Abstract
Background
Hospital admissions for community-acquired infection are increasing rapidly in the United Kingdom, particularly among older individuals, possibly reflecting an increasing prevalence of comorbid conditions such as chronic kidney disease (CKD). This study describes associations between CKD (excluding patients treated by dialysis or transplantation) and community-acquired infection incidence among older people with diabetes mellitus.
Study Design
Retrospective cohort study using primary care records from the Clinical Practice Research Datalink linked to Hospital Episode Statistics admissions data.
Setting & Participants
191,709 patients 65 years or older with diabetes mellitus and no history of renal replacement therapy, United Kingdom, 1997 to 2011.
Predictor
Estimated glomerular filtration rate (eGFR) and history of proteinuria.
Outcomes
Incidence of community-acquired lower respiratory tract infections (LRTIs, with pneumonia as a subset) and sepsis, diagnosed in primary or secondary care, excluding hospital admissions from time at risk.
Measurements
Poisson regression was used to calculate incidence rate ratios (IRRs) adjusted for age, sex, smoking status, comorbid conditions, and characteristics of diabetes. Estimates for associations of eGFR with infection were adjusted for proteinuria, and vice versa.
Results
Strong graded associations between lower eGFRs and infection were observed. Compared with patients with eGFRs ≥ 60 mL/min/1.73 m2, fully adjusted IRRs for pneumonia among those with eGFRs < 15, 15 to 29, 30 to 44, and 45 to 59 mL/min/1.73 m2 were 3.04 (95% CI, 2.42-3.83), 1.73 (95% CI, 1.57-1.92), 1.19 (95% CI, 1.11-1.28), and 0.95 (95% CI, 0.89-1.01), respectively. Associations between lower eGFRs and sepsis were stronger, with fully adjusted IRRs up to 5.56 (95% CI, 3.90-7.94). Those associations with LRTI were weaker but still clinically relevant at up to 1.47 (95% CI, 1.34-1.62). In fully adjusted models, a history of proteinuria remained an independent marker of increased infection risk for LRTI, pneumonia, and sepsis (IRRs of 1.07 [95% CI, 1.05-1.09], 1.26 [95% CI, 1.19-1.33], and 1.33 [95% CI, 1.20-1.47]).
Limitations
Patients without creatinine results were excluded.
Conclusions
Strategies to prevent infection among people with CKD are needed.
Index Words: Community-acquired infections, lower respiratory tract infections (LRTIs), pneumonia, sepsis, non–dialysis-dependent chronic kidney disease (CKD), decreased renal function, estimated glomerular filtration rate (eGFR), proteinuria, diabetes mellitus, aged, elderly, electronic health records
Hospitalization rates for infections in the United Kingdom are increasing rapidly, particularly among older individuals: age-standardized hospital admission rates for community-acquired pneumonia more than doubled between 2000 and 2010.1,2 The driving factors behind this increase are unclear, but a higher prevalence of comorbid conditions in the aging population has been suggested.1,2
One comorbid condition associated with hospitalization for infection is chronic kidney disease (CKD). Patients receiving renal replacement therapy may be at increased infection risk due to their treatment. This study focuses on patients with CKD not treated by dialysis or transplantation, which will be referred to as CKD. A graded association between increasing severity of CKD and higher risk of hospitalization with pneumonia and sepsis has been reported, even at early stages of CKD.3-5 These studies identified CKD by reduced estimated glomerular filtration rate (eGFR). Proteinuria also has been found to indicate an increased risk of infection-related hospitalization among patients with diabetes.6,7 CKD is a risk factor for poor prognosis from infection, so this could be driven by a higher chance of hospital admission for patients with community-acquired infection if they have CKD.8 It is unclear whether CKD is a risk factor for higher incidence of infection in the community. One large case-control study identified CKD as a risk factor for incidence of community-acquired pneumonia in primary care, but relied on routine diagnosis of CKD in the general population and did not exclude patients receiving renal replacement therapy.9
There is a high prevalence of CKD among people with diabetes, particularly older people.10-12 Patients with diabetes are monitored regularly in primary care for CKD, and this has been financially incentivized in the United Kingdom since 2004.13,14 Thus, studying people with diabetes minimizes the potential for ascertainment bias in estimating the association between CKD and infection from routinely collected electronic health records. The subset of the UK population with diabetes mellitus and aged 65 years or older is large and growing and experiences a high burden of infection.15,16 This population also is at higher risk of infectious complications such as acute kidney injury.17 If CKD is a risk factor for infection incidence among older people with diabetes, this could be important to health service planning, as well as to patients and their clinicians.
We aimed to describe, among older people with diabetes, the associations between CKD (excluding patients with a history of renal replacement therapy) and community-acquired lower respiratory tract infection (LRTI), pneumonia (as a subset of LRTI), and sepsis. We used linked health records to identify infections managed in primary or secondary care.
Methods
Data Sources
We used the May 2011 data set of the Clinical Practice Research Datalink (CPRD), a database of anonymized primary care medical records comprising 12.8 million patient records at 627 practices in the United Kingdom.18 Data include patient demographics, health behaviors, test results, diagnoses, and prescriptions. The CPRD population is representative of the general UK population and the validity of recorded diagnoses generally is high.19,20 Linked data are available for patients registered at consenting English practices. For linked patients, this study used linked data for all hospital inpatient admissions to National Health Service hospitals in England from Hospital Episodes Statistics (HES) and socioeconomic status from the Office for National Statistics.21,22
Study Population and Follow-up
All patients in CPRD at any point between April 1997 and March 2011 with diabetes mellitus, aged 65 years or older, with at least one valid serum creatinine result during the study period, and with no history of renal replacement therapy were eligible. The definition of diabetes was based on diagnostic Read codes. “Definite” codes, for example, C10F.00 Type 2 diabetes mellitus, were sufficient evidence of diabetes. “Possible” codes, for example, 90LA.11 Diabetes monitored, required an antidiabetes medication prescription for confirmation. Full code lists were published previously.15 Patients met eligibility criteria at the latest date of diabetes diagnosis, 65th birthday, 1 year after practice registration, practice fulfilling CPRD quality control standards, or April 1, 1997. Their study entry date was their first valid serum creatinine result after the eligibility criteria were met. Patients left the study at the earliest date of death, leaving the practice, last data collection from the practice, renal replacement therapy (dialysis or kidney transplantation), or March 31, 2011.
