Abstract
Closely related ancient endosymbionts may retain minor genomic distinctions through evolutionary time, yet the biological relevance of these small pockets of unique loci remains unknown. The tsetse fly (Diptera: Glossinidae), the sole vector of lethal African trypanosomes (Trypanosoma spp.), maintains an ancient and obligate mutualism with species belonging to the gammaproteobacterium Wigglesworthia. Extensive concordant evolution with associated Wigglesworthia species has occurred through tsetse species radiation. Accordingly, the retention of unique symbiont loci between Wigglesworthia genomes may prove instrumental toward host species-specific biological traits. Genome distinctions between “Wigglesworthia morsitans” (harbored within Glossina morsitans bacteriomes) and the basal species Wigglesworthia glossinidia (harbored within Glossina brevipalpis bacteriomes) include the retention of chorismate and downstream folate (vitamin B9) biosynthesis capabilities, contributing to distinct symbiont metabolomes. Here, we demonstrate that these W. morsitans pathways remain functionally intact, with folate likely being systemically disseminated through a synchronously expressed tsetse folate transporter within bacteriomes. The folate produced by W. morsitans is demonstrated to be pivotal for G. morsitans sexual maturation and reproduction. Modest differences between ancient symbiont genomes may still play key roles in the evolution of their host species, particularly if loci are involved in shaping host physiology and ecology. Enhanced knowledge of the Wigglesworthia-tsetse mutualism may also provide novel and specific avenues for vector control.
INTRODUCTION
Bacteria adapt to specific environments, including host-associated niches, through the retention, rearrangement, gain, or decay of functional capabilities. Research on ancient obligate host associations (i.e., in which bacteria display an extensive concordant evolution with their host) has demonstrated that microbial symbiont genome evolution can be influenced by microbial community dynamics (1–6), in addition to host physiology and ecology (7–9). One extreme case has been described within the mealybug, where dual symbiont species and the host have retained complementary loci that, only when integrated as a symbiotic system (i.e., a holobiont) and not as individual species, are capable of producing specific requisite nutrients (3, 9). Extensive gene purging is characteristic among ancient bacterial symbionts, as they challenge the lower limits of genome size (10, 11). These symbionts typically exhibit tremendous genomic stasis between strains and species, likely due to their environmental isolation, as their genomes encode only those capabilities necessary for the maintenance of the mutualism (reviewed in reference 10). In contrast, the genomes of free-living bacteria allow the bacteria to adapt to their surroundings by encoding a plethora of strain-specific loci, known as dispensable genes (12), contributing to increased habitat occupancy and their successful viability within alternative environments (13, 14). With ancient bacterial symbionts, many fewer pockets of genes unique between species and strains have been described (15, 16), but to date their functional roles and potential contributions to host fitness remain unknown.
The tsetse fly (Diptera: Glossina) and its symbionts together form a valuable model system that can be used to gain deeper insight into the evolution of host-associated symbioses due to the low-complexity microbiota of the tsetse fly. In addition to enhancing our comprehension of host-associated microbial genome evolution, the tsetse symbioses also hold public health significance, as tsetse flies are the obligate vectors of Trypanosoma spp., the causative agents of fatal human African trypanosomiasis (HAT) and nagana, a veterinary wasting disease. Tsetse flies consistently harbor three bacterial symbionts, the obligate mutualist Wigglesworthia spp. (17), the commensal Sodalis glossinidius (18), and members of the parasitic genus Wolbachia (19, 20). Field studies report a more complex and diverse adult microbiota (21–24), although the majority is believed to be transient in nature. Enhanced knowledge of tsetse symbioses may provide novel and specific avenues for vector control, such as through paratransgenesis, in which symbionts deliver effector molecules that interfere with parasite development (25, 26), or the use of a novel class of pesticides aimed at disrupting pivotal symbiont-mediated tsetse fly metabolic processes.
The low complexity of the tsetse microbiota is believed to be maintained by facets of tsetse biology, including a sterile diet (i.e., a vertebrate blood diet by both sexes) and a unique reproductive strategy known as adenotrophic viviparity (i.e., live birth), which excludes larvae from environmental microbial exposure (27). In adenotrophic viviparity, the majority of larval development occurs in utero, where the mother provides nourishment, mostly in the form of amino acids and lipids (28), and vertically transmits the Wigglesworthia and Sodalis symbionts through highly specialized accessory organs, known as milk glands (29, 30). Following the completion of intrauterine larval development, a late 3rd-instar larva is deposited and quickly enters into pupation. The significant maternal investment results in a low number of gonotrophic cycles producing few progeny, which offers promise for tsetse population suppression measures targeting the already low vector reproductive output.
The tsetse-Wigglesworthia association is believed to have formed prior to Glossina species radiation. In support of this suggestion, extensive concordant evolution between each tsetse species and its specific Wigglesworthia symbiont has been observed (31). This ancient symbiont is localized intracellularly within a bacteriome organ at the anterior midgut, while an additional extracellular population is localized within the milk glands (30, 32). The functional roles of Wigglesworthia include nutrient provisioning (33–36), contributing B vitamins typically lacking in blood, and influencing host immunological (37, 38) and microbial community (5, 6, 39) robustness. The importance of the symbiosis is demonstrated by the loss of female fecundity upon the removal of Wigglesworthia (20, 40, 41), which can partially be restored through the provisioning of B vitamins (33) or nutrient-rich yeast extract (20) in blood meals, providing strong evidence for its nutrient supplementation role. The contributory roles of specific symbiont metabolites toward various aspects of host biology, particularly those that differ in retention between different Wigglesworthia species, remain largely understudied.
The annotated genomes of Wigglesworthia harbored within the Glossina morsitans (“Wigglesworthia morsitans”) (35) and Glossina brevipalpis (Wigglesworthia glossinidia) (34) bacteriomes share features with other ancient symbionts (reviewed in reference 10). These characteristics include extreme adenine-thymine (AT) bias and a highly reduced size (∼700 kb), with only those genes believed to be necessary for the maintenance of mutualism being retained. These Wigglesworthia genomes demonstrate extensive chromosomal synteny, despite long-term separation and subsequent host species codiversification (35). Comparative genome analyses between the two Wigglesworthia species enable the development of hypotheses regarding the functional differences between these symbionts. Interestingly, one of the few features unique to the W. morsitans genome relative to the features of the reference W. glossinidia genome is that it encodes the complete chorismate biosynthesis pathway (see Fig. S1 in the supplemental material), which converts phosphoenolpyruvate (PEP) and erythrose 4-phosphate into chorismate, a precursor for the production of the aromatic amino acids and vitamins (42, 43). W. morsitans is then able to incorporate chorismate into the p-aminobenzoate (PABA) biosynthesis branch for downstream folate (vitamin B9) production. Folate is required for all life, as it is involved in DNA synthesis, repair, and methylation (44–46) and may be produced by W. morsitans to supplement the vitamin-poor vertebrate blood diet of its host.
In the study described here, we aimed to functionally characterize the distinct retention of the chorismate and folate biosynthetic potential by W. morsitans, examine its role in tsetse host sexual maturation and reproductive biology, and discuss whether selection likely has preserved these capabilities during host coevolution. To accomplish this, we first performed expression analyses of various W. morsitans loci involved in chorismate and folate production, as well as a corresponding tsetse folate transporter locus within bacteriomes, relative to host sex, physiological development, and pregnancy to determine whether these are actively and differentially transcribed. Assays for the detection of metabolites within tsetse bacteriomes were performed to confirm W. morsitans folate production and to determine if the differences in symbiont biosynthetic locus expression correspond with relative folate abundance. Furthermore, functional studies characterized the impact of inhibiting W. morsitans chorismate and folate production on various aspects of G. morsitans biology, specifically, longevity, digestion, and reproduction. Lastly, we discuss how these distinct biosynthetic capabilities between ancient mutualists may have contributed to tsetse ecological variation.
MATERIALS AND METHODS
Tsetse flies.
G. morsitans morsitans flies were maintained in the Department of Biology insectary at West Virginia University at 24 ± 1°C with 55% relative humidity on a 12-h light/12-h dark schedule. The tsetse flies were fed defibrinated bovine blood (Hemostat Laboratories, Dixon, CA) every 48 h using an artificial membrane feeding system (47).
Reverse transcription analyses of W. morsitans chorismate and folate biosynthetic loci.
To examine the expression of the W. morsitans chorismate and folate biosynthetic pathway loci (see Fig. S1 in the supplemental material), the levels of transcription of aroA (3-phosphoshikimate 1-carboxyvinyltransferase), pabB (aminodeoxychorismate synthase subunit I), and folP (7,8-dihydropteroate synthase) were assessed. Tsetse flies were sacrificed, and bacteriomes were dissected. Total RNA was isolated following the TRIzol protocol (Invitrogen, Carlsbad, CA), treated with DNase I (Ambion), and verified to be free of DNA contamination through PCR using RNA only as the template. First-strand cDNA synthesis was performed with ∼140 ng RNA, a 2 μM primer cocktail of gene-specific 3′ end primers (primers aroAqPCRRev, pabBqPCRRev, folPqPCRRev, and rpsCqPCRRev; Table 1), and SuperScript II reverse transcriptase (Invitrogen). Quantitative PCR (qPCR) was performed with SsoFast EvaGreen supermix (Bio-Rad, Hercules, CA), 0.4 mM gene-specific primers (Table 1), and 2 μl cDNA template in a Bio-Rad CFX96 real-time PCR detection system with 30 amplification cycles. Internal standards were constructed by cloning PCR amplicons from each locus (for the primers, see Table 1) into the pGEM-T vector (Promega, Madison WI). The amplicons were quantified by comparison to the quantities on standard curves with Bio-Rad CFX Manager software (version 2.0), and abundance of the transcripts was normalized to that of the W. morsitans rpsC (30S ribosomal protein S3) transcript. The species specificity of the primers used for reverse transcription was verified by using Sodalis culture DNA as a negative control. At least three bacteriomes were processed per group, with each sample being analyzed in triplicate and the results being averaged. Dissociation curves were included in each run in which a single melting peak was observed. Negative controls were included in all amplification reactions.
