Abstract
It was previously demonstrated that there are no indigenous strains of Bradyrhizobium japonicum forming nitrogen-fixing root nodule symbioses with soybean plants in arable field soils in Poland. However, bacteria currently classified within this species are present (together with Bradyrhizobium canariense) as indigenous populations of strains specific for nodulation of legumes in the Genisteae tribe. These rhizobia, infecting legumes such as lupins, are well established in Polish soils. The studies described here were based on soybean nodulation field experiments, established at the Poznań University of Life Sciences Experiment Station in Gorzyń, Poland, and initiated in the spring of 1994. Long-term research was then conducted in order to study the relation between B. japonicum USDA 110 and USDA 123, introduced together into the same location, where no soybean rhizobia were earlier detected, and nodulation and competitive success were followed over time. Here we report the extra-long-term saprophytic survival of B. japonicum strains nodulating soybeans that were introduced as inoculants 20 years earlier and where soybeans were not grown for the next 17 years. The strains remained viable and symbiotically competent, and molecular and immunochemical methods showed that the strains were undistinguishable from the original inoculum strains USDA 110 and USDA 123. We also show that the strains had balanced numbers and their mobility in soil was low. To our knowledge, this is the first report showing the extra-long-term persistence of soybean-nodulating strains introduced into Polish soils and the first analyzing the long-term competitive relations of USDA 110 and USDA 123 after the two strains, neither of which was native, were introduced into the environment almost 2 decades ago.
INTRODUCTION
Soybean [Glycine max (L.) Merr.] is still an emerging crop in Poland, and soybeans are presently being planted only on about 2,800 ha. However, there has been a slow but systematic increase in production, and this is consistent with estimations made earlier by soybean breeders (1). Grown only on a small scale in a few isolated locations, soybeans still remain interesting for farmers, and their cultivation is considered a potential supplemental source of plant proteins to feed to livestock.
Despite this, there are several registered soybean cultivars well suited to local environmental conditions, including Aldana and Augusta (from Poland), Merlin and Lissabon (from Austria), and Annushka and Mavka (from Ukraine). Recently, qualified seed production of the cultivars mentioned above has dramatically increased from 2 ha in 2009 to almost 600 ha in 2013, which further indicates farmers' interest in soybean production in Poland (Jerzy Nawracała, personal communication).
Relatively little information concerning the presence of symbiotic, nitrogen-fixing bradyrhizobia on soybeans in Poland is available. There are no indigenous Bradyrhizobium japonicum strains forming nitrogen-fixing, root nodule symbioses with soybeans in arable lands in Poland, and thus far, only a few local populations have been identified in a few locations where inocula were applied for growing soybeans (1). It should be mentioned, however, that bacteria initially classified as B. japonicum are present (together with Bradyrhizobium canariense) as indigenous populations of strains specific for legumes of the tribe Genisteae. These rhizobia, infecting lupins, are well established in Polish soils. They have colonized the area, presumably migrating together with their hosts from the Mediterranean after the retreat of glaciers during the last 10,000 years (2). These bacteria, however, cannot nodulate soybeans.
The lack of naturally occurring soybean microsymbionts in Polish soils provides unique opportunities to study the persistence, stability, competitiveness, and dispersal of bradyrhizobia introduced as inocula. It also opens the possibility to analyze the survival of rhizobia as soil saprophytes in the absence of host plants. This issue is particularly relevant since, despite the fact that rhizobia do not form resting-stage spores (3), they are still characterized as persistent autochthonous microbes in soils where they have been introduced. Moreover, there have been relatively few studies on the long-term survival of rhizobia in the absence of a legume host and in foreign environments and those without naturally occurring host plants. For soybeans, a few of these studies were done in France (4, 5) and Brazil (6, 7), but in some there was repeated inoculation due to die off.
The competitiveness of strains infecting soybeans may be strongly dependent on environmental conditions, and if they are indigenous, strains are likely to be more fit than more recent bacterial additions to soils to interact with host plant genotypes. Based on these assumptions, we designed experiments to study the competitive relationship between B. japonicum strains USDA 110 and USDA 123, introduced together into a field location where no soybean rhizobia were previously detected. While it has been shown that USDA 123 outcompeted USDA 110 in North American soils with strains comprised of several Bradyrhizobium serotypes (8–10), no information on how these bacteria compete for nodulation in natural field soils devoid of indigenous soybean-nodulating bradyrhizobia is available.
Here we report the results of field experiments initiated in 1994 where soybeans were sown into the soil after inoculation with B. japonicum USDA 123 and USDA 110, either separately or as a mixed inoculant. Soybeans were then grown in this location for the subsequent 3 years, and the field was used for growing other crops for the next 17 years. After this time, nodulation results showed the extra-long-term saprophytic survival of B. japonicum strains nodulating soybeans and the long-term competitiveness of USDA 110 and USDA 123 in natural soils initially devoid of soybean-nodulating bradyrhizobia.
MATERIALS AND METHODS
Initial field experiments from 1994 to 1997.
A soybean nodulation field experiment was established at the Poznań University of Life Sciences Experiment Station in Gorzyń, Poland, about 80 km northwest from Poznań, Poland, in the spring of 1994 on soils characterized as arenic hapludalfs or albic luvisols (arenic) (11, 12). The selected field site was free of indigenous strains capable of nodulating soybeans (1; unpublished data). Prior to the first sowing of surface-sterilized soybean [G. max (L.) Merr. cv Naviko] seeds, separate plots were inoculated with strain USDA 123, strain USDA 110, or a mixture containing equal cell concentrations of both strains. Inoculation was performed using a peat-based inoculum as previously described (13). The seeds were inoculated using peat inoculum (100 g of inoculum per 1 kg of seeds) and gum arabic as a sticker. The mixed inoculation was performed using equal amounts of USDA 110 and USDA 123 at a final concentration of 2 × 106 cells per seed. In the case of mixed inoculation, half of this number was USDA 110 and half was USDA 123. An uninoculated control plot was also included in the experiment, and each experimental plot was replicated four times (see Fig. S1 in the supplemental material). No inoculations were performed during subsequent experimental seasons. The experimental field is a part of a larger experimental area where field experiments had been conducted for at least 50 years. The crops cultivated in this particular part of the experimental station were wheat, barley, triticale, potatoes, and yellow and narrow-leafed lupins.
