Abstract
Severe aplastic anemia (SAA) is a life-threatening disorder for which allogeneic hematopoietic stem cell transplantation (HSCT) is the current available curative treatment. HSCT from matched sibling donors (MSDs) is the preferred therapy for children with acquired SAA. For patients who lack MSDs, immunosuppressive therapy (IST) is widely accepted as a first-line treatment before considering HCT from an unrelated donor (URD). Given the recent progress in HSCT using URDs for childhood SAA, well-matched URDs became a realistic alternative for pediatric patients who have no suitable related donors and who are refractory to IST. However, it is quite challenging to treat patients with refractory SAA who lack suitable related or URDs. Even though haploidentical HSCT from genetically mismatched family members seemed to be an attractive procedure with the amazing benefit of readily available donors for most patients, early attempts were disappointing because of refractory graft-versus-host disease (GVHD) and excessively high transplant-related mortality. Recent advances with effective ex vivo depletion of T cells or unmanipulated in vivo regulation of T cells, better supportive care, and optimal conditioning regimens have significantly improved the outcome of haploidentical transplant. Besides considerable progress in the treatment of malignant diseases, recent emerging evidences for haploidentical HSCT in SAA has provided additional therapeutic options for patients with refractory diseases. Further improvements to decrease the rates of graft failure, GVHD, and infectious complications will facilitate the emergence of haploidentical HSCT as a front-line therapy for treating acquired SAA in children and adolescents who have no suitably matched donors.
Keywords: Hematopoietic stem cell transplantation, Aplastic anemia, Child, Adolescents
Introduction
Severe aplastic anemia (SAA) is a life-threatening disorder for which allogeneic hematopoietic stem cell transplantation (HSCT) is the current available curative treatment. HSCT from a matched sibling donor (MSD) is the preferred therapy for children with acquired SAA. The outcomes of HSCT from MSDs are excellent with survival rates of 90% or higher1,2,3). In addition, some comparative studies support the superiority of MSD-HSCT over immunosuppressive therapy (IST), given the risk of clonal evolution and the high relapse rate after IST4,5). However, only 1 to 2 out of 10 children with SAA have a MSD. Therefore, IST is widely used as the first-line treatment for patients who lack a MSD before considering HSCT from an unrelated donor (URD)6,7).
Recently, there has been progress in HSCT using URDs for childhood SAA. The best donor selection using molecular typing of human leukocyte antigen (HLA) played a major role, but modifications of the conditioning regimen and improvements in supportive care have also occurred. Nowadays, the survival after well-matched URD HSCT approaches the outcome of MSD transplants8,9,10). A recent randomized study showed that IST with rabbit-antithymocyte globulin (ATG) had an inferior response and survival rate compared with those of horse-ATG11). In addition, the use of IST before HSCT has been associated with lower survival12,13). As horse-ATG is no longer available in Korea, URD HSCT is occasionally recommended as an initial treatment for children with SAA partially because of the limited efficacy of IST using rabbit-ATG.
Given the recent data with favorable outcome, HSCT with well-matched URD has become a realistic option for pediatric patients who have no suitable related donors and who are refractory to IST14). However, it is quite challenging to treat patients with refractory SAA who lack suitable related or URDs. Of course, supportive therapy could be an option and other types of novel immunosuppressive therapies could be a possibility. In addition, alternative donor transplantations using cord blood or haploidentical family donors (HFDs) could be potential treatments for children with refractory SAA in need of frequent transfusions and repeated antibiotic therapy.
This article reviews the status of HLA-haploidentical HSCT from mismatched family donors as an alternative therapeutic approach for children and adolescents with acquired SAA.
History of HSCT from HFD
HSCT is a curative procedure for a wide range of pediatric malignant and nonmalignant diseases. Unfortunately, it is not always possible to find a donor for patients requiring HSCT. Thus, a certain number of patients still could not find a donor, resulting in death without undergoing transplant. The need for alternative donors has pressed on the development of new transplantation approaches such as transplantation from HLA-haploidentical mismatched family donors. HSCT from HFDs has several advantages (Table 1). (1) Virtually all patients who need HSCT can find a donor. (2) Transplantation could be performed immediately, which is critical to patients with high-risk malignant diseases or very SAA requiring urgent treatment. (3) Further access to donors is easy for cellular therapy to treat relapse and infections or to undergo a second transplantation. In addition, HFDs could rescue patients experiencing early graft failure (GF), which is a life-threatening complication requiring prompt intervention after allogeneic HSCT15).
