Abstract
Background
Oroantral fistula may develop as a complication of tooth extraction owing to infection, trauma, or removal of maxillary cyst or tumors. Closure by using a palatal mucoperiosteal flap with the greater palatine vessels is a very traditional and basic technique. The palatal mucosa is thick and is firm. However, deformation can occur with shifting of the mucoperiosteal flap, survival of the flap may be unsuccessful, and patients may have substantial intraoral discomfort felt until healing of the eminence with the arcuation. As a method to relieve these problems, we present a mucoperiosteal tunnel technique for the closure of oroantral fistula by using a palatal mucoperiosteal flap pedicled with the greater palatine vessels.
Method
A 42-year-old man and a 69-year-old woman each had a palatal fistula after palatal tumor resection and tooth extraction, respectively. We designed a mucoperiosteal flap pedicled with the left greater palatine vessel. We ablated the mucoperiosteum between the fistula and the mucoperiosteal flap, and passed this flap under the ablated mucoperiosteum as a tunnel.
Result
One year after surgery, the fistula had not reappeared and the mucoperiosteal flap harvest did not generate dyskinesis of the soft palate.
Conclusion
Tunnel technique for the closure of an oroantral fistula with a pedicled palatal mucoperiosteal flap is obtains good fructification.
Keywords: Tunnel technique, Maxillary sinusitis, Mucoperiosteal tunnel, Oroantral fistula, Pedicled mucoperiosteal flap
Introduction
Oroantral fistulas may develop as a result of infection, trauma, or removal of maxillary cysts or tumors [1, 2]. The size of the fistula depends on the depth and range of the surgical stress. An oroantral fistula <5 mm does not require any intervention and closes spontaneously [3], while oroantral fistulas >5 mm require surgical treatment. The greatest problem with a fistula is the reduction in the quality of life resulting from water leaks into the nasal cavity and maxillary air sinus. A fistula can also result in maxillary sinusitis.
Closure techniques involve the use of a tongue flap, buccal advancement flap, palatal rotation flap or transposition flap with a pedicle, or a free vascularized flap [1–4]. If local surgical treatment (e.g., postextraction fistula) without vascular injury of the greater palatine artery is indicated, closure can be expected by simply covering the fistula with a mucoperiosteal flap. On the other hand, the wound formed after the resection of a tumor may sometimes include a wide vascular resection. In the latter situation, surgeons must be very careful about how and where to form a closure flap. Surgeons often use a mucoperiosteal flap that includes the greater palatine vessels as the vascular pattern for a palatal mucoperiosteal flap. Closure using a palatal mucoperiosteal flap with the greater palatine vessels is a very traditional and basic technique. The palatal mucosa is thick and firm. However, shifting of the mucoperiosteal flap may cause deformation, the survival of the flap may be unsuccessful, and patients may have substantial intraoral discomfort until healing of the eminence with the arcuation. In order to address these problems, we present a mucoperiosteal tunnel technique for the closure of an oroantral fistula by using a palatal mucoperiosteal flap that is pedicled by the greater palatine vessels.
Case 1
A 42-year-old man with a history of en bloc resection of a black lesion at the hard palate complained of water and air leakage into the maxillary sinus. The new mucosa grew after the resection of the tumor, but caused bone resorption at the ablation center, and therefore formed an oroantral fistula (Fig. 1). On the computed tomography (CT) scan, the mucosal fistula was macroscopically 8 mm and the bone defect was 15 mm in diameter.
Fig. 1.
