Abstract
Background
Opioid analgesics and other psychoactive drugs may pose an even greater risk for drug overdose in persons with mental health disorders.
Objective
The purpose of this study was to examine interactions of filled prescriptions for opioids, benzodiazepines, antidepressants, and zolpidem with mental health disorders in regard to drug overdose.
Design
The study was a retrospective cohort review.
Subjects
Subjects were national HMO beneficiaries aged 18–64 years, enrolled at least 1 year (01/2009 to 07/2012), who filled at least two prescriptions for Schedule II or III opioids for non-cancer pain.
Main Measures
The outcome was the first inpatient or outpatient drug overdose after the first filled opioid prescription. Predictors were calculated in 6-month intervals and exactly 6 months before a drug overdose: opioid use (mean daily morphine-equivalent dose), benzodiazepine use (days’ supply), antidepressant use (days’ supply), zolpidem use (days’ supply), mental health disorders (depression, anxiety/PTSD, psychosis), pain-related conditions, and substance use disorders (alcohol, other drug).
Key Results
A total of 1,385 (0.67 %) subjects experienced a drug overdose (incidence rate 421/100,000 person-years). The adjusted odds ratios (AOR) for overdose among all subjects rose monotonically with daily opioid dose, but highest (AOR = 7.06) for persons with depression and a high opioid dose (≥100 mg) versus no depression or opioid use. Longer-term antidepressants (91–180 days) were protective for persons with depression, with 20 % lower AORs for overdose versus short-term (1–30 days) or none. For persons without depression, the AORs of overdose were increased for antidepressant use, but greatest (AOR = 1.98) for short-term use versus none. The AORs of overdose increased with the duration of benzodiazepine therapy among all subjects, with over 2.5-fold higher AORs for 91–180 days versus none.
Conclusions
Opioids and longer-duration benzodiazepines were associated with drug overdose among all subjects, but opioid risk was greatest for persons with depression. Antidepressant use > 90 days reduced the odds of overdose for persons with depression, but all antidepressant use increased the risk for persons without depression.
KEY WORDS: opioid analgesics, overdose, psychotherapeutic drugs
INTRODUCTION
Between 1999 and 2009, U. S. rates of mortality from accidental pharmaceutical drug overdose rose over fourfold for opioid analgesics and threefold for sedative-hypnotics.1 Of the more than 22,000 unintentional pharmaceutical overdose deaths nationally in 2010, three-quarters involved opioid analgesics, while benzodiazepines were identified in one-quarter and antidepressants in nearly 20 %.2 Concurrent use of these drugs appears to carry an even higher risk. The combination of prescribed opioids and benzodiazepines is the most common cause of polysubstance overdose deaths nationally.1 Persons with mental health disorders are more likely to be treated for chronic pain with high-dose opioid therapy that has also been associated with more co-prescribed sedative-hypnotic therapy.3 However, we are unaware of studies examining the risk of drug overdose for complex inter-relationships of multiple drugs commonly used by persons with chronic pain and comorbid mental health disorders.
We hypothesized that patients with mental health disorders who take opioids and other psychotherapeutic drugs would have a significantly greater risk for overdose than those who do not. To examine these hypotheses, we analyzed a longitudinal database from a national health maintenance organization (HMO) of persons with non-cancer pain who filled multiple prescriptions for opioid analgesics. This study offers insights into the risks and potential benefits of opioids, benzodiazepines, antidepressants, and/or zolpidem for persons with mental health disorders.
METHODS
Study Setting
The study utilized patient data from the Aetna Health Maintenance Program that provides comprehensive full-service care to approximately 2.1 million persons nationally. Study data were obtained from enrollment files and claims for services and prescriptions. The study was approved by the Institutional Review Board of the University of Texas Health Science Center at San Antonio.
Study Subjects
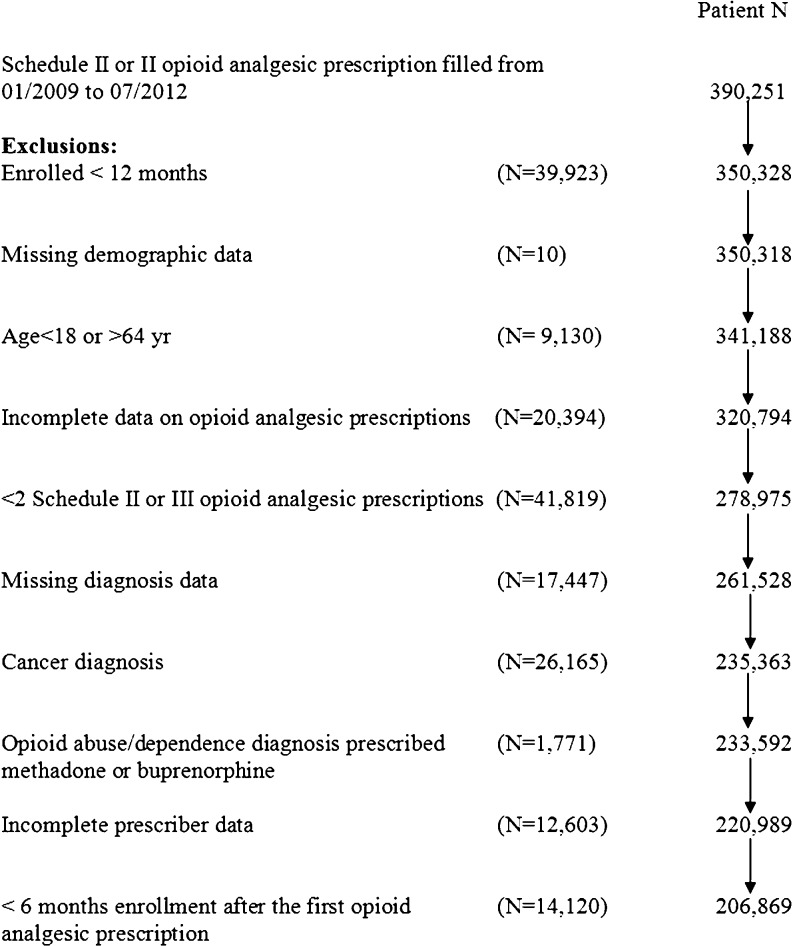
The study cohort included persons aged 18 to 64 years with non-cancer pain who filled at least two Schedule II or III prescriptions for non-injectable opioid analgesics from January 2009 through July 2012. Eligible subjects were continuously enrolled at least 12 months in the Aetna plan and had claims for service utilization at least 6 months before the first drug overdose event. The cohort derivation revealed that the most frequent exclusions were due to incomplete opioid prescription data, missing diagnostic data, or a non-basal cell cancer diagnosis (Fig. 1).
Figure 1.
Derivation of study cohort of persons filling at least two prescriptions for Schedule II or III opioid analgesics.
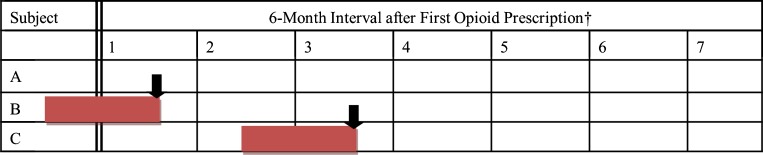
Study subjects’ medications and clinical conditions were examined for each 6-month interval after the first filled opioid prescription, up to a maximum of seven intervals. Incomplete intervals were excluded. Examples of 6-month interval data for three sample patients are shown in Fig. 2. Subject A had no overdose event; therefore, time-varying covariates were examined for each complete 6 month-interval after the first filled opioid prescription until the subject left the plan or the study time frame ended. Subject B had an overdose within the first 6-month interval. To examine a full 6 months before that event, covariates were based on data from services and medications received before and after the first opioid prescription. For subject C, the overdose occurred in the third 6-month interval; therefore, time-varying covariates were calculated for 6-month intervals without an event and from the 6 months before the overdose event. Subjects were censored after the first overdose.
Figure 2.
Calculation of covariates for persons with an overdose event* and comparison group.* Dark arrow shows the date of the overdose event. Persons with an overdose event are censored after the first overdose event. †Date of first opioid prescription indicated by dark line. A If no overdose occurred, up to seven 6-month intervals would be observed. Covariates were examined in each observed 6-month interval. B Overdose occurred within 6 months of the first opioid prescription, covariates determined from 6 months prior to the overdose date. Comparison group: persons with no event in the first interval (such as A and C in interval 1). C Overdose occurred in third 6-month interval, covariates examined from prior 6 months spanning second and third 6-month intervals. Comparison group: persons with no event in the third interval (such as A in interval 3).
Outcome Variable
The study outcome was drug overdose diagnosed from an inpatient or outpatient clinical encounter following the first filled opioid prescription (drug overdose ICD-9-CM codes in Appendix 1). For each subject, this time-varying outcome was measured in each 6-month interval after the first filled opioid prescription until last enrollment or end of the study time frame. For subjects with multiple overdose events in a given 6-month interval, only the first was considered.
Classification of Morphine-Equivalent Dose
Using an approach previously reported by our group,4 a morphine-equivalent dose (MED) for each Schedule II or III opioid prescription (non-injectable formulations) was calculated from the number of pills dispensed multiplied by strength (in milligrams), then multiplied by a morphine-equivalent conversion factor derived from several sources, including published data,5,6 conversion tables from Internet sources, and drug information resources (Table 2 in Appendix 2).7,8 A clinical pharmacist assisted with calculating conversions. We capped the daily dose based on the maximum recommended for that drug.
Table 2.
