Abstract
The epidermis on leaves protects plants from pathogen invasion and provides a waterproof barrier. It consists of a layer of cells that is surrounded by thick cell walls, which are partially impregnated by highly hydrophobic cuticular components. We show that the Arabidopsis T-DNA insertion mutants of REDUCED WALL ACETYLATION 2 (rwa2), previously identified as having reduced O-acetylation of both pectins and hemicelluloses, exhibit pleiotrophic phenotype on the leaf surface. The cuticle layer appeared diffused and was significantly thicker and underneath cell wall layer was interspersed with electron-dense deposits. A large number of trichomes were collapsed and surface permeability of the leaves was enhanced in rwa2 as compared to the wild type. A massive reprogramming of the transcriptome was observed in rwa2 as compared to the wild type, including a coordinated up-regulation of genes involved in responses to abiotic stress, particularly detoxification of reactive oxygen species and defense against microbial pathogens (e.g., lipid transfer proteins, peroxidases). In accordance, peroxidase activities were found to be elevated in rwa2 as compared to the wild type. These results indicate that cell wall acetylation is essential for maintaining the structural integrity of leaf epidermis, and that reduction of cell wall acetylation leads to global stress responses in Arabidopsis.
Keywords: cell wall acetylation, trichomes, cuticles, epidermis, Botrytis cinerea, peroxidase, mRNA sequencing
Introduction
The epidermis of plants forms a protective layer against xenobiotics, ultraviolet light, and pathogens and provides a waterproof barrier (Liu, 2006; Kourounioti et al., 2013). The epidermis consists of a layer of cells surrounded by thick cell walls that are partially impregnated with the cuticle layer. The main cell wall components in the epidermis are complex polysaccharides: celluloses, hemicelluloses, and pectins. Cellulose forms paracrystalline microfibrils and provides the scaffold of the cell wall; hemicelluloses, mainly xyloglucan in growing tissues such as epidermal cells, crosslink with cellulose to provide support to the cellulose network; while pectins not only crosslink these and other cell wall polymers but also serve as hydrated extracellular matrix components (Carpita and Gibeaut, 1993; Somerville et al., 2004). In contrast, the cuticle consists of highly hydrophobic long-chain hydrocarbons (e.g., cutins and waxes). These cuticle components are transported across the hydrophilic cell wall while partially impregnated within the cell wall (Yeats and Rose, 2013).
Pectins and hemicelluloses are subjected to modifications, of which O-acetylation has attracted growing attention in recent years because it significantly impacts a number of industrial applications including food, lumber, and biofuel industries (Klein-Marcuschamer et al., 2010; Gille and Pauly, 2012; Pawar et al., 2013). In pectic homogalacturonan and rhamnogalacturonan (RG) I, O-acetylation occurs at the O-2 and O-3 positions in the backbone galacturonic acid residues (Schols et al., 1990; Ishii, 1997), while in RG II sidechains aceric acid and fucose residues are O-acetylated (Whitcombe et al., 1995). In the hemicellulose xyloglucan, acetylation mainly occurs on the galactose residue in the sidechain with the exception that in Solanaceae and Poaceae glucose residues in the backbones are acetylated (Jia et al., 2005). In the hemicelluloses xylans and gluconomannans O-acetylation occurs in the backbone at O-2 and O-3 position in the xylosyl and mannosyl residues, respectively (Lundqvist et al., 2002; Perrin et al., 2003; Jia et al., 2005; Van Dongen et al., 2011; Gille and Pauly, 2012; Pawar et al., 2013; Xiong et al., 2013). In addition, acetylation of lignins has been reported in some angiosperms (Del Rio et al., 2007; Lu and Ralph, 2008).
Three classes of proteins are known to be involved in O-acetylation of cell wall polysaccharides in the Golgi apparatus. The REDUCED WALL ACETYLATION family proteins (RWA1 through 4 in Arabidopsis) are thought to be responsible for the translocation of acetyl-CoA across the Golgi membrane and appear to supply the acetyl-donor to both pectins and hemicellluloses, because knock-out of individual RWAs impacted the level of acetylation in both pectins and hemicelluloses (Lee et al., 2011; Manabe et al., 2011, 2013). For instance, homozygous rwa2 mutants of Arabidopsis exhibit approximately 20% reduction in the degree of acetylation in both pectins and hemicelluloses (Manabe et al., 2011). The TRICHOME BIREFRINGENCE-LIKE family proteins (TBR1 and TBL1 through 46 in Arabidopsis) are likely to conferr polymer specificity, because different tbl mutants of Arabidopsis show polymer-specific reduction in the level of acetylation: altered xyloglucan 4 (axy4)/tbl27 and axy4L/tbl22 lack O-acetylation of xyloglucan (Gille et al., 2011), while tbl29/eskimo1(esk1) mutants have reduced O-acetylation of xylan and mannan (Gille et al., 2011; Xiong et al., 2013). Recently, in vivo acetyltransferase activity of TBL29 has been demonstrated, lending support to the above notion (Urbanowicz et al., 2014). A gene(s) responsible for pectin-specific acetylation has not yet been identified. Lastly, AXY9, a protein that shows a limited sequence similarity to TBL, was also found to be involved in acetylation of hemicelluloses (Schultink et al., 2015). The reported phenotypes of rwa2, axy4, and tbl29 are diverse; rwa2 shows enhanced resistance to the necrotrophic fungal pathogen Botrytis cinerea (Manabe et al., 2011), axy4 shows enhanced sensitivity to aluminum (Zhu et al., 2014), while tbl29 shows enhanced freezing tolerance and dwarfism (Xin and Browse, 1998; Yuan et al., 2013). These observations indicate that cell wall acetylation plays roles in broad aspects of plant stress responses.
The aim of the present work was to gain a better understanding of the role of cell wall acetylation in plant stress responses. To this end, we have carried out a series of phenotypic, microscopic, biochemical and transcriptomic analyses on the panel of Arabidopsis (ecotype Columbia-0) mutants defective in cell wall acetylation. We discovered that in the rwa2 mutants the architectures of the cell wall-cuticle layer was altered and the trichomes were fragile and collapsed, while the other (tbl29 and axy4-3) mutants appeared similar to the wild type. Furthermore, global transcriptome reprogramming including up-regulation of a large set of stress related genes and the concomitant accumulation of peroxidase activities was observed in the rwa2 mutant. These effects underpin the importance of cell wall acetylation in the leaf surface integrity and stress responses in plants.
Materials and methods
Plant material and growth
Arabidopsis thaliana L. Heyn. ecotype Colombia-0 and mutants were grown in soil in Percival climate chamber with a 12 h dark/light cycle at 22°C and 70% relative humidity. When grown on plates, seeds were surface-sterilized as previously described (Weigel and Glazebrook, 2002). Analysis of root growth inhibition was performed according to Weigel and Glazebrook (2002) on half-strength Murashige Skoog (MS) medium containing 0.5% (v/v) sucrose and 0.8% (v/v) agar in the vertically orientation for 1 week. The seedlings were transferred to new MS-agar plates containing hormones (indole-acetic acid and trans-zeatin in the range between 0.01 and 10 μM; ABA in the range between 0.3 and 1.5 μM; JA, 10 and 25 μM), grown vertically. For quantification, the plates were scanned and the root lengths were measured using the software ImageJ (http://rsbweb.nih.gov/ij/).
Staining and light microscopy
To assess cuticle permeability, detached leaves were floated on the toluidine blue solution, 0.025% (w/v), for 15 min and rinsed with distilled water before imaging with the Stereoscope (Leica EZ4D, Leica, Denmark). Alternatively, soil grown plants were sprayed with the toluidine blue solution, incubated for 45 min and rinsed with excess water to remove the unbound dye.
Electron microscopy
Leaf pieces of approximately 1 × 3 mm were taken from the tip part leaves having approximately 20 mm long leaf blades from three independent plants in each genotype. Samples were fixed for 4 h in Karnovsky's fixative [5% (v/v) glutaraldehyde, 4% (v/v) paraformaldehyde, 0.1 M sodium cacodylate buffer, pH 7.3], washed in the buffer, and post-fixed in 1% (v/v) osmium tetroxide in the 0.1 M sodium cacodylate buffer for 8 h at 4°C. After washing in the buffer and water, the samples were dehydrated in a graded acetone series and embedded in Spurr resin. The resin was polymerised in an oven at 60°C for 8 h. Ultrathin sections (40 nm thick) were cut with a diamond knife using a Reichert-Jung/LKB Supernova ultramicrotome and sections were contrasted with 1% (v/v) uranyl acetate and lead citrate [2.7% (v/v) in 3.5% (v/v) sodium citrate] and examined in a Philips CM 100 TEM at 80 kV. Quantification of cell wall thickness and cuticle thickness was performed with ImageJ. Five regions from the outer cell wall of the epidermis cells, avoiding trichomes, were analyzed for each genotype, and within each region 20 pairs of measurements were made at the magnification of 64,000 folds. For scanning electron microscopy (SEM), leaf sections were fixed and washed as indicated above, and were dehydrated in ascending concentrations of acetone reaching 100% (v/v) acetone at the final step and dried in an EMS 850 CP drier. Specimens were mounted onto metal stubs, sputter-coated with gold:palladium (1:1) in a Polaron SC 7640 (Quorum Technologies, Newhaven, UK) automated sputter coater, and viewed in a Quanta 200 SEM (FEI CompanyTM) at 10 kV. Contrast adjustments were carried out using Adobe Photoshop CS5 and final mounting of images were done with Adobe Illustrator C52.
Water loss assay
Rosette leaves from 4-weeks-old plants were cut at the petiole and immediately weighed in plastic weighing boats. The leaves were incubated at room temperature on the laboratory bench and weighed every 20 min. The amount of water loss was calculated as percentage of weight loss compared to original weight.
Leaf gas exchange
Photosynthesis and transpiration were measured 30 days after germination. A fully expanded leaf, still attached to an intact plant, was placed in the cuvette of a CIRAS-2 portable photosynthesis and transpiration monitor (CIRAS-2 Portable Photosynthesis System; PP Systems, Amesbury, USA). The concentration of CO2 in the cuvette was maintained at 400 ppm, humidity at 80% and light level was 150 μmol photons m−2 s−1. Four independent plants were measured for each line.
Stomatal aperture measurement
Detached rosette leaves from 4-week old plants were floated in opening buffer (5 mM KCl, 10 mM MES, pH 5.6) for 2 h in the light. Hundred microliters of 50 μM ABA in 2% (v/v) ethanol or 2% (v/v) ethanol (control) was added and the leaves were incubated for 2 h. To measure the response to drought, 4-week-old plants were exposed to 100% humidity for 12 h. Rosette leaves were detached from the plant and incubated on the lab bench for 1 h. Following treatment, the leaves were grinded in opening buffer with a polytron and the homogenate was filtered through nylon cloth (30 μm mesh size). The isolated epidermal fragments were transferred to microscope slides and viewed under the microscope (Leica DM750, Leica, Denmark), 400x magnification.