TABLE 1.
Primers used in this studya
| Primer name | Sequence (5′-3′) | Tab (°C) | Length (bp) | ||
|---|---|---|---|---|---|
| aroAqPCRFor | GGT ACT GCT ATG CGT TTG C | 54.3 | 128 | ||
| aroAqPCRRev | GCA CCA CCT TGT CTT AAA GC | ||||
| pabBqPCRFor | CGC AAA TTG GAA CCG TAT CAG | 50.2 | 154 | ||
| pabBqPCRRev | CCC GTA ATT GAC CCA CCT G | ||||
| folPqPCRFor | TTT CTG ATG GTG GAC AGT TTA C | 51.5 | 149 | ||
| folPqPCRRev | TCG TTC TGA TTC TTC AAG TTC G | ||||
| rpsCqPCRFor | CAA GAC CTG GAA TAG TAA TCG G | 50.1 | 198 | ||
| rpsCqPCRRev | CAC GCT TCA TTG CTC TAC G | ||||
| aroAstdFor | TTT TAT TAT CGG CGC AAA CC | 55.0 | 457 | ||
| aroAstdRev | AAT GGG GCC ATG ATG AGT AA | ||||
| pabBstdFor | TAA CTG CGC ACC ATT TTC TG | 52.0 | 468 | ||
| pabBstdRev | CCA CAC CAT GCA TTT CTT CT | ||||
| folPstdFor | AAA TGT CAC ACC GGA TTC GT | 55.0 | 498 | ||
| folPstdRev | CCG GGG TCA ATG ATT ATA CG | ||||
| rpsCstdFor | TGG CGT TCT ACA TGG TAT GC | 55.0 | 702 | ||
| rpsCstdRev | TGC ACG AAA AGT GTG TAG GG | ||||
| GmBtubfor | CCA TTC CCA CGT CTT CAC TT | 55.0 | 151 | ||
| GmBtubrev | GAC CAT GAC GTG GAT CAC AG | ||||
| GmRFCfor | CTC AAA GCC ACC ACC TTG TT | 55.0 | 294 | ||
| GmRFCrev | CAA CGA TGA CAA GAC GGC TA | ||||
The qPCR primers used in this study were designed using Beacon Designer software (version 7.2; Premier Biosoft International), and internal standard primers were created using Primer3 software (http://frodo.wi.mit.edu/).
Ta, annealing temperature.
Folate detection within bacteriomes.
A standard Lactobacillus rhamnosus microbiological assay (48) was used for folate quantification in wild-type (WT) flies and aposymbiotic flies (generated by obtaining progeny from females maintained on blood supplemented with 25 μg/ml tetracycline and 1% [wt/vol] yeast extract [20, 39]). The bacteriomes were dissected, individually placed into 0.1 M K2HPO4-KH2PO4 buffer with 1% ascorbic acid (pH 6.3), and immediately homogenized to release the bacteriocyte contents. To release folate from bound proteins and denature folate-degrading and interconversion enzymes, samples were placed in an H2O bath at 100°C for 5 min. Subsequently, bacteriome samples were incubated with charcoal-pretreated rat plasma (Wistar rat plasma; Innovative Research, Novi, MI), which served as a conjugase of folate polyglutamates to monoglutamates, at 37°C for 30 min. Samples were then sterilized using a 0.22-μm-pore-size filter, and 1:100 dilutions were mixed with folate-deficient Difco folic acid casei medium (Becton, Dickinson and Company, Sparks, MD) supplemented with 20 μg/ml chloramphenicol (Chl; Sigma, St. Louis, MO) and inoculated with log-phase L. rhamnosusChl (chloramphenicol resistant; ATCC 27773) at an optical density at 600 nm of 0.01. The cultures were incubated for 18 h at 37°C without aeration. Standard concentrations of folic acid (10 to 125 fmol/well; Sigma) were also mixed with the L. rhamnosusChl culture to create a standard curve. A positive control consisting of 300 fmol folic acid and a negative control consisting of initial buffer only were subjected to the complete procedure to ensure the retention of the initial folate and a lack of additional folate, respectively. Moreover, an additional negative control included only L. rhamnosusChl lacking folate supplementation. The growth of L. rhamnosus was measured by use of a Biomate3 spectrophotometer (Thermo Fisher Scientific, Madison, WI) and absorbance readings at 600 nm. The folate contents of the bacteriome samples were determined by comparison to the standard curve (for which R2 values of 0.95 to 0.99 were obtained) and expressed as the number of picomoles of folate per bacteriome. At least 4 bacteriomes were sampled for each group.
Determination of bacteriome Wigglesworthia density.
To differentiate whether changes in folate abundance in the bacteriomes were due to either higher transcriptional levels or an increase in symbiont density, tsetse flies were sacrificed and DNA was isolated from the dissected bacteriomes using the protocol of Holmes and Bonner (49). The density of Wigglesworthia (i.e., the number of symbiont genomes per host cell) was determined by qPCR using single-copy Wigglesworthia thiC and G. morsitans chitinase loci, as previously described (6).
Investigation of tsetse folate transporter expression within the bacteriome.
Expression of the G. morsitans folate transporter (VectorBase gene accession number GMOY005445), known as the reduced folate carrier (RFC), within bacteriome samples was examined using semiquantitative reverse transcription-PCR (RT-PCR) analyses. Bacteriome RNA was isolated, and reverse transcription was performed as described above using a cocktail of primers GmBtubrev and GmRFCrev specific for the 3′ end of the gene (Table 1). Primer specificity for G. morsitans RFC was confirmed in silico using the NCBI Primer BLAST and VectorBase programs. Second-strand synthesis was performed with primers specific for the complementary 5′ end of the gene (Table 1) and 2 μl cDNA template for 35 amplification cycles. The products were analyzed using agarose gel electrophoresis and visualized with Kodak One image analysis software. Each time point was examined in triplicate. Negative controls were included in all PCRs. The constitutively expressed G. morsitans β-tubulin gene (GenBank accession number DQ377071) was used as a loading control and to verify RNA integrity.
Impact of chorismate pathway inhibition on G. morsitans biology.
Different concentrations of glyphosate [N-(phosphonomethyl)glycine; Sigma], a specific competitive enzymatic inhibitor of AroA (50–52), were administered by incorporation of glyphosate into G. morsitans blood meals. The impacts of symbiont chorismate pathway inhibition on tsetse longevity, digestion, reproductive output, and progeny development were examined as described below.
Longevity.
The survival of both WT and aposymbiotic adult flies (maintained by treatment with 25 μg/ml tetracycline [40] through adulthood) was monitored to differentiate between the effects of glyphosate treatment on tsetse and symbiont biology. The flies were maintained on diet combinations consisting of blood only or blood supplemented with 10 or 20 mM glyphosate. The number of dead flies was recorded daily for a duration of 60 days.
Digestion.
Flies were maintained on 0 or 10 mM glyphosate-supplemented blood meals. At 2 weeks of age, the flies were offered their respective blood meals for 20 min, and only those with blood-filled abdomens remained in the study. Blood meal digestion was determined by measuring undigested hemoglobin levels using the cyanmethemoglobin method (Sigma), as previously described (40). Briefly, tsetse midguts (n = 3 per time point and treatment) were dissected, homogenized in 2 ml Drabkin's reagent (Sigma), and incubated at ambient temperature in the dark for 15 min. Measurements of the absorbance at 540 nm were taken for each sample. The hemoglobin concentration was determined by comparing the mean absorbance reading for an unknown to the readings on a standard curve (R2 = 0.98) created using bovine hemoglobin (Sigma) and was expressed as the number of milligrams of hemoglobin per gut by converting the hemoglobin concentrations from milligrams per milliliter to the number of milligrams per 20 μl (the average volume consumed by tsetse flies during feeding).
Tsetse reproductive output and progeny development.
To examine whether symbiont chorismate pathway inhibition impacted larval development, virgin females were maintained on defibrinated bovine blood only or defibrinated bovine blood supplemented with 10 mM glyphosate and/or 500 nM folic acid and mated with WT males after 2 feedings. At 20 days of age, the reproductive tracts (including the spermatheca, oocytes, and uterus) were removed using a Leica S6D dissection microscope (Leica Microsystems, Heerbrugg, Switzerland), and images were captured with a Leica DFC420 digital color camera and Leica Application Suite software (version 2.8.0). Measurements of the uterus, which contained a developing larva if the fly was pregnant, were then obtained using ImageJ software (53) and compared to those of age-matched WT virgin and mated females. To examine the effect of chorismate inhibition on pupal deposition and eclosion, virgin females were maintained on the supplemented blood meals described above and mated with WT males after 2 feedings. Pupa deposition and maternal fly mortality were monitored for 45 days (∼2 gonotrophic cycles). Pupal weights, eclosion rates, and teneral wing area (obtained using ImageJ software), used as a measure of body size (54, 55), were also recorded.
Statistical analyses.
The statistical significance of the data was analyzed by Student's t test or the Mann-Whitney U test using Microsoft Excel software, with variances being compared using F tests. JMP software (version 7.0; SAS Institute, Cary, NC, USA) and GraphPad Prism software (version 5.01) were used to perform analyses of variance (ANOVAs) followed by Tukey-Kramer post hoc pairwise comparisons of the means, χ2 tests, and survival analyses. Survival curves were created using the Kaplan-Meier method and compared using the log-rank test (56).