Definition of CKD
We estimated glomerular filtration rate from primary care serum creatinine test results, multiplied by 0.95 to correct for lack of isotope-dilution mass spectrometry standardization, using the CKD-EPI (CKD Epidemiology Collaboration) creatinine equation.23-25 We included adjustment for black ethnicity.26 To reduce misclassification of eGFR from variability in serum creatinine results or acute illness, we used a last-carried-forward method, with eGFR initially defined using the creatinine result that marked entry to the study and updated at each subsequent creatinine result so that eGFR was always defined by the single most recent creatinine result, as previously performed by James et al.3,27 For our main analyses, we used eGFR categories corresponding to those used in diagnosis (<15, 15-29, 30-44, 45-59, and ≥60 mL/min/1.73 m2).25 During the study period, many UK laboratories did not report the specific value of eGFR results if they were ≥60 mL/min/1.73 m2 and so we did not distinguish eGFR categories > 60 mL/min/1.73 m2 in the main analysis. We repeated the final model including additional separate categories for eGFR of 60 to 74, 75 to 89, and ≥90 mL/min/1.73 m2, with patients with eGFRs of 75 to 89 mL/min/1.73 m2 as a reference group.
Either a positive urine protein test result or a diagnosis of proteinuric kidney disease in CRPD defined onset of a history of proteinuria. We excluded urine protein test results that occurred on the same day as a diagnostic Read code for urinary tract infection. We did not count trace results as positive and checked records for internal consistency.
Definition of Infections
We studied 3 acute community-acquired infections: LRTI (which included diagnoses such as influenza and acute bronchitis), pneumonia (as a subset of LRTI), and sepsis. Either diagnostic Read codes in CPRD or any International Classification of Diseases, Tenth Revision code that formed the primary diagnostic code on hospital admission in HES could define an infection. To avoid overestimation from repeat attendances for the same infection, diagnostic codes recorded within 28 days of one another were attributed to a single episode of infection, with index date defined by the first diagnostic code and duration until 28 days after the last diagnostic code. If any LRTI included a pneumonia code, the pneumonia episode was considered to start from the first instance of the pneumonia code and end on the end date of the LRTI within which it occurred. Any infection with onset date during an HES hospitalization spell, within 14 days after hospital discharge, or that included a code for postoperative infection was identified as a hospital-acquired infection and excluded. These methods were described in detail previously.15,28
Time at Risk
Patients were not at risk of a community-acquired infection during an infection (community or hospital acquired), during an HES hospitalization spell, or within 14 days following hospital discharge, and these periods were removed from time at risk. Time at risk was calculated separately for each type of infection; a patient could be at risk of sepsis despite an ongoing LRTI, for example.
Definition of Covariates
Age was defined in 5-year age bands up to a final category of 85 years or older. Socioeconomic status was assigned at practice level using 2007 Office for National Statistics estimates of the Index of Multiple Deprivation, a composite area-level marker of deprivation.22 Smoking status was identified as current, ex-smoker, or nonsmoker from both HES and CPRD data. Smoking cessation products were considered to indicate current smoking because cessation success rates are low.29 The most recent smoking status record by the study entry date defined smoking status at baseline when available; if not recorded, the first subsequent record defined smoking status at baseline. Comorbid conditions (ischemic heart disease, congestive cardiac failure, hypertension, cerebrovascular disease, other dementia, and chronic lung disease) were defined using diagnostic CPRD Read codes. The first diagnostic record at any point in the patient’s records defined onset of the condition. Baseline hemoglobin A1c level was defined by the most recent hemoglobin A1c test result in CPRD prior to (or on) the study entry date. Baseline diabetic medication history was defined using CPRD prescription records.
Data Analysis
Incidence rates were calculated for each infection using Poisson regression with lexis expansions for age and a random-effects model to adjust for multiple infection episodes. Analysis was conducted separately for each type of infection (LRTI, pneumonia, and sepsis) using 3 main regression models.
Negative proteinuria test results tend to be under-recorded in primary care records.30 For comorbid conditions and proteinuria status, absence of a positive record was treated as absence of disease, and for hemoglobin A1c, absence of a recorded result was included as a category of hemoglobin A1c status. We excluded patients with no smoking status available.
Our first model adjusted for age, sex, socioeconomic status at practice level, and date prior to or post April 1, 2004, when Quality Outcomes Framework guidelines introduced financial incentives for recording CKD status among people with diabetes in primary care that are suggested to have improved ascertainment of CKD in primary care.31 Our second model additionally adjusted for confounding by smoking status and comorbid conditions. This second model was run both with all variables assessed at baseline and separately with new onset of comorbid condition time updated during the study (but not smoking status because changes in smoking status are particularly vulnerable to reverse causation). Our final model additionally adjusted for hemoglobin A1c level and diabetic medication history at baseline. All nonbinary covariates were modeled as categorical variables.
Sensitivity analyses repeated the final model with the following adjustments: limiting follow-up to post April 1, 2004; restricting the data set to patients with HES linkage available; and using only the first infection as an outcome.
All estimates for the associations of eGFR with infection were adjusted for proteinuria, and vice versa, so that the effect estimates for eGFR and proteinuria are independent.
We looked for evidence of interaction between eGFR and proteinuria and between eGFR and age (65-74 and ≥75 years) in the final models for LRTI and pneumonia using likelihood ratio tests to assess nested models with and without interaction terms. We did not look for interaction in the sepsis regression model due to the smaller number of events.
Stata, version 13.1 (StataCorp LP), was used for data analyses. All code lists are available on request.
Ethics Approval
The study was approved by the Independent Scientific Advisory Committee of the CPRD (ISAC reference 11_033A) and the London School of Hygiene & Tropical Medicine Ethics Committee (LSHTM reference 6116).
