Abstract
Objective
The aim of this study was to evaluate the clinical behavior and management outcome of recurrent endometrial stromal sarcoma (ESS).
Methods
A retrospective review of charts of 10 patients with recurrent ESS was performed and relapse-free interval, relapse site, treatment, response to treatment, duration of follow-up and clinical outcome extracted. Survival outcome measures used were post-relapse survival which was defined as the time from first evidence of relapse to death from any cause. Living patients were censored at the date of last follow-up.
Results
The median age and median relapse-free interval at the time of initial relapse were 51.5 years and 66.5 months, respectively. The number of relapses ranged from one to five. Sixteen surgical procedures for recurrent disease included nine (56.0%) complete resections. There was no statistically significant difference between initial recurrent tumors and second/subsequent recurrent tumors in the rate of complete surgery (44.4% vs. 71.4%, respectively, p=0.36). Of the eleven evaluable occasions when hormonal therapy was used for recurrent disease, disease control was achieved in eight (72.7%). There was no difference between initial recurrent tumors and second/subsequent recurrent tumors in disease control rate by hormonal therapy (85.7% vs. 50.0%, respectively, p=0.49). The 10-year post-relapse survival rate was 90.0% and the overall median post-relapse survival 119 months (range, 7 to 216 months).
Conclusion
Post-relapse survival of patients with ESS can be expected to be >10 years when treated by repeated surgical resection and hormonal therapy or both.
Keywords: Aromatase Inhibitors; Gonadotropin-Releasing Hormone; Retrospective Studies; Sarcoma, Endometrial Stromal; Survival Rate
INTRODUCTION
According to the World Health Organization classification, there are three categories of endometrial stromal tumors: endometrial stromal nodule, endometrial stromal sarcoma (ESS), and undifferentiated endometrial sarcoma [1]. ESS is the third most common uterine type of sarcoma following leiomyosarcoma and undifferentiated endometrial sarcoma [2]. These tumors, which were previously referred to as low-grade ESS or endolymphatic stromal myosis, have the following distinct characteristics: a young age of onset, high potential for recurrence, indolent clinical course, and hormonal sensitivity with overexpression of estrogen and progesterone receptors. Recurrences develop in 23% to 59% of all patients with ESS [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19]. According to two recent systematic reviews, 15% to 25% of these patients die of recurrent disease [20,21]. All reports of higher mortality rates (19% to 50%) were published more than 30 years ago [4,7,8]. In contrast, recent publications have reported that fewer than 10% of patients with ESS eventually die of this disease [11,14,15,17,18]. High-dose progestins [18,22,23], aromatase inhibitors [24,25], and gonadotropin-releasing hormone (GnRH) agonist [26,27] are reportedly effective forms of hormonal treatment in patients with advanced or recurrent ESS. Thus, the change in mortality rate of ESS may be attributable to the frequent use of hormonal therapy. In contrast, radiation therapy is relatively ineffective against unresectable ESS [28]. There is also insufficient evidence concerning the efficacy of chemotherapy [29]. The aim of this study was to clarify the clinical outcome of recurrent ESS in the hormone-therapy era.
MATERIALS AND METHODS
1. Patients
This retrospective multicenter study included patients with ESS treated at the following hospitals: Hokkaido Cancer Center, Hokkaido University Hospital, Asahikawa Kosei General Hospital, Sapporo Municipal Hospital, Tomakomai Oji General Hospital, Sapporo Kosei General Hospital, Obihiro Kosei General Hospital, and Hakodate Chuo Hospital. Patients were identified for inclusion through institutional database searches up to 2009. Of the 25 patients with ESS identified by participating institutions, 10 had developed recurrent ESS. Their medical records were reviewed and patient age, the International Federation of Gynecology and Obstetrics stage, relapse-free interval (RFI), relapse site, treatment, response to treatment, duration of follow-up, and clinical outcome extracted.