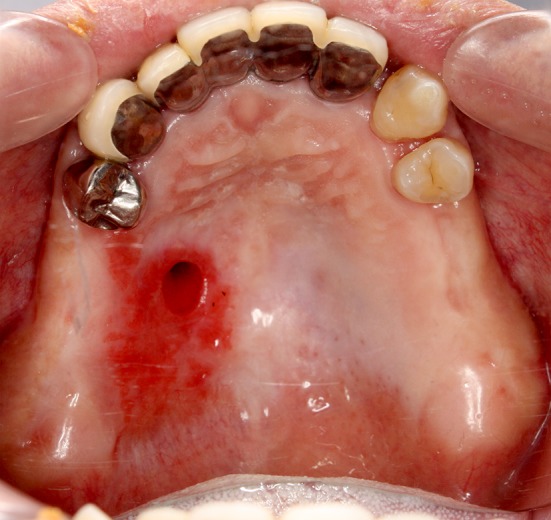
Image obtained before the closure of the palatal fistula in case 1. The fistula exists near the palatal median
The patient wore palatal covering dentures and he felt water leaking to some extent into the maxillary sinus via the fistula, but he was able to consume food. The onset of maxillary sinusitis was gradual, and ultimately a swollen mucous membrane of the maxillary sinus was projected in an edematous form through the palatal fistula to the oral side (Fig. 2); the patient was administered antibiotics. We decided to perform palatal closure of the fistula because the mucous membrane of the maxillary sinus projection from the fistula disappeared and because the perifistular inflammation improved.
Fig. 2.
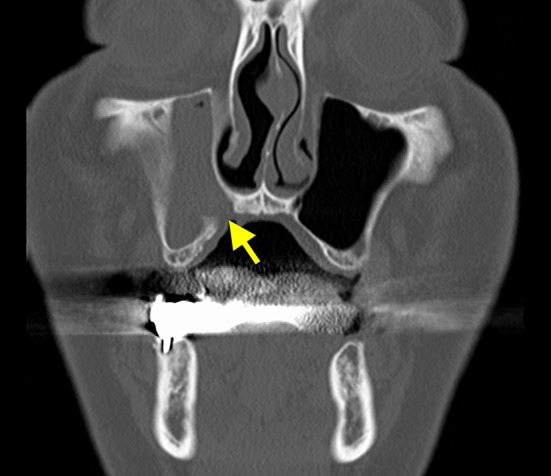
The preclosure operative coronal section computed tomography image shows right maxillary sinusitis. The mucosa of the maxillary sinus is thickened, and there is little aeration (the arrow indicates the fistula)
The right greater palatine artery was resected in the initial operation. We designed a mucoperiosteal flap pedicled with the left greater palatine vessel and confirmed this by using a Doppler acouophone along the path of the greater palatine vessel. The patient was asked to open his mouth wide and mouth care gel was applied to the palate, after which the pulsation of the greater palatine artery was auscultated (Fig. 3). We could clearly hear a beat along the greater palatine artery and were able to confirm its position.