Table 1. Advantages of haploidentical hematopoietic stem cell transplantation.
| Availability for almost all patients |
| Immediate donor accessibility |
| No racial or ethnic restrictions |
| Multiple donors |
| Continued donor access |
Even though haploidentical HSCT seemed to be an attractive procedure with the amazing benefit of readily available donors, early attempts at haploidentical HSCT from genetically haploidentical family members were disappointing because of the development of refractory graft-versus-host disease (GVHD) and excessively high transplant-related mortality (TRM). High rates of graft rejection (GR) and refractory GVHD were major drawbacks to the use of haploidentical HSCT for patients who need transplantation but lack a suitable donor. In addition, delayed immune recovery and the high prevalence of infection are significant obstacles. Initial practice revealed that haploidentical HSCT had a considerably higher incidence of GF and GVHD, resulting in high rates of morbidity and mortality16,17).
Recent advances in haploidentical HSCT
Recent advances in effective ex vivo depletion of T-cells or unmanipulated in vivo regulation of T-cells, better supportive care, and optimal conditioning regimens have significantly improved the outcome of haploidentical transplant. T-cell depletion (TCD) of donor grafts to prevent fatal GVHD is crucial for successful haploidentical HSCT. The methods of TCD could be carried out in vivo (T-cell-replete transplant) or ex vivo (T-cell-depleted transplant). Various approaches have been developed, including ex vivo selection of CD34+ cells with or without megadoses of purified stem cells, ex vivo TCD, in vivo TCD using T-cell antibodies such as ATG, and posttransplant cyclophosphamide (PTCY) (Table 2). The ex vivo techniques to remove T-cells have evolved from the selection of CD34+ hematopoietic stem cell progenitors towards the depletion of CD3+ cells and more recently the depletion of αβ+ T-cells. Compared with the positive selection of CD34+ cells, direct depletion of CD3+ cells has the advantage of increased numbers of natural killer cells, monocytes, and other immunomodulating cells18). The depletion of CD3+ cells is superior to the selection of CD34+ cells in terms of rapid engraftment and immune reconstitution19,20). Moreover, preliminary reports on the new method of αβ+ TCD further showed improved outcomes of T-cell-depleted haploidentical transplants. The depletion of αβ+ T-cells produces grafts containing many γδ+ lymphocytes and other effector cells. While αβ+ T-cells are known to be associated with the initiation of GVHD, γδ+ T-cells can enhance immune reconstitution and are not implicated in GVHD21). Bertaina et al.22) reported the promising results of αβ+ T-cell-depleted haploidentical HSCT for pediatric patients with nonmalignant diseases, with a TRM and an overall survival rate of 9.3% and 91.1%, respectively.
Table 2. Approaches in T-cell depletion for haploidentical hematopoietic stem cell transplantation.
| Ex vivo T-cell depletion |
| Indirect T-cell depletion |
| Selection of CD34+ cells |
| Direct T-cell depletion |
| Depletion of CD3+ T cells |
| Depletion of αβ+ T cells |
| In vivo T-cell depletion |
| Antilymphocyte antibodies |
| Allodepletion with cyclophosphamide |
Besides T-cell-depleted haploidentical HSCT, there have also been remarkable advances in unmanipulated T-cell-replete transplant. Huang et al.23) reported the results of T-cell-replete haploidentical transplant using an intensive preparative regimen containing high doses of ATG. In addition, recent approaches have been developed using PTCY to control GVHD by eliminating allo-activated donor T-cells24). Remarkable advances in haploidentical transplants have enabled successful T-cell-depleted and T-cell-replete transplantation23,24,25).