Analysis of cuticle composition
To extract wax and cutins, 5–10 leaves per plants were used. The extraction and derivatization of wax and cutin monomers were performed as previously described (Bonaventure et al., 2004; Molina et al., 2006). For the GC-FID analysis the following conditions were used. HP-5 capillary column (30 m, 0.32 mm ID, 0.25 μm film thickness) was used and helium carrier gas at the flow rate of 2 ml min−1 with the gradient oven temperature programmed from 140 to 310°C at increment of 3°C min−1 followed by 10 min at 310°C. Samples were injected in split mode (30:1 ratio, 310°C injector temperature) and peaks quantified on the basis of their FID ion current. For GC-MS, the same column was used with helium carrier gas at 2 mL min−1 and a gradient oven temperature programmed from 110 to 300°C at the increment of 10°C min−1. Split-less injection was used and the mass spectrometer operated in scan mode over 40–500 amu (electron impact ionization) with peaks quantified on the basis of their total ion current.
Pathogen infection assay
Detached leaves of 3–4 weeks old plants were inoculated with B. cinerea IK2018 spore solution (5 * 105 spores ml−1 in half strength PDB) as previously described (Denby et al., 2004). To quantify lesion sizes, high quality digital images were acquired and processed with ImageJ.
mRNA sequencing
Wild type and rwa2-3 treated with mock (PDB alone) or infected with B. cinerea in PDB were planted in a randomized complete block design and individual leaves chosen from different plants. Half of the leaves were infected with B. cinerea while the other halves were inoculated with the half strength PDB as a control as previously described (Manabe et al., 2011). Three independent samples per genotype were harvested at 0 h as a control (“untreated”). Thereafter three samples were taken per genotype per treatment at 24 and 48 h post treatment (“mock” and “B. cinerea”). Each sample was independently extracted for total RNA using Spectrum RNA kit (Sigma-Aldrich, Denmark) with on-column DNAase treatment and the RNA integrity checked by gel and quantified with NanoDrop 2000 (ThermoScientific, USA). The RNA was then converted into sequencing libraries and sequenced at the Beijing Genome institute. This provides three independent RNAseq samples per genotype per treatment per timepoint. All reads were mapped against a synthetic transcriptome that combined the A. thaliana Ensembl (TAIR10) transcriptome with the predicted Botrytis cinerea B05.10 transcriptome using TopHat v2.0.8 (Trapnell et al., 2009) and default settings. Mapped reads were counted using HTSeq (Anders et al., 2014) using the setting -m intersection-nonempty. Differential expression was analyzed with EdgeR (Robinson et al., 2010) using a model that accounted for the genotype at RWA2 and directly tested for an interaction of the genotypes with the treatment (mock vs. B. cinerea) and an interaction with time point. All P values were adjusted to a FDR of 0.05 within EdgeR using the factorial model and are presented along with the mean corrected cpm per transcript per genotype (Supplementary Table 1).
Localization of hydrogen peroxide and peroxidase activity
Hydrogen peroxide and peroxidase activity were visualized with the use of 3,3′-diaminobenzidine (DAB; Thordal-Christensen et al., 1997). The DAB precipitate was visualized by light microscopy and stereoscope. Extracellular peroxidase activity was measured using TMB (tetramethylbenzidine) (Barcelo, 1998). Detached rosette leaves from 4-week old plants were either used directly for the assay or incubated with B. cinerea or PDB as described above. The leaves were floated on the TMB solution for 30 min. The TMB solution was removed to cuvettes and the absorbance at 654 nm was recorded.
Expression analysis of the RWA2 promoter
The DNA fragment covering the 1668 base pairs upstream of the RWA2 start codon was selected to be the promoter region (Prwa2) and it was PCR amplified using primers with USER overhangs: forward 5′- GGCTTAAUaaattgccttaaatccagcg-3′ and reverse 5′-GGCTTAAUttccgatcagagaagca-3′. The resulting PCR fragment was inserted in the USER cassette of the pLIFE41 vector containing the Kanamycin resistance gene and the BASTA® resistance gene and the resulting vector was introduced in Agrobacterium tumefaciens. The wild type Arabidopsis was transformed by floral dipping and BASTA® was used to select for positive transformants and the presence of the Prwa2:GUS construct was verified by PCR with the primers: forward 5′-gttcttattacgacaccg-3′ and reverse 5′-ccggcatagttaaagaaatc-3′.
Histochemical staining was performed as previously described (Harholt et al., 2006).
Results
Resistance against B. cinerea, surface permeability, and trichomes of acetylation mutants
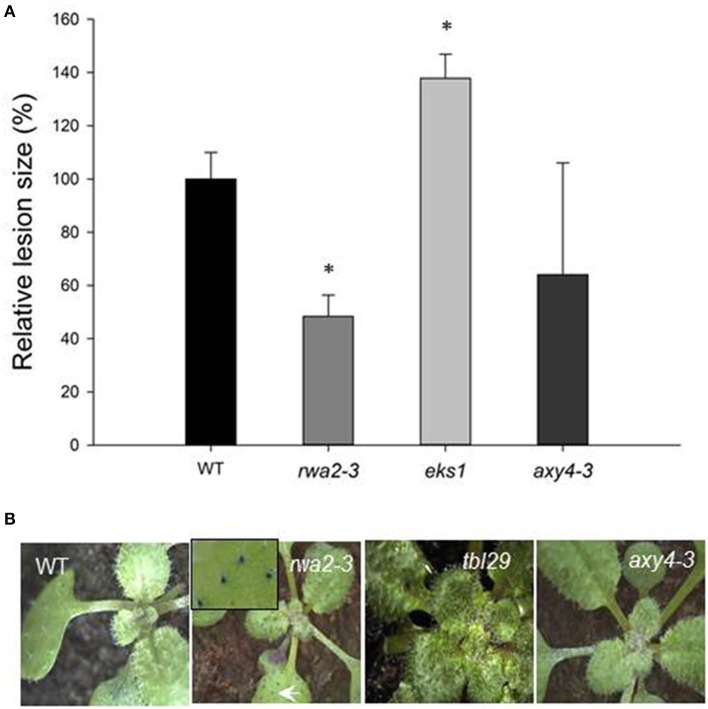
The responses of the wild type, rwa2-3, tbl29, and axy4-3 mutants to B. cinerea were analyzed side-by-side. rwa2-3 developed smaller lesions as compared to the wild type upon infection (Figure 1A) as previously shown (Manabe et al., 2011). In contrast, axy4-3 developed lesions comparable in size and appearance to the wild type, whereas tbl29 developed significantly larger lesions relative to the wild type, indicating that tbl29 is more susceptible to B. cinerea as compared to the wild type.
Figure 1.
Resistance to B. cinerea and toluidine blue staining of wild type and cell wall acetylation mutants (rwa2-3, tbl29, and axy4-3). (A) B. cinerea infection defined by lesion area measured. Relative mean values to the wild type are shown and the error bars represent standard deviations (N > 8). An asterisk indicates statistically significant difference from the wild type by Student's t-test (P < 0.05). (B) Toluidine blue staining pattern of 3-week old plants. The same results were obtained in three independent analyses. The arrowhead indicates the staining of a trichome.
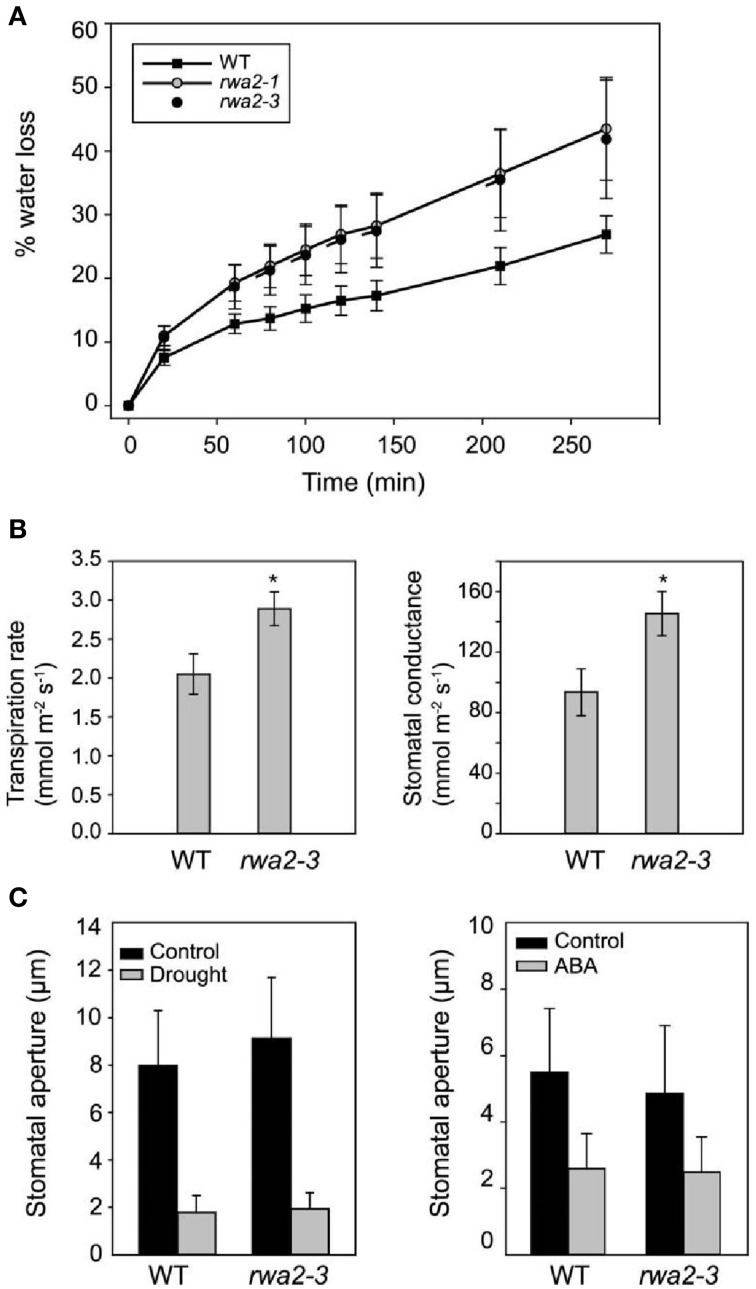
Enhanced resistance against B. cinerea is often observed among mutants defective in assembly or biosynthesis of the cuticle layer (Chassot et al., 2007; Tang et al., 2007; Voisin et al., 2009; Curvers et al., 2010; Bessire et al., 2011; Suo et al., 2013). In order to test the integrity of the cuticular layers in the acetylation mutants, toluidine blue was applied to the leaf surface. Toluidine blue is a cationic dye that does not stain leaves with an intact cuticular layer due to repulsion by the highly hydrophobic cuticle (Tanaka et al., 2004); however it stains cuticle mutants due to irregularity in the cuticle layer. When treated with toluidine blue, rwa2-3 retained the dye predominantly in trichomes (Figure 1B). In contrast, neither the wild type, axy4-3, nor tbl29 retained the dye (Figure 1B). In order to further assess leaf permeability of the rwa2 mutants, detached leaves of rwa2-1 and rwa2-3 were incubated at room temperature and the weight loss was measured over time. The rwa2 mutants lost weight faster than wild type (Figure 2A). Leaf gas exchange measurements were conducted in order to quantify transpiration rate and stomatal conductance across lamina. Transpiration rate is a measure of the actual net water loss, while stomatal conductance is a measure of conductivity for water transport across lamina, and both measures depends on the ratio of open/closed stomata but also on the integrity of the cuticle layer. Since the phenotypes of rwa2-1 and rwa2-3 were largely indistinguishable, only rwa2-3 was analyzed. The stomatal conductance and transpiration rate were increased up to 50% of the wild-type level in rwa2-3 (Figures 2B,C). It should be noted that the increased water loss and transpiration is not due to misregulation of guard cells as no difference in the stomatal aperture was observed between the wild type and rwa2 neither under drought conditions nor upon treatment with abscisic acid (ABA), which is a major regulator of stomata closure (Acharya and Assmann, 2009) (Figure 2C). There was no difference in the stomatal density between the wild type and rwa2-3 (data not shown). These results show that the leaf surface of rwa2, particularly in trichomes, is damaged and more permeable to transpiration.
