Abstract
Objectives
To evaluate the effects of topical hemocoagulase on intra-oral extraction sockets and impact on the healing process as well as to produce a clinico-histological healing score.
Study Design
This prospective study compared two extraction sites in each subject. One site received topical hemocoagulase while other site did not receive it. Both the sites were chosen in the same patient and extraction was done at separate intervals. The biopsy was done on a random basis with the pathologist being blinded to the study. Clinical evaluation was done on days 7, 14 and 21. Biopsy was done either on days 7 or 14 for the case as well as for the control. A clinical as well as histological score was developed and the healing was assessed. Statistical analysis was done using Wilcoxon signed rank test and p value ≤ 0.05 was considered significant and z-score was also calculated.
Results
The clinical score did not show any statistical significance. The histological total score on day 14 and combined overall analysis of days 7 and 14 showed statistical significance. There was an increased incidence (n = 4) of osteoid formation in the hemocoagulase group on day 14.
Conclusion
The application of hemocoagulase may improve and accelerates the process of wound healing in extraction sockets.
Keywords: Topical Haemocoagulase, Extraction socket healing, Healing score, Histological healing, Clinical healing
Introduction
Impaired wound healing is a common clinical problem that occurs due to disordered collagen formation and underlying predisposing conditions [1, 2]. Drugs that arrest bleeding, promote epithelization and provide relief from pain have been widely used to accelerate healing. It is a well known fact that snake venom, one of the most concentrated enzyme sources, is a valuable expedient of the healing process. Botroclot (Jaggat pharmaceuticals)—a topical preparation that is prepared from snake venom contains extracted hemocoagulase. It is used for its healing as well as procoagulant properties. It has been introduced to arrest bleeding at the site of injury.
Botroclot, a nontoxic systemic hemocoagulant fraction of venom is obtained from the Brazilian snake Bothrops-jararaca or atrox. Preparations are available all over the world by different names e.g.: Botroxobin [3]—a WHO approved product, Botropase—a systemic procoagulant. The preparation of Botroclot topical solution in each ml contains: (a) Acqueous solution of hemocoagulase isolated from Bothrops atrox or Bothrops jararaca 0.2 Cu/ml. (b) Chlorhexidine 0.1 % v/v (as a preservative) and (c) Water for injection IPq.s
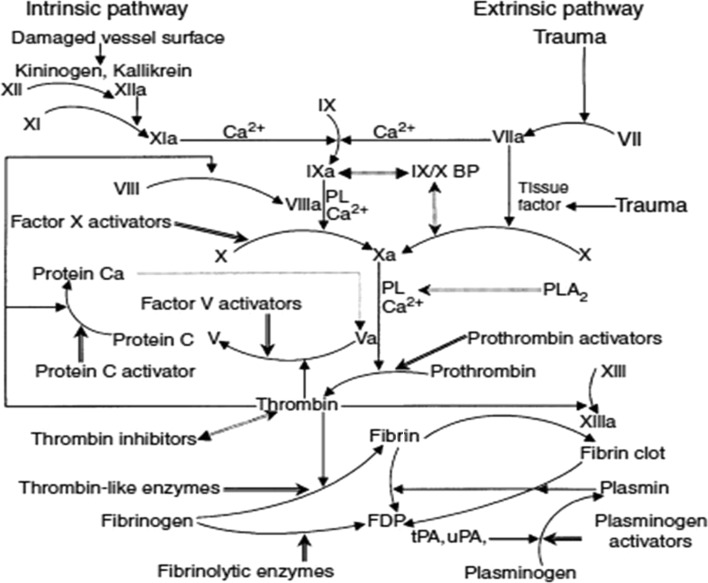
Hemocoagulase has multifaceted procoagulant [4] actions. It accelerates the formation of fibrin monomers and hastens fibrin clot formation. It activates factor Xa and helps in the formation of thrombin at the site of hemorrhage. It is also found to stabilize the fibrin by an action on the Factor XIIIa (Fig. 5). A few reports have suggested that this parenteral preparation acts like a prohealer, enhances epithelization, increases wound tensile strength and the turnover of collagen in the healing of wound [5–7]. There is no data on the efficacy of hemocoagulase topical solution in arresting bleeding from extraction sockets and its effects on tissue repair intra-orally. Hence, this study was undertaken to evaluate the effects of hemocoagulase on intra-oral extraction sockets and healing process of a post-extraction wound. The other objective of this study was to develop a healing score for an extraction socket.
