Abstract
Multidrug-resistant Klebsiella pneumoniae producing the KPC carbapenemase have rapidly spread throughout the world, causing severe healthcare-associated infections with limited antimicrobial treatment options. Dissemination of KPC-producing K. pneumoniae is largely attributed to expansion of a single dominant strain, ST258. In this study, we explore phylogenetic relationships and evolution within ST258 and its clonal group, CG258, using whole genome sequence analysis of 167 isolates from 20 countries collected over 17 years. Our results show a common ST258 ancestor emerged from its diverse parental clonal group around 1995 and likely acquired bla KPC prior to dissemination. Over the past two decades, ST258 has remained highly clonal despite diversity in accessory elements and divergence in the capsule polysaccharide synthesis locus. Apart from the large recombination event that gave rise to ST258, few mutations set it apart from its clonal group. However, one mutation occurs in a global transcription regulator. Characterization of outer membrane protein sequences revealed a profile in ST258 that includes a truncated OmpK35 and modified OmpK37. Our work illuminates potential genomic contributors to the pathogenic success of ST258, helps us better understand the global dissemination of this strain, and identifies genetic markers unique to ST258.
Introduction
Enterobacteriaceae are a common cause of healthcare-associated bacterial infections, including pneumonia, meningitis, sepsis, and other life threatening illness, especially among patients with underlying medical conditions. The recent rise of carbapenem-resistant Enterobacteriaceae (CRE) has left clinicians with limited antimicrobial treatment options for these infections, and has been declared an immediate public health threat that requires urgent and aggressive action by the Centers for Disease Control and Prevention [1]. Klebsiella pneumoniae carbapenemase (KPC)-producing K. pneumoniae are now one of the most widely disseminated CRE pathogens, and are associated with high morbidity and mortality rates [2, 3]. Since their initial identification in 2001 [4], KPC-producing K. pneumoniae have emerged throughout the United States (currently identified in 47 states; CDC unpublished data) and the world, spanning five continents that also include South America, Eurasia, Africa and Australia [5–7].
The rapid, widespread dissemination of KPC-producing K. pneumoniae is largely attributed to the clonal expansion of a single dominant strain, sequence type (ST) 258 as defined by multilocus sequence typing or MLST, currently circulating in over 20 countries [6]. ST258 is a member of the recently designated clonal group (CG) 258 [8], which comprises several other sequence types linked to outbreaks, suggesting that these strains may share genetic features that predispose them to pathogenicity or increased transmissibility. Unlike ST258, other CG258 strains are associated with a variety of carbapenemases including KPC, NDM, VIM, and OXA-48 [9–11]. The transmission of KPC-producing ST258 and other CG258 strains is frequently linked to patient travel or healthcare exposure in known endemic areas, such as the United States, Israel, and Greece [6, 12, 13]. Despite previous genomic analyses of ST258 [14–18], an explanation for its pathogenic success in the healthcare system remains unclear.
Large homologous recombination events frequently shape genomes to result in new emerging pathogens [19]. A sequence of these events has now been documented for CG258 and ST258. Gaiarsa and colleagues, using sequence from Italian isolates and the public database, discovered a putative recombination event that gave rise to CG258. Their evidence shows a donor related to K. pneumoniae ST1628 contributed ~1.3 Mbp to an ancestor of ST11 (CG258) sometime before 1985 [17]. Chen and colleagues used public genomic data to show the ST258 lineage resulted from a ~1.1 Mbp recombination event between ST11 and a strain related to a Brazilian ST442 isolate [20]. DeLeo and colleagues published a whole genome SNP-based phylogeny of ST258 from mostly the northeastern U.S., and concluded that ST258 comprises two distinct lineages which diverged after a homologous recombination event of ~215kb that included the capsule polysaccharide synthesis (cps) locus [15]. Additionally, Wyres and colleagues documented several recombination events involving cps loci in CG258 [18].
We contribute to the rapidly growing knowledge base of the KPC-producing K. pneumoniae pandemic with a geographically, temporally, and genotypically diverse set of isolates, and phylogenetically place ST258 and CG258 within the context of other pathogenic K. pneumoniae. We propose a timeline for the emergence, cps locus divergence, and clinical detection of ST258. We also examine regions of interest of the genomes including mobile elements, single nucleotide polymorphisms (SNPs), cps loci, and outer membrane proteins to compare ST258 to the rest of CG258, and to compare CG258 to other strains, to elucidate factors that may contribute to their pathogenic success.
Results
Genomics of K. pneumoniae
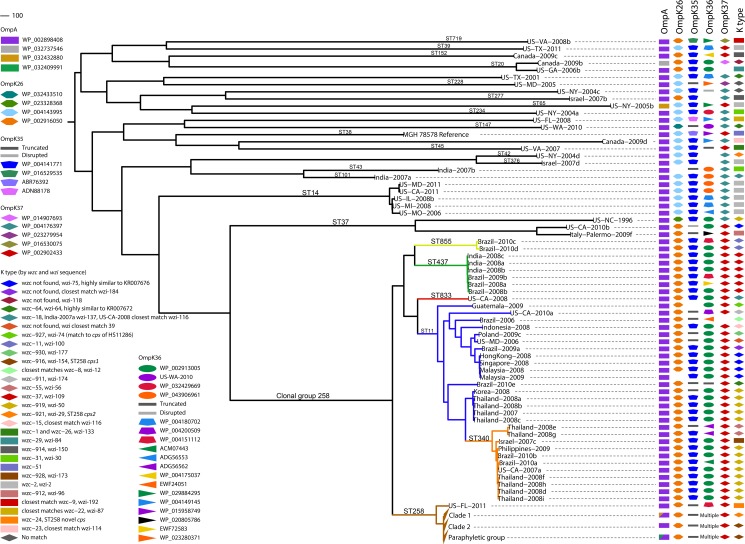
Our whole genome sequence analyses are based on SNPs, which are inherently stable, that fall in the core genome, or only the regions of the genome homologous to all isolates in the sample set (see details in Materials and Methods). Whole genome analysis of our 167 diverse isolates resulted in a core genome size of 2.2 Mbp. After two clear outliers were removed (a ST334 and a novel sequence type that were more than 37,000 SNPs from their closest neighbor), the core was still small at 2.4 Mbp (Table 1) compared to the known K. pneumoniae chromosome size of 5.1 to 5.4 Mbp (from publicly available genomes of clinical isolates). This signifies a lack of genomic overlap among the isolates, likely due to a large number of genes acquired through horizontal gene transfer (HGT) and non-homologous recombination. These calculations show, even with a limited number of isolates outside CG258, that K. pneumoniae is a very diverse species; the average pairwise SNP distance between sequence types is 8,490. The maximum parsimony reconstruction of the phylogeny using the SNP data support this, illustrating diversity even in the homologous regions of the genomes with long branches between isolates (Fig 1)
Table 1. Results of mapping Illumina sequencing reads from K. pneumoniae isolates to relevant reference genomes.
For further definition of the calculations see Materials and Methods.
| Sample set | No. isolates | Reference genome (genome size) | Total no. SNPs | No. SNPs parsimony informative | Total breadth of reference genome coverage (Core genome size) | Breadth of reference genome coverage all samples | Maximum parsimony consistency index | Illustr. |
|---|---|---|---|---|---|---|---|---|
| K. pneumoniae this study | 167 | MGH 78578 (5.32 Mbp) | 163,711 | 54,853 | 40.7% (2.16 Mbp) | >70.8% | 0.53 | Data not shown |
| K. pneumoniae 2 outliers pruned | 165 | MGH 78578 (5.32 Mbp) | 49,094 | 24,735 | 44.6% (2.37 Mbp) | >70.8% | 0.34 | Fig 1 |
| CG258 | 138 | NJST258_1 ~1.1 Mbp masked (4.2 Mbp) | 7,256 | 4,626 | 50.0% (2.1 Mbp) | >77.6% | 0.6 | Fig 2A |
| ST258* | 102 | NJST258_1 ~215kb masked (5.05 Mbp) | 1,425 | 307 | 75.5% (3.82 Mbp) | >93.1% | 0.96 | Figs 2B and3 |
| ST258 from this study* and SRA Study SRP036874** | 185 | NJST258_1 ~215kb masked (5.05 Mbp) | 2,282 | 582 | 72.3% (3.65 Mbp) | >93.1% | 0.96 | S1 Fig |
* Including ST512 and ST1199, both point mutations SLVs of ST258
** Including ST379, ST512, and ST418, all SLVs of ST258 [15]
Fig 1. Genetic diversity of healthcare-associated K. pneumoniae.
A maximum parsimony phylogeny based on 49,094 core genome SNPs in 165 K. pneumoniae isolates and the reference genome MGH 78578 illustrate the diversity of K. pneumoniae pathogens. The consistency index of the phylogeny is 0.34, reflecting a high number of homoplasious SNPs and indicative of high levels of homologous recombination. (Non-homologous DNA is not analyzed, as it is not part of the core genome.) The main branches of the groups within ST258 are collapsed. In CG258, branches are colored by sequence type. Outer membrane protein sequence was matched by BLAST to a Genbank accession number, except in the case of OmpK36 where matches of high similarity were not always found, in which case the sample name was used as the identifier. The cps loci of all strains were characterized by the wzc and wzi sequences [76, 77] and full-length characterization where genome assemblies allowed.
Our analyses provide the resolution to infer evolutionary history and exemplify the limitations of relational inferences from the traditional seven-locus MLST scheme. The adoption of the scgMLST scheme and clonal group nomenclature proposed by Bialek-Davenet et al. [8] address these limitations, however a full conversion from MLST-derived “sequence types” has yet to be proposed. Hundreds of SNPs separate ST258 from most MLST single locus variants (SLVs); the average pairwise SNP distance between ST258 isolates and those of the rest of the clonal group is 304 SNPs. Long branches separating isolates of the same sequence type or within the same clonal group, for example in ST37 and within CG258, often signify homologous recombination events like those documented by Gaiarsa, Chen, and DeLeo [15, 17, 20]. In contrast, ST512 and ST1199 are point mutation SLVs of ST258 and are clearly part of the ST258 lineage, and for ease of reading, are referred to as ST258 throughout. ST258 is a closely related group (average pairwise SNP distance of 13) within its diverse ancestral group (average pairwise SNP distance of the remaining isolates in CG258 is 214). This is evidence that ST258 is a recent emergence from the ancestral CG258 clade (Fig 1).
