Summary
The majority of proteins in eukaryote cells are subjected to amino-terminal acetylation. This co-translational modification can affect the stability of a protein and also regulate its biological function. Amino-terminally acetylated recombinant proteins cannot be produced using prokaryote expression systems, such as E. coli, as these cells lack the appropriate N-α-terminal acetyltransferase complexes. Here we describe a simple protocol that allows the recombinant expression and purification of NatB dependent amino-terminally acetylated proteins from E. coli.
Keywords: NatB, Amino-terminal acetylation, N-α-terminal acetyltransferases, E. coli, fission yeast, recombinant protein
Introduction
Prokaryotes, such as E. coli, are commonly the cell type of choice for recombinant protein production during research and biomedical applications, as they provide a cheap and simple system for producing large quantities of protein. However, one drawback is that these cells lack the necessary machinery to undertaken post-translational modifications of proteins, such as amino-terminal acetylation, that normally occur within a eukaryote cell.
N-terminal acetylation is one of the most common modifications of proteins in eukaryotes, which involves the transfer of an acetyl group from acetyl coenzyme A to the amino-terminal amino acid of a protein [1]. The addition of the acetyl group to the N-terminal residue neutralizes positive charges which can affect the function and stability of the protein, as well as regulate either interactions with other proteins or further post translational modifications such as phosphorylation (e.g. Troponin T) or ubiquitination.
The acetylation of amino terminal amino acids is catalyzed by a group of protein complexes called N-α-terminal acetyltransferases (NATs). The NATs have been extensively studied in the budding yeast Saccharomyces cerevisiae, in which three major classes of NAT have been identified; NatA, NatB and NatC. These complexes have since been shown to be conserved and acetylate the majority of the human proteome [2]. The NatB complex consists of two subunits: a 23kDa catalytic subunit, Naa20, and a 92 kDa auxiliary subunit, Naa25 [3]. NatB target proteins always have an N-terminal methionine residue, which is followed by an acidic or arginine residue and previous studies have shown that all proteins with these sequences are acetylated [1].
Here we describe a simple method developed in this lab for generating amino-terminally acetylated recombinant proteins in E. coli cells [4]. By co-expressing genes encoding for proteins that are normally substrates of the NatB N-α-acetyltransferase complex (i.e. protein sequence starts with M-D-, M-E-, M-N-) together with genes encoding for the fission yeast NatB complex, it is possible to produce large quantities of amino-terminally acetylated proteins in E. coli with significant cost time befits over eukaryote expression and chemical acetylation alternatives.
2. Materials
LB Medium (1 litre): 10 g Tryptone; 10 g Sodium Chloride; 5 g Yeast Extract; 20 g Agar (if solid medium required). Make up to 1 litre with distilled water and autoclave for 15 min. Antibiotics added to medium after autoclaving and cooling.
NZY Medium (1 litre): 10 g NZ Amine Casein Hydrolysate; 5 g Sodium Chloride; 5 g Yeast Extract. Make up to 1 litre with distilled water and autoclave for 15 min. The following were added after autoclaving and cooling, and just prior to use: 12.5 ml of sterile 1 M Magnesium Chloride; 12.5 ml of sterile 1 M Magnesium Sulphate; 20 ml of sterile 20% (w/v) Glucose; appropriate antibiotics.
Antibiotics: For every ml media volume add 1 μl from the appropriate antibiotic stock solutions which are made up at the following concentrations and can be stored in 1 ml aliquots at −20 °C: 50 mg/ml Ampicillin (dissolved in H2O); 38 mg/ml Chloramphenicol (dissolved in ethanol); 50 mg/ml of Kanamycin (dissolved in H2O).
SOC Medium (1 litre): 20 g Tryptone; 5g Yeast extract; 2ml of 5 M Sodium Chloride; 2.5 ml of 1 M Potassium Chloride; 10 ml of 1 M Magnesium Chloride; 10 ml of 1 M Magnesium Sulphate; 30 ml of 1 M Glucose.
E.coli expression strain. e.g. BL21 DE3.
pNatB plasmid: pACYCduet-naa20+-naa25+. See Note 1.
3. Methods
3.1: Preparation of BL21-DE3-pNatB competent cells
Use an aliquot of the pNatB plasmid to transform a lab stock of BL21-DE3 E.coli competent cells to generate the BL21-DE3-pNatB strain.