Results
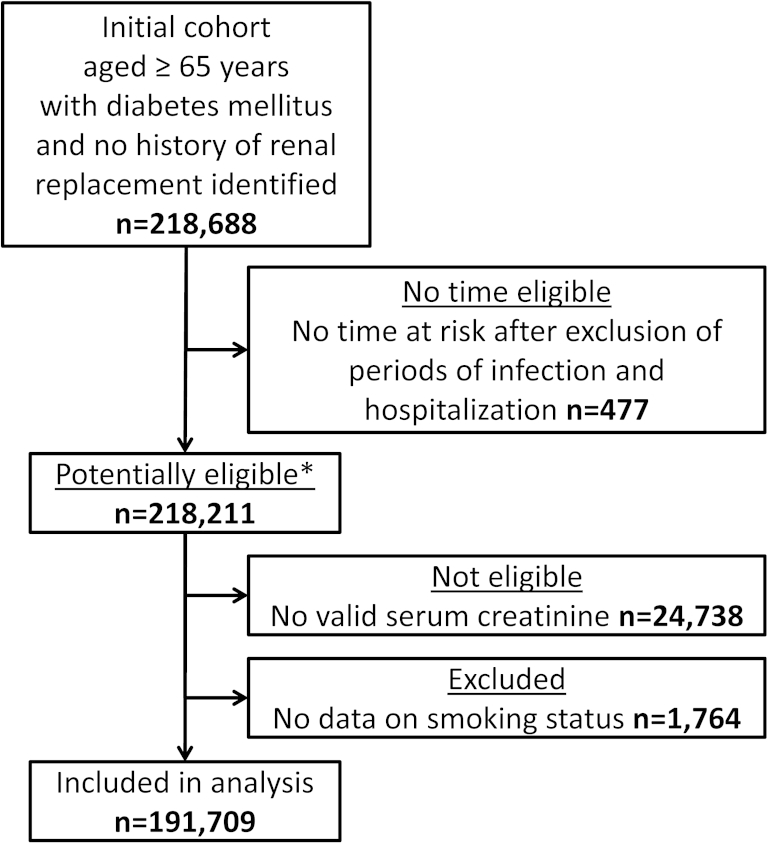
Of 218,211 patients potentially eligible for inclusion, 191,709 (87.9%) had a valid serum creatinine result and complete data available (Fig 1). Study participants were followed up for a median of 4.6 (interquartile range [IQR], 2.3-7.6) years. Median age at study entry was 71 (IQR, 66-78) years. For 113,106 study participants (59.0%), HES linkage was available. The population with no available serum creatinine result had a high prevalence of missing data for both smoking status (2,760 of 24,738 [11.2%]) and hemoglobin A1c results (10,866 of 24,739 [43.9%]), suggesting that this population may not attend primary care services frequently. The population with no available creatinine result had a comorbid condition profile similar to patients with CKD stages 3 to 5 in terms of prevalence of congestive heart failure and cerebrovascular disease (Table 1).
Figure 1.
Flowchart of study eligibility and participation. ∗Baseline characteristics described in Table 1.
Table 1.
Baseline Characteristics of Potentially Eligible Study Population by Baseline eGFR
| No Scr (n = 24,738)a | eGFR ≥ 60 (n = 124,521)b | eGFR < 60 (n = 68,952)b | |
|---|---|---|---|
| Female sex | 12,662 (51.2) | 54,246 (43.6) | 40,907 (59.3) |
| Age category | |||
| 65-69 y | 7,356 (29.7) | 63,364 (50.9) | 15,849 (23.0) |
| 70-74 y | 4,852 (19.6) | 28,549 (22.9) | 13,668 (19.8) |
| 75-79 y | 4,570 (18.5) | 18,689 (15.0) | 15,610 (22.6) |
| 80-84 y | 3,843 (15.5) | 9,297 (7.5) | 12,816 (18.6) |
| ≥85 y | 4,117 (16.6) | 4,622 (3.7) | 11,009 (16.0) |
| SESc by practice | |||
| 1: least deprived | 3,839 (15.5) | 21,818 (17.5) | 12,341 (17.9) |
| 2 | 4,269 (17.3) | 22,312 (17.9) | 12,515 (18.2) |
| 3 | 5,101 (20.6) | 26,136 (21.0) | 13,962 (20.3) |
| 4 | 6,037 (24.4) | 28,355 (22.8) | 15,500 (22.5) |
| 5: most deprived | 5,492 (22.2) | 25,900 (20.8) | 14,634 (21.2) |
| Smoking status | |||
| Current smoker | 3,976 (16.1) | 21,398 (17.2) | 9,127 (13.2) |
| Ex-smoker | 7,034 (28.4) | 51,901 (41.7) | 25,991 (37.7) |
| Nonsmoker | 10,968 (44.3) | 50,551 (40.6) | 32,741 (47.5) |
| Missing | 2,760 (11.2) | 671 (0.5) | 1,093 (1.6) |
| Comorbid conditions | |||
| Ischemic heart disease | 6,381 (25.8) | 30,743 (24.7) | 23,308 (33.8) |
| Congestive heart failure | 3,108 (12.6) | 6,221 (5.0) | 10,122 (14.7) |
| Hypertension | 12,229 (49.4) | 73,263 (58.8) | 45,915 (66.6) |
| Cerebrovascular disease | 4,169 (16.9) | 13,157 (10.6) | 11,500 (16.7) |
| Other dementia | 1,350 (5.5) | 1,651 (1.3) | 1,889 (2.7) |
| Chronic lung disease | 2,344 (9.5) | 9,266 (7.4) | 5,847 (8.5) |
| Antidiabetes medications | |||
| Insulin only | 1,407 (5.7) | 3,936 (3.2) | 3,346 (4.9) |
| Oral medication only | 9,811 (39.7) | 54,635 (43.9) | 27,169 (39.4) |
| Both insulin and oral | 1,473 (5.6) | 8,078 (6.5) | 5,764 (8.4) |
| None recorded | 12,047 (48.7) | 57,872 (46.5) | 32,673 (47.4) |
| Hemoglobin A1cd | |||
| Good | 7,256 (29.3) | 58,177 (46.7) | 31,026 (45.0) |
| Borderline | 5,185 (21.0) | 46,122 (37.0) | 24,157 (35.0) |
| Poor | 1,431 (5.8) | 7,433 (6.0) | 4,142 (6.0) |
| None recorded | 10,866 (43.9) | 12,789 (10.3) | 9,627 (14.0) |
Note: N = 218,211. Values are given as number (percentage). Baseline is date of study entry for study participants (n = 191,709) and date of eligibility for study entry for patients not included in the study due to having no available Scr result or no available smoking status. eGFRs expressed in mL/min/1.73 m2.
Abbreviations: eGFR, estimated glomerular filtration rate; Scr, serum creatinine; SES, socioeconomic status.
These patients had no Scr result available and hence were not included in study.
These patients had an Scr result available and were included in study unless smoking status was missing.
Index of multiple deprivation.
Good, <53 mmol/mol (<7%); borderline, 53-86 mmol/mol (7%-10%); poor, >86 mmol/mol (>10%).
We found good completeness of serum creatinine result recording: only 11% of potentially eligible patients lacked a valid serum creatinine result (Fig 1). The median time for which each creatinine result was carried forward was 137 (IQR, 56-242) days. At study entry, 67,859 (35.4%) participants had CKD stages 3 to 5, defined as eGFR < 60 mL/min/1.73 m2, and 25,433 (13.3%) had a history of proteinuria (Table 2).25
Table 2.
