Abstract
Introduction
Tetanus remains a problem of immense concern worldwide. Management of tetanus with its attendant complications are challenges to the physician and its prevention is the chief priority. The choice of tetanus prophylaxis for patients with wounds depends on obtaining their vaccination history, which has been demonstrated to be unreliable. The tetanus antibody test may avoid inadequate prophylactic measures and reduce costs.
Purpose
This study is conducted to determine the feasibility of rapid tetanus antibody test (SD Bioline) in the accident and emergency department.
Methods
A randomized prospective study was conducted on 50 patients, divided into two groups—A and B with 25 patients each respectively. Group A had patients with a history of tetanus immunization <5 years elapsed from the last booster dose. Group B had patients who did not know the immunization status or more than 5 years elapsed from the last booster dose of tetanus immunization. Groups A and B were further classified into Group 1 and Group 2 based on whether the wound is tetanus prone or a clean wound respectively. Tetanus antibody test was done using whole-blood from the patients by finger prick.
Results
Among the 50 patients, 25 (50 %) were classified as ‘unprotected’ according to the history. Thirteen of the 25 patients showed tetanus antibody positive. In Group A, only 9 of the 25 patients showed tetanus antibody positive.
Conclusion
The rapid tetanus antibody test in the emergency room could make tetanus prevention more accurate with improved technical feasibility. The test may eliminate unnecessary injecting of vaccine, reduce the cost and can be performed at the hospital setting.
Keywords: Tetanus, Emergency, Wounds
Introduction
The care of patients with multiple injuries is a complex multifaceted problem requiring basic knowledge and skills in various surgical disciplines to restore these patients to health. The prevention and treatment of tetanus like-wise, is an added responsibility on the surgeon because of the increasing number of patients sustaining multiple injuries on the one hand, and the large proportion of population not in a condition to express the vaccination status on the other [3].
Tetanus prophylaxis is the cornerstone of disease prevention, which depends on the characteristics of the wound (low or high risk) and on evaluation of the patient’s immunity based on the vaccination history [6]. The history of tetanus immunization may not be accurate, from the patient always, due to various reasons. In a developing country like India, most patients are unaware of the vaccinations. Some patients are ignorant about tetanus vaccination and many others do not remember about the same. Thus, the tetanus antibody test can play a great role in it. This study is conducted to determine the feasibility of rapid tetanus antibody test (SD Bioline) in the accident and emergency department.
The aim of the study is to estimate the tetanus antibody level in patients with maxillofacial injury and to compare the results with the history of the tetanus vaccination.
The study groups are prepared based on the recommendation of tetanus vaccination in wound management as follows:
Recommendations of the advisory committee on immunization practices (ACIP): guide to tetanus prophylaxis in routine wound management among adults aged 19–64 years [5]
| Characteristic | Clean, minor wound | All other woundsa | ||
|---|---|---|---|---|
| History of adsorbed tetanus toxoid (doses) | Tdap or Tdb | TIG | Tdap or Tdb | TIG |
| Unknown or <3 primary doses | Yes | No | Yes | Yes |
| ≥3 Primary doses | Noc | No | Nod | No |
Tdap, includes immunization against diphtheria and pertussis (with the acellular pertussis vaccine), is preferred to Td for adults who require a tetanus toxoid-containing vaccine as part of wound management and who have not previously received Tdap
Diphtheria-tetanus vaccine adsorbed (adult) (Td)—used as the preferred agent for adults and children ≥7 years old. For adults previously vaccinated with Tdap, Td should be used if a tetanus toxoid-containing vaccine is indicated for wound care. A history of a neurologic reaction (e.g., encephalopathy) or an immediate anaphylactic reaction is a contraindication to further Td
Tetanus toxoid fluid (TT)—used in patients who have a contraindication to combined antigens or those who are hypersensitive to the aluminum adjuvant in the adsorbed toxoid
aSuch as, but not limited to, wounds contaminated with dirt, faeces, soil, and saliva; puncture wounds; avulsions; and wounds resulting from missiles, crushing, burns, and frostbite
bTdap is preferred to Td for adults who have never received Tdap. Td is preferred to TT for adults who received Tdap previously or when Tdap is not available. If TT and TIG are both used, Tetanus Toxoid Adsorbed, rather than tetanus toxoid for booster use only (fluid vaccine) should be used
cYes, if ≥10 years since the last tetanus toxoid-containing vaccine dose
dYes, if ≥5 years since the last tetanus toxoid-containing vaccine dose
Materials and Methods
Study Group
Fifty patients of age group 10–64 years with maxillofacial injury before 24 h were selected for the study for the period from June 2009 to August 2010 and divided into two groups.