Set up a 5 ml overnight shaking (220 rpm) culture from a single colony inoculum of BL21-DE3 in LB media supplemented with chloramphenicol.
Add 1 ml of this culture to 0.5 ml of sterile 60% v/v glycerol. Then mix and transfer to a 2 ml cryotube before storing in a - 80 °C freezer.
Streak out BL21-DE3-pNatB E. coli cells from the - 80°C stocks onto a LB + chloramphenicol plate and incubate overnight at 37 °C.
Grow a 5 ml overnight shaking (220 rpm) culture from a single colony inoculum of the BL21-DE3-pNatB cells in LB media supplemented with chloramphenicol.
The next morning use 0.5 ml of this pre-culture to inoculate 50 ml of fresh LB medium (+ chloramphenicol) within a sterile 250 ml Erlenmeyer flask.
Grow cells in a shaking (180 rpm) incubator at 37 °C until the culture reaches a 578nm optical density of 0.6 to 0.8.
Cool the cells on ice for 10 minutes, before centrifuging at 2,700 RCF at 4 °C for 10 minutes.
Place 0.5 ml microfuge tubes on dry ice to chill for aliquots in step 11.
Gently re-suspend the pelleted cells in 10 ml of sterile solution containing 0.1M CaCl2 10% glycerol. Incubate on ice for 15 minutes.
Centrifuge cells at 2,700 RCF at 4 °C for 10 minutes and re-suspend the pelleted cells in 1 ml sterile of cold 0.1 M CaCl2 10% Glycerol solution. Dispense competent cells as 50 μl aliquots into microfuge tubes (which have been resting on dry ice) and then store at - 80 °C.
3.2: Transformation of BL21-DE3-pNatB cells
Defrost an aliquot of BL21-DE3-pNatB competent cells on ice.
Add up to 5 μl of the plasmid from which the protein to be acetylated can be expressed, and mix by gently stirring with a pipette tip.
Incubate cells on ice for 20 min before subjecting to a 90 sec heat shock at 42 °C, and then return immediately onto ice for 2 min.
Add 200 μl of SOC medium to the cells and incubate at 37 °C with shaking (220 rpm) for 1 hr.
Plate cells onto LB agar plate supplemented with chloramphenicol and appropriate antibiotic for plasmid containing gene encoding for amino-terminal acetylation target protein.
3.3: Expression of acetylated recombinant proteins in E. coli
-
Set up a culture for protein expression as soon as colonies have formed on the BL21-DE3-pNatB transformation plate. (See Note 3).
If protein is not toxic to E.coli cells go to go through steps 2-4. However, if protein is toxic, or you find its expression is repressed in the cell go straight to step 5.
Set up a 50ml starter culture in NZY media (See Note 4) supplemented with appropriate antibiotics in a 250 ml Erlenmeyer flask using a single colony inoculum from the BL21-DE3-pNatB transformation plate.
Grow starter culture overnight at 37 °C with shaking (See Note 5)
Inoculate a larger volume (i.e. 1 litre) culture using 1ml inoculum from the starter culture for every 200 ml culture volume (i.e. for a 1 l culture use a 5 ml inoculum). Culture cells at 37 °C with vigorous shaking (220 rpm). Go straight to step 6.
Inoculate 200 ml of NZY supplemented with appropriate antibiotics in a 500 ml Erlenmeyer flask using a single colony inoculum from the BL21-DE3-pNatB transformation plate from step 1.
Culture at 37 °C (See Note 6) until the 595nm optical density reaches value between 0.4 and 0.5.
Induce expression of NatB complex by adding 1 ml of 100 mg/ml IPTG / litre culture volume.
If gene expressing target protein is not under control of T7 promoter, induce expression by addition of appropriate molecule. (See Note 6).
Take samples at hourly time points to check production. (See Note 7)
Grow cells for 2-4 hours from induction and then harvest by centrifugation at 4,000 RCF at 4 °C for 30 minutes. Store pellets at −20 °C until protein purification is undertaken.
3.4: Confirmation of amino-terminal acetylation
Purify target protein using the same method as you normally would use for the unacetylated form.
