Abstract
During a survey of cold-adapted fungi in alpine glaciers on the Qinghai-Tibet Plateau, 1 428 fungal isolates were obtained of which 150 species were preliminary identified. Phoma sclerotioides and Pseudogymnoascus pannorum were the most dominant species. Psychrotolerant species in Helotiales (Leotiomycetes, Ascomycota) were studied in more detail as they represented the most commonly encountered group during this investigation. Two phylogenetic trees were constructed based on the partial large subunit nrDNA (LSU) to infer the taxonomic placements of these strains. Our strains nested in two well-supported major clades, which represented Tetracladium and a previously unknown lineage. The unknown lineage is distant to any other currently known genera in Helotiales. Psychrophila gen. nov. was therefore established to accommodate these strains which are characterised by globose or subglobose conidia formed from phialides on short or reduced conidiophores. Our analysis also showed that an LSU-based phylogeny is insufficient in differentiating strains at species level. Additional analyses using combined sequences of ITS+TEF1+TUB regions were employed to further investigate the phylogenetic relationships of these strains. Together with the recognisable morphological distinctions, six new species (i.e. P. antarctica, P. lutea, P. olivacea, T. ellipsoideum, T. globosum and T. psychrophilum) were described. Our preliminary investigation indicates a high diversity of cold-adapted species in nature, and many of them may represent unknown species.
Keywords: glaciers, Phoma sclerotioides, Pseudogymnoascus pannorum, Psychrophila, psychrotolerant, Tetracladium
INTRODUCTION
Cold-adapted fungi are ubiquitous in cold habitats such as the deep seas, Arctic and Antarctic areas, and glaciers. Cold-adapted fungi have evolved special properties, e.g., cold adapted enzymes, change of membrane fluidity, and other cellular components, to enable them to grow at low temperatures at rates comparable to those of mesophiles at moderate temperatures (D’Amico et al. 2006, Ruisi et al. 2007). During the past two decades, research on cold-adapted fungi has increased, driven by their potential value for application in biotechnology (Margesin & Schinner 1994, 1999). Cold-adapted fungi have become important sources for the discovery of novel bioactive secondary metabolites and enzymes (Flam 1994, Pietra 1997, Biabini & Laatch 1998, Gudjarnnson 1999, Höller et al. 2000, Verbist et al. 2000, Bhadury et al. 2006, Ebel 2006, Blunt et al. 2007, Rateb & Ebel 2011).
Microorganisms living in low temperature environments are generally referred to as psychrophiles or psychrotolerants. Psychrophiles have been defined as species that can grow at or below 0 °C; have optimum growth temperatures (OGT) of ≤ 15 °C and maximum growth temperatures (MGT) of ≤ 20 °C; while psychrotolerants can grow close to 0 °C, have OGT > 15 °C and MGT > 20 °C (Morita 1975). However, these definitions are also ambiguous and may not be applicable for most of the eukaryotes, as some higher organisms known as psychrophiles, such as some algae, plants, insects, marine and terrestrial invertebrates, and fish may have much broader growth-temperature ranges. The terms stenopsychrophile and eurypsychrophile have therefore been proposed to modify the definitions of psychrophilic and psychrotolerant. The ‘steno-’ and ‘eury-’ are referred ecological terms derived from Shelford’s law of tolerance that describe narrow or wide tolerance to an environmental determinant, respectively. The stenopsychrophile (equal to ‘psychrophile’) refers to microorganisms with a restricted growth-temperature range that cannot tolerate higher temperatures. Eurypsychrophile (equal to ‘psychrotolerant microorganisms’) describes microorganisms that ‘like’ permanently cold environments, but can also tolerate a wide range of temperatures extending into the mesophilic range (Cavicchioli 2006).
Since the discovery of bioluminescent bacteria that are able to grow at 0 °C by Forster (1887), a number of psychrophilic bacteria have been discovered from deep ocean sediments, glacier ice, and soils of the polar regions (DeLong et al. 1997, Mountfort et al. 1998, Price 2000, Berestovskaya et al. 2002, Margesin et al. 2003, Bowman et al. 2004, Seo et al. 2005, Zhang et al. 2006, 2008, Grünke et al. 2012). However, the number of known cold-adapted fungi, especially psychrophilic fungi, is relatively low. In recent years, the diversity of filamentous fungi in cold niches has been increasingly investigated, and the number of known species has greatly expanded (Möller & Dreyfuss 1996, Robinson 2001, Blanchette et al. 2004, Arenz et al. 2006, Connell et al. 2006, Held et al. 2006, Malosso et al. 2006, Duncan et al. 2008, Onofri et al. 2008, Selbmann et al. 2008, Arenz & Blanchette 2009, Jurgens et al. 2009). Most species in these studies, however, are psychrotolerant, and only a few were documented as psychrophiles such as Thelebolus microsporus, Mucor strictus, Phoma herbarum, Humicola marvinii, Pseudogymnoascus destructans, and some snow molds (e.g. Sclerotinia borealis, Microdochium nivale, Coprinus psychromorbidus) (Schipper 1967, Dejardin & Ward 1971, Traquair & Smith 1982, Richard et al. 1997, Hsiang et al. 1999, Tronsmo et al. 2001, Singh et al. 2006, Gargas et al. 2009, Hoshino et al. 2010, Anupama et al. 2011, Minnis & Lindner 2013). Species in several yeast genera including Mrakia, Mrakiella and Rhodotorula were usually described as psychrophilic. For example, Mrakia frigida grew well at 15 °C and 4 °C but poorly at 20 °C (Margaret 1966); Mrakia psychrophila from Antarctic soil had an optimal growth temperature of 10 °C and a MGT of 18 °C (Xin & Zhou 2007); Mrakiella cryoconiti, M. aquatica and M. niccombsii from alpine and arctic habitats also exhibited psychrophilic features and failed to grow at temperatures over 20 °C (Margesin & Fell 2008, Robin et al. 2010). Psychrophilic fungi are phylogenetically diverse and we identified the cold-adapted fungi through a polyphasic approach integrating phylogenetic analysis, morphological characterization and cold-adapted features in the present study.
The Qinghai-Tibet Plateau, often called the ‘world’s roof’ or ‘the third pole’, is located in the southwest of China and is the highest and largest low-latitude region with permafrost in the world. The high elevation and low latitude make the Qinghai-Tibet Plateau a unique alpine ecosystem that is sensitive to changes in climate and surface conditions (Cheng 1998). In the last 30 years, the permafrost area on the Qinghai-Tibet Plateau has decreased by over 10 000 km2 (Li & Cheng 1999). Therefore, researchers have been paying more attention to investigate microorganisms on the Qinghai-Tibet Plateau. Although prokaryotes have been extensively investigated in this area (Xiang et al. 2005, 2009, Liu et al. 2006, 2007, 2009a, b, Yao et al. 2006, Zhang et al. 2007, 2009, Yang et al. 2008), fungi have not received much attention. During an investigation of the cold-adapted fungi of the Qinghai-Tibet Plateau, 1 428 fungal isolates were obtained, of which 150 species were preliminarily identified. In this paper, we studied some dominant fungi from Qinghai-Tibet Plateau in Helotiales in detail. A few related isolates from the Antarctic were also included.
MATERIALS AND METHODS
Sample collection
Soil samples were collected from seven glaciers in 2009–2011. The sampling areas were located at the edge or centre of the following glaciers: Midui and Zhadang Glacier in Tibet, Qiyi and Toumingmengke Glacier in Gansu Province, Hailuogou Glacier in Sichuan Province, Yuzhufeng Glacier in Qinghai Province and Mingyong Glacier in Yunnan Province. In addition, some soil samples were also collected from Antarctic, near the Great Wall Station in January 2011 (Table 1). For all sampling, clean hand tools were surface sterilised with 70 % ethanol before use. After the removal of the top 5–10 cm of surface sediment, c. 500 g soil sample was collected from the underlying layer and placed in a fresh Zip-lock plastic bag. The samples were maintained at 4 °C until arrival at the laboratory.
Table 1.
Details of the soil samples collected at 5–10 cm depth for the survey of cold-adapted fungi on the Qinghai-Tibet Plateau and Antarctic.
| Sampling location | Collection date | GPS location | Altitude (m) | Depth of collection (cm) | |
|---|---|---|---|---|---|
| Midui | 16 October 2009 | N29°27' | E96°30' | 3874 | 5–10 |
| Zhadang | 27 September 2010 | N30°28' | E90°38' | 5800 | 5–10 |
| Yuzhufeng | 1 October 2009 | N35°41' | E94°17' | 4658 | 5–10 |
| Toumingmengke | 3 October 2010 | N39°29' | E96°32' | 4545 | 5–10 |
| Qiyi | 7 October 2010 | N39°15' | E97°45' | 4315 | 5–10 |
| Hailuogou | 20 April 2011 | N29°33' | E101°58' | 3461 | 5–10 |
| Mingyong | 4 May 2011 | N28°27' | E98°45' | 2811 | 5–10 |
| Antarctic | 5 January 2011 | S62°12' | W58°57' | 10 | 5–10 |
Isolation of fungi
Fungi were isolated from soil samples as soon as they were taken to the lab using a traditional pour plate method. A 10 g quantity of each soil sample was suspended in sterile-distilled water in a flask. The volume was then increased to 100 mL before the suspension was shaken to disperse soil particles and then serially diluted to 10–2, 10–3 and 10–4. For selection selection of psychrophilic or psychrotolerant fungi, about 0.1 mL of each dilution was placed on the surface of three 90 mm diam Petri plates containing 1/4 PDA (potato dextrose agar plus chloramphenicol at 0.1 mg/mL and streptomycin at 0.1 mg/mL to suppress bacterial growth) and spread evenly. The plates were sealed and incubated at 4, 10 and 20 °C (one plate per temperature). The plates were examined for fungal growth at 1 wk intervals for 4 wk. Colonies that appeared on the plates were transferred to three new plates, which were incubated at 4, 10 and 20 °C as temperature test. The change in colony diameter after 4 wk (growth rate) was determined for each isolate at the three temperatures. The psychrophilic and psychrotolerant fungi isolated in this study were consolidated but not strictly defined by the definition given by Morita (1975). The fungi grew better at 4 and 10 °C than at 20 °C and those that grew better at 20 °C were considered psychrophilic and psychrotolerant. The ex-type specimens (dried culture) were deposited in HMAS (Herbarium Mycologicum Academiae Sinicae), with the living culture in CGMCC (China General Microbiological Culture Collection Center).