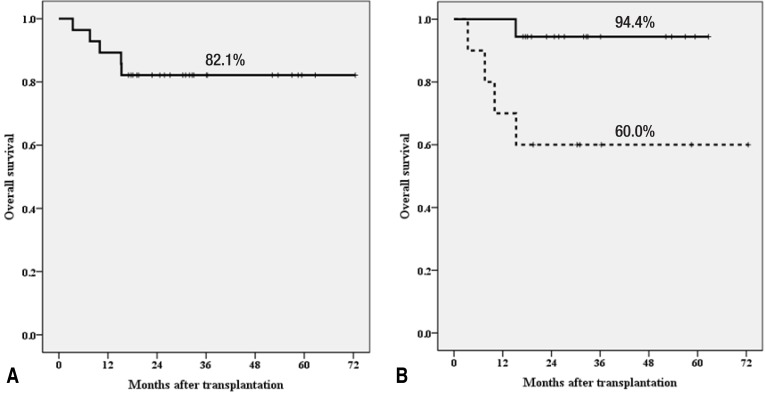
Recently, we reported on haploidentical HSCT using ex vivo CD3-depleted grafts in children and adolescents26). Twenty-eight pediatric patients underwent T-cell-depleted haploidentical HSCT; 10 had malignant diseases and 18 had nonmalignant hematologic diseases including 16 with acquired SAA. Twenty-six patients achieved neutrophil engraftment at a median of 11 days (range, 9 to 15 days). The cumulative incidences of severe grades III-IV acute GVHD and extensive chronic GVHD were 14% and 11%, respectively. Four patients died of non-relapse-related causes (two from cytomegalovirus infection, one from encephalopathy, and one from autoimmune hemolytic anemia) and one of leukemia relapse. The TRM at 100 days, 1 year, and 2 years were 0.0%, 10.7%, and 14.3%, respectively. The overall survival rate of all patients was 82.1% and those for nonmalignant diseases and malignant diseases were 94.4% and 60.0%, respectively (Fig. 1).
Fig. 1. Overall survival rate of all patients (A) and overall survival rate according to disease (B): 94.4% for nonmalignant diseases (n=18) and 60.0% for malignant diseases (n=10) (P=0.019).
Currently, given the low TRM and acceptable occurrence of GVHD, haploidentical HSCT is considered as an established option for children and adults who have diseases curable by HSCT but lack suitable related or URDs.
HSCT from HFD for patients with acquired SAA
Historically, the haploidentical HSCT for patients with SAA was invariably unsuccessful, with high rates of GF and opportunistic infection. Some studies of CD34-selected haploidentical HSCT reported encouraging outcomes, but the number of patients was too small to justify a greater use of this approach27,28). Although haploidentical HSCT is considered an established option for both children and adults with malignant diseases, only a few reports have addressed the use of haploidentical transplant in children with acquired aplastic anemia28,29,30,31). A review by the EBMT SAA Working Party on unmanipulated haploidentical HSCT for 20 patients with acquired SAA revealed a poor outcome with an overall survival rate of 30%32).
Recently, the situation has changed considerably. Several notable reports in recent years have supported haploidentical transplant as a viable option for the treatment of acquired SAA33,34,35,36,37,38). The details of the studies are summarized in Table 3.
Table 3. Haploidentical hematopoietic stem cell transplantation in acquired severe aplastic anemia.
| Source | No. | Age at HSCT (yr), median (range) | Conditioning regimen | Ex vivo T-cell depletion | Primary GF, n (%) | GVHD, n (%) | Death (n) | Cause of death (n) | Survival | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acute≥II | Chronic | GF | GVHD | Infection | ||||||||
| Xu et al.33) (2012) | 19 | 19 (5-33) | BU, CY, ATG | No | 0 (0) | 8 (42) | 9 (56) | 6 | 2 | 2 | 2 | 65% at 2 yr |
| Im et al.34) (2013) | 12 | 13 (3-21) | FLU, CY, ATG±TBI | Yes | 1 (8.3) | 3 (33) | 2 (22) | 0 | 0 | 0 | 0 | 100% at 1 yr |
| Gao et al.35) (2014) | 26 | 25 (18-41) | FLU, CY, ATG | No | 1 (3.8) | 3 (12) | 10 (40) | 4 | 2 | 1 | 1 | 85% at 3 yr |
| Wang et al.36) (2014) | 17 | 10 (4-19) | BU, FLU, CY, ATG | No | 0 (0) | 5 (29) | 4 (27) | 3 | 0 | 1 | 2 | 72% at 1 yr |
| Clay et al.37) (2014) | 8 | 32 (19-57) | FLU, CY, TBI | No | 2 (25) | 1 (13) | 0 (0) | 2 | 2 | 0 | 0 | 75% at 1 yr |
| Steves et al.38) (2015) | 16 | 17 (5-39) | FLU, CY, TBI | No | 1 (6) | 2 (13) | 3 (20) | 5 | 0 | 0 | 5 | 67% at 1 yr |
| Current study | 21 | 14 (3-21) | FLU, CY, ATG±TBI | Yes | 1 (4.8) | 6 (30) | 2 (10) | 1 | 1* | 0 | 0 | 94% at 3 yr |
HSCT, hematopoietic stem cell transplantation; GF, graft failure; GVHD, graft-versus-host disease; BU, busulfan; CY, cyclophosphamide; ATG, antithymocyte globulin; FLU, fludarabine; TBI, total body irradiation.