Morphine Conversion Factors
| gpi_cd | drug_nm | gpi_nm | short_act | max_daily | morph_eqv_fctr | drg_strnth_mg | adj_dose |
|---|---|---|---|---|---|---|---|
| 65991002052020 | APAP/CODEINE SOL 120-12/5 | Acetaminophen w/ Codeine Soln 120–12 MG/5ML | 1 | 150 | 0.15 | 2.4 | 0.36 |
| 65991002051805 | CAPITAL/COD SUS 120-12/5 | Acetaminophen w/ Codeine Susp 120–12 MG/5ML | 1 | 150 | 0.15 | 2.4 | 0.36 |
| 65991002050310 | APAP/CODEINE TAB 300-15MG | Acetaminophen w/ Codeine Tab 300–15 MG | 1 | 13 | 0.15 | 15 | 2.25 |
| 65991002050315 | APAP/CODEINE TAB 300-30MG | Acetaminophen w/ Codeine Tab 300–30 MG | 1 | 12 | 0.15 | 30 | 4.5 |
| 65991002050320 | APAP/CODEINE TAB 300-60MG | Acetaminophen w/ Codeine Tab 300–60 MG | 1 | 6 | 0.15 | 60 | 9 |
| 65991303050120 | TREZIX CAP | Acetaminophen-Caffeine-Dihydrocodeine Cap 356.4-30 | 1 | 10 | 0.25 | 16 | 4 |
| 65991303050340 | APAP/CAFF/DI TAB HYDROCOD | Acetaminophen-Caffeine-Dihydrocodeine Tab 712.8-60 | 1 | 5 | 0.25 | 32 | 8 |
| 65200010100760 | BUPRENORPHIN SUB 2MG | Buprenorphine HCl SL Tab 2 MG (Base Equiv) | 0 | 3 | 75 | 2 | 150 |
| 65200010100780 | BUPRENORPHIN SUB 8MG | Buprenorphine HCl SL Tab 8 MG (Base Equiv) | 0 | 3 | 75 | 8 | 600 |
| 65200010208220 | SUBOXONE MIS 2-0.5MG | Buprenorphine HCl-Naloxone HCl SL Film 2-0.5 MG (B | 0 | 3 | 75 | 2 | 150 |
| 65200010208240 | SUBOXONE MIS 8-2MG | Buprenorphine HCl-Naloxone HCl SL Film 8-2 MG (Bas) | 0 | 3 | 75 | 8 | 600 |
| 65200010200720 | SUBOXONE SUB 2-0.5MG | Buprenorphine HCl-Naloxone HCl SL Tab 2-0.5 MG (Ba) | 0 | 3 | 75 | 2 | 150 |
| 65200010200740 | SUBOXONE SUB 8-2MG | Buprenorphine HCl-Naloxone HCl SL Tab 8-2 MG (Base) | 0 | 3 | 75 | 8 | 600 |
| 65200010008830 | BUTRANS DIS 10MCG/HR | Buprenorphine TD Patch Weekly 10 MCG/HR | 0 | 0.286 | 1800 | 0.01 | 18 |
| 65200010008840 | BUTRANS DIS 20MCG/HR | Buprenorphine TD Patch Weekly 20 MCG/HR | 0 | 0.143 | 1800 | 0.02 | 36 |
| 65200010008820 | BUTRANS DIS 5MCG/HR | Buprenorphine TD Patch Weekly 5 MCG/HR | 0 | 0.286 | 1800 | 0.005 | 9 |
| 65991004100115 | BUT/APAP/CAF CAP CODEINE | Butalbital-Acetaminophen-Caff w/ COD Cap 50-325-40 | 1 | 6 | 0.15 | 30 | 4.5 |
| 65991004300115 | ASCOMP/COD CAP 30MG | Butalbital-Aspirin-Caff w/ Codeine Cap 50-325-40-3 | 1 | 6 | 0.15 | 30 | 4.5 |
| 65100020200305 | CODEINE SULF TAB 15MG | Codeine Sulfate Tab 15 MG | 1 | 13 | 0.15 | 15 | 2.25 |
| 65100020200310 | CODEINE SULF TAB 30MG | Codeine Sulfate Tab 30 MG | 1 | 12 | 0.15 | 30 | 4.5 |
| 65100020200315 | CODEINE SULF TAB 60MG | Codeine Sulfate Tab 60 MG | 1 | 6 | 0.15 | 60 | 9 |
| 65991303100115 | SYNALGOS DC CAP | Dihydrocodeine Compound Cap | 1 | 10 | 0.25 | 16 | 4 |
| 65100025100310 | FENTORA TAB 100MCG | Fentanyl Citrate Buccal Tab 100 MCG (Base Equiv) | 1 | 4 | 125 | 0.1 | 12.5 |
| 65100025100320 | FENTORA TAB 200MCG | Fentanyl Citrate Buccal Tab 200 MCG (Base Equiv) | 1 | 4 | 125 | 0.2 | 25 |
| 65100025100330 | FENTORA TAB 400MCG | Fentanyl Citrate Buccal Tab 400 MCG (Base Equiv) | 1 | 4 | 125 | 0.4 | 50 |
| 65100025100340 | FENTORA TAB 600MCG | Fentanyl Citrate Buccal Tab 600 MCG (Base Equiv) | 1 | 4 | 125 | 0.6 | 75 |
| 65100025100350 | FENTORA TAB 800MCG | Fentanyl Citrate Buccal Tab 800 MCG (Base Equiv) | 1 | 4 | 125 | 0.8 | 100 |
| 65100025108475 | FENTANYL OT LOZ 1200MCG | Fentanyl Citrate Lollipop 1200 MCG | 1 | 4 | 125 | 1.2 | 150 |
| 65100025108485 | FENTANYL OT LOZ 1600MCG | Fentanyl Citrate Lollipop 1600 MCG | 1 | 4 | 125 | 1.6 | 200 |
| 65100025108450 | FENTANYL OT LOZ 200MCG | Fentanyl Citrate Lollipop 200 MCG | 1 | 4 | 125 | 0.2 | 25 |
| 65100025108455 | FENTANYL OT LOZ 400MCG | Fentanyl Citrate Lollipop 400 MCG | 1 | 4 | 125 | 0.4 | 50 |
| 65100025108460 | FENTANYL OT LOZ 600MCG | Fentanyl Citrate Lollipop 600 MCG | 1 | 4 | 125 | 0.6 | 75 |
| 65100025108465 | FENTANYL OT LOZ 800MCG | Fentanyl Citrate Lollipop 800 MCG | 1 | 4 | 125 | 0.8 | 100 |
| 65100025100720 | ABSTRAL SUB 200MCG | Fentanyl Citrate SL Tab 200 MCG (Base Equiv) | 1 | 4 | 125 | 0.2 | 25 |
| 65100025000910 | SUBSYS SPR 100MCG | Fentanyl Sublingual Spray 100 MCG | 1 | 4 | 125 | 0.1 | 12.5 |
| 65100025000920 | SUBSYS SPR 200MCG | Fentanyl Sublingual Spray 200 MCG | 1 | 4 | 125 | 0.2 | 25 |
| 65100025008650 | FENTANYL DIS 100MCG/H | Fentanyl TD Patch 72HR 100 MCG/HR | 0 | 1.5 | 2400 | 0.1 | 240 |
| 65100025008610 | FENTANYL DIS 12MCG/HR | Fentanyl TD Patch 72HR 12 MCG/HR | 0 | 0.5 | 2400 | 0.0125 | 30 |
| 65100025008620 | FENTANYL DIS 25MCG/HR | Fentanyl TD Patch 72HR 25 MCG/HR | 0 | 0.5 | 2400 | 0.025 | 60 |
| 65100025008630 | FENTANYL DIS 50MCG/HR | Fentanyl TD Patch 72HR 50 MCG/HR | 0 | 0.5 | 2400 | 0.05 | 120 |
| 65100025008640 | FENTANYL DIS 75MCG/HR | Fentanyl TD Patch 72HR 75 MCG/HR | 0 | 1 | 2400 | 0.075 | 180 |
| 65991702100353 | HYDROCO/APAP TAB 10–750MG | HYDROCO/APAP TAB 10–750MG | 1 | 5 | 1 | 10 | 10 |
| 65991702100110 | STAGESIC CAP 500-5MG | Hydrocodone-Acetaminophen Cap 5–500 MG | 1 | 8 | 1 | 5 | 5 |
| 65991702102024 | ZOLVIT SOL 10–300MG | Hydrocodone-Acetaminophen Soln 10–300 MG/15ML | 1 | 200 | 1 | 0.67 | 0.67 |
| 65991702102025 | ZAMICET SOL 10–325MG | Hydrocodone-Acetaminophen Soln 10–325 MG/15ML | 1 | 184.6 | 1 | 0.67 | 0.67 |
| 65991702102015 | HYCET SOL 7.5–325 | Hydrocodone-Acetaminophen Soln 7.5–325 MG/15ML | 1 | 184.6 | 1 | 0.5 | 0.5 |
| 65991702102020 | HYDROCODONE/ SOL APAP | Hydrocodone-Acetaminophen Soln 7.5–500 MG/15ML | 1 | 120 | 1 | 0.5 | 0.5 |
| 65991702100375 | XODOL TAB 10–300MG | Hydrocodone-Acetaminophen Tab 10–300 MG | 1 | 13 | 1 | 10 | 10 |
| 65991702100305 | HYDROCO/APAP TAB 10–325MG | Hydrocodone-Acetaminophen Tab 10–325 MG | 1 | 12 | 1 | 10 | 10 |
| 65991702100370 | ZYDONE TAB 10–400MG | Hydrocodone-Acetaminophen Tab 10–400 MG | 1 | 10 | 1 | 10 | 10 |
| 65991702100327 | HYDROCO/APAP TAB 10–500MG | Hydrocodone-Acetaminophen Tab 10–500 MG | 1 | 8 | 1 | 10 | 10 |
| 65991702100345 | HYDROCO/APAP TAB 10–650MG | Hydrocodone-Acetaminophen Tab 10–650 MG | 1 | 6 | 1 | 10 | 10 |
| 65991702100346 | HYDROCO/APAP TAB 10–660MG | Hydrocodone-Acetaminophen Tab 10–660 MG | 1 | 6 | 1 | 10 | 10 |
| 65991702100307 | HYDROCO/APAP TAB 2.5–500 | Hydrocodone-Acetaminophen Tab 2.5–500 MG | 1 | 8 | 1 | 2.5 | 2.5 |
| 65991702100309 | HYDROCO/APAP TAB 5–300MG | Hydrocodone-Acetaminophen Tab 5–300 MG | 1 | 13 | 1 | 5 | 5 |
| 65991702100356 | HYDROCO/APAP TAB 5–325MG | Hydrocodone-Acetaminophen Tab 5–325 MG | 1 | 12 | 1 | 5 | 5 |
