Abstract
C3 glomerulopathy (C3G) defines a group of untreatable ultra-rare renal diseases caused by uncontrolled activation of the alternative complement pathway. Nearly half of patients progress to end stage renal failure within 10 years. Cp40, a second-generation compstatin analog in clinical development, is a 14 amino-acid cyclic peptide that selectively inhibits complement activation in humans and non-human primates by binding to C3 and C3b. We hypothesized that by targeting C3 Cp40 would provide an effective treatment for C3G. By investigating its effects in vitro using multiple assays of complement activity, we show that Cp40 prevents complement-mediated lysis of sheep erythrocytes in sera from C3G patients, prevents complement dysregulation in the presence of patient-derived autoantibodies to the C3 and C5 convertases, and prevents complement dysregulation associated with disease-causing genetic mutations. In aggregate, these data suggest that Cp40 may offer a novel and promising therapeutic option to C3G patients as a disease-specific, targeted therapy. As such, Cp40 could represent a major advance in the treatment of this disease.
Keywords: C3 glomerulopathy, Dense deposit disease, C3 glomerulonephritis, Compstatin, Complement dysregulation
Introduction
The term C3 glomerulopathy (C3G) defines a group of ultra-rare renal diseases in which dysregulation of the complement cascade drives the underlying disease process (Pickering, et al., 2013). The annual incidence of biopsy-proven disease is 1–2 per million, with both sexes affected equally (Medjeral-Thomas, et al., 2014). Patients present with proteinuria, hematuria and often some degree of renal failure (Nester and Smith, 2013). Median age at diagnosis is 21 years and within 10 years nearly half of patients progress to end stage renal failure (ESRF). Post-transplantation, disease recurrence with allograft loss is a common occurrence (50–75%) (Servais, et al., 2012, Lu, et al., 2012).
While the full spectrum of morphological lesions that present as C3G remains to be determined, all are characterized by glomerular changes in which there is C3-dominant staining by immunofluorescence (IF). The two major subgroups of C3G, dense deposit disease (DDD) and C3 glomerulonephritis (C3GN), are differentiated by electron microscopy (EM). DDD is characterized by extremely dense osmiophilic deposits that markedly thicken the glomerular basement membrane (GBM), while C3GN is defined by some combination of mesangial, subepithelial, subendothelial and/or less dense, discontinuous intramembranous deposits (Sethi and Fervenza, 2014).
Caused by dysregulation of the alternative pathway (AP) of complement, the most common acquired drivers of disease are autoantibodies to C3 convertase known as C3 nephritic factors (C3Nefs). Although present in many different disease conditions and reported in normal individuals, most DDD patients (80–85%) and many C3GN patients (~50%) develop these autoantibodies (Servais, et al., 2012, Zhang, et al., 2012), which prolong convertase half-life and promote fluid-phase AP dysregulation (Spitzer, et al., 1969, Daha, et al., 1976). Autoantibodies against other proteins like Factor B (FB) and Factor H (FH) are also occasionally found (Strobel, et al., 2010, Chen, et al., 2011, Goodship, et al., 2012). In both DDD and C3GN, the profile of serological complement biomarkers is consistent with over-activity of the AP and terminal complement cascade (TCC) (Zhang, et al., 2014).
There is no disease-specific treatment for C3G. Current treatment measures are designed to support a patient’s general health and extrapolating from other glomerular diseases, angiotensin converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) are considered as an aid to urine protein management (and also for blood pressure control). Limited C3G-specific data on ACEIs and ARBs suggest that the use of these agents is associated with better renal survival (P <0.0001) (Servais, et al., 2012). Recently, eculizumab, an anti-C5 humanized antibody that prevents terminal complement pathway activity, has been demonstrated to mitigate C3G in some patients (Vivarelli, et al., 2012, McCaughan, et al., 2012, Daina, et al., 2012, Bomback, et al., 2012). However, persistently unchecked C3 convertase activity can lead to disease progression in spite of effective eculizumab-based suppression of the TCC (Gurkan, et al., 2013). These findings suggest that more proximal blockade may be therapeutic. C3 represents an excellent target for intervention, and as such, the compstatin family of peptidic C3 inhibitors is particularly appealing as a possible therapy in C3G (Mastellos, et al., 2015). In this paper, we report the results of in vitro studies using Cp40, a second-generation compstatin analog (Qu, et al., 2013) that has entered clinical development (AMY-101; Amyndas Pharmaceuticals), as a complement inhibitor in patients with DDD and C3GN.
Materials and methods
Patient and control samples
Thirty-four patients with C3G (17 DDD and 17 C3GN) were enrolled in this study under IRB-approved guidelines. The diagnosis of either DDD or C3GN was made by renal biopsy as described previously (Zhang et al., 2014). Whole blood was used as a source of genomic DNA, serum and plasma. Pooled normal serum (PNS, complement preserved) was purchased from Innovative Research, Inc. (Novi, MI).
