Abstract
Introduction
The “Motor Trephine Syndrome (MTS)” also known as the “Sunken brain and Scalp Flap Syndrome” or the “Sinking Skin Flap Syndrome (SSFS)” or the “Syndrome of the trephined” is an unusual syndrome in which neurological deterioration occurs following removal of a large skull bone flap. This syndrome is associated with sensorimotor deficit and neurological deterioration following decompressive craniectomy which is performed for various neurosurgical conditions involving cerebral swelling causing mass effect. The neurological deterioration can be exacerbated or precipitated by CSF diversion procedures like a Ventriculo-Peritoneal shunt.
Objective
It was the objective of this study to observe if any improvement in the patient’s condition, or if any beneficial effects in his sensorimotor deficit could be gained by performing an early cranioplasty as against after the usual delay of one to two years normally allowed for post-craniectomy cases.
Methods
A 52 year old male suffered severe head injury in a road traffic accident and underwent a craniectomy and contusectomy of the left Fronto-Temporo-Parietal (FTP) region for treatment of Acute Subdural hematoma (SDH) as well as hemorrhagic and non-hemorrhagic contusions of the brain with severe mass effect. On recovery from this acute event he was bed bound, on tracheostomy, his GCS was E4VTrM4 with residual right sided hemiparesis. Three months later, he developed Hydrocephalus for which a Right Ventriculo-Peritoneal (V-P) shunt was performed. Following this procedure, severe depression of the skin/scalp flap occurred and the neurological recovery was not as expected. He was diagnosed as a case of “Syndrome of the trephined”. An immediate Cranioplasty was performed, on the third month following the craniectomy procedure, in an attempt to resolve the rapidly deteriorating neurological status of the patient.
Results
In the case presented, following the early Cranioplasty which was performed within three months of the initial craniectomy, the patient’s neurological condition and cognitive functions showed a remarkable, immediate and dramatic improvement. The early Cranioplastic repair led to a remarkable clinical recovery of the patient, with improvement in the cognitive behavior and motor deficit with a rapid reversal of the sensorimotor paresis, reflecting an improvement in brain perfusion.
Conclusion
Patients with the classical “Motor trephine syndrome/ Sinking skin flap syndrome” following large craniectomy defects, may hugely benefit from an early cranioplasty procedure, with a reversal of features of this syndrome and early recovery of their neurological and cognitive functions. Hence, an early cranioplasty can serve as a therapeutic procedure, rather than merely a cosmetic one.
Keywords: Sinking skin flap syndrome (SSFS), Motor trephine syndrome (MTS), Syndrome of the trephined, Fronto-temporo-parietal (FTP) bone flap, Hydrocephalus, Ventriculo-peritoneal (V-P) shunt, Cerebral blood flow (CBF)
Introduction
Decompressive craniectomy has an extremely important role in neurocritical care for the treatment of intractable intracranial hypertension in patients with traumatic brain injury (TBI). The conversion of the cranium from a “closed box” to an “open box” alters the barometric pressure, cerebrospinal fluid (CSF) flow and the cerebral blood flow (CBF). However, this alteration carries benefits as well as risks [1]. Numerous complications may arise as a result of the pathophysiological events that accompany removal of a large piece of skull bone, and these complications arise in a sequential fashion at specific time points following the surgical decompression. Expansion of contusions, new subdural and epidural hematomas contralateral to the decompressed hemisphere, and external cerebral herniation typify early postoperative complications. Within the first week, CSF circulation derangements manifest commonly as subdural hygromas and hydrocephalus. During the later phases of recovery in some craniectomy patients, the forces of atmospheric pressure and gravity overwhelm intracranial pressures, leading to the depression of the scalp flap. Subsequently, there develop new cognitive, sensorimotor, neurological and psychological deficits which are incorporated in the syndrome complex called the sinking skin flap syndrome (SSFS), also called the syndrome of the trephined (ST), or the motor trephine syndrome (MTS). In the longer term, a persistent vegetative state is the most devastating of outcomes of decompressive craniectomy [1, 2].