| 65991702100310 | HYDROCO/APAP TAB 5–500MG | Hydrocodone-Acetaminophen Tab 5–500 MG | 1 | 8 | 1 | 5 | 5 |
| 65991702100322 | XODOL TAB 7.5–300 | Hydrocodone-Acetaminophen Tab 7.5–300 MG | 1 | 13 | 1 | 7.5 | 7.5 |
| 65991702100358 | HYDROCO/APAP TAB 7.5–325 | Hydrocodone-Acetaminophen Tab 7.5–325 MG | 1 | 12 | 1 | 7.5 | 7.5 |
| 65991702100365 | ZYDONE TAB 7.5–400 | Hydrocodone-Acetaminophen Tab 7.5–400 MG | 1 | 10 | 1 | 7.5 | 7.5 |
| 65991702100325 | HYDROCO/APAP TAB 7.5–500 | Hydrocodone-Acetaminophen Tab 7.5–500 MG | 1 | 8 | 1 | 7.5 | 7.5 |
| 65991702100340 | HYDROCO/APAP TAB 7.5–650 | Hydrocodone-Acetaminophen Tab 7.5–650 MG | 1 | 6 | 1 | 7.5 | 7.5 |
| 65991702100350 | HYDROCO/APAP TAB 7.5–750 | Hydrocodone-Acetaminophen Tab 7.5–750 MG | 1 | 5 | 1 | 7.5 | 7.5 |
| 65991702500330 | REPREXAIN TAB 10–200MG | Hydrocodone-Ibuprofen Tab 10–200 MG | 1 | 5 | 1 | 10 | 10 |
| 65991702500310 | REPREXAIN TAB 2.5–200 | Hydrocodone-Ibuprofen Tab 2.5–200 MG | 1 | 5 | 1 | 2.5 | 2.5 |
| 65991702500315 | HYDROCOD/IBU TAB 5–200MG | Hydrocodone-Ibuprofen Tab 5–200 MG | 1 | 5 | 1 | 5 | 5 |
| 65991702500320 | HYDROCOD/IBU TAB 7.5–200 | Hydrocodone-Ibuprofen Tab 7.5–200 MG | 1 | 5 | 1 | 7.5 | 7.5 |
| 65100035100920 | HYDROMORPHON LIQ 1MG/ML | Hydromorphone HCl Liqd 1 MG/ML | 1 | 480 | 4 | 1 | 4 |
| 65100035105205 | HYDROMORPHON SUP 3MG | Hydromorphone HCl Suppos 3 MG | 1 | 12 | 4 | 3 | 12 |
| 65100035100310 | HYDROMORPHON TAB 2MG | Hydromorphone HCl Tab 2 MG | 1 | 16 | 4 | 2 | 8 |
| 65100035100320 | HYDROMORPHON TAB 4MG | Hydromorphone HCl Tab 4 MG | 1 | 8 | 4 | 4 | 16 |
| 65100035100330 | HYDROMORPHON TAB 8MG | Hydromorphone HCl Tab 8 MG | 1 | 4 | 4 | 8 | 32 |
| 65100035107530 | EXALGO TAB 12MG | Hydromorphone HCl Tab SR 24HR 12 MG | 0 | 2 | 4 | 12 | 48 |
| 65100035107540 | EXALGO TAB 16MG | Hydromorphone HCl Tab SR 24HR 16 MG | 0 | 2 | 4 | 16 | 64 |
| 65100035107555 | EXALGO TAB 32MG | Hydromorphone HCl Tab SR 24HR 32 MG | 0 | 4 | 32 | 128 | |
| 65100035107520 | EXALGO TAB 8MG | Hydromorphone HCl Tab SR 24HR 8 MG | 0 | 4 | 4 | 8 | 32 |
| 65100045102060 | MEPERIDINE SOL 50MG/5ML | Meperidine HCl Oral Soln 50 MG/5ML | 1 | 60 | 0.1 | 10 | 1 |
| 65100045100310 | MEPERITAB TAB 100MG | Meperidine HCl Tab 100 MG | 1 | 6 | 0.1 | 100 | 10 |
| 65100045100305 | MEPERITAB TAB 50MG | Meperidine HCl Tab 50 MG | 1 | 12 | 0.1 | 50 | 5 |
| 65993002200110 | MEPROZINE CAP 50-25MG | Meperidine w/ Promethazine Cap 50-25 MG | 1 | 12 | 0.1 | 50 | 5 |
| 65100050101310 | METHADONE CON 10MG/ML | Methadone HCl Conc 10 MG/ML | 1 | 40 | 3 | 10 | 30 |
| 65100050102015 | METHADONE SOL 10MG/5ML | Methadone HCl Soln 10 MG/5ML | 1 | 200 | 3 | 2 | 6 |
| 65100050102010 | METHADONE SOL 5MG/5ML | Methadone HCl Soln 5 MG/5ML | 1 | 400 | 3 | 1 | 3 |
| 65100050100310 | METHADONE TAB 10MG | Methadone HCl Tab 10 MG | 1 | 40 | 3 | 10 | 30 |
| 65100050100305 | METHADONE TAB 5MG | Methadone HCl Tab 5 MG | 1 | 80 | 3 | 5 | 15 |
| 65100050107320 | METHADONE TAB 40MG | Methadone HCl Tab For Oral Susp 40 MG | 1 | 10 | 3 | 40 | 120 |
| 65100055107480 | MORPHINE SUL TAB 200MG ER | MORPHINE SUL TAB 200MG ER | 0 | 1 | 200 | 200 | |
| 65100055102090 | MORPHINE SUL SOL 20MG/ML | Morphine Sulfate (Concentrate) Oral Soln 20 MG/ML | 1 | 36 | 1 | 20 | 20 |
| 65100055207050 | AVINZA CAP 120MG ER | Morphine Sulfate Beads Cap SR 24HR 120 MG | 0 | 13 | 1 | 120 | 120 |
| 65100055207020 | AVINZA CAP 30MG | Morphine Sulfate Beads Cap SR 24HR 30 MG | 0 | 1 | 1 | 30 | 30 |
| 65100055207025 | AVINZA CAP 45MG | Morphine Sulfate Beads Cap SR 24HR 45 MG | 0 | 1 | 1 | 45 | 45 |
| 65100055207030 | AVINZA CAP 60MG CR | Morphine Sulfate Beads Cap SR 24HR 60 MG | 0 | 1 | 1 | 60 | 60 |
| 65100055207035 | AVINZA CAP 75MG | Morphine Sulfate Beads Cap SR 24HR 75 MG | 0 | 21 | 1 | 75 | 75 |
| 65100055207040 | AVINZA CAP 90MG CR | Morphine Sulfate Beads Cap SR 24HR 90 MG | 0 | 17 | 1 | 90 | 90 |
| 65100055107010 | KADIAN CAP 10MG CR | Morphine Sulfate Cap SR 24HR 10 MG | 0 | 1 | 1 | 10 | 10 |
| 65100055107060 | KADIAN CAP 100MG CR | Morphine Sulfate Cap SR 24HR 100 MG | 0 | 1 | 1 | 100 | 100 |
| 65100055107020 | KADIAN CAP 20MG CR | Morphine Sulfate Cap SR 24HR 20 MG | 0 | 2 | 1 | 20 | 20 |
| 65100055107030 | KADIAN CAP 30MG CR | Morphine Sulfate Cap SR 24HR 30 MG | 0 | 1 | 1 | 30 | 30 |
| 65100055107040 | KADIAN CAP 50MG CR | Morphine Sulfate Cap SR 24HR 50 MG | 0 | 1 | 1 | 50 | 50 |
| 65100055107045 | KADIAN CAP 60MG CR | Morphine Sulfate Cap SR 24HR 60 MG | 0 | 1 | 1 | 60 | 60 |
| 65100055107050 | KADIAN CAP 80MG CR | Morphine Sulfate Cap SR 24HR 80 MG | 0 | 1 | 1 | 80 | 80 |
| 65100055102065 | MORPHINE SUL SOL 10MG/5ML | Morphine Sulfate Oral Soln 10 MG/5ML | 1 | 480 | 1 | 2 | 2 |
| 65100055102070 | MORPHINE SUL SOL 20MG/5ML | Morphine Sulfate Oral Soln 20 MG/5ML | 1 | 240 | 1 | 4 | 4 |
| 65100055100310 | MORPHINE SUL TAB 15MG | Morphine Sulfate Tab 15 MG | 1 | 12 | 1 | 15 | 15 |
| 65100055100315 | MORPHINE SUL TAB 30MG | Morphine Sulfate Tab 30 MG | 1 | 6 | 1 | 30 | 30 |
| 65100055107314 | MORPHINE SUL TAB 10MG | Morphine Sulfate Tab Sol 10 MG | 1 | 480 | 1 | 10 | 10 |
| 65100055107460 | MORPHINE SUL TAB 100MG ER | Morphine Sulfate Tab SR 12HR 100 MG | 0 | 3 | 1 | 100 | 100 |
| 65100055107415 | MORPHINE SUL TAB 15MG ER | Morphine Sulfate Tab SR 12HR 15 MG | 0 | 3 | 1 | 15 | 15 |
| 65100055107430 | MORPHINE SUL TAB 30MG CR | Morphine Sulfate Tab SR 12HR 30 MG | 0 | 3 | 1 | 30 | 30 |
| 65100055107445 | MORPHINE SUL TAB 60MG ER | Morphine Sulfate Tab SR 12HR 60 MG | 0 | 3 | 1 | 60 | 60 |
| 65100055700270 | EMBEDA CAP 100-4MG | Morphine-Naltrexone Cap CR 100-4 MG | 0 | 1 | 100 | 100 | |
| 65100055700220 | EMBEDA CAP 20-0.8MG | Morphine-Naltrexone Cap CR 20-0.8 MG | 0 | 4 | 1 | 20 | 20 |
| 65100055700230 | EMBEDA CAP 30-1.2MG | Morphine-Naltrexone Cap CR 30-1.2 MG | 0 | 6 | 1 | 30 | 30 |
| 65100055700240 | EMBEDA CAP 50-2MG | Morphine-Naltrexone Cap CR 50-2 MG | 0 | 2 | 1 | 50 | 50 |
| 65100055700250 | EMBEDA CAP 60-2.4MG | Morphine-Naltrexone Cap CR 60-2.4 MG | 0 | 4 | 1 | 60 | 60 |
| 65100055700260 | EMBEDA CAP 80-3.2MG | Morphine-Naltrexone Cap CR 80-3.2 MG | 0 | 1 | 80 | 80 | |
| 65990002200337 | NARVOX TAB 10–500MG | NARVOX TAB 10–500MG | 1 | 8 | 1.5 | 10 | 15 |
| 65100075100110 | OXYIR CAP 5MG | Oxycodone HCl Cap 5 MG | 1 | 18 | 1.5 | 5 | 7.5 |
| 65100075101320 | ETH-OXYDOSE CON 20MG/ML | Oxycodone HCl Conc 20 MG/ML | 1 | 36 | 1.5 | 20 | 30 |
