Abstract
Cronobacter spp. (formerly defined as Enterobacter sakazakii) are opportunistic bacterial pathogens of both infants and adults. In this study, we analyzed 70 Cronobacter isolates from powdered infant formula (PIF) and an infant formula production facility in China to determine possible contamination routes. The strains were profiled by multilocus sequence typing (MLST), pulsed-field gel electrophoresis (PFGE), PCR-based O-antigen serotyping, and ompA and rpoB sequence analyses. The isolates were primarily Cronobacter sakazakii (66/70) or Cronobacter malonaticus (4/70). The strains were divided into 38 pulsotypes (PTs) using PFGE and 19 sequence types (STs) by MLST. In contrast, rpoB and ompA sequence analyses divided the strains into 10 overlapping clusters each. PCR serotyping of the 66 C. sakazakii and 4 C. malonaticus strains resulted in the identification of four C. sakazakii serotypes (O1, O2, O4, and O7) and a single C. malonaticus serotype, O2. The dominant C. sakazakii sequence types from PIF and an infant formula production factory in China were C. sakazakii clonal complex 4 (CC4) (n = 19), ST1 (n = 14), and ST64 (n = 11). C. sakazakii CC4 is a clonal lineage strongly associated with neonatal meningitis. In the process of manufacturing PIF, the spray-drying, fluidized-bed-drying, and packing areas were the main areas with Cronobacter contamination. C. sakazakii strains with the same pulsotypes (PT3 and PT2) and sequence types (ST1 and ST64) were isolated both from processing equipment and from the PIF finished product.
INTRODUCTION
Cronobacter (formerly defined as Enterobacter sakazakii) is a diverse genus in the family Enterobacteriaceae. These organisms are Gram-negative, motile, facultatively anaerobic, non-spore-forming peritrichous rod-shaped opportunistic bacterial pathogens (1–3). The genus consists of seven species: Cronobacter sakazakii, C. malonaticus, C. turicensis, C. muytjensii, C. dublinensis, C. universalis, and C. condimenti (4). Of these, C. sakazakii has been associated primarily with neonatal infections and C. malonaticus with adult infections (5–7). Cronobacter spp. are adapted to a wide range of environments, such as water, soil, plant material (wheat, rice, herbs, and spices), food production environments, and various food products (8–10). More importantly for neonatal health, Cronobacter spp. have been isolated from powdered infant formula (PIF) and milk powder production factories (11–13). Consequently, they can cause severe neonatal infections through the ingestion of contaminated PIF, especially in low-birth-weight infants (14, 15). Once infected by Cronobacter spp., patients may suffer from fatal necrotizing enterocolitis, septicemia, or meningitis, which can cause severe neurological sequelae, developmental disorders, and even death (16, 17).
Understanding the genetic diversity of the genus Cronobacter can ensure that accurate detection methods are applied for its detection and control, as well as for reliable microbial source tracking of contaminated foods such as infant formula (16–19). Consequently, the genetic diversity of the Cronobacter genus has been a considerable focus of study. An international multilocus sequence typing (MLST) database, containing >350 defined sequence types and metadata for >1,000 strains, has been established for Cronobacter (5, 20) (http://pubmlst.org/cronobacter/). The standard method uses the DNA sequences of 7 loci and recently has been expanded to 53-locus ribosomal MLST as well as 1,865-locus core genome MLST (5, 20). Joseph and colleagues applied the 7-locus method to Cronobacter sp. isolates obtained between 1950 and 2009 and showed that C. sakazakii sequence type 4 (ST4) was the predominant sequence type associated with severe cases of neonatal meningitis but not necrotizing enterocolitis (15, 20). This association between clinical presentation and sequence type was confirmed in 2011, when the isolates from a number of Cronobacter meningitis cases in the United States were shown to belong to C. sakazakii clonal complex 4 (CC4) (21). Furthermore, Cronobacter spp. from PIF and milk powder production factories in several countries had been characterized by MLST. The results showed that the main C. sakazakii sequence type in factories was CC4 (25%), followed by ST1, ST40, ST9, and ST3 (22). However, understanding of the genetic diversity of Cronobacter isolates recovered from PIF and infant formula production factories in China is still very limited. Lu et al. identified and profiled Cronobacter strains isolated from PIF in China by using phenotyping (API 20E), ribotyping, and matrix-assisted laser desorption ionization–time of flight mass spectrometry (23). However, phenotyping and physiochemical techniques do not enable determination of the relatedness of isolates, and commonly used phenotyping databases have not been updated with the changes in Cronobacter taxonomy (5, 24). In contrast, Cui et al. used the 7-locus MLST and multilocus sequence analysis (MLSA) to identify and genotype Cronobacter spp. from clinical, food, and environmental sources in China from 2010 to 2012 (25, 26).
Alternative methods to MLST for Cronobacter typing have been proposed. Although they are not as discriminatory as MLST, they can make significant contributions to our understanding of the organism. Seventeen O-antigen serotypes have been described across the genus, seven of which belong to C. sakazakii; of particular interest is C. sakazakii serotype O2, which often corresponds to C. sakazakii ST4 (5, 27). The associated lipopolysaccharide (LPS) structure could be responsible for the proinflammatory host response to infection. The outer membrane protein OmpA of Cronobacter spp. has been shown to be a potential virulence factor in the crossing of the blood-brain barrier prior to the onset of meningitis, and the sequencing of this gene has been used for identification purposes (28, 29). Sequencing of the housekeeping gene rpoB has also been used for the identification of Cronobacter species (30). However, the method is reported to give false-positive results, including misidentifying strains as Cronobacter during an outbreak (31, 32). Hence, a comparison of the four genotyping methods MLST, O-antigen serotyping, ompA analysis, and rpoB analysis is warranted. The online Cronobacter MLST databases (http://pubmlst.org/cronobacter/) facilitate such comparison, since all four typing schemes are included, and data from previous publications are available through open access.
In our study, 70 Cronobacter strains were isolated from PIF in different parts of China and an infant formula production factory from 2009 to 2012. We determined the genetic diversity of these strains by MLST, pulsed-field gel electrophoresis (PFGE), and O-antigen serotyping, analyzed the phylogenetic relationships of the ompA and rpoB genes, and revealed the possible routes of Cronobacter contamination during PIF manufacture. These results are important for understanding the genetic diversity of Cronobacter spp. in China and for enabling microbial source tracking to control the occurrence of Cronobacter spp. in the PIF processing chain.
MATERIALS AND METHODS
Bacterial strains.
A total of 70 Cronobacter strains were isolated from PIF (n = 43) and an infant formula production factory (n = 27) in China between 2009 and 2012. Details about the isolates are given in Tables 1 and 2. Forty-three Cronobacter sp. isolates had been recovered previously from 1,228 PIF samples from different areas of China. The 27 Cronobacter strains for which data are shown in Table 2 had been isolated from 375 samples taken over a 4-year period from an infant formula factory environment and finished products from the factory. All the isolates had been provisionally identified as Cronobacter spp. using API 20E, and this identification had been confirmed using 16S rRNA gene sequencing (23). All the strains were grown in Luria-Bertani (LB) broth at 37°C for 12 h, streaked onto tryptic soy agar (TSA) plates, and then cultivated at 37°C for 24 h. Single colonies in the TSA plates were inoculated into the LB medium and were cultivated at 37°C for 18 h.