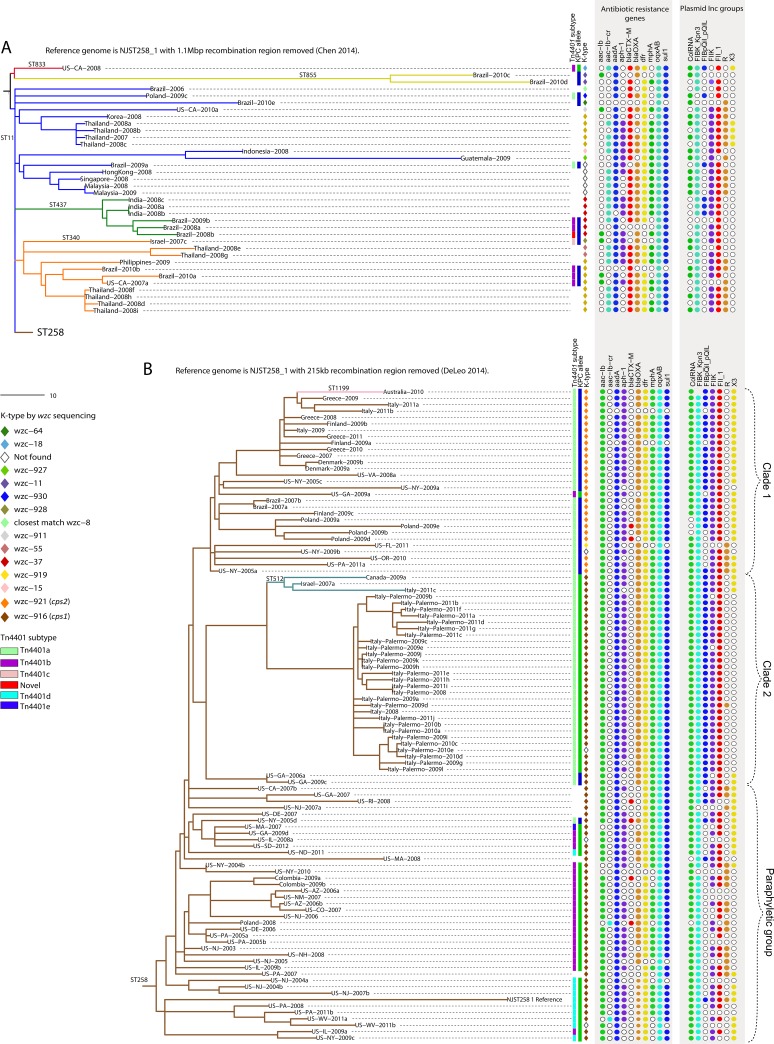
In order to illustrate the evolutionary history of all members of CG258, we masked the large regions of recombination identified in previous studies from the finished chromosome of the ST258 isolate NJST258_1 [15, 20] to filter non-phylogenetically informative SNP loci. Phylogenies of closely related isolates or defined by few SNPs can be heavily influenced by SNPs in recombinant regions leading to false inferences of evolutionary history. Reads from the 137 isolates in CG258 were mapped to NJST258_1 and the 1.06 Mbp recombinant region [20] was masked, resulting in a reference genome of 4.2 Mbp. A significant reduction in the pairwise SNP distance comparison between ST258 and the rest of CG258 resulted; an average of 62 SNPs separate the isolates from the two groups (compared to 304 stated above). The phylogeny from these data illustrates that ST11 is a paraphyletic group with respect to ST437, ST340, and ST258, and only four SNPs distinguish the ST258 lineage from its clonal group (Fig 2A). The core genome in this analysis is 50% of the 4.2 Mbp reference genome, or 2.1 Mbp (Table 1), implying that there are many regions of non-homologous recombination in this sample set.
Fig 2. Phylogenies of CG258 and ST258 with large recombined regions removed.
(A) A maximum parsimony phylogeny based on 1,440 core genome SNPs in 138 CG258 isolates using NJST258_1 with the 1.06 Mbp region of recombination [20] masked as a reference reduces the genomic distance between ST258 and the rest of CG258. The consistency index of the maximum parsimony phylogeny is 0.95, indicating most SNPs in the core are vertically transferred. (B) A maximum parsimony phylogeny based on 1,425 core genome SNPs in 102 ST258 isolates, using NJST258_1 with the 215 kb region of recombination [15] masked as a reference illustrate the clonal nature and evolutionary history of ST258. The consistency index is 0.96 for the ST258 maximum parsimony phylogeny, indicating most SNPs in the core are vertically transferred. Analysis of antibiotic resistance genes, Tn4401, capsule type by wzc sequence, and plasmid incompatibility groups lend insight into the vertical versus horizontal transfer of these genetic elements. Complete SRST2 [65] and PlasmidFinder [71] results are in S2 Table.
When reads from the 101 isolates in the ST258 group were mapped to the complete NJST258_1 (Table 1) chromosome, the ~215kb region of homologous recombination [15] was identified by its high SNP density; 971 out of the total 2,396 SNPs fell in this region. This region was masked from the NJST258_1 reference to generate a refined ST258 SNP matrix. Compared to the entire collection, the core genome of ST258 is considerably larger at 3.8 Mbp (Table 1) due to more genome content in common, emphasizing the clonality of this group. The refined SNP matrix was used in both maximum parsimony and Bayesian (BEAST) analyses (Figs 2B and 3). Resulting phylogenies showed comparable overall topologies with the monophyletic Clades 1 and 2 originally defined by DeLeo et al. [15] conserved, and also sharing a common ancestor of their own. The remaining isolates are paraphyletic with respect to Clades 1 and 2. Within the context of ST258’s genetic relationships with other K. pneumoniae, our data illustrate that ST258 isolates are of a single clonal lineage derived from a recent common ancestor.
Fig 3. Projecting the evolutionary history of ST258.
BEAST analysis based on 1,425 core genome SNPs in 101 ST258 isolates with NJST258_1 reference genome, with the 215 kb region of recombination [15] masked, gives temporal context to the emergence of ST258, with key events and initial reports of KPC-producing K. pneumoniae in different countries plotted. Blue font indicates reports of KPC-producing K. pneumoniae, brown font is ST258. Green shading on the phylogeny shows lines of iterations of Bayesian analyses. The mean mutation rate of K. pneumoniae ST258 is 1.03 x 10−6 (95% HPD 8.09 x 10−7 to 1.24 x 10−6). The TMRCA for the ST258 clade is approximately 20 years ago, around 1995. The plot inset is a root-to-tip analysis of SNP accumulations for each isolate since the MRCA of ST258. The slope of the fit line is 4.66, which is close to the mutation rate calculated by BEAST ((1.03 x 10−6 substitutions per site per year) x (3.8 Mbp core genome size) = 3.9 SNPs per year).
Emergence and evolution of ST258 with KPC
Bayesian analysis estimates the time to most recent common ancestor (TMRCA) of the global ST258 group as 17.2 years before our most recent isolate collected in 2012, or around 1995 (95% highest posterior density [HPD] 12.3 to 23.1 years, Fig 3), slightly different from the conclusions of Gaiarsa et al. [17], who calculated the year 1997. From this study, US-NJ-2003 is the earliest confirmed ST258 to date. Previous reports have linked ST258 to a KPC-producing hospital outbreak in New York City in April, 2000 [21], suggesting that ST258 emerged as clinically significant just 5 years after origination. Notably, the first KPC-producing isolate identified was a ST37 strain collected in 1996 in North Carolina [4], contemporaneous and proximal with our estimates of the first ST258 strains. The estimated time of the recombination event resulting in one of the alternate cps loci [15] is around 2001 to 2003. We observed a strong correlation in the KPC-producing ST258 between cps1-containing isolates with KPC-2 (95%), and cps2-containing isolates (all in Clade 1) with KPC-3 (97%, Fig 2), suggesting the KPC gene point mutation occurred in the common ancestor to Clade 1 between 2001 and 2003 as well. KPC-3-producing K. pneumoniae were first collected around this time [22], likely from ST258 strains [21], supporting this idea.
To enhance our collection of ST258 genomes, published sequence reads from 83 ST258 isolates mostly collected from the northeastern U.S. (NCBI SRA database study SRP036874, [15]) were added to the analysis with the masked NJST258_1 reference genome. A total of 2,282 SNPs were identified among all 186 ST258 isolates and were used in a maximum parsimony analysis and a second BEAST analysis that estimates TMRCA at 16.4 years before the 2012 isolate (95% HPD 12.8 to 20.6 years), both of which corroborate our previous estimates. We also incorporated the sequence data of 22 isolates from the NIH outbreak in 2011 [23] to illustrate the phylogeny of all 208 isolates (S1 Fig). The publicly available genomes fall interspersed with ours throughout the phylogeny, showing that isolates from the northeastern U.S. and Canada genetically reflect a global isolate collection, supporting a northeast U.S. origination or highlighting the region as a hub of global travel. Clade 1 includes 29 publicly available genomes and remains monophyletic with four SNPs common to all. Clade 2 no longer has SNPs in common with the Georgia isolates on a basal branch in Fig 2 (Clade 2 bottom branch, and boxed in S1 Fig), and these isolates were the only KPC-2 producers that fell in Clade 2. The NIH outbreak isolates clustered in their own tight clade, but monophyletic, having five SNPs in common, with the clade of Palermo, Italy, outbreak isolates (S1 Fig).
Our SNP phylogenies corroborate transmission of strains previously suggested to have epidemiologic linkages and highlight previously unrecognized transmission events. In addition, by juxtaposing variable genetic features alongside the SNP-based phylogeny, we obtain further insight into epidemiologic and genetic transmission events. For example, 13 isolates, most collected from patients with a recent history of travel or healthcare exposure in Greece, clustered together despite being collected from diverse locations including Australia, Denmark, Finland, Greece, and Italy (Fig 2, Clade 1) [24–26]. The small distance (average pairwise distance 21 SNPs) found among these isolates and shared genetic features indicate a common source. Also, an isolate collected in 2008 from Florence, Italy, unexpectedly clusters tightly with 27 isolates from a multi-institutional ST258 outbreak in Palermo, Italy. This isolate, Italy-2008, was the first ST258 identified in Italy, and was previously linked to Israel [27].