Group A: 25 Patients with history of tetanus immunization <5 years elapsed from the last booster dose.
Group B: 25 Patients who do not know the immunization status or more than 5 years elapsed from the last booster dose of tetanus immunization.
Group 1: Tetanus Prone Wounds
The wounds that met any one of the following criteria were grouped into contaminated or tetanus prone wounds.
Deep punctured wounds with depth of >1 cm, avulsion, open wound for longer than 6 h, contaminated with foreign bodies like soil, glass or flying objects, crushing, burns and frostbite or the irregular margins with signs of devitalization.
Patients with contaminated/tetanus prone wounds
-
(A)
Less than 5 years since the last booster dose (15 patients)
-
(B)
Five years elapsed since the last booster dose or do not know the previous immunization status (15 patients)
Group 2: Clean or Non-tetanus Prone Wounds
The wounds that met all the following criteria were grouped as clean or non-tetanus prone wounds.
Linear wound, depth is <1 cm/abrasion, with sharp borders, without signs of infection/devitalization or contamination.
-
(A)
Less than 5 years elapsed since the last booster dose of tetanus immunization (10 patients)
-
(B)
More than 5 years passed since the previous booster dose of tetanus immunization or do not know (10 patients)
Informed consent was taken from the patient.
The history of previous tetanus vaccination was obtained from the patient himself or bystander. Thorough clinical examination was done. Age of the wound, Depth, Mechanism of Injury, Contamination and Devitalization were recorded.
Age of the wound was gauged from the time elapsed since the injury and depth was recorded as more than 1 cm if the wound required at least two-layered closure. Any foreign body within the wound was termed as contaminated and wound with inflamed or irregular border was recorded.
Informed consent for the test was taken from every patient. SD Bioline tetanus antibody test was done using whole-blood from the patients by finger prick.
SD Bioline test kit is a chromatographic immunoassay for the qualitative detection of tetanus antibodies in the whole-blood, serum or plasma. It contains a combination of a solid phase coated with tetanus toxoid and tetanus toxoid-dye conjugate (colloidal gold). The operator takes a whole-blood sample from the patient’s fingertip by means of a small pipette provided in the test kit. This sample is placed in the test sample well, to which diluent is added. The diluent flows through the absorbent pad, carrying the toxoid-dye conjugate along the chromatographic strip. A complex with anti-tetanus antibodies forms if such antibodies are present in the blood sample. These complexes react with the immobilized toxoid to form a pink line in the “T” (test) window. In the absence of anti-tetanus antibodies, no line appears in the test window. The excess gold conjugate binds to a control reagent immobilized in the “C” (control) window, forming a purple line, indicating that the test has been carried out correctly. The test should be read 20 min after diluent is added to the blood sample in the device (Fig. 1).
Fig. 1.