Fig. 5.
Coagulation pathway. Physiological blood coagulation and fibrinolytic pathways and the sites of action of venom proteins. Venom proteins are shaded. Straight arrow, activation; dashed straight arrow, inhibition; curved arrow, converting; double-headed arrow, binding (Adapted from: Lu et al. [4])
Materials and Methods
The study was conducted in the department of Oral and Maxillofacial Surgery, Manipal College of Dental Sciences, Mangalore during a 2 year period from January 2004 to December 2005. The study protocol was approved by institutional ethics committee. Patients were included during the study period if they satisfied the inclusion criteria. The study protocol was explained and informed written consent was obtained from all patients who were involved in the study. Patients who could not adhere to the study protocol of follow-up were excluded from the study. Inclusion criteria were: patients aged between 18 and 70 years undergoing multiple tooth extractions (because of grossly decayed or nonrestorable teeth, or severe periodontitis). This ensured that same patient could have both the hemocoagulase and control site. All the sites were chosen such that they were not located in close proximity and extractions were done at different time intervals. Extractions of third molars were not included. This ensured that hemocoagulase did not spread to the control site. The site where hemocoagulase was applied was taken as “test” site where as other site was chosen as the “control” site in the same patient.
Exclusion criteria included patients with known pre-morbid conditions like thromboembolic disorders, hypertension, haemophilia, diabetes mellitus, anticoagulant therapy, pregnant and lactating patients, hypersensitivity to hemocoagulase topical solution and other constituents of the formulation, HIV-positive patients and patients with mental illness. Clinical information with respect to demographic details, reason for extraction was noted.
Once the patients were included in the study, coagulation profile, bleeding time and clotting time were evaluated. Extraction was done under local anaesthesia (2 % lignocaine with 1:2,00,000 adrenaline) and time taken for arrest of bleeding was monitored. At the first site after extraction, once bleeding stopped, 2–3 ml of hemocoagulase solution was topically applied in a gauze piece and the patients were advised to keep the gauze in place for 30–45 min. Patients were taught the manner in which, hemocoagulase should be topically applied twice daily for a total of 7 days. Patients were reminded telephonically to apply hemocoagulase topical solution and were called for follow-up after 7 days.
On day of the follow-up, extraction procedure was carried out at another site which required extraction, as identified on day one. This site was chosen as the control site. Hemocoagulase was not applied to this site. The patients were then followed up again on day 14 and day 21 for each site. Once the extraction was done, clinical healing of the site was noted on every visit.
The biopsy was done by simple randomization either on day 7 or on day 14, on the same day for both control and test sites. The biopsy was sent to the oral pathologist who was blinded to the study and did not know from which site the sample of biopsy was send as well as on which day the biopsy was done. The biopsy was sent to the pathologist in 10 % formaldehyde solution. Histological evaluation was done using hematoxylin and eosin (H&E) staining and Masson’s trichrome staining.
The reports were collected, compiled and analyzed at the end of the study. Clinical healing was based on the investigators clinical observation. This was based on amount of inflammation of the soft tissue, amount of epithelium proliferation surrounding the socket and amount of granulation tissue filling the socket. It was classified into (a) Excellent healing where there was minimal or no inflammation of the soft tissue, good amount proliferating epithelium and socket was completely filled with granulation tissue. (b) Good healing where there was mild to no inflammation of soft tissue, moderate amount of proliferating epithelium and socket was completely filled with granulation tissue. (c) Moderate healing where there was moderate inflammation of the soft tissue, mild amount of proliferating epithelium and when the socket was half to two-third filled with granulation tissue. (d) Poor healing was defined as a socket with moderate to severe inflammation of surrounding soft tissue, exposed bone surface and insufficient amount of granulation tissue. A dry socket was also considered as poor healing.