Fig. 3.
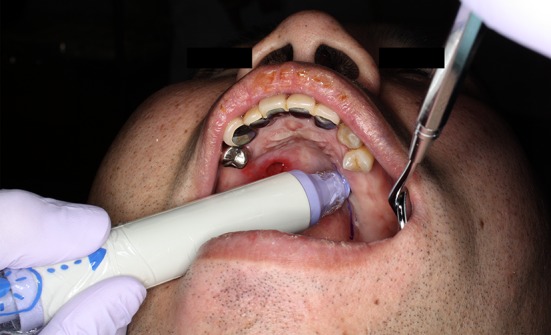
Locating the running of the greater palatine artery using a Doppler acouophone
Surgical Technique
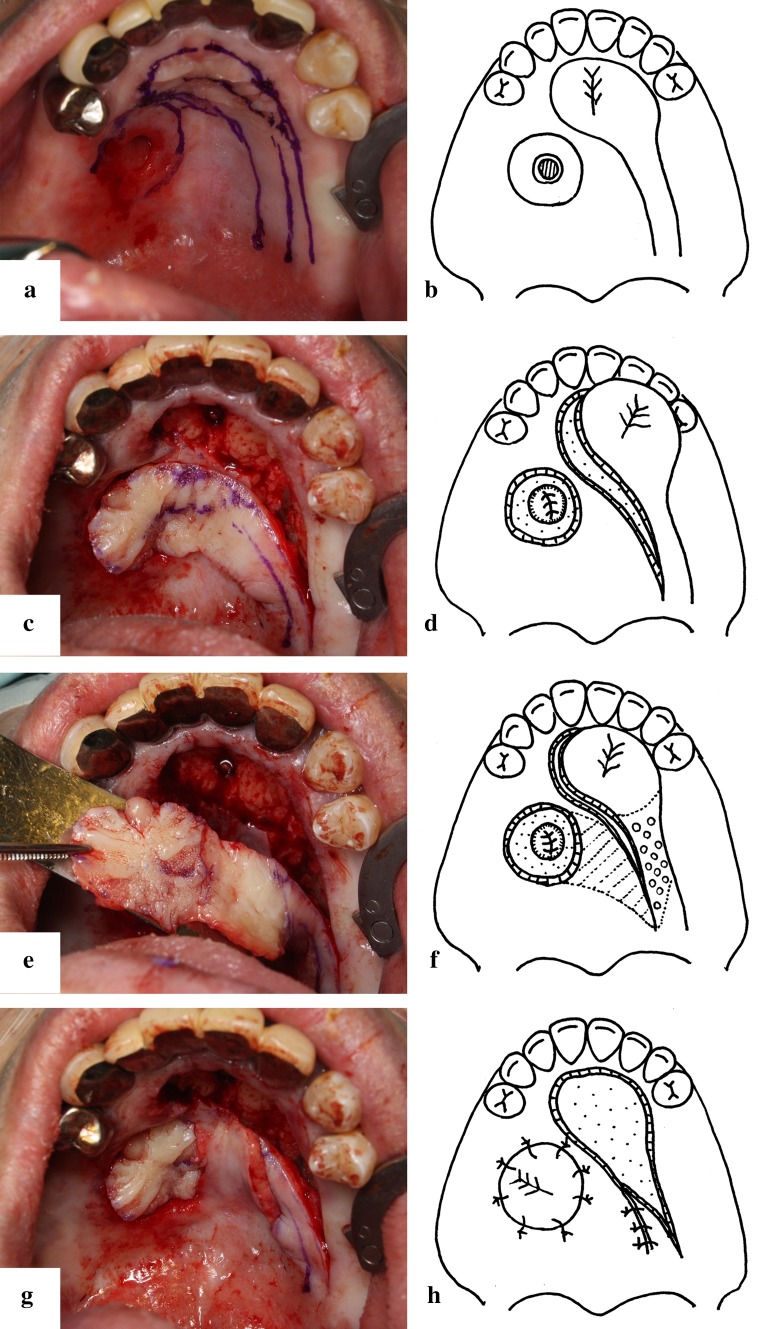
In designing the incision line, we confirmed the bone defect by using a 31-guage anesthesia needle under the mucosa around the oroantral fistula. We removed a 5-mm margin from the fistural bone defect area and incised the mucosa. We also designed a mucoperiosteal flap pedicled with the greater palatine vessel for closing the fistula, but reduced the mucosal arcuation by thinning this pedicle as much as possible when we moved it (Fig. 4a, b). In the mucosal flap, which was incised and ablated, we hung an absorption thread in the wound margin into a wave sewing form, squeezed it, applied it to the perifistular circle, and placed it into the maxillary antrum side as a hinge flap. We furthermore ablated the mucoperiosteum between the fistula and the mucoperiosteal flap, and passed this flap under the ablated mucoperiosteum as a tunnel. In addition, the duplex of the mucoperiosteal flap was denuded (Fig. 4c–h) We finally placed an artificial dermis on the area of the bone that was exposed.
Fig. 4.
