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z, Kim A, Masuch T, Park K, Weng H, Wetzel C, et al. Pirt functions as an endogenous regulator of TRPM8. Nat. Commun. 2013;4:2179. doi: 10.1038/ncomms3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Clark TE, Undem BJ. Ozone activates airway nerves via the selective stimulation of TRPA1 ion channels. J. Physiol. 2010;588:423–433. doi: 10.1113/jphysiol.2009.183301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaka H, Taniguchi J, Satoh J, Mizuno A, Suzuki M. Warm temperature-sensitive transient receptor potential vanilloid 4 (TRPV4) plays an essential role in thermal hyperalgesia. J Biol Chem. 2004;279:35133–35138. doi: 10.1074/jbc.M406260200. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, et al. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Wada M, Masu M. Potentiation of capsaicin receptor activity by metabotropic ATP receptors as a possible mechanism for ATP-evoked pain and hyperalgesia. Proc. Natl. Acad. Sci. 2001;98:6951–6956. doi: 10.1073/pnas.111025298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ufret-Vincenty CA, Klein RM, Hua L, Angueyra J, Gordon SE. Localization of the PIP2 sensor of TRPV1 ion channels. J. Biol. Chem. 2011;286:9688–9698. doi: 10.1074/jbc.M110.192526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urano H, Ara T, Fujinami Y, Hiraoka BY. Aberrant TRPV1 expression in heat hyperalgesia associated with trigeminal neuropathic pain. Int. J. Med. Sci. 2012;9:690–697. doi: 10.7150/ijms.4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanden Abeele F, Zholos A, Bidaux G, Shuba Y, Thebault S, Beck B, et al. Ca2+-independent phospholipase A2-dependent gating of TRPM8by lysophospholipids. J Biol Chem. 2006;281:40174–40182. doi: 10.1074/jbc.M605779200. [DOI] [PubMed] [Google Scholar]
- Voets T, Droogmans G, Wissenbach U, Janssens A, Flockerzi V, Nilius B. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature. 2004;430:748–754. doi: 10.1038/nature02732. [DOI] [PubMed] [Google Scholar]
- Voets T, Owsianik G, Janssens A, Talavera K, Nilius B. TRPM8 voltage sensor mutants reveal a mechanism for integrating thermal and chemical stimuli. Nat. Chem. Biol. 2007;3:174–182. doi: 10.1038/nchembio862. [DOI] [PubMed] [Google Scholar]
- Voight EA, Gomtsyan AR, Daanen JF, Perner RJ, Schmidt RG, Bayburt EK, et al. Discovery of (R)-1-(7-chloro-2,2-bis(fluoromethyl)chroman-4-yl)-3-(3-methylisoquinolin-5-yl)urea (A-1165442): a temperature-neutral transient receptor potential vanilloid-1 (TRPV1) antagonist with analgesic efficacy. J. Med. Chem. 2014;57:7412–7424. doi: 10.1021/jm500916t. [DOI] [PubMed] [Google Scholar]
- Vriens J, Owsianik G, Hofmann T, Philipp SE, Stab J, Chen X, et al. TRPM3 Is a Nociceptor Channel Involved in the Detection of Noxious Heat. Neuron. 2011;70:482–494. doi: 10.1016/j.neuron.2011.02.051. [DOI] [PubMed] [Google Scholar]
- Vriens J, Watanabe H, Janssens A, Droogmans G, Voets T, Nilius B. Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc Natl Acad Sci U A. 2004;101:396–401. doi: 10.1073/pnas.0303329101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner TFJ, Loch S, Lambert S, Straub I, Mannebach S, Mathar I, et al. Transient receptor potential M3 channels are ionotropic steroid receptors in pancreatic β cells. Nat. Cell Biol. 2008;10:1421–1430. doi: 10.1038/ncb1801. [DOI] [PubMed] [Google Scholar]
- Wang YY, Chang RB, Allgood SD, Silver WL, Liman ER. A TRPA1-dependent mechanism for the pungent sensation of weak acids. J. Gen. Physiol. 2011;137:493–505. doi: 10.1085/jgp.201110615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wanrooij SJM, Wouters MM, Van Oudenhove L, Vanbrabant W, Mondelaers S, Kollmann P, et al. Sensitivity testing in irritable bowel syndrome with rectal capsaicin stimulations: role of TRPV1 upregulation and sensitization in visceral hypersensitivity? Am. J. Gastroenterol. 2014;109:99–109. doi: 10.1038/ajg.2013.371. [DOI] [PubMed] [Google Scholar]