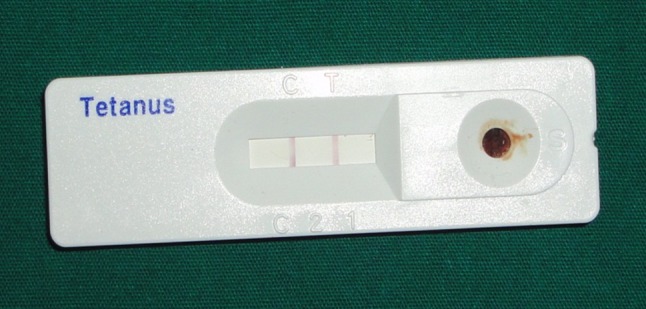
Photograph showing positive test result
The active ingredients of the kit are:
Test strip-tetanus toxoid-gold colloid, mouse IgG-gold colloid
Test line-tetanus toxoid, Control line-goat anti mouse IgG
Assay diluent—100 mM Tris – HCl, sodium azide [11]
Results
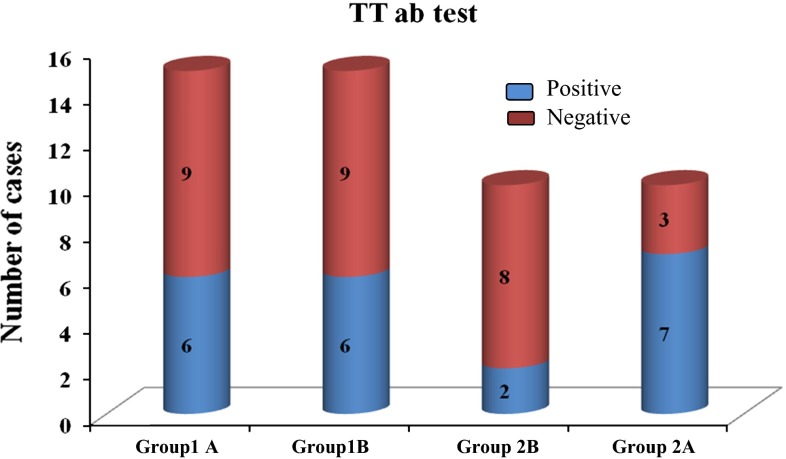
In our study, 15 patients in each group of tetanus prone wounds were evaluated for the tetanus antibody test. In group B, which includes 1B and 2B, 8 patients did not know the tetanus vaccination history and showed positive test result. In the group of clean wounds, 10 patients in each group were evaluated. In Group 2B, 6 patients did not know the tetanus immunization history and showed positive test result (Table 1).
Table 1.
The percentage of tetanus antibody test results among the groups
| Groups | Total | ||||
|---|---|---|---|---|---|
| 1A: tetanus prone wound: immunized <5 years | 1B: tetanus prone wound: not immunized or not known | 2A: clean wound: immunized <5 years | 2B: clean wound, not immunized or not known | ||
| Positive | |||||
| Count | 6 | 6 | 7 | 2 | 21 |
| % | 40.0 | 40.0 | 70.0 | 20.0 | 42.0 |
| Negative | |||||
| Count | 9 | 9 | 3 | 8 | 29 |
| % | 60.0 | 60.0 | 30.0 | 80.0 | 58.0 |
| Total | |||||
| Count | 15 | 15 | 10 | 10 | 50 |
| % | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
p = 0.154 ns
The time taken for interpretation of the test was 3–15 min by a single operator.
Among the 50 patients, 25 (50 %) were classified as ‘unprotected’ according to the history, in fact 8 patients out of 25 patients had a protective antibody level. For these patients, evaluation of immunity by the SD Bioline antibody test instead of vaccination history would have changed the choice of prophylaxis in most cases. In contrast, 13 of the 25 patients considered as protected according to the vaccination history had a positive tetanus antibody result.
The prevalence of tetanus immunity, age distribution, history and depth of wound among the four groups is given in (Figs. 2, 3, 4, 5).
Fig. 2.
Results of tetanus antibody test in tetanus prone wounds and clean wounds
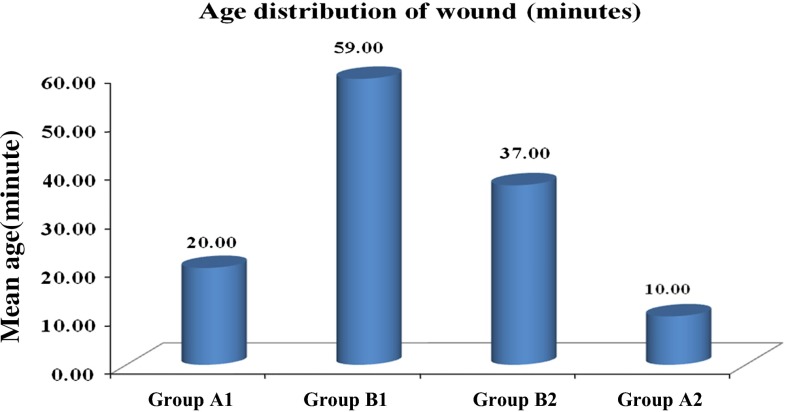
Fig. 3.