Nodulation of soybean plants was analyzed 6 weeks after germination, and both the number and fresh weight of the nodules were measured. The strain occupancy of the nodules was determined by using an immuno-spot-blot method and cross-adsorbed, strain-specific antibodies as previously described (1, 14). The experiment was repeated over four subsequent seasons, until 1997, and after that time, soybeans were not grown at this location.
Detection of soybean-nodulating bradyrhizobia 17 to 20 years after their introduction into Gorzyń soil.
Twelve soil samples were obtained from the original field experiment site in September 2010. Surface-sterilized soybean [G. max (L.) Merr. cv. Augusta] seeds were sown in duplicate pots filled with these soil samples. Four seeds were sown in each pot. The plants were grown in a greenhouse for 5 weeks.
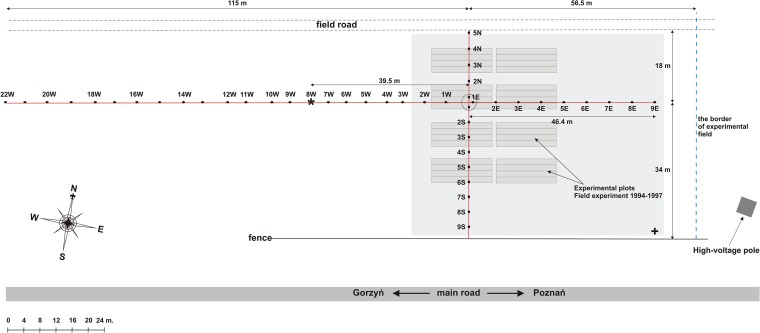
Independently, a trap host approach (15) was used directly in the field to determine the presence of soybean-nodulating bacteria and to determine the distance of their dispersal from their release site. For this purpose, in the spring of 2011, surface-sterilized soybean [G. max (L.) Merr. cv. Augusta] seeds were sown in the spots, forming two perpendicular lines of trap hosts across the site used for the former field experiment in 1994. The plants were harvested, and root nodules were collected 6 weeks after sowing. The experimental design is shown in Fig. 1. In 2014, soybeans were grown on the entire area of the initial experimental site, as indicated above.
FIG 1.
Design and topography of the field experiment of 2011. A number of plant traps were established in order to determine the distance from the original experimental site (gray squares) within which the nodulation of soybeans occurred. Plant traps were aligned east-west and north-south every 4 to 5 m and are marked with black dots, which are numbered starting from the crossing point (1E, 2E, etc.; 1W, 2W, etc.; 2S, 3N, etc.; and 2S, 3S, etc.). About 10 surface-sterilized seeds were sown in each spot. The area shaded light gray indicates the part of the field where soybeans were sown in 2014. An asterisk (at 8W) marks the westward-most spot where soybean bradyrhizobia were detected. The cross indicates the position of Global Positioning System coordinates, which are 52°33′59.3″N, 15°54′47.8″E.
Serological identification of bacteria from soybean root nodules.
Nodules were collected from plants grown in the greenhouse after exposure to soil taken from the field site in September 2010. Four plants were taken from each of 12 soil samples. All nodules from each plant were separately collected and dried at 60°C for 48 h. The 48 samples collectively produced 1,266 nodules, all of which were analyzed for the presence of strains USDA 123 and USDA 110 by using the immuno-spot-blot method and cross-adsorbed, strain-specific antibodies, as previously described (1, 14).
Isolation of bacteria from soybean root nodules.
Bacteria were obtained from the root nodule, which had been surface sterilized with 95% ethanol and 3% sodium hypochlorite, as described by Somasegaran and Hoben (15). Purified bacterial isolates were obtained by streaking nodule suspensions onto AG agar medium (16) containing 50 μl/ml cycloheximide, followed by two to three successive isolations for single pure colonies on the same medium.
DNA isolation and PCR template preparation.
Total genomic DNA was isolated from single bacterial colonies by using a GenElute bacterial genomic DNA kit (Sigma-Aldrich Corporation, St. Louis, MO, USA) following the manufacturer's instructions. Templates for PCR amplification were lysates of crushed soybean nodules stored in 25% glycerol at −80°C. A 50-μl aliquot of PrepMan Ultra buffer (Applied Biosystems, Grand Island, NY, USA) was added to 50 μl of the crushed suspension. The resulting mixture was heated for 5 min at 95°C and centrifuged for 5 min at 14,000 rpm, and the supernatant was used as a template for PCR.
Molecular identification of bacteria from soybean root nodules.
Genomic DNA preparations were obtained from bacteria isolated from nodules from the September 2010 experiment. Three nodules were taken from each soil sample, giving 36 DNA samples. Three independent PCR analyses were performed with DNA from each isolate for the (i) identification of USDA 110-specific sequence (using strain-specific primers), (ii) analysis of recA gene sequences in order to distinguish between B. japonicum strains USDA 123 and USDA 110, and (iii) analysis of strain diversity using RP01 primers.
A B. japonicum USDA 110 strain-specific sequence was selected on the basis of an in silico search of the USDA 110 genome. Genomic positions 17139 to 18371 in the sequence with GenBank accession number BA000040.2 are apparently specific for B. japonicum strains USDA 110 and USDA 6. The PCR primers Bj110F (5′-TAGGTGATGGGATAACGCTCT-3′) and Bj110R (5′-GTCCAGCTTCGCGATCGACCGG-3′) were designed on the basis of this sequence (see Fig. S2 in the supplemental material). The primers amplify a 199-bp DNA fragment between genomic positions 17151 and 17331 encoding a protein of unknown function.