It is worth running a gel to compare the migration of equivalent amounts of unacetylated and acetylated proteins, as differences in migration can give an indication of acetylation efficiency. Acetylation can affect stability of amino-terminal secondary structure, which can be reflected in the ability of a protein to migrate through an SDS-PAGE matrix [5].
Determine size of potentially acetylated protein using electron-spray mass spectroscopy, and compare size with unacetylated protein. Acetylation will increase protein mass by 42 Daltons.
If appropriate you may wish to test acetylation using a functional assay for the target protein, comparing with unacetylated and purified endogenous acetylated protein.
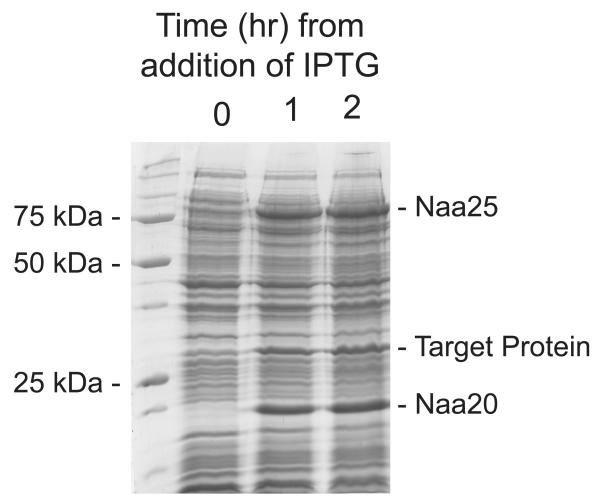
Figure 1.
Coomassie stained SDS-PAGE gel showing induction of the NatB complex and a target protein. Once a fresh culture of BL21-DE3-pNatB pTarget cells had reached an OD595nm of 0.4, IPTG was added to a final concentration 100 μg/ml. Samples of cells were taken at 0, 1, and 2 hours from addition of IPTG, boiled in SDS buffer and run on an SDS-PAGE gel. Intense protein bands can be observed in the each of the post induction samples which have migrated with masses corresponding to the NatB catalytic subunit, Naa20 (20.5 kDa), the NatB regulatory subunit, Naa25 (92.4 kDa) and the target protein.
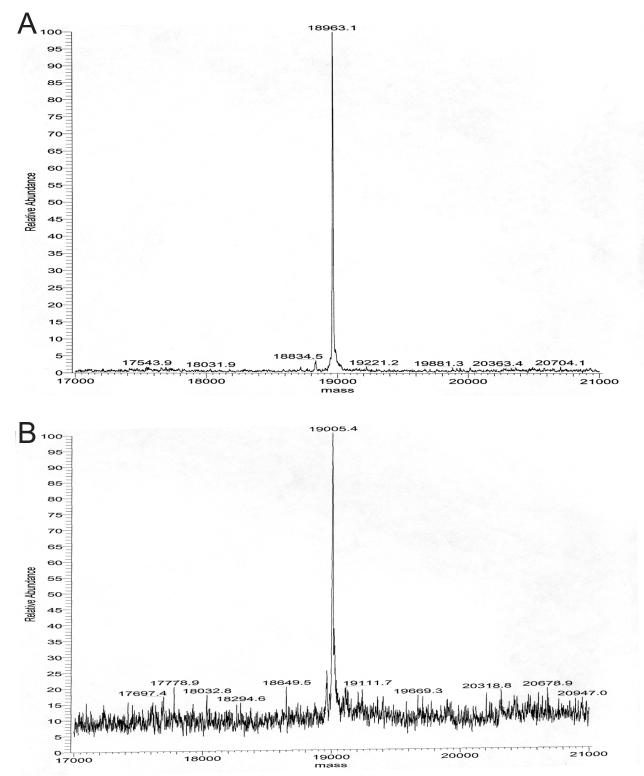
Figure 2.
Deconvolved mass spectra of fission yeast tropomyosin (Cdc8) purified from E. coli either lacking (a) or containing (b) the pNatB plasmid. Using established methods [5] untagged Cdc8 was purified from either (a) BL21-DE3 pJC20cdc8, or (b) BL21-DE3-pNatB pJC20cdc8 cells. Subsequent electron-spray mass spectroscopy of the purified proteins confirmed all of the Cdc8 purified from the BL21-DE3-pNatB cells had an additional 42 Da mass (b), the expected mass of the acetyl group, when compared to Cdc8 from BL21-DE3 cells (a). Amino terminal acetylation was also confirmed by functional assays, western blot analysis and the inability to sequence the peptide from the amino terminus.