Morphological observations
A number of psychrophilic or psychrotolerant fungi were isolated. Among them, Phoma sclerotioides and Pseudogymnoascus pannorum (= Geomyces pannorum) were most frequently encountered (137 and 52 isolates, respectively) and are wellknown cold-adapted species. Sixteen isolates representing some frequently encountered fungi (190 isolates in total) in the Helotiales were studied in more detail. Morphological characteristics were observed, photographed, and measured using material from agar plate and slide culture (Coetzee & Eicker 1990). The colony diameter of fungi growing on PDA plates was measured in two perpendicular directions after 4 wk at different temperatures, and the mean diameter was obtained from five replicate plates cultivated at the same temperatures. Morphological characteristics of colonies including aerial mycelium, density, and pigment production were noted. Microscopic morphology was examined using slide cultures: each isolate was transferred to a 50 mL centrifugal tube and incubated at 10 °C for 3 wk before hyphae, conidiophores, and conidia on water mounts were observed, photographed, and measured with a Nikon 80i microscope with differential interference contrast (DIC) optics.
DNA extraction, PCR amplification, sequencing, phylogenetic analysis and SNP detection
Genomic DNA was extracted from the fungal mycelia on PDA plates following the protocol described by Wang & Zhuang (2004). The primers LROR and LR5 (Vilgalys & Hester 1990) were used to amplify the partial large subunit nrDNA (LSU); ITS1 and ITS4 (White et al. 1990) were used to amplify the internal transcribed spacer region of the nuclear ribosomal RNA gene; EF1–728F and EF1–986R (Carbone & Kohn 1999) were used to amplify partial translation elongation factor 1-α gene (TEF1), and Bt-2a and Bt-2b (Glass & Donaldson 1995) were used to amplify partial β-tubulin gene (TUB). PCR was performed in 25 μL reactions containing DNA template 1.0 μL, each forward and reverse primers 1.0 μL, 2 × MasterMix 12.5 μL (TIANGEN Co. Ltd., Beijing, China) and H2O, using the following parameters: 94 °C for 40 s; followed by 40 cycles at 54 °C for LSU, 53 °C for ITS, 55 °C for TEF1and 52 °C for TUB gene for 50 s and 72 °C for 60 s; and a final extension at 72 °C for 7 min. The PCR products were sequenced with primers mentioned above by Invitrogen Biotechnology Co. Ltd. (Beijing, China). Sequences were compared to accessions in the GenBank database via BLASTn searching to find the most likely taxonomic designation (Table 2).
Table 2.
Fungi and their GenBank accession numbers used in this study. The newly generated sequences in this study are shown in bold
| GenBank no.1 | |||||
|---|---|---|---|---|---|
| Species | Strain number | LSU | ITS | TEF1 | TUB |
| Arachnopeziza variepilosa | M337 | – | EU940163 | – | FJ477045 |
| Ascocoryne sarcoides | OSC #100772 | FJ176886 | – | – | – |
| – | AJ406399 | – | – | – | |
| Botryotinia fuckeliana | LGM002 | – | KC683713 | – | KC6837123 |
| Bulgaria inquinans | ZW-Geo52-Clark | AY789344 | – | – | – |
| CBS 118.31 | DQ470960 | – | – | – | |
| Cadophora fastigiata | DAOM 225754 | JN938877 | – | – | – |
| Cadophora luteo-olivacea | Clo-40 | – | HQ661093 | HQ661078 | HQ661063 |
| ICMP:18096 | HM 116760 | – | – | – | |
| Catenulifera luxurians | CBS 647.75 | – | GU727560 | – | GU727569 |
| Ciborinia camelliae | EFA 1 | – | FJ959095 | – | GQ181121 |
| Cistella spicicola | CBS 731.97 | – | GU727553 | – | GU727565 |
| Cudoniella clavus | OSC 100054 | DQ470944 | – | – | – |
| ILLS60488 | JN012006 | – | – | – | |
| Cudoniella indica | VG 113–4 | GQ477325 | – | – | – |
| VG 112–1 | GQ477324 | – | – | – | |
| Cudoniella tenuispora | ILLS60490 | JN012008 | – | – | – |
| Dermea acerina | CBS 161.38 | DQ247801 | – | – | – |
| Fabrella tsugae | – | AF356694 | – | – | – |
| Hyaloscypha aureliella | M235 | EU940153 | – | – | – |
| M234 | EU940152 | – | – | – | |
| Hyaloscypha daedaleae | ZW-Geo138-Clark | AY789415 | – | – | – |
| Hyaloscypha fuckelii | M233 | EU940154 | – | – | – |
| Hyaloscypha hepaticola | M339 | EU940150 | EU940226 | – | – |
| Hyaloscypha vitreola | M236 | EU940156 | – | – | – |
| M39 | EU940155 | – | – | – | |
| Hymenoscyphus pseudoalbidus | FC-2799 | – | AB705220 | AB705213 | – |
| Hyphodiscus hymeniophilus | CBS 529.87 | GU727555 | – | – | – |
| CBS 602.77 | – | DQ227264 | – | DQ227270 | |
| Leotia lubrica | OSC 100001 | NG 027596 | – | – | – |
| ZW-Geo59-Clark | AY789359 | – | – | – | |
| Loramyces macrosporus | CBS 235.53 | DQ470957 | – | – | – |
| Neofabraea perennans | RGR 90.0107 | – | AF281397 | – | AF281476 |
| Phacidium lacerum | CBS 130.30 | DQ470976 | – | – | – |
| Phialocephala fortinii | K93 395 | – | – | DQ2 74568 | DQ274834 |
| Psychrophila antarctica | ANT80 | KF768459 | JX001628 | KF768425 | KF768438 |
| ANT92 | KF768452 | JX001640 | KF768424 | KF768437 | |
| ANT94 | KF768458 | JX001639 | KF768423 | KF768436 | |
| Psychrophila lutea | HAILUO374 | KF768456 | JX001638 | KF768421 | KF768441 |
| HAILUO407 | KF768455 | JX001615 | KF768422 | KF768439 | |
| HAILUO409 | KF768454 | JX001637 | KF768420 | KF768440 | |
| Psychrophila olivacea | HAILUO368 | KF768457 | JX001618 | KF768427 | KF768443 |
| HAILUO563 | KF768453 | JX001633 | KF768426 | KF768442 | |
| Rhynchosporium orthosporum | H4 | – | HM627471 | HM627456 | KC819296 |
| Rutstroemia firma | CBS 341.62 | DQ470963 | – | – | – |
| Sclerotinia sclerotiorum | CBS 499.50 | AF431951 | – | – | – |
| CBS 499.50 | DQ470965 | – | – | – | |
| WZ0067 | AY789347 | – | – | – | |
| Tetracladium apiense | CCM F-23199 | EU883420 | – | – | – |
| CCM F-23399 | EU883421 | – | – | – | |
| Tetracladium breve | CCM F-10501 | EU883418 | – | – | – |
| Tetracladium ellipsoideum | MIDUI20 | KF768465 | JX029111 | KF768431 | KF768444 |
| MIDUI21 | KF768466 | JX029124 | KF768432 | KF768451 | |
| MIDUI30 | KF768467 | JX029113 | KF768430 | KF768445 | |
| Tetracladium furcatum | CCM F-06983 | EU883428 | – | – | – |
| CCM F-11883 | EU883432 | – | – | – | |
| Tetracladium globosum | HAILUO215 | KF768460 | JX029109 | KF768433 | KF768448 |
| MY24 | KF768461 | JX029118 | KF768434 | KF768449 | |
| MY25 | KF768462 | JX029133 | KF768435 | KF768450 | |
| Tetracladium marchalianum | CCM F-26399 | EU883415 | – | – | – |
| CCM F-11391 | EU883417 | – | – | – | |
| CCM F-19399 | EU883423 | – | – | – | |
| Tetracladium maxilliforme | CCM F-529 | EU883429 | – | – | – |
| CCM F-13186 | EU883430 | – | – | – | |
| Tetracladium palmatum | CCM F-10001 | EU883424 | – | – | – |
| Tetracladium psychrophilum | HAILUO380 | KF768464 | JX029119 | KF768429 | KF768446 |
| MY376 | KF768463 | JX029129 | KF768428 | KF768447 | |
| Tetracladium setigerum | CCM F-19499 | EU883426 | – | – | – |
| CCM F-20987 | EU883425 | – | – | – | |
| Trichoglossum hirsutum | OSC61726 | AY789313 | – | – | – |
| Vibrissea flavovirens | MBH39316 | AY789426 | – | – | – |
| Vibrissea truncorum | CBS #258.91 | FJ176874 | – | – | – |
| CUP 62562 | AY789402 | – | – | – | |
1 LSU: large subunit nrDNA; ITS: Internal transcribed spacers 1 and 2 together with 5.8S nrDNA; TEF1: partial translation elongation factor 1-alpha gene; TUB: partial beta-tubulin gene.