For assessing the quality of healing, histological evaluation of wound tissue was done and following features were observed: (a) Epithelial keratinization: epithelial proliferation, hyperplastic or fully keratinized. (b) Quantity of collagen deposition: nil/mild/moderate/dense. (c) Arrangement of fibers in collagen: haphazard/organized. (d) Fibroblast type: Open phase, closed phase or mixed variety consisting of equal phase fibroblasts. (e) Inflammatory cells: predominance of acute, chronic or mixed type of cells. (f) Fibrosis: nil/mild/moderate or dense. (g) Fibrin mesh work: nil/mild/moderate or extensive. (h) Any other features like osteoid or foci of new bone formation.
Every feature in the histological evaluation was awarded the following scores to grade the quality of post-extraction wound healing:
Epithelium: Epithelial proliferation-1, hyperplastic epithelium-2, keratinised epithelium-3
Collagen quantity: Nil-0, minimal or mild-1, moderate-2, dense-3.
Arrangement of fibers: loose or haphazard arrangement-2, bundle organization-4.
Fibroblast activity: open phase-1, mixed (both open and closed phase)-2, closed phase-3.
Inflammation: acute-1, mixed-2, chronic-3.
Degree of fibrosis: Nil-0, mild-1, moderate-2, dense-3.
Presence of fibrin mesh: extensive-0, moderate-1, minimal-2, nil-3.
Presence of osteoid or foci of new bone formation-2, no bone formation-0
The histological score of healing was finally categorized based on the total score:
Up to 11—poor healing
12–15—Moderate healing
16–18—Good healing
≥19—Very good healing
The clinical healing details were recorded on days 7, 14 and day 21 and appropriate scoring was done. Histological assessment and scoring were done on only days 7 and 14. On day 21, histological biopsy was not done and so scoring was not done.
Statistical Analysis
The clinical as well as histological scoring system was expressed as numerals along with percentages in both the control and test site. Wilcoxon signed rank test was used as test of significance and p value <0.05 was considered significant. The z-score was also calculated.
Results
Fifty-six patients qualified for the study based on the inclusion criteria. Of these only 38 patients could adhere to the protocol and so were taken as the study population. The male to female ratio was 7:31. The mean age of the patients was 47.34 ± 12.29 years. When the mean age and sex in the study population were compared there was statistical significance (p < 0.001) (Table 1).
Table 1.
Distribution of study subjects based on mean age and gender (n = 38)
| Sex | N | Mean age ± Std deviation (in years) | Significance when compared between gender |
|---|---|---|---|
| Male | 7 | 60.57 ± 6.803 | <0.001 |
| Female | 31 | 44.35 ± 11.268 | |
| Total | 38 | 47.34 ± 12.290 |
p value ≤ 0.05 is significant
The clinical scoring of healing was done on day 7, day 14 and day 21. The clinical scoring did not show any statistical significance. The histological scoring was done on day 7, day 14 and a combined overall analysis was done. This is depicted in Table 2. On day 7, biopsy was done only in 16 patients in the control group compared to 18 patients in the hemocoagulase group. It was because in one patient the healing was very poor and there was dry socket whereas in the second patient, healing was good clinically but biopsy sample was insufficient for histological evaluation.
Table 2.