- Watabiki T, Kiso T, Kuramochi T, Yonezawa K, Tsuji N, Kohara A, et al. Amelioration of neuropathic pain by novel transient receptor potential vanilloid 1 antagonist AS1928370 in rats without hyperthermic effect. J. Pharmacol. Exp. Ther. 2011;336:743–750. doi: 10.1124/jpet.110.175570. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Vriens J, Suh SH, Benham CD, Droogmans G, Nilius B. Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J Biol Chem. 2002;277:47044–47051. doi: 10.1074/jbc.M208277200. [DOI] [PubMed] [Google Scholar]
- Wei H, Hämäläinen MM, Saarnilehto M, Koivisto A, Pertovaara A. Attenuation of mechanical hypersensitivity by an antagonist of the TRPA1 ion channel in diabetic animals. Anesthesiology. 2009;111:147–154. doi: 10.1097/ALN.0b013e3181a1642b. [DOI] [PubMed] [Google Scholar]
- Winchester WJ, Gore K, Glatt S, Petit W, Gardiner JC, Conlon K, et al. Inhibition of TRPM8 channels reduces pain in the cold pressor test in humans. J. Pharmacol. Exp. Ther. 2014;351:259–269. doi: 10.1124/jpet.114.216010. [DOI] [PubMed] [Google Scholar]
- Xing H, Chen M, Ling J, Tan W, Gu JG. TRPM8 mechanism of cold allodynia after chronic nerve injury. J Neurosci. 2007;27:13680–13690. doi: 10.1523/JNEUROSCI.2203-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G-Y, Winston JH, Shenoy M, Yin H, Pendyala S, Pasricha PJ. Transient receptor potential vanilloid 1 mediates hyperalgesia and is up-regulated in rats with chronic pancreatitis. Gastroenterology. 2007;133:1282–1292. doi: 10.1053/j.gastro.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Xu H, Ramsey IS, Kotecha SA, Moran MM, Chong JA, Lawson D, et al. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature. 2002;418:181–186. doi: 10.1038/nature00882. [DOI] [PubMed] [Google Scholar]
- Yang F, Cui Y, Wang K, Zheng J. Thermosensitive TRP channel pore turret is part of the temperature activation pathway. Proc. Natl. Acad. Sci. U. S. A. 2010;107:7083–7088. doi: 10.1073/pnas.1000357107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Liu B, Qin F. Modular thermal sensors in temperature-gated transient receptor potential (TRP) channels. Proc. Natl. Acad. Sci. U. S. A. 2011;108:11109–11114. doi: 10.1073/pnas.1105196108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Qin F. Interaction with Phosphoinositides Confers Adaptation onto the TRPV1 Pain Receptor. PLoS Biol. 2009;7:e1000046. doi: 10.1371/journal.pbio.1000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharian E, Cao C, Rohacs T. Gating of transient receptor potential melastatin 8 (TRPM8) channels activated by cold and chemical agonists in planar lipid bilayers. J. Neurosci. Off. J. Soc. Neurosci. 2010;30:12526–12534. doi: 10.1523/JNEUROSCI.3189-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Mak S, Li L, Parra A, Denlinger B, Belmonte C, et al. Direct inhibition of the cold-activated TRPM8 ion channel by Gαq. Nat. Cell Biol. [Internet] 2012 doi: 10.1038/ncb2529. Available from: http://www.nature.com/ncb/journal/vaop/ncurrent/full/ncb2529.html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Isami K, Nakamura S, Shirakawa H, Nakagawa T, Kaneko S. Acute cold hypersensitivity characteristically induced by oxaliplatin is caused by the enhanced responsiveness of TRPA1 in mice. Mol. Pain. 2012;8:55. doi: 10.1186/1744-8069-8-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P, Lieu T, Barlow N, Metcalf M, Veldhuis NA, Jensen DD, et al. Cathepsin S causes inflammatory pain via biased agonism of PAR2 and TRPV4. J. Biol. Chem. 2014;289:27215–27234. doi: 10.1074/jbc.M114.599712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurborg S, Yurgionas B, Jira JA, Caspani O, Heppenstall PA. Direct activation of the ion channel TRPA1 by Ca(2+) Nat Neurosci [Internet] 2007 doi: 10.1038/nn1843. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17259981. [DOI] [PubMed] [Google Scholar]
- Zygmunt PM, Högestätt ED. TRPA1. Handb. Exp. Pharmacol. 2014;222:583–630. doi: 10.1007/978-3-642-54215-2_23. [DOI] [PubMed] [Google Scholar]