Further, after craniectomy, some patients who suffer from hydrocephalus [3], possibly due to disturbance of CSF flow around the cerebral convexities, are managed by CSF diversion procedures. These include ventriculo-peritoneal (V-P) shunt, lumbar drainage or extra ventricular drainage. However, these procedures paradoxically can cause an exacerbated and marked depression of the skin at the craniectomy site with further neurological deterioration. MTS is thus defined as a contralateral hemiparesis and secondary neurological deficits and deterioration, with impairment of general status, in the presence of a concave deformity and a sinking, relaxed skin flap, that develops as a reversible complication in patients treated with decompressive hemicraniectomy. Decreased skull resistance with large hemispheric decompressions, changes in CSF hydrodynamics with CSF circulation dysfunction and decompensation, with associated decrements in CBF and brain metabolism; and longer times to cranioplasty repair have all been hypothesized as possible causes of MTS. It has been hypothesized that this condition is rapidly reversible following cranioplasty repair [2, 3].
The pathophysiology of MTS includes CSF hypovolemia and development of an atmospheric pressure gradient that may be precipitated or aggravated by CSF diversion, dehydration and postural change [4, 5]. The symptoms of SSFS include sensorimotor deficits, headache, vertigo, tinnitus, fatigue, loss of concentration, loss of memory, depression, dysphagia, apraxia, paresis of extremities and epileptic seizures. There often occur delayed motor deficits; hence the condition has also been designated as MTS [3, 6]. The term MTS has been used to describe a delayed motor deficit occurring after decompressive hemicraniectomy, which reverses rapidly following cranioplasty.
Pathophysiology of this syndrome also includes compression of the underlying cortex by an infolded scalp, changes in CBF, changes in the CSF hydrodynamics and a disturbed brain perfusion [3, 4]. Yamamura and Makino [4] found normalization of CSF pressure after cranial repair, suggesting that the intracranial relationships were reformed, including the ventricular system and subarachnoid space over the convexity following the cranioplasty.
A case of a patient with SSFS showing remarkable recovery from neurological sensorimotor deficits after an early cranioplasty, is presented.
Case Report
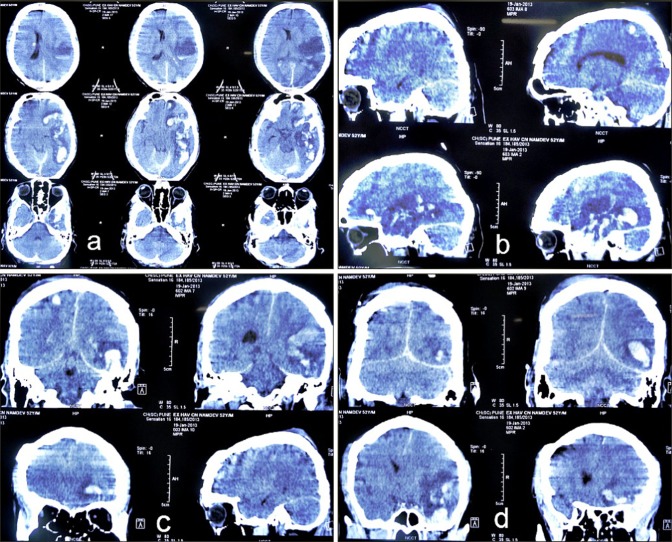
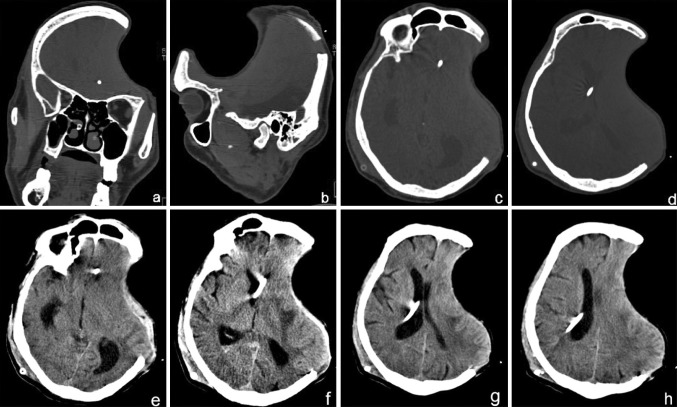
A 55 year old male patient sustained a severe head injury, following an RTA on 17 Jan 2013. His GCS was E3V2M5 and he had complete hemiparesis of the right side with Rt facial nerve paresis as well. Non-contrast computed tomography (NCCT) of the head (Fig. 1) revealed an acute left convexity subdural hematoma (SDH) with significant mass effect with midline shift to the right. There were several small hemorrhagic and non-hemorrhagic contusions in bilateral temporal and right parietal regions. There was generalized post traumatic cerebral edema causing effacement of the cortical sulci and cisternae (Fig. 