Comparison of clinical healing score and histological healing score compared between the Botroclot and control groups
| Poor | Moderate | Good | Excellent | Test of significance* | ||
|---|---|---|---|---|---|---|
| z-score | p value | |||||
| Clinical scoring on day 7, day 14 and day 21 | ||||||
| Day 7 | ||||||
| Control (n = 38) | 1 (2.6 %) | 16 (42.1 %) | 21 (55.3 %) | – | −0.209 | 0.835 |
| Botroclot (n = 38) | 1 (2.6 %) | 17 (44.7 %) | 20 (52.6 %) | – | ||
| Day 14 | ||||||
| Control (n = 38) | 1 (2. %) | 13 (34.2 %) | 23 (60.5 %) | 1 (2.6 %) | −1.706 | 0.088 |
| Botroclot (n = 38) | – | 8 (21.1 %) | 28 (73.7 %) | 2 (5.3 %) | ||
| Day 21 | ||||||
| Control (n = 38) | 1(2.6 %) | 5 (13.2 %) | 31 (81.6 %) | 1 (2.6 %) | −1.459 | 0.145 |
| Botroclot (n = 38) | – | 3 (7.9 %) | 32 (84.2 %) | 3 (7.9 %) | ||
| Histological scoring on day 7, day 14 and a combined analysis | ||||||
| Day 7 | ||||||
| Control (n = 16) | 7 (43.8 %) | 8 (50 %) | 1 (6.3 %) | 1.565 | 0.118 | |
| Botroclot (n = 18) | 8 (44.4 %) | 3 (16.7 %) | 6 (33.3 %) | 1 (5.6 %) | ||
| Day 14 | ||||||
| Control (n = 20) | – | 10 (50 %) | 8 (40 %) | 2 (10 %) | 2.511 | 0.012 |
| Botroclot (n = 19) | – | 2 (10.5 %) | 6 (31.6 %) | 11 (57.9 %) | ||
| Combined overall analysis | ||||||
| Control (n = 36) | 7 (19.4 %) | 18 (50 %) | 9 (25 %) | 2 (5.5 %) | 2.898 | 0.004* |
| Botroclot (n = 37) | 8 (21.6 %) | 5 (13.5 %) | 12 (32.4 %) | 12 (32.4 %) | ||
* Wilcoxon signed rank test (p ≤ 0.05 = significant)
For day 14 postoperative evaluation, the hemocoagulase group had 19 patients where as the control site had 20 patients. This was because in one biopsy sample from hemocoagulase site the tissue was insufficient for histological evaluation. The histological total scoring on day 7 did not show statistical significance, but day 14 and overall combined analysis was statistically significant p = 0.012 and p = 0.004 respectively.
The comparison of both the groups with respect to components of the histological scoring was done on day 7, day 14 and a combined overall scoring was done. This is depicted in Tables 3, 4 and 5.
Table 3.
Histological comparison between the control and the Botroclot site on day 7
| Histological parameters | Control score (n = 16) | Botroclot score (n = 18) | z-value | p value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 0 | 1 | 2 | 3 | 4 | |||
| Epithelium | – | 6 (37.5 %) | 10 (62.5 %) | – | – | – | 2 (11.1 %) | 14 (77.8 %) | 2 (11.1 %) | – | −1.897 | 0.058 |
| Collagen quantity | 1 (6.3 %) | 13 (81.2 %) | 2 (12.5 %) | – | – | 1 (5.6 %) | 10 (55.6 %) | 7 (38.9 %) | – | – | −0.791 | 0.429 |
| Collagen organization | 1 (6.3 %) | – | 13 (81.2 %) | – | 2 (12.5 %) | 1 (5.6 %) | – | 10 (55.6 %) | – | 7 (38.9 %) | −1.897 | 0.058 |
| Fibroblast activity | – | 10 (62.5 %) | 6 (37.5 %) | – | – | – | 15 (83.3 %) | 2 (11.1 %) | 1 (5.6 %) | – | −1.732 | 0.083 |
| Inflammation | – | 6 (37.5 %) | 9 (56.2 %) | 1 (6.3 %) | – | – | 6 (33.3 %) | 8 (44.4 %) | 4 (22.2 %) | – | −0.707 | 0.480 |
| Inflammatory cells | – | 1 (6.3 %) | 13 (81.2 %) | 2 (12.5 %) | – | – | – | 17 (94.4 %) | 1 (5.6 %) | – | 0.000 | 1.000 |
| Degree of fibrosis | – | 6 (37.5 %) | 8 (50 %) | 2 (12.5 %) | – | – | 3 (16.7 %) | 9 (50 %) | 6 (33.3 %) | – | −1.115 | 0.265 |
| Presence of fibrin mesh | 7 (43.7 %) | 4 (25 %) | 3 (18.8 %) | 2 (12.5 %) | – | 5 (27.8 %) | 6 (33.3 %) | 7 (38.9 %) | – | – | −0.321 | 0.748 |
p value ≤ 0.05 is significant
Table 4.