Mobile elements
The KPC gene, bla KPC, is carried on a highly mobile transposable element, Tn4401 [28] that is passed both vertically through bacterial clonal expansion and horizontally between unrelated strains. Tn4401 subtypes have different deletions upstream of bla KPC that confer different promoter regions to the gene [29]. Tn4401b is the full-length Tn4401 element; different deletion events result in conversion to a, c, d, and e subtypes. Sequential deletions could be responsible for subtype conversions; the most plausible based on deletion size are conversions from a or d to c or e, and c to e. However, selection may be against conversions from Tn4401a or d as strongest bla KPC expression occurs with the promoters present in these subtypes [29]. Vertical transfer of bla KPC within a limited period of time and on a local or regional level has been observed previously [30, 31]. Parsimony analysis of our data suggests that the vertical transfer of Tn4401 in ST258 has played a significant role in bla KPC dissemination (Fig 2B). The paraphyly that characterizes isolates outside of Clades 1 and 2 suggests that the ST258 MRCA carried the full length Tn4401b with bla KPC-3 and that deletion events in Tn4401 and point mutations in bla KPC occurred before various clades diverged. Occasionally, independent acquisition by horizontal transfer is evident where an isolate carries Tn4401b while the majority in its clade have another subtype, as is the case for US-GA-2009a (Fig 2B, Clade 1), which also differs in bla KPC type from its closest relatives, and possibly for US-IL-2009a (Fig 2B bottom, cluster containing Tn4401d). US-NY-2005a carries both a Tn4401a and Tn4401b. This isolate may still be carrying the ancestral b and acquired a, or may have acquired b in addition to its a, or this may be the result of a transposon duplication event. Independent Tn4401 acquisition in ST258 is indicative of persistent selective pressure for this dominant strain to harbor bla KPC or genes that may be acquired along with it (i.e. other antibiotic resistance genes carried on the same mobile element).
Conservation of plasmid incompatibility groups within ST258 may reflect the successful vertical transmission of particular plasmids. The predominant incompatibility groups in ST258 are FIBK (pKPN3, in 96% of isolates), ColRNA (in 95%), FII (in 86%), and FIIK (in 82%). The KPC-encoding plasmid pKpQIL described in ST258 outbreaks in Israel and Italy [32, 33] and pKpQIL-like plasmids in New Jersey and New York isolates [34] are multi-replicon plasmids of both incompatibility groups FIIK and FIB (pKpQIL). Within Clades 1 and 2, nine isolates do not have these plasmid types, one of which is US-GA-2009a that appears to have lost and reacquired Tn4401 (Fig 2B). Our phylogeny suggests that the MRCA to Clades 1 and 2 harbored a pKpQIL-like plasmid that was then lost as few as five times. The FIB type of pKpQIL plasmids is absent from most isolates outside of these clades. The plasmid type profiles of Clades 1 and 2 are very similar overall; most isolates have five of the seven types illustrated in Fig 2B. IncX appears to have been lost in the Italy-Palermo isolates (and also appears to have been lost in another clade in the paraphyletic group). Incompatibility types are diverse in the paraphyletic group of isolates. Plasmids, therefore, likely add considerably to the K. pneumoniae species pan-genome. The seven bla KPC-negative ST258 isolates may have lost a KPC-encoding plasmid, as has occurred before [35], however, no clear incompatibility group pattern correlates strongly with its loss. Three of them appear to carry a pKpQIL-like plasmid. Likewise, outside of ST258 in CG258, three Tn4401-negative isolates in ST437 also appear to carry a pKpQIL-like plasmid. And again, no clear incompatibility group pattern correlates strongly with Tn4401 carriage. These observations are not surprising as Tn4401 is associated with many different plasmid types owing to its high mobility [36–39].
An abundance of resistance genes are harbored by CG258, presumably on various plasmids (though indeterminate from these data, Fig 2). These genes encode mechanisms of resistance to quinolones (aac-Ib-cr or oqxAB identified in 100% of isolates), aminoglycosides (aac-Ib, aadA, or aph-1, in 99% of isolates), β-lactams (by β-lactamases encoded by bla KPC, bla CTX-M or bla OXA, in 96%), trimethoprim and sulfonamides (dfr or sul1 in 93% and 94%), and macrolides (mphA, in 81%), many of which were also identified, although less frequently, in unrelated K. pneumoniae (S2 Table). The antibiotic resistance profile these genes confer is highly similar in ST258 and the rest of CG258. At the gene level, ST258 differs from the rest of CG258 in bla KPC and bla CTX-M status, and in aac-Ib and aac-Ib-cr status. The high frequency of bla CTX-M in CG258-non-ST258 may be due to sampling bias, however these sequence types are known to often carry β-lactamase genes [9–11]. Interestingly, the quinolone resistance gene aac-Ib-cr, in many of the CG258-non-ST258 isolates but in only two ST258 isolates, is two nucleotides different from the aminoglycoside resistance gene aac-Ib, present in almost all ST258 and in only six of the rest of CG258 isolates. Both of the aac-Ib-cr-positive ST258s are aac-Ib-negative, and vice versa for the aac-Ib-positive CG258-non-ST258s, suggesting that these two genes are not independently acquired in the two groups, but the MRCA to all carried one and point mutations result in the other. If this is the case, the point mutations have happened in more than one lineage in both groups.
The frequent point mutations observed in CG258 in aac-Ib and aac-Ib-cr are interesting in that all CG258 isolates have the fluoroquinolone resistance-conferring mutations in GyrA (Ser83 to Ile) and ParC (Ser80 to Ile), the DNA gyrase and topoisomerase enzymes on which fluoroquinolones act, and almost all have another aminoglycoside resistance gene, aadA or aph-1. This resistance is important considering fluoroquinolones and aminoglycosides are drugs of choice for urinary tract infections (UTIs), the major pathology caused by ST258. Also, all CG258 isolates carry the genes for the OqxAB efflux pumps, generally conferring low-level resistance to fluoroquinolones. The apparent selection pressure for multiple mechanisms of similar resistance may be due to the slightly different phenotypes conferred by each.
We screened our isolate genome assemblies for several virulence genes recently described in the highly virulent capsule type K2 Kp52.145 isolate [40] to determine their potential contribution to pathogenic success. Within CG258, we found several instances of colibactin genes, which encode a genotoxin that induces host DNA damage, and conjugation machinery of type IV secretion systems (T4SS), which is not surprising given CG258’s plethora of plasmids. Of note, we found two isolates that carry genes similar to the newly described pld1 gene encoding a phospholipase D protein involved in lipid metabolism [40]. PLD1 was found to be prevalent in highly virulent K. pneumoniae isolates or those known to cause severe infections [40]. In our collection, ST39 (US-TX-2011) and ST719 (US-VA-2008b), but no CG258, carried genes similar to pld1.
Factors impacting extracellular interaction
Among the four SNPs that separate all ST258 from the rest of CG258, one is non-synonymous in a gene encoding a transcriptional regulator protein in the multiple antibiotic resistance repressor (MarR) family. Members of this family such as SlyA in Salmonella and MarR in E. coli and K. pneumoniae (different from this MarR family protein) have a helix-turn-helix motif and form homodimers that bind DNA at marboxes to block expression of genes (S2 Fig). MarR family proteins also bind stimulatory ligands thought to result in a conformational change averting its bond with DNA. In this way, MarR family proteins mediate metabolic responses to a cell’s environment. Mar regulons have many regulatory functions in many taxa, including multidrug efflux pump and outer membrane porin production, stress tolerance, toxin degradation, and many other virulence factors [41]. MarR is a repressor of the pleiotropic marRAB regulon. MarA is a gene expression activator that in E. coli is involved in regulation of over 60 genes [42, 43], and binds intrinsic copper released upon disruption of cellular membrane processes [44]. MarA is closely associated with and interacts with several other pleiotropic transcription regulators, including SoxS, Rob, and RamA, which all contribute to regulation of the AcrAB-TolC efflux pump genes implicated in fluoroquinolone and tigecycline resistance [42, 45–47]. Overexpression of RamA results in lipopolysaccharide modifications that alter the outer layer of the cell, decreasing its susceptibility to host-derived antimicrobial peptides as well as polymyxins, and increasing its evasion of phagocytosis by host macrophages [46]. The SNP in the marR-family gene in ST258 isolates, which appears to be 100% specific and 100% sensitive to ST258 by in silico validation, confers Ser34 to Phe amino acid change in the homodimerization region of the protein (S2 Fig). This substitution may affect the proteins’ ability to form the homodimer, which in turn would affect its ability to bind ligands or marboxes. It is conceivable that, considering the potential interconnection of this regulator with others, this amino acid substitution may result in significant metabolic changes in ST258. Indeed, this MarR family protein is very highly conserved among K. pneumoniae, signifying its functional importance. The only other amino acid changes we found in the species are in KP5-1 and 342, both plant-associated isolates, and interestingly, in all of CG258. The Arg4 to Ser mutation in CG258 occurs in a seemingly insignificant domain of the MarR family protein (S2 Fig); however this change may affect protein folding and therefore function. Additionally, isolates in CG258 share a synonymous nucleotide substitution in the gene (another rare occurrence), C408 to T, which appears to be 100% specific and 100% sensitive to CG258 by in silico validation. Although it appears that CG258 inherited this gene from a relative of ST1628 in the 1.3 Mbp recombination event [17], the ST1628 isolate does not share this marR-family gene SNP or the Arg4 to Ser amino acid change with CG258.
We capitalized on the specificity of these SNPs in the marR-family gene by developing assays to target them (Table 2). These assays could be used in real-time PCR or in amplicon sequencing to detect a CG258 and/or ST258 strain. We screened them as dual-probe real-time PCR assays across a subset of our collection, and found them to be 100% specific and 100% sensitive, correctly typing 48 CG258 and 24 ST258. The ST258 assay was also robust to K. pneumoniae, correctly typing 49 non-ST258 isolates comprising more than 20 different sequence types. The CG258 assay detected 13/15 non-CG258 isolates. The two it missed are the divergent US-PA-2001 and US-GA-2009b; these two isolates contain SNPs in the marR-family gene in the assay region.
Table 2. SNP mutations and real-time PCR assays to detect CG258 and ST258.