Sequence data of the four genes were aligned with Clustal X (Thompson et al. 1997). Further manual alignment was carried out with MEGA v. 5 (Tamura et al. 2011) and alignments were deposited in TreeBASE (www.treebase.org, submission no. S16864). Maximum Parsimony (MP) analyses were conducted using PAUP v. 4.0b10 (Swofford 2002) and Bayesian analysis using MrBayes v. 3.1.2 (Altekar et al. 2004). For the MP analysis, ambiguously aligned regions were excluded from all analyses. An unweighted parsimony (UP) analysis was performed. Trees were inferred using the heuristic search option with TBR branch swapping and 1 000 random sequence additions. Branches of zero length were collapsed and all equally most parsimonious trees were saved. Descriptive tree statistics such as tree length (TL), consistency index (CI), retention index (RI), rescaled consistency index (RC) and homoplasy index (HI), were calculated for trees generated. Clade stability was assessed using bootstrap analysis with 1 000 replicates, each with 10 replicates of random stepwise addition of taxa. For the Bayesian analyses, the models of evolution were estimated by using MrModeltest v. 2.3 (Nylander 2004). Posterior probabilities (PP) (Rannala & Yang 1996, Zhaxybayeva & Gogarten 2002) were determined by Markov Chain Monte Carlo sampling (MCMC). Six simultaneous Markov chains were run for 1 000 000 generations and trees were sampled every 100th generation (resulting in 10 000 total trees). The first 2 000 trees represented the burn-in phase of the analyses and were discarded and the remaining 8 000 trees were used for calculating posterior probabilities (PP) in the majority rule consensus tree. Trees were visualised in TreeView v. 1.6.6 (Page 1996).
Unique fixed nucleotide positions are used to characterise and describe several sterile species (see applicable species notes). For the sterile species that was described, the closest phylogenetic neighbour(s) were selected from Fig. 3 and 4, and this focused dataset was subjected to SNP analyses. These single nucleotide polymorphisms (SNPs) were determined for each aligned data partition using DnaSP v. 5.00.07 (Librado & Rozas 2009).
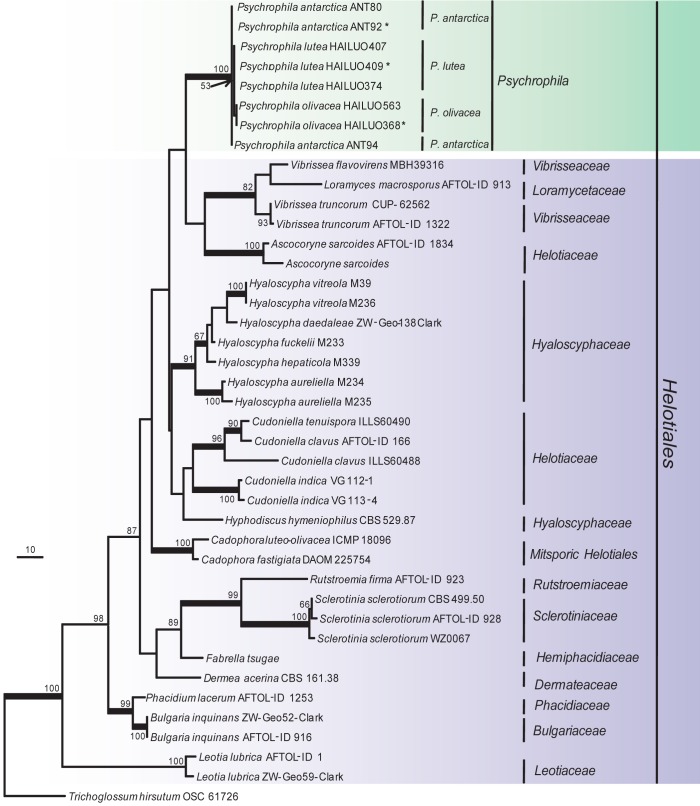
Fig. 3.
Phylogenetic tree derived from maximum parsimony analysis based on combined ITS+TEF1+TUB sequences (TL = 2295, CI = 0.7085, RI = 0.7600, HI = 0.2915 and RC = 0.5384). Phialocephala fortinii K93 395 was used as outgroup. The length of the three genes alignment was 1 383 characters, with 746 phylogenetically informative positions. Bootstrap values of more than 50 % are shown on the respective branches and significant Bayesian posterior probability (≥ 95 %) are indicated as bold branches. Ex-type cultures are marked with asterisks (*).
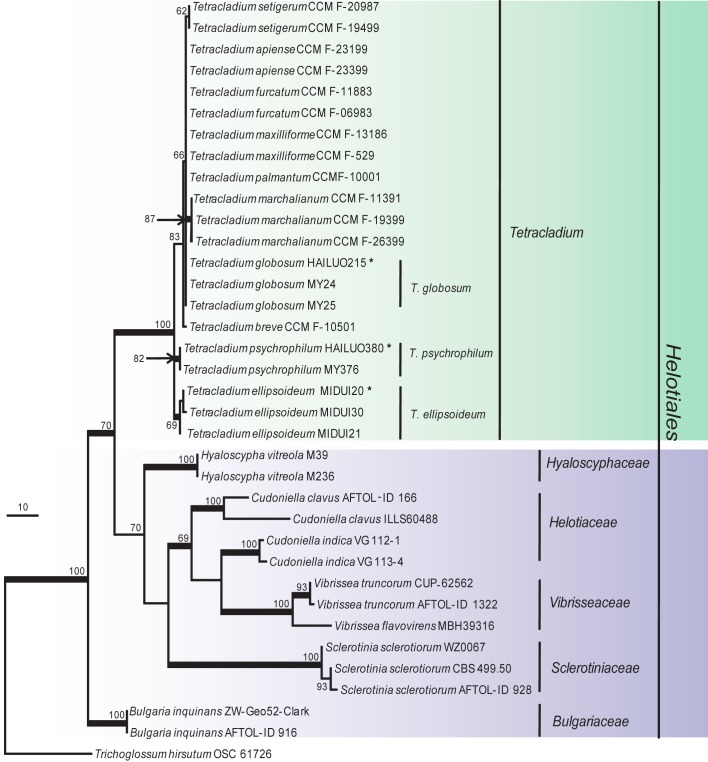
Fig. 4.
Phylogenetic tree derived from maximum parsimony analysis based on combined ITS+TEF1+TUB sequences (TL = 2881, CI = 0.6977, RI = 0.7565, HI = 0.3023 and RC = 0.5278). Phialocephala fortinii K93 395 was used as outgroup. The length of the three genes alignment was 1 577 characters, with 1 043 phylogenetically informative positions. Bootstrap values of more than 50 % are shown on the respective branches and significant Bayesian posterior probability (≥ 95 %) are indicated as bold branches. Ex-type cultures are marked with asterisks (*).
RESULTS
In the current investigation, 1 428 fungal isolates were obtained from 350 samples, which were mainly collected from seven glaciers on the Qinghai-Tibet Plateau; a few specimens were collected from Antarctica. Isolates were preliminarily identified to belong to 78 genera representing 150 species. About onetenth of these isolates were psychrophilic (stenopsychrophile), mostly belonging to the genera Pseudogymnoascus, Phoma, Tetracladium and Psychrophila, the new genus described in this paper. Based on the preliminary identification, 16 isolates belonging to Helotiales, which were the most frequently encountered cold-adapted fungi, were selected to study in more detail.
Phylogenetic analysis and SNP detection
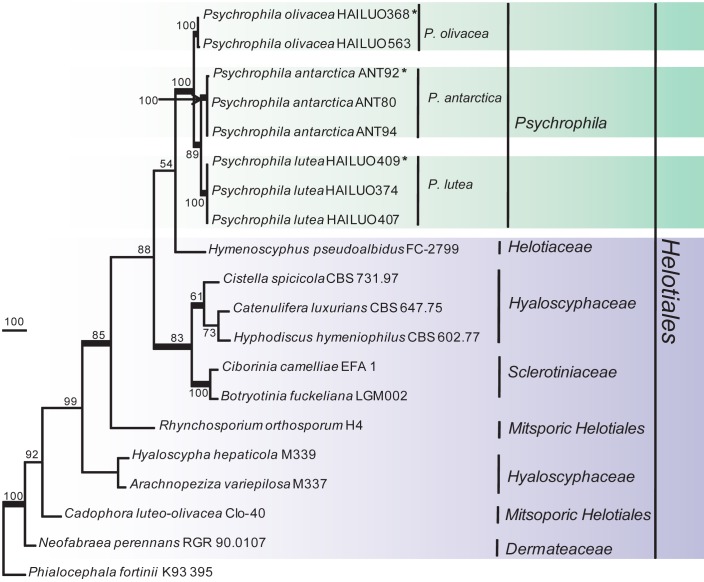
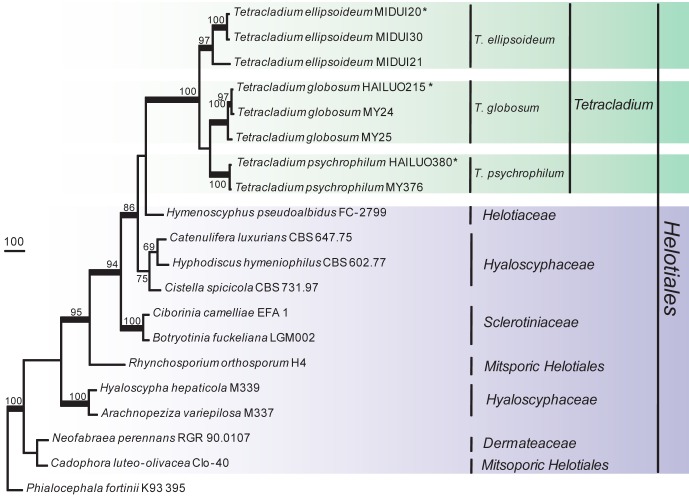
The phylogenetic relationships were determined for 16 isolates. According to the phylogenetic trees based on the partial large subunit nrDNA (LSU), 16 isolates clustered into two independent clades, one was strongly supported and well separated from other known genera in the Helotiales (Fig. 1) and should represent a new genus and the other eight isolates clustered within the Tetracladium clade (Fig. 2). In the phylogenetic trees (Fig. 3, 4) generated from combined sequences of ITS+TEF1+TUB, the isolates in Fig. 1 clustered into one clade comprising three subclades that were well supported and separated from each other. Based on phylogenetic relationships and morphological characteristics, a new genus, Psychrophila, is proposed to accommodate these three new species (P. antarctica, P. lutea and P. olivacea).