Figure 2.
Enhanced surface permeability in rwa2. (A) Water loss from detached leaves of wild type, rwa2-1 and rwa2-3 plants. Rosette leaves were detached from 4-weeks old plants and incubated on the lab bench. Water loss was determined as percentage weight loss compared to time zero. The data are average ± SD of four leaves per time point. (B) Water relations of wild type and rwa2-3 leaves measured by gas exchange analysis. Values are means ± SD of four leaves from individual plants. (C) The aperture of guard cells in response to exogenous ABA and drought. The apertures of guard cells were measured in epidermal peels from leaves treated with 10 μM ABA (left) or in epidermal peels from detached leaves that had been incubated on the bench for 1 h (right) (from plants incubated at high humidity over-night). The data is average of 40 guard cells ± SD. The experiment was repeated three times with similar result. An asterisk indicate a statistically significance difference from the wild type by Student's t-test (P < 0.05).
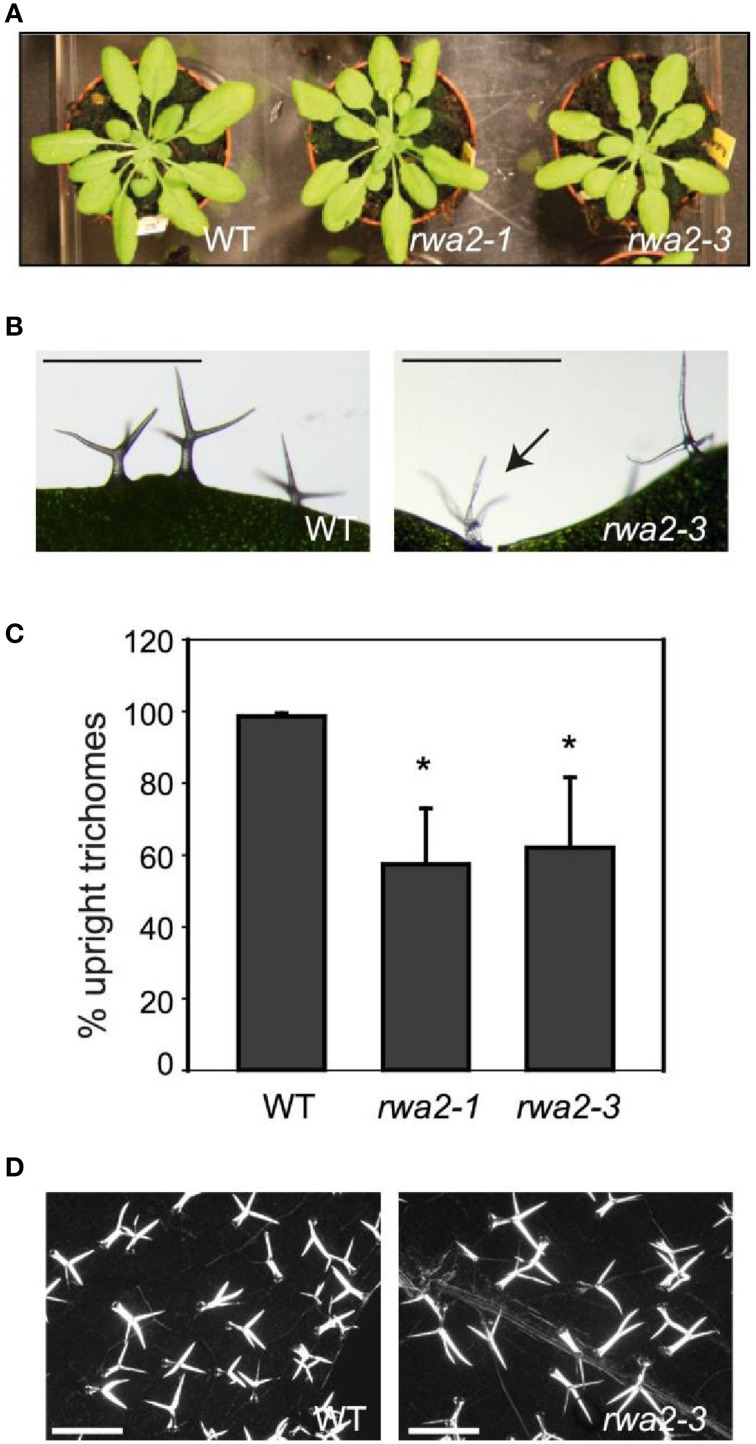
Impairment in cuticle integrity is also known to cause collapsed trichomes. Many trichomes on rwa2 leaves were found to be thinner and more transparent as compared to the wild type and were often partially or fully collapsed (Figures 3B–D). The number of fully collapsed trichomes was about 30% of the total number of leaf trichomes in rwa2 while no collapsed trichomes were observed on wild-type leaves (Figure 3C). The remaining 70% of the rwa2 trichomes were not collapsed but often appeared fragile. Macroscopically the rwa2 mutant plants (rwa2-1 and rwa2-3) appear comparable to the wild-type plants under the standard growth conditions in the green house and growth chambers (Figure 3A). Collapsed trichomes has also been seen in the tbr-2 mutant (Suo et al., 2013), wherein the deposition of paracrystalline cellulose is impaired in trichomes, resulting in loss of trichome birefringence under UV light (Potikha and Delmer, 1995). Trichome birefringence of rwa2 appeared indistinguishable from that of wild type under the conditions tested (Figure 3D). Apart from these morphological differences, trichomes on rwa2 leaves develop normally and have the typical three-branched structure with a similar height to those in the wild type.
Figure 3.
Trichome integrity is altered in rwa2. (A) Macroscopic appearance of 3-weeks old wild type, rwa2-1 and rwa2-3 grown in growth chamber with 12 h dark/light cycle. (B) Trichomes of the wild type (right) and rwa2-3 (left). The arrow indicates a collapsed trichome on rwa2-3. The scale bar equals 0.5 mm. (C) The percentage of “normal looking” trichomes [as depicted in (B left)] on the leaves of wild type, rwa2-1 and rwa2-3. Leaves were destained in ethanol followed by quantification of the total number of trichomes and the number of collapsed trichomes when viewed under the microscope. The data is the average of three independent experiments ± SD. The number of non-collapsed trichomes on rwa2-1 and rwa2-3 leaves are significant different from wild type as tested by Student's t-test (P < 0.05) as indicated by the asterisks. (D) Trichome birefringence of the wild type (left) and rwa2-3 (right) leaves. Scale bar equals 1 mm.
Cuticle and cell wall architectures and trichome morphology are altered in rwa2
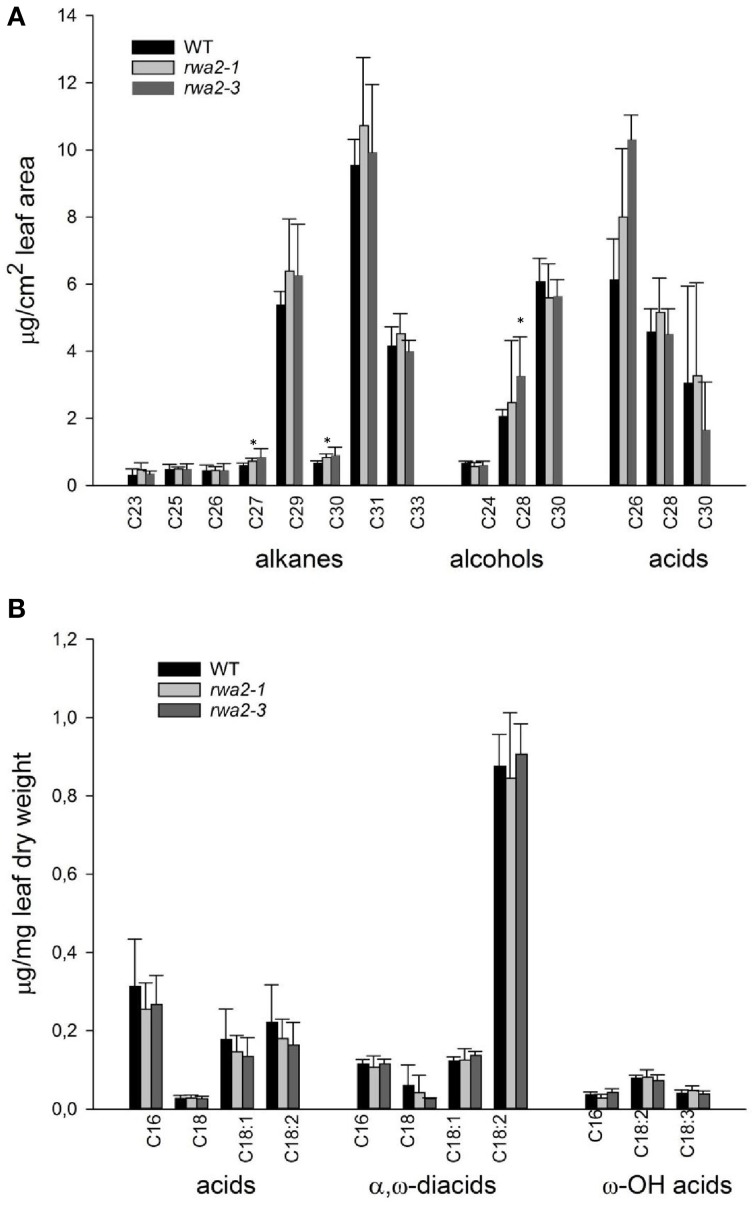
To test if cuticle content and/or composition have been affected in rwa2, the chemical composition of the cuticle was determined by gas chromatography (Bessire et al., 2007). No significant difference in the composition or content of the cuticular wax and cutin monomers was observed between the wild type and the rwa2 mutants (Figures 4A,B). Therefore, biosynthesis and delivery of the wax and cutin components appear to occur normally in rwa2.