TABLE 1.
MLST, PFGE, O-antigen serotyping, and ompA and rpoB analyses of Cronobacter strains isolated from PIF in different parts of China from 2009 to 2012a
| Cronobacter species | Strain no. | ID | Regionb | ST | CC | PT | OT | ompA allele designation | rpoB cluster |
|---|---|---|---|---|---|---|---|---|---|
| C. sakazakii | CE9 | 874 | NE China | 4 | 4 | 1 | O2 | 6 | 1 |
| C. sakazakii | CE10 | 875 | NE China | 4 | 4 | 1 | O2 | 6 | 1 |
| C. sakazakii | CE11 | 876 | E China | 4 | 4 | 1 | O2 | 6 | 1 |
| C. sakazakii | CE12 | 877 | E China | 4 | 4 | 1 | O2 | 6 | 1 |
| C. sakazakii | CE17 | 878 | N China | 4 | 4 | 1 | O2 | 6 | 1 |
| C. sakazakii | CE19 | 880 | N China | 4 | 4 | 1 | O2 | 6 | 1 |
| C. sakazakii | CE20 | 881 | N China | 4 | 4 | 1 | O2 | 6 | 1 |
| C. sakazakii | CE22 | 882 | N China | 4 | 4 | 1 | O2 | 6 | 1 |
| C. sakazakii | CE23 | 883 | N China | 4 | 4 | 1 | O2 | 6 | 1 |
| C. sakazakii | CE18 | 879 | N China | 4 | 4 | 1 | O2 | 21 | 1 |
| C. sakazakii | CE1 | 872 | NE China | 4 | 4 | 15 | O2 | 6 | 1 |
| C. sakazakii | CE7 | 873 | NE China | 4 | 4 | 16 | O2 | 6 | 1 |
| C. sakazakii | CE27 | 884 | NE China | 4 | 4 | 17 | O2 | 6 | 1 |
| C. sakazakii | CE48 | 885 | NE China | 4 | 4 | 19 | O2 | 6 | 1 |
| C. sakazakii | CE49 | 886 | NE China | 4 | 4 | 20 | O2 | 6 | 1 |
| C. sakazakii | CE69 | 865 | NE China | 1 | 1 | 3 | O1 | 3 | 2 |
| C. sakazakii | CE21 | 858 | NE China | 1 | 1 | 8 | O1 | 3 | 2 |
| C. sakazakii | CE24 | 859 | NE China | 1 | 1 | 6 | O1 | 3 | 2 |
| C. sakazakii | CE43 | 860 | NE China | 1 | 1 | 10 | O1 | 3 | 2 |
| C. sakazakii | CE47 | 861 | NE China | 1 | 1 | 11 | O1 | 54 | 2 |
| C. sakazakii | CE25 | 905 | NE China | 64 | 64 | 33 | O2 | 6 | 3 |
| C. sakazakii | CE34 | 910 | NE China | 64 | 64 | 35 | O2 | 6 | 3 |
| C. sakazakii | CE54 | 912 | NE China | 64 | 64 | 37 | O2 | 6 | 3 |
| C. sakazakii | CE51 | 897 | NW China | 21 | 21 | 5 | O1 | 6 | 6 |
| C. sakazakii | CE53 | 899 | NW China | 21 | 21 | 5 | O1 | 6 | 6 |
| C. sakazakii | CE52 | 898 | NW China | 21 | 21 | 28 | O1 | 6 | 6 |
| C. sakazakii | CE41 | 892 | NE China | 12 | 4 | O2 | 5 | 4 | |
| C. sakazakii | CE44 | 893 | NE China | 12 | 4 | O4 | 5 | 4 | |
| C. sakazakii | CE38 | 894 | NE China | 12 | 25 | O4 | 5 | 4 | |
| C. sakazakii | CE16 | 707 | N China | 259 | 24 | ND | 6 | 1 | |
| C. sakazakii | CE28 | 895 | NE China | 17 | 17 | 26 | O2 | 6 | 6 |
| C. sakazakii | CE15 | 900 | N China | 22 | 29 | O2 | 6 | 1 | |
| C. sakazakii | CE50 | 901 | NE China | 22 | 30 | O2 | 6 | 1 | |
| C. sakazakii | CE56 | 902 | NE China | 31 | 31 | O2 | 23 | 5 | |
| C. sakazakii | CE29 | 903 | NE China | 40 | 45 | 32 | O4 | 6 | 7 |
| C. sakazakii | CE8 | 917 | N China | 83 | 83 | 38 | O7 | 6 | 5 |
| C. sakazakii | CE26 | 708 | NE China | 268 | 4 | 36 | O2 | 6 | 1 |
| C. sakazakii | CE55 | 709 | NE China | 260 | ND | O1 | 6 | 2 | |
| C. sakazakii | CE13 | 890 | N China | 8 | 8 | ND | O1 | 5 | 5 |
| C. sakazakii | CE14 | 891 | N China | 8 | 8 | ND | O1 | 5 | 5 |
| C. malonaticus | CMa2 | 706 | S China | 258 | 7 | MaO2 | 24 | 8 | |
| C. malonaticus | CMa35 | 918 | NE China | 201 | 7 | 9 | MaO2 | 8 | 9 |
| C. malonaticus | CMa3 | 919 | NE China | 258 | 18 | MaO2 | 24 | 9 |
ID, strain identification code in the Cronobacter MLST databases; ST, sequence type; CC, clonal complex (defined as clusters of sequence types with single-locus variants); PT, pulsotype; OT, O-antigen serotype; ND, not detected.
E, east; N, north; S, south; NE, northeast; NW, northwest.
TABLE 2.