a Drawing the incision line. b The schema of the flap design. The incision line of the circumference of the fistula designed as a hinge flap. The pedicle of the mucosal flap with the greater palatine artery is thinned as much as possible. c Elevation of the mucoperiosteal flap. d The schema of mucoperiosteal flap elevation. The base of the sinus is reconstructed by the extrophy of the fistula circumference mucosa as a hinge flap. e The duplex of the denuded mucoperiosteal flap. f The schema of the preparation before passing the mucoperiosteal flap. The part of the mucoperiosteal flap under the tunnel is denuded (the part containing the small circles in the schema). The mucosa between the fistula and the mucoperiosteal flap is ablated under the periosteum for passing the mucoperiosteal flap (the oblique line in the schema). g Passing the mucoperiosteal flap through the tunnel. The deformation or floating of the proximal region of the mucoperiosteal flap is minimized. h The schema of passing the mucoperiosteal flap through the tunnel and suturing the mucoperiosteal flap
Follow-Up
The circulation of the mucoperiosteal flap was sufficient, and the mucoperiosteal flap was completely engrafted. Appropriate tension of the mucoperiosteal flap was obtained by making the tunnel and seemed to contribute to the stability of flap. Ruptured sutures and wound infection did not occur. Two months after surgery, the mucoperiosteal flap donor site where we placed the artificial dermis was epithelized (Fig. 5). At 2 weeks, the postoperative CT image showed signs of improvement, and at 2 months, it showed completely improved sinusitis (Fig. 6).
Fig. 5.
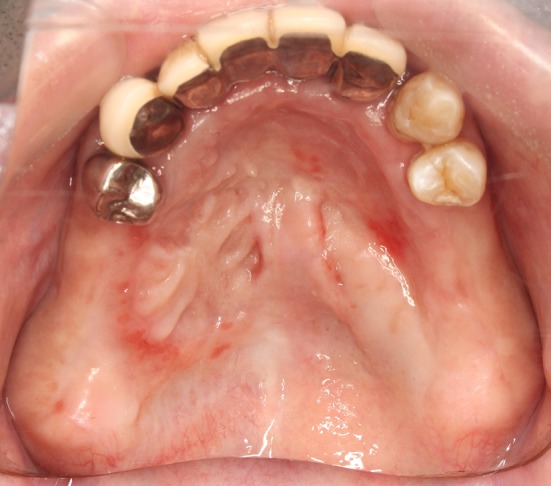
Two months after the closure operation. The mucoperiosteal flap is completely engrafted. The donor site where we placed the artificial dermis is also epithelized
Fig. 6.

The postoperative computed tomography image shows that the maxillary sinusitis is cured
Case 2
A 69-year-old woman had an oroantral fistula after a first molar tooth extraction (Fig. 7). The bone defect was 10 mm in diameter, and we confirmed maxillary sinusitis on CT imaging (Fig. 8). We refreshed the mucosa of the rim of the fistula and tried a simple suture closure. However, because the fistula reappeared, we planned a closure operation using a mucoperiosteal flap pedicled with the greater palatine vessels.
Fig. 7.
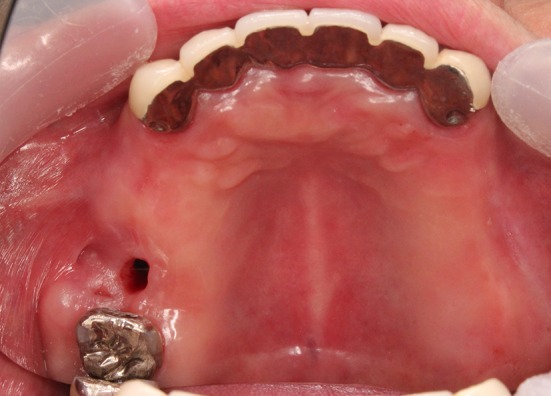
Image obtained before closure of the palatal fistula in case 2. The fistula occurred after the first molar tooth extraction
Fig. 8.
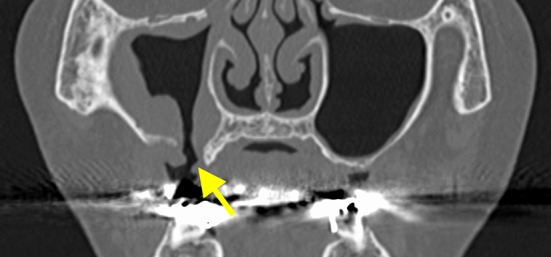
The preclosure operative coronal section computed tomography image shows right maxillary sinusitis. Hypertrophy of the consecutive mucous membrane of the maxillary sinus is detected from the fistula (the arrow indicates the fistula)
Surgical Technique
We identified the greater palatine vessel by Doppler acouophone and designed a mucoperiosteal flap (Fig. 9a, b). The mucosa of the circumference of the fistula was refreshed. We ablated the mucoperiosteum between the fistula and the mucoperiosteal flap, and passed this flap under the ablated mucoperiosteum as a tunnel (Fig. 9c–f). We placed an artificial dermis on the donor site.