Fig. 1.
Phylogenetic tree derived from maximum parsimony analysis based on LSU rDNA sequences (TL = 610, CI = 0.5607, RI = 0.7609, HI = 0.4393 and RC = 0.4266). Trichoglossum hirsutum OSC61726 was used as outgroup. The LSU alignment consists of 851 characters, with 184 phylogenetically informative positions. Bootstrap values of more than 50 % are shown on the respective branches and significant Bayesian posterior probability (≥ 95 %) are indicated as bold branches. Ex-type cultures are marked with asterisks (*).
Fig. 2.
Phylogenetic tree derived from maximum parsimony analysis based on LSU rDNA sequences (TL = 325, CI = 0.7815, RI = 0.9015, HI = 0.2185 and RC = 0.7046). Trichoglossum hirsutum OSC61726 was used as outgroup. The LSU alignment consists of 845 characters, with 151 phylogenetically informative positions. Bootstrap values of more than 50 % are shown on the respective branches and significant Bayesian posterior probability (≥ 95 %) are indicated as bold branches. Ex-type cultures are marked with asterisks (*).
Tetracladium is one of the three aquatic genera with tetraradiate conidia that were described by de Wildeman (1893, 1894, 1895). According to our phylogenetic trees (Fig. 2, 4), isolates in the present study formed three independent subclades that could not be assigned to any known species.
LSU regions had relatively few informative sites for the studied strains and were therefore not selected as good markers at species level. The remaining three loci had varied success for species identification and all of the sterile new species described here could be identified by all three loci.
Taxonomy
PsychrophilaM.M. Wang & Xing Z. Liu, gen. nov. — MycoBank MB801296
Etymology. Psychrophila means cold-loving and is referring to those fungi well adapted to low temperature habitats.
Type species. Psychrophila antarctica M.M. Wang & Xing Z. Liu.
Colonies on PDA slow-growing, cream-white, yellowish or darkolive to dark-brown, with sparse aerial mycelium; vegetative hyphae hyaline, smooth, thick-walled, transversely septate, most agglomerate to bundles, or swollen to moniliform. The cells of aerial hyphae often aggregated in dense clumps, hyphae deep immerged into the agar. Conidiogenous cells phialidic, enteroblastic, hyaline, flask-shaped, apically tapering into a broad funnel, bottleneck-like constriction; the collarette wedge-shaped to campanulate and widely flaring. Conidiophores reduced to conidiogenous cells, sometimes short, or much differentiated. Conidia hyaline, smooth, aseptate, pyriform to globose, within a single conidiogenous locus.
Habitat — Cold environments.
Notes — Species with phialophora-like asexual morphs in the Helotiales include: Ascocoryne with Coryne asexual morphs, which have hyaline, more or less penicillate conidiophores and phialides that lack visible collarettes; asexual morphs of some species of the Dermateaceae, such as Mollisia and Pyrenopeziza, might be accommodated in Cadophora, which has more or less pigmented vegetative hyphae, pale to hyaline phialides and collarettes (Gams 2000); the asexual morph of Hyphodiscus hymeniophilus is Catenulifera rhodogena, which has cylindrical to ampulliform phialides, long and cylindrical collarette, and conidia born in chains or in droplets (Hosoya 2002), in contrast, species in the new genus Psychrophila have hyaline vegetative hyphae, phialides, and collarettes; conidiophores are reduced to conidiogenous cells, sometimes short, or much differentiated; collarettes are wedge-shaped to campanulate and widely flaring; and conidia are hyaline, pyriform to globose. The combination of a cold-adapted nature, morphological characters, and phylogenetic relationships well supports the establishment of the new genus Psychrophila (Fig. 1).
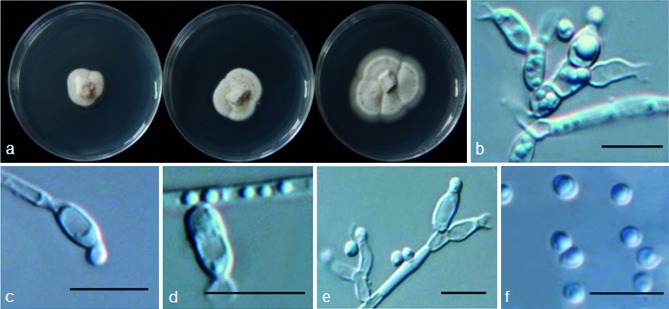
Psychrophila antarctica M.M. Wang & Xing Z. Liu, sp. nov. — MycoBank MB801298; Fig. 5
Fig. 5.
Psychrophila antarctica (from strain ANT92) a. Colony morphology at three temperatures after 4 wk (left-to-right: 4, 10 and 20 °C); b–e. conidiophores and conidiogenous cells; f. conidia. — Scale bars = 10 μm.
Etymology. Antarctica refers to the type locality of this fungus.
Colony on PDA at 10 °C attaining 25 mm diam after 4 wk, OGT 20 °C, eurypsychrophile; colonies cream white, aerial mycelium less abundant or sparse on the surface of the colony. Conidiophores sometimes short, or much differentiated, conidiogenesis phialidic, phialides short, hyaline, flask-shaped, 5.1–8.0 × 2.5–4.5 μm (mean ± s.d. = 6.4 ± 0.89 × 3.5 ± 0.77 μm, n = 30), apically tapering into a broad funnel, bottlenecklike constriction; the collarette 2.1–4 μm (mean ± S.D. = 2.9 ± 0.56 μm, n = 30), wedge-shaped, widely flaring; vegetative hyphae hyaline, sometimes agglomerate to bundles or swollen to irregular shapes, 2–4 μm. Conidia hyaline, 1-celled, smooth, mostly globose, 2.1–3.5 μm diam (mean ± S.D. = 2.7 ± 0.47 μm, n = 30).
Specimen examined. ANTARCTIC, Great Wall Station, S62°12' W58°57', from soil, Jan. 2011, T. Zhang (dried culture HMAS244374 holotype, living culture ex-type CGMCC315133 (ANT92)).
Other isolates examined. ANTARCTIC, Great Wall Station, S62°12' W58°57', from soil, Jan. 2011, T. Zhang, living cultures ANT80, ANT94.
Notes — Psychrophila antarctica is a psychrotolerant fungus with an OGT of 20 °C. This species is known from both Antarctica and the Qinghai-Tibet Plateau, whose origin and evolution deserve further studies.
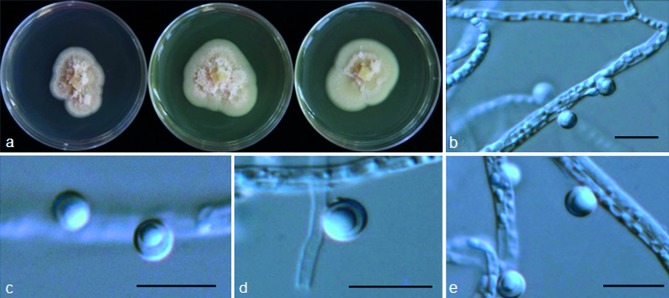
Psychrophila lutea M.M. Wang & Xing Z. Liu, sp. nov. — MycoBank MB801299; Fig. 6
Fig. 6.
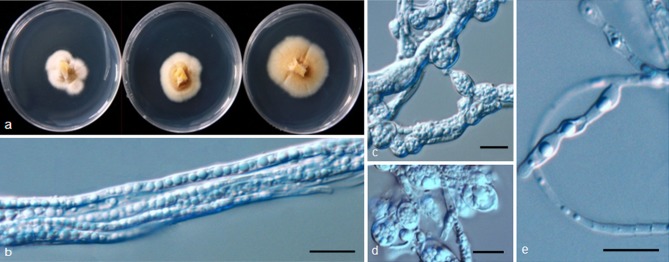
Psychrophila lutea (from strain HAILUO409). a. Colony morphology at three temperatures after 4 wk (left-to-right: 4, 10 and 20 °C); b–e. swollen and aggregated hyphae. — Scale bars = 10 μm.
Etymology. Lutea refers to the yellow colour of the colony.
Cultures sterile. Psychrophila lutea differs from its closest phylogenetic neighbour, P. antarctica (Fig. 3), by unique fixed alleles in three loci based on alignments of the separate loci deposited in TreeBASE as study S16864: ITS positions 76 (A), 316 (C), 413 (C), 416 (A), 449 (T) and 454 (T); TUB positions 134 (T), 138 (A), 146 (A), 147 (A), 170 (A), 206 (C), 294 (T), 303 (C), 305 (G), 314 (T) and 356 (G); TEF1 positions 174 (C), 208 (C), 247 (C), 283 (G), 297 (A) and 327 (A).
Colony on PDA at 10 °C attaining 15 mm diam after 4 wk, OGT 20 °C, eurypsychrophile; bright to brown-yellow, part of the colonies submerged in the medium, hyphae above the medium compacted densely, aerial mycelium absent or sparse, hyaline; vegetative hyphae yellow or brown, smooth-walled, 2–8 μm; aggregated in dense clumps or bundles, sometimes swollen to irregular shapes. Conidiophores and conidia absent.