*The patient died of complications after a booster infusion of CD34+ cells for poor graft function.
Three studies of transplants without ex vivo TCD reported survival rates of 65% to 85%33,35,36). Two of these three studies used unmanipulated peripheral blood stem cells after non-total-body-irradiation (TBI) conditioning with ATG at a dose of 10 mg/kg of recipient weight33,35). The remaining study of 17 children and adolescents with refractory SAA employed a distinctive approach using peripheral blood and G-CSF-primed bone marrow stem cells with additional mesenchymal stem cells derived from an URD and basiliximab to further reduce GVHD36). Severe III-IV acute GVHD only occurred in one patient.
A study evaluated haploidentical HSCT with ex vivo TCD in 12 pediatric patients. All 12 patients survived and were transfusion-independent at a median follow-up of 14.3 months34). A report on haploidentical HSCT using reduced-intensity conditioning (RIC) with high-dose PTCY showed that six of eight patients with refractory SAA survived with full donor chimerism37). Another recent study using the same approach with RIC and PTCY reported overall survival rates of 67.1% in 16 patients with SAA38).
These recent emerging evidences for haploidentical HSCT in SAA has provided additional therapeutic options to consider in patients who experience refractory diseases but do not have a suitable related or URD.
Update of our experience with haploidentical HSCT for children and adolescents with acquired SAA (unpublished data)
We have published our preliminary experience with T-cell-depleted haploidentical transplant for 12 patients with acquired SAA34). Up until February 2015, 21 patients with acquired SAA received haploidentical transplants using ex vivo T-cell-depleted grafts at our center. Sixteen patients received CD3-depleted grafts and five received αβ-depleted stem cells. Conditioning regimens consisted of fludarabine (FLU), cyclophosphamide (CY), and r-ATG with or without low-dose total body irradiation (LD-TBI).
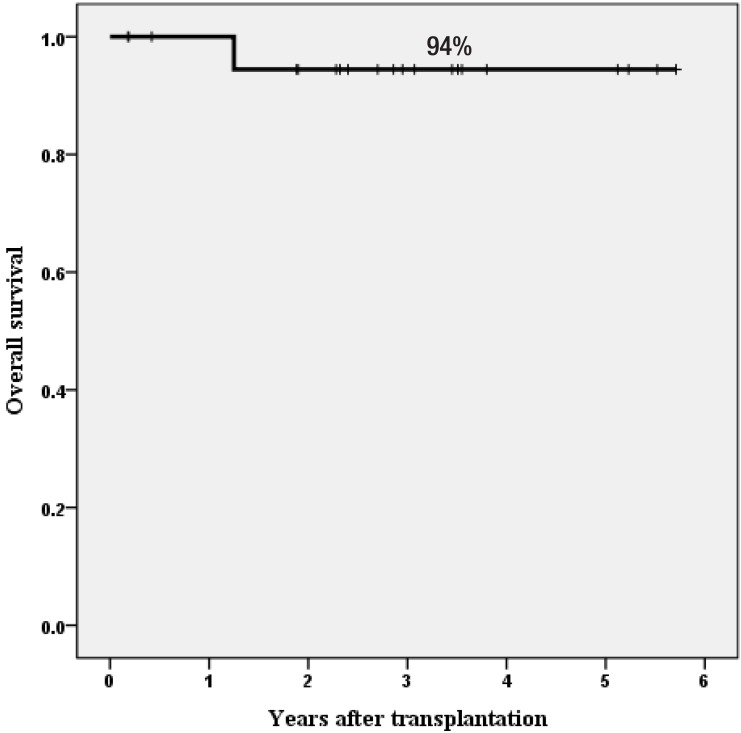
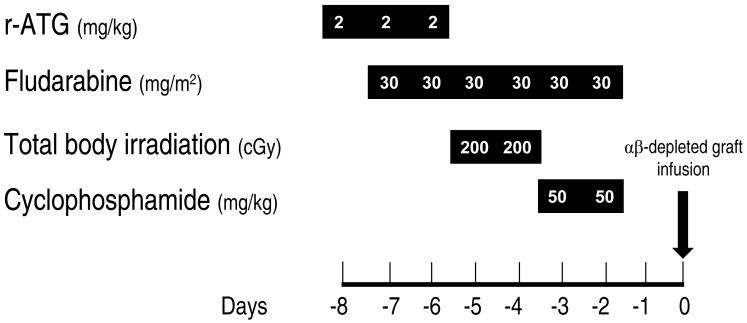
Only one patient died of severe autoimmune hemolytic anemia and pure red cell aplasia after a booster infusion of CD34+ cells for poor graft function at 457 days. All 20 surviving patients were transfusion-independent. As of April 15, 2015, with a median follow-up of 35 months (range, 2 to 70 months), the estimated overall survival rate at 3 years was 94% (Fig. 2). This study strongly suggests that HSCT with HFDs could be offered to children and adolescents with acquired SAA who lack suitable related or URDs. The current protocol comprised FLU, CY, r-ATG, and LD-TBI of 4 Gy with αβ-depleted grafts (Fig. 3).