Figure 4.
Epicuticular wax and cutin composition analysis of leaves of 4-weeks old wild type, rwa2-1 and rwa2-3 plants. (A) Composition of epicuticular waxes extracted by dipping in chloroform and derivatized with BSTFA. (B) Composition of depolymerized residual-bound lipids extracted extensively with methanol/chloroform followed by NaOMe-catalyzed acetylation. Values are means of at least five independent samples and the error bars represent the standard deviations. An asterisk indicate a statistically significance difference from the wild type by Student's t-test (P < 0.05).
In order to gain insights into the ultrastructure of the cuticle and cell wall, transmission electron microscopy (TEM) was performed on the leaf epidermal cells. In the wild type, the cuticle appeared compact and highly electron dense while the cell wall layer underneath was relatively electron opaque (Figure 5A). The cuticle layers in rwa2-1 and rwa2-3 appeared notably more diffused than that in the wild type (Figures 5B,C) with the average thickness increased by approximately 50% of that of the wild type (Table 1). The similar thickness values and the difference between the genotypes were observed in two independent experiments using independently grown plants. Apart from the change in the cuticle layer, the cell wall layer in rwa2 showed marked difference from the wild type. The cell wall layer in rwa2 was interspersed with electron-dense deposits, which were rarely seen in the wild type (Table 1). In addition, the average cell wall thickness in rwa2 was approximately 30–50% larger than that in the wild type (Table 1). Hence, we conclude that both the cuticle and cell wall ultrastructures were altered in rwa2.
Figure 5.
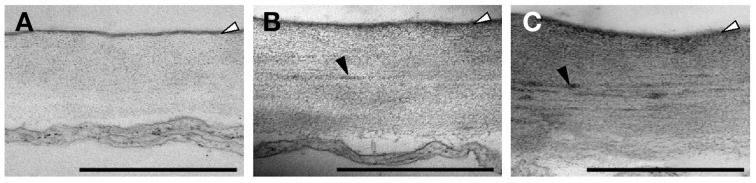
Ultrastructural organization of the epidermis. Electron microscopic images of ultra-thin sections of the outer cell wall in the upper leaf epidermis were obtained for the wild type (A), rwa2-1 (B), and rwa2-3 (C). The cuticle layer is indicated by the white arrowheads. The electron dense deposits are indicated by black arrowheads. All scale bars equal 500 nm.
Table 1.
Ultrastructure of the cell wall and cuticle layer in the wild type and the rwa2 mutants.
| Strain | aThickness (nm) | bNumber of electron dense deposits per image | cThickness of the trichome base (nm) | |
|---|---|---|---|---|
| Cell wall | Cuticle layer | |||
| Wild type | 417 ± 93 | 19 ± 5.0 | 0.83 ± 1.2 | 67 ± 0.67 |
| rwa2-1 | 558 ± 109* | 30 ± 8.0* | 7.3 ± 4.2* | ND |
| rwa2-3 | 642 ± 154* | 32 ± 8.0* | 11 ± 8.6* | 100 ± 13* |
Three hundred randomly selected positions derived from three independent plants were analyzed.
Six independent, equal sized images obtained at the same magnification were analyzed.
Three independent sections, each cutting through the middle of a trichome, were analyzed.
Student's t-test, P < 0.05. ND, not determined.
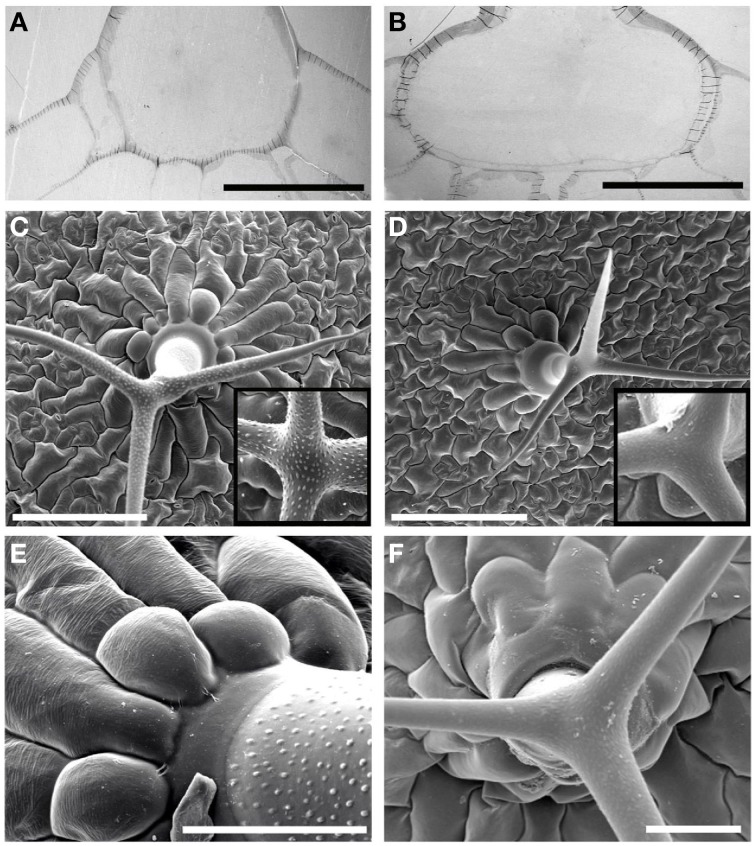
Given the enhanced permeability and structural impairments of rwa2 trichomes (Figures 1B, 3B), the traverse sections of trichomes were analyzed by TEM. It was noticed that the base of the trichomes in rwa2-3 was notably larger than that in the wild type (Figures 6A,B). The average width of three traverse sections, each cutting through the middle of a trichome increased in rwa2-3 by 50% of the wild type level (Table 1). The increase in size of the rwa2 trichome base was confirmed by SEM of trichomes (Figures 6C,D). SEM analysis revealed additional anatomical differences between the wild-type and rwa2-3; the papillae were either entirely missing or reduced in size in rwa2-3 as compared to the wild type (Figures 6C–F). Furthermore, in some cases the trichome base in rwa2-3 was sunken (Figure 6F).
Figure 6.
Ultrastructural organization of the trichomes. Transverse ultra-thin sections of the trichomes in the wild type (A), and rwa2-3 (B). Scale bars equal 50 μm. Lines across the cell wall are artifacts due to wrinkling of the ultra-thin sections. Scanning electron micrographic images of trichomes in the wild type (C) and the rwa2-3 (D). Scale bars equal 200 μm. Inserts (100 × 100 μm) represent close-up images of trichome branches. Close-up images of the trichome base in the wild type (E) and rwa2-3 (F). Scale bars equal 50 μm. All images were acquired by electron microscopy.
Global transcriptomic reprogramming occurred in rwa2
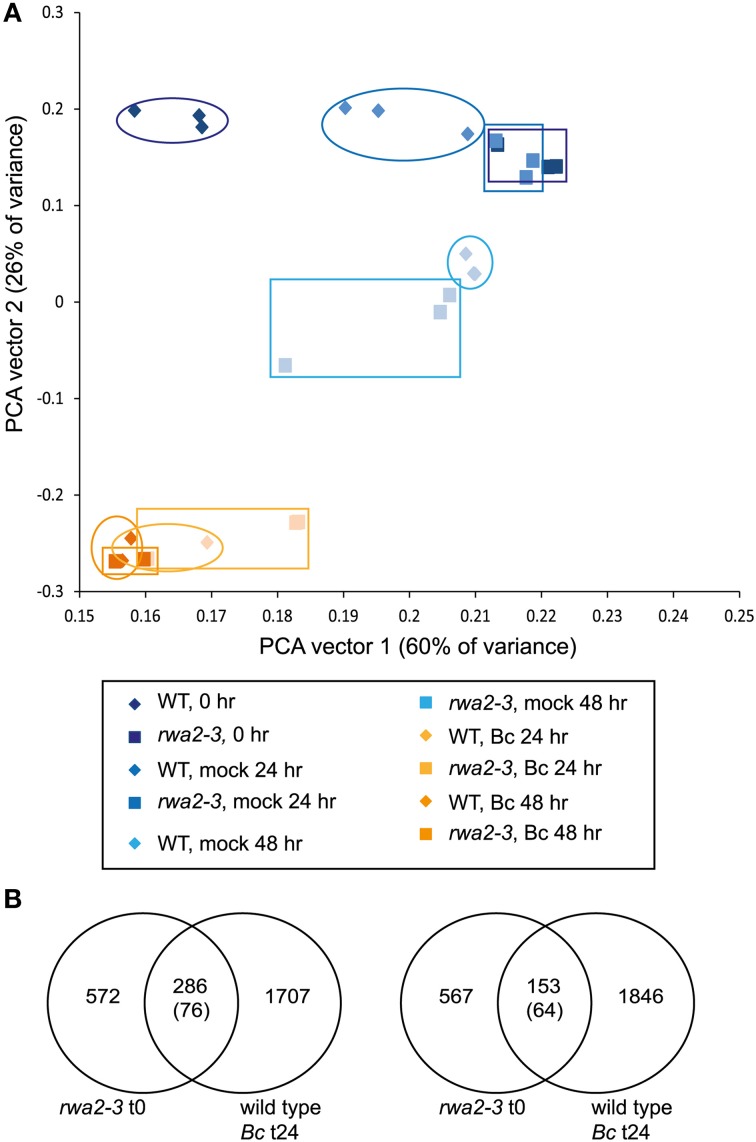
We hypothesized that the surface damage caused by the rwa2 mutation could have a significant impact on plant stress responses and that transcriptome profiling would shed light on which stress response(s) is affected. Wild-type and rwa2-3 leaves were either untreated or treated with mock [potato dextrose broth (PDB) only] or B. cinerea spore solution in PDB for 24 and 48 h and mRNA sequencing was performed (Supplementary Table 1). The largest difference in the transcriptome profile was observed between the untreated wild type and rwa2-3, indicating that the untreated rwa2-3 perceives the environment differently from the wild type (Table 2). Out of 21,178 transcripts that were sequenced in both the wild-type and rwa2-3, 1650 transcripts showed statistically significant differential abundance: 857 genes up-regulated and 763 genes down-regulated [log2 fold ≥ 2, false discovery rate (FDR) ≤ 0.05]. The mock and B. cinerea treatments led to smaller differences between rwa2-3 and the wild type in terms of the number of genes with altered expression levels at both 24 h and 48 h after infection (Table 2). Principal component analysis showed that genotype and treatments describe 86% of the total transcriptomic variance detected (Figure 7). The first vector (PCA vector 1) largely describes the differences between the genotypes at the untreated and early mock treated samples (untreated wild type vs. rwa2-3), while the second vector (PCA vector 2) largely describes the response of the genotypes to infection with B. cinerea (untreated, mock, and B. cinerea treatments). In the wild type, the mock treatment (detachment of leaves followed by incubation in a water-agar medium and application of PDB) caused a notable change in the transcriptome profiles. Interestingly, both the untreated and mock-treated rwa2-3 for 24 h showed a significant overlap with the mock-treated wild type. Upon treatment with B. cinerea, the transcriptomes of the wild type and rwa2-3 showed an even higher degree of overlap (Figure 7). This indicates that the rwa2-3 responds to B. cinerea similarly to the wild type even though their initial transcriptomes are highly divergent.