Histological comparison between the control and the Botroclot site on day 14
| Histological parameters | Control (n = 20) score | Botroclot (n = 19) score | z-value | p value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 0 | 1 | 2 | 3 | 4 | |||
| Epithelium type | – | 1 (5 %) | 18 (90 %) | 1 (5 %) | – | – | – | 10 (52.6 %) | 9 (47.4 %) | – | −2.496 | 0.013 |
| Collagen quantity | – | 9 (45 %) | 11 (55 %) | – | – | – | 1 (5.3 %) | 14 (73.7 %) | 4 (21.1 %) | – | −3.051 | 0.002 |
| Collagen organization | – | – | 12 (60 %) | – | 8 (40 %) | – | – | 5 (26.3 %) | – | 14 (73.7 %) | −2.449 | 0.014 |
| Fibroblast activity | – | 15 (75 %) | 5 (25 %) | – | – | – | 7 (36.8 %) | 10 (52.6 %) | 2 (10.5 %) | – | −2.179 | 0.029 |
| Inflammation | – | 3 (15 %) | 16 (80 %) | 1 (5 %) | – | – | 1 (5.3 %) | 12 (63.2 %) | 6 (31.6 %) | – | −2.449 | 0.014 |
| Inflammatory cells | – | – | 4 (20 %) | 16 (80 %) | – | – | – | 9 (47.4 %) | 10 (52.6 %) | – | −1.732 | 0.083 |
| Degree of fibrosis | – | 9 (45 %) | 11 (55 %) | – | – | – | 2 (10.5 %) | 16 (84.2 %) | 1 (5.3 %) | – | −2.121 | 0.034 |
| Presence of fibrin mesh | 2 (10 %) | 5 (25 %) | 10 (50 %) | 3 (15 %) | – | – | 1 (5.3 %) | 12 (63.2 %) | 6 (31.6 %) | – | −2.066 | 0.039 |
p value ≤ 0.05 is significant
Table 5.
Histological comparison between the control and the botroclot site combined on day 7 and day 14
| Histological parameters | Control (n = 36) score | Botroclot (n = 37) score | z-value | p value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 0 | 1 | 2 | 3 | 4 | |||
| Epithelium | – | 7 (19.4 %) | 28 (77.8 %) | 1 (2.8) | – | – | 2 (5.4 %) | 24 (64.9 %) | 11 (29.7 %) | – | −3.120 | 0.002 |
| Collagen quantity | 1 (2.8 %) | 22 (61.1 %) | 13 (36.1 %) | – | – | 1 (2.7 %) | 11 (29.7 %) | 21 (56.8 %) | 4 (10.8 %) | – | −2.688 | 0.007 |
| Collagen organization | 1 (2.8 %) | – | 25 (69.4 %) | – | 10 (27.8 %) | 1 (2.7 %) | – | 15 (40.5 %) | – | 21 (56.8 %) | −3.000 | 0.003 |
| Fibroblast activity | – | 25 (69.4 %) | 11 (30.6 %) | – | – | – | 22 (59.5 %) | 12 (32.4 %) | 3 (8.1 %) | – | −1.355 | 0.175 |
| Inflammation | – | 9 (25 %) | 25 (69.4 %) | 2 (5.6 %) | – | – | 7 (18.9 %) | 20 (54.1 %) | 10 (27 %) | – | −2.138 | 0.33 |
| Inflammatory cells | – | 1 (2.8 %) | 17 (47.2 %) | 18 (50 %) | – | – | – | 26 (70.3 %) | 11 (29.7 %) | – | −1.604 | 0.109 |
| Degree of fibrosis | – | 15 (41.6 %) | 19 (52.8 %) | 2 (5.6 %) | – | – | 5 (13.5 %) | 25 (67.6 %) | 7 (18.9 %) | – | −2.082 | 0.037 |
| Presence of fibrin mesh | 9 (25 %) | 9 (25 %) | 13 (36.1 %) | 5 (13.9 %) | – | 5 (13.5 %) | 7 (18.9 %) | 19 (51.4 %) | 6 (16.2 %) | – | −1.576 | 0.115 |
| Presence of osteoid bone formation | – | – | – | – | – | – | – | 4 (10.81 %) | – | – | – | – |
p value ≤ 0.05 is significant
When the histological scoring was compared in the both the groups on day 7 there was no statistical significance (Table 3). Similarly, when both groups were compared for histological components on day 14 there was statistical significance in the epithelium (p = 0.0013), collagen quantity (p = 0.002), collagen organization (p = 0.014), fibroblast activity (p = 0.029), inflammation (p = 0.014), degree of fibrosis (p = 0.034) and fibrin mesh (p = 0.039) (Table 4).