1). An emergency left FTP craniectomy with evacuation of the SDH and associated contusectomy was performed under general anesthesia. The excised calvarial bone flap was buried subcutaneously in the abdominal wall. The patient recovered from the surgery and was haemodynamically stable. Due to anticipated prolonged ventilation, an elective percutaneous tracheostomy was performed after 48 h. In the days following, his condition gradually improved, he was weaned off ventilator and was shifted to the sub acute ward. His GCS was E4VtrM4, with Rt hemiparesis. The patient was maintained on tracheostomy, feeds were provided through the Ryles nasogastric tube, and he underwent regular chest and limb physiotherapy. He was gradually recovering, however, after 2 months his neurological status showed a sudden deterioration, the scalp defect became tense and an urgent NCCT brain showed hydrocephalus, for which an emergency right V-P shunt was performed under GA. His neurological status and mentation showed a rapid deterioration and he also developed a disfiguring, indrawn and sucked in left fronto-temporo-parietal (FTP) scalp flap, over the craniectomy defect, at an acute angle to the skull margins of the defect, creating a non-pulsatile gorge-like pit, and the overlying skin was unpinchable (Fig. 2). Plain CT brain showed the cranial cavity in the form of a kidney bean shape with a concavo-convex surface, with the concave surface facing the skull defect, as if held in position by vacuum (Fig. 3). In view of the above he was diagnosed to have SSFP syndrome and was planned for an early cranioplasty. He was therefore rehydrated well and prepared for cranioplasty and replacement of the FTP bone flap that had been preserved in the subcutaneous layer of his abdominal wall. Although the usual period of time recommended before cranioplasty is carried out after severe head injury, is 6 months, it was anticipated that the general condition of the patient, and the motor functions would improve following replacement of the excised bone flap, as a result of negation of the deleterious trephination effect on the hitherto unprotected brain. The atmospheric pressure was presently compressing the unprotected brain on the left owing to the absence of a protective cranial covering in the region. So, he was planned for replacement of the FTP bone flap to relieve this pressure effect and to allow the collapsed brain to expand in volume to its normal size within the cranium, as well as to provide the protective cranial covering for the brain before discharging the patient from the hospital.
Fig. 1.
Post trauma NCCT head of a 55 year old male patient, revealing multiple hemorrhagic and non-hemorrhagic contusions in the left temporal and bilateral parietal regions, with generalized post traumatic cerebral edema causing effacement of the cortical sulci and cisternae
Fig. 2.
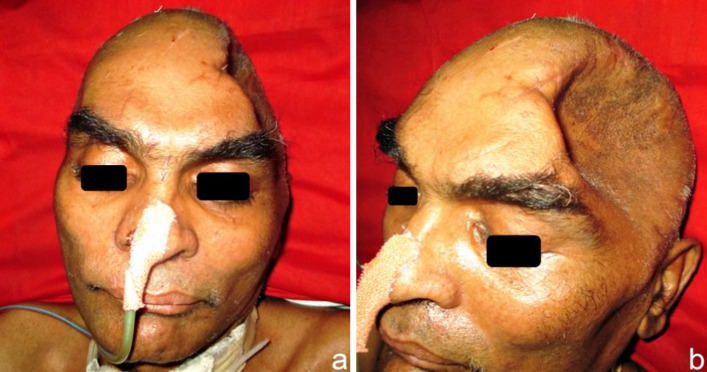
A disfiguring, indrawn and sucked-in left FTP scalp flap, over the craniectomy defect, creating a non-pulsatile gorge-like pit with unpinchable overlying skin, typical of the SSFS
Fig. 3.