| 65100075102005 | OXYCODONE SOL 5MG/5ML | Oxycodone HCl Soln 5 MG/5ML | 1 | 360 | 1.5 | 1 | 1.5 |
| 65100075100320 | OXYCODONE TAB 10MG | Oxycodone HCl Tab 10 MG | 1 | 12 | 1.5 | 10 | 15 |
| 65100075100325 | OXYCODONE TAB 15MG | Oxycodone HCl Tab 15 MG | 1 | 12 | 1.5 | 15 | 22.5 |
| 65100075100330 | OXYCODONE TAB 20MG | Oxycodone HCl Tab 20 MG | 1 | 12 | 1.5 | 20 | 30 |
| 65100075100340 | OXYCODONE TAB 30MG | Oxycodone HCl Tab 30 MG | 1 | 24 | 1.5 | 30 | 45 |
| 65100075100310 | OXYCODONE TAB 5MG | Oxycodone HCl Tab 5 MG | 1 | 18 | 1.5 | 5 | 7.5 |
| 65100075107410 | OXYCONTIN TAB 10MG CR | Oxycodone HCl Tab SR 12HR 10 MG | 0 | 3 | 1.5 | 10 | 15 |
| 65100075107415 | OXYCONTIN TAB 15MG CR | Oxycodone HCl Tab SR 12HR 15 MG | 0 | 3 | 1.5 | 15 | 22.5 |
| 65100075107420 | OXYCONTIN TAB 20MG CR | Oxycodone HCl Tab SR 12HR 20 MG | 0 | 3 | 1.5 | 20 | 30 |
| 65100075107430 | OXYCONTIN TAB 30MG CR | Oxycodone HCl Tab SR 12HR 30 MG | 0 | 3 | 1.5 | 30 | 45 |
| 65100075107440 | OXYCONTIN TAB 40MG CR | Oxycodone HCl Tab SR 12HR 40 MG | 0 | 3 | 1.5 | 40 | 60 |
| 65100075107460 | OXYCONTIN TAB 60MG CR | Oxycodone HCl Tab SR 12HR 60 MG | 0 | 3 | 1.5 | 60 | 90 |
| 65100075107480 | OXYCONTIN TAB 80MG CR | Oxycodone HCl Tab SR 12HR 80 MG | 0 | 8 | 1.5 | 80 | 120 |
| 65990002200120 | OXYCOD/APAP CAP 5–500MG | Oxycodone w/ Acetaminophen Cap 5–500 MG | 1 | 8 | 1.5 | 5 | 7.5 |
| 65990002202005 | ROXICET SOL 5-325/5 | Oxycodone w/ Acetaminophen Soln 5–325 MG/5ML | 1 | 65 | 1.5 | 1 | 1.5 |
| 65990002200333 | PRIMLEV TAB 10–300MG | Oxycodone w/ Acetaminophen Tab 10–300 MG | 1 | 13 | 1.5 | 10 | 15 |
| 65990002200335 | OXYCOD/APAP TAB 10–325MG | Oxycodone w/ Acetaminophen Tab 10–325 MG | 1 | 12 | 1.5 | 10 | 15 |
| 65990002200336 | MAGNACET TAB 10–400MG | Oxycodone w/ Acetaminophen Tab 10–400 MG | 1 | 10 | 1.5 | 10 | 15 |
| 65990002200340 | OXYCOD/APAP TAB 10–650MG | Oxycodone w/ Acetaminophen Tab 10–650 MG | 1 | 6 | 1.5 | 10 | 15 |
| 65990002200305 | OXYCOD/APAP TAB 2.5–325 | Oxycodone w/ Acetaminophen Tab 2.5–325 MG | 1 | 12 | 1.5 | 2.5 | 3.75 |
| 65990002200308 | PRIMALEV TAB 5–300 | Oxycodone w/ Acetaminophen Tab 5–300 MG | 1 | 12 | 1.5 | 5 | 7.5 |
| 65990002200310 | OXYCOD/APAP TAB 5–325MG | Oxycodone w/ Acetaminophen Tab 5–325 MG | 1 | 12 | 1.5 | 5 | 7.5 |
| 65990002200315 | MAGNACET TAB 5–400MG | Oxycodone w/ Acetaminophen Tab 5–400 MG | 1 | 10 | 1.5 | 5 | 7.5 |
| 65990002200325 | PRIMALEV TAB 7.5–300 | Oxycodone w/ Acetaminophen Tab 7.5–300 MG | 1 | 13 | 1.5 | 7.5 | 11.25 |
| 65990002200327 | OXYCOD-APAP TAB 7.5–325 | Oxycodone w/ Acetaminophen Tab 7.5–325 MG | 1 | 12 | 1.5 | 7.5 | 11.25 |
| 65990002200328 | MAGNACET TAB 7.5–400 | Oxycodone w/ Acetaminophen Tab 7.5–400 MG | 1 | 10 | 1.5 | 7.5 | 11.25 |
| 65990002200330 | OXYCOD/APAP TAB 7.5–500 | Oxycodone w/ Acetaminophen Tab 7.5–500 MG | 1 | 8 | 1.5 | 7.5 | 11.25 |
| 65990002220320 | PERCODAN TAB | Oxycodone w/ Aspirin Tab Full Strength | 1 | 12 | 1.5 | 4.8355 | 7.25325 |
| 65990002220340 | OXYCOD/ASA TAB | Oxycodone-Aspirin Tab 4.8355-325 MG | 1 | 12 | 1.5 | 4.8355 | 7.25325 |
| 65990002260320 | OXYCOD/IBU TAB 5–400MG | Oxycodone-Ibuprofen Tab 5–400 MG | 1 | 4 | 1.5 | 5 | 7.5 |
| 65100080100310 | OXYMORPHONE TAB HCL 10MG | Oxymorphone HCl Tab 10 MG | 1 | 24 | 3 | 10 | 30 |
| 65100080100305 | OXYMORPHONE TAB HCL 5MG | Oxymorphone HCl Tab 5 MG | 1 | 24 | 3 | 5 | 15 |
| 65100080107410 | OPANA ER TAB 10MG | Oxymorphone HCl Tab SR 12HR 10 MG | 0 | 3 | 3 | 10 | 30 |
| 65100080107465 | OPANA ER TAB 10MG | Oxymorphone HCl Tab SR 12HR 10 MG (Crush Resistant) | 0 | 3 | 3 | 10 | 30 |
| 65100080107415 | OPANA ER TAB 15MG | Oxymorphone HCl Tab SR 12HR 15 MG | 0 | 3 | 3 | 15 | 45 |
| 65100080107420 | OPANA ER TAB 20MG | Oxymorphone HCl Tab SR 12HR 20 MG | 0 | 3 | 3 | 20 | 60 |
| 65100080107475 | OPANA ER TAB 20MG | Oxymorphone HCl Tab SR 12HR 20 MG (Crush Resistant) | 0 | 3 | 3 | 20 | 60 |
| 65100080107430 | OPANA ER TAB 30MG | Oxymorphone HCl Tab SR 12HR 30 MG | 0 | 3 | 3 | 30 | 90 |
| 65100080107480 | OPANA ER TAB 30MG | Oxymorphone HCl Tab SR 12HR 30 MG (Crush Resistant) | 0 | 3 | 3 | 30 | 90 |
| 65100080107440 | OPANA ER TAB 40MG | Oxymorphone HCl Tab SR 12HR 40 MG | 0 | 6 | 3 | 40 | 120 |
| 65100080107485 | OPANA ER TAB 40MG | Oxymorphone HCl Tab SR 12HR 40 MG (Crush Resistant) | 0 | 6 | 3 | 40 | 120 |
| 65100080107405 | OPANA ER TAB 5MG | Oxymorphone HCl Tab SR 12HR 5 MG | 0 | 3 | 3 | 5 | 15 |
| 65100080107455 | OPANA ER TAB 5MG | Oxymorphone HCl Tab SR 12HR 5 MG (Crush Resistant) | 0 | 3 | 3 | 5 | 15 |
| 65100080107407 | OXYMORPHONE TAB 7.5MG ER | Oxymorphone HCl Tab SR 12HR 7.5 MG | 0 | 3 | 3 | 7.5 | 22.5 |
| 65100091100340 | NUCYNTA TAB 100MG | Tapentadol HCl Tab 100 MG | 1 | 6 | 0.4 | 100 | 40 |
| 65100091100320 | NUCYNTA TAB 50MG | Tapentadol HCl Tab 50 MG | 1 | 12 | 0.4 | 50 | 20 |
| 65100091100330 | NUCYNTA TAB 75MG | Tapentadol HCl Tab 75 MG | 1 | 8 | 0.4 | 75 | 30 |
| 65100091107430 | NUCYNTA ER TAB 100MG | Tapentadol HCl Tab SR 12HR 100 MG | 0 | 3 | 0.4 | 100 | 40 |
| 65100091107440 | NUCYNTA ER TAB 150MG | Tapentadol HCl Tab SR 12HR 150 MG | 0 | 3 | 0.4 | 150 | 60 |
| 65100091107450 | NUCYNTA ER TAB 200MG | Tapentadol HCl Tab SR 12HR 200 MG | 0 | 2 | 0.4 | 200 | 80 |
| 65100091107460 | NUCYNTA ER TAB 250MG | Tapentadol HCl Tab SR 12HR 250 MG | 0 | 2 | 0.4 | 250 | 100 |
| 65100091107420 | NUCYNTA ER TAB 50MG | Tapentadol HCl Tab SR 12HR 50 MG | 0 | 3 | 0.4 | 50 | 20 |
The total MED was computed by summing the MEDs for all opioid prescriptions within a given 6-month interval. The mean daily MED in a 6-month interval was calculated by dividing the total MED by days’ supply for all prescriptions in that interval, excluding overlapping days. We examined five categories for the mean daily MED (i.e., 0, 1–19, 20–49, 50–99, and ≥100 mg), similar to other studies.9,10 For the first overdose, the mean daily MED was based on data from exactly 6 months before that event (Fig. 2).
Classification of Psychoactive Medications
For each 6-month interval, we summed the days’ supply for antidepressants (i.e., SSRIs, SNRIs, and tricyclics), benzodiazepines, and zolpidem. The duration of each drug class or drug was categorized for analysis as follows: 0, 1–30, 31–90, and 91–180 days. Similar to opioid therapy, these time-varying covariates were measured for each 6-month interval, as well as the 6 months before the first overdose event if applicable.
