Synopsis
Despite its growing pervasiveness, the health challenges prompted by obesity in the older adult population are poorly recognized and under-studied. A defined treatment for geriatric obesity is difficult to establish, as it must take into account biological heterogeneity, age-related co-morbidities, and functional limitations (sarcopenia/dynapenia). This restrospective article highlights our current understanding of the optimal body mass index (BMI) in later life, addressing appropriate recommendations based on BMI category, age, and health history. As the findings of randomized control trials of weight loss/maintenance interventions continue to accumulate, we are moving closer to evidence-based and appropriately individualized recommendations for body weight management in older adults.
Keywords: Obesity, overweight, body mass index, physical function, muscle mass, weight reduction, weight maintenance
Introduction: Nature of the Problem
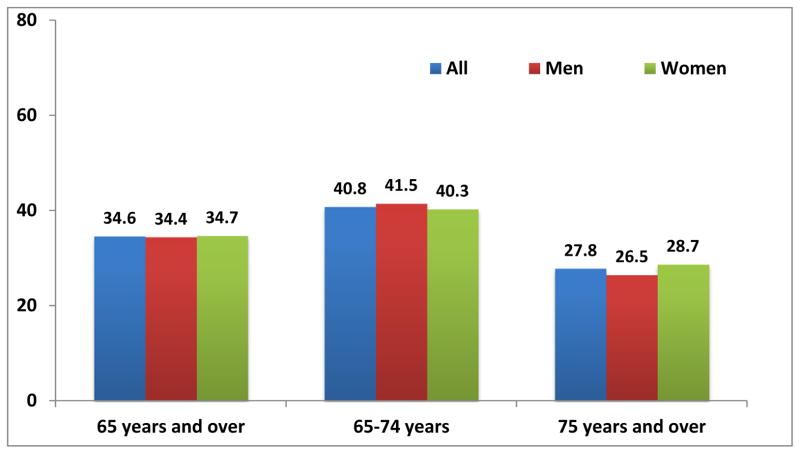
The pervasiveness of the obesity epidemic in the older adult population is poorly recognized and under-studied. Yet, as illustrated in Figure 1, more than 1/3rd of U.S. adults ≥60 years of age have body weights in the obese (body mass index (BMI) ≥30 kg/m2) range[1]; the prevalence of geriatric obesity has steadily increased in recent decades and is expected to continue to rise.[2] Obesity, inactivity, and aging are each independent risk factors for detrimental metabolic changes leading to conditions like cardiovascular disease and impaired glucose intolerance, so the obese older person is especially vulnerable to such derangements.[3 4] However, the loss of function is often the most distressful to elders because it threatens independence and dignity during the later years of life.[5–7] These “fat and frail” older adults have insufficient muscle strength relative to body size to remain fully active. The contribution of obesity to impaired function is progressive, as excessive adiposity and loss of muscle strength/mass are cyclically reinforcing.[8]
Figure 1.
Prevalence of obesity among adults aged 65 and over, by sex: United States 2007–2010
Source: CDC/NCHS, National Health and Nutrition Examination Survey, 2007–2010.[9]
Therapeutic Options for Optimizing Body Mass
Table 1 lists categories of BMI level and corresponding lifestyle (diet and exercise) recommendations to optimize health and longevity in older adults based on the best evidence currently available.
Table 1.
Lifestyle recommendations for older adults should be individualized by BMI and age
| BMI (kg/m2) | Weight Status | Diet Recommendation | Exercise Recommendation |
|---|---|---|---|
| <23.0 | Under-weight | High nutrient dense diet with sufficient kcal to gradually increase weight and muscle mass | Emphasize resistance training to build muscle; also aerobic exercise, balance, and flexibility training |
| 23.0 to 29.9 | Normal/“Overweight” | High nutrient dense diet with sufficient kcal to maintain weight | Combination of aerobic exercise, resistance, balance and flexibility training |
| ≥30 Age ≤80 | Obese | Modest calorie reduction (500 to <1000) to achieve gradual weight loss; emphasize high nutrient density | Combination of aerobic exercise, resistance, balance, and flexibility training |
| ≥30 Age >80 | Obese | High nutrient dense diet with sufficient kcal to maintain weight | Resistance, balance, and flexibility training, aerobic exercise as tolerated |
Underweight
For older adults, a BMI in the range of 23.0 to 29.9 kg/m2 is associated with optimal longevity.[10 11] A meta-analysis by Winter et al.[10] indicated that, within the “normal weight” category of 18 to 24.9 kg/m2, mortality was better at a BMI ≥23.0 kg/m2. Therefore, we recommend that older adults with a BMI <23.0 kg/m2 be encouraged to use a combination of nutrient and calorie dense foods and exercise to gradually increase their BMI, ideally using resistance training to increase muscle mass.