Reagents
Gelatin veronal buffer (GVB)-EDTA (5 mM Barbital, 145 mM NaCl, 10 mM EGTA and 0.1% Gelatin, pH 7.2), GVB-EGTA-Mg2+(5 mM Barbital, 145 mM NaCl, 0.5 mM MgCl2, 10 mM EGTA and 0.1% Gelatin, pH 7.2) and DGVB++ buffer (5 mM Barbital, 140 mM Glucose, 71 mM NaCl, 0.5 mM MgCl2, 0.15 mM CaCl2 and 0.1% Gelatin, pH 7.4) were purchased from Boston Bioproducts, Inc. (Worcester, MA). GVB containing Ca2+-TTHA (triethylenetetramine-N,N,N′,N″,N‴,N‴-hexa-acetic acid, 5 mM Barbital, 140 mM Glucose, 5 mM TTHA, 71 mM NaCl, 0.3 mM CaCl2 and 0.1% Gelatin, pH 7.4) was prepared as previously described (Nagaki, et al., 1974). Human complement proteins C2, FB and FD and human factor H-depleted (FH-dpl) serum were purchased from Complement Technology, Inc. (Tyler, TX). Non-sensitized sheep erythrocytes (E) and antibody-sensitized sheep erythrocytes (EA, both in Alsever’s solution) were purchased from Colorado Serum Co. (Denver, CO) and Complement Technology Inc. (Tyler, TX), respectively. Rat serum was obtained from Pel-Freez Biologicals, Inc. (Rogers, AR). Compstatin analog Cp40 (D-Tyr-Ile-[Cys-Val-1MeTrp-Gln-Asp-Trp-Sar-Ala-His-Arg-Cys]-meIle) was prepared by solid phase peptide synthesis and purified as acetate salt using reversed-phase HPLC as described previously (Qu, et al., 2013).
IgG purification
Patient IgG was purified using the Melon Gel IgG Purification Kit (Thermo Scientific, Rockford, IL) and adjusted to 1mg/ml.
C3Nef assay
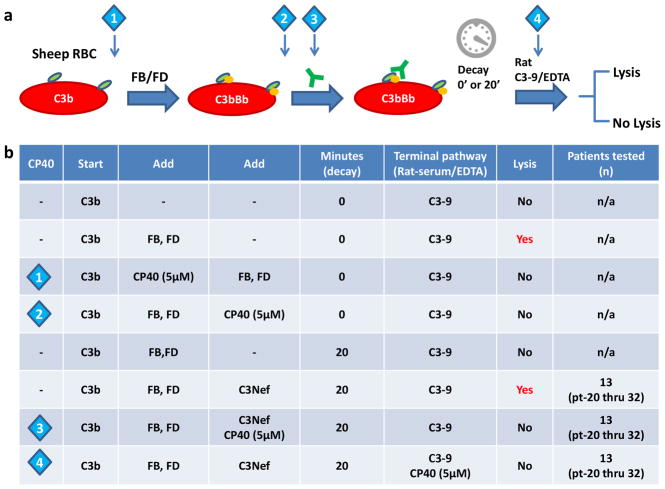
All C3Nef assays were performed as described previously (Zhang, et al., 2012). Briefly, C3b was deposited on non-sensitized sheep erythrocytes by FH-dpl/FB partial inactivated serum (FH-dpl serum was treated 50°C for 5 minutes). After washes with GVB-EGTA-Mg2+ buffer, C3 convertase (C3bBb) was constructed by adding purified FB and FD. C3 convertase-bearing cells were incubated with patient-purified IgG. After allowing convertase to decay at 30°C for 0 and 20 minutes, any remaining C3 convertase was developed by adding rat serum in GVB-EDTA buffer (1:9 dilution) as a source of C3-C9. Cp40 (5μM) was introduced at different stages of this assay as indicated in Figure 3a.
Figure 3.
Cp40 abrogates complement dysregulation induced by C3Nefs in vitro. (a) Illustration of C3Nef assay (C3Nefs, green Y). Cp40 is introduced at time points as indicated by the diamonds. (b) Cp40 inhibits C3Nef-mediated complement dysregulation in patients who are C3Nef positive (n=13).