2. Treatment
Treatments received by eligible patients included surgery, radiotherapy, chemotherapy, and hormonal therapy. All treatments were performed at the discretion of the attending physicians. Hormonal agents used in these patients included medroxyprogesterone acetate (MPA), leuprolide acetate, and dydrogesterone. MPA was administered orally in a daily dose of 400 to 600 mg, leuprolide acetate intramuscularly in a dose of 3.75 mg every 28 days, and dydrogesterone orally in a dose of 15 mg/day. Surgical treatment was classified as complete or incomplete. Response to other treatments was assessed by the Response Evaluation Criteria in Solid Tumors. Thus, complete response (CR) was defined as disappearance of all targets and no evidence of new lesions for at least 4 weeks. Partial response (PR) was defined as at least a 30% decrease in the sum of the longest dimensions of all target measurable lesions, taking as reference the baseline sum of the longest dimensions with no unequivocal progression of non-target lesions and no new lesions. Progressive disease (PD) required at least a 20% increase in the sum of the longest dimensions of target lesions or the appearance of new lesions or death due to disease. Stable disease (SD) was any condition not meeting the above criteria. Response to chemotherapy or hormonal therapy following complete surgery was regarded as not evaluable (NE). Where chemotherapy and hormonal therapy or radiotherapy and hormonal therapy had been used concurrently, the response to each individual therapy was also regarded as NE. The response rate was defined as the sum of CR and PR occupied on all evaluable occasions (CR+PR/CR+PR+SD+PD). Disease control rate was defined as the sum of CR, PR, and SD occupied on all evaluable occasions (CR+PR+SD/CR+PR+SD+PD).
3. Survival analysis
The survival outcome measures used were RFI and post-relapse survival (PRS). RFI was defined as the time from initial treatment of primary tumor to first evidence of relapse. PRS was defined as the time from first evidence of relapse to death from any cause. Living patients were censored at the date of last follow-up.
4. Statistical analysis
Correlation of variables was evaluated with Fisher exact test. Survival rates were estimated by the Kaplan-Meier method. The level of statistical significance was set at 0.05. Statistical analyses were performed with StatView J-5.0 (SAS Institute, Cary, NC, USA).
RESULTS
Table 1 shows the clinical characteristics of the 10 study patients. Their median age at the time of initial relapse was 51.5 years (range, 43 to 69 years). All patients were diagnosed as having stage I disease at the time of initial treatment and all underwent surgery including hysterectomy. Eight patients did not undergo bilateral salpingo-oophorectomy at the time of initial surgery. Although complete resection was achieved in all patients, two patients received adjuvant chemotherapy and three adjuvant hormonal therapies. The most frequent site of initial relapse was the ovary (60.0%), followed by the pelvis (40%). Lymph node recurrence occurred in two patients (20.0%). Six of the eight patients (75.0%) who had not undergone surgical castration had ovarian relapses.
Table 1. Clinical characteristics of patients with recurrent endometrial stromal sarcoma.
| Characteristic | No. (n=10) |
|---|---|
| Age* (yr), median (range) | 51.5 (43-69) |
| Stage | |
| I | 10 |
| II-IV | 0 |
| Initial treatment (surgery) | |
| Total hysterectomy alone | 7 |
| Total hysterectomy+BSO | 1 |
| Total hysterectomy+BSO+LND+OMT | 1 |
| Total hysterectomy+PLN sampling | 1 |
| Initial treatment (adjuvant therapy) | |
| Chemotherapy | |
| Cyclophosphamide+cisplatin | 1 |
| Ifosfamide+epirubicin+cisplatin | 1 |
| Hormonal therapy | |
| Medroxyprogesterone acetate | 3 |
| Relapse-free interval prior to 1st relapse (mo), median (range) | 66.5 (28-159) |
| Initial failure site | |
| Ovary | 6 |
| Pelvis | 4 |
| Omentum | 3 |
| Lymph node | 2 |
| Stump | 2 |
| Mesentery | 1 |
| Small bowel | 1 |
| Appendix | 1 |
| Retroperitoneum | 1 |
| Subcutaneous | 1 |
| Lung | 1 |
BSO, bilateral salpingo-oophorectomy; LND, lymph node dissection; OMT, omentectomy; PLN, pelvic lymph node.
*Age at the time of initial relapse.
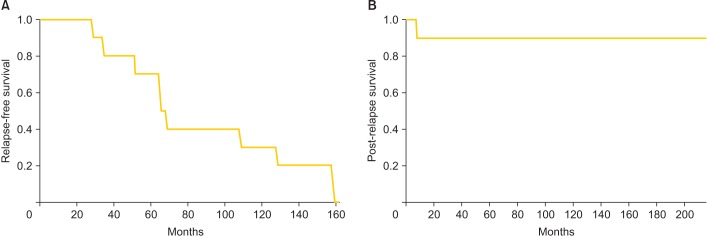
Table 2 shows the clinical outcome of the 10 study patients. The median duration of follow-up from initial treatment of primary tumor was 223.5 months (range, 72 to 349 months). Eight patients (80.0%) were alive at the last time of their follow-up. Two patients had died, one at 72 and the other at 280 months. The median RFI prior to initial relapse was 66.5 months (range, 28 to 159 months). The 10-year PRS rate was 90.0% and the median PRS 119 months (range, 7 to 216 months). Fig. 1 shows the RFI curve and the PRS curve for patients with recurrent ESS.