Fig. 9.
a The drawing of the incision line. b The schema of the flap design. The pedicle part of mucoperiosteal flap is designed moderately thin. c Elevation of the mucoperiosteal flap. d The schema of mucoperiosteal flap elevation and preparation before passing the mucoperiosteal flap. The perifistular mucosa is denuded. The pedicle part of mucoperiosteal flap is also denuded (the part containing the small circles in the schema). The tunnel under the mucoperiosteum is formed (the oblique line in the schema). e Passing the mucoperiosteal flap through the tunnel. The deformation or floating of the proximal region of the mucoperiosteal flap is minimized. f The schema of passing the mucoperiosteal flap through the tunnel and suturing the mucoperiosteal flap
Follow-Up
The mucoperiosteal flap was completely engrafted. There were no ruptured sutures or wound infection. Two months later, we confirmed the complete epithelization of the artificial dermis in the area (Fig. 10). The manifestation of maxillary sinusitis also improved.
Fig. 10.
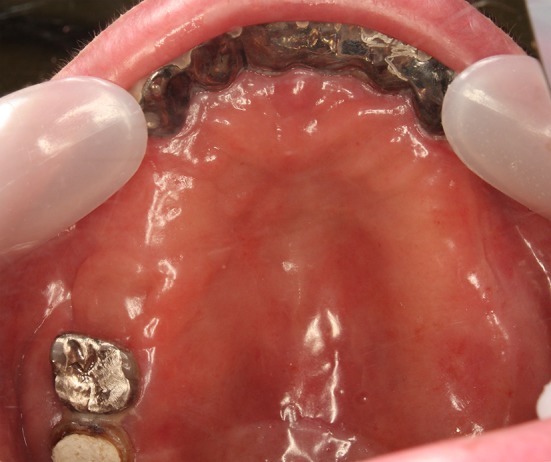
Two months after the closure operation. The mucoperiosteal flap was completely engrafted. We confirmed the complete epithelization of the donor part
Discussion
A palatal fistula, even if it is small, causes dysphagia and dyslalia because of the escape of food and the entry of air into the maxillary antrum and the nasal cavity. A prosthesis or surgical treatment is used to close the fistula. An appropriate method should be used, depending on the situation. In Case 1, we had employed a prosthetic intervention for the fistula for approximately 2 years. However, insertion of a prosthesis is troublesome and frequent adjustment may be necessary. Because of the complication of maxillary sinusitis, we performed palatal closure of the fistula. Closure techniques include the use of a tongue flap, buccal advancement flap, palatal rotation or transposition flap with pedicle, or free vascularized flap. The tongue flap has a stable circulation and may be a reliable flap [5]. In Case 1, the greater palatine vessels on the diseased side were included in the resection of the tumor, and the palate fistula circumference healed as a scar. Therefore, a tongue flap may not be obtained with sufficient vascular flow from the palatal substance of the circumference if a fistula existed in the front or on the lateral side of the palate; however, in Case 1, the fistula was present in the median post [6].
The free flap is not the first choice from the viewpoint of the invasion of the donor in such a small fistula. We believe that it is a suitable tissue for the transplantation of the same or similar modality in the defect, and there is little damage to the harvest area [7, 8]; a grafted mucoperiosteal flap was therefore the first choice. A palatal mucoperiosteal flap pedicled by the greater palatine vessels is conventionally designed without thinning the vascular pedicle part (Fig. 11a, c). The palatal mucosa is thick, and the operability of a shift in the mucoperiosteal flap is not good. Shifting of the mucosal flap is often complicated. With a shift, deformation and floating often occur in the proximal region of the flap (Fig. 11b, d). The deformation is minimized if the stem is slimmed as much as possible. However, the pedicle may be damaged if it is slimmed too much. We confirmed the path of the greater palatine artery by using Doppler acouophone. Using the Doppler auscultation method, we could confirm vascular running surely, easily, and noninvasively. Therefore, we slimmed the proximal region of the pedicle to control deformation. If there is slight deformation of the mucoperiosteal flap, it comes into closer contact with the bones. In addition, because flotation and upsurge are minimized in the proximal region of the mucoperiosteal flap, postoperative discomfort can be relieved.