C4Nef assay
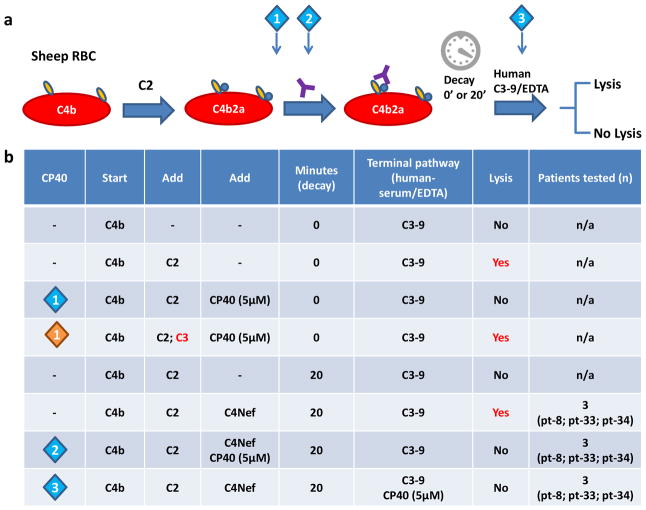
The C4Nef assay was performed as described previously, with modifications (Ohi and Yasugi, 1994). Briefly, C4b was deposited on EA using PNS in GVB-Ca2+-TTHA buffer. After washes, the classical pathway C3 convertase (C4b2a) was made by adding purified C2. C4b2a-bearing cells were incubated with patient-purified IgG. After allowing this convertase to decay at 30°C for either 0 or 20 minutes, any remaining C4b2a was developed by adding human FH-dpl serum in GVB-EDTA buffer (1:5 dilution) as a source of C3-C9. Cp40 (5μM) was introduced at different stages of this assay as indicated in Figure 4a.
Figure 4.
Cp40 abrogates complement dysregulation induced by C4Nefs in vitro. (a) Illustration of C4Nef assay (C4Nefs, purple Y). Cp40 is introduced at time points as indicated by the diamonds. (b) Cp40 prevents formation of C5 convertase (C4b2a3b), thus inhibiting C4Nef-mediated complement dysregulation in patients who are C4Nef positive (n=3).
Alternative pathway activity by hemolytic assay
The ability of Cp40 to inhibit the AP on cell surfaces was assessed in a hemolytic assay using non-sensitized sheep erythrocytes in GVB-EGTA buffer or in GVB-EDTA buffer as a control for spontaneous lysis. Serum (20 μl) with or without Cp40 was mixed with 5 × 108 cells to a total volume of 100 μl and incubated at 37°C for 30 minutes. The reaction was stopped by adding μl of 150 ice-cold GVB-EDTA buffer. Non-lysed cells were removed by centrifugation at 1000g for 10 minutes. Optical density (OD) of the supernatant was recorded at 414 nm, calculating percentage lysis as: [ODsample − ODblank]/[OD(total lysis) − ODblank]. Total lysis was obtained by using an equivalent volume of water.
Gel electrophoresis
Patient and PNS were mixed at 1:1 under GVB-EGTA buffer (AP activation possible) or GVB-EDTA buffer (AP activation not possible) and incubated at 37°C for 45 minutes. C3 degradation products were resolved by electrophoresis on pre-casted agarose Titan gels (Helena Laboratories, Beaumont, TX) and immune-precipitated using a polyclonal anti-human C3 antibody (MP Biomedical Inc., Pittsburgh, PA). Gels were stained with acid blue and quantitated using AlphaEaseFC software (Cell Biosciences Inc., Santa Clara, CA). Cp40 (at a final concentration of 10μM) was introduced with the serum.
Results
Cp40 prevents abnormal hemolysis in C3GN and DDD
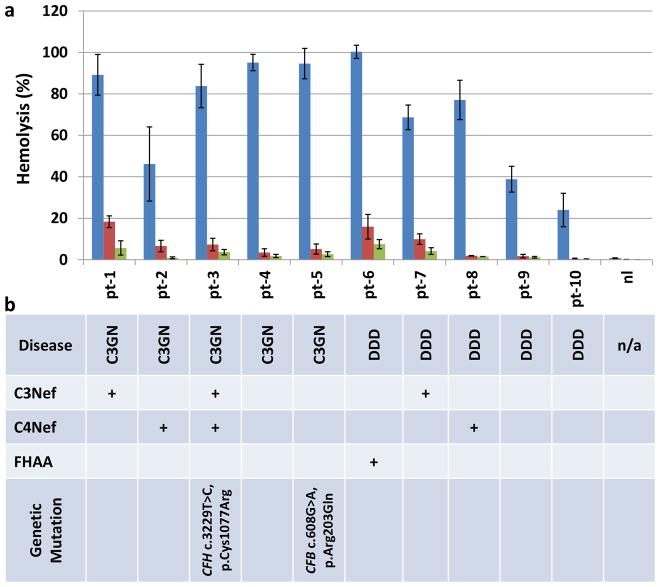
Sheep erythrocytes present a non-activating surface to human complement – when normal human serum is mixed with sheep erythrocytes, lysis does not occur (20% by volume in Mg2+-EGTA buffer to ensure that AP activation is possible). Since exogenous C3 is not supplemented in this assay and C3G patients often have very low C3, most C3G patients are negative for this assay. However, lysis is seen in approximately 12% of C3G patients with moderately reduced C3. This finding implies the presence of serum factors or genetic mutations that impede the interaction of factor H with C3 convertase. When added to this assay, Cp40 suppressed lysis (final concentrations tested, 5μM and 10 μM) in the presence of autoantibodies and genetic mutations (Fig. 1a and b).