In order to reconfirm the identification of the USDA 110 genotype versus the USDA 123 genotype, a common phylogenetic marker, the sequence of recA, was used. The nucleotide sequences of the recA genes from USDA 110 and USDA 123 (collection strains) were determined. Alignment of their 559-bp-long fragments allowed design of specific forward primers recA110 (5′-GGGCTCGACATTGCGCTC-3′) and recA123 (5′-GGGCTCGACATTGCACTG-3′), whose sequences are complementary to the fragment from positions 135 to 151 of the recA sequence (see Fig. S3 in the supplemental material). A common reverse primer, recA549R (5′-TTGCGCAGCGCCTGGCTCAT-3′), specific for the conserved regions of many bradyrhizobia (nucleotide positions 530 to 549) was designed.
The verification of the specificity of the recA primers was done for a collection of USDA 110 and USDA 123 strains and for randomly selected strains isolated from Gorzyń soil. The diversity of isolates identified to be related to strain USDA 110 or USDA 123 was analyzed using PCR with primer RP01 (5′-AATTTTCAAGCGTCGTGCCA-3′) as described by Richardson et al. (17). The PCR was carried out using Allegro Taq DNA polymerase (Novazym, Poznań, Poland) under the following conditions: initial denaturation at 95°C for 2 min; 30 cycles of 30 s at 95°C, 30 s at 56°C for Bj110F, Bj110R, recA110, recA123, and recA549R or 52°C for RP01, and 1 min at 72°C; and a final elongation step of 5 min at 72°C. The PCR products were analyzed on 1% agarose gels.
Enumeration of bacteria by using MPN analyses.
Determination of the number of bacteria capable of nodulating soybeans was performed by using the most-probable-number (MPN) technique as described by Somasegaran and Hoben (15). Soil samples were collected in Gorzyń on 25 October 2012 and stored at 4°C. An equivalent of 100 g (dry mass) of soil (fresh weight, 109.4 g) was suspended in 900 ml of water, and the mixture was shaken intensively for 5 min. The resulting suspension was diluted, and 1 ml of each dilution was added to surface-sterilized soybean seeds sown in Leonard jar assemblies (15). Five replicates were used for each dilution, and a negative control consisting of uninoculated surface-sterilized seeds was used. Nodulation was recorded after 34 days, and MPNs were determined as previously described (15).
Enumeration of bacteria in soils using strain-specific FAs.
The identification and counting of the B. japonicum USDA 110 and USDA 123 cells were done using strain-specific fluorescent antibodies (FAs) as described by Somasegaran and Hoben (15) and Schmidt et al. (18). Two soil samples were analyzed, and two filters were prepared by filtering 10 ml of solution from each sample (see File S1 in the supplemental material). The number of bacteria visible in 30 microscopic fields on each filter was determined three times using a Zeiss Axiovert 200 inverted microscope with a 100× immersion lens and filter set 09 (excitation bandpass [BP], 450 to 490 nm; dichroic beam splitter Farbteiler [FT], 510; emission longpass [LP], 515 nm). No cross-reacting soil bacteria were observed.
Statistical analyses.
Results of all studies were evaluated for significance by using the R statistical software (version 3.1.2) (19). Analysis of variance tests were followed by post hoc analysis with Fisher's least-significant-difference (LSD) test from the Agricolae package (20), and the significance level was set at an α value of 0.05.
RESULTS
Detection of soybean-nodulating bacteria 20 years after their introduction into Gorzyń soil.
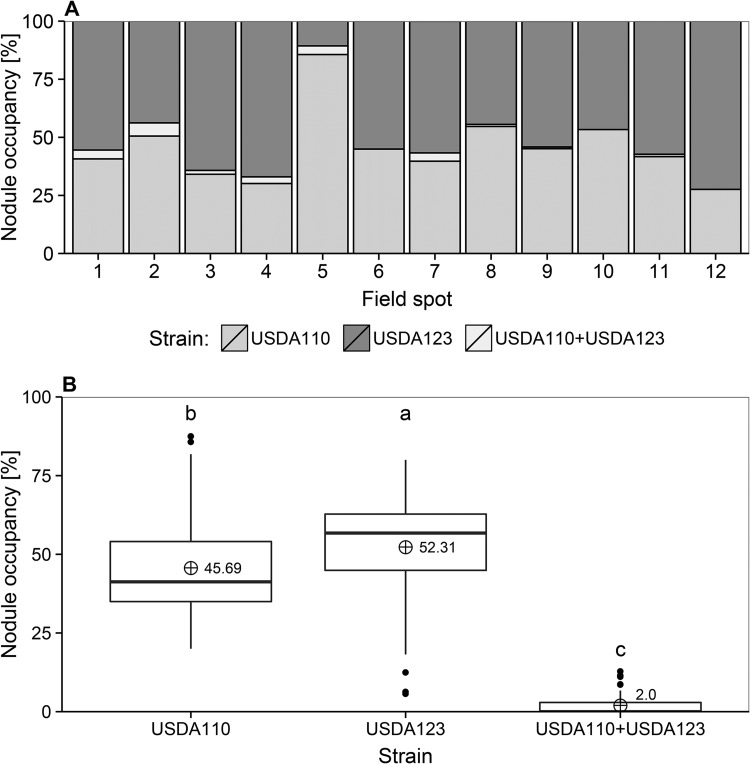
All soil samples taken from the exact same location where the field experiment was conducted in 1994 contained soybean rhizobia. Root nodules were found on all soybean plants grown in the greenhouse in 24 pots containing 12 soil samples. Results of immuno-spot-blot analyses revealed the presence of strains USDA 110 and USDA 123 in balanced numbers. The data shown in Fig. 2A indicate that USDA 123 prevailed in soils taken from five locations (locations [field spots] 1, 3, 4, 6, and 12), with considerable domination in location 12 (72.4%). The soil samples from locations 5, 8, and 10 contained more USDA 110 cells than USDA 123 cells. Location 5 had a significant prevalence of USDA 110 cells (85.6%). The remaining samples (from locations 2, 7, 9, and 11) contained about equal numbers of cells of both strains. Taken together, 587 nodules (46.4%) were occupied by strain USDA 110, 653 nodules (51.6%) were occupied by USDA 123, and 26 nodules (2%) contained both strains (Fig. 2B). Only three locations did not contain any doubly occupied nodules (see Table S1 in the supplemental material). Another 153 nodules were saved as crushed suspensions. Thirty-six suspensions, representing nodules from three plants from each of the 12 soil samples, were randomly chosen and used to obtain pure isolates. These isolates (UPP401 to UPP436) were stored in 50% glycerol at −80°C.