Fig. 2. Overall survival rate for 21 patients with acquired severe aplastic anemia.
Fig. 3. Transplantation protocol for acquired severe aplastic anemia. ATG, antithymocyte globulin.
Future perspectives of haploidentical HSCT for SAA in pediatric patients
In spite of promising evidence for haploidentical HSCT in acquired SAA, there are still several obstacles to be overcome in the future for its universal use. Unresolved issues include conditioning regimen, methods to regulate the donor T-cells, stem cell source, donor selection, and management of GF or poor graft function. Future clinical trials with larger numbers of patients will help to establish the best conditioning regimen and the optimal regulation of donor T-cells leading to maximized outcomes of this novel approach. After all, the major causes of treatment failure after haploidentical HSCT for SAA are GF, including GR, severe GVHD, and infections. GF and GR are life-threatening complications after allogeneic HSCT and are more common in nonmalignant diseases including SAA, especially heavily transfused cases, than in acute leukemia39,40). The management of GF, especially that occurring in early posttransplantation, is often challenging. Although a second haploidentical HSCT with successful rescue has been reported, GF is a frustrating situation and therefore it is desirable to minimize the rate of GF15,41). Delayed immune reconstitution and subsequent infection are not uncommon and are major causes of death after haploidentical transplant. New depletion techniques or adoptive transfer of immune effector cells could enhance the recovery of immune functions after haploidentical HSCT42,43). Our experience with αβ+ T-cell-depleted haploidentical transplants, presented at the annual winter meeting of the Korean Society of Blood and Marrow Transplantation in 2014, showed faster immune recovery compared with CD3-depleted transplants.
Conclusion
Currently, substantial progress in haploidentical HSCT has been achieved for the treatment of patients with both nonmalignant diseases, including SAA, and malignant diseases. Haploidentical transplant could provide a chance to save the lives and restore the normal lifestyles of pediatric patients with SAA in need of frequent transfusions and repeated antibiotic therapy to maintain their lives.
Further improvements to decrease the rates of GF, GVHD, and infectious complications as well as to appropriately deal with poor graft function will facilitate the emergence of haploidentical HSCT as a front-line therapy for treating acquired SAA in children and adolescents who have no suitably matched donors.
Acknowledgments
This study was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (HI10C2020).