RESULTS
W. morsitans chorismate and folate biosynthetic loci exhibit differential expression between tsetse sexes during development and pregnancy.
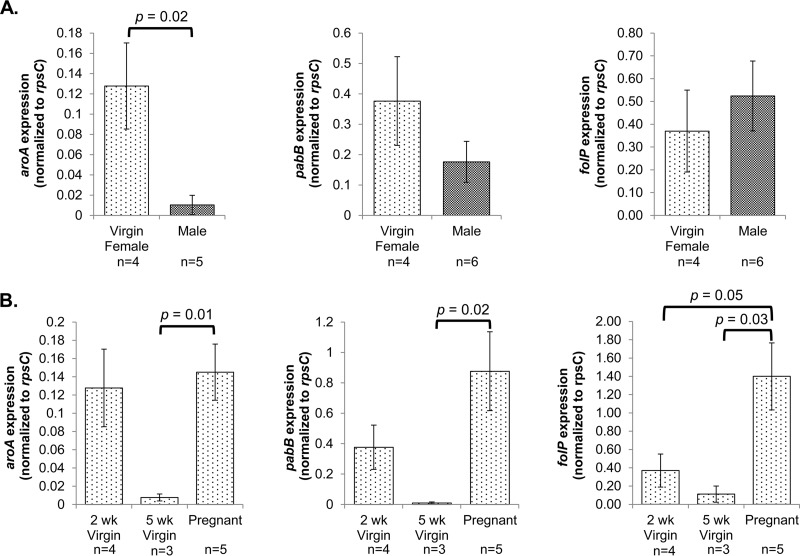
To begin investigating W. morsitans chorismate and folate biosynthetic gene expression within bacteriomes relative to host sex, development, and gestation, the abundance of the aroA, pabB, and folP transcripts was determined by qPCR, and the levels in male and female tsetse flies were compared (Fig. 1). Comparison of 2-week-old tsetse flies revealed higher aroA and pabB transcript levels within the bacteriomes of virgin females than in those of age-matched males, although statistical significance was observed only with the former (P = 0.02, Student's t test), and similar levels of folP expression were observed between the sexes (Fig. 1A). To further investigate the role of W. morsitans chorismate and folate provisioning during female adulthood and pregnancy, the transcriptional profiles of these loci from the bacteriomes of 5-week-old virgin flies and similarly aged adult flies during late gestation (i.e., while carrying a 3rd-instar larva) were also examined. The expression of all three loci was significantly higher in the bacteriomes of pregnant flies than in those of similarly aged virgin females (Fig. 1B; for aroA, P = 0.01, Mann-Whitney U test; for pabB, P = 0.02, Mann-Whitney U test; for folP, P = 0.03, Student's t test). Additionally, the W. morsitans folP transcript abundance was also significantly higher in pregnant females than 2-week-old virgins (Fig. 1B; P = 0.05, Student's t test). Further investigation into chorismate pathway expression during distinct gonotrophic cycles revealed that variation in expression between pregnancies, with higher levels of expression of loci occurring during the second gonotrophic cycle than during the first one (see Fig. S2 in the supplemental material). These data indicate that W. morsitans not only actively transcribes chorismate and folate biosynthesis loci but also increases their expression during early sexual maturation and pregnancy.
FIG 1.
W. morsitans chorismate and folate biosynthetic loci exhibit differential expression within bacteriomes between the tsetse sexes at age 2 weeks (A) and through female adulthood and pregnancy (B). Graphs represent the normalized transcript abundance, with error bars signifying 1 standard error of the mean (SEM). Student's t tests and Mann-Whitney U tests were performed when variances were equal and unequal, respectively, and statistically significant differences are indicated above the bars. aroA, 3-phosphoshikimate 1-carboxyvinyltransferase; pabB, aminodeoxychorismate synthase subunit I; folP, 7,8-dihydropteroate synthase; rpsC, 30S ribosomal protein S3. The sample size (n) is indicated below each group.
The bacteriome folate content is the highest during early female adulthood and pregnancy.
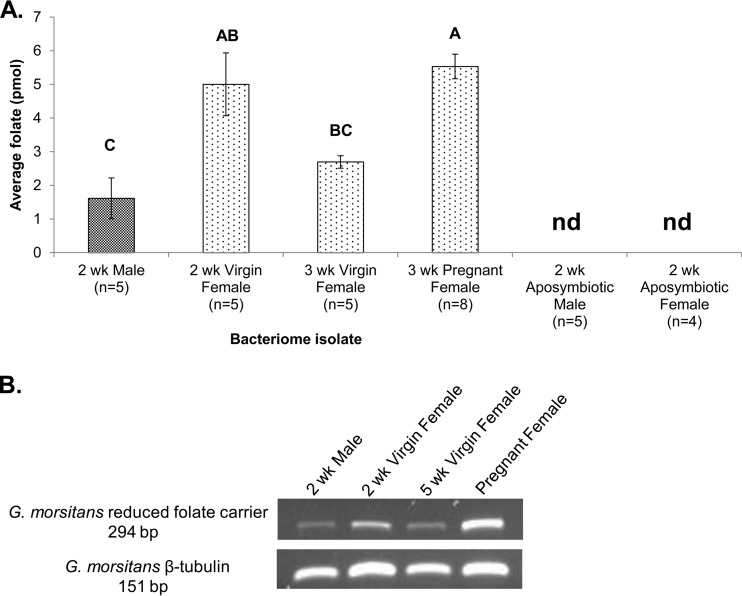
To move beyond gene expression analyses, a standard L. rhamnosus microbiological assay was employed to enable both the detection of folate in single bacteriomes and the quantification of folate abundance from single bacteriomes. Comparison of the bacteriomes within 2-week-old male and virgin female adults demonstrated that there are sex-specific differences in folate content, with virgin female bacteriomes containing significantly more folate (Fig. 2A; P = 0.0006, ANOVA). To determine whether the higher folate content in 2-week-old virgin females resulted from increased transcription or differences in symbiont density, comparisons of the density of W. morsitans within bacteriomes were performed by qPCR. No significant difference in the W. morsitans density between 2-week-old males and virgin females (P = 0.196, Student's t test; data not shown) was observed. Previous research has reported that at age 2 weeks, virgin female tsetse flies harbor a significantly larger population of W. morsitans bacteria than males (57), yet this study examined symbiont densities in whole flies, which includes the female milk gland W. morsitans population. Therefore, the folate content in 2-week-old virgin female bacteriomes is due to an increase in gene activity, rather than heightened symbiont densities. Notably, folate was not detected in the bacteriome samples from 2-week-old aposymbiotic male and virgin female flies (Fig. 2A), supporting the production of this nutrient exclusively by W. morsitans within bacteriomes. To further explore folate production within bacteriomes during reproduction, bacteriome folate contents in age-matched pregnant and virgin female were also compared, and it was revealed that a significantly higher abundance of folate was harbored within the bacteriomes of pregnant individuals (Fig. 2A; P = 0.0006, ANOVA). In support of the results of previous research, which showed that mating does not influence symbiont population density within females (57), W. morsitans densities within 3-week-old virgin and mated bacteriomes were found to be comparable (P = 0.311, Student's t test; data not shown). Therefore, the independent observations of the greater abundance of transcripts for W. morsitans chorismate and folate biosynthesis loci (Fig. 1A and B) as well as higher folate quantities (Fig. 2A) in the bacteriomes of young females and pregnant females indicate that W. morsitans folate production may play crucial roles during early female sexual development and gestation.
FIG 2.
Folate content and host transporter expression within G. morsitans bacteriomes. (A) Total folate quantity was determined using an L. rhamnosus microbiological assay. Statistically significant differences between groups, determined using ANOVA (P = 0.0006), are indicated above the bars using different letters. Error bars represent 1 SEM. The sample size (n) is indicated below each group. nd, samples in which folate was not detected. (B) Semiquantitative RT-PCR analysis of G. morsitans RFC expression, with G. morsitans β-tubulin serving as a loading control. The results for representative samples (n ≥ 3) are shown for each group.
The G. morsitans folate transporter is expressed within bacteriomes.
To examine the activity of the G. morsitans folate transporter (58), known as the reduced folate carrier (RFC), within the bacteriome, semiquantitative RT-PCR was performed. The expression of the RFC is dynamic within adult tsetse bacteriomes (Fig. 2B), as demonstrated by comparison of age-matched males and females as well as virgin and pregnant females. The abundance of the G. morsitans RFC transcript corresponded with the amount of folate detected within the bacteriomes of the respective samples, which was the highest in the bacteriomes of pregnant females (Fig. 2A). Lastly, the bacteriomes of aposymbiotic flies exhibited no RFC expression (data not shown), suggesting that transporter abundance may be directly associated with folate levels.
Chorismate pathway inhibition impacts tsetse biology.
As W. morsitans appears to be actively synthesizing folate within bacteriomes, with higher levels of production occurring during early female adulthood and pregnancy, we further examined the role of this nutrient provisioning in various tsetse fitness parameters, specifically, tsetse host longevity, blood meal digestion, reproduction, and intrauterine larval development, by enzymatically inhibiting chorismate biosynthesis through blood meal glyphosate supplementation.
Tsetse host longevity.