Table 2. Clinical outcome of 10 patients with recurrent endometrial stromal sarcoma.
| Case no. | Age (yr)* | RFI prior to 1st relapse (mo) | RFI prior to 2nd relapse (mo) | RFI prior to 3rd relapse (mo) | RFI prior to 4th relapse (mo) | RFI prior to 5th relapse (mo) | PRS (mo) | OS (mo) | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 45 | 68 | - | - | - | - | 163 | 231 | NED |
| 2 | 53 | 159 | 14 | 65 | 41 | 42 | 190 | 349 | NED |
| 3 | 58 | 64 | 76 | 106 | Persist | Persist | 216 | 280 | Dead |
| 4 | 55 | 108 | 36 | Persist | Persist | Persist | 121 | 229 | AWD |
| 5 | 43 | 65 | Persist | Persist | Persist | Persist | 7 | 72 | DOD |
| 6 | 61 | 128 | - | - | - | - | 117 | 245 | NED |
| 7 | 50 | 28 | - | - | - | - | 82 | 110 | NED |
| 8 | 69 | 51 | Persist | Persist | Persist | Persist | 102 | 153 | AWD |
| 9 | 50 | 34 | 150 | Persist | Persist | Persist | 184 | 218 | AWD |
| 10 | 45 | 158 | - | - | - | - | 26 | 184 | NED |
AWD, alive with disease; DOD, died of disease; NED, no evidence of disease; OS, overall survival; PRS, post-relapse (initial relapse) survival; RFI, relapse-free interval.
*Age at the time of initial relapse.
Fig. 1. (A) Relapse-free interval prior to initial relapse and (B) post-relapse survival curves for patients with recurrent endometrial stromal sarcoma.
Information regarding initial relapse is shown in Table 3. As to treatments for initial relapse, surgery was performed in nine cases and heavy ion radiotherapy in one. Complete resection of tumors was achieved in four of the nine patients who underwent surgery (44.4%). However, complete remission was finally achieved in eight cases (80.0%).
Table 3. Treatment and response to treatment of first relapse.
| Case no. | Initial relapse site | Sequential treatment for 1st relapse | Response to treatment | |||
|---|---|---|---|---|---|---|
| RT | CTX | HT | Overall | |||
| 1 | Pelvis, lung | Surgery, incomplete→CTX (TC)→HT (MPA) | - | SD | CR | Complete remission |
| 2 | Right ovary | Surgery, complete→CTX (CAP)→CTX (CYVADIC) | - | NE | - | Complete remission |
| 3 | Left ovary | Surgery, complete→CTX (CYVADIC) | - | NE | - | Complete remission |
| 4 | Ovaries, pelvis, subcutaneous | Surgery, incomplete→CTX (DC) and HT (GnRHa)*→HT (DDG)† | - | NE | PR*→CR† | Complete remission |
| 5 | Pelvis, mesentery, omentum | Surgery, incomplete→CTX (CYVADIC) and HT (GnRHa) | - | PD | PD | Persistent disease |
| 6 | Right ovary, stump, omentum, retroperitoneum | Surgery, incomplete→CTX (AD)→HT (MPA) | - | PR | CR | Complete remission |
| 7 | Ovaries, PLN, omentum, appendix | Surgery, incomplete→CTX (IEP)→HT (MPA) | - | PR | CR | Complete remission |
| 8 | Pelvis, lung | Pelvic HIRT→HT (MPA) | PR | - | PR | Persistent disease |
| 9 | Stump, PLN, PAN | Surgery, complete→CTX (IEP) | - | NE | - | Complete remission |
| 10 | Ovaries, small bowel | Surgery, complete | - | - | - | Complete remission |
AD, adriamucin+dacarbazine; CAP, cyclophosphamide+adriamycin+cisplatin; CR, complete response; CT, chemotherapy; CYVADIC, cyclophosphamide+vincristine+adriamycin+dacarbazine; DC, docetaxel+carboplatin; DDG, dydrogesterone; GnRHa, gonadotropin-releasing hormone agonists; HIRT, heavy ion radiotherapy; HT, hormonal therapy; IEP, ifosfamide+epirubicin+cisplatin; MPA, medroxyprogesterone acetate; NE, not evaluable; PAN, para-aortic lymph node; PD, progressive disease; PLN, pelvic lymph node; PR, partial response; RT, radiation therapy; S, surgery; SD, stable disease; TC, paclitaxel+carboplatin.