Figure 1.
Cp40 restores complement control in both DDD and C3GN. (a) In vitro hemolysis is prevented in patients with DDD and C3GN (blue, 0μM Cp40; red, 5μM Cp40; green, 10μM Cp40). Shown are the mean values and SEMs of three experiments. (b) Identified disease drivers. C3Nef: C3 Nephritic factors; C4Nef: C4 Nephritic factors; FHAA: Factor H autoantibodies.
Cp40 prevents abnormal C3 conversion
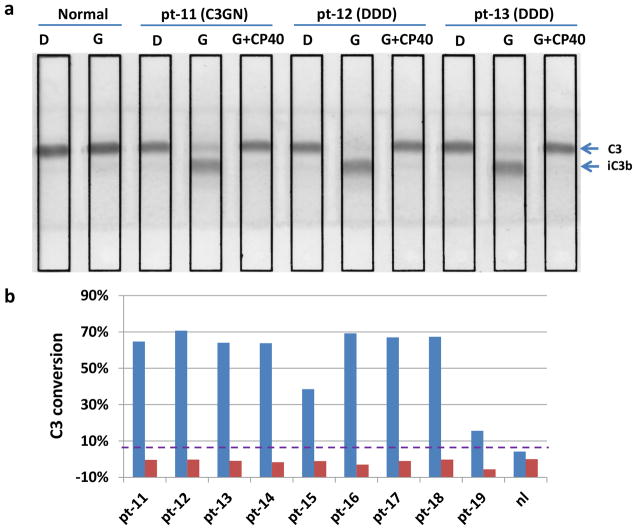
Incubation of PNS with C3G patient sera provides an exogenous source of C3 (C3G patients can have exceedingly low levels of serum C3). In the presence of uncontrolled convertase activity (under Mg2+-EGTA conditions (G) but not under EDTA conditions (D)), the result is the generation of C3 degradation products, which can be resolved by gel electrophoresis. The addition of Cp40 (final concentration 10 μM) to this assay prevented lysis (n=9; 7 DDD, 2 C3GN) (Fig. 2A and B).
Figure 2.
Cp40 prevents abnormal C3 breakdown as assayed by immunofixation electrophoresis (IFE). (a) IFE gel: incubation of PNS with patient sera generates large amounts of iC3b under Mg2+-EGTA conditions (G) but not under EDTA conditions (D). The addition of Cp40 (final 10μM) prevents lysis. Purple dashed line, normal cutoff. (b) Percent C3 conversion, C3 conversion was quantified as: %iC3b(G) − %iC3b(D) or %iC3b(G+CP40) − %iC3b(D).
Cp40 and convertase formation
Compstatin analogs, including Cp40, bind to human but not rodent C3 (Mastellos, et al., 2015), a property that allowed us to study interaction between Cp40 and convertase formation. We built C3 convertase on C3b-bearing cells (E) by providing FB and FD. Cell lysis occurred upon addition of C3-9 (rat serum-EDTA). We then tested whether Cp40 could prevent proteolytic activity by introducing it after the formation of C3bBb. Lysis was prevented (Fig. 3; diamond 2).
Formation of C5 convertases requires the addition of C3b to existing C3 convertases (C3bBb of the AP or C4b2a of the CP). To test whether Cp40 prevents this step from occurring, we used the CP C3 convertase, C4b2a, since this convertase does not have a target for Cp40 (there is no C3b). After building C4b2a on erythrocytes, we added PNS diluted in GVB-EDTA buffer as a source of C3-9. Cell lysis occurred after 1hr incubation at 37°C. When Cp40 (5μM) was introduced after C2 (Fig. 4; diamond 1 blue), no lysis was observed. Lysis did occur if we developed cells with rat serum-EDTA (data not shown) or added human C3 together with C2. These findings mean that Cp40 prevents C5 convertase formation but has no effect on the activity of existing C5 convertase (Fig. 4b; diamond 1 orange).
Cp40 and C3Nef
Most C3G patients circulate C3 convertase-stabilizing autoantibodies called C3Nefs. To investigate whether Cp40 restores complement regulation in the presence of these autoantibodies, we added Cp40 at different time points in the hemolytic assay (either with patient-purified IgG or with rat serum-EDTA). Addition of Cp40 (5μM) prevented hemolysis, implying that Cp40 prevents the cleavage activity of C3Nef-stabilized C3 convertase [n=13 (5 DDD and 8 C3GN)] (Fig. 3b).