Lowercase letters in the probes indicate the targeted SNP state.
| Gene | SNP | SNP Specificity | Assay | Primer/Probe Sequence | Assay Sensitivity/ Specificity |
|---|---|---|---|---|---|
| marR-family | C101 to T | ST258 | ST258_F | ATGGTGGTGCGCCAGTG | To ST258: 100%/100% |
| ST258_R | GCTGACCGAGACGTTGTC | To non-ST258: 100%/100% | |||
| ST258-T_FAMBHQ+ | CATTATTGACTtCGCTATCA | ||||
| nonST258-C-TETBHQ+ | CCATTATTGACTcCGCTAT | ||||
| marR-family | C408 to T | CG258 | CG258_F | ACGGCAGGCGATTTGATTTAACG | To CG258: 100%/100% |
| CG258_R | AGCTGCGTGATCGAGACCTATC | To non-CG258: 87%/100% | |||
| CG258-A_FAMBHQ+ | CGCTGAAGGTaGCGAGAT | ||||
| nonCG258-G_TETBHQ+ | CTGAAGGTgGCGAGATC | ||||
| marR-family | A12 to C | CG258 | Not designed | ||
| oqxR | T389 to C | Most CG258 | Not designed |
A second transcription regulator that potentially shapes the CG258 phenotype is the repressor OqxR of the OqxAB efflux pump genes. The oqxAB locus, originally described on a plasmid in E. coli [48], is widely reported in K. pneumoniae [49]. Bialek et al. recently described a mutation in OqxR that results in overexpression of OqxAB, which contributed to antibiotic resistance in K. pneumoniae clinical isolates, and showed that various classes of antibiotics (fluoroquinolones, chloramphenicol, and β-lactams) are among the OqxAB pump substrates [50]. Zhong et al. also associate OqxAB with tigecycline resistance [47]. We found a mutation in OqxR in CG258, Val130 to Ala, due to SNP T389 to C that appears to be specific to our CG258. (ST11 HS11286, Genbank accession CP003200, does not have the oqxAB locus, however, so it does not appear to be 100% sensitive to CG258.) Veleba et al. found Val130 to Ala, but did not associate this mutation with increased or decreased repression citing confounding effects of other metabolic regulators; however, they mention this mutation is part of current experiments [51]. Italy-Palermo-2009g (ST258) also has a deletion of 13 bases that results in a premature stop codon, likely resulting in a defective repressor protein. Three other isolates outside CG258 also have deletions resulting in truncated proteins (two ST14 isolates and US-GA-2009b of novel sequence type), indicating that mutation of this repressor may be a common mechanism of increased antibiotic resistance in clinical isolates.
We characterized the cps locus and outer membrane protein (OMP) profiles of our collection, two complex systems that encode multiple proteins in direct contact with the extracellular environment and potential antigenic targets for the host immune system. K. pneumoniae capsules play an important role in virulence, and CPS modification has been described in other species to allow evasion of host immune detection [52, 53]. We found a remarkable degree of cps diversity in our collection, with over 35 different variants falling into over 25 different K-types (Fig 1). CG258 alone contains 18 different variants, of which only seven have been characterized [15, 18], and several have no exact match in the wzc and wzi gene sequence databases. Recombination in the cps region is apparent throughout the phylogeny where K-types (by wzc and wzi sequence) match between distantly related isolates (Fig 1). In one case, three distantly related isolates, Brazil-2010e (ST11), US-WA-2010 (ST147), and US-TX-2001 (novel ST), have the same full length cps sequence highly similar to Genbank accession number KR007672 from another ST11 isolate [18]. Interestingly, characterization of the full cps loci showed that all three of our ST37 isolates are characterized by different cps loci, and each is shared with CG258 strains; two are shared among isolates in this study (Fig 1), and one cps locus was characterized in a ST11 isolate previously [18]. In more than one case, we observed K-type matches by wzc or wzi sequence, but sequence divergence in other regions of the cps locus. Two distantly related isolates share a wzc sequence but not wzi (Fig 1), and in two cases wzc and wzi sequences matched between isolates but the full locus did not match.
The majority of our ST258 maintained either cps1 or cps2. Our genomic data second the suggestion by DeLeo and colleagues [15] that a ST258 lineage recombined with DNA from a ST42 strain and acquired the cps1 locus primarily found in ST258 Clade 1. The ST42 isolate in our collection was collected from a Brooklyn hospital in 2004, and the ST42 isolates described in the DeLeo study were collected from New York City hospitals in 2001 to 2002. Our Bayesian analysis calls the cps1 clade monophyletic; the recombination event that introduced cps1 in ST258 likely occurred once in a common ancestor to the clade around 2002 (Fig 3), possibly in the New York City area. However, our observation of the strong correlation between cps1-containing ST258 with KPC-2, and cps2-containing ST258 with KPC-3 (Fig 2), taken in the context of the entire ST258 phylogeny, leads us to hypothesize the bla KPC point mutation occurred around the time of the cps recombination, rather than from independent acquisition of Tn4401, as DeLeo and colleagues suggest [15]. Within Clade 1, our analysis also identified a third ST258 cps locus in US-FL-2011. This locus is identical to part of the capsule type K23 isolate, 2812/50 (GenBank accession no. AB742229), but is disrupted by an IS5-like element in its 5’ end, is missing galF and orf2, and in part resembles HS11286 (Fig 4). US-FL-2011 was collected as part of a hospital outbreak investigation, suggesting these additional cps modifications do not impact ST258 success.
Fig 4. Characterization of three cps loci found in ST258 isolates.
Regions of identity are shaded and GenBank BLAST matches labeled. Putative glycosyltransferases are in green and hypothetical proteins are in blue. The IS5-like element disrupting the 5’ region of the US-FL-2011 cps locus is in red and yellow.
K. pneumoniae outer membrane proteins not only provide structure to the membrane and allow transport of iron, nutrients, and antimicrobial agents via their pores, but also contain extracellular loops that affect surface adhesion and invasion, biofilm formation, and host immune detection [54–56]. We examined sequence of the major porin proteins KpOmpA, OmpK26, OmpK35, OmpK36, and OmpK37 to explore differences in our collection (Fig 1). KpOmpA demonstrated little variation regardless of sequence type; 95% matched GenBank accession WP_002898408. Only five other variants comprised the rest. KpOmpA interacts with plasmid conjugation machinery; its presence increases frequency of conjugation [56]. KpOmpA can also form two different conformations, resulting in two different membrane pore sizes, offering a form of variation in the protein [56]. OmpK26 was also conserved among isolates; 86% matched GenBank accession WP_002916050, and only five other variants comprised the rest. OmpK26 is indispensable to a cell when OmpK35 and OmpK36 proteins are deficient [57].
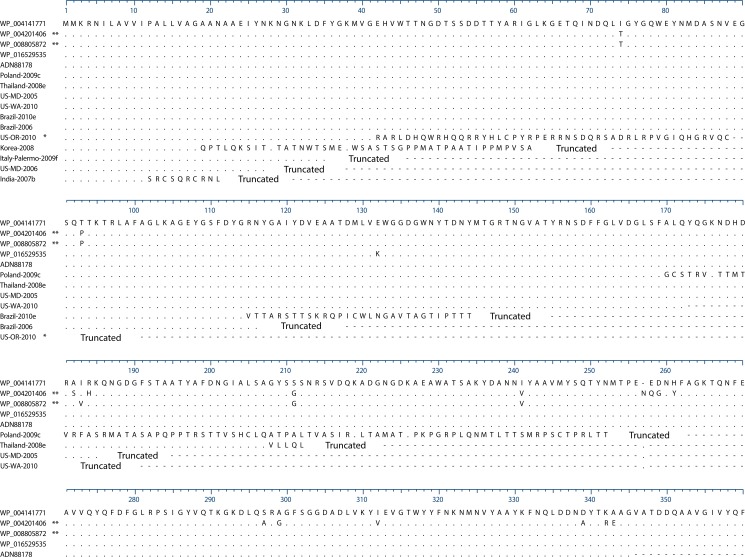
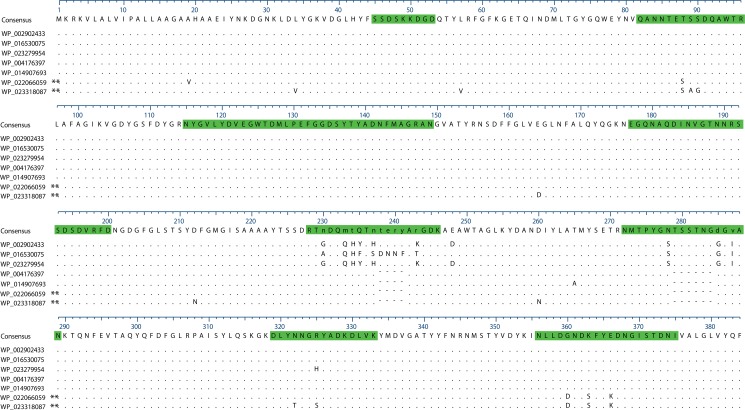
All ST258 isolates in our collection shared an OmpK35 sequence containing a frame-shift that results in a premature stop codon and truncated protein. Although this mutation has been reported previously [58], we found it exclusive to our ST258 group (Fig 1). The resulting outer membrane porin loss increases β-lactam resistance [59] and in combination with a β-lactamase results in high levels of β-lactam resistance [60]. Several other isolates harbored truncated OmpK35 proteins (Figs 1 and 5), likely owing to OmpK35’s allowance of carbapenem antibiotics across the cell membrane, and all harbored a β-lactamase gene, bla KPC (n = 5 outside ST258), bla CTX-M (n = 4), or bla VIM (n = 1). OmpK36 displayed the most diversity (Fig 1, S2 Table); 40 different variants were found, with amino acid variations concentrated in the extracellular loop regions of the protein (Fig 6), presumably diversifying K. pneumoniae’s interactions with the environment and potentially influencing host immune response and adherence of the cells to host surfaces [55]. Most CG258 shared a similar OmpK36 matching GenBank accession WP_002913005 (76% of ST258 and 76% of CG258), with the remainder sharing 12 variants (Fig 6). In the 155 isolates for which a complete OmpK36 protein was characterized, the average pairwise distance is 3 amino acids. Each unique variant differs by an average of 13 amino acids. Seven isolates have a premature stop codon and putatively non-functioning protein, five of which are ST258. In one isolate, an IS4-family insertion disrupts the 5’ end of the gene. These five mutations do not appear to have clonally spread, as each is unique and these isolates do not fall in the same clades. This may reflect selection against OmpK36 truncation; indeed previous reports associate OmpK36 loss with increased susceptibility to phagocytosis [55]. The OmpK37 sequences of 100% of CG258 isolates match Genbank accession WP_002902433, whose amino acid sequence shows extensions in extracellular loop regions (Fig 7). Outside CG258, 26% of isolates carried this protein, and 66% match Genbank accession WP_004176397 or WP_014907693, which have shorter extracellular loops in regions L5 and L6. The two divergent isolates not shown in Fig 1 both had unique protein sequences for all five proteins. The combination of the extended loop regions in OmpK37 and the absence of a functional OmpK35 are not unique to the ST258 group in our study, however only nine isolates outside ST258 have this profile. The combination of this profile with other characteristic features in ST258 likely impacts extracellular interaction with the environment.