FIG 2.
Nodule occupancy of B. japonicum strains in the experimental field locations (A) and in the entire experimental field (B). Different lowercase letters (a, b, c) indicate significant differences among means (represented by crossed circles) according to Fisher's LSD test (LSD = 5.92; P < 0.05).
A plant-trap approach was used to determine the distance from the exact site of initial inoculation where bradyrhizobia could still be found. As shown in Fig. 1 and Table 1, successful nodulation was observed in all soil samples tested but not those located farther than about 40 m westward from the edge of the former experimental site. This result suggests that there was rather poor dispersal of the inoculum strains. In 2014, soybean cultivation was considered to serve as the ultimate confirmation of the presence of soybean-nodulating bradyrhizobia. Nodulation was detected within the entire experimental area. No further analyses were performed.
TABLE 1.
Identification of bacteria inducing nodules on soybean trap plants grown in the field locations shown in Fig. 1
| Location | No. of nodules analyzed | No. (%) of nodules containing strain: |
|
|---|---|---|---|
| USDA 110a | USDA 123b | ||
| 1N | 10 | 6 (60) | 4 (40) |
| 2N | 9 | 6 (66.7) | 3 (33.3) |
| 3N | 5 | 2 (40) | 3 (60) |
| 4N | 3 | 2 (66.7) | 1 (33.3) |
| 5N | 0 | ||
| Total | 27 | 16 | 11 |
| 1S | 9 | 0 | 9 (100) |
| 2S | 9 | 3 (33.3) | 6 (66.7) |
| 3S | 9 | 8 (88.9) | 1 (11.1) |
| 4S | 9 | 3 (33.3) | 6 (66.7) |
| 5S | 9 | 2(22.2) | 7(77.8) |
| 6S | 9 | 5 (55.5) | 4 (44.5) |
| 7S | 9 | 3 (33.3) | 6 (66.7) |
| 8S | 9 | 8 (88.9) | 1 (11.1) |
| 9S | 9 | 7 (77.8) | 2 (22.2) |
| Total | 81 | 39 | 42 |
| 1W | NDc | ||
| 2W | 9 | 6 (66.7) | 3 (33.3) |
| 3W | 9 | 7 (77.8) | 2 (22.2) |
| 4W | 9 | 7 (77.8) | 2 (22.2) |
| 5W | 6 | 2 (33.3) | 4 (66.7) |
| 6W | 9 | 5 (55.5) | 4 (44.5) |
| 7W | 1 | 1 | 0 |
| 8W | 3 | 2 | 1 |
| 9W | 0 | ||
| 10W–22W | 0 | ||
| Total | 46 | 33 | 16 |
| 1E | 9 | 4 (44.5) | 5 (55.5) |
| 2E | 9 | 1 (11.1) | 8 (88.9) |
| 3E | 9 | 1 (11.1) | 8 (88.9) |
| 4E | 9 | 3 (33.3) | 6 (66.7) |
| 5E | 9 | 2 (22.2) | 7 (77.8) |
| 6E | 9 | 8 (88.9) | 1 (11.1) |
| 7E | 9 | 8 (88.9) | 1 (11.1) |
| 8E | 9 | 9 (100) | 0 (0) |
| 9E | 9 | 9 (100) | 0 (0) |
| Total | 81 | 45 | 36 |
Identification is based on PCR analyses done using USDA 110-specific primers (Bj110F and Bj110R).
Bacteria which did not reveal the presence of a USDA 110-specific amplicon. The nucleotide sequences of recA from five randomly picked isolates were determined, showing their identity with USDA 123.
ND, not done (no plants germinated in this location).
The identification of strains occupying nodules was performed using the PCR assay with USDA 110-specific primers. The data in Table 1 show that both strains were found to be dispersed within the entire area, revealing a fairly balanced proportion of both bacterial genotypes.
Molecular identification of bacteria from soybean root nodules.