MLST, PFGE, O-antigen serotyping, and ompA and rpoB analyses of Cronobacter strains isolated from an infant formula production factory in China from 2009 to 2012a
| Cronobacter species | Strain no. | ID | Source | ST | CC | PT | OT | ompA allele designation | rpoB cluster |
|---|---|---|---|---|---|---|---|---|---|
| C. sakazakii | CE61 | 887 | Final product | 4 | 4 | 21 | O2 | 55 | 1 |
| C. sakazakii | CE64 | 888 | Final product | 4 | 4 | 22 | O2 | 6 | 1 |
| C. sakazakii | CE67 | 889 | Final product | 4 | 4 | 23 | O2 | 6 | 1 |
| C. sakazakii | CE60 | 863 | U valve tube | 1 | 1 | 3 | O1 | 3 | 2 |
| C. sakazakii | CE63 | 864 | Powder lumps after spray drying | 1 | 1 | 3 | O1 | 3 | 2 |
| C. sakazakii | CE70 | 866 | Final product | 1 | 1 | 3 | O1 | 3 | 2 |
| C. sakazakii | CE71 | 867 | Final product | 1 | 1 | 3 | O1 | 3 | 2 |
| C. sakazakii | CE72 | 868 | Final product | 1 | 1 | 3 | O1 | 3 | 2 |
| C. sakazakii | CE73 | 869 | Final product | 1 | 1 | 3 | O1 | 3 | 2 |
| C. sakazakii | CE74 | 870 | Powder lumps on fluidized bed | 1 | 1 | 3 | O1 | 3 | 2 |
| C. sakazakii | CE59 | 862 | Raw material | 1 | 1 | 12 | O1 | 3 | 2 |
| C. sakazakii | CE79 | 871 | Powder lumps on fluidized bed | 1 | 1 | 14 | O1 | 3 | 2 |
| C. sakazakii | CE30 | 906 | Final product | 64 | 64 | 2 | O2 | 6 | 3 |
| C. sakazakii | CE31 | 908 | Final product | 64 | 64 | 2 | O2 | 6 | 3 |
| C. sakazakii | CE36 | 911 | Powder lumps on fluidized bed | 64 | 64 | 2 | O2 | 6 | 3 |
| C. sakazakii | CE62 | 913 | Powder lumps on fluidized bed | 64 | 64 | 2 | O2 | 6 | 3 |
| C. sakazakii | CE68 | 914 | Powder lumps on fluidized bed | 64 | 64 | 2 | O2 | 6 | 3 |
| C. sakazakii | CE77 | 915 | Final product | 64 | 64 | 2 | O2 | 6 | 3 |
| C. sakazakii | CE78 | 916 | Final product | 64 | 64 | 2 | O2 | 6 | 3 |
| C. sakazakii | CE75 | 711 | Final product | 261 | 64 | 2 | O2 | 6 | 3 |
| C. sakazakii | CE76 | 922 | Final product | 261 | 64 | 2 | O2 | 6 | 3 |
| C. sakazakii | CE33 | 909 | Raw material | 64 | 64 | 34 | O2 | 6 | 3 |
| C. sakazakii | CE58 | 896 | Raw material | 17 | 17 | 27 | O2 | 22 | 6 |
| C. sakazakii | CE32 | 904 | Final product | 50 | 2 | O2 | 21 | 3 | |
| C. sakazakii | CE65 | 710 | Fixed bed | 269 | 13 | O7 | 6 | 10 | |
| C. sakazakii | CE66 | 921 | Fixed bed | 269 | 6 | O7 | 6 | 10 | |
| C. malonaticus | CMa5 | 920 | Raw material | 258 | 7 | MaO2 | 24 | 9 |
ID, strain identification code in the Cronobacter MLST databases; ST, sequence type; CC, clonal complex (defined as clusters of sequence types with single-locus variants); PT, pulsotype; OT, O-antigen serotype.
For accurate determination of the interspecific phylogenetic relationships, C. sakazakii ATCC 29544T, ATCC BAA-894, ATCC 29004, and ATCC 12868 were used as the C. sakazakii species reference strains. C. malonaticus CDC 105877T, C. dublinensis LMG 23823T, C. turicensis LMG 23827T, C. universalis NCTC 9529T, C. condimenti LMG 26250T, and C. muytjensii ATCC 51329T were used as the type strains of the remaining species.
MLST analysis.
Genomic DNA was extracted by the TIANamp Bacteria DNA kit (Tiangen Biotech [Beijing] Co., Ltd., Beijing, China) and was amplified using the 7 primer pairs described previously (24). The PCR products were sequenced by Life Technologies Limited (Shanghai, China). All allele profiles and ST assignments can be obtained from the Cronobacter MLST open-access database (http://pubmlst.org/cronobacter/). The phylogenetic relationship of the concatenated sequences (3,036 bp) of the seven housekeeping genes (atpD, fusA, glnS, gltB, gyrB, infB, and ppsA) was analyzed using the maximum-likelihood algorithm in MEGA, version 6, with 1,000 bootstrap replicates. The nucleotide diversity indices were determined using DnaSP software, version 5.0.
PFGE analysis.
The pulsed-field gel electrophoresis (PFGE) scheme for Cronobacter spp. was that described by previous studies (11, 16). Electrophoresis was performed with the auto algorithm model on a CHEF DR-II electrophoresis system (Bio-Rad Laboratories, Hercules, CA, USA) with an initial switch time of 2.16 s, a final switch time of 63.80 s, a condensation temperature of 14°C, and the electric field alternates of 120°. A lambda ladder PFGE marker (New England BioLabs) was used as the DNA size marker for standard analysis. After electrophoresis for 18 h, the gels were stained for 30 min with 10 mg ml−1ethidium bromide and were then destained for 30 min with distilled water. The PFGE gels were visualized and photographed under a UV transilluminator. The gel images were analyzed using the Dice coefficient and the unweighted pair group method with arithmetic means (UPGMA) in GelCompar II software, version 5.1 (Applied Maths, Sint-Martens-Latem, Belgium), with a 1.5% band position tolerance. The intraspecific diversity was reflected with the Shannon-Weiner index.
O-antigen serotype analysis.
Using multiplex serotyping PCR, the serotypes of all 70 Cronobacter isolates were determined with the primers and reaction conditions given in previous publications (33, 34). The mixed primers for C. sakazakii are composed of seven pairs of primers encoding the gene sequences of C. sakazakii serotypes O1 to O7, and the mixed primers for C. malonaticus consist of two pairs of primers encoding the sequences of C. malonaticus serotypes O1 and O2. The resulting PCR products were sequenced (Life Technologies Limited, China) and were analyzed using the neighbor-joining algorithm in MEGA, version 6.
ompA and rpoB sequence analyses.
The specific PCR amplifications of ompA and rpoB were performed as described by Mohan Nair and Venkitanarayanan (28) and Li et al. (30), respectively. The PCR products were sequenced (Life Technologies Ltd., China). The ompA sequences were submitted to the MLST Cronobacter databases for allele designation, and the rpoB sequences were submitted to the NCBI. The phylogenetic trees of ompA and rpoB were constructed using the maximum-likelihood algorithm in MEGA, version 6, with 1,000 bootstrap replicates.
Nucleotide sequence accession numbers.
The rpoB sequences determined in this study have been submitted to the NCBI under GenBank accession numbers KP192773 to KP192846.
RESULTS
MLST analysis of Cronobacter spp. isolated from powdered infant formula and the production environment.
Initial experiments using 16S rRNA gene sequencing had identified the Cronobacter isolates as either C. sakazakii or C. malonaticus. By use of 7-locus MLST, the 70 Cronobacter strains clustered into 19 sequence types, shown in Tables 1 and 2. Seventeen sequence types were in the species C. sakazakii, and the remaining two were in C. malonaticus. Overall, C. sakazakii was the dominant (66/70 [94.29%]) species isolated from both PIF and its production environment. The main C. sakazakii sequence types were C. sakazakii ST4 (18/66 [27.27%]), ST1 (14/66 [21.21%]), and ST64 (11/66 [16.67%]). Only four strains of C. malonaticus were isolated: three strains from PIF (C. malonaticus ST258) and one from the manufacturing plant (C. malonaticus ST258). Of the 19 sequence types isolated, 6 had not been reported before and therefore were assigned new sequence types in the Cronobacter MLST databases: ST258, ST259, ST268, ST260, ST269, and ST261. The new allele numbers assigned were atpD89, glnS107, glnS108, gltB127, gltB128, gyrB125, ppsA160, and ppsA161.