Demographic and Clinical Variables
Study subject demographic data included age as of July 2012, sex, and U.S. region of residence from among four categories as defined by the Centers for Disease Control and Prevention. Time-varying indicators for pain-related conditions were created for each 6-month interval using diagnosis codes for outpatient and hospital encounters, and included the following: back pain, large joint arthritis/other musculoskeletal disorders, neuropathic pain, unspecified chronic pain, and chronic headache (ICD-9-CM codes). Time-varying indicators were also created for mental health/substance use disorders, including anxiety or post-traumatic stress disorder (PTSD), depression, psychosis, drug abuse, and alcohol abuse. Anxiety and PTSD were combined, as the latter was uncommon (<1 %), and these conditions often overlap. Because psychiatric conditions and substance use disorders are usually not transient, once a diagnosis occurred within a 6-month interval, it was considered to persist subsequently. The ICD-9-CM codes used to identify clinical conditions are available upon request.
Analyses
Study cohort characteristics were summarized using descriptive statistics. Differences in characteristics of patients with and without a drug overdose event were examined using the chi-square test for categorical variables and the two-sample t test with an unequal variance assumption for continuous variables. Patterns of treatment with drugs of interest were examined descriptively for each 6-month interval. Using repeated-measures logistic regression with the generalized estimating equations method under an unstructured correlation matrix, we examined the effects of daily opioid dose and duration of filled prescriptions for psychoactive drugs on the odds of overdose, adjusting for time-fixed covariates (i.e., age, sex, and region) as well as time-varying covariates (i.e., chronic pain conditions, mental health, and substance use disorders). Clinical judgment guided a backward model selection procedure. We started with a full model including demographics, clinical conditions, substance use disorders (i.e., drug or alcohol), and medications, as well as all possible interactions of daily opioid dose with each of the three psychoactive drug groups (i.e., benzodiazepines, antidepressants, and zolpidem), and interactions of each of these drugs with mental health conditions (i.e., anxiety/PTSD, depression, and psychotic disorder). The final model included clinically important and/or statistically significant factors. We conducted a post hoc descriptive analysis of combination therapy in each 6-month interval separately for persons diagnosed with depression or anxiety/PTSD. All statistical tests were performed with a two-sided significance level of 0.05 and analyses conducted using SAS software (Version 9.3).
RESULTS
The study cohort comprised over 206,000 subjects; 57 % were women, and the average age was 44 years (Table 1). Nearly half of the cohort resided in southern states, reflecting the distribution of the HMO plan. The most common non-cancer pain-related conditions were musculoskeletal, including large joint arthritis/other musculoskeletal disorders, and back pain-related conditions. With regard to mental health conditions, anxiety/PTSD and depression occurred in 15 and 13 % of the cohort, respectively, while psychosis and alcohol and other substance use disorders were each diagnosed in less than 3 %.
Table 1.
Study Cohort Characteristics
| Characteristics | Any drug overdose event* | Total | |
|---|---|---|---|
| No (N = 205,484) | Yes (N = 1385) | (N = 206,869) | |
| Demographics | |||
| Women, n (%) | 116,585 (56.7) | 887 (64.0) | 117,472 (56.8) |
| Age, mean (SD) | 44.1 (12.0) | 42.5 (12.4) | 44.1 (12.0) |
| U.S. Region, n (%) | |||
| Midwest | 11,950 (5.8) | 78 (5.6) | 12,028 (5.8) |
| Northeast | 60,146 (29.3) | 421 (30.4) | 60,567 (29.3) |
| South | 96,413 (46.9) | 659 (47.6) | 97,072 (46.9) |
| West | 36,975 (18.0) | 227 (16.4) | 37,202 (18.0) |
| Clinical conditions†, n (%) | |||
| Non-cancer pain conditions | |||
| Large joint arthritis, other musculoskeletal‡ | 101,577 (49.4) | 739 (53.4) | 102,316 (49.5) |
| Back pain | 79,801 (38.8) | 722 (52.1) | 80,523 (38.9) |
| Neuropathy | 1762 (0.9) | 26 (1.9) | 1788 (0.9) |
| Chronic pain (unspecified) | 13,874 (6.8) | 337 (24.3) | 14,211 (6.9) |
| Headache | 14,460 (7.0) | 175 (12.6) | 14,635 (7.1) |
| Mental health and substance use disorders | |||
| Anxiety or post-traumatic stress disorder | 30,308 (14.8) | 579 (41.8) | 30,887 (14.9) |
| Depression | 25,466 (12.4) | 757 (54.7) | 26,223 (12.7) |
| Psychosis | 5268 (2.6) | 335 (24.2) | 5603 (2.7) |
| Alcohol abuse | 4283 (2.1) | 354 (25.6) | 4637 (2.2) |
| Other substance abuse | 4044 (2.0) | 376 (27.2) | 4420 (2.1) |
* P value for comparison of all variables was <0.001 except for region (0.43)
† Clinical conditions diagnosed at any point in study time frame. ICD-9-CM codes available from authors
‡ Arthritis, arthralgia, fracture, sprains
Over the course of 3.5 years, 1,385 of 206,869 (0.67 %) subjects were diagnosed with a drug overdose (Table 1). With the exception of region of residence, persons with an overdose event differed significantly in all observed patient characteristics. Persons who experienced a drug overdose were more likely to be women and of a younger age than those that did not. The greatest clinical differences appeared in patients diagnosed with back pain, chronic pain (unspecified), mental health conditions, and substance use disorders.
Across all 658,280 6-month intervals observed for the cohort, the incidence rate for drug overdose was 421 per 100,000 person-years. The proportion of subjects with a drug overdose was highest (0.06 %) in the first 6-month interval, declining in subsequent intervals from 0.04 to 0.01 %. After the first interval, during which all subjects filled at least one opioid prescription, the proportion receiving opioids subsequently stabilized at 42 to 50 %, with the exception of the last interval, when it was approximately 60 %. In the first interval, over one-third of subjects received higher-daily-dose opioids (≥50 mg), which then declined to about 16 %, except for the last interval, when nearly 30 % received higher-dose opioids.