Information regarding second/subsequent relapses is shown in Table 4. Of the eight patients with complete remissions after treatments for initial relapse, four (50.0%) had second relapses, which took the form of intra-abdominal recurrence in all of them. As to treatments for second relapse, surgery was performed in three cases and hormonal therapy alone in one. Complete resection of tumors was achieved in two of the three patients who underwent surgery (66.7%). Complete remission was finally achieved in two cases (50.0%) after treatment for second relapse. Both patients with complete remissions had a third relapse; one developed tumor embolism in the inferior vena cava and the other multiple lung metastases. One of these patients underwent repeated complete surgical resections on each occasion of subsequent relapse.
Table 4. Treatment and response to treatment of second/subsequent relapse.
| Case no. | Relapse site | Sequential treatment for relapse | Response to treatment | ||
|---|---|---|---|---|---|
| CTX | HT | Overall | |||
| Second relapse | |||||
| 2 | Pelvis, mesentery, diaphragm | Surgery, complete→CTX (CPM) and HT (DDG) | NE | NE | Complete remission |
| 3 | Right ovary | Surgery, complete | - | - | Complete remission |
| 4 | Pelvis | HT (MPA) | - | PR | Persistent disease |
| 9 | Intra-abdomen, subcutaneous, lung | Surgery, incomplete→HT (MPA) | - | PD | Persistent disease |
| Third relapse | |||||
| 2 | Right ovarian vessel, inferior vena cava | Surgery, complete | Complete remission | ||
| 3 | Lung | CTX (CYVADIC)→HT(MPA) | SD | SD | Persistent disease |
| Forth relapse | |||||
| 2 | Liver, omentum | Surgery, complete→HT (MPA) | - | NE/PD | Complete remission |
| Fifth relapse | |||||
| 2 | Intra-abdomen | CTX (GD)→Surgery, complete→HT (MPA) | PD | NE | Complete remission |
CTX, chemotherapy; CPM, cyclophosphamide; CYVADIC, cyclophosphamide+vincristine+adriamycin+dacarbazine; DC, docetaxel+carboplatin; DDG, dydrogesterone; GD, gemcitabine+docetaxel; HT, hormonal therapy; MPA, medroxyprogesterone acetate; NE, not evaluable; PD, progressive disease; PR, partial response; S, surgery.
Complete resection was achieved on 9/16 occasions (56.0%) when surgery was performed for recurrent disease (Supplementary Table 1). There was no statistically significant difference between initial recurrent tumors and second/subsequent recurrent tumors in the rate of complete resection (44.4% vs. 71.4%, respectively; p=0.36). Of the 11 evaluable occasions of hormone therapy for recurrent disease, the agent used was MPA in eight instances, leuprolide acetate in two, and dydrogesterone in one. The median duration of hormonal therapy was 17 months (range, 2 to 121 months). The response rate was 63.6% and disease control rate 72.7%. One patient had SD for the 68 months, during which she received 600 mg/day MPA as treatment for a third relapse. There was no difference in disease control rate between initial recurrent tumors and second/subsequent recurrent tumors (85.7% vs. 50.0%, respectively; p=0.49). A higher response rate was achieved with initial recurrent tumors than with second/subsequent recurrent tumors (85.7% vs. 25.0%, respectively; p=0.08).
DISCUSSION
The post-recurrence clinical course of ESS has not so far been well described because a long period of time is needed to assess it. The median durations of follow-up in earlier reports ranged from 46 to 130 months [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19]. In contrast, our median follow-up of 223.5 months (range, 72 to 349 months) is markedly longer than those of previously published studies. The followings are the three major findings of our study. First, the median PRS of our patients with recurrent ESS was >10 years. It was actually 119 months; however, it must be 10 years or more because only 20% of eligible patients had died by the time of final analysis. Second, hormonal therapy was extremely effective against initial recurrent tumors, achieving CRs in four of the five patients (80.0%) with unresectable initial recurrent tumors. Third, hormonal therapy was also clinically effective against second/subsequent recurrent tumors. Two-thirds of the patients with second recurrent tumors who received MPA achieved PRs. ESS has been described as an indolent tumor with a tendency to late recurrence. Our data show that recurrent ESS also behaves indolently. Mortality rates in ESS seem to have decreased during the last three decades (Table 5) [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19]. The frequent use of hormonal therapy may be largely responsible for this change in mortality rate.