Post craniectomy NCCT head showing the cranial defect, with a significant mass effect and midline shift to the right, and evidence of the SSFS. The cranial contents appear in the form of a kidney bean shape with a concavo-convex surface, with the concave surface facing the skull defect, as if held in position by vacuum
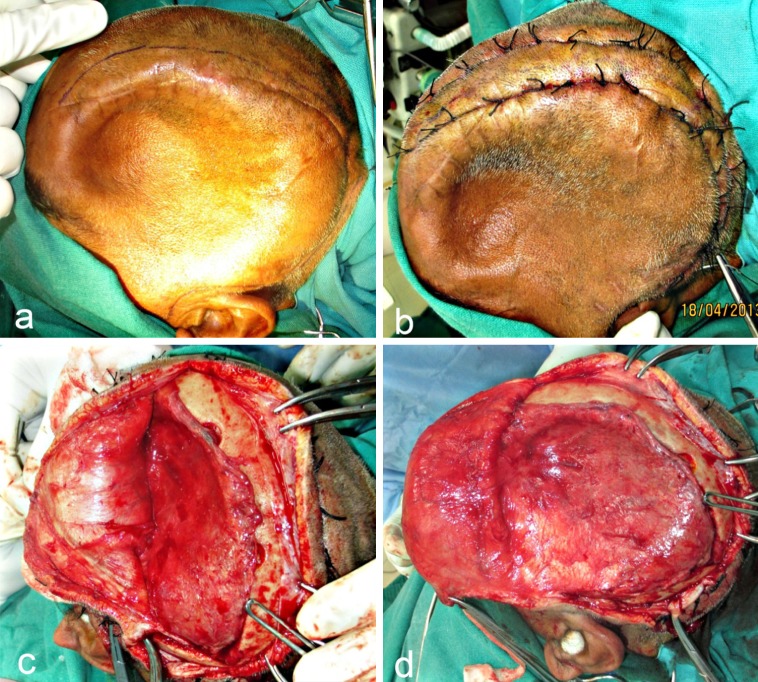
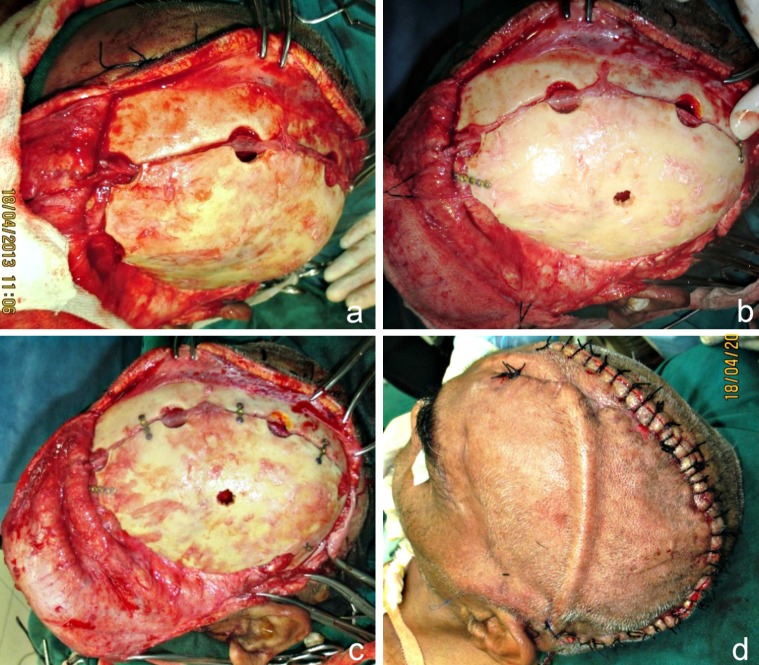
The patient was taken up for surgery under GA. After the patient was scrubbed and draped, the incision line was marked keeping approximately a distance of 1 cm from the edges of the previous operative scar (Fig. 4a). 1: 80,000 adrenaline was infiltrated and haemostatic sutures (using Black Braided Silk 1/0) were placed on either side of the proposed incision line (Fig. 4b). Incision was placed through the skin and subcutaneous tissue, the galea and the pericranium down to the bone. Periosteal elevators were used to expose bone at the periphery of the defect, except at the infratemporal and zygomatic arch regions. An avascular plane between the dura and the overlying galea adhering to it, in the region of the defect, was identified and blunt dissection was carried out along this plane, exposing the entire defect (Fig. 4c, d). There was no dural breach or CSF leak encountered. The scalp flap thus raised, was overturned over a rolled gauze pad and secured anteriorly with traction sutures.