Specimen examined. CHINA, Sichuan, Hailuogou Glacier, N29°33' E101°58', from soil, 20 Apr. 2011, M. Wang (dried culture HMAS244372 holotype, living ex-type culture CGMCC315134 = HAILUO409).
Other isolates examined. CHINA, Sichuan, Hailuogou Glacier, N29°33' E101°58', from soil, 20 Apr. 2011, M. Wang, living cultures HAILUO374, HAILUO407.
Notes — We have used some low nutrient media such as corn meal agar (CMA) and water agar (WA) to induce strains of P. lutea to sporulate without success. Phylogenetic analyses showed that it formed a distinct clade most closely related to P. antarctica (Fig. 1, 3) but could be differentiated from the later by SNP analysis.
Psychrophila olivacea M.M. Wang & Xing Z. Liu, sp. nov. — MycoBank MB801300; Fig. 7
Fig. 7.
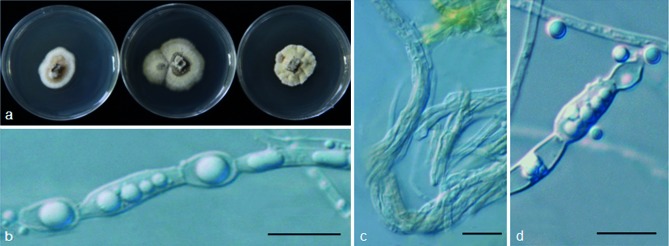
Psychrophila olivacea (from strain HAILUO368). a. Colony morphology at three temperatures after 4 wk (left-to-right: 4, 10 and 20 °C); b–d. aggregated hyphae. — Scale bars = 10 μm.
Etymology. Olivacea refers to the olive colour of the colony.
Cultures sterile. Psychrophila olivacea differs from its closest phylogenetic neighbour, P. antarctica and P. lutea (Fig. 3), by unique fixed alleles in three loci based on alignments of the separate loci deposited in TreeBASE as study S16864.
P. antarctica: ITS positions 113 (C), 116 (A), 133 (A), 308 (C), 343 (A), 346 (G), 364 (C), 411 (G), 413 (C), 425 (G), 431 (G), 439 (G), 451 (A) and 454 (T); TUB positions 116 (C), 137 (T), 151 (G), 163 (T), 198 (T), 199 (A), 220 (C), 223 (T), 295 (C), 304 (G), 306 (C), 328 (C) and 371 (T); TEF1 positions 174 (C), 195 (G), 203 (A), 208 (C), 247 (C), 252 (C), 297 (A), 306 (T), 307 (T), 308 (G), 313 (A), 327 (C), 333 (T) and 340 (T).
P. lutea: ITS position 113 (C), 116 (A), 308 (C), 316 (T), 317 (C), 333 (C), 343 (A), 346 (G), 364 (C), 411 (G), 416 (G), 425 (G), 431 (G), 439 (G), 449 (T), 451 (A) and 454 (T); TUB positions 134 (C), 138 (G), 151 (G), 163 (T), 170 (G), 199 (A), 206 (T), 223 (T), 294 (A), 295 (C), 303 (T), 304 (G), 305 (G), 306 (C), 314 (G), 316 (A), 356 (A) and 371 (T); TEF1 positions 195 (G), 203 (A), 252 (C), 283 (A), 306 (T), 307 (T), 308 (G), 313 (A), 327 (C), 333 (T) and 340 (T).
Colony on PDA at 10 °C attaining 10–15 mm diam after 4 wk, growth rate similar at 10 and 20 °C, stenopsychrophile; light to dark olive, sometimes appearing light grey on the surface because of some young aerial hyphae; part of the colonies immerged in the medium, some hyphae above the medium compact densely, colony surface sometimes furrowed; aerial hyphae sparse, hyaline or olive, vegetative hyphae, olive to dark olive, smooth-walled, 2–7 μm; aggregate in dense clumps or rhizomorphs, sometimes swollen to irregular shapes. Conidiophores and conidia absent.
Specimen examined. CHINA, Sichuan, Hailuogou Glacier, N29°33' E101°58', from soil, 20 Apr. 2011, M. Wang (dried culture HMAS244375 holotype, living culture ex-type CGMCC315135 = HAILUO368).
Other isolate examined. CHINA, Sichuan, Hailuogou Glacier, N29°33' E101°58', from soil, 20 Apr. 2011, M. Wang, living culture HAILUO563.
Notes — No conidia or conidiophores were observed for P. olivacea on PDA, CMA and WA. Psychrophila olivacea differs from P. lutea in the colony morphology and OGT.
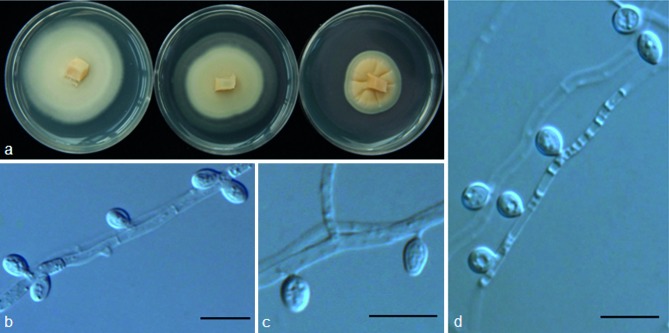
Tetracladium globosum M.M. Wang & Xing Z. Liu, sp. nov. — MycoBank MB801301; Fig. 8
Fig. 8.
Tetracladium globosum (from strain HAILUO215). a. Colony morphology at three temperatures after 4 wk (left-to-right: 4, 10 and 20 °C); b–e. conidia and hyphae. — Scale bars = 10 μm.
Etymology. Globosum refers to its globose conidia.
Colonies on PDA at 10 °C attaining 30–45 mm diam after 4 wk, pale yellow to light pinkish, OGT 10 °C, stenopsychrophile; part of the colony immerged in the medium, aerial hyphae sparse and hyaline; vegetative hyphae hyaline, smooth, thin-walled, transversely septate, 1–4 μm. Conidia 1-celled, hyaline, globose, smooth-walled, 3.0–5.5 μm (mean ± S.D. = 4.4 ± 0.81 μm, n = 30), attaching to the hyphae with very short conidiophores, which are not obvious.
Specimens examined. CHINA, Sichuan, Hailuogou Glacier, N29°33' E101°58', from soil, 20 Apr. 2011, Manman Wang, dried culture specimen HMAS244377 holotype, living culture ex-type CGMCC315136 = HAILUO215.
Other isolates examined. CHINA, Yunnan, Mingyong Glacier, N28°27' E98°45', from soil, 4 May 2011, M. Wang, living cultures MY24, MY25.
Notes — Species described in the genus Tetracladium are all aquatic and mostly inhabit decaying litter in streams and rivers (Bärlocher 1992). Tetracladium species produce tetraradiate conidia, which are thought to aid in their colonisation of substrates (Read et al. 1992). Unlike the previously described species, T. globosum has globose conidia, indicating that tetraradiate conidia may be an ecologically adapted characteristic. The OGT is 10 °C but the fungus can also grow at 20 °C. It is interesting that the OGT of T. globosum varied among different isolates. This phenomenon has also been observed in other fungi such as Pseudogymnoascus pannorum, indicating psychrophily may be an adapted character (Kochkina et al. 2007).
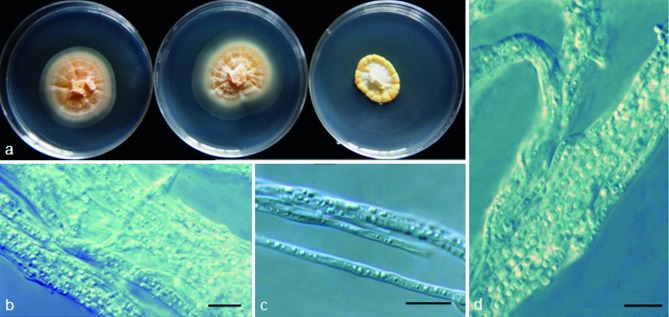
Tetracladium ellipsoideum M.M. Wang & Xing Z. Liu, sp. nov. — MycoBank MB801302; Fig. 9
Fig. 9.
Tetracladium ellipsoideum (from strain MIDUI20). a. Colony morphology at three temperatures after 4 wk (left-to-right: 4, 10 and 20 °C); b–d. conidia, conidiogenous cells and hyphae. — Scale bars = 10 μm.
Etymology. Ellipsoideum refers to the shape of the conidia.
Colony on PDA at 10 °C attaining 30–40 mm diam after 4 wk, pale to bright yellow, OGT at 10 °C, stenopsychrophile; aerial hyphae absent or sparse; vegetative hyphae hyaline, smooth, thin-walled, transversely septate, 1–3 μm. Conidia borne on short, undifferentiated or sessile pedicels (up to 1 μm long), 1-celled, hyaline, ellipsoid, smooth-walled, 4–6.8 × 2–3.4 μm (mean ± S.D. = 5.3 ± 0.69 × 3.7 ± 0.67 μm, n = 30).
Specimen examined. CHINA, Tibet, Midui Glacier, N29°27' E96°30', from soil, 16 Oct. 2009, Manman Wang (dried culture specimen HMAS244378 holotype, culture ex-type CGMCC315137 = MIDUI20).
Other isolates examined. CHINA, Tibet, Midui Glacier, N29°27' E96°30', from soil, 16 Oct. 2009, M. Wang, living cultures MIDUI30, MIDUI21.