Overweight
In the case of older adults, a BMI in the range typically categorized as “overweight” (25.0 to 29.9 kg/m2) is not associated with adverse mortality outcomes.[12] Flegal and colleagues[11] analyzed 97 studies (of 2.88 million individuals) and found that being overweight was associated with the lowest mortality across all age groups, as well as in older adults specifically. In their analysis, being obese (BMI ≥30) was associated with higher mortality across all ages, although not in older adults. These findings indicate that the protective effects of overweight on survival occur independently of age and that in the case of obesity detrimental effects on survival are somewhat blunted in later life.
Obese, <80 years
Until recently, weight reduction therapies for obesity in older adults have been deemed controversial.[13–15] With traditional weight loss regimens, the loss of lean muscle mass can constitute 25% or more of the total amount of weight lost.[16] Bone mineral density is also slightly reduced when weight loss occurs.[13] Thus, there is tension between the need to minimize the negative side effects of weight loss and the many advantages of reducing excess body weight. However, for older adults who are obese and experiencing metabolic and/or functional challenges, recent evidence argues that beneficial and safe body weight reduction can be achieved, especially when exercise is included as part of the treatment.[17] Based on studies in obese older adults who are physically able to participate, both aerobic and resistance exercise training can help to protect lean mass and preserve function during weight loss.[18–20] While weight loss interventions for older adults remain controversial, per the accumulating evidence from randomized controlled trials illustrated in the next section, weight reduction in this population can benefit muscle quality, physical function, inflammatory status, and metabolic profiles. [13][17][2]
Obese, 80+ years and/or with complicating circumstances
There are essentially no studies of obesity reduction in adults 80 year of age or older. In these individuals, we would therefore advocate for weight maintenance with an emphasis on a healthy diet and exercise as tolerated. This would also be the case for any older adult with a terminal illness, those with severe chronic medical conditions, and persons with moderate to severe dementia.
Clinical Outcomes
Weight-loss therapy that minimizes loss of muscle and bone mass is recommended for older persons who are obese and who have functional impairments or medical complications that can benefit from weight reduction.[21] Table 2 presents the findings to date of clinical intervention trials for obesity reduction in older adults.
Table 2.
Non-Pharmacological Weight Loss Interventions
| Studies | Study population | Intervention | Outcomes | Important Findings |
|---|---|---|---|---|
| Beavers et al. 2015[31] |
N= 24 Mean age: 68.4±5.5 yrs Gender: 88% women BMI: 35.1±4.3 kg/m2 Health: Abdominal obesity (WC ≥102 men 88 cm for men and women) |
Design: RCT Arms: WL+Soy (n=12) WL+Non-soy (n=12) Control (n = 93) Duration : 3 mo |
Body composition by DXA; cardiometabolic biomarkers (systolic and diastolic BP, glucose, serum insulin, total cholesterol, triglycerides, LDL, HDL, and hsCRP); SPPB; 400-m walk; knee extensor strength using dynamometer; grip strength. |