Fig. 4.
a Incision line marked approximately 1.5 cm from the palpable margins of the bony defect. b Hemostatic sutures place on either side of the proposed incision line. c, d Blunt dissection carried out along the avascular plane between the dura and overlying galea, exposing the entire defect. The “sunken in” appearance of dura and underlying brain tissue is obvious
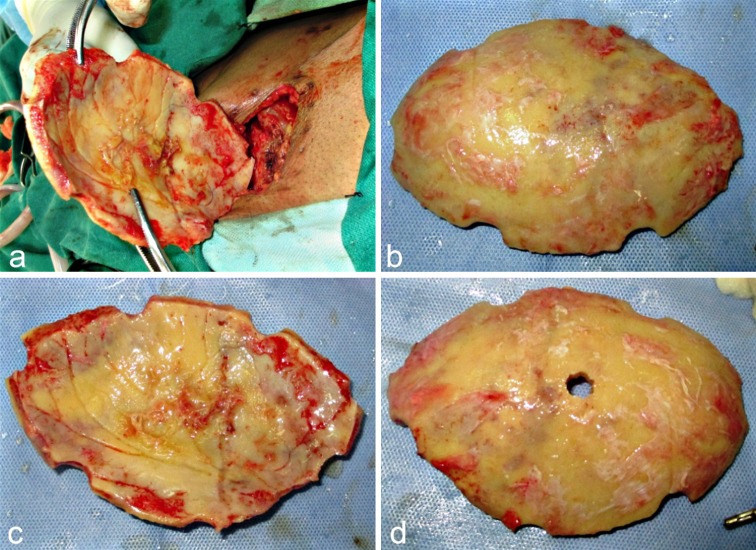
The excised calvarial FTP bone flap that was implanted in the subcutaneous layer of the abdominal wall was now approached using the old surgical scar over the right side of the abdomen (Fig. 5a–c). Dissection was carried out carefully exposing the bone flap. It was meticulously dissected free of all the adherent soft tissues, particularly from its inferior aspect, taking care not to cause any breach in the anterior abdominal wall. Soft tissue closure was carried out in layers. A 5 mm diameter bur hole was drilled through the center of the bone flap to allow for aspiration of any epidural collection post-operatively, if the need arose (Fig. 5d). The flap was placed over the defect and was fixed using microplates and screws after ensuring hemostasis (Fig. 6a–c). A vacuum assisted closed suction drain was placed before turning back the scalp flap back in place, and closure was accomplished, using Vicryl 2-0 for the galea and subcutaneous tissue and Black Braided Silk 1-0 for the skin (Fig. 6d).