Table 2.
Number of genes with altered expression in rwa2 as compared to the wild type as identified by mRNA sequencing (log2 fold ≥ 2, FDR ≤ 0.05).
| Treatment | Up-regulated | Down-regulated | |
|---|---|---|---|
| Untreated | 857 | 793 | |
| Mock treatment | 24 h | 320 | 40 |
| 48 h | 134 | 10 | |
| Botrytis treatment | 24 h | 415 | 319 |
| 48 h | 32 | 5 |
Figure 7.
Global transcript analysis. (A) Shown is a principal component analysis of the entire dataset with the individual samples plotted. Wild type samples are shown as diamonds and rwa2 samples are shown as squares with the blue to orange transition showing the time course of the experiment. The first two PCA vectors are utilized that explain 86% of the total variance. (B) VENN diagrams showing the overlap of genes differentially expressed (left side: upregulated genes, right side repressed genes) in rwa2 control vs. wild type control, and wild type infected with B. cinerea vs. wild type mock across the time points. The genes that are differentially expressed in rwa2 control vs. the corresponding wild-type samples have a high overlap with the expression profile of genes in wild type infected with B. cinerea. The numbers in brackets represent the overlap expected by chance. The overlaps are highly significant both for induced (P < 0.001) and for repressed genes (P < 0.001) as determined by χ2-test.
Because the major difference was found in the untreated rwa2-3 and wild type, further analysis focused on these samples. Analysis of gene ontology (GO) categories by the AmiGo software (Ashburner et al., 2000) showed that a large fraction of the up-regulated genes in rwa2-3 belong to categories relating to both abiotic and biotic stress responses, with notable examples of responses to and transport of organic and inorganic substances, as well as detoxification processes and oxidative stress responses (Supplementary Table 2). Several of the genes that are up-regulated in untreated rwa2-3 have been shown to be important for resistance against B. cinerea (Table 3 and references therein). This includes lipid transfer proteins (AT4g12470, AT4G12480, AT4g12490) and peroxidases (AT2g37130, AT5g39580, and AT5g64129) that were up-regulated in a cutinase-overexpressing Arabidopsis transgenic line as compared to the wild type (Chassot et al., 2007). Moreover, overexpression of each of these transcripts in the wild-type background was sufficient to cause enhanced resistance against B. cinerea (Chassot et al., 2007). Hence the enhanced resistance of rwa2-3 against B. cinerea can be explained by the constitutive and coordinated up-regulation of these defense related genes.
Table 3.
Defense related genes upregulated in the untreated rwa2-3 as compared to the untreated wild type (N = 3, FDR < 0.05).
| Gene description | Locus tag | Fold change rel. to WT |
|---|---|---|
| Lipid transfer proteins | At4g12480a,b | 301 |
| At4g12490a | 492 | |
| At2g38530a | 10 | |
| At4g12470/AZI1a | 37 | |
| At2g37870c | 111 | |
| Peroxidases | At2g37130a | 5.5 |
| At5g64120a | 6.5 | |
| Defensins | At5g44430/PDF1.2Cd | 4.5 |
| At1g75830/PDF1.1d | 315 | |
| Protease inhibitors | At2g38870a | 5 |
| At5g43580e | 256 | |
| Trypsin inhibitors | At2g43510a | 10 |
| At1g73260f | 12 | |
| ELI-3 defensive protein | At4g37990g | 10.5 |
| PR4 | At3g04720h | 3 |
| GDSL lipase 1 (GLIP1) | At5g40990i | 96 |
| PROPEP3 | At5g64905j | 12 |
| Glycolate oxidase 3 (GOX3) | At4g18360k | 41 |
In contrast to the up-regulated GO categories, the most overrepresented biological process category among the down-regulated genes was “response to hormones” (Supplementary Table 3). Manual inspection of genes that specifically respond to treatment with auxin, ABA, brassinosteroid (BR), cytokinin (CK), ethylene, gibberellic acid, and jasmonic acid (JA) (Nemhauser et al., 2006) showed that several transcripts that are up-regulated upon treatment with CK, auxin, and JA were coordinately down-regulated in rwa2-3 untreated leaves (Supplementary Figure 1). However, the rwa2-3 mutant was found to retain the wild-type level of response to these hormones; when seedlings were grown on nutrient agar plates supplemented with the hormones JA, CK, auxin or ABA no difference in general growth or root inhibition was observed between wild type and rwa2-3 (Supplementary Figure 2).
The transcript profile of the untreated rwa2-3 relative to the untreated wild type was compared to publically available microarray data by using Signature Tool in the Genevestigator software (Zimmermann et al., 2004). The 300 genes that showed the highest differential expression as compared to the wild type and with the lowest FDR values, hence highest confidence, were subjected to the analysis. Among the top 30 transcript datasets that showed similarities to the rwa2-3 transcript profile, experiments inducing oxidative stress (growth under high light, cold, drought; CAT2HP1 overexpressor; catalase2-1) were highly represented (Supplementary Table 4). In addition, consistent with the GO analysis, transcript profiles upon changes in lipid metabolism or signaling (suppressor of SA insensitivity-1, application of phytoprostane A1), exogenous application of xenobiotics (phenanthrene, fenclorin, sulfometuron methyl), alteration of phytohormones (ARR22 overexpression, application of salicylic acid), and microbial infection (Golovinomyces cichoracearum, Alternaria brassicicola) were found.
Extracellular peroxidases accumulate in the rwa2 mutant
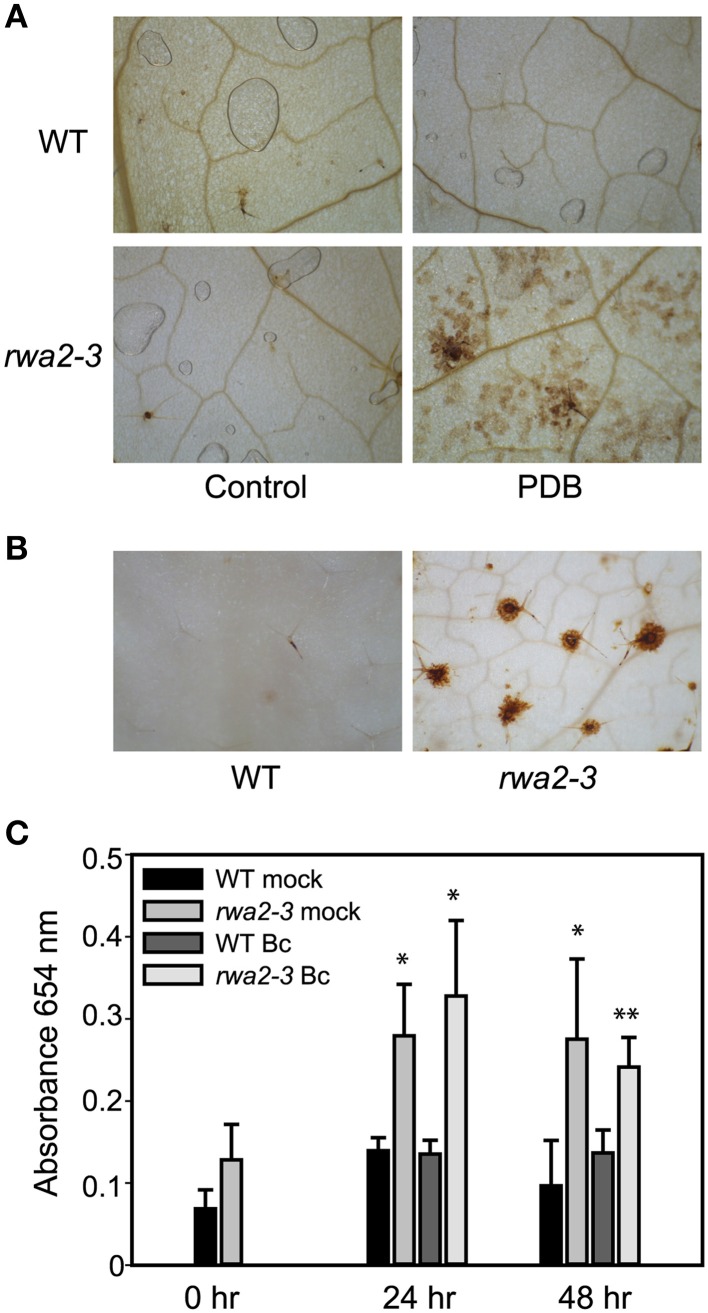
To test if rwa2 experiences increased oxidative stress, we conducted 3,3′-diaminobenzidine (DAB) staining to visualize H2O2 production (Thordal-Christensen et al., 1997). DAB staining did not show notable accumulation of H2O2 on the leaf surface of the wild type and the rwa2 mutants (Figure 8A). On the other hand, the mock treatment (PDB) resulted in an elevated H2O2 production in rwa2-3 leaves as compared to the wild type (Figure 8A). To test peroxidase activities, the leaves from the same developmental stage were incubated with DAB in the presence of H2O2 as previously described (Thordal-Christensen et al., 1997). Intense DAB staining around the trichome base was observed for untreated rwa2-3 leaves whereas no notable staining was observed for the wild type (Figure 8B). Consistently, the measurement of extracellular peroxidase activity with the tetramethylbenzidine assay (Barcelo, 1998) shows that the rwa2-3 mutant possesses a slightly higher extracellular peroxidase activity as compared to the wild type (Figure 8C, 0 h), and the activity increased further by 24 h upon treatment with mock (PDB alone) or B. cinerea spores in PDB to the comparable levels. It should be noted that the leaves at the same developmental stage were used and there is no difference in leaf sizes between the wild type (1.77 ± 0.23 cm2, N = 10) and the rwa2-3 mutant (1.92 ± 0.33 cm2, N = 11). Therefore, the rwa2 mutant accumulates a higher level of peroxidase activity around the trichome base and the activity is further induced upon treatment by components in PDB.