Table 5. A literature review of recurrence/death in primary endometrial stromal sarcoma.
| Study | No. of patient | Recurrence (%) | Death (%) | Median follow-up period (mo) |
|---|---|---|---|---|
| Norris et al. (1966) [3] | 20 | 7 (35) | 1 (5) | NA |
| Krieger et al. (1973) [4] | 182 | 91 (50) | 35 (19) | NA |
| Piver et al. (1984) [5] | 52 | 25 (48) | NA | NA |
| Mansi et al. (1990) [6] | 8 | 3 (38) | 1 (13) | 63 |
| Berchuck et al. (1990) [7] | 22 | 13 (59) | 6 (27) | 63 |
| Larson et al. (1990) [8] | 12 | 6 (50) | 6 (50) | 96 |
| Goff et al. (1993) [9] | 10 | 3 (33) | 0 | NA |
| Gadducci et al. (1996) [10] | 26 | 6 (23) | 3 (12) | 92 |
| Chu et al. (2003) [11] | 22 | 10 (46) | NA | 100 |
| Li et al. (2005) [12] | 36 | 14 (39) | 3 (8) | 35 (case), 60 (control) |
| Leath et al. (2007) [13] | 72 | 27 (38) | 9 (12) | 80 |
| Amant et al. (2007) [14] | 31 | 8 (26) | 5 (16) | 56 |
| Nam et al. (2008) [15] | 22 | 5 (23) | 1 (5) | 55 |
| Kim et al. (2008) [16] | 22 | 10 (46) | 2 (9) | 77 |
| Beck et al. (2012) [17] | 42 | 16 (38) | NA | 130 |
| Mizuno et al. (2012) [18] | 13 | 4 (31) | 1 (8) | 117 |
| Yoon et al. (2014) [19] | 114 | 33 (29) | 10 (9) | 46 |
NA, not available.
One of the issues we have identified is of some concern. Although hormonal agents are extremely effective against initial recurrent tumors, we observed no CRs to these agents in patients with second/subsequent recurrences and no complete or PR in those with third/subsequent recurrences. These findings suggest that hormonal agents are more effective against initial recurrent tumors than against second/subsequent recurrences.
Because ESS is characteristically indolent and hormonal therapy is remarkably effective, long-term administration of hormonal agents is a topic that requires further investigation. It is well known that the adverse effects of high-dose progestin therapy include thromboembolic complications, weight gain, and depression [30]. Although high-dose progestin therapy has been considered to be well tolerated, in earlier studies oral MPA was only administered for short periods for treatment of advanced or recurrent endometrial cancer [31]. Mizuno et al. [18] evaluated the effects of long-term, high-dose MPA with an anti-platelet agent (aspirin, 100 mg/day) and observed no thromboembolic complications over a median duration of 63 months (range, 28 to 92 months). Use of an anti-platelet agent may prevent thromboembolic events during high-dose MPA therapy. The adverse effects of GnRH agonists are caused by a lack of estrogen, long-term use of GnRH agonists causes osteoporosis [32,33]. Adverse effects of aromatase inhibitors are also associated with a deficiency of estrogen; these include musculoskeletal stiffness and pain, fatigue, hot flushes, and nausea [24,34]. There is reportedly a high rate of non-compliance with letrozole therapy in breast cancer patients, the overall probability of non-compliance being 18.4% over this study's 2.5 years of follow-up [34]. The most frequent adverse events are musculoskeletal-related symptoms [34]. Dydrogesterone is a hormonally active, non-androgenic synthetic steroid that was developed in the 1950s [35]. Its molecular structure is almost identical to that of natural progesterone, which accounts for the lack of estrogenic, androgenic glucocorticoid, and mineralocorticoid properties [36]. Therefore, dydrogesterone is preferable to other progestins such as MPA because of the lack of adverse effects of long-term medication or repeated courses of treatment [36]. One patient in the present study (Case 4) experienced troublesome adverse effects of a GnRH agonist; subsequent use of dydrogesterone was effective [37].
Hysterectomy with bilateral salpingo-oophorectomy is generally considered a standard surgical treatment. However, it has been shown that ovary-sparing surgery does not worsen survival outcome [10,11,12,14,16,38,39]. In the present study, the median overall survival time of the eight patients who did not undergo surgical castration at the time of initial surgery was 230 months (range, 72 to 349 months), despite six of these patients (75.0%) subsequently developing ovarian recurrences. The present findings encourage us to perform ovarian-sparing surgeries, especially in women with ESS of reproductive age. On the other hand, in large population-based studies lymph node metastases have been noted in less than 10% of patients undergoing lymphadenectomy [38,39]. While the prognostic significance of lymph node involvement is controversial, lymphadenectomy does not improve the prognosis of ESS [14,38,39]. In this study, lymph nodes were one of the initial failure sites in two patients. Although these patients were found to have nodal recurrences relatively soon after their initial surgery, both remain alive with PRS times of 82 and 184 months. Thus, our findings do not support routine lymphadenectomy at the time of initial treatment.
In this retrospective study, there were too few patients to draw any definitive conclusions. However, it will always be difficult to conduct a prospective study aimed at assessing the long-term clinical course of recurrent ESS because of the rarity of ESS and its indolent clinical course. PRS of patients with recurrent ESS can be expected to be >10 years when treated by repeated surgical resection and hormonal therapy or both.