Fig. 11.
a, c The schema of the mucoperiosteal flap using the traditional approach. The pedicle part is widened. b, d At the shift of the mucoperiosteal flap, deformation easily occurs in the flap (indicated by the arrow) and the axial part of the flap easily floats
To acquire the distance of the mucoperiosteal flap, it was necessary to design the distal portion of the flap from the incisive foramen across the median to turn it primarily around the greater palatine foramen. We were concerned about the circulation, but we were able to confirm sufficient distal blood flow after the harvest of the mucoperiosteal flap.
The mucosa remains between the perifistular-trimmed mucosa and the slimmed mucoperiosteal flap. When shifting occurs in the mucoperiosteal flap, the residual mucosal area overlaps with this flap. We denuded the mucoperiosteal flap and ablated the residual mucoperiosteal bottom and pulled the flap under this. If the proximal region of the mucoperiosteal flap deforms easily when the shifting occurs through the mucoperiosteal tunnel, the approximal surfaces of the bones and the mucoperiosteal flap increase. In addition, the proximal region of the mucoperiosteal flap is moderately suppressed and does not float up, and the mucoperiosteal flap seems to be well anchored.
Conclusion
We successfully closed an oroantral fistula with a hard palatal mucoperiosteal flap pedicled with the greater palatine vessels. The flap is passed through a mucoperiosteal tunnel and obtains good fructification.
References
- 1.Yilmaz T, Suslu AE, Gursel B. Treatment of oroantral fistula: experience with 27 cases. Am J Otolaryngol. 2003;24:221–223. doi: 10.1016/S0196-0709(03)00027-9. [DOI] [PubMed] [Google Scholar]
- 2.Visscher SH, van Minnen B, Bos RR. Closure of oroantral communications: a review of literature. J Oral Maxillofac Surg. 2010;68:1384–1391. doi: 10.1016/j.joms.2009.07.044. [DOI] [PubMed] [Google Scholar]
- 3.Lazow SK. Surgical management of the oroantral fistula: flap procedures. Oper Tech Otolaryngol Head Neck Surg. 1999;10:148–152. doi: 10.1016/S1043-1810(99)80037-2. [DOI] [Google Scholar]
- 4.Singh J, Prasad K, Lalitha RM, Ranganath K. Buccal pad of fat and its applications in oral and maxillofacial surgery: a review of published literature (February) 2004 to (July) 2009. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110:698–705. doi: 10.1016/j.tripleo.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 5.Kim YK, Yeo HH, Kim SG. Use of the tongue flap for intraoral reconstruction: a report of 16 cases. J Oral Maxillofac Surg. 1998;56:716–719. doi: 10.1016/S0278-2391(98)90803-5. [DOI] [PubMed] [Google Scholar]
- 6.Nakakita N, Maeda K, Ando S, Ojimi H, Utsugi R. Use of a buccal musculomucosal flap to close palatal fistulae after cleft palate repair. Br J Plast Surg. 1990;43:452–456. doi: 10.1016/0007-1226(90)90012-O. [DOI] [PubMed] [Google Scholar]
- 7.Ito O, Suzuki S, Park S, Kawazoe T, Sato M, Saso Y, Iwasaki Y, Hata Y. Eyelid reconstruction using a hard palate mucoperiosteal graft combined with a V-Y subcutaneously pedicled flap. Br J Plast Surg. 2001;54:106–111. doi: 10.1054/bjps.2000.3480. [DOI] [PubMed] [Google Scholar]
- 8.Kim JW, Kikkawa DO, Lemke BN. Donor site complications of hard palate mucosal grafting. Ophthal Plast Reconstr Surg. 1997;13:36–39. doi: 10.1097/00002341-199703000-00007. [DOI] [PubMed] [Google Scholar]