Figure 8.
Increased peroxidase activity and hydrogen peroxide accumulation in rwa2. (A) DAB staining for hydrogen peroxide in untreated (left) and in response to treatment with potato dextrose broth (PDB) (right). Five micro liters of 2% (v/v) PDB was placed on each side of the mid vain for 48 h followed by DAB staining. Chlorophylls were extracted in 96% (v/v) ethanol. (B) Peroxidase activities accumulate at trichome bases in uninfected rwa2 leaves. Leaves were stained with DAB in the presence of 0.1% (v/v) H2O2 for 1 h. Chlorophylls were extracted in 96% (v/v) ethanol. (C) Extracellular peroxidase activity in the wild type and rwa2-3 leaves. Detached leaves were treated with mock (PDB) or B. cinerea for 24 and 48 h. To measure peroxidase activity, the leaves were floated with the adaxial side downwards in TMB and H2O2. Peroxidase activity was measured as absorbance at 654 nm. The data is average of three samples ± SD. The asterisks indicate significant difference between wild type and rwa2-3 as determined by Two-Way ANOVA test (*P < 0.05; **P < 0.01).
The promoter of RWA2 is active in trichomes
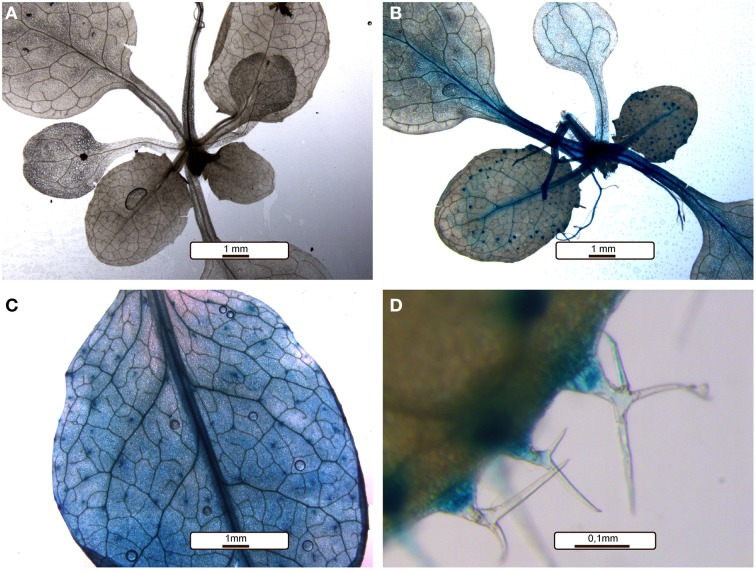
RWA2 is expressed in root, stem, leaf and flower (Lee et al., 2011; Manabe et al., 2011) with specific expression in the xylem in roots and secondary xylem in stem (Lee and Rose, 2011; Manabe et al., 2011). However, tissue-type specific expression pattern of RWA2 in leaves has not yet been reported. Ten independent lines of transgenic lines expressing a transcriptional fusion of the RWA2 promoter region (from −1600 to +50 bp relative to A in the start codon) and the reporter gene β-glucuronidase (GUS) were analyzed. As shown in Figure 9, histochemical staining with 5-Bromo-4-chloro-3-indolyl-β-d-glucuronide revealed promoter RWA2:GUS expression in leaves and notably in trichomes. In light of the rwa2 trichome phenotypes, strong GUS expression in this tissue supports the notion that RWA2 expression is required for structural integrity of leaf surface and particularly trichome bases.
Figure 9.
RWA2 is expressed abundantly in leaves, particularly in trichomes in Arabidopsis. RWA2 expression was analyzed by GUS staining in 3-weeks old Arabidopsis plants, 10 independent transformed lines were tested. (A) The negative control (promoter-less GUS line). (B) A representative RWA2 promoter:GUS fusion line. (C,D) Close-up images of leaves and trichomes in a representative RWA2 promoter:GUS fusion line.
Discussion
We discovered that the structural integrity of Arabidopsis leaf surface was severely impaired in rwa2 mutants. The cell wall layer in the epidermis was morphologically distinct from that of the wild type with accumulation of numerous electron-dense deposits (Figure 5), indicative of abnormal cell wall architecture. Notably, rwa2 showed enhanced permeability to the cationic dye toluidine blue exclusively in trichomes (Figure 1), and trichomes appeared fragile; the base of the trichome was enlarged, often either ruptured or sunken (Figure 6) and a large fraction of the trichomes on rwa2 was collapsed (Figure 3). In addition, the papillae on the trichome surface were dramatically reduced in size (Figure 6). These phenotypes are often associated with mutants that are defective in cuticle integrity and biosynthesis (i.e., transgenic CUTE plants that overexpresses a cutinase and lacs2, lcr, fdh, pec1, and gl1 mutants) (Serrano et al., 2014). Although the cutin monomer and wax compositions were not altered in rwa2 as compared to the wild type, the cuticle layer in epidermis was notably diffused and thicker (Table 1, Figure 5). Moreover, mRNA sequencing revealed a massive reprogramming of the transcriptome in the rwa2-3 mutant characterized by coordinated induction of genes related to abiotic and biotic stress responses (Table 3, Figure 7). These results demonstrate that cell wall acetylation plays an important role in maintaining the surface structural integrity in Arabidopsis leaves and its impairment results in global stress responses in plants. Because it has previously been proposed that RWA2 functions as an acetyl-CoA transporter localizing to the Golgi apparatus (Manabe et al., 2011), it was possible that the cytosolic acetyl-CoA pool had been altered in rwa2 and this might have caused the observed phenotypes. In the cytosol, acetyl-CoA is converted to malonyl-CoA that feeds into the synthesis of a range of phytochemicals including wax and cutins (Oliver et al., 2009). It has been shown that overexpression of an ATP citrate lysase that synthesizes acetyl-CoA in the cytosol of Arabidopsis resulted in 30% increase in wax loading and the cutin monomer octadecadie-1,18-dioic acid content (Xing et al., 2014). In contrast, rwa2 contained the wild-type level of cutin and wax components (Figure 4). Therefore, it was concluded that the cytosolic acetyl-CoA pool is unlikely to be altered at a significant extent and to cause the observed phenotypes.
Because the increased permeability and enhanced resistance to B. cinerea were only observed in rwa2 and not in tbl29 and axy4-3 (Figure 1), we speculate that the reduced acetyl groups in pectins contribute to the observed phenotypes by interfering with the normal cell wall and cuticle assembly. Earlier studies have shown that during the development of the cuticle, cutin polymers become progressively impregnated with the cell wall polysaccharides, particularly pectins (Schieferstein and Loomis, 1959). Pectinase treatment has been reported effective for releasing the cuticles (Orgell, 1955; Baker and Procopiou, 1975) and isolated cuticle from pear leaf stains with ruthenium red, a stain widely used to detect pectins (Norris and Bukovac, 1968). Treatment with EDTA and oxalic acid or ammonium oxalate, which are often used for isolation of pectins, have also been shown to be effective in isolating cuticles (Huelin and Gallop, 1951). In rwa2, the excess hydroxyl groups, as a result of reduced acetylation, may form atypical crosslinking with the cutins leading to abnormal cuticle assembly. Alternatively, though mutually nonexclusive, cell wall acetylation may impact the transport of cutin and wax across the cell wall. Both cutins and waxes are long chain fatty acid derivatives and must cross the highly hydrophilic cell wall layer and the transport process is thought to involve apoplastic carriers, possibly lipid transport proteins (LTPs) (Yeats and Rose, 2013; Hurlock et al., 2014). Notably, the expression levels of 10 genes coding for LTPs and LTP like proteins were shown to be up-regulated in rwa2 as compared to the wild type, which may represent a compensatory response (Table 3).
Transcriptomic profile of the rwa2-3 mutant was dramatically different from that of the wild type (Table 2, Figure 7) with a coordinate up-regulation genes involved in responses to detoxification and oxidative stress (Supplementary Table 2). Trichomes are involved in a range of protective mechanisms from adverse environmental conditions including protection from UV and excessive light (Karabourniotis and Bornman, 1999; Franke et al., 2005), and heavy metals detoxification (Freeman et al., 2006; Sarret et al., 2009; Marmiroli et al., 2010). Moreover, disturbance of cuticle biosynthesis or overexpression of a cutinase, as well as mechanical stress of leaf epidermis (i.e., wounding by forceps, soft rubbing by fingers), have been shown to induce reactive oxygen species and resistance to B. cinerea (L'Haridon et al., 2011; Benikhlef et al., 2013). It is likely that altered cell wall and cuticle assembly, as discussed above, causes structural and functional impairments in the trichomes and cuticle layer causing the induction of detoxification and oxidative stress responses, and this may ultimately lead to enhanced resistance against B. cinerea in rwa2. This notion is further supported by the previous results that ectopic overexpression of individual peroxidase genes induced in rwa2-3 was sufficient to cause enhanced resistance against B. cinerea in Arabidopsis (Chassot et al., 2007). Peroxidases are thought to cause cell wall stiffening in the presence of H2O2 through oxidative formation of covalent bonds between aromatic cell wall components (Francoz et al., 2015 and reference therein). It is possible that the induced levels of H2O2 and peroxidases (both transcripts and activities) (Figure 8, Table 3) might cause an enhanced cell wall fortification in and around the trichomes in the rwa2 mutants, causing restricted infection by the fungus.
Author contributions
All authors contributed to either the conception, design of the work or the acquisition, analysis and interpretation of data, drafted and approved the manuscript.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We acknowledge the Center for Advanced Bioimaging (CAB), Faculty of Science, University of Copenhagen for microscopy facilities and Catherine Skrzynski Nielsen and Piotr Binczycki for skilled preparation of the material for TEM. This work was supported by the Danish Advanced Technology Foundation [Biomass for the 21st century, grant number 001-2011-4]; the Danish Council for Strategic Research [Plant Power, grant number 12-131834]; EU FP7 People Programme Marie Curie Actions [PHOTO.COMM, grant number 317184]; The Villum Foundation [grant number VKR023371]; the U.S. National Science foundation [IOS grant numbers 1339125 and 1021861]; the USDA National Institute of Food and Agriculture [Hatch project number CA-D-PLS-7033-H]; the Danish National Research Foundation [DNRF99]; and the U.S. Department of Energy, Office of Science, Office of Biological and Environmental Research [contract no. DE–AC02–05CH11231].