ACKNOWLEDGEMENT
The authors gratefully acknowledge the assistance of Rifumi Hattori, Masaki Azuma, Eiji Nomura, Daisuke Akashi, Tsunemaro Kojo, Soromon Kataoka, Hidemichi Watari, Shinichiro Minobe, Yoko Ohba, and Kazuhira Okamoto in collecting data.
Footnotes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
Supplementary Material
Supplementary Table 1. Results of surgery and hormonal therapy by the number of recurrence.
| Variable | Number of recurrence | |
|---|---|---|
| Initial recurrence | Second/subsequent recurrence | |
| Surgery | ||
| Complete surgery | 4 | 5 |
| Incomplete surgery | 5 | 2 |
| Hormonal therapy | ||
| Complete response | 4 | 0 |
| Progressive response | 2 | 1 |
| Stable disease | 0 | 1 |
| Progressive disease | 1 | 2 |
References
- 1.Hendrickson MR, Tavassoli FA, Kempson RL, McCluggage WG, Haller U, Kubik-Huch RA. Mesenchymal tumours and related lesions. In: Tavassoli FA, Devilee P, editors. World Health Organization classification of tumours. Pathology and genetics of tumours of the breast and female genital organs. Lyon: IARC Press; 2003. pp. 233–244. [Google Scholar]
- 2.Boll D, Verhoeven RH, van der Aa MA, Pauwels P, Karim-Kos HE, Coebergh JW, et al. Incidence and survival trends of uncommon corpus uteri malignancies in the Netherlands, 1989-2008. Int J Gynecol Cancer. 2012;22:599–606. doi: 10.1097/IGC.0b013e318244cedc. [DOI] [PubMed] [Google Scholar]
- 3.Norris HJ, Taylor HB. Mesenchymal tumors of the uterus. I. A clinical and pathological study of 53 endometrial stromal tumors. Cancer. 1966;19:755–766. doi: 10.1002/1097-0142(196606)19:6<755::aid-cncr2820190604>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 4.Krieger PD, Gusberg SB. Endolymphatic stromal myosis: a grade I endometrial sarcoma. Gynecol Oncol. 1973;1:299–313. [Google Scholar]
- 5.Piver MS, Rutledge FN, Copeland L, Webster K, Blumenson L, Suh O. Uterine endolymphatic stromal myosis: a collaborative study. Obstet Gynecol. 1984;64:173–178. [PubMed] [Google Scholar]
- 6.Mansi JL, Ramachandra S, Wiltshaw E, Fisher C. Endometrial stromal sarcomas. Gynecol Oncol. 1990;36:113–118. doi: 10.1016/0090-8258(90)90120-a. [DOI] [PubMed] [Google Scholar]
- 7.Berchuck A, Rubin SC, Hoskins WJ, Saigo PE, Pierce VK, Lewis JL., Jr Treatment of endometrial stromal tumors. Gynecol Oncol. 1990;36:60–65. doi: 10.1016/0090-8258(90)90109-x. [DOI] [PubMed] [Google Scholar]
- 8.Larson B, Silfversward C, Nilsson B, Pettersson F. Endometrial stromal sarcoma of the uterus. A clinical and histopathological study. The Radiumhemmet series 1936-1981. Eur J Obstet Gynecol Reprod Biol. 1990;35:239–249. doi: 10.1016/0028-2243(90)90168-z. [DOI] [PubMed] [Google Scholar]
- 9.Goff BA, Rice LW, Fleischhacker D, Muntz HG, Falkenberry SS, Nikrui N, et al. Uterine leiomyosarcoma and endometrial stromal sarcoma: lymph node metastases and sites of recurrence. Gynecol Oncol. 1993;50:105–109. doi: 10.1006/gyno.1993.1172. [DOI] [PubMed] [Google Scholar]
- 10.Gadducci A, Sartori E, Landoni F, Zola P, Maggino T, Urgesi A, et al. Endometrial stromal sarcoma: analysis of treatment failures and survival. Gynecol Oncol. 1996;63:247–253. doi: 10.1006/gyno.1996.0314. [DOI] [PubMed] [Google Scholar]
- 11.Chu MC, Mor G, Lim C, Zheng W, Parkash V, Schwartz PE. Low-grade endometrial stromal sarcoma: hormonal aspects. Gynecol Oncol. 2003;90:170–176. doi: 10.1016/s0090-8258(03)00258-0. [DOI] [PubMed] [Google Scholar]
- 12.Li AJ, Giuntoli RL, 2nd, Drake R, Byun SY, Rojas F, Barbuto D, et al. Ovarian preservation in stage I low-grade endometrial stromal sarcomas. Obstet Gynecol. 2005;106:1304–1308. doi: 10.1097/01.AOG.0000185511.91694.1e. [DOI] [PubMed] [Google Scholar]