Age of the wound at the time of test in tetanus prone and clean wound groups
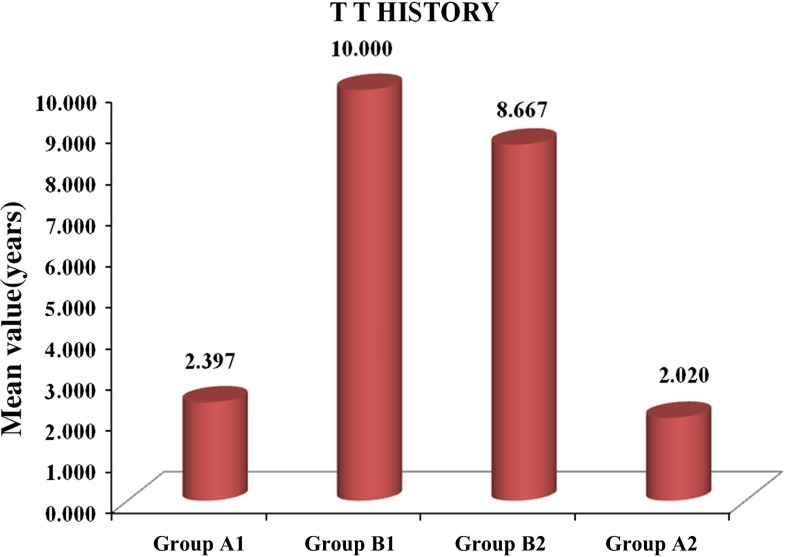
Fig. 4.
Distribution of tetanus vaccination history among the groups
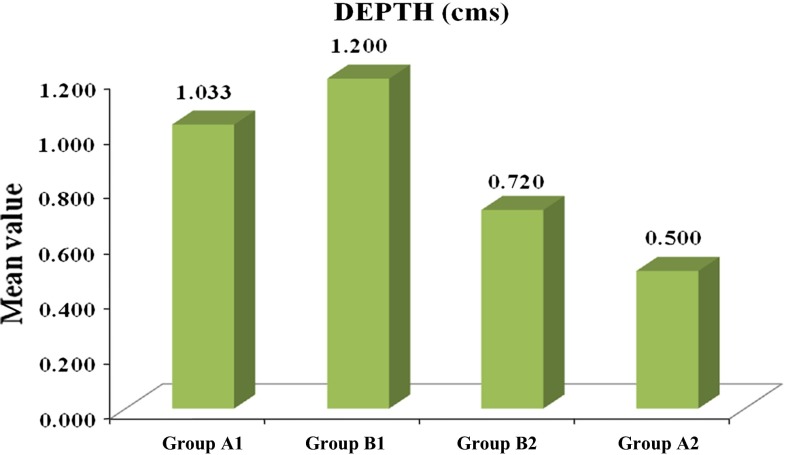
Fig. 5.
Average depth of the wound distributed among the groups
Discussion
Tetanus remains a problem of immense concern worldwide. Management of tetanus with its attendant complications are challenges to the physician. Prevention is the chief priority and it is absolutely essential that people are educated about this potentially serious, but extremely preventable disorder, for this is the only step towards global eradication [1]. According to WHO, the protective level of immunity against tetanus is assay specific. With in vivo neutralization tests or modified ELISA assays, serum antitoxin concentrations exceeding 0.01 IU/ml are usually considered protective, whereas antitoxin concentrations of at least 0.1–0.2 IU/ml are defined as positive when standard ELISA techniques are used for this assessment [10]. The main disadvantage of ELISA is the time required for the test to perform. The test is normally conducted in laboratory than at the point of care. It is not feasible at the hospital setting. Tetanus antibody test can be an adjunct to it. The SD Bioline rapid tetanus test will measure the antitoxin level in the whole blood more than 0.2 IU/ml [11]. The performances of other rapid antibody tests have been generally validated against ELISA [2].