Cp40 and C4Nef
To investigate whether Cp40 prevents C4Nef-mediated complement dysregulation, we tested the classical pathway C3 convertase, C4b2a, stabilized for at least 20 minutes using C4Nef derived from 3 patients (1 DDD and 2 C3GN). In this assay, C4b2a-bound cells were developed using PNS-EDTA. The effect of Cp40 on C4Nef-stabilized C4b2a was evaluated by adding Cp40 either with patient-purified IgG or with PNS serum-EDTA. At both time points, Cp40 (5μM) prevented hemolysis. When the assay was developed using rat serum-EDTA as a source of C3-9, hemolysis occurred, consistent with the inability of Cp40 to bind to rodent C3 (data not shown). These data imply that Cp40 corrects C4Nef- mediated complement dysregulation.
Discussion
Patients with C3G have hematuria, proteinuria and low serum C3 at clinical presentation; most also have hypertension and edema. Although a renal biopsy is required for an unequivocal diagnosis (Pickering, et al., 2013), once a diagnosis is confirmed, treatment options to prevent progression to ESRF are limited. For this reason, as data implicating complement dysregulation in C3G have become more robust, clinicians have turned to available anti-complement drugs as potential therapy. There are now a few reports describing the efficacy of eculizumab in C3G (Vivarelli, et al., 2012, McCaughan, et al., 2012, Daina, et al., 2012, Bomback, et al., 2012, Le Quintrec, et al., 2015).
Eculizumab binds C5 and prevents its cleavage by C5 convertase. The direct result is two-fold: the potent anaphylatoxin, C5a, is not generated and the production of C5b-9 is prevented. Thus, eculizumab should be an effective in the subset of C3G patients with dysregulation of C5 convertase (Zhang, et al., 2014). In patients with dysregulation of both C3 and C5 convertases, or exclusively at the level of C3 convertase, C5 blockade does not prevent disease progression (Bomback, et al., 2012, Gurkan, et al., 2013, Berthe-Aucejo, et al., 2014). These observations are consistent with murine models of C3G (Cfh−/−C5−/−) in which disease is ameliorated but not completely corrected (Pickering, et al., 2006). These data also confirm the need for more proximal inhibition of complement in C3G patients.
Several therapies that target C3 or C3 convertase are under development. These drugs can be classified broadly as antibody-based therapeutics, which aim to control complement by blocking specific components of C3 convertase to prevent its formation and/or function (e.g., anti-C3b monoclonal antibody (Paixao-Cavalcante, et al., 2014); anti-FB antibody (Subias, et al., 2014); anti-properdin antibody (Pauly, et al., 2014)), and regulation-based strategies, which aim to control complement by augmenting control of the C3 convertase (e.g., soluble CR1 (Zhang, et al., 2013); mini-FH (directly links the regulatory and surface targeting domains of FH) (Hebecker, et al., 2013); TT30 (combines the regulatory domain of FH with the iC3b/C3d-binding domain of CR2) (Risitano, et al., 2012)). Clinical experience with these new drugs in C3G patients is nearly non-existent (Zhang, et al., 2013).
Unique amongst C3/C3 convertase-targeting therapies is Cp40, a small peptide that binds to C3. Since physiological concentrations of plasma C3 are very high (~1.3 g/L) and its turnover is rapid, sustained effective inhibition of C3 convertase may require comparatively large doses of any therapeutic to achieve sustained target saturation. In this regard, Cp40 is attractive. Cp40 is the most recent compstatin analog to be developed. As compared to the original compstatin, it shows significant improvement in binding affinity to human C3 (6,000-fold), enhanced inhibitory potency, and an extended in vivo half-life (~12 hours after single intravenous injection) (Qu, et al., 2013).
To gain insight into the possibility of AP regulation with Cp40 in C3G, we performed a battery of tests using patient sera and plasma samples. Since not all patients are positive for all tests, we selected 34 samples (17 DDD and 17 C3GN) from our registry. We typically use a direct hemolytic assay with non-sensitized sheep erythrocytes as a screening test to determine whether on-going AP dysregulation is present. Although only a small percentage of patients are positive on this assay (~12%) Cp40 prevented AP dysregulation independent of the underlying disease trigger in all positive patients (Figure 1).
IFE is a fluid phase assay used most frequently as an indirect test for C3Nefs, autoantibodies that extend the half-life of C3 convertase several fold. Cp40 arrested abnormal C3 turnover in all C3G we tested (Figure 2). Finally, direct interaction between CP40 and purified nephritic factors (C3Nefs and C4Nefs from 16 patients) strongly support our therapeutic concept: virtual C3 depletion can be an effective treatment strategy for C3G regardless of the presence of C3Nefs or C4Nefs (Figures 3 and 4). As down-stream complement control occurs in the presence of Cp40, the formation of C5 convertase and the generation of C5a, soluble C5b-9, and lytic membrane attack complex are also prevented.