Multilocus sequence analysis (MLSA).
The nucleotide diversity (π) of the seven housekeeping genes was analyzed using DnaSP software, version 5.0 (Table 3). The GC contents of all alleles ranged from 53.61% (fusA) to 62.84% (ppsA), averaging 58.84%, a level similar to the whole-genome GC content of C. sakazakii BAA-894 (57%) (2). The number of alleles ranged from 10 (atpD) to 16 (gyrB and ppsA). The proportion of fragments found as polymorphic sites (expressed as a percentage) ranged from 2.74% (fusA) to 9.71% (gyrB), averaging 6.13% (186 polymorphic sites) of the concatenated 7 alleles (total length, 3,036 nucleotides). The nucleotide diversity of the 3,036 nucleotides was 0.0176, ranging from 0.0084 (atpD) to 0.0300 (gyrB and ppsA) per individual gene, suggesting that the nucleotide diversity of atpD was the lowest and the nucleotide diversity values of gyrB and ppsA were higher than those for other housekeeping genes. The ratio of nonsynonymous to synonymous mutations (Ka/Ks) of the concatenated sequences was 0.0014 and ranged from 0 (atpD, gltB, gyrB, and infB) to 0.0471 (fusA). The Ka/Ks ratios of all seven housekeeping genes were less than 1.
TABLE 3.
Polymorphism of the 7 MLST housekeeping genes for 66 C. sakazakii and 4 C. malonaticus isolates
| Locus | Size (bp) | No. of alleles | GC content (%) | No. (%) of polymorphic sites | Ksa | Kab | Ka/Ks | πc |
|---|---|---|---|---|---|---|---|---|
| atpD | 390 | 10 | 59.51 | 11 (2.82) | 0.0305 | 0 | 0 | 0.0084 |
| fusA | 438 | 12 | 53.61 | 12 (2.74) | 0.0705 | 0.0033 | 0.0471 | 0.0183 |
| glnS | 363 | 14 | 57.56 | 21 (5.79) | 0.0727 | 0.0005 | 0.0071 | 0.0163 |
| gltB | 507 | 15 | 61.72 | 42 (8.28) | 0.0918 | 0 | 0 | 0.0219 |
| gyrB | 402 | 16 | 56.95 | 39 (9.71) | 0.1309 | 0 | 0 | 0.0300 |
| infB | 441 | 13 | 58.52 | 14 (3.17) | 0.0542 | 0 | 0 | 0.0129 |
| ppsA | 495 | 16 | 62.84 | 47 (9.49) | 0.1391 | 0.0003 | 0.0022 | 0.0300 |
| Concatenated sequence | 3,036 | 19 | 58.84 | 186 (6.13) | 0.0739 | 0.0001 | 0.0014 | 0.0176 |
Ks, number of synonymous substitutions.
Ka, number of nonsynonymous substitutions.
π, nucleotide diversity.
Phylogenetic relationship of C. sakazakii and C. malonaticus isolates.
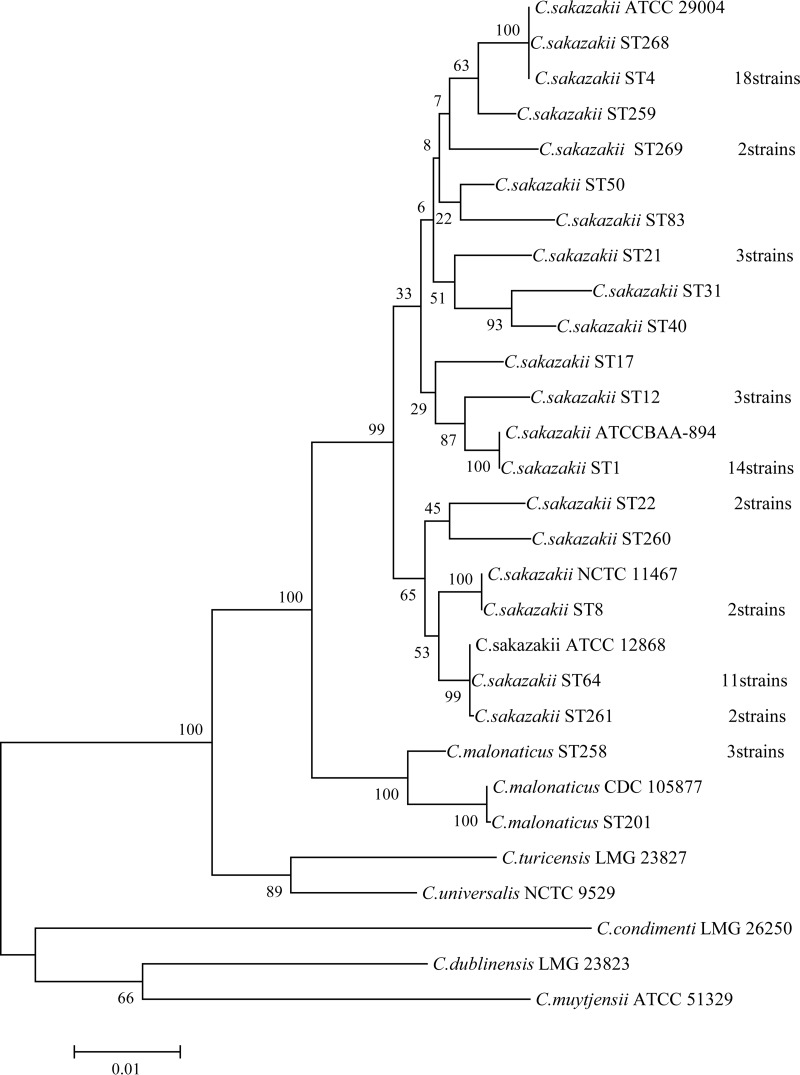
A phylogenetic tree based on the concatenated sequences of the seven housekeeping genes (total length, 3,036 bp) for the C. sakazakii and C. malonaticus isolates and 10 reference strains was constructed (Fig. 1). The 66 C. sakazakii strains clustered in the same clade with >95% similarity. C. malonaticus was closer to C. sakazakii than the other five Cronobacter species.
FIG 1.
Maximum-likelihood tree of the spliced sequences of the 7 loci (3,036 bp) for the 80 strains. Seventy Cronobacter strains isolated from PIF or an infant formula production factory, 4 reference strains (C. sakazakii ATCC BAA-894, C. sakazakii ATCC 29004, C. sakazakii ATCC 29544, and C. sakazakii ATCC 12868), and 6 type strains (C. malonaticus CDC 105877T, C. dublinensis LMG 23823T, C. turicensis LMG 23827T, C. universalis NCTC 9529T, C. condimenti LMG 26250T, and C. muytjensii ATCC 51329T) were included. The tree was obtained using MEGA, version 6.0, with 1,000 bootstrap replicates.