There have been conflicting reports on the toxic effects of glyphosate on animals (59–63). Glyphosate inhibits chorismate production by competitively binding to AroA (3-phosphoshikimate 1-carboxyvinyltransferase), preventing the incorporation of PEP (52) and 3-phosphoshikimate to create 5-O-(1-carboxyvinyl)-3-phosphoshikimate, which is subsequently converted into chorismate (see Fig. S1 in the supplemental material). While the W. morsitans genome encodes the chorismate and folate biosynthesis pathways, searches for the respective enzymes within the G. morsitans genome (58) did not produce any homologous hits, supporting the belief that animals generally lack these capabilities (42, 64). Therefore, glyphosate should directly impact symbiont rather than tsetse fly biology, with any negative consequences resulting from treatment likely being alleviated by decreasing symbiont populations.
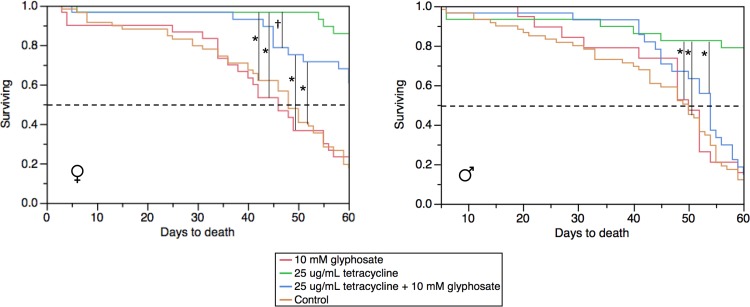
To differentiate between a direct toxic effect of glyphosate on the tsetse host versus symbiont biology, we examined the survival of tsetse maintained on glyphosate-supplemented (10 and 20 mM) and/or tetracycline-supplemented (25 μg/ml) blood. Previously, tetracycline treatments have been shown to nearly clear symbionts within tsetse individuals (37, 40). Interestingly, only treatments with tetracycline significantly increased tsetse longevity (for males, P < 0.0001, log-rank test; for females, P < 0.0001, log-rank test) (Fig. 3), possibly due to the reduction in the costs required for maintaining the symbiotic association, particularly within a colony context. The supplementation of 10 mM glyphosate did not have adverse effects on longevity, as the survival curves were similar to those for the controls fed blood only (for males, P = 0.7547, log-rank test; for females, P = 0.9463, log-rank test) (Fig. 3). Female tsetse flies administered tetracycline and 10 mM glyphosate had significantly higher survival rates than those fed blood alone (P = 0.0001, log-rank test) or blood with 10 mM glyphosate supplementation (P < 0.0001, log-rank test). Although the trend was not statistically significant, a similar trend was observed in male flies maintained on both tetracycline- and 10 mM glyphosate-supplemented blood, which had increased survival compared to those fed 10 mM glyphosate (P = 0.4612, log-rank test) and blood alone (P = 0.2057, log-rank test) (Fig. 3). The increased survival of tsetse flies maintained on both tetracycline and glyphosate compared to that of tsetse flies maintained on glyphosate only suggests that glyphosate mostly impacts symbiont biology. In contrast to the longevity cost with 10 mM glyphosate supplementation, 20 mM glyphosate supplementation did result in a longevity cost in WT flies (see Fig. S3 in the supplemental material), suggesting that there may be adverse, toxic effects on the host at a concentration this high. Tsetse maintained on 20 mM glyphosate supplemented blood had significantly shorter survival than controls (for males, P < 0.0001, log-rank test; for females, P < 0.0001, log-rank test) (see Fig. S3 in the supplemental material). As 10 mM glyphosate does not appear to be toxic toward the host with respect to longevity, this concentration was used to further investigate the inhibition of chorismate biosynthesis on other aspects of tsetse biology.
FIG 3.
Survival curves, created in JMP software (version 7.0) using the Kaplan-Meier method, of WT or aposymbiotic G. morsitans flies maintained on 10 mM glyphosate-supplemented blood for 60 days. Data are for ≥30 individuals per treatment. Significant differences between treatment groups, determined using the log-rank test, are indicated. †, P = 0.035; *, P ≤ 0.0001.
Digestion.
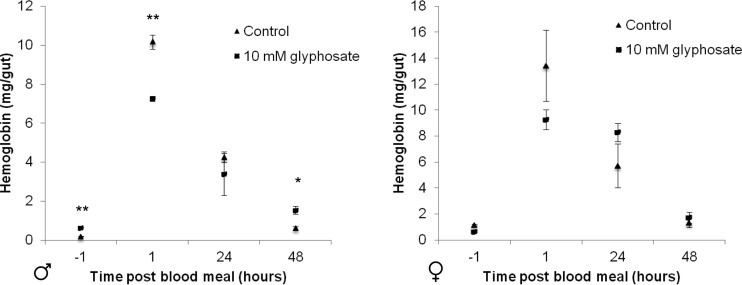
During the survival study, visual inspection of tsetse abdomens indicated that individuals administered glyphosate had prolonged blood meal digestion (Fig. 4). Therefore, we further examined the impact of glyphosate (10 mM) on digestion by measuring the hemoglobin levels in the midguts of treated 2-week-old flies. Interestingly, flies maintained on blood alone took larger blood meals, as represented by higher hemoglobin levels within the abdomens at 1 h postfeeding, although statistical significance was observed only in males (Fig. 4; P = 0.001, Student's t test). Even though they took smaller blood meals, males maintained on 10 mM glyphosate contained significantly more hemoglobin within their midguts at 48 h postfeeding (P = 0.01, Student's t test), indicating compromised digestive capabilities, with a similar trend being observed in females, although that trend lacked significance.
FIG 4.
Concentrations of hemoglobin, representing undigested blood, from 2-week-old G. morsitans flies. Significant differences between control and glyphosate-treated flies at each time point are indicated. *, P < 0.05; **, P < 0.01. Error bars indicate 1 SEM (n = 3 per time point and treatment group).
Host reproduction.
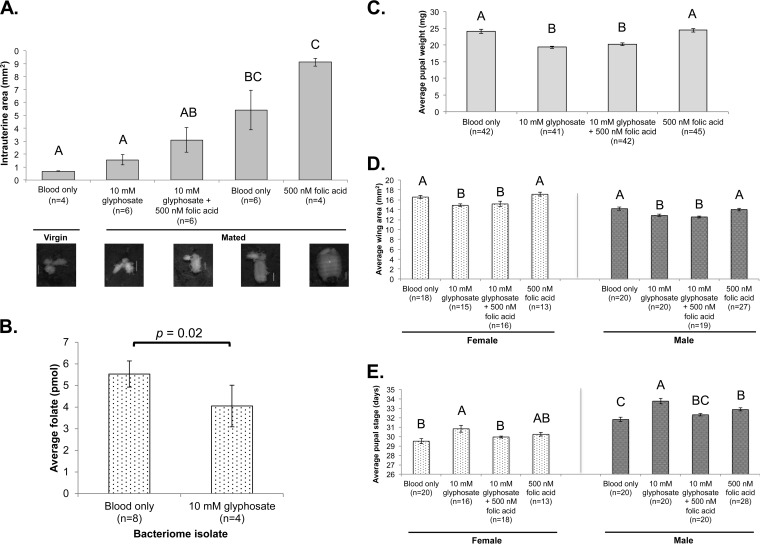
To examine whether chorismate pathway inhibition and the consequent suppression of downstream folate production influence intrauterine larval development, mated females were maintained on specific blood meal regimens (Fig. 5A) and the reproductive tracts were dissected at 20 days postemergence, a time point associated with late intrauterine larval development during the 1st gonotrophic cycle within mated WT females (65). The mean intrauterine area of the group maintained on 10 mM glyphosate-supplemented blood was significantly smaller than that of the mated controls (Fig. 5A; P < 0.0001, ANOVA) and was similar to that of virgins, suggesting that chorismate biosynthesis is critical for larval development. Notably, the bacteriome folate content, determined using the L. rhamnosus microbiological assay, of age-matched, mated females maintained on 10 mM glyphosate-supplemented blood was significantly lower than that of mated females fed blood alone (P = 0.02, Student's t test) (Fig. 5B). These data demonstrate that glyphosate supplementation of the blood meal impacts W. morsitans chorismate production within bacteriocytes, resulting in decreased downstream folate production, which significantly hampers in utero larval development. When 500 nM folic acid was used to rescue the reproductive impact of glyphosate administration, there was an increase in the intrauterine area in mated females with glyphosate supplementation of the blood meal, which was significantly larger than that for virgins (P < 0.0001, ANOVA) and similar to that for mated controls, further supporting the downstream utilization of chorismate for folate biosynthesis by W. morsitans and the importance of the folate supply for intrauterine larval development. Folic acid supplementation alone resulted in larger uterine areas in mated females than in age-matched WT flies, further supporting a role for this nutrient during pregnancy and indicating a deficiency of vitamin B9 even within typical blood meals. These data demonstrate that although females maintained on glyphosate remain fecund, glyphosate has negative impacts on intrauterine larval development as a result of W. morsitans chorismate pathway inhibition.
FIG 5.
Inhibition of W. morsitans chorismate biosynthesis impacts progeny development. (A) Comparison of uterine areas of age-matched (20-day-old) females (containing developing larva) maintained on the indicated treatments (P < 0.0001, ANOVA). Representative images for each treatment are included at the bottom. (B) Folate content within bacteriomes of 3-week-old mated females maintained on blood with or without 10 mM glyphosate supplementation. (C to E) Pupal weight (P < 0.0001, ANOVA) (C), teneral wing area (for females, P = 0.0002, ANOVA; for males, P < 0.0001, ANOVA) (D), and length of the pupal life stage (E) (for females, P = 0.0035, ANOVA; for males, P < 0.0001, ANOVA) of progeny deposited from females maintained on the specified blood meal supplementation. Statistically significant differences, determined using Student's t test or ANOVA, are indicated above the bars. The different letters indicate statistically significant differences between samples. Error bars signify 1 SEM. n, sample size.