Prevalence of Markers of CKD at Baseline for Study Participants
| eGFR | Proteinuria Absent | History of Proteinuria | Total |
|---|---|---|---|
| <15 | 307 | 234 (43.3) | 541 |
| 15-29 | 3,373 | 1,205 (26.3) | 4,578 |
| 30-44 | 14,857 | 3,417 (18.7) | 18,274 |
| 45-59 | 38,672 | 5,794 (13.0) | 44,466 |
| 60-74 | 52,168 | 6,726 (11.4) | 58,894 |
| 75-89 | 41,446 | 5,379 (11.5) | 46,825 |
| ≥90 | 15,453 | 2,678 (14.8) | 18,131 |
| Total | 166,276 | 25,433 (13.3) | 191,709 |
Note: n = 191,709. Markers of CKD are eGFR and history of proteinuria. Values are given as number or number (row percentage). eGFR categories expressed in mL/min/1.73 m2.
Abbreviations: CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate.
We observed 115,080 LRTIs among 56,076 patients, 7,870 episodes of pneumonia among 7,095 patients, and 1,980 episodes of sepsis among 1,902 patients. Crude incidence rates were as follows: LRTI, 155.8 (95% confidence interval [CI], 154.3-157.4)/1,000 person-years; pneumonia, 10.3 (95% CI, 10.1-10.6)/1,000 person-years; and sepsis, 2.5 (95% CI, 2.4-2.6)/1,000 person-years (Table 3).
Table 3.
Infection Rates With Corresponding Rate Ratios by eGFR
| Infection | No. of Events | Time at Risk (person-y) | Crude Rate/1,000 Person-y (95% CI) | Minimally Adjusteda,b | Adjusted for Comorbid Conditions at Baselineb,c | Adjusted for Time-Updated Comorbid Conditionsb,d | Adjusted for Characteristics of Diabetesb,e |
|---|---|---|---|---|---|---|---|
| LRTI | 115,080 | 808,194 | 155.8 (154.3-157.4) | ||||
| eGFR < 15 | 607 | 2,532 | 295.3 (265.8-324.8) | 1.78 (1.61-1.96) | 1.67 (1.51-1.85) | 1.52 (1.38-1.68) | 1.47 (1.34-1.62) |
| eGFR 15-29 | 5,153 | 25,016 | 228.0 (219.8-236.2) | 1.38 (1.33-1.43) | 1.28 (1.23-1.33) | 1.20 (1.15-1.24) | 1.17 (1.13-1.22) |
| eGFR 30-44 | 16,557 | 96,214 | 188.7 (184.8-192.6) | 1.19 (1.16-1.22) | 1.13 (1.10-1.15) | 1.09 (1.07-1.12) | 1.08 (1.05-1.10) |
| eGFR 45-59 | 29,783 | 204,866 | 159.9 (157.4-162.4) | 1.06 (1.05-1.08) | 1.04 (1.03-1.06) | 1.03 (1.02-1.05) | 1.03 (1.01-1.04) |
| eGFR ≥ 60 | 62,980 | 479,565 | 143.0 (141.3-144.7) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Pneumoniaf | 7,870 | 816,517 | 10.3 (10.1-10.6) | ||||
| eGFR < 15 | 99 | 2,570 | 52.8 (40.7-65.0) | 4.26 (3.37-5.38) | 3.69 (2.92-4.65) | 3.25 (2.58-4.10) | 3.04 (2.42-3.83) |
| eGFR 15-29 | 650 | 25,362 | 30.6 (27.8-33.3) | 2.29 (2.07-2.53) | 2.01 (1.82-2.23) | 1.82 (1.65-2.01) | 1.73 (1.57-1.92) |
| eGFR 30-44 | 1,523 | 97,360 | 17.4 (16.4-18.4) | 1.42 (1.33-1.53) | 1.31 (1.22-1.40) | 1.23 (1.15-1.32) | 1.19 (1.11-1.28) |
| eGFR 45-59 | 1,980 | 207,025 | 10.2 (9.7-10.7) | 1.01 (0.95-1.07) | 0.98 (0.93-1.04) | 0.96 (0.91-1.02) | 0.95 (0.89-1.01) |
| eGFR ≥ 60 | 3,618 | 484,200 | 7.8 (7.6-8.1) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Sepsis | 1,980 | 816,826 | 2.5 (2.4-2.6) | ||||
| eGFR < 15 | 41 | 2,572 | 17.8 (11.8-23.9) | 7.40 (5.19-10.55) | 6.93 (4.86-9.90) | 6.19 (4.34-8.82) | 5.56 (3.90-7.94) |
| eGFR 15-29 | 186 | 25,382 | 8.1 (6.8-9.3) | 3.29 (2.75-3.94) | 3.01 (2.52-3.61) | 2.69 (2.25-3.23) | 2.50 (2.08-3.00) |
| eGFR 30-44 | 387 | 97,416 | 4.2 (3.7-4.6) | 1.80 (1.58-2.06) | 1.70 (1.49-1.95) | 1.60 (1.39-1.83) | 1.51 (1.32-1.73) |
| eGFR 45-59 | 499 | 207,103 | 2.5 (2.2-2.7) | 1.19 (1.06-1.34) | 1.17 (1.04-1.32) | 1.14 (1.02-1.29) | 1.11 (0.99-1.25) |
| eGFR ≥ 60 | 867 | 484,352 | 1.8 (1.7-2.0) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
Note: Unless otherwise indicated, values are given as rate ratio (95% CI). eGFR categories expressed in mL/min/1.73 m2.
Abbreviations: CI, confidence interval; eGFR, estimated glomerular filtration rate; LRTI, lower respiratory tract infection.
Adjusted for proteinuria (updated), age (updated), sex, socioeconomic status by practice, and financial year prior to or post 2004.
P < 0.001 for all rate ratios. Likelihood ratio test for inclusion of eGFR as a categorical variable in the model.
Adjusted for proteinuria (updated), age (updated), sex, socioeconomic status by practice, financial year prior to or post 2004, ischemic heart disease, congestive cardiac failure, hypertension, cerebrovascular disease, other dementia, chronic lung disease, and smoking at baseline.
Adjusted for proteinuria (updated), age (updated), sex, socioeconomic status by practice, financial year prior to or post 2004, ischemic heart disease (updated), congestive cardiac failure (updated), hypertension (updated), cerebrovascular disease (updated), other dementia (updated), chronic lung disease (updated), and smoking (baseline).