Notes — The morphology of T. ellipsoideum is very similar to that of T. globosum. Tetracladium ellipsoideum produces conidia that are pyriform to ellipsoid rather than globose as observed for T. globosum. Conidiophores are somewhat differentiated and obvious for T. ellipsoideum but not obvious for T. globosum.
Tetracladium psychrophilum M.M. Wang & Xing Z. Liu, sp. nov. — MycoBank MB801304; Fig. 10
Fig. 10.
Tetracladium psychrophilum (from strain HAILUO380). a. Colony morphology at three temperatures after 4 wk (left-to-right: 4, 10 and 20 °C); b–d. hyphae. — Scale bars = 10 μm.
Etymology. Psychrophilum refers to the cold-loving character of the species.
Cultures sterile. Tetracladium psychrophilum differs from its closest phylogenetic neighbour, T. globosum (Fig. 4), by unique fixed alleles in three loci based on alignments of the separate loci deposited in TreeBASE as study S16864: ITS positions 97 (C), 108 (A), 310 (C), 336 (C), 337 (C), 397 (A), 416 (G), 427 (C) and 430 (G); TUB positions 125 (C), 131 (T), 140 (A), 152 (G), 157 (C), 185 (A), 186 (T) and 196 (C); TEF1 positions 78 (C), 80 (G), 84 (T), 99 (C), 153 (T), 161 (T) and 166 (C).
Colony slow-growing, attaining about 10–15 mm diam on PDA at 10 °C after 4 wk, cream-white to pale yellow; OGT 10 °C, MGT 20 °C, stenopsychrophile; aerial mycelium sparse or absent, conidia absent; vegetative hyphae hyaline, often aggregate, 2–14 μm. Conidia and conidiophores absent.
Specimen examined. CHINA, Sichuan, Hailuogou Glacier, N29°33' E101°58', from soil, 20 Apr. 2011, M. Wang (dried culture HMAS244371 holotype, living ex-type culture CGMCC315139 = HAILUO380).
Other isolate examined. CHINA, Yunnan, Mingyong Glacier, N28°27' E98°45', from soil, 4 May 2011, M. Wang, living culture MY376.
Notes — Tetracladium psychrophilum grows slowly with OGT at 10 °C and MGT at 20 °C. Unlike T. globosum and T. ellipsoideum, T. psychrophilum did not produce conidia or conidiophores.
DISCUSSION
The rising of the Qinghai-Tibet Plateau was an important geological event in the Quaternary period when the average rate of rising was 1.0–1.1 mm/year. In the last 10 000 years, the plateau has raised 300–700 m and is still rapidly rising (Li & Wang 1983). This steady rising of the Qinghai-Tibet Plateau may have resulted in gradual environmental changes and niches that are inhabited by cold-adapted fungi.
Species of Geomyces and Phoma are widespread and especially common in northern temperate regions or Arctic and Antarctic permafrost soils. Traditionally, Geomyces is characterized by short but distinctly branched conidiophores that have spore chains formed directly from the conidiogenous cells. Members of the genus are psychrotolerant and have been reported from Arctic and Antarctic permafrost soils (Kirk et al. 2008, Blehert et al. 2009). Phylogenetic analyses indicate Geomyces and allied genera such as Gymnostellatospora and Pseudogymnoascus should be classified in the family Pseudeurotiaceae (Minnis & Lindner 2013). The best-known psychrophilic fungus in this group is perhaps Pseudogymnoascus destructans, which causes white nose syndrome and high mortality of bats (Blehert et al. 2009, Gargas et al. 2009). In our study, Pseudogymnoascus pannorum was found to be dominant on the Qinghai-Tibet Plateau, accounting nearly 10 % of all isolates obtained. Interestingly, although the morphological characteristics and temperature profile differ among isolates of this species, all isolates presented identical ITS and SSU rDNA sequences. Phoma sclerotioides is known as a snow mould and as the causal agent of brown root rot of alfalfa and other perennial forage legumes in temperate regions with harsh winters (Wunsch & Bergstrom 2011). It was also the most frequently isolated species from a decomposing high arctic moss Schistidium apocarpum (Leung et al. 2011). In this survey, we obtained 52 P. sclerotioides isolates in a total of 1 428 isolates from Qinghai-Tibet Plateau, with all of the strains of P. sclerotioides isolated from soil and identified based on comparison of morphologies and ITS and SSU rDNA sequences. Phoma sclerotioides has been divided into seven intraspecific varieties, which differ in their morphology, temperature adaptation, and plant hosts (Sanford 1933, Berkenkamp & Baenziger 1969, Wunsch & Bergstrom 2011). Whether these varieties satisfy the phylogenetic concept of species should be considered in future research.
Psychrophila antarctica produces phialidic conidiogenous cells, which is also shared by some species in genera such as Ascocoryne, Cadophora and Catenulifera in the Helotiales. Although there are no special morphological characters in Psychrophila, the cold-adaptation of all isolates and species and the phylogenetic distinction from other genera well support its establishment as novel genus.
Tetracladium species are common in aquatic habitats, and they produced tetraradiate conidia that may facilitate their attachments to the substrate and provide a stable base for rapid germination (Read et al. 1992). Tetracladium species are primary agents of leaf litter and wood decay in streams and rivers. Some aquatic fungi including Tetracladium species are distributed worldwide (Descals 1997, Shearer et al. 2007, Wurzbacher et al. 2010) and in lotic habitats from the equator to the Arctic (Shearer et al. 2007). Tetracladium species have been documented from streams of alpine glaciers and from snow-covered soil (Robinson et al. 2000, Kuhnert et al. 2012), and are likely to be cold-adapted. The genus is rather homogeneous in terms of cultural characters and conidiogenesis. In addition to the type species, T. marchalianum, seven other species have been reported in the genus, e.g. T. apiense (Sinclair & Eicker 1981), T. breve (Roldán et al. 1989), T. furcatum (Descals & Webster 1983), T. maxilliforme (Ingold 1942), T. nainitalense (Sati et al. 2009), T. palmatum (Roldán et al. 1989) and T. setigerum (Ingold 1942). Recent phylogenetic analyses suggested that the genus is monophyletic and affiliated with Helotiales (Nikolcheva & Bärlocher 2002, Baschien et al. 2006, Letourneau et al. 2010, Seena et al. 2010). The three new species described here are paraphylogenetically clustered with aquatic Tetracladium species. Tetracladium globosum and T. ellipsoideum produce simple globose or clavate conidia on very short conidiophores or on the hypha (sessile), and T. psychrophilum does not produce conidia. All three are clearly different from previously described aquatic species in this genus and may reflect their adaptation to glacial niches with little free water. The three new species can grow well at temperatures below 20 °C and produce colonies that are light or bright yellow or light pink. Their colonies are often flat, with sparse or no aerial mycelium, which may be beneficial for cold-adaptation.
Acknowledgments
This study was supported by the Ministry of Science and Technology of China (No. 2012ZX09301002–003) and 973 Programme (Grants No. 20009CB522302). The authors thank professor B. Jaffee (University of California, Davis) for his critical comments and English correction and editing.