Supplementary material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2015.00550
References
- Acharya B. R., Assmann S. M. (2009). Hormone interactions in stomatal function. Plant Mol. Biol. 69, 451–462. 10.1007/s11103-008-9427-0 [DOI] [PubMed] [Google Scholar]
- Anders S., Pyl P. T., Huber W. (2014). HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169. 10.1093/bioinformatics/btu638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner M., Ball C. A., Blake J. A., Botstein D., Butler H., Cherry J. M., et al. (2000). Gene ontology: tool for the unification of biology. The gene ontology consortium. Nat. Genet. 25, 25–29. 10.1038/75556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker E. A., Procopiou J. (1975). Cuticles of citrus species - composition of intracuticular lipids of leaves and fruits. J. Sci. Food Agric. 26, 1347–1352. 10.1002/jsfa.2740260913 [DOI] [Google Scholar]
- Barcelo R. A. (1998). Hydrogen peroxide production is a general property of the lignifying xylem from vascular plants. Ann. Bot. 82, 97–103. 10.1006/anbo.1998.0655 [DOI] [Google Scholar]
- Benikhlef L., L'Haridon F., Abou-Mansour E., Serrano M., Binda M., Costa A., et al. (2013). Perception of soft mechanical stress in Arabidopsis leaves activates disease resistance. BMC Plant Biol. 13:133. 10.1186/1471-2229-13-133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessire M., Borel S., Fabre G., Carraca L., Efremova N., Yephremov A., et al. (2011). A Member of the PLEIOTROPIC DRUG RESISTANCE family of ATP binding cassette transporters is required for the formation of a functional cuticle in Arabidopsis. Plant Cell 23, 1958–1970. 10.1105/tpc.111.083121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessire M., Chassot C., Jacquat A. C., Humphry M., Borel S., Petetot J. M. C., et al. (2007). A permeable cuticle in Arabidopsis leads to a strong resistance to Botrytis cinerea. EMBO J. 26, 2158–2168. 10.1038/sj.emboj.7601658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaventure G., Beisson F., Ohlrogge J., Pollard M. (2004). Analysis of the aliphatic monomer composition of polyesters associated with Arabidopsis epidermis: occurrence of octadeca-cis-6, cis-9-diene-1,18-dioate as the major component. Plant J. 40, 920–930. 10.1111/j.1365-313X.2004.02258.x [DOI] [PubMed] [Google Scholar]
- Carpita N. C., Gibeaut D. M. (1993). Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J. 3, 1–30. 10.1111/j.1365-313X.1993.tb00007.x [DOI] [PubMed] [Google Scholar]
- Chassot C., Nawrath C., Metraux J. P. (2007). Cuticular defects lead to full immunity to a major plant pathogen. Plant J. 49, 972–980. 10.1111/j.1365-313X.2006.03017.x [DOI] [PubMed] [Google Scholar]
- Curvers K., Seifi H., Mouille G., de Rycke R., Asselbergh B., Van Hecke A., et al. (2010). Abscisic acid deficiency causes changes in cuticle permeability and pectin composition that influence tomato resistance to Botrytis cinerea. Plant Physiol. 154, 847–860. 10.1104/pp.110.158972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Coninck B. M. A., Sels J., Venmans E., Thys W., Goderis I. J. W. M., Carron D., et al. (2010). Arabidopsis thaliana plant defensin AtPDF1.1 is involved in the plant response to biotic stress. New Phytol. 187, 1075–1088. 10.1111/j.1469-8137.2010.03326.x [DOI] [PubMed] [Google Scholar]
- Del Rio J. C., Marques G., Rencoret J., Martinez A. T., Gutierrez A. (2007). Occurrence of naturally acetylated lignin units. J. Agric. Food Chem. 55, 5461–5468. 10.1021/jf0705264 [DOI] [PubMed] [Google Scholar]
- Denby K. J., Kumar P., Kliebenstein D. J. (2004). Identification of Botrytis cinerea susceptibility loci in Arabidopsis thaliana. Plant J. 38, 473–486. 10.1111/j.0960-7412.2004.02059.x [DOI] [PubMed] [Google Scholar]
- Francoz E., Ranocha P., Nguyen-Kim H., Jamet E., Burlat V., Dunand C. (2015). Roles of cell wall peroxidases in plant development. Phytochemistry 112, 15–21. 10.1016/j.phytochem.2014.07.020 [DOI] [PubMed] [Google Scholar]
- Franke R., Briesen I., Wojciechowski T., Faust A., Yephremov A., Nawrath C., et al. (2005). Apoplastic polyesters in Arabidopsis surface tissues–a typical suberin and a particular cutin. Phytochemistry 66, 2643–2658. 10.1016/j.phytochem.2005.09.027 [DOI] [PubMed] [Google Scholar]
- Freeman J. L., Zhang L. H., Marcus M. A., Fakra S., McGrath S. P., Pilon-Smits E. A. (2006). Spatial imaging, speciation, and quantification of selenium in the hyperaccumulator plants Astragalus bisulcatus and Stanleya pinnata. Plant Physiol. 142, 124–134. 10.1104/pp.106.081158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gille S., de Souza A., Xiong G. Y., Benz M., Cheng K., Schultink A., et al. (2011). O-Acetylation of Arabidopsis hemicellulose xyloglucan requires AXY4 or AXY4L, proteins with a TBL and DUF231 domain. Plant Cell 23, 4041–4053. 10.1105/tpc.111.091728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gille S., Pauly M. (2012). O-acetylation of plant cell wall polysaccharides. Front. Plant Sci. 3:12. 10.3389/fpls.2012.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harholt J., Jensen J. K., Sørensen S. O., Orfila C., Pauly M., Scheller H. V. (2006). ARABINAN DEFICIENT 1 is a putative arabinosyltransferase involved in biosynthesis of pectic arabinan in Arabidopsis. Plant Physiol. 140, 49–58. 10.1104/pp.105.072744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Blanco C., Feng D. X., Hu J., Sanchez-Vallet A., Deslandes L., Llorente F., et al. (2007). Impairment of cellulose synthases required for Arabidopsis secondary cell wall formation enhances disease resistance. Plant Cell 19, 890–903. 10.1105/tpc.106.048058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelin F. E., Gallop R. A. (1951). Studies in the natural coating of apples.1. Preparation and Properties of Fractions. Aust. J. Sci. Res. Ser. B Biol. Sci. 4, 526–532. [Google Scholar]
- Huffaker A., Pearce G., Ryan C. A. (2006). An endogenous peptide signal in Arabidopsis activates components of the innate immune response. Proc. Natl. Acad. Sci. U.S.A. 103, 10098–10103. 10.1073/pnas.0603727103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlock A. K., Roston R. L., Wang K., Benning C. (2014). Lipid trafficking in plant cells. Traffic 15, 915–932. 10.1111/tra.12187 [DOI] [PubMed] [Google Scholar]
- Ishii T. (1997). O-acetylated oligosaccharides from pectins of potato tuber cell walls. Plant Physiol. 113, 1265–1272. 10.1104/pp.113.4.1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Z. H., Cash M., Darvill A. G., York W. S. (2005). NMR characterization of endogenously O-acetylated oligosaccharides isolated from tomato (Lycopersicon esculentum) xyloglucan. Carbohydr. Res. 340, 1818–1825. 10.1016/j.carres.2005.04.015 [DOI] [PubMed] [Google Scholar]
- Karabourniotis G., Bornman J. F. (1999). Penetration of UV−A, UV−B and blue light through the leaf trichome layers of two xeromorphic plants, olive and oak, measured by optical fibre microprobes. Physiol. Plant. 105, 655–661. 10.1034/j.1399-3054.1999.105409.x [DOI] [Google Scholar]
- Kiedrowski S., Kawalleck P., Hahlbrock K., Somssich I. E., Dangl J. L. (1992). Rapid activation of a novel plant defense gene is strictly dependent on the Arabidopsis RPM1 disease resistance locus. EMBO J. 11, 4677–4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein-Marcuschamer D., Oleskowicz-Popiel P., Simmons B. A., Blanch H. W. (2010). Technoeco-nomic analysisofbiofuels, Awiki-based platform for lignocellulosic biorefineries. Biomass Bioenergy 34, 1914–1921. 10.1016/j.biombioe.2010.07.033 [DOI] [Google Scholar]
- Kourounioti R. L. A., Band L. R., Fozard J. A., Hampstead A., Lovrics A., Moyroud E., et al. (2013). Buckling as an origin of ordered cuticular patterns in flower petals. J. R. Soc. Interface 10:20120847. 10.1098/rsif.2012.0847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laluk K., Mengiste T. (2011). The Arabidopsis extracellular UNUSUAL SERINE PROTEASE INHIBITOR functions in resistance to necrotrophic fungi and insect herbivory. Plant J. 68, 480–494. 10.1111/j.1365-313X.2011.04702.x [DOI] [PubMed] [Google Scholar]
- Lee C., Teng Q., Zhong R., Ye Z. H. (2011). The four Arabidopsis reduced wall acetylation genes are expressed in secondary wall-containing cells and required for the acetylation of xylan. Plant Cell Physiol. 52, 1289–1301. 10.1093/pcp/pcr075 [DOI] [PubMed] [Google Scholar]
- Lee S.-J., Rose J. K. C. (2011). Characterization of the plant cell wall proteome using high-throughput screens in The Plant Cell Wall, ed Popper Z. (Humana Press; ), 255–272. 10.1007/978-1-61779-008-9_18 [DOI] [PubMed] [Google Scholar]
- L'Haridon F., Besson-Bard A., Binda M., Serrano M., Abou-Mansour E., Balet F., et al. (2011). A permeable cuticle is associated with the release of reactive oxygen species and induction of innate immunity. PLoS Pathog. 7:e1002148. 10.1371/journal.ppat.1002148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Brader G., Palva E. T. (2008). Kunitz trypsin inhibitor: an antagonist of cell death triggered by phytopathogens and fumonisin b1 in Arabidopsis. Mol. Plant 1, 482–495. 10.1093/mp/ssn013 [DOI] [PubMed] [Google Scholar]
- Li L., Zhang C., Xu D., Schlappi M., Xu Z. Q. (2012). Expression of recombinant EARLI1, a hybrid proline-rich protein of Arabidopsis, in Escherichia coli and its inhibition effect to the growth of fungal pathogens and Saccharomyces cerevisiae. Gene 506, 50–61. 10.1016/j.gene.2012.06.070 [DOI] [PubMed] [Google Scholar]
- Liu Z. Q. (2006). Leaf epidermal cells: a trap for lipophilic xenobiotics. J. Integr. Plant Biol. 48, 1063–1068. 10.1111/j.1744-7909.2006.00301.x [DOI] [Google Scholar]