Fig. 5.
a–c Cranial bone flap retrieved from the abdominal wall, where it had been implanted following craniectomy. d A 5 mm bur hole drilled through the flap to allow for subsequent aspiration of epidural collection, if any, in the post-operative period
Fig. 6.
a–c Fronto-temporo-parietal bone flap positioned over the cranial defect and fixed using micro plates and screws. d A vacuum assisted closed suction drain placed prior to closure
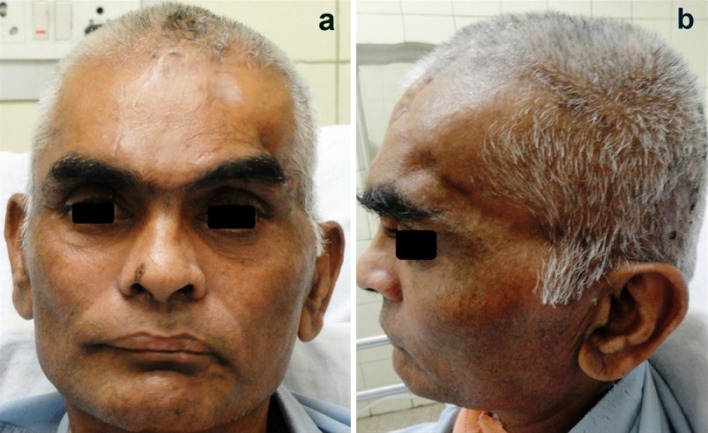
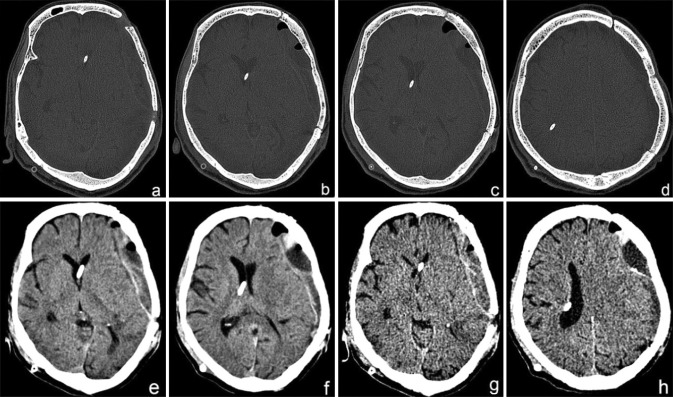
Post operatively, the patient was placed on a protocol of antibiotics, pain relief and other supportive medications. The postoperative recovery of the patient proceeded quickly, smoothly and uneventfully. There was a dramatic recovery of his GCS score deficit. On the second day following the cranioplasty, he was able to move his right hand and leg. Over the next 3 days, he recovered ability to follow simple commands, such as hand grasping and leg elevation. He was able to sit up with some support and improved in cognitive functions as well. His memory improved drastically and he was able to recognize his wife and daughter and began to understand and respond to what was being said to him. There was an excellent restoration of the contour of the calvarium with complete resolution of features of the SSFS (Fig. 7). He could be weaned off tracheostomy on the fifth post op day and was able to take feeds orally. A post-cranioplasty check CT scan brain (Fig. 8) proved the imaging recovery of the brain and cranial defect. He was gradually mobilized and his Foley’s catheter replaced with U drain. There were no seizures during the hospital stay and he was discharged on the 11th post-operative day.
Fig. 7.
a, b A good restoration of the cranial contour following the early cranioplasty and rapid resolution of features of the sinking skin flap/MTS with drastic improvement in GCS score, motor and cognitive functions, alertness and memory by the 5th postoperative day
Fig. 8.
a–h Post cranioplasty NCCT showing restoration of integrity of the cranial vault and resolution of the SSFS with a well appreciable expansion of the brain back to its original contour. An epidural collection is visible, which has been aspirated through the bur hole placed pre-operatively through the bone flap
Discussion
SSFS was first reported in 1997 by Yamamura et al. [3, 4], who defined this condition as objective neurological abnormalities that could be explained solely due to the concavity of the skin flap and the pressure of the atmosphere on the underlying brain tissue. Along with other authors [5, 6], they differentiated this condition from “the post-traumatic syndrome” [7], which were related to subjective symptoms and not objective neurological deficits. Fodstad et al. [8] believed that only symptoms reduced or relieved by cranioplasty should be included in the definition of ST. He observed that some patients with a depressed skin flap after external cerebral decompression showed improved neurologic symptoms after cranioplasty to correct tissue formation under the flap [3, 9]. Even in our patient with SSFS there was seen a rapid improvement of neurologic symptoms, motor functions, cognitive behavior as well as an improvement in memory, after cranioplasty.