Fig 5. OmpK35 alignment of all alleles found in the 167 isolates.
Sequences are labeled by Genbank accession number when they’re an exact match. WP_004141771 was the most frequently found complete protein in our isolates, and was used as the reference in the alignment. Dots are conserved sites, dashes are sites downstream of a premature stop codon. * US-OR-2010 represents all ST258 isolates in the study. ** These variants are in the divergent isolates US-PA-2001 and US-GA-2009b, not shown in Fig 1.
Fig 6. OmpK36 alignment of all alleles found in the 167 isolates.
100% identity BLAST matches were not found for several sequences; sample names are used for these sequences. WP_002913005 was the most frequently found protein so was used as the reference. Sequence in green represents the extracellular loop regions of the protein. Dots are conserved sites, dashes are gaps or represent sites downstream of a premature stop codon. * This variant is not shown in Fig 1; it occurs in ST258 isolate US-GA-2007. ** These variants are in the divergent isolates US-PA-2001 and US-GA-2009b, not shown in Fig 1.
Fig 7. OmpK37 alignment of all alleles found in the 167 isolates.
Sequence in green represents the extracellular loop regions of the protein assumed from the structure of OmpF by Doménech-Sánchez et al. [59]. Dots are conserved sites, dashes are gaps. ** These variants are in the divergent isolates US-PA-2001 and US-GA-2009b, not shown in Fig 1.
Discussion
Perfect examples of large homologous recombination events between unrelated strains resulting in new, more successful pathogens [61] are the evolutionary events that generated CG258 [17] and the ST258 strains [20], both results of single events that encompassed approximately 20% of the K. pneumoniae genome. Whether these events are the secrets to CG258 and ST258’s success is unclear. Over the past decade, CG258 strains carrying carbapenemase genes, especially KPC-producing K. pneumoniae ST258, have become some of the most successful multidrug-resistant bacterial pathogens in healthcare settings throughout the world. Reports describe ST258’s ability to overtake a previously established carbapenemase-producing K. pneumoniae strain within an institution in Greece [62], and to rapidly disseminate throughout a country and surpass a pre-existing KPC-producing K. pneumoniae population in Israel [13, 21]. As other KPC-producing K. pneumoniae preceded ST258, this suggests that KPC alone is not the driver of ST258’s success, and that ST258 has other evolutionary advantages.
The combination of our parsimony analysis and the coincident emergence of ST258 and KPC around 1995–1996 leads us to propose that ST258’s common ancestor acquired KPC-encoding Tn4401 prior to dissemination. KPC-producing ST258 probably originated in the northeastern U.S., clinically emerging in hospital outbreaks as early as 2000. The subsequent success of ST258 has played a large role in the global dissemination of KPC through vertical transmission. We noted two instances where it appears ST258 replaced its Tn4401 element through horizontal transmission. Why ST258 is closely linked to Tn4401 is unknown. Given that ST258 is a healthcare-associated pathogen, a likely contributor to its selection is the heavy use of carbapenem antibiotics. In the late 1980s and early 1990s, clinicians relied on carbapenems as a last resort to battle the increasing number of Enterobacteriaceae producing extended-spectrum beta-lactamases (ESBLs). While carbapenem use continued during the time of ST258’s origination, a recent study by the Veteran’s Health Administration noted 102% increase in carbapenem use in their acute care facilities between 2005 and 2009 [63]. This increase was also noted in other U.S. hospitals, and mirrors the rapid expansion of ST258. The use of other antibiotics within the healthcare setting, particularly fluoroquinolones and aminoglycosides used to treat urinary tract infections (UTIs), could also act as a positive selective force, considering that ST258 causes UTIs and typically carries resistance mechanisms to several other classes of antibiotics.
The global spread pKpQIL-like plasmids, responsible for much of ST258’s Tn4401 carriage [32–34] demonstrates the tenacity of particular plasmids. This plasmid analysis suggests the most recent common ancestor (MRCA) of ST258 Clades 1 and 2 carried a pKpQIL-like plasmid and it is highly persistent. It is impossible to determine whether pKpQIL-like plasmids date back further than the MRCA of Clades 1 and 2 from our data. It may have been in older ancestors and lost, or acquired multiple times. The vertical fidelity those plasmid types show in Clades 1 and 2 suggests it is not easily lost.
The antibiotic resistance gene patterns of CG258 do not illuminate a particular profile responsible for ST258 clonal success. CG258 isolates are similar, with the exception of the SNP variant genes’ aac-Ib and aac-Ib-cr mutual exclusivity. CG258 non-ST258 isolates have aac-Ib-cr, conferring fluoroquinolone resistance, while most ST258 have aac-Ib, conferring aminoglycoside resistance. Both groups carry at least one other aminoglycoside resistance gene and have the fluoroquinolone resistance mutations in gyrA and parC. Both aminoglycosides and fluoroquinolones are a highly used drug for UTIs, a common pathology of CG258 strains, and these multiple mechanisms for resistance to the same classes of antibiotics may offer higher resistance levels or resistance to different drugs within the same classes. Additionally, the amino acid change in the OqxAB repressor protein OqxR found in this study specific to CG258 isolates (Val130 to Ala) could suppress its repressor functions, resulting in overexpression of the OqxAB efflux system and high-level fluoroquinolone resistance. A similar mutation, Val102 to Gly, was responsible for a multidrug resistance phenotype in K. pneumoniae clinical isolates [50]. The deletion mutations that result in truncated OqxR proteins in three other clinical isolates studied here suggest a non-functional OqxR is not lethal and may offer a fitness advantage in certain circumstances. Further experiments are needed to test these hypotheses.
Two other potentially important mutations documented in this study, one in CG258 and the other specific to ST258, occur in a MarR family transcription repressor protein. MarR family proteins have been described in many species as responders to environmental stimuli such as host immune factors, toxins, antibiotics, and stress factors [41]. All CG258 isolates share a SNP in the marR-family gene resulting in amino acid change Arg4 to Ser. Although this change occurs outside of functionally characterized domains of the protein, it may affect protein structure and therefore function. The mutation specific to ST258 results from one of the four point mutations in the core genome separating ST258 from the rest of CG258. The resulting amino acid change, Ser34 to Phe, occurs in the homodimerization region of the protein. Given that this protein is highly conserved in the K. pneumoniae species, these amino acid changes may be significant. The Ser34 to Phe mutation may affect the ability to form a complete functional protein, bind stimuli ligands, or bind DNA to repress transcription. It is conceivable that suppression of the MarR family protein results in overexpression of systems that give ST258 a fitness advantage in particular environments. Further experiments to test this are underway. The SNP in ST258 that confers the amino acid change is now the target of a sensitive and specific assay to detect ST258. Additionally, another SNP in the marR-family gene encompassing all CG258 is the target of a second assay to detect CG258. SNPs are stable mutations, especially in highly conserved genes, and can be detected using a variety of molecular methods. Here we’ve shown that real-time PCR can be used for rapid detection and typing of K. pneumoniae. Both assays show 100% sensitivity and specificity, so are ideal for easy, cost-effective surveillance for ST258 and CG258.
Capsule modification allows adaptation to changing environments [53], and the variety of cps genotypes in our collection indicates that the capsule locus is highly mobile. IS elements reside within the cps region of some strains, potentiating the formation of new capsule types [18]. CG258 has at least 23 different capsule types, 11 uncharacterized, and ST258 at least three; one of which, firstly described in this study, lacks two highly conserved cps genes apparently deleted by integration of an IS element. Despite this disparity, the success of the strain does not appear to be affected. Some isolates had inconsistent genotypes in the capsule genes wzc and wzi: two shared a wzc but had different wzi genotypes, and some had identical genotypes but clearly different capsule types. These data, considered with the discovery of IS elements in several cps loci [18], should factor into interpretations of capsule typing by wzc and wzi sequencing. Several different capsule types characterize successful K. pneumoniae clinical pathogens, and as more isolates are sequenced, more and more types will undoubtedly be found. The limited number of capsule types characterizing ST258 make the capsule a good target for a vaccine, however surveillance will be critical to detect any future recombination events.
The functional repertoire of outer membrane proteins in K. pneumoniae is vast and complex, but undoubtedly includes functions critical to environmental adaptation. Depending on the allele, OmpA may cause more or less invasive capacity, immune evasion, adherence to particular cells or surfaces, and can affect frequency of plasmid conjugal receipt from donors [56]. The conservation in OmpA sequence in our collection may reflect selection against mutation. Likewise, OmpK26 was conserved despite the isolate diversity, suggesting selection against mutation. OmpK26 compensates for dual OmpK35 and OmpK36 loss in clinical isolates [57], and may play a role in compensating for OmpK35 loss alone, which we found is common in this collection of isolates. Conversely, we found 40 different OmpK36 sequences in our isolates, with amino acid variation concentrated in the extracellular loop regions. Selection for variation in OmpK36 may slow host recognition, or allow colonization of new tissues, as these are functions in the OmpK36 repertoire [55]. Outer membrane protein analysis revealed a profile in all ST258 that includes a truncated OmpK35, which would be expected to have deleterious effects on fitness, but may provide a degree of positive selection in a host environment where OmpK35 is not typically expressed [64]. In our analyses, protein truncation was much more frequent in OmpK35 than in other outer membrane proteins. The OmpK37 sequence found in our ST258 and CG258 isolates contains insertions in the extracellular loop regions, which again may impact interaction with its environment. ST258’s OMP profile, including OmpK35 loss and OmpK37 extended loops, could contribute to its enhanced ability to persist in a host or healthcare environment.
Our data underscore the usefulness of whole genome sequencing in epidemiology, evolutionary history, and specific genetic attributes of pathogens. The genomic analyses of KPC-producing K. pneumoniae that we present in this study provide further insight into the evolution and rapid spread of the globally dominant strain, ST258. We show that in addition to the large recombination events that gave rise to CG258 and ST258 [17, 20], key point mutations may also play a significant role in the evolution of these strains. Based on these SNPs, the limited number of cps variations and the OMP profile that is conserved within ST258, this work also provides information important to surveillance and to development of a vaccine to specifically target ST258 and contain the KPC-producing K. pneumoniae pandemic.