- Lu F., Ralph J. (2008). Novel tetrahydrofuran structures derived from beta-beta-coupling reactions involving sinapyl acetate in Kenaf lignins. Org. Biomol. Chem. 6, 3681–3694. 10.1039/b809464k [DOI] [PubMed] [Google Scholar]
- Lundqvist J., Teleman A., Junel L., Zacchi G., Dahlman O., Tjerneld F., et al. (2002). Isolation and characterization of galactoglucomannan from spruce (Picea abies). Carbohydr. Polym. 48, 29–39. 10.1016/S0144-8617(01)00210-723067113 [DOI] [Google Scholar]
- Manabe Y., Nafisi M., Verhertbruggen Y., Orfila C., Gille S., Rautengarten C., et al. (2011). Loss-of-function mutation of REDUCED WALL ACETYLATION2 in Arabidopsis leads to reduced cell wall acetylation and increased resistance to Botrytis cinerea. Plant Physiol. 155, 1068–1078. 10.1104/pp.110.168989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manabe Y., Verhertbruggen Y., Gille S., Harholt J., Chong S. L., Pawar P. M., et al. (2013). Reduced wall acetylation proteins play vital and distinct roles in cell wall O-acetylation in Arabidopsis. Plant Physiol. 163, 1107–1117. 10.1104/pp.113.225193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmiroli M., Gonnelli C., Maestri E., Gabbrielli R., Marmiroli N. (2010). Localisation of nickel and mineral nutrients Ca, K, Fe, Mg by scanning electron microscopy microanalysis in tissues of the nickel-hyperaccumulator Alyssum bertolonii Desv. and the non-accumulator Alyssum montanum L. Plant Biosyst. 138, 231–243. 10.1080/11263500400011126 [DOI] [Google Scholar]
- Molina I., Bonaventure G., Ohlrogge J., Pollard M. (2006). The lipid polyester composition of Arabidopsis thaliana and Brassica napus seeds. Phytochemistry 67, 2597–2610. 10.1016/j.phytochem.2006.09.011 [DOI] [PubMed] [Google Scholar]
- Nemhauser J. L., Hong F., Chory J. (2006). Different plant hormones regulate similar processes through largely nonoverlapping transcriptional responses. Cell 126, 467–475. 10.1016/j.cell.2006.05.050 [DOI] [PubMed] [Google Scholar]
- Norris R. F., Bukovac M. J. (1968). Structure of pear leaf cuticle with special reference to cuticular penetration. Am. J. Bot. 55, 975–983. 10.2307/2440563 [DOI] [Google Scholar]
- Oh I. S., Park A. R., Bae M. S., Kwon S. J., Kim Y. S., Lee J. E., et al. (2005). Secretome analysis reveals an Arabidopsis lipase involved in defense against Alternaria brassicicola. Plant Cell 17, 2832–2847. 10.1105/tpc.105.034819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D. J., Nikolau B. J., Wurtele E. S. (2009). Acetyl CoA - life at the metabolic nexus. Plant Sci. 176, 597–601. 10.1016/j.plantsci.2009.02.005 [DOI] [Google Scholar]
- Orgell W. H. (1955). The isolation of plant cuticle with pectic enzymes. Plant Physiol. 30, 78–80. 10.1104/pp.30.1.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawar P. M., Koutaniemi S., Tenkanen M., Mellerowicz E. J. (2013). Acetylation of woody lignocellulose: significance and regulation. Front. Plant Sci. 4:118. 10.3389/fpls.2013.00118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin R. M., Jia Z., Wagner T. A., O'Neill M. A., Sarria R., York W. S., et al. (2003). Analysis of xyloglucan fucosylation in Arabidopsis. Plant Physiol. 132, 768–778. 10.1104/pp.102.016642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potikha T., Delmer D. P. (1995). A mutant of Arabidopsis thaliana displaying altered patterns of cellulose deposition. Plant J. 7, 453–460. 10.1046/j.1365-313X.1995.7030453.x [DOI] [Google Scholar]
- Robinson M. D., McCarthy D. J., Smyth G. K. (2010). edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140. 10.1093/bioinformatics/btp616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas C. M., Senthil-Kumar M., Wang K., Ryu C. M., Kaundal A., Mysore K. S. (2012). Glycolate oxidase modulates reactive oxygen species-mediated signal transduction during nonhost resistance in Nicotiana benthamiana and Arabidopsis. Plant Cell 24, 336–352. 10.1105/tpc.111.093245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarret G., Willems G., Isaure M. P., Marcus M. A., Fakra S. C., Frerot H., et al. (2009). Zinc distribution and speciation in Arabidopsis halleri x Arabidopsis lyrata progenies presenting various zinc accumulation capacities. New Phytol. 184, 581–595. 10.1111/j.1469-8137.2009.02996.x [DOI] [PubMed] [Google Scholar]
- Schieferstein R. H., Loomis W. E. (1959). Development of the cuticular layers in angiosperm leaves. Am. J. Bot. 46, 625–635. 10.2307/2439666 [DOI] [Google Scholar]
- Schols H. A., Posthumus M. A., Voragen A. G. J. (1990). Structural features of hairy regions of pectins isolated from apple juice produced by the liquefaction process. Carbohydr. Res. 206, 117–129. 10.1016/0008-6215(90)84011-i [DOI] [Google Scholar]
- Schultink A., Naylor D., Dama M., Pauly M. (2015). The role of the plant-specific ALTERED XYLOGLUCAN9 protein in Arabidopsis cell wall polysaccharide O-acetylation. Plant Physiol. 167, 1271–1283. 10.1104/pp.114.256479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano M., Coluccia F., Torres M., L'Haridon F., Metraux J. P. (2014). The cuticle and plant defense to pathogens. Front. Plant Sci. 5:274. 10.3389/fpls.2014.00274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville C., Bauer S., Brininstool G., Facette M., Hamann T., Milne J., et al. (2004). Toward a systems approach to understanding plant cell walls. Science 306, 2206–2211. 10.1126/science.1102765 [DOI] [PubMed] [Google Scholar]
- Suo B., Seifert S., Kirik V. (2013). Arabidopsis GLASSY HAIR genes promote trichome papillae development. J. Exp. Bot. 64, 4981–4991. 10.1093/jxb/ert287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Tanaka H., Machida C., Watanabe M., Machida Y. (2004). A new method for rapid visualization of defects in leaf cuticle reveals five intrinsic patterns of surface defects in Arabidopsis. Plant J. 37, 139–146. 10.1046/j.1365-313X.2003.01946.x [DOI] [PubMed] [Google Scholar]
- Tang D., Simonich M. T., Innes R. W. (2007). Mutations in LACS2, a long-chain acyl-coenzyme A synthetase, enhance susceptibility to avirulent Pseudomonas syringae but confer resistance to Botrytis cinerea in Arabidopsis. Plant Physiol. 144, 1093–1103. 10.1104/pp.106.094318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomma B. P., Eggermont K., Penninckx I. A., Mauch-Mani B., Vogelsang R., Cammue B. P., et al. (1998). Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc. Natl. Acad. Sci. U.S.A. 95, 15107–15111. 10.1073/pnas.95.25.15107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thordal-Christensen H., Zhang Z., Wei Y., Collinge D. B. (1997). Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley—powdery mildew interaction. Plant J. 11, 1187–1194. 10.1046/j.1365-313X.1997.11061187.x [DOI] [Google Scholar]
- Trapnell C., Pachter L., Salzberg S. L. (2009). TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25, 1105–1111. 10.1093/bioinformatics/btp120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanowicz B. R., Peña M. J., Moniz H. A., Moremen K. W., York W. S. (2014). Two Arabidopsis proteins synthesize acetylated xylan in vitro. Plant J. 80, 197–206. 10.1111/tpj.12643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dongen F. E. M., Van Eylen D., Kabel M. A. (2011). Characterization of substituents in xylans from corn cobs and stover. Carbohydr. Polym. 86, 722–731. 10.1016/j.carbpol.2011.05.007 [DOI] [Google Scholar]
- Voisin D., Nawrath C., Kurdyukov S., Franke R. B., Reina-Pinto J. J., Efremova N., et al. (2009). Dissection of the complex phenotype in cuticular mutants of Arabidopsis reveals a role of SERRATE as a mediator. PLoS Genet. 5:e1000703. 10.1371/journal.pgen.1000703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel D., Glazebrook J. (2002). Arabidopsis. A Laboratory Manual. New York, NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Whitcombe A. J., O'neill M. A., Steffan W., Albersheim P., Darvill A. G. (1995). Structural characterization of the pectic polysaccharide, rhamnogalacturonan-II. Carbohydr. Res. 271, 15–29. 10.1016/0008-6215(94)00002-W [DOI] [PubMed] [Google Scholar]
- Xin Z., Browse J. (1998). Eskimo1 mutants of Arabidopsis are constitutively freezing-tolerant. Proc. Natl Acad. Sci. U.S.A. 95, 7799–7804. 10.1073/pnas.95.13.7799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing S., van Deenen N., Magliano P., Frahm L., Forestier E., Nawrath C., et al. (2014). ATP citrate lyase activity is post-translationally regulated by sink strength and impacts the wax, cutin and rubber biosynthetic pathways. Plant J. 79, 270–284. 10.1111/tpj.12559 [DOI] [PubMed] [Google Scholar]
- Xiong G. Y., Cheng K., Pauly M. (2013). Xylan O-acetylation impacts xylem development and enzymatic recalcitrance as indicated by the Arabidopsis mutant tbl29. Mol. Plant 6, 1373–1375. 10.1093/mp/sst014 [DOI] [PubMed] [Google Scholar]
- Yeats T. H., Rose J. K. C. (2013). The formation and function of plant cuticles. Plant Physiol. 163, 5–20. 10.1104/pp.113.222737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y., Teng Q., Zhong R., Ye Z. H. (2013). The Arabidopsis DUF231 domain-containing protein ESK1 mediates 2-O- and 3-O-acetylation of xylosyl residues in xylan. Plant Cell Physiol. 54, 1186–1199. 10.1093/pcp/pct070 [DOI] [PubMed] [Google Scholar]
- Zhu X. F., Sun Y., Zhang B. C., Mansoori N., Wan J. X., Liu Y., et al. (2014). TRICHOME BIREFRINGENCE-LIKE27 affects aluminum sensitivity by modulating the O-acetylation of xyloglucan and aluminum-binding capacity in Arabidopsis. Plant Physiol. 166, 181–189. 10.1104/pp.114.243808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann P., Hirsch-Hoffmann M., Hennig L., Gruissem W. (2004). GENEVESTIGATOR. Arabidopsis microarray database and analysis toolbox. Plant Physiol. 136, 2621–2632. 10.1104/pp.104.046367 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.