Footnotes
Conflict of interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Kikuchi A, Yabe H, Kato K, Koh K, Inagaki J, Sasahara Y, et al. Long-term outcome of childhood aplastic anemia patients who underwent allogeneic hematopoietic SCT from an HLA-matched sibling donor in Japan. Bone Marrow Transplant. 2013;48:657–660. doi: 10.1038/bmt.2012.205. [DOI] [PubMed] [Google Scholar]
- 2.Schrezenmeier H, Passweg JR, Marsh JC, Bacigalupo A, Bredeson CN, Bullorsky E, et al. Worse outcome and more chronic GVHD with peripheral blood progenitor cells than bone marrow in HLA-matched sibling donor transplants for young patients with severe acquired aplastic anemia. Blood. 2007;110:1397–1400. doi: 10.1182/blood-2007-03-081596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim HK. Aplastic anemia. Korean J Pediatr. 2007;50:519–523. [Google Scholar]
- 4.Kojima S, Horibe K, Inaba J, Yoshimi A, Takahashi Y, Kudo K, et al. Long-term outcome of acquired aplastic anaemia in children: comparison between immunosuppressive therapy and bone marrow transplantation. Br J Haematol. 2000;111:321–328. doi: 10.1046/j.1365-2141.2000.02289.x. [DOI] [PubMed] [Google Scholar]
- 5.Yoshida N, Kobayashi R, Yabe H, Kosaka Y, Yagasaki H, Watanabe K, et al. First-line treatment for severe aplastic anemia in children: bone marrow transplantation from a matched family donor versus immunosuppressive therapy. Haematologica. 2014;99:1784–1791. doi: 10.3324/haematol.2014.109355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guinan EC. Acquired aplastic anemia in childhood. Hematol Oncol Clin North Am. 2009;23:171–191. doi: 10.1016/j.hoc.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 7.Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood. 2006;108:2509–2519. doi: 10.1182/blood-2006-03-010777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bacigalupo A, Socie' G, Lanino E, Prete A, Locatelli F, Locasciulli A, et al. Fludarabine, cyclophosphamide, antithymocyte globulin, with or without low dose total body irradiation, for alternative donor transplants, in acquired severe aplastic anemia: a retrospective study from the EBMT-SAA Working Party. Haematologica. 2010;95:976–982. doi: 10.3324/haematol.2009.018267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang HJ, Shin HY, Park JE, Chung NG, Cho B, Kim HK, et al. Successful engraftment with fludarabine, cyclophosphamide, and thymoglobulin conditioning regimen in unrelated transplantation for severe aplastic anemia: a phase II prospective multicenter study. Biol Blood Marrow Transplant. 2010;16:1582–1588. doi: 10.1016/j.bbmt.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 10.Yagasaki H, Takahashi Y, Hama A, Kudo K, Nishio N, Muramatsu H, et al. Comparison of matched-sibling donor BMT and unrelated donor BMT in children and adolescent with acquired severe aplastic anemia. Bone Marrow Transplant. 2010;45:1508–1513. doi: 10.1038/bmt.2009.378. [DOI] [PubMed] [Google Scholar]
- 11.Scheinberg P, Nunez O, Weinstein B, Scheinberg P, Biancotto A, Wu CO, et al. Horse versus rabbit antithymocyte globulin in acquired aplastic anemia. N Engl J Med. 2011;365:430–438. doi: 10.1056/NEJMoa1103975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ades L, Mary JY, Robin M, Ferry C, Porcher R, Esperou H, et al. Long-term outcome after bone marrow transplantation for severe aplastic anemia. Blood. 2004;103:2490–2497. doi: 10.1182/blood-2003-07-2546. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi R, Yabe H, Hara J, Morimoto A, Tsuchida M, Mugishima H, et al. Preceding immunosuppressive therapy with antithymocyte globulin and ciclosporin increases the incidence of graft rejection in children with aplastic anaemia who underwent allogeneic bone marrow transplantation from HLA-identical siblings. Br J Haematol. 2006;135:693–696. doi: 10.1111/j.1365-2141.2006.06352.x. [DOI] [PubMed] [Google Scholar]
- 14.Scheinberg P, Young NS. How I treat acquired aplastic anemia. Blood. 2012;120:1185–1196. doi: 10.1182/blood-2011-12-274019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park JA, Koh KN, Choi ES, Jang S, Kwon SW, Park CJ, et al. Successful rescue of early graft failure in pediatric patients using T-cell-depleted haploidentical hematopoietic SCT. Bone Marrow Transplant. 2014;49:270–275. doi: 10.1038/bmt.2013.163. [DOI] [PubMed] [Google Scholar]