With regard to psychoactive medications, 19 to 24 % of the cohort filled one or more antidepressant prescriptions in each 6-month interval, with 11 to 16 % filling over 90 days’ supply in a given interval. Fifteen to twenty-five percent of the cohort filled at least one benzodiazepine prescription, and 7 to 13 % received more than 90 days’ supply. At least one zolpidem prescription was filled by 8 to 12 % of the cohort, with 4 to 7 % filling more than 90 days’ supply.
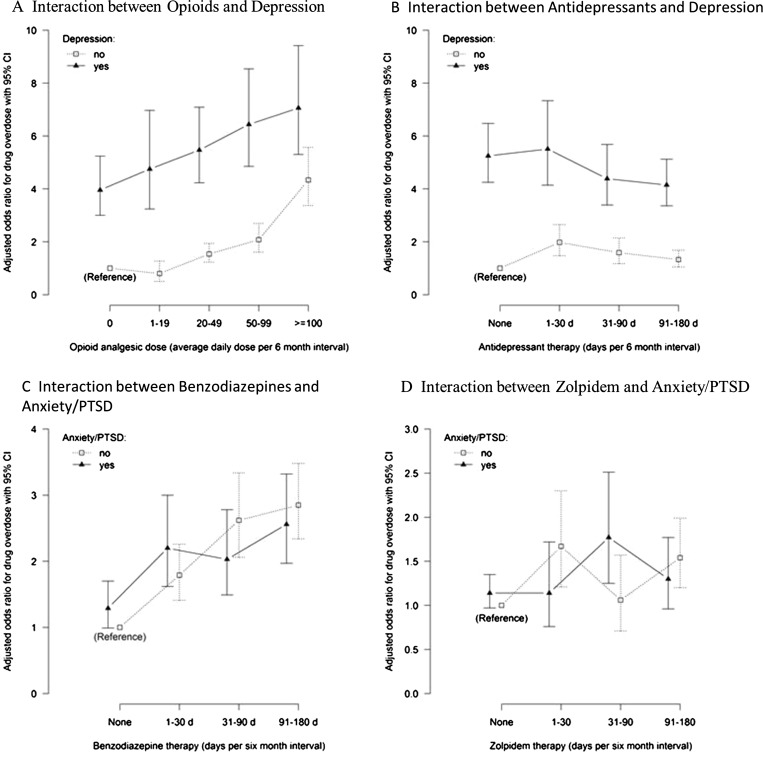
In the fully adjusted model predicting drug overdose, the following significant interactions were observed: opioid therapy with depression (chi(4) = 36.53, p < 0.001); antidepressant therapy with depression (chi(3) = 20.20, p < 0.001); benzodiazepine therapy with anxiety/PTSD (chi(3) = 10.36, p = 0.016) and zolpidem therapy with anxiety/PTSD diagnosis (chi(3) = 8.79, p = 0.03) (Fig. 3; full model-fitting results in Appendix 3). In the interaction between opioid therapy and depression (Fig. 3a), the adjusted odds of overdose were significantly higher for persons with depression than those without this diagnosis, regardless of opioid dose (all p < 0.001). The adjusted odds of drug overdose were 7.06 (95 % CI: 5.30 to 9.42) for the combined effect of depression and very high opioid dose (≥100 mg) versus no depression or opioid use. Among persons without depression, drug overdose was associated with increasing opioid dose (chi2(4) = 161.4, p < 0.001), but the risk rose gradually with all but a very high daily dose, for which the adjusted odds were 4.34 (95 % CI: 3.37 to 5.57) versus no opioids. Among persons with depression, an increasing opioid dose monotonically increased the likelihood of drug overdose (chi2(4) = 24.20, p < 0.001), with adjusted odds of 1.78 (95 % CI: 1.39 to 2.29) for a very high opioid dose versus no opioids.
Figure 3.
Adjusted odds ratios for drug overdose associated with significant interactions of opioid analgesics and selected psychotherapeutic drugs with mental health conditions. Other variables in the model include: daily morphine-equivalent dose category, time interval, age, gender, region, five chronic non-cancer pain conditions, anxiety/PTSD, depression, psychotic disorder, alcohol abuse, drug abuse, antidepressant therapy, benzodiazepine therapy, and zolpidem per 6-month interval (medications analyzed in four levels: none, 1–30 days, 31–90 days, 91–180 days).
An interaction between antidepressant therapy and depression (Fig. 3b) showed that regardless of antidepressant therapy, the risk of drug overdose was significantly greater for persons diagnosed with depression than for those who were not (all p < 0.001). For persons with depression, long-term antidepressant therapy (91–180 days) was associated with overdose events (chi2(3) = 8.49, p = 0.037), but this association was protective, with an adjusted odds ratio of 0.79 (95 % CI: 0.66 to 0.95) versus none. On the other hand, shorter-term therapy (≤ 90 days) had no benefit (p = 0.72 for 1–30 days; p = 0.133 for 31–90 days). For persons without a diagnosis of depression, antidepressant therapy increased the likelihood of overdose (chi2(3) = 28.10, p < 0.001); this effect was greatest for short-term (1–30 days) antidepressant use, with adjusted odds of 1.98 (95 % CI: 1.48 to 2.65), declining to 1.33 (95 % CI: 1.05 to 1.68, p = 0.018) for long-term antidepressants (91–180 days) versus none.
Among all subjects, the likelihood of overdose was similarly increased by benzodiazepine therapy, regardless of duration (all p > 0.06) (Fig. 3c). An interaction showed that duration of benzodiazepine therapy among persons with anxiety/PTSD had a non-monotonic association with drug overdose, but the association was monotonic among persons without anxiety/PTSD. The adjusted odds for drug overdose was highest [OR = 2.85 (95 % CI: 2.34 to 3.48)] for persons without anxiety/PTSD who received long-term (91–180 days) benzodiazepine therapy versus none.
Zolpidem use similarly increased the odds of overdose for persons with or without anxiety/PTSD (all p > 0.10), except for 31–90 days (p = 0.05). Among persons with anxiety/PTSD, duration of zolpidem use was not associated with drug overdose events (chi2(3) = 6.52, p = 0.089), but among those without anxiety/PTSD, the association was significant (chi2(3) = 18.92, p < 0.0001). Among persons without anxiety/PTSD, the adjusted odds of overdose were 1.67 (95 % CI: 1.21 to 2.30) for short-term therapy (1–30 days) versus none, and 1.54 (95 % CI: 1.20 to 1.99) for long-term therapy (91–180 days) versus none. However, the odds of overdose were highest for persons with anxiety/PTSD and 31–90 days of zolpidem therapy (OR = 1.77, 95 % CI: 1.25 to 2.51) versus no anxiety/PTSD and no zolpidem.
A post hoc analysis revealed that among persons diagnosed with depression, treatment with multiple medications was most likely to have occurred in the first 6-month interval, when all subjects filled opioid prescriptions and 69 % also received antidepressants, 45 % received benzodiazepines, and 20 % received zolpidem (Table 5 in Appendix 4). Prescriptions for all four classes of drugs were filled by 5 to 10 % of patients with depression, depending on the 6-month interval. Among patients with anxiety/PTSD, over half also filled prescriptions for antidepressants or benzodiazepines in the first 6 month-interval, and about 25 % continued both opioids and antidepressants in subsequent intervals, while about 30 % continued both opioids and benzodiazepines.
Table 5.