There were four biopsies in the hemocoagulase group which showed presence of osteoid formation and none in the control group. When both the groups were compared after combining the day 7 and day 14 findings there was statistical significance noted in epithelium (p = 0.002), collagen quantity (p = 0.007) and collagen organization (p = 0.003) and degree of fibrosis (p = 0.037) (Table 5). The histological slide depicting poor, moderate, good and excellent healing is shown in Figs. 1, 2, 3 and 4 respectively.
Fig. 1.
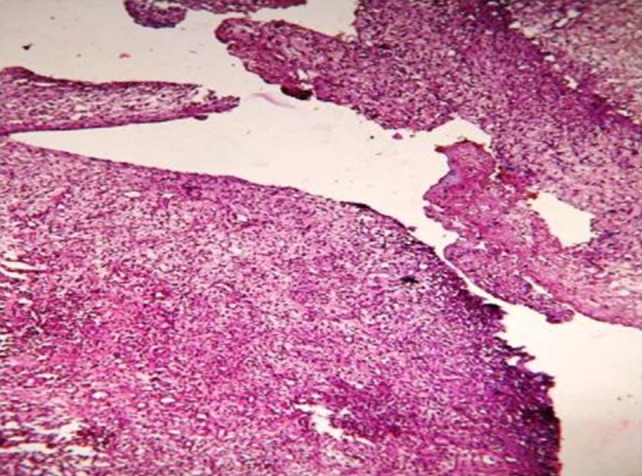
Slide with poor healing of the extraction socket
Fig. 2.

Slide with moderate healing of the extraction socket
Fig. 3.

Slide with biopsy of the socket showing good healing
Fig. 4.
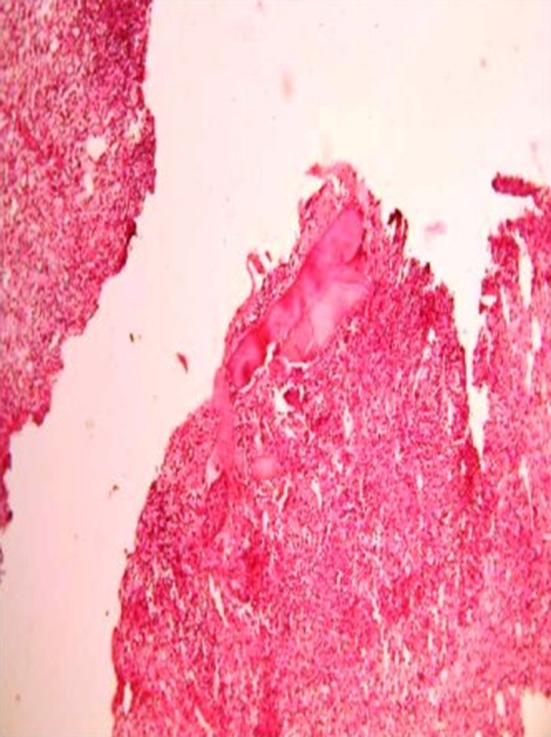
Slide with biopsy of the socket showing excellent healing
Discussion
The most commonly performed procedure in oral surgery is tooth extraction; healing of which is a multifactorial process and is determined by systemic and local factors that influence the outcome. Only a few studies [8–11] have documented the histology of extraction-socket healing in humans.