- 16.Beatty PG, Clift RA, Mickelson EM, Nisperos BB, Flournoy N, Martin PJ, et al. Marrow transplantation from related donors other than HLA-identical siblings. N Engl J Med. 1985;313:765–771. doi: 10.1056/NEJM198509263131301. [DOI] [PubMed] [Google Scholar]
- 17.Sykes M, Preffer F, McAfee S, Saidman SL, Weymouth D, Andrews DM, et al. Mixed lymphohaemopoietic chimerism and graft-versus-lymphoma effects after non-myeloablative therapy and HLA-mismatched bone-marrow transplantation. Lancet. 1999;353:1755–1759. doi: 10.1016/S0140-6736(98)11135-2. [DOI] [PubMed] [Google Scholar]
- 18.Lang P, Schumm M, Greil J, Bader P, Klingebiel T, Muller I, et al. A comparison between three graft manipulation methods for haploidentical stem cell transplantation in pediatric patients: preliminary results of a pilot study. Klin Padiatr. 2005;217:334–338. doi: 10.1055/s-2005-872529. [DOI] [PubMed] [Google Scholar]
- 19.Bethge WA, Faul C, Bornhauser M, Stuhler G, Beelen DW, Lang P, et al. Haploidentical allogeneic hematopoietic cell transplantation in adults using CD3/CD19 depletion and reduced intensity conditioning: an update. Blood Cells Mol Dis. 2008;40:13–19. doi: 10.1016/j.bcmd.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Handgretinger R, Chen X, Pfeiffer M, Mueller I, Feuchtinger T, Hale GA, et al. Feasibility and outcome of reduced-intensity conditioning in haploidentical transplantation. Ann N Y Acad Sci. 2007;1106:279–289. doi: 10.1196/annals.1392.022. [DOI] [PubMed] [Google Scholar]
- 21.Daniele N, Scerpa MC, Caniglia M, Bernardo ME, Rossi C, Ciammetti C, et al. Transplantation in the onco-hematology field: focus on the manipulation of αβ and γδ T cells. Pathol Res Pract. 2012;208:67–73. doi: 10.1016/j.prp.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Bertaina A, Merli P, Rutella S, Pagliara D, Bernardo ME, Masetti R, et al. HLA-haploidentical stem cell transplantation after removal of αβ+ T and B cells in children with nonmalignant disorders. Blood. 2014;124:822–826. doi: 10.1182/blood-2014-03-563817. [DOI] [PubMed] [Google Scholar]
- 23.Huang XJ, Liu DH, Liu KY, Xu LP, Chen H, Han W, et al. Treatment of acute leukemia with unmanipulated HLA-mismatched/haploidentical blood and bone marrow transplantation. Biol Blood Marrow Transplant. 2009;15:257–265. doi: 10.1016/j.bbmt.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 24.Luznik L, O'Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lang P, Teltschik HM, Feuchtinger T, Muller I, Pfeiffer M, Schumm M, et al. Transplantation of CD3/CD19 depleted allografts from haploidentical family donors in paediatric leukaemia. Br J Haematol. 2014;165:688–698. doi: 10.1111/bjh.12810. [DOI] [PubMed] [Google Scholar]
- 26.Im HJ, Koh KN, Suh JK, Lee SW, Choi ES, Jang S, et al. Refinement of treatment strategies in ex vivo T-cell-depleted haploidentical SCT for pediatric patients. Bone Marrow Transplant. 2015;50:225–231. doi: 10.1038/bmt.2014.232. [DOI] [PubMed] [Google Scholar]
- 27.Woodard P, Cunningham JM, Benaim E, Chen X, Hale G, Horwitz E, et al. Effective donor lymphohematopoietic reconstitution after haploidentical CD34+-selected hematopoietic stem cell transplantation in children with refractory severe aplastic anemia. Bone Marrow Transplant. 2004;33:411–418. doi: 10.1038/sj.bmt.1704358. [DOI] [PubMed] [Google Scholar]
- 28.Lacerda JF, Martins C, Carmo JA, Lourenço F, Juncal C, Ismail S, et al. Haploidentical stem cell transplantation with purified CD34+ cells after a chemotherapy-alone conditioning regimen in heavily transfused severe aplastic anemia. Biol Blood Marrow Transplant. 2005;11:399–400. doi: 10.1016/j.bbmt.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 29.Passweg JR, Perez WS, Eapen M, Camitta BM, Gluckman E, Hinterberger W, et al. Bone marrow transplants from mismatched related and unrelated donors for severe aplastic anemia. Bone Marrow Transplant. 2006;37:641–649. doi: 10.1038/sj.bmt.1705299. [DOI] [PubMed] [Google Scholar]