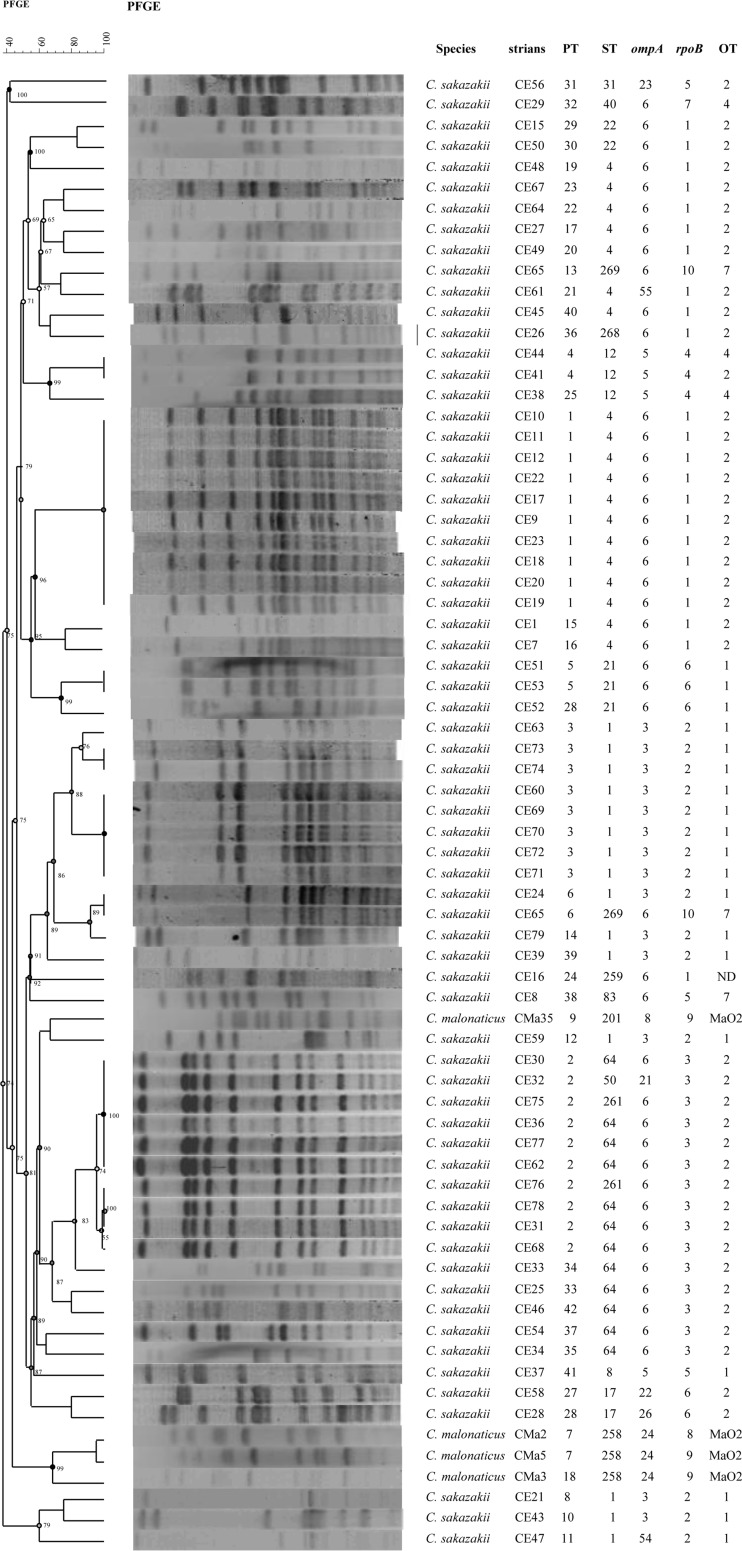
PFGE analysis of C. sakazakii and C. malonaticus isolates.
A total of 74 Cronobacter sp. strains, comprising the 70 Cronobacter isolates and 4 C. sakazakii reference strains, were analyzed using PFGE. Three C. sakazakii strains (CE13, CE14, and CE55) could not be digested with XbaI; therefore, only 67 isolates were analyzed using UPGMA in GelCompar II software, version 5.1 (Fig. 2). By using 95% similarity as the critical threshold, the 67 Cronobacter strains formed 38 pulsotypes. The major pulsotypes were PT1 (10/71 [14%]), PT2 (10/71 [14%]), and PT3 (8/71 [11%]). Strains belonging to the same sequence type corresponded to a number of pulsotypes. For example, C. sakazakii ST4 was further divided into 10 pulsotypes (Shannon-Weiner index, 2.50); C. sakazakii ST1 contained 8 pulsotypes (Shannon-Weiner index, 2.31); and C. sakazakii ST64 consisted of 5 pulsotypes (Shannon-Weiner index, 1.58). The C. sakazakii PT1 strains were mainly from northern China.
FIG 2.
Dendrogram based on XbaI-mediated PFGE profiles of 71 Cronobacter spp. The tree was drawn using UPGMA and the Dice coefficient with 1.5% tolerance.
ompA and rpoB analysis of C. sakazakii and C. malonaticus.
C. sakazakii and C. malonaticus strains were distinguishable by both ompA and rpoB sequences, each of which formed 10 clusters that overlapped (Tables 1 and 2). The majority of strains had ompA6 (42/70 [60%]) and belonged to nine sequence types: ST4, ST64, ST17, ST21, ST40, ST259, ST260, ST261, and ST269. There were eight different rpoB gene clusters across 67 C. sakazakii isolates, and two further clusters for the 3 C. malonaticus strains. The C. sakazakii rpoB cluster 1 strains (24/70 [34.28%]), which belonged to ST4, ST22, ST250, ST260, ST268, and ST269, overlapped with some of those in ompA6.
O-antigen serotype analysis of Cronobacter sp. isolates.
A neighbor-joining tree of the C. sakazakii and C. malonaticus serotypes is shown in Fig. 3. According to the different sequence sizes of target genes, all strains were divided into five serotypes. Each serotype was composed of a number of STs (Fig. 3; Tables 1 and 2). The largest number of strains belonged to C. sakazakii serotype O2 (39/70 [55.71%]) and included eight C. sakazakii sequence types: ST4, ST17, ST21, ST31, ST50, ST64, ST261, and ST268. C. sakazakii serotype O1 (20/70 [28.57%]) contained C. sakazakii ST1, ST8, ST21, and ST260. C. sakazakii serotype O4 (4/70 [5.71%]) was composed of C. sakazakii strains belonging to ST12 and ST40. C. sakazakii serotype O7 was composed of three strains, which belonged to ST83 and ST269 (two strains). Finally, C. malonaticus serotype O2 (4/70 [5.71%]) contained C. malonaticus ST201 and ST258. No sequence types were found in more than one O-antigen serotype. Furthermore, C. sakazakii serotype O2 (55.71%) and C. sakazakii serotype O1 (28.57%) were the dominant serotypes isolated from PIF and an infant formula production factory.