To further investigate the necessity of W. morsitans-produced folate in tsetse reproduction, flies were maintained on the supplemented blood meals described above and larval deposition was observed for 45 days. There were no significant differences between the treatment groups in the time to first larval deposition (P = 0.28, ANOVA), the level of larval deposition per mated female (P = 0.9, ANOVA), or pupal eclosion rates (P = 0.7, chi-square test) over the period of observation (data not shown). The weight of pupae (Fig. 5C; ANOVA, P < 0.0001) and the wing areas of teneral progeny (Fig. 5D; for females, P = 0.0002, ANOVA; for males, P < 0.0001, ANOVA) from females maintained on blood only or 500 nM folic acid were significantly greater than those deposited by mothers fed 10 mM glyphosate with or without 500 nM folic acid, suggesting the importance of W. morsitans folate production on these host features, in addition to the effect of insufficient complementation with 500 nM folic acid on these specific traits. Interestingly, progeny from the group treated with 10 mM glyphosate had a significantly longer pupal life stage than progeny from the groups treated with blood only or 10 mM glyphosate plus 500 nM folic acid (Fig. 5E; for females, P = 0.0035, ANOVA; for males, P < 0.0001, ANOVA), again reflecting a lag in development due to symbiont folate biosynthesis inhibition (Fig. 5C). These results, coupled with the intrauterine area measurements, suggest that W. morsitans folate production is pivotal for the developmental progression of tsetse progeny. In addition to a significantly longer pupal period, the progeny from females administered glyphosate were physically smaller in regard to both pupal weight and teneral wing area, showing that lengthening of the developmental time within the puparium fails to recover offspring size.
DISCUSSION
Ancient bacterial symbionts from closely related host species have highly similar genomic contents, yet they also possess small pockets of unique loci (35, 66). The preservation of these symbiont metabolome distinctions through evolutionary time, particularly given the strong reductive genome pressures encountered by these symbionts (10), suggests that these may have been selected for and play a vital part in the biology and evolution of their host species. Here, we used the tsetse fly, specifically, G. morsitans, and its obligate mutualist, W. morsitans, to investigate the significance of the effect of one small set of symbiont species-specific genes on host biology. Approximately 40% of the coding sequences that are encoded by the W. morsitans genome and that are absent in the W. glossinidia reference genome are involved in chorismate biosynthesis and the downstream incorporation of chorismate into folate production. Although these represent a relatively small number of genes, given the significance of chorismate and folate as precursors in thymidylate (dTMP), purine, and amino acid synthesis and their pivotal role in biological processes, such as DNA production and cell growth (44, 45, 64), the retention of these loci results in distinctive symbiont metabolomes between tsetse fly species (35). Here, we demonstrate that the W. morsitans chorismate and subsequent folate biosynthesis pathways not only have remained functionally intact but also maintain significance for multiple tsetse host fitness parameters. These studies provide the first pieces of evidence supporting the actual production of the vitamin folate within the bacteriome by this ancient obligate mutualist and a potential means of dissemination through a synchronously expressed host transporter.
The transcriptional abundance of W. morsitans chorismate and folate biosynthetic loci during female development and pregnancy corresponds with the relative amounts of folate detected within the bacteriomes of females, which appear to be the highest during intrauterine larval development. Additionally, the differences in W. morsitans chorismate biosynthetic locus expression observed between gonotrophic cycles (see Fig. S2 in the supplemental material) suggest the depletion of nutrients during each pregnancy and the necessity for W. morsitans to replenish these resources in its host following larviposition. Currently, the signals regulating W. morsitans folate biosynthesis, as well as their origin (i.e., Wigglesworthia or tsetse host), remain unknown. The amount of folate within aposymbiotic flies was nondetectable, providing additional evidence for the production of this nutrient within the bacteriome solely by the W. morsitans symbiont. These findings further highlight the importance of this mutualism for tsetse host reproduction, as W. morsitans contributes essential nutrients aiding in the growth and development of intrauterine larvae (20, 33–35). The role of additional Wigglesworthia-produced metabolites in tsetse physiology is also becoming more evident. For example, Wigglesworthia vitamin B6 biosynthesis is required for the recycling of proline (the main energy source for tsetse) from alanine during the generation of ATP via the tricarboxylic acid (TCA) cycle and through the female lactation period to enable successful larval development (36).
The sequencing and annotation of the G. morsitans genome (58) resulted in the identification of a single folate transporter, known as the RFC (58). Studies performed with other animal models have shown that the RFC maintains bidirectional properties (67), being used for both the import and export of folate. The transcriptional profile of the G. morsitans RFC within bacteriome samples, in sync with folate abundance, suggests that this transporter may be used to systemically disseminate W. morsitans-produced folate, although further functional characterization is required.
To explore the role of symbiont chorismate and downstream folate production on host biology, flies were administered glyphosate, a chorismate biosynthesis pathway inhibitor, within their blood meals. The administration of a low concentration of glyphosate (10 mM) did not impact tsetse longevity compared to that of flies fed blood only (Fig. 3). At this concentration, there mostly appeared to be Wigglesworthia-specific effects, as symbiont removal enhanced host survival (Fig. 3), suggesting that an energy cost is required for maintenance of the mutualism, at least within colony flies. Additionally, glyphosate treatment resulted in significantly decreased folate levels within the bacteriomes (Fig. 5B), indicating that this compound is able to enter bacteriocytes and impact W. morsitans biology.
Inhibition of symbiont chorismate production negatively impacted the host in various ways, supporting the hypothesis that these unique W. morsitans loci retain importance for tsetse biology. First, inhibition of the W. morsitans chorismate biosynthesis pathway resulted in decreased digestion by tsetse, which is similar to the findings of a previous study describing the phenotype of W. morsitans-free flies (40), providing additional evidence for this symbiont's role in host blood meal digestion. In Drosophila, digestion has been associated with midgut cell turnover (68). The inhibited digestive capabilities seen in tsetse maintained on glyphosate may be a consequence of cell turnover impairment, as folate, whose supply is disrupted in the symbiont, is required for cell generation. The negative effects of glyphosate treatment may be due to decreased nutrient availability but could also be a result of the accumulation of shikimate intermediates (specifically, shikimate-3-phosphate), as glyphosate inhibits the action of AroA, likely contributing to the bloodier abdomens of treated tsetse individuals over time. Additional studies are needed to more comprehensively understand the cause of inhibition of digestive capabilities following the administration of glyphosate. Glyphosate treatment also negatively impacted intrauterine larval development. Although the larvae from glyphosate-treated females were deposited at the same rate as those from the WT group, the pupae were smaller and had a significantly longer pupal stage. The reduced progeny size and longer developmental time may have negative impacts on their biology by even further exaggerating the already slow reproductive cycle of tsetse. These findings correspond with those of past research demonstrating the importance of folate in the diet of insects for successful reproduction and growth (69, 70). Interestingly, antifolates administered within the diet of the buffalo fly (Haematobia irritans exigua), which also feeds on blood, resulted in adult female sterility and longevity costs (71). Methotrexate and aminopterin (antifolate and folate biosynthetic inhibitors, respectively) have also been shown to inhibit larval development and induce the sterility of various dipterans (71–73), but these effects can be rescued by the supplementation of exogenous folate (73). Similar to the results of this study, increasing the concentration of folic acid within the diet of the housefly (Musca domestica) also caused an increase in larval growth (73). This study contributes to the body of work demonstrating the importance of folate for all animals during reproduction and supports a role for bacterial symbionts in its production. At an applied angle, the disruption of pivotal symbiont-mediated metabolic processes within tsetse offers novel control targets for public health measures.
While the Sodalis genome also retains the ability to produce chorismate and folate, W. morsitans appears to be primarily responsible for this nutrient provisioning. Evidence includes the findings of past research which showed that flies lacking W. morsitans but still retaining Sodalis are reproductively sterile (40), demonstrating that Sodalis cannot compensate for the decrease in provisioned nutrients within the fly. The absence of W. morsitans through tsetse host generations (in which the host is capable of reproducing when maintained on yeast extract-supplemented blood meals) also resulted in the eventual loss of Sodalis (39), supporting a metabolic dependence of Sodalis on W. morsitans, which has previously been shown with thiamine (vitamin B1) (5). Additionally, in the field, Sodalis is not present in all tsetse populations or individuals (23, 74), indicating that this secondary symbiont has been established more recently and does not play a role as pivotal as that of W. morsitans in tsetse fitness. To ensure that this study solely examined the contributions of W. morsitans, folate production within the bacteriome organ, which is densely packed with W. morsitans cells, was measured, and the study also utilized primers that specifically amplify W. morsitans loci. Future studies should investigate the Sodalis chorismate and folate biosynthetic pathways within G. morsitans to determine whether they retain functionality or are in the process of being purged from the genome. In support of the latter hypothesis, the Sodalis genome, encoding a high number of pseudogenes (75, 76), has undergone a drastic reduction during the course of adapting to a symbiotic lifestyle (5, 77, 78). Additional research may also examine whether these loci retain more significant roles in tsetse species whose Wigglesworthia symbionts have lost these functional capabilities, leading to further genomic complementation of biosynthetic capabilities, as reported for ancient coresident symbionts (1, 3, 4, 79).