- 13.Leath CA, 3rd, Huh WK, Hyde J, Jr, Cohn DE, Resnick KE, Taylor NP, et al. A multi-institutional review of outcomes of endometrial stromal sarcoma. Gynecol Oncol. 2007;105:630–634. doi: 10.1016/j.ygyno.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 14.Amant F, De Knijf A, Van Calster B, Leunen K, Neven P, Berteloot P, et al. Clinical study investigating the role of lymphadenectomy, surgical castration and adjuvant hormonal treatment in endometrial stromal sarcoma. Br J Cancer. 2007;97:1194–1199. doi: 10.1038/sj.bjc.6603986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nam EJ, Kim JW, Lee DW, Jang SY, Hong JW, Kim YT, et al. Endometrial stromal sarcomas: a retrospective analysis of 28 patients, single center experience for 20 years. Cancer Res Treat. 2008;40:6–10. doi: 10.4143/crt.2008.40.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim WY, Lee JW, Choi CH, Kang H, Kim TJ, Kim BG, et al. Low-grade endometrial stromal sarcoma: a single center's experience with 22 cases. Int J Gynecol Cancer. 2008;18:1084–1089. doi: 10.1111/j.1525-1438.2007.01159.x. [DOI] [PubMed] [Google Scholar]
- 17.Beck TL, Singhal PK, Ehrenberg HM, Rose PG, Lele SB, Krivak TC, et al. Endometrial stromal sarcoma: analysis of recurrence following adjuvant treatment. Gynecol Oncol. 2012;125:141–144. doi: 10.1016/j.ygyno.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 18.Mizuno M, Yatabe Y, Nawa A, Nakanishi T. Long-term medroxyprogesterone acetate therapy for low-grade endometrial stromal sarcoma. Int J Clin Oncol. 2012;17:348–354. doi: 10.1007/s10147-011-0299-y. [DOI] [PubMed] [Google Scholar]
- 19.Yoon A, Park JY, Park JY, Lee YY, Kim TJ, Choi CH, et al. Prognostic factors and outcomes in endometrial stromal sarcoma with the 2009 FIGO staging system: a multicenter review of 114 cases. Gynecol Oncol. 2014;132:70–75. doi: 10.1016/j.ygyno.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 20.Rauh-Hain JA, del Carmen MG. Endometrial stromal sarcoma: a systematic review. Obstet Gynecol. 2013;122:676–683. doi: 10.1097/AOG.0b013e3182a189ac. [DOI] [PubMed] [Google Scholar]
- 21.Gadducci A, Cosio S, Romanini A, Genazzani AR. The management of patients with uterine sarcoma: a debated clinical challenge. Crit Rev Oncol Hematol. 2008;65:129–142. doi: 10.1016/j.critrevonc.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 22.Reich O, Regauer S. Hormonal therapy of endometrial stromal sarcoma. Curr Opin Oncol. 2007;19:347–352. doi: 10.1097/CCO.0b013e3281a7ef3a. [DOI] [PubMed] [Google Scholar]
- 23.Pink D, Lindner T, Mrozek A, Kretzschmar A, Thuss-Patience PC, Dorken B, et al. Harm or benefit of hormonal treatment in metastatic low-grade endometrial stromal sarcoma: single center experience with 10 cases and review of the literature. Gynecol Oncol. 2006;101:464–469. doi: 10.1016/j.ygyno.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 24.Maluf FC, Sabbatini P, Schwartz L, Xia J, Aghajanian C. Endometrial stromal sarcoma: objective response to letrozole. Gynecol Oncol. 2001;82:384–388. doi: 10.1006/gyno.2001.6238. [DOI] [PubMed] [Google Scholar]
- 25.Krauss K, Bachmann C, Hartmann JT, Siegmann K, Sotlar K, Wallwiener D, et al. Management of late recurrence of a low-grade endometrial stromal sarcoma (LGESS): treatment with letrozole. Anticancer Res. 2007;27(5B):3477–3480. [PubMed] [Google Scholar]
- 26.Mesia AF, Demopoulos RI. Effects of leuprolide acetate on low-grade endometrial stromal sarcoma. Am J Obstet Gynecol. 2000;182:1140–1141. doi: 10.1067/mob.2000.103939. [DOI] [PubMed] [Google Scholar]
- 27.Burke C, Hickey K. Treatment of endometrial stromal sarcoma with a gonadotropin-releasing hormone analogue. Obstet Gynecol. 2004;104(5 Pt 2):1182–1184. doi: 10.1097/01.AOG.0000133533.05148.aa. [DOI] [PubMed] [Google Scholar]