The work done by Amler et al. [9] have shown the step-wise process of extraction socket healing. Additionally, Boyne [10] studied extraction-socket alveolar bone biopsies and, more recently, Devlin and Sloan [11] studied extraction-socket biopsies harvested during resective cancer surgery. Normal healing of the extraction site is dependent on blood clot formation, the progression of that clot to an organized matrix, and the formation of bone. Inability to heal will lead to development of dry socket.
The process of extraction socket healing can be accelerated using various products. These include platelet rich plasma, phenytoin, snake venom and various artificially created biological products. Of these, snake venom contains a variety of proteolytic enzymes affecting the coagulation process. They are denominated as snake venom thrombin like enzymes (SVTLE) [12]. These SVTLE are procoagulants and convert fibrinogen to fibrin. They have been extensively studied by both basic researchers and clinicians because of their potential therapeutic uses in myocardial infarction, ischemic stroke and thrombotic diseases. Snake venom proteins not only influence coagulation but also help in platelet plug formation. These proteins are potentially useful as reagents for clinical and basic research of thrombosis and hemostasis.
The wounds of the oral cavity heal rapidly. With obvious exception, infection of the oral wound is rare. Rich vascularity and salivary factors are believed to be involved in the faster rate of oral tissue repair. Occasionally, the management of the oral wound requires medication. The implications of a procoagulant, antiseptic, anti-inflammatory and anti-infective drug application on the healing wound are quite substantial, drawing attention from clinicians. However, the medication used should not affect the reparative process adversely. Snake venom has all the above properties and so it was considered for the study. This is the first study that demonstrates the use of intra-oral topical hemocoagulase—a processed snake venom preparation (Botroclot–Jaggat Pharmaceuticals) to accelerate the process of healing in extracted sockets (Fig. 5). In this study we have analyzed the process of healing in these extraction sockets and also studied the effect of processed snake venom on the same.
We have clinically as well as histologically evaluated the process of healing, both on the control and topical hemocoagulase applied sockets by doing random biopsy on day 7 and day 14. This has allowed us to develop a clinical as well as histological score for the healing process. The clinical score was based on the investigators clinical observation. This did not show any statistical significance on all the 3 days. Histological score was based on various factors and this was done on day 7 and day 14 as well as a combined score of days 7 and 14 was computed. This showed statistical significance. Biopsies were not done on day 21 since most of the wounds heal within 21 days and logistically it was not possible to call patients for a second biopsy.
In our study, patients with multiple extraction sites were chosen as candidates for the study. The control and test sites were chosen at different times in same patient, thereby preventing spread of hemocoagulase from one side to other. This also ensured that baseline characteristics of the patient with respect to healing did not change when both the sites were compared. Furthermore, our pathologist was blinded to specimen and so the results were not biased. Practically, it is not necessary to biopsy every socket that gets extracted since invariably, most of the extraction sockets heal. The biopsies in our study helped us to understand the process of healing and how the use of hemocoagulase helped in accelerating the process of healing.
The study has also led us to develop a unique score for healing of the sockets both clinically and histologically. Clinically, it is not possible to determine how exactly the healing process is occurring based on inspection of the extraction site. Random biopsies were done on day 7 and day 14 to determine the process of healing. Each patient was subjected for the biopsy for test and control site either on day 7 or day 14. The presence of osteoid formation in the histological study was considered as one of the important pointer to the healing process. This was seen in 4 patients, all in the hemocoagulase group on day 14. Similarly, parameters that showed statistical significance include epithelium, collagen quantity and organization, degree of fibrosis. We are aware that this is a small sample and it would be difficult to extra polate these results as applicable on a larger basis. Nevertheless, it can be suggested that topical use of hemocoagulase for extraction wounds is advantageous in two ways: that it arrests bleeding, and secondly, improves the structural integrity of the newly laid scar tissue. Further research is necessary to elucidate the exact mechanism of action of hemocoagulase on wound healing.