Acknowledgement
Work in these labs is supported by the BBSRC, Royal Society and Wellcome Trust.
4. Notes
The pNatB (pACYCduet-naa20+-naa25+) plasmid [4], allowing co-expression of the catalytic (Naa20) and regulatory (Naa25) subunits of the fission yeast NatB complex, is freely available upon request to researchers working in non-profit organisations. Send requests to: d.p.mulvihill@kent.ac.uk.
Ensure plasmid from which target protein is expressed confers resistance to an antibiotic which is distinct from that of pNatB (i.e. an antibiotic other than chloramphenicol).
In our experience, expression and acetylation is most efficient when freshly transformed cells are used.
Expression and acetylation efficiency can be affected by choice of media. For many proteins yield is improved by growing cells in NZY when compared to LB media. However we have received reports that in some cases minimal media improves the solubility and acetylation efficiency for some proteins.
We have found that even expression of minimal quantities of some acetylated proteins can be toxic to the E. coli cell (i.e. from leaky expression from many un-induced promoters) and prolonged growth / culture leads to dramatic reduction in protein yields. Therefore for many proteins acetylation and expression yield can be dramatically improved by leaving out the starter culture step and instead inoculating a large volume culture with a single colony from a plate of freshly transformed BL21-DE3-pNatB cells.
Solubility and expression of acetylated proteins is sometimes improved by growing bacterial cells at lower temperatures (i.e. 20 or 25 °C). Undertake small-scale test inductions at different temperatures if you need to improved yield of acetylated protein.
Using T7 promoters to control expression of target protein and NatB complex allows simultaneous and efficient induction. However sequential induction may be desirable if acetylation yield is low. Therefore you may wish to consider putting the target gene under the control of a different promoter (e.g. arabinose) and induce its expression ~ 30 min after the NatB complex has been expressed.
Confirm that the expression of the NatB complex does not abolish the expression of the target protein. You may find that expression is reduced, however this is to be expected.
This method has been used successfully to produce recombinant amino-terminally acetylated proteins from E. coli with each of the three NatB recognition sequences (i.e. M-E-, M-D-, and M-N-) [4] + unpublished results).
If you find you normally obtain higher protein yields when expressing your target protein of interest in a specific E.coli strain (e.g. BL21-Star or Rosetta DE3), it is likely you will obtain higher yield of the acetylated form in the same strain co-transformed with the pNatB plasmid.
Remember, if you are using affinity tags during the purification of your protein, ensure you tag the protein at the carboxyl terminus and not at the amino terminus you are attempting to acetylate.
References
- 1.Polevoda B, Sherman F. N-terminal acetyltransferases and sequence requirements for N-terminal acetylation of eukaryotic proteins. J Mol Biol. 2003;325:595–622. doi: 10.1016/s0022-2836(02)01269-x. [DOI] [PubMed] [Google Scholar]
- 2.Arnesen T, Van Damme P, Polevoda B, Helsens K, Evjenth R, Colaert N, Varhaug JE, Vandekerckhove J, Lillehaug JR, Sherman F, Gevaert K. Proteomics analyses reveal the evolutionary conservation and divergence of N-terminal acetyltransferases from yeast and humans. Proc Natl Acad Sci U S A. 2009;106:8157–8162. doi: 10.1073/pnas.0901931106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polevoda B, Arnesen T, Sherman F. A synopsis of eukaryotic Nalpha-terminal acetyltransferases: nomenclature, subunits and substrates. BMC Proc. 2009;3(Suppl 6):S2. doi: 10.1186/1753-6561-3-S6-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson M, Coulton AT, Geeves MA, Mulvihill DP. Targeted amino-terminal acetylation of recombinant proteins in E. coli. PLoS One. 2010;5:e15801. doi: 10.1371/journal.pone.0015801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skoumpla K, Coulton AT, Lehman W, Geeves MA, Mulvihill DP. Acetylation regulates tropomyosin function in the fission yeast Schizosaccharomyces pombe. J Cell Sci. 2007;120:1635–1645. doi: 10.1242/jcs.001115. [DOI] [PubMed] [Google Scholar]