Materials and Methods
Strain collection
This study’s K. pneumoniae isolated in the United States (n = 72) were selected from the CDC’s collection, which primarily comprises isolates submitted for reference testing or as part of an outbreak investigation in which the CDC was involved. Selection criteria were based on PFGE profiles, MLST sequence types when available, geography, year of isolation, and KPC status. U.S. isolates were selected with a focus on ST258, followed by other CG258 and non-CG258 isolates. Isolates from other countries (n = 95) were generously donated upon a request to various countries with recent reports of KPC-producing ST258 or CG258 strains (see Acknowledgments). S1 and S2 Tables describe the isolate collection.
Sequencing, MLST, and SNP detection
Genome libraries were prepared with a 500 base pair insert size using a KAPA Library Preparation Kit with Standard PCR Library Amplification (Kapa Biosystems, Wilmington, MA) and sequenced on a 101 bp read, paired-end Illumina GAIIx run. SRST2 [65] was used to determine multilocus sequence types. NASP, a pipeline developed by TGen North (https://github.com/TGenNorth/NASP), was used to detect SNPs. In brief, reads were aligned to the finished K. pneumoniae genomes MGH 78578 (GenBank accession no. CP000647) or the ST258 reference genome NJST258_1 (GenBank CP006923) using Novoalign (Novocraft.com) and SNPs called with GATK [66]. Data filtered out included SNP loci with less than 10X coverage or with less than 90% consensus in any one sample, regions duplicated in the reference genome as identified by Nucmer, and SNP loci that were not present in all genomes in the dataset. The results were output in a SNP matrix from a core genome common to all isolates in the analysis. Core genome size is expressed as the size of the reference genome (or percentage of the total reference genome size) excluding repeated regions and covered by reads at 10X or higher depth by all samples, or the length of the DNA that all samples in a given set have in common after filtering based on the above criteria. Read data were deposited in the NCBI SRA database under BioProject ID PRJNA252957.
Phylogenetic analysis
Phylogenetic trees were generated from the SNP matrices using the maximum parsimony method with 1000 bootstraps in MEGA 5.2 [67] [68]and subsequently plotted by means of ITOL v2 [69]. The genome of a K. oxytoca isolate (GenBank accession no. CP003218) was used as the outgroup to root an initial K. pneumoniae tree. The isolates with the basal-most branch, or the isolates with the branch closest to the outgroup, was used as the outgroup to root the following tree without K. oxytoca. All subsequent trees to analyze a progressively smaller number of isolates used the isolates with the basal-most branch from the previous tree as the root.
Bayesian evolutionary analysis was performed in BEAST v1.7.4 [70] using the SNP matrix generated by NASP to compute evolutionary rates and divergence times using the GTR model of nucleotide substitution and an uncorrelated lognormal relaxed clock. A tree prior of exponential growth was used along with a random starting tree and an exponential growth rate set to random walk. Isolates were dated based on the year of isolation and were run with 50 million generations and a burn-in phase of 5 million. Three independent Markov Chain Monte Carlo analyses were completed and combined in order for all parameters’ effective sample size values to be larger than 500.
Targeted genome analysis
Plasmid incompatibility groups were detected in silico by uploading read data to PlasmidFinder [71]. Known horizontally transferred antibiotic resistance genes were detected with SRST2 [65, 72]. Selected genes were also aligned to a reference gene with SeqMan NGen (DNASTAR, Madison, WI) to confirm their presence and type in read data. Reads were assembled using SPAdes Genome Assembler [73] after trimming Illumina adaptors with Trimmomatic [74]. Porin sequences were analyzed using SSTAR (https://github.com/tomdeman-bio/Sequence-Search-Tool-for-Antimicrobial-Resistance-SSTAR-) and Geneious [75], and cps loci and Tn4401 were characterized using Geneious [75] and SeqMan NGen (DNASTAR, Madison, WI). Capsule types were assigned using the wzc and wzi sequence databases in BIGSdb ([76, 77], http://bigsdb.web.pasteur.fr/klebsiella/).
SNP assays
Real-time PCR assays targeting the SNPs specific to ST258 and CG258 were designed with Biosearch Technologies’ RealTimeDesign software (Biosearch Technologies, Petaluma, CA). Assays were run in 10uL reactions on the 7900HT instrument (Life Technologies, Carlsbad, CA) with 1X PerfeCTa qPCR FastMix II (Quanta Biosciences, Gaithersburg, MD), 600 nM forward and reverse primers, 200 nM each probe, and 1 μL DNA template (approximately 0.5ng). Thermal conditions included denaturation for 4 min at 95°C followed by 40 cycles of 15 s at 95°C and 1 min at 60°C.
Supporting Information
101 isolates are from this study, 83 are from DeLeo et al. [15] and were retrieved from the SRA database of NCBI (Study no. SRP036874,), 22 are from the outbreak at the National Institutes of Health described by Snitkin et al. [23] and were retrieved as assemblies from Genbank. The US-GA isolates that were in Clade 2 previously but fall outside Clade 2 in this phylogeny are in the dotted box. Consistency index = 0.97.
(PDF)
Figure recreated from Wilkinson and Grove [78], with the addition of the MarR family amino acid sequence described in this study (bottom sequence). The amino acid substitution specific to ST258 in the α1 region is boxed. Light and dark shading indicates >70% similarity or >70% identity at that position respectively. α = alpha helices, β = beta turns, W = wing. The helix-turn-helix motif corresponds to helices α3 and α4, and helices α1, α5, and α6 form the dimerization domain [78].
(PDF)
(DOCX)
(XLSX)
(XLSX)
Acknowledgments
We greatly thank the following researchers for the generous contribution of global K. pneumoniae isolates for this study: M.A. Miller and S. Lévesque, Laboratoire de santé publique du Québec, Canada; A. Mammerum and F. Hansen, Statens Serum Institut, Copenhagen, Denmark; P. Giakkoupi and A.C. Vatopoulos, Hellenic Republic Ministry of Health and Welfare, Athens, Greece; P. Huntington, PaLMS Royal North Shore Hospital, St. Leonards, Australia; R. Cantón and T.M. Coque, Hospital Ramón y Cajal, Madrid, Spain; M. Gniadkowski and R. Izdebski, National Medicines Institute, Warsaw, Poland; Y. Carmeli, Division of Epidemiology, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel; C. Mammina, Dept. of Sciences for Health Promotion “G. D’Alessandro,” Palermo, Italy; G.M. Rossolini and T. Giani, Università degli Studi di Siena, Siena, Italy; Asia Pacific Foundation for Infectious Diseases, Asian Bacterial Bank, Seoul, Korea; M. Österblad, National Institute for Health and Welfare, Turku, Finland; A. Correa Bermúdez and M. Fernanda Mojica, Bacterial Resistance Group, CIDEIM, Cali, Colombia; M. Dutra Asensi, Instituto Oswaldo Cruz-Fiocruz, Rio de Janeiro, Brazil; J. Yan and Y. Yu, Sir Run Run Shaw Hospital, Zhejiang University, China. We also extend our appreciation to A. Carattoli and H. Hasman for generously opening up the PlasmidFinder database for our use.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Data Availability
All sequence data files are available from the NCBI SRA database BioProject ID PRJNA252957.
Funding Statement
This work was supported by award number R01AI090782 from the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2013 Centers for Disease Control and Prevention, 2013. [Google Scholar]
- 2. Jacob JT, Klein E, Laxminarayan R, Beldavs Z, Lynfield R, Kallen AJ, et al. Vital Signs: Carbapenem-Resistant Enterobacteriaceae. MMWR Morb Mortal Wkly Rep. 2013;62. [PMC free article] [PubMed] [Google Scholar]
- 3. Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infection control and hospital epidemiology: the official journal of the Society of Hospital Epidemiologists of America. 2008;29(12):1099–106. 10.1086/592412 . [DOI] [PubMed] [Google Scholar]
- 4. Yigit H, Queenan AM, Anderson GJ, Domenech-Sanchez A, Biddle JW, Steward CD, et al. Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother. 2001;45(4):1151–61. Epub 2001/03/21. 10.1128/AAC.45.4.1151-1161.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brink AJ, Coetzee J, Clay CG, Sithole S, Richards GA, Poirel L, et al. Emergence of New Delhi metallo-beta-lactamase (NDM-1) and Klebsiella pneumoniae carbapenemase (KPC-2) in South Africa. J Clin Microbiol. 2012;50(2):525–7. 10.1128/JCM.05956-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Munoz-Price LS, Poirel L, Bonomo RA, Schwaber MJ, Daikos GL, Cormican M, et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. The Lancet infectious diseases. 2013;13(9):785–96. 10.1016/S1473-3099(13)70190-7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen LF, Anderson DJ, Paterson DL. Overview of the epidemiology and the threat of Klebsiella pneumoniae carbapenemases (KPC) resistance. Infection and drug resistance. 2012;5:133–41. 10.2147/IDR.S26613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bialek-Davenet S, Criscuolo A, Ailloud F, Passet V, Jones L, Delannoy-Vieillard AS, et al. Genomic definition of hypervirulent and multidrug-resistant Klebsiella pneumoniae clonal groups. Emerg Infect Dis. 2014;20(11):1812–20. 10.3201/eid2011.140206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Voulgari E, Gartzonika C, Vrioni G, Politi L, Priavali E, Levidiotou-Stefanou S, et al. The Balkan region: NDM-1-producing Klebsiella pneumoniae ST11 clonal strain causing outbreaks in Greece. J Antimicrob Chemother. 2014. 10.1093/jac/dku105 . [DOI] [PubMed] [Google Scholar]
- 10. Pena I, Picazo JJ, Rodriguez-Avial C, Rodriguez-Avial I. Carbapenemase-producing Enterobacteriaceae in a tertiary hospital in Madrid, Spain: high percentage of colistin resistance among VIM-1-producing Klebsiella pneumoniae ST11 isolates. Int J Antimicrob Agents. 2014. 10.1016/j.ijantimicag.2014.01.021 . [DOI] [PubMed] [Google Scholar]