Weight change: WL+Soy: −7.2±7.8 kg; WL+Non-soy: −8.4±3.7 kg Body Comp: No difference between arms on all body composition measures. All body composition measures were significantly decreased in both groups except thigh intermuscular fat volume. Total body fat was reduced by 5.3±2.4 kg and lean mass by 2.5±1.9 kg in both arms combined. Function: No difference between interventions. Combined, there was no change in 400-m walk time, SPPB, and grip strength; while knee extensor strength was significantly reduced (−5.09±9.4 N/m). When knee extensor strength was corrected for thigh muscle volume there was no longer a significant change (−0.00±0.02 N/m). Cardiometabolic biomarkers: Diastolic BP was significantly reduced in the WL+Non-soy (66.7±2.7 mmHg) compared to WL+Soy (75.3±2.7 mmHg). No other cardiometabolic biomarkers differed between interventions. Combined, both interventions had significant reductions in systolic BP (−9.4±45.6 mmHg), total cholesterol (−24.6±20.6 mg/dL), LDL (−14.0±20.0 mg/dL), and insulin (−4.4±6.0 μIU/mL) measures. |
| Verreijen et al. 2015[25] |
N= 60 Mean age: 63.0±5.6 yrs Gender: 53% women BMI: 33.0±4.4 kg/m2 Health: BMI ≥30 kg/m2 or BMI ≥28 kg/m2 and abdominal obesity (WC ≥102 men 88 cm for men and women |
Design: RCT Arms: EX+WL+Protein (n=30) EX + WL (n=30) Duration : 13 wk |
Body composition by DXA; handgrip strength; 4-m gait speed; 400-walk speed; chair strength. |
Weight change: WL+Protein+EX: −3.4±3.6 kg; WL+EX: −2.8±2.8 kg, no difference between arms. Body Comp: Fat mass was decreased in both arms (WL+Protein+EX: −3.2±3.1 kg; WL+EX: −2.5±2.4 kg). Appendicular and leg muscle mass differed between arms (WL+Protein+EX: 0.4±1.2 kg; 0.3±1.2 kg vs. WL+EX: −0.5±2.1 kg; −0.6±1.2 kg). Function: No difference between interventions. All functional measures significantly improved in both arms. |
| Beavers et al. 2014[32]; Beavers et al. 2013[33]; Rajeski et al. 2011[34] |
N= 288 Mean age: 67±5yrs Gender: 67% women BMI: 32.8±3.8 kg/m2 Health: CVD or cardiometabolic dysfunction and self-reported functional limitations |
Design: RCT Arms: WL+EX (n=98) EX (n=97) Control (n = 93) Duration: 18 mo |
Body composition by DXA; cardiometabolic biomarkers (systolic and diastolic BP, glucose, serum insulin, total cholesterol, triglycerides, LDL, HDL, HOMA-IR); inflammatory markers (adiponectin, leptin, high-sensitivity IL-6, IL6sR, and Stnfr1); 400-m walk. |
Weight change: WL+EX: −7.3±7.1 kg; EX: −1.3±5.1 kg; Control: −1.0±6.2 kg Body Comp: Fat mass was significantly reduced in the WL+EX arm compared to EX and Control arms. Lean mass decreased greater in WL+EX arm (−2.5±2.8 kg) compared to EX (−0.7±2.2 kg) and Control (−0.8±2.4 kg) arms. Function: Walking speed increased more in WL+EX (323.3 [3.7] seconds) compared to EX (336.3 [3.9] seconds) and Control (341.3 [3.9] seconds) arms. Cardiometabolic Biomarkers: Decreased body mass was significantly associated with improvements in diastolic BP, triglycerides, glucose, insulin, HOMA-IR, and HDL. Inflammatory Biomarkers: Leptin and hsIL-6 were significantly lower in WL+EX (21.3 (19.7, 22.9) ng/mL; 2.1 (1.9, 2.3) pg/mL) compared to EX (29.3 (26.9, 31.8) ng/mL; 2.5 (2.3, 2.7) pg/mL) and Control (30.3 (27.9, 32.8) ng/mL; 2.4 (2.2, 2.6) pg/mL) arms. |
| Beavers et al. 2014[35] |
N= 284 Mean age: 66±6 yrs Gender: 67% women BMI: 33.4±3.7 kg/m2 Health: OA in one or both knees |
Design: RCT Arms: WL+EX (n=101) WL (n=88) EX (n = 95) Duration: 18 mo |
Regional BMD and T-scores at hip and spine; Body composition by DXA. |
Weight change: WL+EX: −10.4±8.0 kg; WL: −9.1±8.6 kg: EX: −1.3±4.5 kg BMD: Significant reduction in BMD at hip and femoral neck in both WL+EX (−19.5 (−25.8, −13.2); −12.5 (−18.1, −6.8)) and WL (−23.7 (−30.3, −17.2); −13.2 (−19.1, −7.3)) arms compared to EX (−2.0 (−8.5, 4.4); −0.8 (−6.6, 5.0)). |
| Solomon et al. 2013[36] |
N= 15 Mean age: 65±1yrs Gender: 55% women BMI: 35.0±1.0 kg/m2 Health: Sedentary |
Design: RCT Arms: WL low GI+EX (n=4) WL high GI+EX (n= 11) Duration: 3 mo |
Muscle lipid composition by 3H-MRS of soleus muscle. |