As folate is essential for all life (44–46), the absence of the chorismate and folate biosynthesis pathways within the W. glossinidia genome (34) brings into question how G. brevipalpis obtains this required nutrient. The loss of the chorismate and folate biosynthesis capabilities within the W. glossinidia genome may have initially resulted from stochastic mutations in pathway genes arising from high levels of genetic drift due to extreme population bottlenecks during transmission (10). Once the biosynthetic pathways were no longer intact, additional loci may have then been purged from the genome through further relaxed selection. One alternative mechanism for folate acquisition may be through cross feeding with Sodalis or another member (albeit minor) of the gut microbiota, such as Serratia, which has been reported in multiple tsetse species (22–24). Folate secretion has been reported for multiple Serratia strains (80) within other gastrointestinal systems. For example, Serratia grimesii within termite hindguts is believed to provision the tetrahydrofolate (THF) essential for the growth of both the insect host and a cohabitating CO2-reducing homoacetogenic Treponema primitia spirochete (81). Nutrient production may also involve the host, as observed with the mealybug mutualism and the production of phenylalanine (3, 9). It is also worth noting that within the bedbug (Cimex lectularius), Wolbachia symbionts are localized to bacteriomes and are associated with nutritional mutualism (82), in stark contrast to the reproductive parasitism resulting from these infections in most other insects. Further, significant portions of the Wolbachia genome have been inserted into the G. morsitans chromosomal DNA (58, 83). Future analyses should examine whether these horizontal transfer events are associated with metabolic activity and the extent of these transfers in other tsetse fly species. Alternatively, due to the concordant evolution of Wigglesworthia and its tsetse host species (31), differences in the feeding ecology between tsetse species may have resulted from the loss or retention of specific symbiont capabilities. Multiple lines of blood meal analyses have identified distinct feeding patterns for the various tsetse fly species (84–89). The Suidae, particularly warthogs, have been identified to be the main host of G. morsitans, while hippopotamus is preferred by G. brevipalpis. Since the folate content in blood has been shown to vary between animals (90, 91) and is largely dependent on diet (91), measurement of folate levels within vertebrate blood types may provide support for this hypothesis. Additionally, while G. brevipalpis is known to have a lower vector competency than G. morsitans (92–96), the contributing factors resulting in this phenotype remain unknown but may include these symbiont metabolome distinctions. The retention of capabilities within specific Wigglesworthia species, such as folate production, may have influenced evolutionary and phenotypic variation within the Glossina lineage.
This study describes the metabolic interdependence of W. morsitans chorismate and folate production on G. morsitans biology. Here, we show that the retention of these nutrient biosynthesis capabilities by W. morsitans retains functionality and is important for host biology, specifically during female sexual maturation and intrauterine development. The loss of chorismate and folate biosynthetic capabilities within W. glossinidia may have been due to either stochastic losses, possibly influencing the nutritional ecology of the tsetse host, or the opposite situation, where G. brevipalpis feeding behavior may have influenced the loss of these pathways. The evolution of symbiont genomes, which would impact their metabolic capabilities, may therefore contribute to the phenotypic and ecological distinctions among associated host species.
Supplementary Material
ACKNOWLEDGMENTS
We thank Brett Clark and Vivian Delgado for their technical assistance.
This work was supported by a NASA West Virginia Space Grant Consortium graduate fellowship (to A.K.S.) and the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number R01AI118789 (to R.V.M.R.).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00553-15.
REFERENCES
- 1.McCutcheon JP, McDonald BR, Moran NA. 2009. Convergent evolution of metabolic roles in bacterial co-symbionts of insects. Proc Natl Acad Sci U S A 106:15394–15399. doi: 10.1073/pnas.0906424106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCutcheon JP, Moran NA. 2010. Functional convergence in reduced genomes of bacterial symbionts spanning 200 My of evolution. Genome Biol Evol 2:708–718. doi: 10.1093/gbe/evq055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCutcheon JP, von Dohlen CD. 2011. An interdependent metabolic patchwork in the nested symbiosis of mealybugs. Curr Biol 21:1366–1372. doi: 10.1016/j.cub.2011.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu D, Daugherty SC, Van Aken SE, Pai GH, Watkins KL, Khouri H, Tallon LJ, Zaborsky JM, Dunbar HE, Tran PL, Moran NA, Eisen JA. 2006. Metabolic complementarity and genomics of the dual bacterial symbiosis of sharpshooters. PLoS Biol 4:e188. doi: 10.1371/journal.pbio.0040188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Snyder AK, Deberry JW, Runyen-Janecky L, Rio RVM. 2010. Nutrient provisioning facilitates homeostasis between tsetse fly (Diptera: Glossinidae) symbionts. Proc Biol Sci 277:2389–2397. doi: 10.1098/rspb.2010.0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Snyder AK, McLain C, Rio RVM. 2012. The tsetse fly obligate mutualist Wigglesworthia morsitans alters gene expression and population density via exogenous nutrient provisioning. Appl Environ Microbiol 78:7792–7797. doi: 10.1128/AEM.02052-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rio RV, Lefevre C, Heddi A, Aksoy S. 2003. Comparative genomics of insect-symbiotic bacteria: influence of host environment on microbial genome composition. Appl Environ Microbiol 69:6825–6832. doi: 10.1128/AEM.69.11.6825-6832.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nyholm SV, Stewart JJ, Ruby EG, McFall-Ngai MJ. 2009. Recognition between symbiotic Vibrio fischeri and the haemocytes of Euprymna scolopes. Environ Microbiol 11:483–493. doi: 10.1111/j.1462-2920.2008.01788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Husnik F, Nikoh N, Koga R, Ross L, Duncan RP, Fujie M, Tanaka M, Satoh N, Bachtrog D, Wilson AC, von Dohlen CD, Fukatsu T, McCutcheon JP. 2013. Horizontal gene transfer from diverse bacteria to an insect genome enables a tripartite nested mealybug symbiosis. Cell 153:1567–1578. doi: 10.1016/j.cell.2013.05.040. [DOI] [PubMed] [Google Scholar]
- 10.McCutcheon JP, Moran NA. 2012. Extreme genome reduction in symbiotic bacteria. Nat Rev Microbiol 10:13–26. doi: 10.1038/nrmicro2670. [DOI] [PubMed] [Google Scholar]
- 11.Lopez-Madrigal S, Latorre A, Porcar M, Moya A, Gil R. 2011. Complete genome sequence of “Candidatus Tremblaya princeps” strain PCVAL, an intriguing translational machine below the living-cell status. J Bacteriol 193:5587–5588. doi: 10.1128/JB.05749-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scholle MD, Gerdes SY. 2008. Whole-genome detection of conditionally essential and dispensable genes in Escherichia coli via genetic footprinting. Methods Mol Biol 416:83–102. doi: 10.1007/978-1-59745-321-9_6. [DOI] [PubMed] [Google Scholar]
- 13.Lukjancenko O, Wassenaar TM, Ussery DW. 2010. Comparison of 61 sequenced Escherichia coli genomes. Microb Ecol 60:708–720. doi: 10.1007/s00248-010-9717-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oh S, Buddenborg S, Yoder-Himes DR, Tiedje JM, Konstantinidis KT. 2012. Genomic diversity of Escherichia isolates from diverse habitats. PLoS One 7:e47005. doi: 10.1371/journal.pone.0047005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang Z, Jones DH, Khuri S, Tsinoremas NF, Wyss T, Jander G, Wilson AC. 2013. Comparative analysis of genome sequences from four strains of the Buchnera aphidicola Mp endosymbion of the green peach aphid, Myzus persicae. BMC Genomics 14:917. doi: 10.1186/1471-2164-14-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lamelas A, Gosalbes MJ, Moya A, Latorre A. 2011. New clues about the evolutionary history of metabolic losses in bacterial endosymbionts, provided by the genome of Buchnera aphidicola from the aphid Cinara tujafilina. Appl Environ Microbiol 77:4446–4454. doi: 10.1128/AEM.00141-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aksoy S. 1995. Wigglesworthia gen. nov. and Wigglesworthia glossinidia sp. nov., taxa consisting of the mycetocyte-associated, primary endosymbionts of tsetse flies. Int J Syst Bacteriol 45:848–851. doi: 10.1099/00207713-45-4-848. [DOI] [PubMed] [Google Scholar]
- 18.Dale C, Maudlin I. 1999. Sodalis gen. nov. and Sodalis glossinidius sp. nov., a microaerophilic secondary endosymbiont of the tsetse fly Glossina morsitans morsitans. Int J Syst Bacteriol 49:267–275. doi: 10.1099/00207713-49-1-267. [DOI] [PubMed] [Google Scholar]