A non-pulsatile, concave, horizontally across sunken-in brain under an unpinchable scalp at a craniectomy site (Fig. 2), is a sign of vacuum-sucked underlying brain. By the law of Physics, the vacuum or negative pressure causing suction of one side of the brain across and toward the opposite side can be created due to two possible reasons. The first possible reason can be a loss of volume and pressure in the air-tight closed space of the cranial cavity, which under normal circumstances, contains a fixed volume of brain, blood and CSF. The second reason could be a high atmospheric pressure brought to bear upon the brain at the craniectomy site, resulting in a pressure gradient across the skin flap. This would act directly on the brain resulting in tissue compression and depression with a shift of the scalp and brain to the opposite side [10, 11]. In our case both the above factors probably had an equal role to play.
Langfitt [12] had theorized in 1968 that atmospheric pressure is directly transmitted to the intracranial cavity, causing inward shifting of the scalp over the craniectomy site. Accordingly, neurological improvement has been reported when this pressure disturbance is normalized after cranioplasty repair. Several authors have proposed that a negative gradient between atmospheric and intracranial pressure, which is aggravated by changes in the CSF compartment following CSF hypovolemia, to be the mechanism of this syndrome after craniectomy [13]. Prolonged dehydration and an upright posture may also precipitate this phenomenon. CSF drainage (for e.g.: a V-P shunt or a lumbar puncture) performed in a patient suffering from hydrocephalus, as in this case, exacerbates this effect by creating a pressure gradient through the craniectomy site.
There are various pathophysiological theories for the cause of the neurological and motor deficits seen in SSFS/MTS [14]: (a) direct cortical compression; (b) hydrodynamically disturbed CSF parameters; (c) haemodynamically reduced CBF, cerebrovascular reserve capacity and venous return due to pressure on the vasculature and brain tissue; (d) disturbed metabolism [15].
The goal of treatment in patients with this syndrome, with its attendant neurological deterioration, is restoration of the pressure exerted by depression of the craniectomy site [15]. This is achieved by cranioplasty as it improves the neurological deficits and corrects abnormal CSF dynamics. The cranioplasty may also improve the postural blood flow regulation, cerebrovascular reserve capacity and cerebral glucose metabolism [17], with improvement in brain perfusion and hemodynamics [16, 17].
There was observed a remarkable clinical improvement in the cognitive behavior, memory and motor deficit in our patient following early cranioplasty, with a rapid reversal of all features of MTS.
Conclusion
Contralateral monoparesis and secondary neurological deterioration in the presence of a sinking skin flap, also known as the MTS, sometimes develops as a reversible complication in patients treated with decompressive hemicraniectomy. This happens as a result of three main factors: Direct atmospheric pressure on the unprotected brain, CBF disturbances and CSF circulation abnormalities. Upon replacement of the excised bone flap, CSF and edema fluid changes within the parenchyma and CBF normalize, leading to improvements in the patient’s motor function. In the case presented, an early cranioplastic repair led to a remarkable clinical recovery of the patient. There was seen a dramatic improvement in the patient’s cognitive behavior and motor deficit and a rapid reversal of his sensorimotor paresis. This reflected an improvement in brain perfusion and hence served as a therapeutic procedure, rather than merely a cosmetic one.