All complement-inhibition strategies increase susceptibility to infection, which is a major clinical concern. However, pre-treatment vaccination can substantially reduce the risk of infection. Long-term eculizumab use (over 2 years) reports only 2 patients of 195 who developed meningococcal infections caused by non-vaccinated strains; these patients were treated with antibiotics (Hillmen, et al., 2013). Although long-term inhibition at the level of C3 has not been evaluated clinically to date, inherited deficiencies of C3 are reported and suggest that infection risk for infection is increased only to a limited set of pathogens in younger persons (Reis, et al., 2006). Thus Cp40 therapy will require prophylactic vaccination against encapsulated bacteria, including meningococci, pneumococci and haemophilus influenza.
To date, Cp40 has been evaluated in several disease models, including paroxysmal nocturnal hemoglobinuria, hemodialysis-induced inflammation and periodontal disease (Maekawa, et al., 2014, Reis, et al., 2014, Risitano, et al., 2014). To this list we now add C3G. If Cp40 is able to offer to C3G patients a disease-specific, targeted therapy, this drug will represent a major advancement for these patients.
Acknowledgments
This work was supported by grants from the National Institutes of Health (AI068730, AI030040, EY020633, and GM097747) and the European Community’s Seventh Framework Programme under grant agreement number 602699 (DIREKT).
Footnotes
Conflicts of interest
J.D.L. and D.R. are the inventors of patents and/or patent applications that describe the use of complement inhibitors for therapeutic purposes. J.D.L. is the founder of Amyndas Pharmaceuticals, which is developing complement inhibitors for clinical applications. The remaining authors declare no competing financial interests.
References
- Pickering MC, D’Agati VD, Nester CM, Smith RJ, Haas M, Appel GB, Alpers CE, Bajema IM, Bedrosian C, Braun M, Doyle M, Fakhouri F, Fervenza FC, Fogo AB, Fremeaux-Bacchi V, Gale DP, Goicoechea de Jorge E, Griffin G, Harris CL, Holers VM, Johnson S, Lavin PJ, Medjeral-Thomas N, Paul Morgan B, Nast CC, Noel LH, Peters DK, Rodriguez de Cordoba S, Servais A, Sethi S, Song WC, Tamburini P, Thurman JM, Zavros M, Cook HT. C3 glomerulopathy: consensus report. Kidney Int. 2013;84:1079. doi: 10.1038/ki.2013.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medjeral-Thomas NR, O’Shaughnessy MM, O’Regan JA, Traynor C, Flanagan M, Wong L, Teoh CW, Awan A, Waldron M, Cairns T, O’Kelly P, Dorman AM, Pickering MC, Conlon PJ, Cook HT. C3 glomerulopathy: clinicopathologic features and predictors of outcome. Clin J Am Soc Nephrol. 2014;9:46. doi: 10.2215/CJN.04700513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nester CM, Smith RJ. Diagnosis and treatment of C3 glomerulopathy. Clin Nephrol. 2013;80:395. doi: 10.5414/CN108057. [DOI] [PubMed] [Google Scholar]
- Servais A, Noel LH, Roumenina LT, Le Quintrec M, Ngo S, Dragon-Durey MA, Macher MA, Zuber J, Karras A, Provot F, Moulin B, Grunfeld JP, Niaudet P, Lesavre P, Fremeaux-Bacchi V. Acquired and genetic complement abnormalities play a critical role in dense deposit disease and other C3 glomerulopathies. Kidney Int. 2012;82:454. doi: 10.1038/ki.2012.63. [DOI] [PubMed] [Google Scholar]
- Lu DF, Moon M, Lanning LD, McCarthy AM, Smith RJ. Clinical features and outcomes of 98 children and adults with dense deposit disease. Pediatr Nephrol. 2012;27:773. doi: 10.1007/s00467-011-2059-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi S, Fervenza FC. Pathology of renal diseases associated with dysfunction of the alternative pathway of complement: C3 glomerulopathy and atypical hemolytic uremic syndrome (aHUS) Semin Thromb Hemost. 2014;40:416. doi: 10.1055/s-0034-1375701. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Meyer NC, Wang K, Nishimura C, Frees K, Jones M, Katz LM, Sethi S, Smith RJ. Causes of alternative pathway dysregulation in dense deposit disease. Clin J Am Soc Nephrol. 2012;7:265. doi: 10.2215/CJN.07900811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer RE, Vallota EH, Forristal J, Sudora E, Stitzel A, Davis NC, West CD. Serum C’3 lytic system in patients with glomerulonephritis. Science. 1969;164:436. doi: 10.1126/science.164.3878.436. [DOI] [PubMed] [Google Scholar]