- 19.Zhou WG, Rousset F, O'Neill S. 1998. Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proc Biol Sci 265:509–515. doi: 10.1098/rspb.1998.0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alam U, Medlock J, Brelsfoard C, Pais R, Lohs C, Balmand S, Carnogursky J, Heddi A, Takac P, Galvani A, Aksoy S. 2011. Wolbachia symbiont infections induce strong cytoplasmic incompatibility in the tsetse fly Glossina morsitans. PLoS Pathog 7:e1002415. doi: 10.1371/journal.ppat.1002415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geiger A, Fardeau ML, Grebaut P, Vatunga G, Josenando T, Herder S, Cuny G, Truc P, Ollivier B. 2009. First isolation of Enterobacter, Enterococcus, and Acinetobacter spp. as inhabitants of the tsetse fly (Glossina palpalis palpalis) midgut. Infect Genet Evol 9:1364–1370. doi: 10.1016/j.meegid.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 22.Geiger A, Fardeau ML, Falsen E, Ollivier B, Cuny G. 2010. Serratia glossinae sp. nov., isolated from the midgut of the tsetse fly Glossina palpalis gambiensis. Int J Syst Evol Microbiol 60:1261–1265. doi: 10.1099/ijs.0.013441-0. [DOI] [PubMed] [Google Scholar]
- 23.Lindh JM, Lehane MJ. 2011. The tsetse fly Glossina fuscipes fuscipes (Diptera: Glossina) harbours a surprising diversity of bacteria other than symbionts. Antonie Van Leeuwenhoek 99:711–720. doi: 10.1007/s10482-010-9546-x. [DOI] [PubMed] [Google Scholar]
- 24.Aksoy E, Telleria EL, Echodu R, Wu Y, Okedi LM, Weiss BL, Aksoy S, Caccone A. 2014. Analysis of multiple tsetse fly populations in Uganda reveals limited diversity and species-specific gut microbiota. Appl Environ Microbiol 80:4301–4312. doi: 10.1128/AEM.00079-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Vooght L, Caljon G, Stijlemans B, De Baetselier P, Coosemans M, Van Den Abbeele J. 2012. Expression and extracellular release of a functional anti-trypanosome Nanobody® in Sodalis glossinidius, a bacterial symbiont of the tsetse fly. Microb Cell Fact 11:23. doi: 10.1186/1475-2859-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rio RVM, Hu Y, Aksoy S. 2004. Strategies of the home-team: symbioses exploited for vector-borne disease control. Trends Microbiol 12:325–336. doi: 10.1016/j.tim.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Leak S. 1999. Tsetse biology and ecology. CABI Publishing, Nairobi, Kenya. [Google Scholar]
- 28.Tobe SS, Langley PA. 1978. Reproductive physiology of Glossina. Annu Rev Entomol 23:283–307. doi: 10.1146/annurev.en.23.010178.001435. [DOI] [PubMed] [Google Scholar]
- 29.Ma WC, Denlinger DL. 1974. Secretory discharge and microflora of milk gland in tsetse flies. Nature 247:301–303. doi: 10.1038/247301a0. [DOI] [Google Scholar]
- 30.Balmand S, Lohs C, Aksoy S, Heddi A. 2013. Tissue distribution and transmission routes for the tsetse fly endosymbionts. J Invertebr Pathol 112(Suppl):S116–S122. doi: 10.1016/j.jip.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen XA, Li S, Aksoy S. 1999. Concordant evolution of a symbiont with its host insect species: molecular phylogeny of genus Glossina and its bacteriome-associated endosymbiont, Wigglesworthia glossinidia. J Mol Evol 48:49–58. doi: 10.1007/PL00006444. [DOI] [PubMed] [Google Scholar]
- 32.Cheng Q, Aksoy S. 1999. Tissue tropism, transmission and expression of foreign genes in vivo in midgut symbionts of tsetse flies. Insect Mol Biol 8:125–132. doi: 10.1046/j.1365-2583.1999.810125.x. [DOI] [PubMed] [Google Scholar]
- 33.Nogge G. 1981. Significance of symbionts for the maintenance of an optimal nutritional state for successful reproduction in hematophagous arthropods. Parasitology 82:101–104. [Google Scholar]
- 34.Akman L, Yamashita A, Watanabe H, Oshima K, Shiba T, Hattori M, Aksoy S. 2002. Genome sequence of the endocellular obligate symbiont of tsetse flies, Wigglesworthia glossinidia. Nat Genet 32:402–407. doi: 10.1038/ng986. [DOI] [PubMed] [Google Scholar]
- 35.Rio RV, Symula RE, Wang J, Lohs C, Wu YN, Snyder AK, Bjornson RD, Oshima K, Biehl BS, Perna NT, Hattori M, Aksoy S. 2012. Insight into the transmission biology and species-specific functional capabilities of tsetse (Diptera: glossinidae) obligate symbiont Wigglesworthia. mBio 3(1):e00240-11. doi: 10.1128/mBio.00240-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michalkova V, Benoit JB, Weiss BL, Attardo GM, Aksoy S. 2014. Vitamin B6 generated by obligate symbionts is critical for maintaining proline homeostasis and fecundity in tsetse flies. Appl Environ Microbiol 80:5844–5853. doi: 10.1128/AEM.01150-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weiss BL, Wang JW, Aksoy S. 2011. Tsetse immune system maturation requires the presence of obligate symbionts in larvae. PLoS Biol 9:e1000619. doi: 10.1371/journal.pbio.1000619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weiss B, Aksoy S. 2011. Microbiome influences on insect host vector competence. Trends Parasitol 27:514–522. doi: 10.1016/j.pt.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang J, Brelsfoard C, Wu Y, Aksoy S. 2013. Intercommunity effects on microbiome and GpSGHV density regulation in tsetse flies. J Invertebr Pathol 112(Suppl):S32–S39. doi: 10.1016/j.jip.2012.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pais R, Lohs C, Wu YN, Wang JW, Aksoy S. 2008. The obligate mutualist Wigglesworthia glossinidia influences reproduction, digestion, and immunity processes of its host, the tsetse fly. Appl Environ Microbiol 74:5965–5974. doi: 10.1128/AEM.00741-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nogge G. 1976. Sterility in tsetse flies (Glossina morsitans Westwood) caused by loss of symbionts. Experientia 32:995–996. doi: 10.1007/BF01933932. [DOI] [PubMed] [Google Scholar]
- 42.Herrmann KM, Weaver LM. 1999. The shikimate pathway. Annu Rev Plant Physiol Plant Mol Biol 50:473–503. doi: 10.1146/annurev.arplant.50.1.473. [DOI] [PubMed] [Google Scholar]
- 43.Dosselaere F, Vanderleyden J. 2001. A metabolic node in action: chorismate-utilizing enzymes in microorganisms. Crit Rev Microbiol 27:75–131. doi: 10.1080/20014091096710. [DOI] [PubMed] [Google Scholar]
- 44.Koury MJ, Ponka P. 2004. New insights into erythropoiesis: the roles of folate, vitamin B12, and iron. Annu Rev Nutr 24:105–131. doi: 10.1146/annurev.nutr.24.012003.132306. [DOI] [PubMed] [Google Scholar]
- 45.Lucock M. 2004. Is folic acid the ultimate functional food component for disease prevention? BMJ 328:211–214. doi: 10.1136/bmj.328.7433.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suzuki HTM, Sato H, Sonoda T, Sakauchi F, Mori M. 2006. Roles and causes of abnormal DNA methylation in gastrointestinal cancers. Asian Pac J Cancer Prev 7:177–185. [PubMed] [Google Scholar]
- 47.Moloo SK. 1971. An artificial feeding technique for Glossina. Parasitology 63:507–512. doi: 10.1017/S0031182000080021. [DOI] [PubMed] [Google Scholar]
- 48.Arcot J, Shrestha A. 2005. Folate: methods of analysis. Trends Food Sci Technol 16:253–266. doi: 10.1016/j.tifs.2005.03.013. [DOI] [Google Scholar]
- 49.Holmes DS, Bonner J. 1973. Preparation, molecular-weight, base composition, and secondary structure of giant nuclear ribonucleic-acid. Biochemistry 12:2330–2338. doi: 10.1021/bi00736a023. [DOI] [PubMed] [Google Scholar]
- 50.Steinrucken HC, Amrhein N. 1980. The herbicide glyphosate is a potent inhibitor of 5-enolpyruvyl-shikimic-acid-3-phosphate synthase. Biochem Biophys Res Commun 94:1207–1212. doi: 10.1016/0006-291X(80)90547-1. [DOI] [PubMed] [Google Scholar]
- 51.Boocock MR, Coggins JR. 1983. Kinetics of 5-enolpyruvylshikimate-3-phosphate synthase inhibition by glyphosate. FEBS Lett 154:127–133. doi: 10.1016/0014-5793(83)80888-6. [DOI] [PubMed] [Google Scholar]
- 52.Steinrucken HC, Amrhein N. 1984. 5-Enolpyruvylshikimate-3-phosphate synthase of Klebsiella pneumoniae 2. Inhibition by glyphosate [N-(phosphonomethyl)glycine]. Eur J Biochem 143:351–357. [DOI] [PubMed] [Google Scholar]
- 53.Rasband WS. 2012. ImageJ. National Institutes of Health, Bethesda, MD: http://imagej.nih.gov/ij/. [Google Scholar]
- 54.Robertson FW. 1959. Studies in quantitative inheritance. XII. Cell size and number in relation to genetic and environmental variation of body size in Drosophila. Genetics 44:869–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carron A. 2007. Correlation between wing measurements and dry body weight in male and female Ochlerotatus (Ochlerotatus) caspius (Pallas, 1771) (Diptera: Culicidae). Eur Mosquito Bull 24:4–8. [Google Scholar]
- 56.Bewick V, Cheek L, Ball J. 2004. Statistics review 12: survival analysis. Crit Care 8:389–394. doi: 10.1186/cc2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rio RV, Wu YN, Filardo G, Aksoy S. 2006. Dynamics of multiple symbiont density regulation during host development: tsetse fly and its microbial flora. Proc Biol Sci 273:805–814. doi: 10.1098/rspb.2005.3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.International Glossina Genome Initiative. 2014. Genome sequence of the tsetse fly (Glossina morsitans): vector of African trypanosomiasis. Science 344:380–386. doi: 10.1126/science.1249656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kaya B, Creus A, Yanikoglu A, Cabre O, Marcos R. 2000. Use of the Drosophila wing spot test in the genotoxicity testing of different herbicides. Environ Mol Mutagen 36:40–46. doi:. [DOI] [PubMed] [Google Scholar]