FIG 3.
Neighbor-joining tree of O-antigen sequences from 18 Cronobacter isolates. The tree was structured by MEGA, version 6, with 1,000 bootstrap replicates. The 18 Cronobacter isolates represent 18 different STs of Cronobacter spp.
Microbial source tracking.
The powdered infant formula production environment and finished products of a production factory had been monitored continuously from 2009 to 2012. Twenty-six C. sakazakii isolates and one C. malonaticus isolate were recovered; details are given in Table 2. The isolates were recovered from raw material, a U valve tube, powder lumps after spray drying, powder lumps on a fluidized bed, a fixed bed, and finished products. It is noteworthy that eight C. sakazakii PT2 (ST64) strains and seven C. sakazakii PT3 (ST1) strains were isolated over a 3-month period from both processing equipment and finished products. Three C. sakazakii ST4 strains were recovered from the PIF finished product but not from environmental samples. In total, one-third (9/27) of C. sakazakii isolates belonged to ST1. C. sakazakii ST17, ST50, ST261, and ST269 and C. malonaticus ST258 were not detected in the finished products.
DISCUSSION
It is well known that Cronobacter spp. can cause severe infections in neonates and infants through ingestion of contaminated infant formula (3, 5, 9, 35). China is a major market for PIF, and considerable attention is given to its safety. However, our current knowledge of this organism in PIF and the manufacturing environment in China is limited. Thus, it is necessary to improve our understanding of the diversity of this genus and possible routes of PIF contamination.
Initial identification of these strains using phenotyping (API 20E) gave a presumptive identification of Enterobacter sakazakii. However, the use of API 20E for identification of this organism is very limited, because the corresponding database has not been updated with the Cronobacter genus. Furthermore, 16S rRNA gene sequence analysis could not determine whether the strains were C. sakazakii or C. malonaticus. This issue is well known and is due to errors in GenBank and microheterogeneities within the multiple copies of 16S rRNA in the Cronobacter genome (24). ompA and rpoB sequence analyses were able to correctly identify the species of the isolates but did not distinguish between isolates within the species as much as MLST or PFGE (Fig. 2). The lack of reliable power to distinguish between C. sakazakii and C. malonaticus by 16S rRNA gene sequencing has been reported previously and has led to the adoption of fusA DNA sequencing as a suitable alternative (5, 20). This also has the advantage of being 1 of the 7 loci used in the MLST scheme. Consequently, all Cronobacter spp. can be identified using the fusA allele and then fully typed using the remaining 6 loci of the MLST scheme (Fig. 1) (5, 20). The phylogenetic tree of concatenated sequences (3,036 bp) from the seven housekeeping genes reflects the whole-genome phylogeny of the Cronobacter genus (4). To date, the curated Cronobacter MLST databases have >1,000 strains, >350 defined 7-locus STs, and >100 searchable whole genomes as well as metadata (http://pubmlst.org/cronobacter/). They also contain the profiles for other typing schemes, such as the ompA and rpoB schemes.
PFGE is a well-established means of profiling bacterial strains for epidemiological purposes but is not used for species identification. In this study, PFGE distinguished between strains within the same sequence type. For example, C. sakazakii ST4 strains were divided into 10 pulsotypes. This is a very important observation given the life-threatening meningitis infections associated with this sequence type and the application of PFGE in epidemiological investigations, as well as in source tracking in a PIF production facility. However, as reported by other researchers, not all strains can be analyzed by PFGE, and the method cannot be used to identify Cronobacter isolates (2, 11). Therefore, a stepwise analysis by MLST followed by PFGE may be suitable for comprehensive profiling of Cronobacter isolates.
In previous studies, ST4, ST1, ST40, ST9, and ST3 were shown to be the main C. sakazakii sequence types isolated from PIF and milk powder production factories in several countries (5, 21). In this study, ST4, ST1, and ST64 were recovered from PIF and an infant formula production factory. Although the genetic basis of Cronobacter virulence has yet to be established for different clinical presentations, certain Cronobacter sequence types have been found to be associated with particular infections: C. sakazakii CC4 is strongly associated with cases of neonatal meningitis, C. sakazakii ST12 with necrotizing enterocolitis, and C. malonaticus CC7 with adult infections (5). Among the 19 sequence types isolated in this study, C. sakazakii ST268 differed from ST4 at only one locus (gltB, position 144, C or T, respectively) and is therefore within C. sakazakii CC4. C. malonaticus ST201 is in CC7, since it differed by only one locus (gltB, position 256, C or T, respectively) from the ST7 profile. Therefore, MLST not only identified and genotyped isolates but also reflected the potential clinical significance of neonatal infection and enabled accurate source tracking and/or attribution.
The Ka/Ks values (0 to 0.0471) showed that the 7 MLST alleles were in phase-stabilizing or purifying selection, which is consistent with the characteristics of housekeeping genes. The strong clonality within the C. sakazakii and C. malonaticus species, as given by the stability of the clonal group equivalents using 7-locus MLST, 54-locus ribosomal MLST, and 1,865-locus core genome MLST (5), should be noted.
Although ompA and rpoB sequence analyses have been used for identification purposes, neither method gave greater discrimination between strains than either MLST or PFGE. Typing of strains according to their O antigen was commonly used for bacterial pathogens such as Salmonella spp., Escherichia coli, and Listeria monocytogenes before DNA-sequencing methods became more accessible. Though of interest due to the considerable knowledge of the O antigen in other members of the Enterobacteriaceae, O-antigen serotyping of Cronobacter spp. has revealed only 17 distinguishing profiles across the seven species in the genus (33). The method also requires the species of the isolates to be identified before serotyping, due to the overlap of serotypes across different Cronobacter species (33, 34). C. sakazakii serotype O2 is the primary serotype of isolates from powdered infant formula from Chinese retail markets, and this serotype often corresponds with C. sakazakii ST4, which causes neonatal meningitis (15, 21). However, C. sakazakii serotype O2 is also found to comprise several sequence types that are not yet of such clinical importance. In this study, these sequence types were ST17, ST21, ST31, ST50, ST64, ST261, and ST268. This broad range of sequence types is also shown in the Cronobacter MLST databases, where 28 sequence types are given as C. sakazakii serotype O2. The O antigen does not follow the phylogeny of the Cronobacter genus; the DNA sequence for the same serotype occurs in more than one Cronobacter species, and even in E. coli O29 and O103 (34).