REFERENCES
- Altekar G, Dwarkadas S, Huelsenbeck JP, et al. 2004. Parallel Metropolis-coupled Markov chain Monte Carlo for Bayesian phylogenetic inference. Bioinformatics 20: 407–415. [DOI] [PubMed] [Google Scholar]
- Anupama PD, Praveen KD, Singh RK, et al. 2011. Psychrophilic and halotolerant strain of Thelebolus microsporus from Pangong Lake, Himalaya. Mycosphere 2: 601–609. [Google Scholar]
- Arenz BE, Blanchette RA. 2009. Investigations of fungal diversity in wooden structures and soils at historic sites on the Antarctic Peninsula. Canadian Journal of Microbiology 55: 46–56. [DOI] [PubMed] [Google Scholar]
- Arenz BE, Held BW, Jurgens JA, et al. 2006. Fungal diversity in soils and historic wood from the Ross Sea Region of Antarctica. Soil Biology & Biochemistry 38: 3057–3064. [Google Scholar]
- Bärlocher F. 1992. Research on aquatic hyphomycetes: historical background and overview. In: Bärlocher F. (ed), The ecology of aquatic hyphomycetes: 1–15. Springer-Verlag, Berlin. [Google Scholar]
- Baschien C, Marvanová L, Szewzyk U. 2006. Phylogeny of selected aquatic hyphomycetes based on morphological and molecular data. Nova Hedwigia 83: 311–352. [Google Scholar]
- Berestovskaya YY, Vasil’eva L, Chestnykh O, et al. 2002. Methanotrophs of the psychrophilic microbial community of the Russian Arctic tundra. Mikrobiologiia 71: 538–544. [PubMed] [Google Scholar]
- Berkenkamp B, Baenziger H. 1969. The reaction of sweet clover varieties to brown root rot. Canadian Journal of Plant Science 49: 181–183. [Google Scholar]
- Bhadury P, Mohammed BT, Wright PC. 2006. The current status of natural products from marine fungi and their potential as anti-infective agents. Journal of Industrial Microbiology and Biotechnology 33: 325–337. [DOI] [PubMed] [Google Scholar]
- Biabini MAF, Laatch H. 1998. Advances in chemical studies of low-molecular weight metabolites of marine fungi. Journal für praktische Chemie 340: 589–607. [Google Scholar]
- Blanchette RA, Held BW, Jurgens JA, et al. 2004. Wood destroying soft-rot fungi in the historic expeditions huts of Antarctica. Applied and Environmental Microbiology 70: 1328–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blehert DS, Hicks AC, Behr M, et al. 2009. Bat white-nose syndrome: An emerging fungal pathogen? Science 323 (5911): 227. [DOI] [PubMed] [Google Scholar]
- Blunt JW, Copp BR Hu WP, et al. 2007. Marine natural products. Natura Product Reports 24: 31–86. [DOI] [PubMed] [Google Scholar]
- Bowman JP, Gosink JJ, McCammon SA, et al. 2004. Psychropiezophilic microorganisms. Cell and Molecular Biology 50: 429–436. [PubMed] [Google Scholar]
- Carbone I, Kohn LM. 1999. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91: 553–556. [Google Scholar]
- Cavicchioli R. 2006. Cold-adapted archaea. Nature Reviews Microbiology 4: 331–343. [DOI] [PubMed] [Google Scholar]
- Cheng G. 1998. Glaciology and geocryology of China in the past 40 years: progress and prospect. Journal of Glaciology and Geocryology 20, 3: 213–226. [Google Scholar]
- Coetzee JC, Eicker A. 1990. A simple slide culture technique facilitating the viewing of growing fungi. Phytophylactica 22: 361–362. [Google Scholar]
- Connell L, Redman R, Craig S, et al. 2006. Distribution and abundance of fungi in the soils of Taylor Valley, Antarctica. Soil Biology & Biochemistry 38: 3083–3094. [Google Scholar]
- D’Amico S, Collins T, Marx JC, et al. 2006. Psychrophilic microorganisms: challenges for life. EMBO Reports 7: 385–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejardin RA, Ward EWB. 1971. Growth and respiration of psychrophilic species of the genus Typhula. Canadian Journal of Botany 49: 339–347. [Google Scholar]
- DeLong EF, Franks DG, Yayanos AA. 1997. Evolutionary relationship o cultivated psychrophilic and barophilic deep-sea bacteria. Applied and Environmental Microbiology 63: 2105–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descals E. 1997. Ingoldian fungi: some field and laboratory techniques. Bolletín de la Societat d’Història Natural de les Balears 40: 169–221. [Google Scholar]
- Descals E, Webster J. 1983. Four new staurosporous hyphomycetes from mountain streams. Transactions of the British Mycological Society 80: 67–75. [Google Scholar]
- Duncan SM, Minasaki R, Farrell RL, et al. 2008. Screening fungi isolated from historic Discovery Hut on Ross Island, Antarctica for cellulose degradation. Antarctic Sciences 20: 463–470. [Google Scholar]
- Ebel R. 2006. Secondary metabolites from marine-derived fungi. In: Proksch P, Muller WEG. (eds), Frontiers in marine biotechnology: 73–143. Horizon Bioscience, London. [Google Scholar]
- Flam F. 1994. Chemical prospectors scour the seas for promising drugs. Science 226: 1324–1325. [DOI] [PubMed] [Google Scholar]
- Forster J. 1887. Über einige Eigenschaften leuchtender Bakterien. Zentbl. Bakt. ParasitKde 2: 337–340. [Google Scholar]
- Gams W. 2000. Phialophora and some similar morphologically little-differentiated anamorphs of divergent ascomycetes. Studies in Mycology 45: 187–199. [Google Scholar]
- Gargas A, Trest MT, Christensen M, et al. 2009. Geomyces destructans sp. nov. associated with bat white-nose syndrome. Mycotaxon 108: 147–154. [Google Scholar]
- Glass NL, Donaldson G. 1995. Development of primer sets designed for use with PCR to amplify conserved genes from filamentous ascomycetes. Applied and Environmental Microbiology 61: 1323–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grünke S, Lichtschlag A, Beer D de, et al. 2012. Mats of psychrophilic thiotrophic bacteria associated with cold seeps of the Barents Sea. Biogeosciences Discussions 9: 3917–3948. [Google Scholar]
- Gudjarnnson S. 1999. Bioactive marine natural products. Rit Fiskideildar 16: 107–110. [Google Scholar]
- Held BW, Jurgens JA, Duncan SM, et al. 2006. Assessment of fungal diversity and deterioration in a wooden structure at New Harbor, Antarctica. Polar Biology 29: 526–531. [Google Scholar]
- Höller U, Wright AD, Matthee GF, et al. 2000. Fungi from marine sponges: Diversity, biological activity and secondary metabolites. Mycological Research 104: 1354–1365. [Google Scholar]
- Hoshino YT, Terami F, Tkachenko OB, et al. 2010. Mycelial growth of the snow mold fungus, Sclerotinia borealis, improved at low water potentials: an adaptation to frozen environment. Mycoscience 51: 98–103. [Google Scholar]
- Hosoya T. 2002. Hyaloscyphaceae in Japan (6): the genus Hyphodiscus in Japan and its anamorph Catenulifera gen. nov. Mycoscience 43: 47–57. [Google Scholar]
- Hsiang T, Matsumoto N, Millett SM. 1999. Biology and management of Typhula snow molds of turfgrass. Plant Disease 83: 788–798. [DOI] [PubMed] [Google Scholar]
- Ingold CT. 1942. Aquatic hyphomycetes of decaying alder leaves. Transactions of the British Mycological Society 25: 339–417. [Google Scholar]
- Jurgens JA, Blanchette RA, Filley TR. 2009. Fungal diversity and deterioration in mummified woods from the ad Astra Ice Cap region in the Canadian High Arctic. Polar Biology 32: 751–758. [Google Scholar]
- Kirk PM, Cannon PF, Minter DW, et al. (eds). 2008. Ainsworth & Bisby’s Dictionary of the Fungi. 10th ed. CABI Publishing. [Google Scholar]
- Kochkina GA, Ivanushkina NE, Akimov VN, et al. 2007. Halo- and psychrotolerant Geomyces fungi from Arctic cryopegs and marine deposits. Microbiology 76, 1: 31–38. [PubMed] [Google Scholar]
- Kuhnert R, Oberkofler I, Peintner U. 2012. Fungal growth and biomass development is boosted by plants in snow-covered soil. Microbial Ecology 64, 1: 79–90. [DOI] [PubMed] [Google Scholar]
- Letourneau A, Seena S, Marvanová L, et al. 2010. Potential use of barcoding to identify aquatic hyphomycetes. Fungal Diversity 40: 51–64. [Google Scholar]
- Leung G, Robson GD, Robinson CH. 2011. Characterisation of cold-tolerant fungi from a decomposing High Arctic moss. Soil Biology & Biochemistry 43: 1975–1979. [Google Scholar]
- Li BY, Wang FB. 1983. Tibet Quaternary geology. Science Publication House, P.R. China. [Google Scholar]
- Li X, Cheng GD. 1999. A GIS aided response model of high altitude permafrost to global change. Science in China Series D 42, 1: 72–79. [Google Scholar]
- Librado P, Rozas J. 2009. DnaSP v. 5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25: 1451–1452. [DOI] [PubMed] [Google Scholar]
- Liu YQ, Yao TD, Jiao NZ, et al. 2009a. Culturable bacteria in glacial meltwater at 6,350 m on the east Rongbuk glacier, Mount Everest. Extremophiles 13: 89–99. [DOI] [PubMed] [Google Scholar]
- Liu YQ, Yao TD, Jiao NZ, et al. 2009b. Bacterial diversity in the snow over Tibetan plateau glaciers. Extremophiles 13: 411–423. [DOI] [PubMed] [Google Scholar]
- Liu YQ, Yao TD, Kang SC, et al. 2006. Seasonal variation of snow microbial community structure in the East Rongbuk glacier. Mt Everest. Chinese Science Bulletin 51: 1476–1486. [Google Scholar]
- Liu YQ, Yao TD, Kang SC, et al. 2007. Microbial community structure in major habitats above 6,000 m on Mount Everest. Chinese Science Bulletin 52: 2350–2357. [Google Scholar]
- Malosso E, Waite IS, English L, et al. 2006. Fungal diversity in maritime Antarctic soils determined using a combination of culture isolation, molecular fingerprinting and cloning techniques. Polar Biolology 29: 552–561. [Google Scholar]
- Margaret E. 1966. Three new yeasts from Antarctic soils: Candida nivalis, Candida gelida and Candida frigida spp.n. Antonie van Leeuwenhoek 32: 25–28. [DOI] [PubMed] [Google Scholar]
- Margesin R, Fell JW. 2008. Mrakiella cryoconiti gen. nov., sp. nov., a psychrophilic, anamorphic, basidiomycetous yeast from alpine and arctic habitats. International Journal of Systematic and Evolutionary Microbiology 58: 2977–2982. [DOI] [PubMed] [Google Scholar]
- Margesin R, Schinner F. 1994. Properties of cold-adapted microorganisms and their potential role in biotechnology. Journal of Biotechnology 33: 1–14. [Google Scholar]
- Margesin R, Schinner FY.(eds). 1999. Biotechnological Applications of Cold-Adapted Organisms. Springer, Berlin. [Google Scholar]