- 60.Li AP, Long TJ. 1988. An evaluation of the genotoxic potential of glyphosate. Fundam Appl Toxicol 10:537–546. doi: 10.1016/0272-0590(88)90300-4. [DOI] [PubMed] [Google Scholar]
- 61.Lipok J. 2009. Dual action of phosphonate herbicides in plants affected by herbivore—model study on black bean aphid Aphis fabae rearing on broad bean Vicia faba plants. Ecotoxicol Environ Saf 72:1701–1706. doi: 10.1016/j.ecoenv.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 62.Schneider MI, Sanchez N, Pineda S, Chi H, Ronco A. 2009. Impact of glyphosate on the development, fertility and demography of Chrysoperla externa (Neuroptera: Chrysopidae): ecological approach. Chemosphere 76:1451–1455. doi: 10.1016/j.chemosphere.2009.05.029. [DOI] [PubMed] [Google Scholar]
- 63.Evans SC, Shaw EM, Rypstra AL. 2010. Exposure to a glyphosate-based herbicide affects agrobiont predatory arthropod behaviour and long-term survival. Ecotoxicology 19:1249–1257. doi: 10.1007/s10646-010-0509-9. [DOI] [PubMed] [Google Scholar]
- 64.de Crecy-Lagard V, El Yacoubi B, de la Garza RD, Noiriel A, Hanson AD. 2007. Comparative genomics of bacterial and plant folate synthesis and salvage: predictions and validations. BMC Genomics 8:245. doi: 10.1186/1471-2164-8-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Attardo GM, Guz N, Strickler-Dinglasan P, Aksoy S. 2006. Molecular aspects of viviparous reproductive biology of the tsetse fly (Glossina morsitans morsitans): regulation of yolk and milk gland protein synthesis. J Insect Physiol 52:1128–1136. doi: 10.1016/j.jinsphys.2006.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van Ham RCHJ, Kamerbeek J, Palacios C, Rausell C, Abascal F, Bastolla U, Fernandez JM, Jimenez L, Postigo M, Silva FJ, Tamames J, Viguera E, Latorre A, Valencia A, Moran F, Moya A. 2003. Reductive genome evolution in Buchnera aphidicola. Proc Natl Acad Sci U S A 100:581–586. doi: 10.1073/pnas.0235981100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Matherly LH, Goldman DI. 2003. Membrane transport of folates. Vitam Horm 66:403–456. [DOI] [PubMed] [Google Scholar]
- 68.Ohlstein B, Spradling A. 2006. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature 439:470–474. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- 69.Fraenkel G, Blewett M. 1946. Folic acid in the nutrition of certain insects. Nature 157:697. doi: 10.1038/157697b0. [DOI] [PubMed] [Google Scholar]
- 70.Fraenkel G, Blewett M. 1947. The importance of folic acid and unidentified members of the vitamin B complex in the nutrition of certain insects. Biochem J 41:469–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Elvin CM, Liyou NE, Pearson R, Kemp DH, Dixon NE. 2003. Molecular cloning and expression of the dihydrofolate reductase (DHFR) gene from adult buffalo fly (Haematobia irritans exigua): effects of antifolates. Insect Mol Biol 12:173–183. doi: 10.1046/j.1365-2583.2003.00399.x. [DOI] [PubMed] [Google Scholar]
- 72.Affleck JG, Neumann K, Wong L, Walker VK. 2006. The effects of methotrexate on Drosophila development, female fecundity, and gene expression. Toxicol Sci 89:495–503. doi: 10.1093/toxsci/kfj036. [DOI] [PubMed] [Google Scholar]
- 73.Perry AS, Miller S. 1965. The essential role of folic acid and the effect of antimetabolites on growth and metamorphosis of housefly larvae Musca domestica L. J Insect Physiol 11:1277–1287. doi: 10.1016/0022-1910(65)90121-6. [DOI] [PubMed] [Google Scholar]
- 74.Farikou O, Njiokou F, Mbida JAM, Njitchouang GR, Djeunga HN, Asonganyi T, Simarro PP, Cuny G, Geiger A. 2010. Tripartite interactions between tsetse flies, Sodalis glossinidius and trypanosomes—an epidemiological approach in two historical human African trypanosomiasis foci in Cameroon. Infect Genet Evol 10:115–121. doi: 10.1016/j.meegid.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 75.Toh H, Weiss BL, Perkin SAH, Yamashita A, Oshima K, Hattori M, Aksoy S. 2006. Massive genome erosion and functional adaptations provide insights into the symbiotic lifestyle of Sodalis glossinidius in the tsetse host. Genome Res 16:149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Belda E, Moya A, Bentley S, Silva FJ. 2010. Mobile genetic element proliferation and gene inactivation impact over the genome structure and metabolic capabilities of Sodalis glossinidius, the secondary endosymbiont of tsetse flies. BMC Genomics 11:449. doi: 10.1186/1471-2164-11-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weiss BL, Wu YN, Schwank JJ, Tolwinski NS, Aksoy S. 2008. An insect symbiosis is influenced by bacterium-specific polymorphisms in outer-membrane protein A. Proc Natl Acad Sci U S A 105:15088–15093. doi: 10.1073/pnas.0805666105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maltz MA, Weiss BL, O'Neill M, Wu Y, Aksoy S. 2012. OmpA-mediated biofilm formation is essential for the commensal bacterium Sodalis glossinidius to colonize the tsetse fly gut. Appl Environ Microbiol 78:7760–7768. doi: 10.1128/AEM.01858-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McCutcheon JP, Moran NA. 2007. Parallel genomic evolution and metabolic interdependence in an ancient symbiosis. Proc Natl Acad Sci U S A 104:19392–19397. doi: 10.1073/pnas.0708855104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Iwai K, Kobashi M, Fujisawa H. 1970. Occurrence of Crithidia factors and folic acid in various bacteria. J Bacteriol 104:197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Graber JR, Breznak JA. 2005. Folate cross-feeding supports symbiotic homoacetogenic spirochetes. Appl Environ Microbiol 71:1883–1889. doi: 10.1128/AEM.71.4.1883-1889.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hosokawa T, Koga R, Kikuchi Y, Meng XY, Fukatsu T. 2010. Wolbachia as a bacteriocyte-associated nutritional mutualist. Proc Natl Acad Sci U S A 107:769–774. doi: 10.1073/pnas.0911476107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brelsfoard C, Tsiamis G, Falchetto M, Gomulski LM, Telleria E, Alam U, Doudoumis V, Scolari F, Benoit JB, Swain M, Takac P, Malacrida AR, Bourtzis K, Aksoy S. 2014. Presence of extensive Wolbachia symbiont insertions discovered in the genome of its host Glossina morsitans morsitans. PLoS Negl Trop Dis 8:e2728. doi: 10.1371/journal.pntd.0002728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Weitz B. 1960. Feeding habits of bloodsucking arthropods. Exp Parasitol 9:63–82. doi: 10.1016/0014-4894(60)90011-4. [DOI] [Google Scholar]
- 85.Glasgow JP, Weitz B. 1956. The natural hosts of some species of Glossina in East Africa. Trans R Soc Trop Med Hyg 50:593–612. doi: 10.1016/0035-9203(56)90065-7. [DOI] [PubMed] [Google Scholar]
- 86.Weitz B, Jackson CHN. 1955. The host-animals of Glossina morsitans at Daga-Iloi. Bull Entomol Res 46:531–538. doi: 10.1017/S0007485300039523. [DOI] [Google Scholar]
- 87.Moloo SK. 1993. The distribution of Glossina species in Africa and their natural hosts. Int J Trop Insect Sci 14:511–527. [Google Scholar]
- 88.Weitz B. 1963. The feeding habits of Glossina. Bull World Health Organ 28:711–729. [PMC free article] [PubMed] [Google Scholar]
- 89.Clausen PH, Adeyemi I, Bauer B, Breloeer M, Salchow F, Staak C. 1998. Host preferences of tsetse (Diptera: Glossinidae) based on bloodmeal identifications. Med Vet Entomol 12:169–180. doi: 10.1046/j.1365-2915.1998.00097.x. [DOI] [PubMed] [Google Scholar]
- 90.Schweigert BS, Pearson PB. 1947. The folic acid content of blood from various species. Am J Physiol 148:319–322. [DOI] [PubMed] [Google Scholar]
- 91.Leamon CP, Reddy JA, Dorton R, Bloomfield A, Emsweller K, Parker N, Westrick E. 2008. Impact of high and low folate diets on tissue folate receptor levels and antitumor responses toward folate-drug conjugates. J Pharmacol Exp Ther 327:918–925. doi: 10.1124/jpet.108.143206. [DOI] [PubMed] [Google Scholar]
- 92.Harley JM. 1971. Comparison of the susceptibility of infection with Trypanosoma rhodesiense of Glossina pallidipes, G. morsitans, G. fuscipes and G. brevipalpis. Ann Trop Med Parasitol 65:185–189. [DOI] [PubMed] [Google Scholar]
- 93.Moloo SK, Kutuza SB. 1988. Comparative study on the susceptibility of different Glossina species to Trypanosoma brucei brucei infection. Trop Med Parasitol 39:211–213. [PubMed] [Google Scholar]
- 94.Moloo SK, Kabata JM, Sabwa CL. 1994. A study on the maturation of procyclic Trypanosoma brucei brucei in Glossina morsitans centralis and G. brevipalpis. Med Vet Entomol 8:369–374. doi: 10.1111/j.1365-2915.1994.tb00100.x. [DOI] [PubMed] [Google Scholar]
- 95.Moloo SK, Okumu IO, Kuria NM. 1998. Comparative susceptibility of Glossina longipennis and G. brevipalpis to pathogenic species of Trypanosoma. Med Vet Entomol 12:211–214. doi: 10.1046/j.1365-2915.1998.00095.x. [DOI] [PubMed] [Google Scholar]
- 96.Moloo SK, Orinda GO, Sabwa CL, Minja SH, Masake RA. 1999. Study on the sequential tsetse-transmitted Trypanosoma congolense, T. brucei brucei and T. vivax infections to African buffalo, eland, waterbuck, N′Dama and Boran cattle. Vet Parasitol 80:197–213. doi: 10.1016/S0304-4017(98)00209-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.