Many reports are available regarding the tetanus antibody evaluation by ELISA. A multicentre study in 2004, assessing compliance with anti-tetanus immunization protocols in Emergency Departments in the United States revealed that physicians provided inadequate prophylaxis to approximately 35 % of patients with wounds [5].
Sedgwick et al. [8] in 1983 reported the study of a rapid quantitative micro enzyme-linked immunosorbent assay for the detection of immunoglobulin G antibodies to tetanus toxoid. The assay is performed in 2.5 h and detects antibody levels of between 0.1 and 6 U/ml.
Colombet et al. [7] in 2005 reported that the feasibility and accuracy of the Tetanos Quick Stick (TQS) rapid finger prick test in the multicentre emergency department by comparing with ELISA with the sensitivity and specificity of the blood/lab-TQS test were 84 % (95 % CI 82–86) and 99 % (95 % CI 92–100), respectively, and found that TQS is better than clinical history at preventing unnecessary treatment. Its use in emergency departments should not be considered without a quality assurance process and close collaboration between biologists and clinicians.
A comparative study by Stubbe et al. [6] in 2007 of TQS with ELISA concluded that TQS could be a valuable diagnostic tool in selected patients in emergency department.
In 2007 at Korea, Lee et al. [9] reported the evaluation of SD Bioline test kit. A total of 326 peripheral blood specimens (whole-blood, 319; serum, 326) from healthy subjects and patients were used. SD Bioline tetanus kit was evaluated for precision, accuracy, effect of specimens, operator variance, and the total processing time. The results from SD Bioline tetanus kit were compared with those from two quantitative ELISA kits. SD Bioline tetanus kit showed an excellent analytical performance with sensitivity of 88–97 %, specificity of 87–92 %, positive predictive value of 81–89 %, negative predictive value of 90–98 %. With its rapid turnaround time and the ease of handling and interpretation, SD Bioline tetanus kit seems appropriate for the evaluation of tetanus immunization status as a point of care testing device. However, education for operators and standardized guidelines for result interpretation should be emphasized.
Study conducted by Paulke-Korinek et al. in 2008 on randomly selected patient sera concluded that TQS could determine the tetanus immunization status where fast decision was needed, but the sensitivity was 55 % [2]. He reported the study of Protetanus test (rapid test for tetanus antibody in UK) having the specificity of 100 %.
Cooke in 2009 had done literature review on cost savings attributable to Pro tetanus following Stubbe’s model, revealed the need of further investigation [2].
A prospective concordance study was conducted by Elkharrat et al. in 2010 in France on 1,080 patients blindly comparing TQS with ELISA and concluded that TQS could help identify patients who mistakenly claimed that they have been vaccinated and were at a risk of receiving no tetanus prophylaxis. It was suggested that the TQS be added into current A&E unscheduled tetanus guideline [2].
We have evaluated the tetanus antibody test in accident and emergency department for the determination of tetanus immunization status in trauma patients, based on the tests performed and previous reports, the sensitivity being 96.5 % and specificity being 87 %.
In the present study, though the sample size was less it could detect the necessity of tetanus immunization in all samples, which are found to be protective in 40 % of the contaminated wound group (both Groups A1 and B1) and in 70 % of the patients who received the last tetanus booster vaccination before 5 years and in 20 % of the patients who did not know the last dose of non-contaminated wound group. The diagnosis of protected wounds is often limited and can be improved by the use of rapid diagnostic tests in the emergency department. More widespread use of this test would reduce inappropriate prescription of immunoglobulin in protected patients and would limit the proportion of unprotected patients receiving no preventive treatment.
The time taken for interpretation of the test was 3–15 min by a single operator. That may be the reason for the non-existence of error in the test procedure.