- Margesin R, Sproer C, Schumann P, et al. 2003. Pedobacter cryoconitis sp. nov., a facultative psychrophile from alpine glacier cryoconite. International Journal of Systematic and Evolutionary Microbiology 53: 1291–1296. [DOI] [PubMed] [Google Scholar]
- Minnis AM, Lindner DL. 2013. Phylogenetic evaluation of Geomyces and allies reveals no close relatives of Pseudogymnoascus destructans, comb. nov.,in bat hibernacula of eastern North America. Fungal Biology 117: 638–649. [DOI] [PubMed] [Google Scholar]
- Möller C, Dreyfuss MM. 1996. Microfungi from Antarctic lichens, mosses and vascular plants. Mycologia 88: 922–933. [Google Scholar]
- Morita RY. 1975. Psychrophilic bacteria. Bacteriological Reviews 39: 144– 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountfort DO, Rainey FA, Burghardt J, et al. 1998. Psychromonas antarcticus gen. nov., sp. nov., a new aerotolerant anaerobic, halophilic psychrophile isolated from pond sediment of the McMurdo ice shelf, Antarctica. Archives of Microbiology 169: 231–238. [DOI] [PubMed] [Google Scholar]
- Nikolcheva LG, Bärlocher F. 2002. Phylogeny of Tetracladium based on 18S rDNA. Czech Mycology 53: 285–295. [Google Scholar]
- Nylander JAA. 2004. MrModeltest v2. Program distributed by the author. Evolutionary Biology Centre, Uppsala University. [Google Scholar]
- Onofri S, Barreca D, Selbmann L, et al. 2008. Resistance of Antarctic black fungi and cryptoendolithic communities to simulated space and Martian conditions. Studies in Mycology 61: 99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page RDM. 1996. TreeView: An application to display phylogenetic trees on personal computers. Computer Application of Biosciences 12, 4: 357–358. [DOI] [PubMed] [Google Scholar]
- Pietra F. 1997. Secondary metabolites from marine microorganisms – bacteria, protozoa, algae and fungi – achievements and prospectives. Natural Product Reports 14: 453–464. [DOI] [PubMed] [Google Scholar]
- Price PB. 2000. A habitat for psychrophiles in deep Antarctic ice. Proceedings of the National Academy of Sciences USA 97: 1247–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rannala B, Yang Z. 1996. Probability distribution of molecular evolutionary trees: a new method of phylogenetic inference. Journal of Molecular Evolution 43: 304–311. [DOI] [PubMed] [Google Scholar]
- Rateb ME, Ebel R. 2011. Secondary metabolites of fungi from marine habitats. Natural Product Reports 28: 290–344. [DOI] [PubMed] [Google Scholar]
- Read SJ, Moss ST, Jones EBG. 1992. Attachment and germination of conidia. In: Bärlocher F. (ed), The ecology of aquatic hyphomycetes: 135–151. Springer-Verlag, Berlin. [Google Scholar]
- Richard WN, Palm ME, Johnstone K, et al. 1997. Ecological and physiological characterization of Humicola marvinii, a new psychrophilic fungus from Fellfield soils in the Maritime Antarctic. Mycologia 89, 5: 705–711. [Google Scholar]
- Robin S, Hall T, Turchetti B, et al. 2010. Cold-adapted yeasts from Antarctica and the Italian Alps – description of three novel species: Mrakia robertii sp. nov., Mrakia blollopis sp. nov. and Mrakiella niccombsii sp. nov. Extremophiles 14: 47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson CH. 2001. Cold adaptation in Arctic and Antarctic fungi. New Phytologist 151: 341–353. [Google Scholar]
- Robinson CT, Gessner MO, Callies KA, et al. 2000. Larch needle breakdown in contrasting streams of an alpine glacial floodplain. Journal of the North American Benthological Society 19, 2: 250–262. [Google Scholar]
- Roldán A, Descals E, Honrubia M. 1989. Pure culture studies on Tetracladium. Mycological Research 93: 452–465. [Google Scholar]
- Ruisi S, Barreca D, Selbmann L, et al. 2007. Fungi in Antarctica. Reviews of Environmental Science and Biotechnology 6: 127–141. [Google Scholar]
- Sanford GB. 1933. A root rot of sweet clover and related crops caused by Plenodomus meliloti Dearness & Sanford. Canadian Journal of Research, Section C 8: 337–348. [Google Scholar]
- Sati SC, Arya P, Belwal M. 2009. Tetracladium nainitalense sp. nov., a root endophyte from Kumaun Himalaya, India. Mycologia 101, 5: 692–695. [DOI] [PubMed] [Google Scholar]
- Schipper MA. 1967. Mucor strictus hagem, a psychrophilic fungus, and Mucor falcatus sp.n. Antonie Van Leeuwenhoek 33, 2: 189–195. [DOI] [PubMed] [Google Scholar]
- Seena S, Pascoal C, Marvanová L, et al. 2010. DNA barcoding of fungi: a case study using ITS sequences for identifying aquatic hyphomycete species. Fungal Diversity 44: 77–87. [Google Scholar]
- Selbmann L, Hoog GS de, Zucconi L, et al. 2008. Drought meets acid: three new genera in a dothidealean clade of extremotolerant fungi. Studies in Mycology 61: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo HJ, Bae SS, Lee JH, et al. 2005. Photobacterium frigidiphilum sp. nov., a psychrophilic, lipolytic bacterium isolated from deep-sea sediments of Edison Seamount. International Journal of Systematic and Evolutionary Microbiology 55: 1661–1666. [DOI] [PubMed] [Google Scholar]
- Shearer CA, Descals E, Kohlmeyer B, et al. 2007. Fungal biodiversity in aquatic habitats. Biodiversity Conservation 16: 49–67. [Google Scholar]
- Sinclair RC, Eicker A. 1981. Tetracladium apiense, a new aquatic species from South Africa. Transactions of British Mycological Society 76: 515–517. [Google Scholar]
- Singh SM, Puja G, Bhat DJ. 2006. Psychrophilic fungi from Schirmacher Oasis, East Antarctica. Current Science 90, 10: 1388–1392. [Google Scholar]
- Swofford DL. 2002. PAUP*. Phylogenetic Analysis Using Parsimony (and other methods). Version 4. Sinauer Associates, Sunderland, Massachusetts. [Google Scholar]
- Tamura K, Peterson D, Peterson N, et al. 2011. MEGA5: Molecular Evolutionary Genetics Analysis using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Molecular Biology and Evolution 28: 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, et al. 1997. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research 24: 4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traquair JA, Smith DJ. 1982. Sclerotial strains of Coprinus psychromorbidus, a snow mold basidiomycete. Canadian Journal of Plant Pathology 4, 1: 27–36. [Google Scholar]
- Tronsmo AM, Hsiang T, Okuyama H, et al. 2001. Low temperature diseases caused by Microdochium nivale. In: Iriki N, Gaudet DA, Tronsmo AM, et al. (eds), Low temperature plant microbe interactions under snow: 75–86. Sapporo, Hokkaido National Agricultural Experimental Station. [Google Scholar]
- Verbist JF, Sallenave C, Pruchus YF. 2000. Marine fungal substances. Studies in Natural Products Chemistry 24: 979–1092. [Google Scholar]
- Vilgalys R, Hester M. 1990. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zhuang WY. 2004. Designing primer sets for amplification of partial calmodulin genes from penicillia. Mycosystema 23: 466–473. [Google Scholar]
- White TJ, Bruns T, Lee J, et al. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, et al. (eds), PCR protocols: a guide to methods and applications: 315–322. Academic Press, San Diego, California, USA. [Google Scholar]
- Wildeman E de. 1893. Note mycologiques. Fasc. II. Annales de la Societé Belge de Microscopie 17: 35–68. [Google Scholar]
- Wildeman E de. 1894. Note mycologiques. Fasc. II. Annales de la Societé Belge de Microscopie 17: 135–161. [Google Scholar]
- Wildeman E de. 1895. Note mycologiques. Fasc. VI. Annales de la Societé Belge de Microscopie 17: 191–206. [Google Scholar]
- Wunsch MJ, Bergstrom GC. 2011. Genetic and morphological evidence that Phoma sclerotioides, causal agent of brown root rot of alfalfa, is composed of a species complex. Phytopathology 101: 594–610. [DOI] [PubMed] [Google Scholar]
- Wurzbacher CM, Bärlocher F, Grossart HP. 2010. Fungi in lake ecosystems. Aquatic Microbial Ecology 59: 125–149. [Google Scholar]
- Xiang S, Yao T, An L, et al. 2005. 16S rRNA sequences and difference in bacteria isolated Muztag Ata Glacier at increasing depths. Applied and Environmental Microbiology 71: 4619–4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang SR, Shang TC, Chen Y, et al. 2009. Dominant bacteria and biomass in the Kuytun 51 Glacier. Applied and Environmental Microbiology 75: 7287–7290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin MX, Zhou PJ. 2007. Mrakia psychrophila sp. nov., a new species isolated from Antarctic soil. Journal of Zhejiang University Science B 8, 4: 260–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang DQ, Wang JH, Bai Y, et al. 2008. Diversity and distribution of the prokaryotic community in near-surface permafrost sediments in the Tianshan Mountains, China. Canadian Journal of Microbiology 54: 270–280. [DOI] [PubMed] [Google Scholar]
- Yao T, Xiang S, Zhang X, et al. 2006. Microorganisms in the Malan ice core and their relation to climatic and environmental changes. Global Biogeochemical Cycles 20, 1: GB1004. [Google Scholar]
- Zhang DC, Li HR, Xin YH, et al. 2008. Phaeobacter arcticus sp. nov., a psychrophilic bacterium isolated from the Arctic. International Journal of Systematic and Evolutionary Microbiology 58: 1384–1387. [DOI] [PubMed] [Google Scholar]
- Zhang DC, Wang HX, Liu HC, et al. 2006. Flavobacterium glaciei sp. nov., a psychrophilic bacterium isolated from the China No.1 glacier. International Journal of Systematic and Evolutionary Microbiology 56: 2921–2925. [DOI] [PubMed] [Google Scholar]
- Zhang GS, Ma XJ, Niu FJ, et al. 2007. Diversity and distribution of alkaliphilic psychrotolerant bacteria in the Qinghai-Tibet Plateau permafrost region. Extremophiles 11: 415–424. [DOI] [PubMed] [Google Scholar]
- Zhang XJ, Ma XJ, Wang NL, et al. 2009. New subgroup of Bacteroidetes and diverse microorganisms in Tibetan plateau glacial ice provide a biological record of environmental conditions. FEMS Microbiology Ecology 67: 21–29. [DOI] [PubMed] [Google Scholar]
- Zhaxybayeva O, Gogarten JP. 2002. Bootstrap, Bayesian probability and maximum likelihood mapping: exploring new tools for comparative genome analyses. Genomics 3: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]