- 11. Lascols C, Peirano G, Hackel M, Laupland KB, Pitout JD. Surveillance and molecular epidemiology of Klebsiella pneumoniae isolates that produce carbapenemases: first report of OXA-48-like enzymes in North America. Antimicrob Agents Chemother. 2013;57(1):130–6. 10.1128/AAC.01686-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Woodford N, Turton JF, Livermore DM. Multiresistant Gram-negative bacteria: the role of high-risk clones in the dissemination of antibiotic resistance. FEMS microbiology reviews. 2011;35(5):736–55. 10.1111/j.1574-6976.2011.00268.x . [DOI] [PubMed] [Google Scholar]
- 13. Grundmann H, Livermore DM, Giske CG, Canton R, Rossolini GM, Campos J, et al. Carbapenem-non-susceptible Enterobacteriaceae in Europe: conclusions from a meeting of national experts. Euro surveillance: bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2010;15(46). . [DOI] [PubMed] [Google Scholar]
- 14. Chmelnitsky I, Shklyar M, Hermesh O, Navon-Venezia S, Edgar R, Carmeli Y. Unique genes identified in the epidemic extremely drug-resistant KPC-producing Klebsiella pneumoniae sequence type 258. J Antimicrob Chemother. 2013;68(1):74–83. Epub 2012/10/09. 10.1093/jac/dks370 . [DOI] [PubMed] [Google Scholar]
- 15. Deleo FR, Chen L, Porcella SF, Martens CA, Kobayashi SD, Porter AR, et al. Molecular dissection of the evolution of carbapenem-resistant multilocus sequence type 258 Klebsiella pneumoniae. Proc Natl Acad Sci U S A. 2014. Epub 2014/03/19. 10.1073/pnas.1321364111 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Adler A, Khabra E, Chmelnitsky I, Giakkoupi P, Vatopoulos A, Mathers AJ, et al. Development and validation of a multiplex PCR assay for identification of the epidemic ST-258/512 KPC-producing Klebsiella pneumoniae clone. Diagn Microbiol Infect Dis. 2014;78(1):12–5. 10.1016/j.diagmicrobio.2013.10.003 . [DOI] [PubMed] [Google Scholar]
- 17. Gaiarsa S, Comandatore F, Gaibani P, Corbella M, Dalla Valle C, Epis S, et al. Genomic epidemiology of Klebsiella pneumoniae: the Italian scenario, and novel insights into the origin and global evolution of resistance to carbapenem antibiotics. Antimicrob Agents Chemother. 2014. 10.1128/AAC.04224-14 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wyres KL, Gorrie C, Edwards DJ, Wertheim HF, Hsu LY, Van Kinh N, et al. Extensive capsule locus variation and large-scale genomic recombination within the Klebsiella pneumoniae clonal group 258. Genome Biol Evol. 2015. 10.1093/gbe/evv062 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Croucher NJ, Harris SR, Grad YH, Hanage WP. Bacterial genomes in epidemiology—present and future. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2013;368(1614):20120202 10.1098/rstb.2012.0202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen L, Mathema B, Pitout JD, DeLeo FR, Kreiswirth BN. Epidemic Klebsiella pneumoniae ST258 Is a Hybrid Strain. MBio. 2014;5(3). 10.1128/mBio.01355-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Navon-Venezia S, Leavitt A, Schwaber MJ, Rasheed JK, Srinivasan A, Patel JB, et al. First report on a hyperepidemic clone of KPC-3-producing Klebsiella pneumoniae in Israel genetically related to a strain causing outbreaks in the United States. Antimicrob Agents Chemother. 2009;53(2):818–20. Epub 2008/11/26. 10.1128/AAC.00987-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Woodford N, Tierno PM Jr., Young K, Tysall L, Palepou MF, Ward E, et al. Outbreak of Klebsiella pneumoniae producing a new carbapenem-hydrolyzing class A beta-lactamase, KPC-3, in a New York Medical Center. Antimicrob Agents Chemother. 2004;48(12):4793–9. Epub 2004/11/25. doi: 48/12/4793 [pii] 10.1128/AAC.48.12.4793-4799.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Snitkin ES, Zelazny AM, Thomas PJ, Stock F, Group NCSP, Henderson DK, et al. Tracking a hospital outbreak of carbapenem-resistant Klebsiella pneumoniae with whole-genome sequencing. Science translational medicine. 2012;4(148):148ra16 10.1126/scitranslmed.3004129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huntington P, Coatsworth N, Hardiman R, Hudson B, Kotsiou G, Fernandes C, editors. Klebsiella pneumoniae carbapenemase in Australia: detection of a KPC-producing clinical isolate at a Sydney hospital. The Australian Society for Microbiology 2011 Annual Conference; 2011; Hobart, Tasmania, Australia: The Australian Society for Microbiology 2011 Annual Conference.
- 25. Osterblad M, Kirveskari J, Koskela S, Tissari P, Vuorenoja K, Hakanen AJ, et al. First isolations of KPC-2-carrying ST258 Klebsiella pneumoniae strains in Finland, June and August 2009. Euro surveillance: bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2009;14(40). . [PubMed] [Google Scholar]
- 26. Hammerum AM, Hansen F, Lester CH, Jensen KT, Hansen DS, Dessau RB. Detection of the first two Klebsiella pneumoniae isolates with sequence type 258 producing KPC-2 carbapenemase in Denmark. Int J Antimicrob Agents. 2010;35(6):610–2. 10.1016/j.ijantimicag.2010.01.024 . [DOI] [PubMed] [Google Scholar]
- 27. Giani T, D'Andrea MM, Pecile P, Borgianni L, Nicoletti P, Tonelli F, et al. Emergence in Italy of Klebsiella pneumoniae sequence type 258 producing KPC-3 Carbapenemase. J Clin Microbiol. 2009;47(11):3793–4. 10.1128/JCM.01773-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cuzon G, Naas T, Nordmann P. Functional characterization of Tn4401, a Tn3-based transposon involved in blaKPC gene mobilization. Antimicrob Agents Chemother. 2011;55(11):5370–3. Epub 2011/08/17. doi: AAC.05202-11 [pii] 10.1128/AAC.05202-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Naas T, Cuzon G, Truong HV, Nordmann P. Role of ISKpn7 and deletions in blaKPC gene expression. Antimicrob Agents Chemother. 2012. Epub 2012/06/27. doi: AAC.00334-12 [pii] 10.1128/AAC.00334-12 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Endimiani A, Hujer AM, Perez F, Bethel CR, Hujer KM, Kroeger J, et al. Characterization of blaKPC-containing Klebsiella pneumoniae isolates detected in different institutions in the Eastern USA. J Antimicrob Chemother. 2009;63(3):427–37. Epub 2009/01/22. 10.1093/jac/dkn547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gomez SA, Pasteran FG, Faccone D, Tijet N, Rapoport M, Lucero C, et al. Clonal dissemination of Klebsiella pneumoniae ST258 harbouring KPC-2 in Argentina. Clin Microbiol Infect. 2011;17(10):1520–4. 10.1111/j.1469-0691.2011.03600.x . [DOI] [PubMed] [Google Scholar]
- 32. Garcia-Fernandez A, Villa L, Carta C, Venditti C, Giordano A, Venditti M, et al. Klebsiella pneumoniae ST258 producing KPC-3 identified in Italy carries novel plasmids and OmpK36/OmpK35 porin variants. Antimicrob Agents Chemother. 2012. Epub 2012/01/19. doi: AAC.05308-11 [pii] 10.1128/AAC.05308-11 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leavitt A, Chmelnitsky I, Carmeli Y, Navon-Venezia S. Complete nucleotide sequence of KPC-3-encoding plasmid pKpQIL in the epidemic Klebsiella pneumoniae sequence type 258. Antimicrob Agents Chemother. 2010;54(10):4493–6. Epub 2010/08/11. doi: AAC.00175-10 [pii] 10.1128/AAC.00175-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen L, Chavda KD, Melano RG, Jacobs MR, Koll B, Hong T, et al. Comparative Genomic Analysis of KPC-Encoding pKpQIL-Like Plasmids and Their Distribution in New Jersey and New York Hospitals. Antimicrob Agents Chemother. 2014;58(5):2871–7. 10.1128/AAC.00120-14 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Adler A, Paikin S, Sterlin Y, Glick J, Edgar R, Aronov R, et al. A Swordless Knight: the epidemiology and molecular characteristics of the blaKPC-negative sequence-type 258 Klebsiella pneumoniae clone. J Clin Microbiol. 2012. Epub 2012/07/21. doi: JCM.00987-12 [pii] 10.1128/JCM.00987-12 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Frasson I, Lavezzo E, Franchin E, Toppo S, Barzon L, Cavallaro A, et al. Antimicrobial treatment and containment measures for an extremely drug-resistant Klebsiella pneumoniae ST101 isolate carrying pKPN101-IT, a novel fully sequenced bla(KPC-2) plasmid. J Clin Microbiol. 2012;50(11):3768–72. Epub 2012/09/14. 10.1128/JCM.01892-12 JCM.01892-12 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mataseje LF, Boyd DA, Willey BM, Prayitno N, Kreiswirth N, Gelosia A, et al. Plasmid comparison and molecular analysis of Klebsiella pneumoniae harbouring bla(KPC) from New York City and Toronto. J Antimicrob Chemother. 2011;66(6):1273–7. Epub 2011/03/17. doi: dkr092 [pii] 10.1093/jac/dkr092 . [DOI] [PubMed] [Google Scholar]
- 38. Jiang Y, Yu D, Wei Z, Shen P, Zhou Z, Yu Y. Complete nucleotide sequence of Klebsiella pneumoniae multidrug resistance plasmid pKP048, carrying blaKPC-2, blaDHA-1, qnrB4, and armA. Antimicrob Agents Chemother. 2010;54(9):3967–9. Epub 2010/06/16. doi: AAC.00137-10 [pii] 10.1128/AAC.00137-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Almeida AC, de Sa Cavalcanti FL, Vilela MA, Gales AC, de Morais MA Jr., Camargo de Morais MM. Escherichia coli ST502 and Klebsiella pneumoniae ST11 sharing an IncW plasmid harbouring the bla(KPC-2) gene in an Intensive Care Unit patient. Int J Antimicrob Agents. 2012;40(4):374–6. Epub 2012/07/24. 10.1016/j.ijantimicag.2012.05.022 . [DOI] [PubMed] [Google Scholar]
- 40. Lery LM, Frangeul L, Tomas A, Passet V, Almeida AS, Bialek-Davenet S, et al. Comparative analysis of Klebsiella pneumoniae genomes identifies a phospholipase D family protein as a novel virulence factor. BMC Biol. 2014;12:41 10.1186/1741-7007-12-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Perera IC, Grove A. Molecular mechanisms of ligand-mediated attenuation of DNA binding by MarR family transcriptional regulators. Journal of molecular cell biology. 2010;2(5):243–54. 10.1093/jmcb/mjq021 . [DOI] [PubMed] [Google Scholar]