Adjusted for proteinuria (updated), age (updated), sex, socioeconomic status by practice, financial year prior to or post 2004, ischemic heart disease (updated), congestive cardiac failure (updated), hypertension (updated), cerebrovascular disease (updated), other dementia (updated), chronic lung disease (updated), smoking (baseline), hemoglobin A1c level (baseline), and antidiabetes medication history (baseline).
Subset of LRTI.
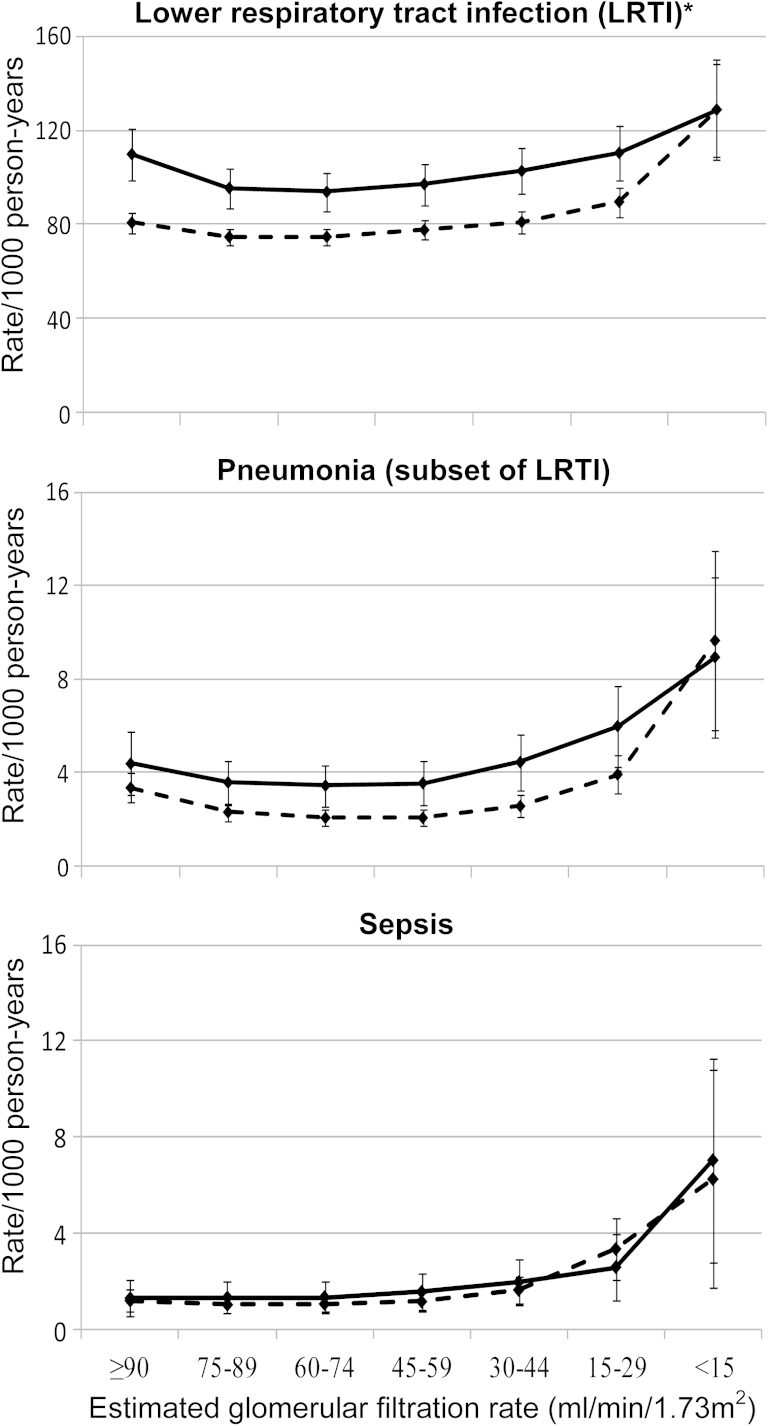
Both eGFR and proteinuria were independent risk markers for incidence of LRTI, pneumonia, and sepsis (P < 0.001 for each analysis). A high incidence of infection was observed among patients with CKD. For example, crude LRTI rates were 228.0 (95% CI, 219.8-236.2)/1,000 person-years among patients with eGFRs of 15 to 29 mL/min/1.73 m2 compared to 143.0 (95% CI, 141.3-144.7)/1,000 person-years among patients with eGFRs ≥ 60 mL/min/1.73 m2. The association between eGFR and infection incidence was graded, with increased infection incidence even at early stages of CKD. Strong and graded associations between reduced eGFR and infection remained after adjustment for age, sex, smoking status, comorbid conditions, and characteristics of diabetes. Compared to eGFR ≥ 60 mL/min/1.73 m2, fully adjusted incidence rate ratios (IRRs) for pneumonia were 3.04 (95% CI, 2.42-3.83), 1.73 (95% CI, 1.57-1.92), 1.19 (95% CI, 1.11-1.28), and 0.95 (95% CI, 0.89-1.01) for eGFRs < 15, 15 to 29, 30 to 44, and 45 to 59 mL/min/1.73 m2, respectively. The associations between reduced eGFR and sepsis were stronger, with fully adjusted IRRs up to 5.56 (95% CI, 3.90-7.94), while those with LRTI were less strong but still clinically relevant, with fully adjusted IRRs up to 1.47 (95% CI, 1.34-1.62; Table 3). Fully adjusted rates and rate ratios using patients with eGFRs of 75 to 89 mL/min/1.73 m2 as a reference group suggested a J-shaped relationship between eGFR and LRTI and pneumonia incidence (Fig 2; Table S1 [available as online supplementary material]).
Figure 2.
Fully adjusted infection rates/1,000 person-years (with 95% confidence intervals) against category of estimated glomerular filtration rate, by proteinuria status. Solid line, patients with a history of proteinuria; dashed line, patients with no history of proteinuria. Rates adjusted for age (updated), sex, socioeconomic status by practice, financial year prior to or post 2004, ischemic heart disease (updated), congestive cardiac failure (updated), hypertension (updated), cerebrovascular disease (updated), other dementia (updated), chronic lung disease (updated), smoking (baseline), hemoglobin A1c level (baseline), and antidiabetes medication history (baseline). ∗LRTI y-axis scale is 10-fold greater than the pneumonia and sepsis scales.