Maxillofacial injuries are largely due to road traffic accidents or fall where there is more likelihood of contamination of soil or rusted materials. The risk of tetanus is mainly determined by the contamination of wound and the history of the tetanus immunization. The condition of the patient will not aid the patient to provide the history. Tetanus antibody test may be supportive to provide accurate diagnosis.
There is limited evidence available on the clinical and economic benefits of the rapid tetanus antibody test. To our knowledge, no reports are available related to this test after injury in India. The tetanus antibody test is found to be cost effective when compared to the tetanus immunoglobulin (250 IU) which costs around Rs. 1,000 and the test costs only Rs. 135. Use of tetanus antibody test has a cost benefit only for patients presenting with tetanus-prone wounds and is considered from the vaccination history to be unprotected without primary vaccination and in older individuals where the injection of the tetanus immunoglobulin is mandatory.
Limitations of this study may be the sample size and the recall visits of the patient after the tetanus antibody test.
Conclusion
The history of tetanus immunization cannot be elicited from the patient always due to various reasons. The test represents a useful tool in the evaluation of tetanus immunity for the patients with soft tissue injuries because it is rapid, simple, diagnostic and cost effective, eliminates the unnecessary injection of vaccine and may be performed at the hospital settings. Further, study with large sample size related to the test performance to compare with ELISA may be required to know the accuracy of the test.
Acknowledgments
Authors thank S.D. Bio Standard Diagnostics Pvt. Ltd. for test kit and Manipal University for providing the facilities during the course of study.
Conflict of interest
None.
Contributor Information
A. Chithra, Phone: +91820 2922215, Email: chithramds@gmail.com
K. M. Cariappa, Phone: +91820 2922215
Abhay Taranath Kamath, Phone: +91820 2922215.
Adarsh Kudva, Phone: +91820 2922215.
References
- 1.Bhatia R, Prabhakar S, Grover VK. Tetanus. Neurol India. 2002;50:398–407. [PubMed] [Google Scholar]
- 2. Lu B, Craven M, Morgan S, Clark D (2010) Evidence review rapid test for tetanus immunity CEP10001. nhscep.useconnect.co.uk
- 3.Kurtz LH. Tetanus in treatment of patients with multiple trauma. J Natl Med Assoc. 1964;56:178. [PMC free article] [PubMed] [Google Scholar]
- 4.Maria P-K, Pamela R-W, Michael K, Batya T (2008) Pretravel consultation: rapid dipstick test as a decision guidance for the application of tetanus booster vaccinations. J Trav Med 15:437–441 [DOI] [PubMed]
- 5.Moran GJ, Talan DA, Abrahamian FM. Antimicrobial prophylaxis for wound and procedures in the emergency department. Infect Dis Clin N Am. 2008;22:117–143. doi: 10.1016/j.idc.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Stubbe M, Mortelmans L, Desruelles D, Swinnen R, Vranckx M, Brasseuret E, et al. Improving tetanus prophylaxis in the emergency department: a prospective, double-blind cost-effectiveness study. Emerg Med J. 2007;24:648–653. doi: 10.1136/emj.2007.048520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colombet I, Saguez C, Pors M, Coudert B, Chatellier G, Espinozaet P, et al. Diagnosis of tetanus immunization status: multicenter assessment of a rapid biological test. Clin Diagn Lab Immun. 2005;12:1057–1062. doi: 10.1128/CDLI.12.9.1057-1062.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sedgwick AK, Ballow M, Sparks K, Tilto RC. Rapid quantitative microenzyme-linked immunosorbent assay for tetanus antibodies. J Clin Microbiol. 1983;18:104–109. doi: 10.1128/jcm.18.1.104-109.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JE, Lee SY. Evaluation of SD BIOLINE tetanus kit. Korean J Lab Med. 2007;27:192–196. doi: 10.3343/kjlm.2007.27.3.192. [DOI] [PubMed] [Google Scholar]
- 10.The immunological basis for immunization series module 3: tetanus update (2006) http://whqlibdoc.who.int/publications/2007/9789241595551_eng.pdf
- 11.Suppliers’ manual, S.D. Bio standard Diagnostics PVT. Ltd. Available at, http://www.standardia.com