- 42. Grkovic S, Brown MH, Skurray RA. Regulation of bacterial drug export systems. Microbiology and molecular biology reviews: MMBR. 2002;66(4):671–701, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li XZ, Plesiat P, Nikaido H. The challenge of efflux-mediated antibiotic resistance in Gram-negative bacteria. Clin Microbiol Rev. 2015;28(2):337–418. 10.1128/CMR.00117-14 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hao Z, Lou H, Zhu R, Zhu J, Zhang D, Zhao BS, et al. The multiple antibiotic resistance regulator MarR is a copper sensor in Escherichia coli. Nature chemical biology. 2014;10(1):21–8. 10.1038/nchembio.1380 . [DOI] [PubMed] [Google Scholar]
- 45. Wang X, Chen H, Zhang Y, Wang Q, Zhao C, Li H, et al. Genetic characterisation of clinical Klebsiella pneumoniae isolates with reduced susceptibility to tigecycline: Role of the global regulator RamA and its local repressor RamR. Int J Antimicrob Agents. 2015. 10.1016/j.ijantimicag.2014.12.022 . [DOI] [PubMed] [Google Scholar]
- 46. De Majumdar S, Yu J, Fookes M, McAteer SP, Llobet E, Finn S, et al. Elucidation of the RamA Regulon in Klebsiella pneumoniae Reveals a Role in LPS Regulation. PLoS pathogens. 2015;11(1):e1004627 10.1371/journal.ppat.1004627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhong X, Xu H, Chen D, Zhou H, Hu X, Cheng G. First emergence of acrAB and oqxAB mediated tigecycline resistance in clinical isolates of Klebsiella pneumoniae pre-dating the use of tigecycline in a Chinese hospital. PLoS One. 2014;9(12):e115185 10.1371/journal.pone.0115185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hansen LH, Johannesen E, Burmolle M, Sorensen AH, Sorensen SJ. Plasmid-encoded multidrug efflux pump conferring resistance to olaquindox in Escherichia coli. Antimicrob Agents Chemother. 2004;48(9):3332–7. 10.1128/AAC.48.9.3332-3337.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Perez F, Rudin SD, Marshall SH, Coakley P, Chen L, Kreiswirth BN, et al. OqxAB, a quinolone and olaquindox efflux pump, is widely distributed among multidrug-resistant Klebsiella pneumoniae isolates of human origin. Antimicrob Agents Chemother. 2013;57(9):4602–3. 10.1128/AAC.00725-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bialek-Davenet S, Lavigne JP, Guyot K, Mayer N, Tournebize R, Brisse S, et al. Differential contribution of AcrAB and OqxAB efflux pumps to multidrug resistance and virulence in Klebsiella pneumoniae. J Antimicrob Chemother. 2015;70(1):81–8. 10.1093/jac/dku340 . [DOI] [PubMed] [Google Scholar]
- 51. Veleba M, Higgins PG, Gonzalez G, Seifert H, Schneiders T. Characterization of RarA, a novel AraC family multidrug resistance regulator in Klebsiella pneumoniae. Antimicrob Agents Chemother. 2012;56(8):4450–8. 10.1128/AAC.00456-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Frank CG, Reguerio V, Rother M, Moranta D, Maeurer AP, Garmendia J, et al. Klebsiella pneumoniae targets an EGF receptor-dependent pathway to subvert inflammation. Cellular microbiology. 2013;15(7):1212–33. 10.1111/cmi.12110 . [DOI] [PubMed] [Google Scholar]
- 53. Segura M. Fisher scientific award lecture—the capsular polysaccharides of Group B Streptococcus and Streptococcus suis differently modulate bacterial interactions with dendritic cells. Canadian journal of microbiology. 2012;58(3):249–60. 10.1139/w2012-003 . [DOI] [PubMed] [Google Scholar]
- 54. Lin J, Huang S, Zhang Q. Outer membrane proteins: key players for bacterial adaptation in host niches. Microbes and infection / Institut Pasteur. 2002;4(3):325–31. . [DOI] [PubMed] [Google Scholar]
- 55. March C, Cano V, Moranta D, Llobet E, Perez-Gutierrez C, Tomas JM, et al. Role of bacterial surface structures on the interaction of Klebsiella pneumoniae with phagocytes. PLoS One. 2013;8(2):e56847 10.1371/journal.pone.0056847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Smith SG, Mahon V, Lambert MA, Fagan RP. A molecular Swiss army knife: OmpA structure, function and expression. FEMS microbiology letters. 2007;273(1):1–11. 10.1111/j.1574-6968.2007.00778.x . [DOI] [PubMed] [Google Scholar]
- 57. Garcia-Sureda L, Domenech-Sanchez A, Barbier M, Juan C, Gasco J, Alberti S. OmpK26, a novel porin associated with carbapenem resistance in Klebsiella pneumoniae. Antimicrob Agents Chemother. 2011;55(10):4742–7. 10.1128/AAC.00309-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kitchel B, Rasheed JK, Endimiani A, Hujer AM, Anderson KF, Bonomo RA, et al. Genetic factors associated with elevated carbapenem resistance in KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2010;54(10):4201–7. Epub 2010/07/28. doi: AAC.00008-10 [pii] 10.1128/AAC.00008-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Domenech-Sanchez A, Martinez-Martinez L, Hernandez-Alles S, del Carmen Conejo M, Pascual A, Tomas JM, et al. Role of Klebsiella pneumoniae OmpK35 porin in antimicrobial resistance. Antimicrob Agents Chemother. 2003;47(10):3332–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kaczmarek FM, Dib-Hajj F, Shang W, Gootz TD. High-level carbapenem resistance in a Klebsiella pneumoniae clinical isolate is due to the combination of bla(ACT-1) beta-lactamase production, porin OmpK35/36 insertional inactivation, and down-regulation of the phosphate transport porin phoe. Antimicrob Agents Chemother. 2006;50(10):3396–406. 10.1128/AAC.00285-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Croucher NJ, Klugman KP. The emergence of bacterial "hopeful monsters". MBio. 2014;5(4):e01550–14. 10.1128/mBio.01550-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Souli M, Galani I, Antoniadou A, Papadomichelakis E, Poulakou G, Panagea T, et al. An outbreak of infection due to beta-Lactamase Klebsiella pneumoniae Carbapenemase 2-producing K. pneumoniae in a Greek University Hospital: molecular characterization, epidemiology, and outcomes. Clin Infect Dis. 2010;50(3):364–73. 10.1086/649865 . [DOI] [PubMed] [Google Scholar]
- 63.America TSfHEo. Large Veteran Health Administration Study Shows 'Last Resort' Antibiotics Use on the Rise Dallas, TX2011 [cited 2014 Aug 12]. Available: http://www.shea-online.org/View/smid/428/ArticleID/72.aspx.
- 64. Tsai YK, Fung CP, Lin JC, Chen JH, Chang FY, Chen TL, et al. Klebsiella pneumoniae outer membrane porins OmpK35 and OmpK36 play roles in both antimicrobial resistance and virulence. Antimicrob Agents Chemother. 2011;55(4):1485–93. 10.1128/AAC.01275-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Holt K. SRST2 Short Read Sequence Typing for Bacterial Pathogens: GitHub; 2013 [updated Feb 6, 2014; cited 2014 March 1]. Available: https://github.com/katholt/srst2.
- 66. McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20(9):1297–303. 10.1101/gr.107524.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28(10):2731–9. Epub 2011/05/07. doi: msr121 [pii] 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Paradis E, Claude J, Strimmer K. APE: Analyses of Phylogenetics and Evolution in R language. Bioinformatics. 2004;20(2):289–90. . [DOI] [PubMed] [Google Scholar]
- 69. Letunic I, Bork P. Interactive Tree Of Life v2: online annotation and display of phylogenetic trees made easy. Nucleic acids research. 2011;39(Web Server issue):W475–8. 10.1093/nar/gkr201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Drummond AJ, Suchard MA, Xie D, Rambaut A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol. 2012;29(8):1969–73. Epub 2012/03/01. 10.1093/molbev/mss075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Carattoli A, Zankari E, Garcia-Fernandez A, Volby Larsen M, Lund O, Villa L, et al. PlasmidFinder and pMLST: in silico detection and typing of plasmids. Antimicrob Agents Chemother. 2014. 10.1128/AAC.02412-14 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, Lund O, et al. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother. 2012;67(11):2640–4. 10.1093/jac/dks261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. Journal of computational biology: a journal of computational molecular cell biology. 2012;19(5):455–77. 10.1089/cmb.2012.0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bolger AM, Lohse M, Usadel B. Trimmomatic: A flexible trimmer for Illumina Sequence Data. Bioinformatics. 2014. 10.1093/bioinformatics/btu170 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Geneious. Available from Biomatters Ltd. Available: http://www.geneious.com. 2013.
- 76. Pan YJ, Lin TL, Lin YT, Su PA, Chen CT, Hsieh PF, et al. Identification of Capsular Types in Carbapenem-Resistant Klebsiella pneumoniae Strains by wzc Sequencing and Implications for Capsule Depolymerase Treatment. Antimicrob Agents Chemother. 2015;59(2):1038–47. 10.1128/AAC.03560-14 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Brisse S, Passet V, Haugaard AB, Babosan A, Kassis-Chikhani N, Struve C, et al. wzi Gene sequencing, a rapid method for determination of capsular type for Klebsiella strains. J Clin Microbiol. 2013;51(12):4073–8. 10.1128/JCM.01924-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wilkinson SP, Grove A. Ligand-responsive transcriptional regulation by members of the MarR family of winged helix proteins. Current issues in molecular biology. 2006;8(1):51–62. . [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
101 isolates are from this study, 83 are from DeLeo et al. [15] and were retrieved from the SRA database of NCBI (Study no. SRP036874,), 22 are from the outbreak at the National Institutes of Health described by Snitkin et al. [23] and were retrieved as assemblies from Genbank. The US-GA isolates that were in Clade 2 previously but fall outside Clade 2 in this phylogeny are in the dotted box. Consistency index = 0.97.
(PDF)
Figure recreated from Wilkinson and Grove [78], with the addition of the MarR family amino acid sequence described in this study (bottom sequence). The amino acid substitution specific to ST258 in the α1 region is boxed. Light and dark shading indicates >70% similarity or >70% identity at that position respectively. α = alpha helices, β = beta turns, W = wing. The helix-turn-helix motif corresponds to helices α3 and α4, and helices α1, α5, and α6 form the dimerization domain [78].
(PDF)
(DOCX)
(XLSX)
(XLSX)
Data Availability Statement
All sequence data files are available from the NCBI SRA database BioProject ID PRJNA252957.