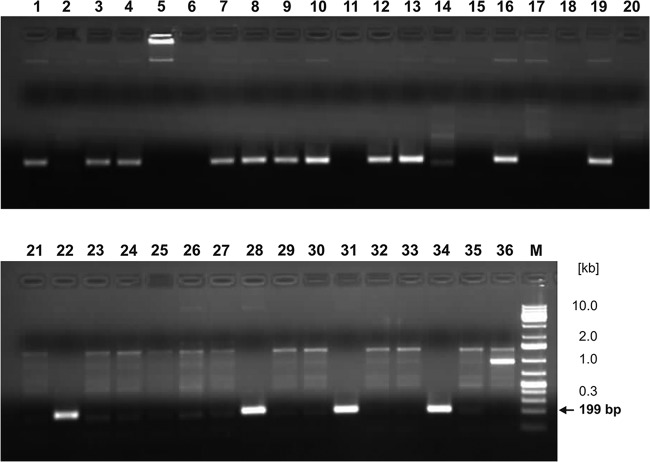
The identity and diversity of each of the 36 purified isolates were determined by using PCR and USDA 110 strain-specific and RP01 primers. Results verifying the specificity of the USDA 110-specific primers are shown in Fig. 3. The amplicon of the expected size of 199 bp appeared when lysed cells of B. japonicum USDA 110 were used as the template for PCR amplification (Fig. 3, lane 1). No specific products were generated with DNAs of the other bacteria tested, including B. japonicum USDA 123 (Fig. 3, lane 2) and lupin-nodulating strains B. japonicum UPP323 and UPP344 (2), Bradyrhizobium sp. strains UPP331 and UPP370, Bradyrhizobium sp. strain USDA 3045 (Lupinus), as well as Bradyrhizobium elkanii, resembling strain UPP372, and Bradyrhizobium yuanmingense, resembling strain UPP253. On the basis of these data, primers Bj110F and Bj110R were considered to be specific for B. japonicum strain USDA 110, and they were used to characterize purified isolates obtained from the root nodules of soybean plants grown in soil from Gorzyń. The PCR results in Fig. 4 show that 15 of 36 isolates (42%) could be presumed to be the descendants of the original inoculum strain B. japonicum USDA 110.
FIG 3.
The PCR amplification products obtained using primers Bj110F and Bj110R from the lysates of B. japonicum USDA 110 (lane 1), B. japonicum USDA 123 (lane 2), Bradyrhizobium sp. strain USDA 3045 (Lupinus) (lane 3), Bradyrhizobium sp. USDA 3045 (Lupinus) genomic DNA (lane 4), B. japonicum UPP323 (lane 5), Bradyrhizobium sp. UPP331 (lane 6), B. japonicum UPP344 (lane 7), B. elkanii UPP372 (lane 8), Bradyrhizobium sp. UPP370 (lane 9), and Bradyrhizobium yuanmingense UPP253 (lane 10). Lane M, molecular markers (DirectLoad wide-range DNA marker; Sigma, St. Louis, MO).
FIG 4.
PCR profiling, using primers Bj110F and Bj110R, of strains isolated from the nodules of soybeans grown in a greenhouse in pots filled with soil taken from the field site in Gorzyń on 23 September 2010. The particular lanes of the gels contain the amplification products obtained with the template of DNA from B. japonicum, as follows: 1, UPP401; 2, UPP402; 3, UPP403; 4, UPP404; 5, UPP405; 6, UPP406; 7, UPP407; 8, UPP408; 9, UPP409; 10, UPP410; 11, UPP411; 12, UPP412; 13, UPP413; 14, UPP414; 15, UPP415; 16, UPP416; 17, UPP417; 18, UPP418; 19, UPP419; 20, UPP420; 21, UPP421; 22, UPP422; 23, UPP423; 24, UPP424; 25, UPP425; 26, UPP426; 27, UPP427; 28, UPP428; 29, UPP429; 30, UPP430; 31, UPP431; 32, UPP432; 33, UPP433; 34, UPP434; 35, UPP435; and 36, UPP436. Lane M, molecular markers (DirectLoad wide-range DNA marker; Sigma, St. Louis, MO).
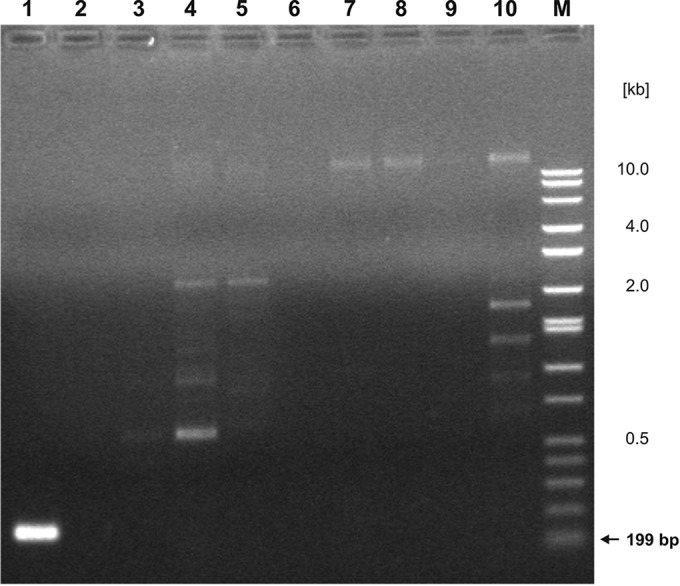
PCR amplifications done with recA-specific primers were used to reconfirm the identities of the B. japonicum isolates. The data in Fig. 5 show the specificity and usefulness of these primers. The DNA of the USDA 123 collection strain and isolates identified to be the presumed descendants of this strain (UPP405 and UPP406; Fig. 4; compare lanes 5 and 6) did not generate PCR amplification products when the recA110 primer was used (Fig. 4, lanes 4 to 6). Conversely, the recA123 primer did not produce PCR amplification products with template DNA from USDA 110 and its presumed descendants (UPP401 and UPP403).
FIG 5.
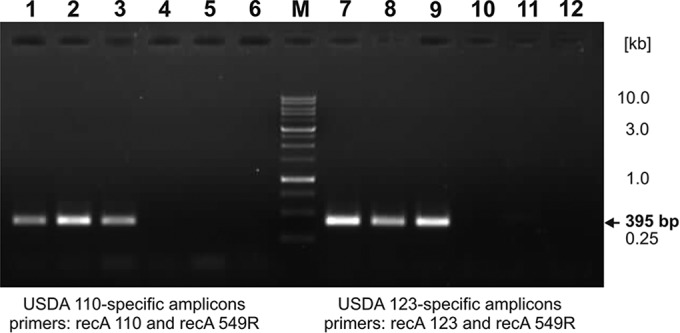
PCR profiles obtained using primers specific for the fragment of the recA gene. The lysates of bacteria of collection strains and soil isolates were used as DNA templates. Lanes 1 to 6 contain the PCR products obtained using primers recA110 and recA549R and the following strains: USDA 110 (collection strain) (lane 1) UPP401 (lane 2), UPP403 (lane 3), USDA 123 (collection strain) (lane 4), UPP405 (lane 1), and UPP406 (lane 6). Lanes 7 to 12 contain the PCR products obtained using primers recA123 and recA549R and the following strains: USDA 123 (collection strain) (lane 7), UPP405 (lane 8), UPP406 (lane 9), USDA 110 (collection strain) (lane 10), UPP401 (lane 11), and UPP403 (lane 12). Lane M, molecular markers (Nova 1-kb ladder; Novazym).