Combination Drugs Filled by Persons with Depression or Anxiety/PTSD by 6-Month Interval
| Characteristic | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Total |
|---|---|---|---|---|---|---|---|---|
| All subjects, N | 206,869 | 162,706 | 118,660 | 80,602 | 53,562 | 30,801 | 5080 | 658,280 |
| Subjects with depression, N (%) | 14,088 (6.8) | 15,456 (9.5) | 13,813 (11.6) | 10,657 (13.2) | 7941 (14.8) | 5331 (17.3) | 1182 (23.3) | 68,468 (10.4) |
| Opioids+Antidepressants, N (%)* | 9667 (68.6) | 6276 (40.6) | 5027 (36.4) | 3576 (33.6) | 2538 (32) | 1750 (32.8) | 482 (40.8) | 29,316 (42.8) |
| Opioids+Benzodiazepines, N (%)* | 6329 (44.9) | 4547 (29.4) | 3733 (27) | 2711 (25.4) | 1995 (25.1) | 1416 (26.6) | 431 (36.5) | 21,162 (30.9) |
| Opioids + zolpidem, N (%)* | 2818 (20.0) | 2010 (13.0) | 1659 (12.0) | 1248 (11.7) | 896 (11.3) | 645 (12.1) | 189 (16.0) | 9465 (13.8) |
| Opioids + Antidepressants+Benzodiazepines, N (%)* | 4856 (34.5) | 3319 (21.5) | 2640 (19.1) | 1899 (17.8) | 1368 (17.2) | 940 (17.6) | 286 (24.2) | 15,308 (22.4) |
| Opioids+Benzodiazepines+Zolpidem, N (%)* | 1677 (11.9) | 1232 (8.0) | 1007 (7.3) | 749 (7.0) | 515 (6.5) | 370 (6.9) | 108 (9.1) | 5658 (8.3) |
| Opioids +Antidepressants+Benzodiazepines+ Zolpidem, N (%)* | 1368 (9.7) | 956 (6.2) | 782 (5.7) | 591 (5.6) | 374 (4.7) | 266 (5.0) | 79 (6.7) | 4416 (6.5) |
| Subjects with anxiety/PTSD, N (%) | 15,532 (7.5) | 17,764 (10.9) | 16,274 (13.7) | 12,933 (16.1) | 9626 (18) | 6317 (20.5) | 1346 (26.5) | 79,792 (12.1) |
| Opioids+Antidepressants, N (%)† | 7766 (50) | 5204 (29.3) | 4298 (26.4) | 3127 (24.2) | 2265 (23.5) | 1542 (24.4) | 431 (32) | 24,633 (30.9) |
| Opioids+Benzodiazepines, N (%)† | 8715 (56.1) | 5970 (33.6) | 5007 (30.8) | 3671 (28.4) | 2663 (27.7) | 1810 (28.7) | 530 (39.4) | 28,366 (35.6) |
| Opioids + Zolpidem, N (%)* | 2770 (17.8) | 1976 (11.1) | 1708 (10.5) | 1306 (10.1) | 939 (9.8) | 646 (10.2) | 179 (13.3) | 9524 (11.9) |
| Opioids +Antidepressants +Benzodiazepines, N (%)† | 4922 (31.7) | 3295 (18.6) | 2685 (16.5) | 1947 (15.1) | 1414 (14.7) | 952 (15.1) | 289 (21.5) | 15,504 (19.4) |
| Opioids +Antidepressants+Benzodiazepines, N (%)† | 4922 (31.7) | 3295 (18.6) | 2685 (16.5) | 1947 (15.1) | 1414 (14.7) | 952 (15.1) | 289 (21.5) | 15,504 (19.4) |
| Opioids +Benzodiazepines+Zolpidem, N (%)† | 1938 (12.5) | 1390 (7.8) | 1161 (7.1) | 859 (6.6) | 602 (6.3) | 419 (6.6) | 116 (8.6) | 6485 (8.1) |
| Opioids +Antidepressants+Benzodiazepines + Zolpidem, N (%)† | 1270 (8.2) | 877 (4.9) | 732 (4.5) | 572 (4.4) | 357 (3.7) | 252 (4) | 70 (5.2) | 4130 (5.2) |
*Entries are N(%) among subjects with depression
†Entries are N(%) among subjects with anxiety/PTSD
DISCUSSION
Among over 200,000 HMO beneficiaries with non-cancer pain who filled multiple prescriptions for Schedule II or III opioids, complex interactions with regard to risk of drug overdose appeared between mental health disorders and treatment with opioids or other psychotherapeutic drugs. The adjusted odds of drug overdose in a 6-month interval for high daily morphine-equivalent dose of ≥100 mg increased sevenfold for persons with depression and fourfold for persons without depression. These data are consistent with studies reporting up to a ninefold greater risk of opioid overdose for high-dose opioid therapy (≥100 mg),10 although our analyses more clearly distinguish the risk for persons with depression.
A novel finding in this study among persons diagnosed with depression was a significant protective effect with regard to drug overdose for longer-term (> 90 days) antidepressant therapy compared to no antidepressant use. Depression is highly prevalent in persons with chronic pain, ranging from 18 to 56 % in a systematic review.11 A mood disorder may precede or often follows the development of chronic pain and is associated with greater pain severity.12 Long-term antidepressant therapy can offer dual benefits of improving mood and, as reported in a recent systematic review, improved pain-related outcomes for diverse types of painful conditions.13
On the other hand, among persons without depression, antidepressant therapy was associated with significantly greater odds of drug overdose, which was greatest for short-term (1–30 days) therapy. This is unlikely due to suicide attempts after initiating antidepressants, which has primarily been a concern for children and adolescents,14 or to poisoning by these drugs, as it is relatively rare.15 Several studies have reported no benefit for short-term antidepressant therapy in the treatment of depression, but less is known about the effects on pain.16,17 Short-term antidepressant therapy may be an indicator for non-adherence16 to this medication and increased reliance on riskier medications for pain management. Alternatively, in an analysis of root causes of opioid overdose deaths, Webster and colleagues suggested that antidepressant use could pose risks due to central nervous system depressant effects, and recommended structured care for persons with mental health disorders who are treated with opioids for pain.18 Unfortunately, psychiatric care is lacking in the management of many chronic pain patients.19
Calcaterra and colleagues reported that opioids plus benzodiazepines were the most common cause of polysubstance overdose deaths in a national study from 1999 to 2009, and increased the risk of drug overdose associated with opioids.1 In our cohort, benzodiazepines significantly increased the risk of overdose but did not disproportionately affect persons with mental health disorders. However, we did find that the odds of overdose progressively rose with longer duration of benzodiazepine therapy. In our national HMO cohort, 45 % of depressed patients were receiving concurrent opioids and benzodiazepines at any one time, and this dangerous combination additively increased the likelihood of overdose. Depending on duration of use, zolpidem increased the odds of overdose by as much as 77 %, and was received by 10 to 20 % of patients with opioids. These data add to the body evidence of risks for zolpidem use along with other frequently misused drugs such as opioids.20
This study has several limitations to acknowledge. First, we relied on coded diagnoses to identify mental health disorders that have limited sensitivity, although this misclassification should attenuate our findings, because persons with depression would be included among those without. We also relied on coded diagnoses to identify drug overdose events that miss overdoses occurring out of network or resulting in outpatient death. However, the rate of drug overdose events in this cohort (421/100,000 person-years) in inpatient and outpatient health care settings was predictably higher than the rate of 238.1/100,000 person-years for drug overdose from an analysis of only emergency room visits in North Carolina in 2011.21 Second, we assumed that patients were taking drugs after they filled prescriptions, but non-adherence for opioids in particular is a major concern.22,23 In addition, total and average daily opioid dose were computed using days’ supply data that may not be reliable. Third, we examined patients’ medications and overdose events in 6-month intervals in order to establish a tractable but reasonably long time frame to examine receipt of drugs and this outcome. By design, our analysis only considers prescriptions filled before the overdose event. Reassuringly, our observed associations between opioid use and overdose are similar to those from longitudinal studies of drugs prescribed before an opioid-related overdose death.24 Fourth, drug overdoses in this cohort may have been due to illicit drug use. According to the CDC, however, opioid painkillers have been involved in the majority of unintentional drug overdose deaths nationally since 2003.25 Fifth, we were unable to distinguish unintentional overdose from suicide attempts. Sixth, although we adjusted for alcohol and other drug abuse disorders, these conditions likely further complicate observed interactions of opioids and other psychotropic medications with mental health disorders.
This study has several notable strengths. We examined all drug overdose events, instead of only deaths, which represent only a small fraction of these events. We used data for filled prescriptions instead of prescriptions from an electronic medical record that may or may not have been filled. Our study also has several implications for clinical practice. Among persons with depression, opioid use had a dose–response association with increased odds of drug overdose, and thus dose minimization is essential. Among persons without depression, the odds of overdose rose rapidly for a daily opioid dose of 100 mg or higher. These data reinforce recommendations to use non-pharmacologic pain management approaches such as cognitive behavioral therapy as a means to limit opioid use.26 We also found that duration of benzodiazepine use was linearly associated with greater overdose risk, but even short-term treatment was positively associated with overdose. Concurrent use of these drugs is common, and represents an important risk. Although the effect of zolpidem was lower, it nonetheless additively increased the risk of overdose. With regard to risk mitigation, our study found a significant protective effect with antidepressant therapy for longer than 90 days among persons diagnosed with depression. Unfortunately, antidepressants appeared to increase the risk of overdose for persons without depression. Overall, these data reinforce the complexities of medication management for chronic pain and support expert recommendations to employ non-drug approaches to pain control while minimizing the risks associated with these drugs.27
ACKNOWLEDGEMENTS AND CONFLICT OF INTEREST
The work on this project was supported by an intramural grant from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 1UL TR001120. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
APPENDIX 1. ICD-9-CM CODE FOR DIAGNOSIS OF DRUG OVERDOSE
ICD-9-CM code, 965.0, 965.00, 965.02, 965.09, 965.1, 965.4, 965.61, 965.69, 965.8, 965.9, 967.6, 967.8, 967.9, 969.4, 977.9, E850.1-E850.6, E850.8, E850.9, E852.8, E852.9, E853.2, E950.0 or E950.2.
APPENDIX 2
APPENDIX 3: RESULTS FOR THE FINAL MODEL
Table 3.
Type III Sums of Squares Table
| Source | DF | Chi-square | Pr > ChiSq |
|---|---|---|---|
| Opioid analgesic dose | 4 | 120.88 | <0.0001 |
| Interval | 1 | 2.14 | 0.1439 |
| Age | 1 | 38.26 | <0.0001 |
| Gender | 1 | 8.15 | 0.0043 |
| U.S. region | 3 | 1.83 | 0.6076 |
| Back pain | 1 | 0.16 | 0.6897 |
| Large joint arthritis, other musculoskeletal | 1 | 5.15 | 0.0233 |
| Neuropathy | 1 | 1.11 | 0.2921 |
| Chronic pain (unspecified) | 1 | 39.03 | <0.0001 |
| Headache | 1 | 0.11 | 0.7406 |
| Depression | 1 | 102.29 | <0.0001 |
| Anxiety or PTSD | 1 | 0.06 | 0.8135 |
| Psychosis | 1 | 66.59 | <0.0001 |
| Alcohol abuse | 1 | 152.28 | <0.0001 |
| Other substance abuse | 1 | 122.12 | <0.0001 |
| Antidepressant therapy | 3 | 11.77 | 0.0082 |
| Benzodiazepine therapy | 3 | 127.39 | <0.0001 |
| Zolpidem therapy | 3 | 11.2 | 0.0107 |
| Opioid × Depression | 4 | 36.53 | <0.0001 |
| Antidepressant therapy × Depression | 3 | 20.2 | 0.0002 |
| Benzodiazepine therapy × Anxiety or PTSD | 3 | 10.36 | 0.0157 |
| Zolpidem therapy × Anxiety or PTSD | 3 | 8.79 | 0.0300 |
APPENDIX 3: RESULTS FOR THE FINAL MODEL (CONT.)