Tracking the sources of Cronobacter strains can reveal the possible intrinsic contamination routes in the production of PIF, enabling the reduction of contamination with Cronobacter spp. Craven et al. investigated the Cronobacter contamination of five Australian milk powder factories by PFGE. They suggested that Cronobacter strains are spread in the milk powder production environment by the movements of air, milk powder dust particles, and personnel (11). Sonbol et al. further studied the Cronobacter strains from the study of Craven et al. by MLST profiling. They found that C. sakazakii ST4 was present in tanker bays, factory roofs, the milk powder processing environment, and the outside grounds of five milk powder factories in a 1-year period (22). In a survey of PIF and follow-up formulas (FUF), it was reported that 49 of 399 samples were contaminated with Cronobacter spp. (36). The authors speculated that nutrient addition during PIF and FUF production increased the risk of intrinsic product contamination. However, in our results, the strains isolated from the raw materials and nutrients were not found in the finished products. Instead, the spray-drying, fluidized-bed, and packing areas were regarded as the major contamination sites. These results indicate that these areas of the plant should be considered the higher-risk processing areas and should be subjected to enhanced surveillance activity.
Dust particles in the air of a manufacturing plant can be a vector of Cronobacter dispersal, and higher concentrations are found during bagging and the final packing of the PIF (37, 38). Thus, during PIF production, especially in the spray-drying, fluidized-bed, and packing areas, once the air is contaminated with Cronobacter spp., the strains may have an opportunity to contaminate the final product. To reduce contamination by Cronobacter spp. in the production of PIF, some measures should be taken, such as keeping the air humidity low, reducing the number of dust particles in the air, cleaning production equipment frequently, and treating waste powder effectively. In addition, the population of airborne microorganisms was closely related to the climate and was higher in the winter than in the summer (38). Finally, it should be noted that contamination of powdered infant formula can also occur due to extrinsic contamination from the preparation equipment and personnel (3, 19).
This study has improved our understanding of the genetic diversity of Cronobacter spp. isolated from PIF and the production environment of PIF in China and has provided guidance for reducing Cronobacter contamination in the production of PIF.
ACKNOWLEDGMENTS
This study was supported by the National Key Technology Support Program (2013BAD18B11, 2012BAD29B07), the Science Foundation for Distinguished Young Scholars of Heilongjiang Province (JC201415), the National Natural Science Foundation of China (31171718), the National Science and Technology Project (2011AA100902), and the Promotion Program for Innovation of Scientific Research in Heilongjiang Province (YC13D005).
REFERENCES
- 1.Gurtler JB, Kornacki JL, Beuchat LR. 2005. Enterobacter sakazakii: a coliform of increased concern to infant health. Int J Food Microbiol 104:1–34. doi: 10.1016/j.ijfoodmicro.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Kucerova E, Clifton SW, Xia X-Q, Long F, Porwollik S, Fulton L, Fronick C, Minx P, Kyung K, Warren W, Fulton R, Feng D, Wollam A, Shah N, Bhonagiri V, Nash WE, Hallsworth-Pepin K, Wilson RK, McClelland M, Forsythe SJ. 2010. Genome sequence of Cronobacter sakazakii BAA-894 and comparative genomic hybridization analysis with other Cronobacter species. PLoS One 5:e9556. doi: 10.1371/journal.pone.0009556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yan QQ, Condell O, Power K, Butler F, Tall BD, Fanning S. 2012. Cronobacter species (formerly known as Enterobacter sakazakii) in powdered infant formula: a review of our current understanding of the biology of this bacterium. J Appl Microbiol 113:1–15. doi: 10.1111/j.1365-2672.2012.05281.x. [DOI] [PubMed] [Google Scholar]
- 4.Joseph S, Desai P, Ji Y, Cummings CA, Shih R, Degoricija L, Rico A, Brzoska P, Hamby SE, Masood N, Hariri S, Sonbol H, Chuzhanova N, McClelland M, Furtado MR, Forsythe SJ. 2012. Comparative analysis of genome sequences covering the seven Cronobacter species. PLoS One 7:e49455. doi: 10.1371/journal.pone.0049455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forsythe SJ, Dickins B, Jolley KA. 2014. Cronobacter, the emergent bacterial pathogen Enterobacter sakazakii comes of age; MLST and whole genome sequence analysis. BMC Genomics 15:1121. doi: 10.1186/1471-2164-15-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holý O, Forsythe S. 2014. Cronobacter spp. as emerging causes of healthcare-associated infection. J Hosp Infect 86:169–177. doi: 10.1016/j.jhin.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Holý O, Petrzelova J, Hanulik V, Chroma M, Matouskova I, Forsythe SJ. 2014. Epidemiology of Cronobacter spp. isolates from patients admitted to the Olomouc University Hospital (Czech Republic). Epidemiol Mikrobiol Imunol 63:69–72. [PubMed] [Google Scholar]
- 8.Friedemann M. 2007. Enterobacter sakazakii in food and beverages (other than infant formula and milk powder). Int J Food Microbiol 116:1–10. doi: 10.1016/j.ijfoodmicro.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 9.Iversen C, Forsythe S. 2004. Isolation of Enterobacter sakazakii and other Enterobacteriaceae from powdered infant formula milk and related products. Food Microbiol 21:771–777. doi: 10.1016/j.fm.2004.01.009. [DOI] [Google Scholar]
- 10.Joseph S, Cetinkaya E, Drahovska H, Levican A, Figueras MJ, Forsythe SJ. 2012. Cronobacter condimenti sp. nov., isolated from spiced meat, and Cronobacter universalis sp. nov., a species designation for Cronobacter sp. genomospecies 1, recovered from a leg infection, water and food ingredients. Int J Syst Evol Microbiol 62:1277–1283. doi: 10.1099/ijs.0.032292-0. [DOI] [PubMed] [Google Scholar]
- 11.Craven HM, McAuley CM, Duffy LL, Fegan N. 2010. Distribution, prevalence and persistence of Cronobacter (Enterobacter sakazakii) in the nonprocessing and processing environments of five milk powder factories. J Appl Microbiol 109:1044–1052. doi: 10.1111/j.1365-2672.2010.04733.x. [DOI] [PubMed] [Google Scholar]
- 12.Fu S, Gao J, Li Y, Chen H. 2011. Isolation of Cronobacter spp. isolates from infant formulas and their survival in the production process of infant formula. Czech J Food Sci 29:391–399. [Google Scholar]