- Daha MR, Fearon DT, Austen KF. C3 nephritic factor (C3NeF): stabilization of fluid phase and cell-bound alternative pathway convertase. J Immunol. 1976;116:1. [PubMed] [Google Scholar]
- Strobel S, Zimmering M, Papp K, Prechl J, Jozsi M. Anti-factor B autoantibody in dense deposit disease. Mol Immunol. 2010;47:1476. doi: 10.1016/j.molimm.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Chen Q, Muller D, Rudolph B, Hartmann A, Kuwertz-Broking E, Wu K, Kirschfink M, Skerka C, Zipfel PF. Combined C3b and factor B autoantibodies and MPGN type II. N Engl J Med. 2011;365:2340. doi: 10.1056/NEJMc1107484. [DOI] [PubMed] [Google Scholar]
- Goodship TH, Pappworth IY, Toth T, Denton M, Houlberg K, McCormick F, Warland D, Moore I, Hunze EM, Staniforth SJ, Hayes C, Cavalcante DP, Kavanagh D, Strain L, Herbert AP, Schmidt CQ, Barlow PN, Harris CL, Marchbank KJ. Factor H autoantibodies in membranoproliferative glomerulonephritis. Mol Immunol. 2012;52:200. doi: 10.1016/j.molimm.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Nester CM, Martin B, Skjoedt MO, Meyer NC, Shao D, Borsa N, Palarasah Y, Smith RJ. Defining the complement biomarker profile of C3 glomerulopathy. Clin J Am Soc Nephrol. 2014;9:1876. doi: 10.2215/CJN.01820214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivarelli M, Pasini A, Emma F. Eculizumab for the treatment of dense-deposit disease. N Engl J Med. 2012;366:1163. doi: 10.1056/NEJMc1111953. [DOI] [PubMed] [Google Scholar]
- McCaughan JA, O’Rourke DM, Courtney AE. Recurrent dense deposit disease after renal transplantation: an emerging role for complementary therapies. Am J Transplant. 2012;12:1046. doi: 10.1111/j.1600-6143.2011.03923.x. [DOI] [PubMed] [Google Scholar]
- Daina E, Noris M, Remuzzi G. Eculizumab in a patient with dense-deposit disease. N Engl J Med. 2012;366:1161. doi: 10.1056/NEJMc1112273. [DOI] [PubMed] [Google Scholar]
- Bomback AS, Smith RJ, Barile GR, Zhang Y, Heher EC, Herlitz L, Stokes MB, Markowitz GS, D’Agati VD, Canetta PA, Radhakrishnan J, Appel GB. Eculizumab for dense deposit disease and C3 glomerulonephritis. Clin J Am Soc Nephrol. 2012;7:748. doi: 10.2215/CJN.12901211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurkan S, Fyfe B, Weiss L, Xiao X, Zhang Y, Smith RJ. Eculizumab and recurrent C3 glomerulonephritis. Pediatr Nephrol. 2013;28:1975. doi: 10.1007/s00467-013-2503-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastellos DC, Yancopoulou D, Kokkinos P, Huber-Lang M, Hajishengallis G, Biglarnia AR, Lupu F, Nilsson B, Risitano AM, Ricklin D, Lambris JD. Compstatin: a C3-targeted complement inhibitor reaching its prime for bedside intervention. Eur J Clin Invest. 2015 doi: 10.1111/eci.12419. [DOI] [PMC free article] [PubMed]
- Qu H, Ricklin D, Bai H, Chen H, Reis ES, Maciejewski M, Tzekou A, DeAngelis RA, Resuello RR, Lupu F, Barlow PN, Lambris JD. New analogs of the clinical complement inhibitor compstatin with subnanomolar affinity and enhanced pharmacokinetic properties. Immunobiology. 2013;218:496. doi: 10.1016/j.imbio.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaki K, Iida K, Inai S. A new method for the preparation of EAC14 cell with human or guinea-pig serum. J Immunol Methods. 1974;5:307. doi: 10.1016/0022-1759(74)90117-3. [DOI] [PubMed] [Google Scholar]
- Ohi H, Yasugi T. Occurrence of C3 nephritic factor and C4 nephritic factor in membranoproliferative glomerulonephritis (MPGN) Clin Exp Immunol. 1994;95:316. doi: 10.1111/j.1365-2249.1994.tb06530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Quintrec M, Lionet A, Kandel C, Bourdon F, Gnemmi V, Colombat M, Goujon JM, Fremeaux-Bacchi V, Fakhouri F. Eculizumab for treatment of rapidly progressive c3 glomerulopathy. Am J Kidney Dis. 2015;65:484. doi: 10.1053/j.ajkd.2014.09.025. [DOI] [PubMed] [Google Scholar]