The diversity of isolates identified to be related to strain USDA 110 or USDA 123 was analyzed by using PCR with primer RP01 and appeared to be low, since only two types of profiles consistently representing the two strains identified were found in Fig. 6. Among the 36 strains analyzed, the profiles for strains UPP403, UPP404, UPP407, UPP408, UPP401, UPP409, UPP410, UPP412, UPP413, UPP416, UPP419, UPP422, UPP428, UPP431, and UPP434 (shown in lanes 1, 2, 5, 6, 9, 12, 13, 17, 18, 19, 20, 22, 28, 31, and 34, respectively) represented the profile typical for strain USDA 110. The remaining profiles were characteristic of B. japonicum strain USDA 123. The fragments in lane 16 are likely due to a nodule (used as the template source) that was occupied by both strains.
FIG 6.
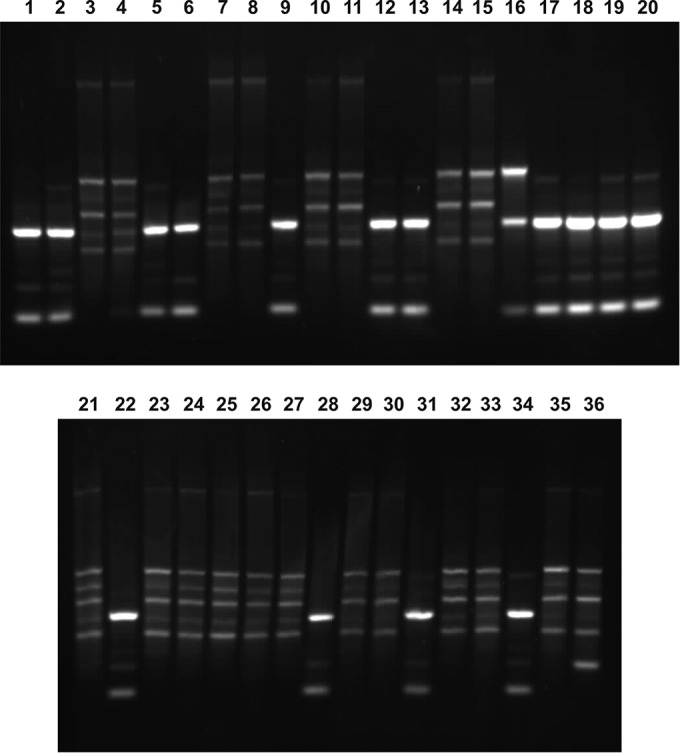
PCR profiling, using primer RP01, of strains isolated from soybean nodules grown in a greenhouse in pots filled with soil taken from the field site in Gorzyń on 23 September 2010. The particular lanes of the gels contain the amplification products obtained with the template of DNA from B. japonicum, as follows: 1, UPP403; 2, UPP404; 3, UPP405; 4, UPP406; 5, UPP407; 6, UPP408; 7, UPP417; 8, UPP418; 9, UPP401; 10, UPP411; 11, UPP420; 12, UPP409; 13, UPP410; 14, UPP402; 15, UPP415; 16, UPP414; 17, UPP412; 18, UPP413; 19, UPP416; 20, UPP419; 21, UPP421; 22, UPP422; 23, UPP423; 24, UPP424; 25, UPP425; 26, UPP426; 27, UPP427; 28, UPP428; 29, UPP429; 30, UPP430; 31, UPP431; 32, UPP432; 33, UPP433; 34, UPP434; 35, UPP435; 36, UPP436.
Enumerating bradyrhizobia in soils.
The MPN technique was used to quantify the number of bradyrhizobia in Gorzyń soil samples capable of nodulating soybeans. The results indicated that this soil sample contained about 1 × 103 bradyrhizobia per gram (dry weight) of soil. The number of bradyrhizobia in duplicate Gorzyń soil samples was also evaluated by using direct immunofluorescence (18). Results of fluorescent-antibody (FA) analyses indicated that, on average, in 2012, the soil contained 1.2 × 104 and 6.9 × 103 cells per gram of USDA 123 and USDA 110, respectively, after 15 years of no host plants (see Table S2 in the supplemental material).
DISCUSSION
The experiments described here were designed to study the interrelationship of well-known competing B. japonicum strains (USDA 123 and USDA 110) introduced into a foreign environment from 1994 to 1997. The mixed-inoculation experiment revealed that USDA 110 initially prevailed over USDA 123. About 70% of the nodules were occupied by this strain, as established by using an immuno-spot-blot method and cross-adsorbed strain-specific antibodies. However, good performance by USDA 110 was also observed during the following years. For natural reasons, the areas occupied by both strains expanded, leading to an overlap of their initial sites of inoculation. The domination of USDA 110 was not as pronounced during the 3rd and 4th years after inoculation, but both strains showed a good survival rate (unpublished data). These observations allowed us to determine (i) the persistence (survival) of symbiotic bacteria (bradyrhizobia) without a host legume, (ii) the genetic stability of inoculant bacteria in the soil environment, and (iii) the mobility of particular genotypes, that is, the spatial range of bacteria spread from the site of their introduction into soil.