Proteinuria was an independent risk marker for infection incidence after adjustment for eGFR (Table 4). In minimally adjusted analyses, patients with a history of proteinuria had a higher incidence of LRTI, pneumonia, and sepsis (IRRs of 1.13 [95% CI, 1.10-1.15], 1.37 [95% CI, 1.30-1.45], and 1.44 [95% CI, 1.30-1.59], respectively) compared with patients without a history of proteinuria. These associations were diminished but persisted after adjustment for time-updated comorbid conditions and characteristics of diabetes for LRTI, pneumonia, and sepsis (IRRs of 1.07 [95% CI, 1.05-1.09], 1.26 [95% CI, 1.19-1.33], and 1.33 [95% CI, 1.20-1.47], respectively). The effect of proteinuria did not vary by eGFR category (Fig 2).
Table 4.
Infection Rates With Corresponding Rate Ratios by Proteinuria Status
| Infection | No. of Events | Time at Risk (person-y) | Crude Rate/1,000 Person-y (95% CI) | Minimally Adjusteda,b | Adjusted for Comorbid Conditions at Baselineb,c | Adjusted for Time-Updated Comorbid Conditionsb,d | Adjusted for Characteristics of Diabetesb,e |
|---|---|---|---|---|---|---|---|
| LRTI | 115,080 | 808,194 | 155.8 (154.3-157.4) | ||||
| Proteinuria | 31,823 | 202,658 | 178.7 (175.7-181.8) | 1.13 (1.10-1.15) | 1.15 (1.13-1.17) | 1.09 (1.07-1.11) | 1.07 (1.05-1.09) |
| No proteinuria | 83,257 | 605,536 | 148.9 (147.3-150.5) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Pneumoniaf | 7,870 | 816,517 | 10.3 (10.1-10.6) | ||||
| Proteinuria | 2,646 | 204,908 | 14.4 (13.8-15.1) | 1.37 (1.30-1.45) | 1.40 (1.33-1.48) | 1.31 (1.24-1.39) | 1.26 (1.19-1.33) |
| No proteinuria | 5,224 | 611,609 | 9.0 (8.8-9.3) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Sepsis | 1,980 | 816,826 | 2.5 (2.4-2.6) | ||||
| Proteinuria | 712 | 205,002 | 3.6 (3.3-3.9) | 1.44 (1.30-1.59) | 1.46 (1.32-1.61) | 1.41 (1.27-1.56) | 1.33 (1.20-1.47) |
| No proteinuria | 1,268 | 611,824 | 2.1 (2.0-2.2) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
Note: Unless otherwise indicated, values are given as rate ratio (95% CI).
Abbreviations: CI, confidence interval; eGFR, estimated glomerular filtration rate; LRTI, lower respiratory tract infection.
Adjusted for eGFR (updated), age (updated), sex, socioeconomic status by practice, and financial year prior to or post 2004.
P < 0.001 for all rate ratios. Likelihood ratio test for inclusion of eGFR as a binary variable in the model.
Adjusted for eGFR (updated), age (updated), sex, socioeconomic status by practice, financial year prior to or post 2004, ischemic heart disease, congestive cardiac failure, hypertension, cerebrovascular disease, other dementia, chronic lung disease, and smoking at baseline.
Adjusted for eGFR (updated), age (updated), sex, socioeconomic status by practice, financial year prior to or post 2004, ischemic heart disease (updated), congestive cardiac failure (updated), hypertension (updated), cerebrovascular disease (updated), other dementia (updated), chronic lung disease (updated), and smoking (baseline).
Adjusted for eGFR (updated), age (updated), sex, socioeconomic status by practice, financial year prior to or post 2004, ischemic heart disease (updated), congestive cardiac failure (updated), hypertension (updated), cerebrovascular disease (updated), other dementia (updated), chronic lung disease (updated), smoking (baseline), hemoglobin A1c level (baseline), and antidiabetes medication history (baseline).
Subset of LRTI.
No clinically important interaction between age and eGFR was observed for LRTI or pneumonia. Sensitivity analyses limiting follow-up to post April 2004, restricting the data set to patients with HES linkage available, or using only the first infection as an outcome found similar results to the main analysis.
Discussion
In our study population of older patients with diabetes, there was a high burden of community-acquired LRTI, pneumonia, and sepsis among those with CKD (manifested as reduced eGFR and/or history of proteinuria). Reduced eGFR and history of proteinuria represented independent risk markers for incidence of LRTI, pneumonia, and sepsis. Associations between eGFR and infection incidence were graded, with increased infection incidence at more severe stages of CKD. These associations persisted after adjustment for comorbid conditions, smoking status, and characteristics of diabetes mellitus. The association between eGFR and infection was not modified by age. Effect sizes were larger for sepsis than for pneumonia, and for pneumonia than for LRTI.
The strengths of the study include the following: first, consideration of both eGFR and proteinuria in mutually adjusted analyses; second, frequent monitoring of serum creatinine and proteinuria, allowing good ascertainment of CKD status; third, the detailed methods used to define infections, including exclusion of time in hospital from time at risk and treating recurrent consultations for infection within 28 days as a single episode; and fourth, inclusion of infections managed in primary care, not just those resulting in hospitalization or death.
The study is limited by the nature of routinely collected data; we may have underascertained proteinuria or comorbid conditions. The high prevalence of proteinuria and comorbid conditions observed in the study population suggests that neither is an extensive problem. Proteinuria monitoring has been financially incentivized in primary care for this population since 2004.32 A small percentage of the potential study population had no available creatinine results and therefore were not included. These people did not appear to seek regular care, which may have led to us underestimating the association between CKD and infection. We do not have formal validation study data for our outcomes, but the advantage of linked data is capturing a more complete ascertainment of infections than in the stand-alone data sets used previously.
The complex relationships between CKD, infection, and cardiovascular disease limit interpretation of the direction of any causal association between CKD and infection. The same comorbid conditions, such as cardiovascular events, may both cause and be caused by CKD. Adjustment for baseline comorbid conditions risks underestimation of the association between CKD and infection by residual confounding from new-onset comorbid conditions that may reflect baseline risk factors for these comorbid conditions, such as poorly treated hypertension prior to CKD. Adjustment for time-updated comorbid conditions is vulnerable to overadjusting for events that mediate any effect of CKD on infection. We present both models. The true association between CKD and infection is likely to lie between the 2 results. We avoided time-updating hemoglobin A1c levels and smoking status because these are vulnerable to reverse causality from infection (eg, pneumonia may motivate smoking cessation). We also addressed the risk of reverse causality by conducting a sensitivity analysis in which we limited follow-up to the first infection, which found results similar to the main analysis. We did not adjust for vaccination status because inter-relationships between CKD status, receipt of vaccination, and infection are likely to be complex and looking formally for vaccine effectiveness was beyond the scope of this report.