- 13.Mullane NR, Whyte P, Wall PG, Quinn T, Fanning S. 2007. Application of pulsed-field gel electrophoresis to characterise and trace the prevalence of Enterobacter sakazakii in an infant formula processing facility. Int J Food Microbiol 116:73–81. doi: 10.1016/j.ijfoodmicro.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 14.Drudy D, Mullane NR, Quinn T, Wall PG, Fanning S. 2006. Enterobacter sakazakii: an emerging pathogen in powdered infant formula. Clin Infect Dis 42:996–1002. doi: 10.1086/501019. [DOI] [PubMed] [Google Scholar]
- 15.Joseph S, Forsythe SJ. 2011. Predominance of Cronobacter sakazakii sequence type 4 in neonatal infections. Emerg Infect Dis 17:1713–1715. doi: 10.3201/eid1709.110260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caubilla-Barron J, Hurrell E, Townsend S, Cheetham P, Loc-Carrillo C, Fayet O, Prere MF, Forsythe SJ. 2007. Genotypic and phenotypic analysis of Enterobacter sakazakii strains from an outbreak resulting in fatalities in a neonatal intensive care unit in France. J Clin Microbiol 45:3979–3985. doi: 10.1128/JCM.01075-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lehner A, Stephan R. 2004. Microbiological, epidemiological, and food safety aspects of Enterobacter sakazakii. J Food Prot 67:2850–2857. [DOI] [PubMed] [Google Scholar]
- 18.Skovgaard N. 2007. New trends in emerging pathogens. Int J Food Microbiol 120:217–224. doi: 10.1016/j.ijfoodmicro.2007.07.046. [DOI] [PubMed] [Google Scholar]
- 19.Kucerova E, Joseph S, Forsythe S. 2011. The Cronobacter genus: ubiquity and diversity. Quality Assurance Saf Crops Foods 3:104–122. doi: 10.1111/j.1757-837X.2011.00104.x. [DOI] [Google Scholar]
- 20.Joseph S, Sonbol H, Hariri S, Desai P, McClelland M, Forsythe SJ. 2012. Diversity of the Cronobacter genus as revealed by multilocus sequence typing. J Clin Microbiol 50:3031–3039. doi: 10.1128/JCM.00905-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hariri S, Joseph S, Forsythe SJ. 2013. Cronobacter sakazakii ST4 strains and neonatal meningitis, United States. Emerg Infect Dis 19:175–177. doi: 10.3201/eid1901.120649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sonbol H, Joseph S, McAuley CM, Craven HM, Forsythe SJ. 2013. Multilocus sequence typing of Cronobacter spp. from powdered infant formula and milk powder production factories. Int Dairy J 30:1–7. doi: 10.1016/j.idairyj.2012.11.004. [DOI] [Google Scholar]
- 23.Lu Y, Chen Y, Lu XA, Lv J, Man CX, Chai YL, Jiang YJ. 2014. Comparison of methods for the microbiological identification and typing of Cronobacter species in infant formula. J Dairy Sci 97:632–641. doi: 10.3168/jds.2013-7147. [DOI] [PubMed] [Google Scholar]
- 24.Baldwin A, Loughlin M, Caubilla-Barron J, Kucerova E, Manning G, Dowson C, Forsythe S. 2009. Multilocus sequence typing of Cronobacter sakazakii and Cronobacter malonaticus reveals stable clonal structures with clinical significance which do not correlate with biotypes. BMC Microbiol 9:223. doi: 10.1186/1471-2180-9-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cui J, Du X, Liu H, Hu G, Lv G, Xu B, Yang X, Li W, Cui Z. 2014. The genotypic characterization of Cronobacter spp. isolated in China. PLoS One 9:e102179. doi: 10.1371/journal.pone.0102179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cui JH, Du XL, Wei RJ, Zhou HJ, Li W, Forsythe S, Cui ZG. 2015. Multilocus sequence typing analysis of Cronobacter spp. isolated from China. Arch Microbiol 197:665–672. doi: 10.1007/s00203-015-1097-0. [DOI] [PubMed] [Google Scholar]
- 27.Jarvis KG, Yan QQ, Grim CJ, Power KA, Franco AA, Hu L, Gopinath G, Sathyamoorthy V, Kotewicz ML, Kothary MH, Lee C, Sadowski J, Fanning S, Tall BD. 2013. Identification and characterization of five new molecular serogroups of Cronobacter spp. Foodborne Pathog Dis 10:343–352. doi: 10.1089/fpd.2012.1344. [DOI] [PubMed] [Google Scholar]
- 28.Mohan Nair MK, Venkitanarayanan K. 2007. Role of bacterial OmpA and host cytoskeleton in the invasion of human intestinal epithelial cells by Enterobacter sakazakii. Pediatr Res 62:664–669. doi: 10.1203/PDR.0b013e3181587864. [DOI] [PubMed] [Google Scholar]
- 29.Singamsetty VK, Wang Y, Shimada H, Prasadarao NV. 2008. Outer membrane protein A expression in Enterobacter sakazakii is required to induce microtubule condensation in human brain microvascular endothelial cells for invasion. Microb Pathog 45:181–191. doi: 10.1016/j.micpath.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Cao L, Zhao J, Cheng Q, Lu F, Bie X, Lu Z. 2012. Use of rpoB gene sequence analysis for phylogenetic identification of Cronobacter species. J Microbiol Methods 88:316–318. doi: 10.1016/j.mimet.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Jackson EE, Sonbol H, Masood N, Forsythe SJ. 2014. Genotypic and phenotypic characteristics of Cronobacter species, with particular attention to the newly reclassified species Cronobacter helveticus, Cronobacter pulveris, and Cronobacter zurichensis. Food Microbiol 44:226–235. doi: 10.1016/j.fm.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 32.Jackson EE, Flores JP, Fernandez-Escartin E, Forsythe SJ. 2015. Reevaluation of a suspected Cronobacter sakazakii outbreak in Mexico. J Food Prot 78:1191–1196. doi: 10.4315/0362-028X.JFP-14-563. [DOI] [PubMed] [Google Scholar]
- 33.Sun Y, Wang M, Wang Q, Cao B, He X, Li K, Feng L, Wang L. 2012. Genetic analysis of the Cronobacter sakazakii O4 to O7 O-antigen gene clusters and development of a PCR assay for identification of all C. sakazakii O serotypes. Appl Environ Microbiol 78:3966–3974. doi: 10.1128/AEM.07825-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jarvis KG, Grim CJ, Franco AA, Gopinath G, Sathyamoorthy V, Hu L, Sadowski JA, Lee CS, Tall BD. 2011. Molecular characterization of Cronobacter lipopolysaccharide O-antigen gene clusters and development of serotype-specific PCR assays. Appl Environ Microbiol 77:4017–4026. doi: 10.1128/AEM.00162-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention. 2002. Enterobacter sakazakii infections associated with the use of powdered infant formula—Tennessee, 2001. MMWR Morb Mortal Wkly Rep 51:297–300. [PubMed] [Google Scholar]
- 36.Xu X, Wu Q, Zhang J, Ye Y, Yang X, Dong X. 2014. Occurrence and characterization of Cronobacter spp. in powdered formula from Chinese retail markets. Foodborne Pathog Dis 11:307–312. doi: 10.1089/fpd.2013.1657. [DOI] [PubMed] [Google Scholar]
- 37.Mullane N, Healy B, Meade J, Whyte P, Wall PG, Fanning S. 2008. Dissemination of Cronobacter spp. (Enterobacter sakazakii) in a powdered milk protein manufacturing facility. Appl Environ Microbiol 74:5913–5917. doi: 10.1128/AEM.00745-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brandl H, Fricker-Feer C, Ziegler D, Mandal J, Stephan R, Lehner A. 2014. Distribution and identification of culturable airborne microorganisms in a Swiss milk processing facility. J Dairy Sci 97:240–246. doi: 10.3168/jds.2013-7028. [DOI] [PubMed] [Google Scholar]