Addressing the first issue, our findings clearly indicate that both strains introduced as the inocula remained viable and active as soybean microsymbionts for over 20 years after the only inoculation in May 1994 and the subsequent growth of soybean for an additional 3 years (1994 to 1997). This result is in contrast to that of Dunigan et al. (21), who showed that repeated inoculation was required for 3 years to establish B. japonicum in a Louisiana soil. The number of bacterial cells capable of nodulating soybeans long after inoculation was also surprisingly high for the introduced strains.
Their abundance, estimated by the MPN method (∼103 g−1), was very similar to the number of Bradyrhizobium sp. strains nodulating lupins and native to Polish soils. Martyniuk et al. (22) classified soils containing over 1,000 cells in 1 g of soil to be highly populated by these rhizobia. This observation should be interpreted by considering particular groups of diazotrophic, bacterial symbionts. One should remember that their presence in the soil is usually detected by the plant-trap method. The difference in our results and those of previous studies may be due to the fact that others have introduced rhizobia into soils already containing an indigenous population of these bacteria.
Our estimate of cell numbers based on the FA method suggested that the number of rhizobial cells was 20 times more than that determined by the MPN method. This may be due to the presence of viable but not culturable (infectible) bradyrhizobia. On the other hand, the immunofluorescence technique tends to overestimate the population sizes of soil bacteria (23). This is perhaps the reason for the relatively common opinion that the presence of host plants is necessary for the survival of their respective microsymbionts. The chromosomal localization of genes regulating symbiosis in the genus Bradyrhizobium may explain why bacteria belonging to this group persist relatively well in soil. However, the survival period of B. japonicum as saprophytes was reported to be 8 to 13 years (4, 5), and environmental conditions also remain an important factor governing the presence of indigenous strains. The very poor survival of inoculant strains of a Bradyrhizobium sp. (Cajanus) was reported (24). This was most probably due to the high soil temperature and fluctuating soil moisture content. None of these factors were present in the Polish soil used in this study, and there were no indigenous strains of soybean-nodulating B. japonicum. Thus, as has been pointed out by others and is standard practice in some countries (e.g., New Zealand), decisions on the application and type of inoculum strain should be made carefully. As has been discussed earlier (25, 26), both edaphic conditions and existing bacterial populations strongly affect inoculation success.
Considering the second issue, isolates which were obtained from the root nodules of soybean plants grown in the soil from the experimental site in this study were very likely descendants of the original inoculum strains (B. japonicum USDA 110 and USDA 123). Thus, these strains appear to be very stable in their new environment. Similar observations were reported for soils in France (5), whereas there was great variability in B. japonicum and B. elkanii survivors 7 years after their introduction as inoculants into Brazilian soils (6). Interestingly, none of the tested strains outcompeted the other strain after long-term persistence in the Polish soil without host plants, and the abundance of the strains in the soil was almost equal. This is in contrast to previous reports for U.S. soils showing the dominance of strain USDA 123 over strain USDA 110 (8–10). This may in part be due to a lack of other competing strains and strain compatibility with the local environment, including the local microbiota.
With respect to the issue of mobility, that is, the spatial range of bacterial spread from the site of inoculation, it was rather remarkable that soybean-nodulating bacteria were limited to a rather small area. No soybean bradyrhizobia were found about 50 m from the original experimental plots, as determined by using the MPN method. It should be noted, however, that the original experiment in 1994 was done within the larger area of an experimental field, where cultivation (plowing and harrowing) was done in different directions along the entire field. Moreover, even the short distance of mobility of the rhizobia appeared to be limited to mechanical movement (e.g., by harrowing) of soil particles, so the spatial range likely increased only about twice during the course of the experiment. This is in contrast to the findings for symbiotic strains with characteristics similar to those of the inoculant strains found in uncropped soils in Brazil (7). While the spread of soil through the air has been considered one mechanism for the movement of compatible bacteria outside the area of host growth (27), this did not appear to be the case at our field site.
In summary, the results of this study show that soybean-specific B. japonicum strains remained viable and symbiotically competent in a foreign environment that was originally free of indigenous soybean microsymbionts for at least 20 years after they were introduced into the soil as inocula. This occurred even though soybeans were not grown in the location for the next 17 years. We also found that the surviving isolates, which were determined by using molecular and immunochemical methods, were undistinguishable from the original inoculant strains, USDA 110 and USDA 123. Moreover, these competing strains could be detected in fairly high and balanced numbers, suggesting that the competitive dominance of strain USDA 123 in U.S. soils may be due to situations where the inoculant strain was introduced into an environment where well-established populations of indigenous strains exist. Alternately, abiotic factors may have favored one strain over another. Lastly, the mobility of bradyrhizobia was relatively low and nodulation-competent soybean microsymbionts could be detected no farther than about 40 m from the limits of the original area of inoculation, despite intensive soil cultivation at the study site. Taken together these results show that B. japonicum strains maintain their symbiotic competence for decades in the absence of a legume host.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported, in part, by previous grant HRN-5600-G-00-2074-00 from the U.S. Agency for International Development (to M.J.S. and C.J.M.), by the University of Minnesota Agricultural Experiment Station (to M.J.S.), and by Poznań University of Life Sciences projects 508.181.03 and 508.181.00 (to C.J.M.).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.01399-15.