References
- 1.Stiver SI. Complications of decompressive craniectomy for traumatic brain injury. Neurosurg Focus. 2009;26:27–29. doi: 10.3171/2009.4.FOCUS0965. [DOI] [PubMed] [Google Scholar]
- 2.Stiver SL, Wintermark M, Manley GT. Motor trephine syndrome: a mechanistic hypothesis. Acta Neurochir Suppl. 2008;102:273–277. doi: 10.1007/978-3-211-85578-2_51. [DOI] [PubMed] [Google Scholar]
- 3.Isago T, Nozaki M, Kikuchi Y. Sinking skin flap syndrome: a case of improved cerebral blood flow after cranioplasty. Ann Plast Surg. 2004;53:288–292. doi: 10.1097/01.sap.0000106433.89983.72. [DOI] [PubMed] [Google Scholar]
- 4.Yamamura A, Makino H. Neurological deficits in the presence of the sinking skin flap following decompressive craniectomy. Neurol Med Chir (Tokyo) 1997;17:43–53. doi: 10.2176/nmc.17pt1.43. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura T, Takashima T, Isobe K, Yamaura A. Rapid neurological alteration associated with concave deformity of the skin flap in a craniectomized patient. Case report. Neurol Med Chir (Tokyo) 1980;20:89–93. doi: 10.2176/nmc.20.89. [DOI] [PubMed] [Google Scholar]
- 6.Grant FC, Norcross NC. Repair of cranial defects by cranioplasty. Ann Surg. 1939;110:488–512. doi: 10.1097/00000658-193910000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grantham EC, Landis HP. Cranioplasty and the post-traumatic syndrome. J Neurosurg. 1948;5:19–22. doi: 10.3171/jns.1948.5.1.0019. [DOI] [PubMed] [Google Scholar]
- 8.Fodstad H, Love JA, Ekstedt J, Friden H, Liliequist B. Effect of cranioplasty on cerebrospinal fluid hydrodynamics in patients with the syndrome of the trephined. Acta Neurochir (Wien) 1984;70:21–30. doi: 10.1007/BF01406039. [DOI] [PubMed] [Google Scholar]
- 9.Kelly GR, Johnson PL, Sinking skin flap syndrome Craniotomy can precipitate brainstem herniation in CSF hypovolemia. Neurology. 2004;62:436–471. doi: 10.1212/wnl.62.1.157. [DOI] [PubMed] [Google Scholar]
- 10.Liao CC, Kao MC. Cranioplasty for patients with severe depressed skull bone defect after cerebrospinal fluid shunting. J Clin Neurosci. 2002;9:533–555. doi: 10.1054/jocn.2002.1116. [DOI] [PubMed] [Google Scholar]
- 11.Akins PT, Guppy KH. Sinking skin flaps, paradoxical herniation and external brain tamponade: a review of decompressive craniectomy management. Neurocrit Care. 2008;9:269–276. doi: 10.1007/s12028-007-9033-z. [DOI] [PubMed] [Google Scholar]
- 12.Langfitt TW. Increased intracranial pressure. Clin Neurosurg. 1968;16:436–471. doi: 10.1093/neurosurgery/16.cn_suppl_1.436. [DOI] [PubMed] [Google Scholar]
- 13.Schwab S, Erbuguth F. Paradoxical herniation after decompressive trephining. Nervenartz. 1998;69:896–900. doi: 10.1007/s001150050360. [DOI] [PubMed] [Google Scholar]
- 14.Dujovny M, Agner C, Aviles A. Syndrome of the trephined: theory and facts. Crit Rev Neurosurg. 1999;9:271–278. doi: 10.1007/s003290050143. [DOI] [PubMed] [Google Scholar]
- 15.Bhat AR, Kirmani AR. “Sunken brain and scalp flap” syndrome following decompressive “extra-craniectomy”. Indian J Neurotrauma. 2011;8:105–108. doi: 10.1016/S0973-0508(11)80009-2. [DOI] [Google Scholar]
- 16.Kemmling A, Duning T, Lemke L. Case report of MR perfusion imaging in sinking skin flap syndrome: growing evidence for haemodynamic impairment. BMC Neurol. 2010;10:80–83. doi: 10.1186/1471-2377-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winkler PA, Stummer W. Influence of cranioplasty on postural blood flow regulation, cerebrovascular reserve capacity and cerebral glucose metabolism. J Neurosurg. 2000;93:53–61. doi: 10.3171/jns.2000.93.1.0053. [DOI] [PubMed] [Google Scholar]