Conclusion
Our study has documented that the application of hemocoagulase may improve and accelerate the process of wound healing in extraction sockets. In the process, we have not only compared this with healing in sockets where nothing was applied but have also shown that the healing is definitely accelerated. In addition, we have taken into consideration the various histological parameters and developed a healing score which can be used to assess the process of healing in extraction sockets.
Acknowledgements
The authors wish to thank Dr Sai Kumar, Consultant Public Health, WHO, Geneva, Dr Rajeev, Department of Community Medicine, Kasturba Medical College, Mangalore and Dr Rekha Prabhu, Department of Community Dentistry, Yenopaya Dental College, Mangalore for their help during the study.
References
- 1.Kumar V, Abbas AK, Fausto N, Aster JC (2010) Tissue renewal, regeneration and repair In: Kumar V, Abbas AK, Fausto N, Aster JC (Eds) Robins and Cotran pathological basis of disease, 8th edn. pennsylvania: Saunders, pp 79–110
- 2.Sultana J, Molla MR, Kamal M, et al. Histological differences in wound healing in maxillofacial region in patients with or without risk factors. Bangladesh J Pathol. 2009;24(1):3–8. [Google Scholar]
- 3.Stocker K, Barlow GH. The coagulant enzyme from Bothrops atrox venom (Batroxobin) Methods Enzymol. 1976;45:214–223. doi: 10.1016/S0076-6879(76)45021-8. [DOI] [PubMed] [Google Scholar]
- 4.Lu Q, Clemetson JM, Clemetson KJ. Snake venoms and hemostasis. J Thromb Haemost. 2005;3:1791–1799. doi: 10.1111/j.1538-7836.2005.01358.x. [DOI] [PubMed] [Google Scholar]
- 5.Ramesh KV, Rao CM, Bairy KL, et al. Effects of procoagulants on wound healing. Indian J Exp Biol. 1990;28:43–45. [PubMed] [Google Scholar]
- 6.Udupa SL, Shaila HP, Udupa AL, et al. Effects of botropase on wound healing. Biochem Arch. 1991;7:207–212. [Google Scholar]
- 7.Ramesh KV, Kulkarni DR, Bairy KL, et al. Effects of botropase on wound healing. Indian J Surg. 1990;5:218–221. [Google Scholar]
- 8.Steiner GG, Francis W, Burrel R, et al. The healing socket and socket regeneration. Compend Contin Educ Dent. 2008;29(2):114–124. [PubMed] [Google Scholar]
- 9.Amler MH, Johnson PL, Salman I. Histological and histochemical investigation of human alveolar socket healing in undisturbed extraction wounds. J Am Dent Assoc. 1960;61(7):32–44. doi: 10.14219/jada.archive.1960.0152. [DOI] [PubMed] [Google Scholar]
- 10.Boyne PJ. Osseous repair of the postextraction alveolus in man. Oral Surg Oral Med Oral Pathol. 1966;21(6):805–813. doi: 10.1016/0030-4220(66)90104-6. [DOI] [PubMed] [Google Scholar]
- 11.Devlin H, Sloan P. Early bone healing events in the human extraction socket. Int J Oral Maxillofac Surg. 2002;31(6):641–645. doi: 10.1054/ijom.2002.0292. [DOI] [PubMed] [Google Scholar]
- 12.Castro HC, Zingali RB, Albuquerque MG, et al. Snake venom thrombin-like enzymes: from reptilase to now. Cell Mol Life Sci. 2004;61(7–8):843–856. doi: 10.1007/s00018-003-3325-z. [DOI] [PMC free article] [PubMed] [Google Scholar]