Our results may not be generalizable to the general population without diabetes because there may be a particular relationship between CKD and infection among older people with diabetes. However, older people with diabetes are a large and growing population with a high burden of CKD and infection, and so our findings in this population are important.
The associations we observed of preexisting eGFR with sepsis and pneumonia were similar to those observed between eGFR with bloodstream infections and pneumonia diagnoses in hospital records among the general population 65 years and older (although different outcome definitions mean the absolute rates are not comparable).3,5 A large case-control study in the United Kingdom identified CKD as a risk factor for primary care diagnosis of pneumonia (adjusted odds ratio, 1.72; 95% CI, 1.3-2.07), but the unclear definition of CKD may have included patients with an increased risk of infection from renal replacement therapy and did not permit stage-specific rate ratios.9 James et al3 and Dalrymple et al33 observed a J-shaped association between eGFR and infection risk, which was not present using cystatin C–based eGFR. Our results suggested that patients with eGFRs ≥ 90 mL/min/1.73 m2 had a slightly increased risk of LRTI or pneumonia compared with those with eGFRs of 75 to 89 mL/min/1.73 m2, but we did not observe a J shape for the association of eGFR with sepsis. James et al found that the association of eGFR with hospitalization for pneumonia was weaker among older age groups.3 We did not observe an interaction between eGFR and age in the present study, which may be due partly to our study population being limited to older people. In a previous study identifying albuminuria as a risk factor for infection-related hospitalization among patients with diabetes, eGFR was not an independent risk marker for infection, and infections managed in primary care were not included.6 To our knowledge, our finding that proteinuria is a risk marker for incidence of LRTI, pneumonia, and sepsis, independently of eGFR, and including infections managed in primary care, is novel.
This study found that the associations of reduced eGFR and history of proteinuria with infection appeared to be due in part to underlying accrued cardiovascular and cerebrovascular comorbid conditions, but a strong graded association remained after adjustment for these. It has been suggested that CKD as a risk marker for infection may be a surrogate for chronicity of diabetes or poor glycemic control.6 Adjustments for cardiovascular and cerebrovascular comorbid conditions will reflect chronicity and severity of diabetes to a certain extent, but further adjustment for severity and control of diabetes had little effect on the associations between CKD and infection, suggesting that the associations between CKD and infections are not explained fully by these factors. Stronger independent associations with CKD were observed for pneumonia than LRTI, and stronger still for sepsis, consistent with a view of CKD as a risk factor for poorer prognosis and greater incidence of infection. For clinicians managing older people with diabetes, our findings may help identify patients at increased risk of infection and inform discussions about infection risk and vaccination.30 For policy makers, the association of CKD with a high burden of morbidity from infection is important for health service planning because the populations with diabetes and CKD are predicted to grow.34
Several unanswered questions remain. More research is needed to identify the biological mechanisms underlying the associations of proteinuria and eGFR with infection and to improve infection prevention strategies. For example, better understanding of vaccine effectiveness among people with CKD could inform whether pneumococcal and influenza vaccination recommendations should include people with proteinuria.35
Acknowledgements
Support: Dr Thomas reports a Career Development Fellowship grant (CDF 2010-03-32) from the National Institute for Health Research during the conduct of the study. Dr McDonald reports a PhD studentship grant from Kidney Research UK (grant reference ST2/2011) during the conduct of the study, for which Drs Thomas and Nitsch wrote the studentship application. The study sponsors had no role in the design or conduct of the study; the collection, management, analysis, or interpretation of data; the preparation, review, or approval of the manuscript; or the decision to submit it for publication. The views expressed in this publication are those of the authors and not necessarily those of the UK National Health Service, the National Institute for Health Research, the Department of Health, or Kidney Research UK.
Financial Disclosure: Dr Nitsch reports subcontract work for grants received by BMJ Informatica for the Healthcare Quality Improvement Partnership national CKD audit. The remaining authors declare that they have no other relevant financial interests.
Contributions: Research idea and study design: HIM, SLT, ERCM, DN; data acquisition: SLT; data analysis/interpretation: HIM, SLT, ERCM, DN; statistical analysis: HIM, ERCM; supervision or mentorship: SLT, DN. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. HIM takes responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Footnotes
Table S1: Sensitivity analysis of association between eGFR and infection incidence.
Note: The supplementary material accompanying this article (http://dx.doi.org/10.1053/j.ajkd.2014.11.027) is available at www.ajkd.org
Supplementary Material
Sensitivity analysis of association between eGFR and infection incidence.
References
- 1.Bardsley M., Blunt I., Davies S., Dixon J. Is secondary preventive care improving? Observational study of 10-year trends in emergency admissions for conditions amenable to ambulatory care. BMJ Open. 2013;3(1):e002007. doi: 10.1136/bmjopen-2012-002007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trotter C.L., Stuart J.M., George R., Miller E. Increasing hospital admissions for pneumonia, England. Emerg Infect Dis. 2008;14(5):727–733. doi: 10.3201/eid1405.071011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.James M.T., Quan H., Tonelli M. CKD and risk of hospitalization and death with pneumonia. Am J Kidney Dis. 2009;54(1):24–32. doi: 10.1053/j.ajkd.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Collins A.J., Foley R.N., Herzog C. US Renal Data System 2010 Annual Data Report. Am J Kidney Dis. 2011;57(1 suppl 1):A8. doi: 10.1053/j.ajkd.2010.10.007. e1-e526. [DOI] [PubMed] [Google Scholar]
- 5.James M.T., Laupland K.B., Tonelli M., Manns B.J., Culleton B.F., Hemmelgarn B.R. Risk of bloodstream infection in patients with chronic kidney disease not treated with dialysis. Arch Intern Med. 2008;168(21):2333–2339. doi: 10.1001/archinte.168.21.2333. [DOI] [PubMed] [Google Scholar]