- 28.Weitmann HD, Knocke TH, Kucera H, Potter R. Radiation therapy in the treatment of endometrial stromal sarcoma. Int J Radiat Oncol Biol Phys. 2001;49:739–748. doi: 10.1016/s0360-3016(00)01369-9. [DOI] [PubMed] [Google Scholar]
- 29.Sutton G, Blessing JA, Park R, DiSaia PJ, Rosenshein N. Ifosfamide treatment of recurrent or metastatic endometrial stromal sarcomas previously unexposed to chemotherapy: a study of the Gynecologic Oncology Group. Obstet Gynecol. 1996;87(5 Pt 1):747–750. doi: 10.1016/0029-7844(96)00003-8. [DOI] [PubMed] [Google Scholar]
- 30.Jordan VC. Medroxyprogesterone acetate and metastases: of mice and (wo)men. J Natl Cancer Inst. 2005;97:619–621. doi: 10.1093/jnci/dji126. [DOI] [PubMed] [Google Scholar]
- 31.Thigpen JT, Brady MF, Alvarez RD, Adelson MD, Homesley HD, Manetta A, et al. Oral medroxyprogesterone acetate in the treatment of advanced or recurrent endometrial carcinoma: a dose-response study by the Gynecologic Oncology Group. J Clin Oncol. 1999;17:1736–1744. doi: 10.1200/JCO.1999.17.6.1736. [DOI] [PubMed] [Google Scholar]
- 32.Sugimoto AK, Hodsman AB, Nisker JA. Long-term gonadotropin-releasing hormone agonist with standard postmenopausal estrogen replacement failed to prevent vertebral bone loss in premenopausal women. Fertil Steril. 1993;60:672–674. doi: 10.1016/s0015-0282(16)56220-7. [DOI] [PubMed] [Google Scholar]
- 33.Paoletti AM, Serra GG, Cagnacci A, Vacca AM, Guerriero S, Solla E, et al. Spontaneous reversibility of bone loss induced by gonadotropin-releasing hormone analog treatment. Fertil Steril. 1996;65:707–710. [PubMed] [Google Scholar]
- 34.Fontein DB, Nortier JW, Liefers GJ, Putter H, Meershoek-Klein Kranenbarg E, van den Bosch J, et al. High non-compliance in the use of letrozole after 2.5 years of extended adjuvant endocrine therapy. Results from the IDEAL randomized trial. Eur J Surg Oncol. 2012;38:110–117. doi: 10.1016/j.ejso.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 35.Reerink EH, Scholer HF, Westerhof P, Querido A, Kassenaar AA, Diczfalusy E, et al. A new class of hormonally active steroids. Nature. 1960;186:168–169. doi: 10.1038/186168a0. [DOI] [PubMed] [Google Scholar]
- 36.Schindler AE. Progestational effects of dydrogesterone in vitro, in vivo and on the human endometrium. Maturitas. 2009;65(Suppl 1):S3–S11. doi: 10.1016/j.maturitas.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 37.Akashi D, Todo Y, Shimada C, Okamoto K, Minobe S, Kato H. Successful use of dydrogesterone as maintenance therapy in recurrent endometrial stromal sarcoma: a case report. Jpn J Clin Oncol. 2013;43:1145–1149. doi: 10.1093/jjco/hyt142. [DOI] [PubMed] [Google Scholar]
- 38.Chan JK, Kawar NM, Shin JY, Osann K, Chen LM, Powell CB, et al. Endometrial stromal sarcoma: a population-based analysis. Br J Cancer. 2008;99:1210–1215. doi: 10.1038/sj.bjc.6604527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shah JP, Bryant CS, Kumar S, Ali-Fehmi R, Malone JM, Jr, Morris RT. Lymphadenectomy and ovarian preservation in low-grade endometrial stromal sarcoma. Obstet Gynecol. 2008;112:1102–1108. doi: 10.1097/AOG.0b013e31818aa89a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Results of surgery and hormonal therapy by the number of recurrence.
| Variable | Number of recurrence | |
|---|---|---|
| Initial recurrence | Second/subsequent recurrence | |
| Surgery | ||
| Complete surgery | 4 | 5 |
| Incomplete surgery | 5 | 2 |
| Hormonal therapy | ||
| Complete response | 4 | 0 |
| Progressive response | 2 | 1 |
| Stable disease | 0 | 1 |
| Progressive disease | 1 | 2 |