Table 4.
Adjusted Odds Ratios for Drug Overdose Associated with Significant Interactions of Opioid Analgesics and Selected Psychotherapeutic Drugs with Mental Health Conditions
| Adjusted odds ratio (95 % CI)* | ||
|---|---|---|
| Opioid analgesic dose (average daily dose per 6-month interval) | Depression=no | Depression=yes |
| 0 mg | 1 | 3.96 (3.00, 5.24) |
| 1–19 mg | 0.80 (0.50, 1.27) | 4.75 (3.24, 6.97) |
| 20–49 mg | 1.54 (1.23, 1.94) | 5.47 (4.23, 7.09) |
| 50–99 mg | 2.08 (1.61, 2.69) | 6.44 (4.85, 8.54) |
| ≥100 mg | 4.34 (3.37, 5.57) | 7.06 (5.30, 9.42) |
| Antidepressant therapy (days per 6-month interval) | Depression=no | Depression=yes |
| None | 1 | 5.25 (4.25, 6.48) |
| 1–30 day | 1.98 (1.48, 2.65) | 5.51 (4.14, 7.34) |
| 31–90 day | 1.59 (1.18, 2.15) | 4.39 (3.39, 5.68) |
| 91–180 day | 1.33 (1.05, 1.68) | 4.15 (3.36, 5.12) |
| Benzodiazepine therapy (days per 6-month interval) | Anxiety/PTSD=no | Anxiety/PTSD=yes |
| None | 1 | 1.29 (0.99, 1.70) |
| 1–30 day | 1.79 (1.41, 2.26) | 2.20 (1.62, 3.00) |
| 31–90 day | 2.62 (2.06, 3.34) | 2.03 (1.49, 2.78) |
| 91–180 day | 2.85 (2.34, 3.48) | 2.56 (1.97, 3.32) |
| Zolpidem therapy (days per 6-month interval) | Anxiety/PTSD=no | Anxiety/PTSD=yes |
| None | 1 | 1.14 (0.97, 1.35) |
| 1–30 | 1.67 (1.21, 2.3) | 1.14 (0.76, 1.72) |
| 31–90 | 1.06 (0.71, 1.57) | 1.77 (1.25, 2.51) |
| 91–180 | 1.54 (1.2, 1.99) | 1.30 (0.96, 1.77) |
*Other variables in the model include: opioid daily MED category, time interval, age, gender, region, five chronic non-cancer pain conditions, anxiety/PTSD, depression, psychotic disorder, alcohol abuse, drug abuse, antidepressant therapy, benzodiazepine therapy and zolpidem per 6-month interval (four levels: none, 1–30 days, 31–90 days, 91–180 days)
APPENDIX 4: COMBINATION DRUGS FILLED BY PERSONS WITH DEPRESSION OR ANXIETY/PTSD BY 6-MONTH INTERVAL
REFERENCES
- 1.Calcaterra S, Glanz J, Binswanger IA. National trends in pharmaceutical opioid related overdose deaths compared to other substance related overdose deaths: 1999–2009. Drug Alcohol Depend. 2013;131(3):263–70. doi: 10.1016/j.drugalcdep.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States, 2010. JAMA. 2013;309(7):657–9. doi: 10.1001/jama.2013.272. [DOI] [PubMed] [Google Scholar]
- 3.Kobus AM, Smith DH, Morasco BJ, et al. Correlates of higher-dose opioid medication use for low back pain in primary care. J Pain. 2012;13(11):1131–8. doi: 10.1016/j.jpain.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang Y, Turner BJ. Assessing risk for drug overdose in a national cohort: role for both daily and total opioid dose? J Pain. 2014 doi: 10.1016/j.jpain.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foley KM. The treatment of cancer pain. N Engl J Med. 1985;313(2):84–95. doi: 10.1056/NEJM198507113130205. [DOI] [PubMed] [Google Scholar]
- 6.Vissers KC, Besse K, Hans G, Devulder J, Morlion B. Opioid rotation in the management of chronic pain: where is the evidence? Pain Pract. 2010;10(2):85–93. doi: 10.1111/j.1533-2500.2009.00335.x. [DOI] [PubMed] [Google Scholar]
- 7.Agency Medical Director’s Group. Web-Based Opioid Dose Calculator. Available at http://agencymeddirectors.wa.gov/mobile.html. Accessed April 7, 2014
- 8.Hallenbeck JL. Palliative Care Perspectives. Oxford: Oxford University Press; 2003. pp. 36–74. [Google Scholar]
- 9.Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patters and opioid overdose-related deaths. JAMA. 2011;305(13):1315–21. doi: 10.1001/jama.2011.370. [DOI] [PubMed] [Google Scholar]
- 10.Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85–92. doi: 10.7326/0003-4819-152-2-201001190-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163(20):2433–45. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- 12.Gerrits MM, van Oppen P, van Marwijk HW, Penninx BW, van der Horst HE. Pain and the onset of depressive and anxiety disorders. Pain. 2014;155(1):53–9. doi: 10.1016/j.pain.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Mercier A, Auger-Aubin I, Lebeau JP, et al. Evidence of prescription of antidepressants for non-psychiatric conditions in primary care: an analysis of guidelines and systematic reviews. BMC Fam Pract. 2013;4:14–55. doi: 10.1186/1471-2296-14-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bridge JA, Iyengar S, Salary CB, Barbe RP, Birmaher B, Pincus HA, Ren L, Brent DA. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA. 2007;297(15):1683–96. doi: 10.1001/jama.297.15.1683. [DOI] [PubMed] [Google Scholar]
- 15.McKenzie MS, McFarland BH. Trends in antidepressant overdoses. Pharmacoepidemiol Drug Saf. 2007;16(5):513–23. doi: 10.1002/pds.1355. [DOI] [PubMed] [Google Scholar]
- 16.Stewart JA, Deliyannides DA, Hellerstein DJ, McGrath PJ, Stewart JW. Can people with nonsevere major depression benefit from antidepressant medication? J Clin Psychiatry. 2012;73:518–25. doi: 10.4088/JCP.10m06760. [DOI] [PubMed] [Google Scholar]
- 17.Serna MC, Cruz I, Real J, Gascó E, Galván L. Duration and adherence of antidepressant treatment (2003 to 2007) based on prescription database. Eur Psychiatry. 2010;25:206–13. doi: 10.1016/j.eurpsy.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 18.Webster LR, Cochella S, Dasgupta N, Fakata KL, Fine PG, Fishman SM, Grey T, Johnson EM, Lee LK, Passik SD, Peppin J, Porucznik CA, Ray A, Schnoll SH, Stieg RL, Wakeland W. An analysis of the root causes for opioid-related overdose deaths in the United States. Pain Med. 2011;12(Suppl 2):S26–35. doi: 10.1111/j.1526-4637.2011.01134.x. [DOI] [PubMed] [Google Scholar]
- 19.Howe CQ, Sullivan MD. The missing ‘P’ in pain management: how the current opioid epidemic highlights the need for psychiatric services in chronic pain care. Gen Hosp Psychiatry. 2014;36(1):99–104. doi: 10.1016/j.genhosppsych.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Zosel A, Osterberg EC, Mycyk MB. Zolpidem misuse with other medications or alcohol frequently results in intensive care unit admission. Am J Ther. 2011;18(4):305–8. doi: 10.1097/MJT.0b013e3181d169ed. [DOI] [PubMed] [Google Scholar]
- 21.Harmon KJ, Proescholdbell S, Marshall S, Waller A. Utilization of emergency department data for drug overdose surveillance in North Carolina. Online J Public Health Inform. 2014;6(1):e174. doi: 10.5210/ojphi.v6i1.5200. [DOI] [Google Scholar]
- 22.Matteliano D, Chang YP. Describing prescription opioid adherence among individuals with chronic pain using urine drug testing. Pain Manag Nurs. 2014 Jun. [DOI] [PubMed]
- 23.Anastassopoulos KP, Chow W, Tapia CI, Baik R, Moskowitz B, Kim MS. Reported side effects, bother, satisfaction, and adherence in patients taking hydrocodone for non-cancer pain. J Opioid Manag. 2013;9(2):97–109. doi: 10.5055/jom.2012.0151. [DOI] [PubMed] [Google Scholar]
- 24.Bohnert AS, Ilgen MA, Ignacio RV, McCarthy JF, Valenstein M, Blow FC. Risk of death from accidental overdose associated with psychiatric and substance use disorders. Am J Psychiatry. 2012;169(1):64–70. doi: 10.1176/appi.ajp.2011.10101476. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention (CDC) CDC grand rounds: prescription drug overdoses - a U.S. epidemic. MMWR Morb Mortal Wkly Rep. 2012;61(1):10–3. [PubMed] [Google Scholar]
- 26.Windmill J, Fisher E, Eccleston C, Derry S, Stannard C, Knaggs R, Moore RA. Interventions for the reduction of prescribed opioid use in chronic non-cancer pain. Cochrane Database Syst Rev. 2013;9 doi: 10.1002/14651858.CD010323.pub2. [DOI] [PubMed] [Google Scholar]
- 27.Washington State Agency Medical Directors’ Group. Interagency Guideline on Opioid Dosing for Chronic Non-cancer Pain: An Educational Aid to Improve Care and Safety with Opioid Treatment. Olympia (WA): Washington State Department of Labor and Industries; 2010 (http://www.guideline.gov/content.aspx?id=23792)