- 6.Hamilton E.J., Martin N., Makepeace A., Sillars B.A., Davis W.A., Davis T.M. Incidence and predictors of hospitalization for bacterial infection in community-based patients with type 2 diabetes: the Fremantle Diabetes Study. PLoS One. 2013;8(3):e60502. doi: 10.1371/journal.pone.0060502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karunajeewa H., McGechie D., Stuccio G., Stingemore N., Davis W.A., Davis T.M. Asymptomatic bacteriuria as a predictor of subsequent hospitalisation with urinary tract infection in diabetic adults: the Fremantle Diabetes Study. Diabetologia. 2005;48(7):1288–1291. doi: 10.1007/s00125-005-1794-3. [DOI] [PubMed] [Google Scholar]
- 8.Viasus D., Garcia-Vidal C., Cruzado J.M. Epidemiology, clinical features and outcomes of pneumonia in patients with chronic kidney disease. Nephrol Dial Transplant. 2011;26(9):2899–2906. doi: 10.1093/ndt/gfq798. [DOI] [PubMed] [Google Scholar]
- 9.Vinogradova Y., Hippisley-Cox J., Coupland C. Identification of new risk factors for pneumonia: population-based case-control study. Br J Gen Pract. 2009;59(567):e329–e338. doi: 10.3399/bjgp09X472629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.New J.P., Middleton R.J., Klebe B. Assessing the prevalence, monitoring and management of chronic kidney disease in patients with diabetes compared with those without diabetes in general practice. Diabet Med. 2007;24(4):364–369. doi: 10.1111/j.1464-5491.2007.02075.x. [DOI] [PubMed] [Google Scholar]
- 11.De Lusignan S. Identification and management of chronic kidney disease. Prescriber. 2008;19(10):10–18. [Google Scholar]
- 12.Collins A.J., Foley R.N., Chavers B. United States Renal Data System 2011 annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States. Am J Kidney Dis. 2012;59(1 suppl 1):e1–e420. doi: 10.1053/j.ajkd.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 13.The NHS Information Centre Prescribing and Primary Care Services . The NHS Information Centre; Leeds, UK: 2011. Quality and Outcomes Framework Achievement Data 2010/11. [Google Scholar]
- 14.Campbell S.M., Reeves D., Kontopantelis E., Sibbald B., Roland M. Effects of pay for performance on the quality of primary care in England. N Engl J Med. 2009;361(4):368–378. doi: 10.1056/NEJMsa0807651. [DOI] [PubMed] [Google Scholar]
- 15.McDonald H.I., Nitsch D., Millett E.R., Sinclair A., Thomas S.L. New estimates of the burden of acute community-acquired infections among older people with diabetes mellitus: a retrospective cohort study using linked electronic health records. Diabet Med. 2013;31(5):606–614. doi: 10.1111/dme.12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holman N., Forouhi N.G., Goyder E., Wild S.H. The Association of Public Health Observatories (APHO) diabetes prevalence model: estimates of total diabetes prevalence for England, 2010-2030. Diabet Med. 2011;28(5):575–582. doi: 10.1111/j.1464-5491.2010.03216.x. [DOI] [PubMed] [Google Scholar]
- 17.Finlay S., Bray B., Lewington A.J. Identification of risk factors associated with acute kidney injury in patients admitted to acute medical units. Clin Med. 2013;13(3):233–238. doi: 10.7861/clinmedicine.13-3-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.CPRD. The Clinical Practice Research Datalink-CPRD. 2012. http://www.cprd.com/intro.asp. Accessed August 30, 2012.
- 19.Herrett E., Thomas S.L., Schoonen W.M., Smeeth L., Hall A.J. Validation and validity of diagnoses in the General Practice Research Database: a systematic review. Br J Clin Pharmacol. 2010;69(1):4–14. doi: 10.1111/j.1365-2125.2009.03537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walley T., Mantgani A. The UK General Practice Research Database. Lancet. 1997;350(9084):1097–1099. doi: 10.1016/S0140-6736(97)04248-7. [DOI] [PubMed] [Google Scholar]
- 21.The Health and Social Care Information Centre. HESonline. 2012. http://www.hscic.gov.uk/hes. Accessed September 14, 2012.
- 22.Office for National Statistics. 2012. www.ons.gov.uk. Accessed September 14, 2012.
- 23.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsushita K., Mahmoodi B.K., Woodward M. Comparison of risk prediction using the CKD-EPI equation and the MDRD Study equation for estimated glomerular filtration rate. JAMA. 2012;307(18):1941–1951. doi: 10.1001/jama.2012.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Kidney Foundation: K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 suppl 1):S1–S266. [PubMed] [Google Scholar]
- 26.Mathur R., Bhaskaran K., Chaturvedi N. Completeness and usability of ethnicity data in UK-based primary care and hospital databases. J Public Health (Oxf) 2014;36(4):684–692. doi: 10.1093/pubmed/fdt116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Lusignan S., Tomson C., Harris K., van Vlymen J., Gallagher H. Creatinine fluctuation has a greater effect than the formula to estimate glomerular filtration rate on the prevalence of chronic kidney disease. Nephron Clin Pract. 2011;117(3):c213–c224. doi: 10.1159/000320341. [DOI] [PubMed] [Google Scholar]
- 28.Millett E.R., Quint J.K., Smeeth L., Daniel R.M., Thomas S.L. Incidence of community-acquired lower respiratory tract infections and pneumonia among older adults in the United Kingdom: a population-based study. PLoS One. 2013;8(9):e75131. doi: 10.1371/journal.pone.0075131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferguson J., Bauld L., Chesterman J., Judge K. The English smoking treatment services: one-year outcomes. Addiction. 2005;100(suppl 2):59–69. doi: 10.1111/j.1360-0443.2005.01028.x. [DOI] [PubMed] [Google Scholar]
- 30.Anandarajah S., Tai T., de Lusignan S. The validity of searching routinely collected general practice computer data to identify patients with chronic kidney disease (CKD): a manual review of 500 medical records. Nephrol Dial Transplant. 2005;20(10):2089–2096. doi: 10.1093/ndt/gfi006. [DOI] [PubMed] [Google Scholar]
- 31.Hobbs H., Stevens P., Klebe B. Referral patterns to renal services: what has changed in the past 4 years? Nephrol Dial Transplant. 2009;24(11):3411–3419. doi: 10.1093/ndt/gfp289. [DOI] [PubMed] [Google Scholar]
- 32.NHS Employers. Quality and outcomes framework. 2012. http://www.nhsemployers.org/payandcontracts/generalmedicalservicescontract/qof/pages/qualityoutcomesframework.aspx. Accessed September 12, 2012.
- 33.Dalrymple L.S., Katz R., Kestenbaum B. The risk of infection-related hospitalization with decreased kidney function. Am J Kidney Dis. 2012;59(3):356–363. doi: 10.1053/j.ajkd.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Go A.S., Chertow G.M., Fan D., McCulloch C.E., Hsu C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 35.Kausz A.T., Gilbertson D.T. Overview of vaccination in chronic kidney disease. Adv Chronic Kidney Dis. 2006;13(3):209–214. doi: 10.1053/j.ackd.2006.04.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sensitivity analysis of association between eGFR and infection incidence.