- Berthe-Aucejo A, Sacquepee M, Fila M, Peuchmaur M, Perrier-Cornet E, Fremeaux-Bacchi V, Deschenes G. Blockade of alternative complement pathway in dense deposit disease. Case Rep Nephrol. 2014;2014:201568. doi: 10.1155/2014/201568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering MC, Warren J, Rose KL, Carlucci F, Wang Y, Walport MJ, Cook HT, Botto M. Prevention of C5 activation ameliorates spontaneous and experimental glomerulonephritis in factor H-deficient mice. Proc Natl Acad Sci U S A. 2006;103:9649. doi: 10.1073/pnas.0601094103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paixao-Cavalcante D, Torreira E, Lindorfer MA, Rodriguez de Cordoba S, Morgan BP, Taylor RP, Llorca O, Harris CL. A humanized antibody that regulates the alternative pathway convertase: potential for therapy of renal disease associated with nephritic factors. J Immunol. 2014;192:4844. doi: 10.4049/jimmunol.1303131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subias M, Tortajada A, Gastoldi S, Galbusera M, Lopez-Perrote A, de Lopez LJ, Gonzalez-Fernandez FA, Villegas-Martinez A, Dominguez M, Llorca O, Noris M, Morgan BP, Rodriguez de Cordoba S. A novel antibody against human factor B that blocks formation of the C3bB proconvertase and inhibits complement activation in disease models. J Immunol. 2014;193:5567. doi: 10.4049/jimmunol.1402013. [DOI] [PubMed] [Google Scholar]
- Pauly D, Nagel BM, Reinders J, Killian T, Wulf M, Ackermann S, Ehrenstein B, Zipfel PF, Skerka C, Weber BH. A novel antibody against human properdin inhibits the alternative complement system and specifically detects properdin from blood samples. PLoS One. 2014;9:e96371. doi: 10.1371/journal.pone.0096371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Nester CM, Holanda DG, Marsh HC, Hammond RA, Thomas LJ, Meyer NC, Hunsicker LG, Sethi S, Smith RJ. Soluble CR1 therapy improves complement regulation in C3 glomerulopathy. J Am Soc Nephrol. 2013;24:1820. doi: 10.1681/ASN.2013010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebecker M, Alba-Dominguez M, Roumenina LT, Reuter S, Hyvarinen S, Dragon-Durey MA, Jokiranta TS, Sanchez-Corral P, Jozsi M. An engineered construct combining complement regulatory and surface-recognition domains represents a minimal-size functional factor H. J Immunol. 2013;191:912. doi: 10.4049/jimmunol.1300269. [DOI] [PubMed] [Google Scholar]
- Risitano AM, Notaro R, Pascariello C, Sica M, del Vecchio L, Horvath CJ, Fridkis-Hareli M, Selleri C, Lindorfer MA, Taylor RP, Luzzatto L, Holers VM. The complement receptor 2/factor H fusion protein TT30 protects paroxysmal nocturnal hemoglobinuria erythrocytes from complement-mediated hemolysis and C3 fragment. Blood. 2012;119:6307. doi: 10.1182/blood-2011-12-398792. [DOI] [PubMed] [Google Scholar]
- Hillmen P, Muus P, Roth A, Elebute MO, Risitano AM, Schrezenmeier H, Szer J, Browne P, Maciejewski JP, Schubert J, Urbano-Ispizua A, de Castro C, Socie G, Brodsky RA. Long-term safety and efficacy of sustained eculizumab treatment in patients with paroxysmal nocturnal haemoglobinuria. Br J Haematol. 2013;162:62. doi: 10.1111/bjh.12347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis ES, Falcao DA, Isaac L. Clinical aspects and molecular basis of primary deficiencies of complement component C3 and its regulatory proteins factor I and factor H. Scand J Immunol. 2006;63:155. doi: 10.1111/j.1365-3083.2006.01729.x. [DOI] [PubMed] [Google Scholar]
- Maekawa T, Abe T, Hajishengallis E, Hosur KB, DeAngelis RA, Ricklin D, Lambris JD, Hajishengallis G. Genetic and intervention studies implicating complement C3 as a major target for the treatment of periodontitis. J Immunol. 2014;192:6020. doi: 10.4049/jimmunol.1400569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis ES, DeAngelis RA, Chen H, Resuello RR, Ricklin D, Lambris JD. Therapeutic C3 inhibitor Cp40 abrogates complement activation induced by modern hemodialysis filters. Immunobiology. 2014 doi: 10.1016/j.imbio.2014.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risitano AM, Ricklin D, Huang Y, Reis ES, Chen H, Ricci P, Lin Z, Pascariello C, Raia M, Sica M, Del Vecchio L, Pane F, Lupu F, Notaro R, Resuello RR, DeAngelis RA, Lambris JD. Peptide inhibitors of C3 activation as a novel strategy of complement inhibition for the treatment of paroxysmal nocturnal hemoglobinuria. Blood. 2014;123:2094. doi: 10.1182/blood-2013-11-536573. [DOI] [PMC free article] [PubMed] [Google Scholar]