- 30.Koh KN, Im HJ, Kim BE, Choi ES, Jang S, Kwon SW, et al. Haploidentical haematopoietic stem cell transplantation using CD3 or CD3/CD19 depletion and conditioning with fludarabine, cyclophosphamide and antithymocyte globulin for acquired severe aplastic anaemia. Br J Haematol. 2012;157:139–142. doi: 10.1111/j.1365-2141.2011.08924.x. [DOI] [PubMed] [Google Scholar]
- 31.Kremens B, Basu O, Grosse-Wilde H, Sauerwein W, Schaefer UW, Havers W. Transplantation of CD34-enriched peripheral stem cells from an HLA-haplotype mismatched donor to a patient with severe aplastic anemia. Bone Marrow Transplant. 2001;27:111–113. doi: 10.1038/sj.bmt.1702748. [DOI] [PubMed] [Google Scholar]
- 32.Ciceri F, Lupo-Stanghellini MT, Korthof ET. Haploidentical transplantation in patients with acquired aplastic anemia. Bone Marrow Transplant. 2013;48:183–185. doi: 10.1038/bmt.2012.231. [DOI] [PubMed] [Google Scholar]
- 33.Xu LP, Liu KY, Liu DH, Han W, Chen H, Chen YH, et al. A novel protocol for haploidentical hematopoietic SCT without in vitro T-cell depletion in the treatment of severe acquired aplastic anemia. Bone Marrow Transplant. 2012;47:1507–1512. doi: 10.1038/bmt.2012.79. [DOI] [PubMed] [Google Scholar]
- 34.Im HJ, Koh KN, Choi ES, Jang S, Kwon SW, Park CJ, et al. Excellent outcome of haploidentical hematopoietic stem cell transplantation in children and adolescents with acquired severe aplastic anemia. Biol Blood Marrow Transplant. 2013;19:754–759. doi: 10.1016/j.bbmt.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 35.Gao L, Li Y, Zhang Y, Chen X, Gao L, Zhang C, et al. Long-term outcome of HLA-haploidentical hematopoietic SCT without in vitro T-cell depletion for adult severe aplastic anemia after modified conditioning and supportive therapy. Bone Marrow Transplant. 2014;49:519–524. doi: 10.1038/bmt.2013.224. [DOI] [PubMed] [Google Scholar]
- 36.Wang Z, Zheng X, Yan H, Li D, Wang H. Good outcome of haploidentical hematopoietic SCT as a salvage therapy in children and adolescents with acquired severe aplastic anemia. Bone Marrow Transplant. 2014;49:1481–1485. doi: 10.1038/bmt.2014.187. [DOI] [PubMed] [Google Scholar]
- 37.Clay J, Kulasekararaj AG, Potter V, Grimaldi F, McLornan D, Raj K, et al. Nonmyeloablative peripheral blood haploidentical stem cell transplantation for refractory severe aplastic anemia. Biol Blood Marrow Transplant. 2014;20:1711–1716. doi: 10.1016/j.bbmt.2014.06.028. [DOI] [PubMed] [Google Scholar]
- 38.Esteves I, Bonfim C, Pasquini R, Funke V, Pereira NF, Rocha V, et al. Haploidentical BMT and post-transplant Cy for severe aplastic anemia: a multicenter retrospective study. Bone Marrow Transplant. 2015;50:685–689. doi: 10.1038/bmt.2015.20. [DOI] [PubMed] [Google Scholar]
- 39.Champlin RE, Horowitz MM, van Bekkum DW, Camitta BM, Elfenbein GE, Gale RP, et al. Graft failure following bone marrow transplantation for severe aplastic anemia: risk factors and treatment results. Blood. 1989;73:606–613. [PubMed] [Google Scholar]
- 40.Woodard P, Tong X, Richardson S, Srivastava DK, Horwitz EM, Benaim E, et al. Etiology and outcome of graft failure in pediatric hematopoietic stem cell transplant recipients. J Pediatr Hematol Oncol. 2003;25:955–959. doi: 10.1097/00043426-200312000-00010. [DOI] [PubMed] [Google Scholar]
- 41.Lang P, Mueller I, Greil J, Bader P, Schumm M, Pfeiffer M, et al. Retransplantation with stem cells from mismatched related donors after graft rejection in pediatric patients. Blood Cells Mol Dis. 2008;40:33–39. doi: 10.1016/j.bcmd.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 42.Feuchtinger T, Opherk K, Bethge WA, Topp MS, Schuster FR, Weissinger EM, et al. Adoptive transfer of pp65-specific T cells for the treatment of chemorefractory cytomegalovirus disease or reactivation after haploidentical and matched unrelated stem cell transplantation. Blood. 2010;116:4360–4367. doi: 10.1182/blood-2010-01-262089. [DOI] [PubMed] [Google Scholar]
- 43.Oevermann L, Lang P, Feuchtinger T, Schumm M, Teltschik HM, Schlegel P, et al. Immune reconstitution and strategies for rebuilding the immune system after haploidentical stem cell transplantation. Ann N Y Acad Sci. 2012;1266:161–170. doi: 10.1111/j.1749-6632.2012.06606.x. [DOI] [PubMed] [Google Scholar]