REFERENCES
- 1.Mądrzak CJ, Golińska B, Króliczak J, Pudełko K, Łażewska D, Lampka B, Sadowsky MJ. 1995. Diversity among field populations of Bradyrhizobium japonicum in Poland. Appl Environ Microbiol 61:1194–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stępkowski T, Żak M, Moulin L, Króliczak J, Golińska B, Narożna D, Safronova VI, Mądrzak CJ. 2011. Bradyrhizobium canariense and Bradyrhizobium japonicum are the two dominant Rhizobium species in root nodules of lupin and serradella plants growing in Europe. Syst Appl Microbiol 34:368–375. doi: 10.1016/j.syapm.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch AM. 2010. How rhizobia survive in the absence of a legume host, a stressful world indeed, p 377–391. In Seckbach J, Grube M. (ed), Symbioses and stress: joint ventures in biology. Springer, Netherlands, Dordrecht, The Netherlands. [Google Scholar]
- 4.Brunel B, Cleyet-Marel JC, Normand P, Bardin R. 1988. Stability of Bradyrhizobium japonicum inoculants after introduction into soil. Appl Environ Microbiol 54:2636–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Obaton M, Bouniols A, Guillaume P, Guillaume P, Vadez V. 2002. Are Bradyrhizobium japonicum stable during a long stay in soil? Plant Soil 245:315–326. doi: 10.1023/A:1020447928911. [DOI] [Google Scholar]
- 6.Batista JSS, Hungria M, Barcellos FG, Ferreira MC, Mendes IC. 2007. Variability in Bradyrhizobium japonicum and B. elkanii seven years after introduction of both the exotic microsymbiont and the soybean host in a cerrados soil. Microb Ecol 53:270–284. doi: 10.1007/s00248-006-9149-2. [DOI] [PubMed] [Google Scholar]
- 7.Ferreira MC, Hungria M. 2002. Recovery of soybean inoculant strains from uncropped soils in Brazil. Field Crops Res 79:139–152. doi: 10.1016/S0378-4290(02)00119-3. [DOI] [Google Scholar]
- 8.Keyser HH, Cregan PB. 1987. Nodulation and competition for nodulation of selected genotypes among Bradyrhizobium japonicum serogroup 123 isolates. Appl Environ Microbiol 53:2631–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kosslak RM, Bohlool BB. 1985. Influence of environmental factors on interstrain competition in Rhizobium japonicum. Appl Environ Microbiol 49:1128–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lohrke SM, Orf JH, Martínez-Romero E, Sadowsky MJ. 1995. Host-controlled restriction of nodulation by Bradyrhizobium japonicum strains in serogroup 110. Appl Environ Microbiol 61:2378–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.IUSS Working Group WRB. 2006. World reference base for soil resources. Word soil resources reports no. 103. FAO, Rome, Italy. [Google Scholar]
- 12.Natural Resources Conservation Service, US Department of Agriculture. 1999. Soil taxonomy: a basic system of soil classification for making and interpreting a soil surveys, 2nd ed Agricultural handbook 436. US Government Printing Office, Washington, DC. [Google Scholar]
- 13.Singleton PW, Somasegaran P, Nakao PL, Keyser HH, Hoben HJ, Ferguson PI. 1990. Applied BNF technology. A practical guide for extension specialists. NifTAL Project, University of Hawaii, US Agency for International Development, Honolulu, HI. [Google Scholar]
- 14.Cregan PB, Keyser HH, Sadowsky MJ. 1989. Host plant effects on nodulation and competitiveness of the Bradyrhizobium japonicum serotype strains constituting serocluster 123. Appl Environ Microbiol 55:2532–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Somasegaran P, Hoben HJ. 1994. Handbook for rhizobia: methods in legume-rhizobium technology. Springer-Verlag, New York, NY. [Google Scholar]
- 16.Sadowsky MJ, Tully RE, Cregan PB, Keyser HH. 1987. Genetic diversity in Bradyrhizobium japonicum serogroup 123 and its relation to genotype-specific nodulation of soybean. Appl Environ Microbiol 53:2624–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richardson AE, Viccars LA, Watson JM, Gibson AH. 1995. Differentiation of Rhizobium strains using the polymerase chain reaction with random and directed primers. Soil Biol Biochem 27:515–524. doi: 10.1016/0038-0717(95)98626-Y. [DOI] [Google Scholar]
- 18.Schmidt EL, Bankole RO, Bohlool BB. 1968. Fluorescent antibody approach to study of rhizobia in soil. J Bacteriol 95:1987–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Core Team R. 2014. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 20.De Mendiburu F. 2014. agricolae: statistical procedures for agricultural research. R package version 1.2-1. http://CRAN.R-project.org/package=agricolae. [Google Scholar]
- 21.Dunigan EP, Bollich PK, Hutchinson RL, Hicks PM, Zaunbrecher FC, Scott SG, Mowers RP. 1984. Introduction and survival of an inoculant strain of Rhizobium japonicum in soil. Agron J 76:463–466. doi: 10.2134/agronj1984.00021962007600030023x. [DOI] [Google Scholar]
- 22.Martyniuk S, Oroń J, Martyniuk M. 2005. Diversity and numbers of root nodule bacteria (rhizobia) in Polish soils. Acta Soc Bot Pol 74:83–86. [Google Scholar]
- 23.Tong Z, Sadowsky MJ. 1994. A selective medium for isolation and quantification of Bradyrhizobium japonicum and Bradyrhizobium elkanii strains from soils and inoculants. Appl Environ Microbiol 60:581–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dudeja SS, Khurana AL. 1989. Persistence of Bradyrhizobium sp. (Cajanus) in a sandy loam. Soil Biol Biochem 21:709–713. doi: 10.1016/0038-0717(89)90068-0. [DOI] [Google Scholar]
- 25.Botha WJ, Jaftha JB, Bloem JF, Habig JH, Law IJ. 2004. Effect of soil bradyrhizobia on the success of soybean inoculant strain CB 1809. Microbiol Res 159:219–231. doi: 10.1016/j.micres.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Brockwell J, Bottomley PJ, Thies JE. 1995. Manipulation of rhizobia microflora for improving legume productivity and soil fertility: a critical assessment. Plant Soil 174:143–180. doi: 10.1007/BF00032245. [DOI] [Google Scholar]
- 27.Hirsch PR. 1996. Population dynamics of indigenous and genetically modified rhizobia in the field. New Phytol 133:159–171. doi: 10.1111/j.1469-8137.1996.tb04351.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.