Weight change: −8.6 ± 1.1% for both groups Muscle Lipid Comp: Unchanged. |
| Armamento-Villareal et al. 2012[37]; Villareal et al. 2011[17]; Shah et al. 2011[38] |
N= 93 Age: Control: 69±4; WL, EX, and WL+EX:70±4 yrs Gender: Control: 67% women; WL: 65% women; EX: 62% women; WL+EX: 57% women BMI: control: 37.3±4.7; WL: 37.2±4.5; EX: 36.9±5.4; WL+EX: 37.2±5.4 kg/m2 Health: Mild to moderate frailty Sedentary lifestyle |
Design: RCT Arms: Control (n=27) WL (n=26) EX (n=26) WL+EX (n=28) Duration: 12 mo |
BMD; serum sclerostin; hip geometry; C-terminal telopeptide of type collagen 1; osteocalcin; N-terminal propeptide of type 1 procollagen, serum estradiol, IGF-1; Vitamin D; serum PTH; PPT; VO2 max; FSQ score; 1-RM; dynamic balance (obstacle course); static balance (single limb leg stance time); gait speed; SF-36; body composition by DXA; anthropometrics |
Weight change: Control: −0.1±3.5 kg, <1%; WL: −9.7±5.4 kg, 10%; EX: −0.5±3.6 kg, 1%; WL+EX: −8.6±3.8 kg, 9% Function: WL+EX and EX arms significantly improved in all functional measures compared to control. WL significantly improved compared to control in all functional measures except strength and gait speed. PPT and VO2 improved more in WL+EX than WL or EX. Similar improvements were seen between WL+EX and EX arms in FSQ, 1-RM and gait speed, and between WL+EX and WL in single limb leg stance time. Body Comp: Significant and similar decreased in BW and FM in WL and WL+EX arm. FFM increased in EX and decreased less in the WL+EX compared to WL. BMD: Increased in EX (1.5±1.6%) and decreased in WL (−2.6±2.1%) and WL+EX (−1.1±2.7%). Bone Metabolism: Sclerostin increased in diet arm (10.5±1.9%) and unchanged in all other arms. Serum CTX and osteocalcin increased in the WL arm (24±32%), decreased in the EX arm (−13±31%), and remained unchanged in the WL+EX arm. Serum 25(OH)D increased in all arms. Serum leptin and estradiol remained unchanged in EX and control arms while decreasing in the WL (−25±31%; −15±18%) and WL+EX (−38±26%; −13±30%), arms. Hip Geometry: Significant decreases in cross sectional area, and cortical thickness and increases in bucking ratio at narrow neck, intertrochanter and femoral shaft in all arms except WL-EX arm. |
| Anton et al. 2011[18] |
N= 34 Age: 63.7±4.5 yrs Gender: Women only BMI: Control: 35.8±6.8 kg/m2, WL + EX: 37.8±5.5 kg/m2 Health: Mild to moderate functional impairment |
Design: RCT Arms: Control (n=17) WL+EX (n=17) Duration: 6 mo |
Walking speed; SPPB; knee extension isokinetic; anthropometrics |
Weight change: Control: −0.23±4.08 kg; WL+EX: −5.95±4.08 kg Function: Walking speed increased more in WL+EX compared to control (0.16±0.03 m/s vs. 0.02±0.03 m/s); WL+EX and control increased SPPB scores, with greater increased in WL+EX (1.82±1.24 vs. 0.80±1.20) Other: WL+EX decreased BW greater than control (5.95±0.992 vs. 0.23±0.99 kg) |
| Dube et al. 2011[39] |
N= 16 Mean age: 67.2±4.0 yrs Gender: 56.2% women BMI: 30.6 ± 0.8 kg/m2 Health: Impaired glucose tolerance or elevated FBS |
Design: RCT Arms: Aerobic EX (n = 8) WL (n = 8) Duration: 4 mo |
Muscle lipid composition by histochemistry of muscle biopsy. Body composition by DXA. |
Weight change: WL: −8.5±1.5%; EX: −1.8±0.9% Muscle Lipid Comp: Decreased in WL (−16.0±3.2%); increased in EX (40.8±18.2%) Body Comp: Fat mass decreased greater in WL than in EX. FFM decreased in WL, increased slightly in EX. |
| Haus et al. 2011[40] |
N= 36 Mean age: 66±1 yrs Gender: 83.3 % women BMI: 32.9±3.2 kg/m2 Health: Sedentary |
Design: RCT Arms: WL low GI+EX (n=7) WL high GI+EX (n=8) Duration: 7 days |
Muscle lipid composition by 3H-MRS of soleus muscle. |
Weight change: WL low GI±EX: −1.7±0.6 kg; WL high GI+EX: −1.9±0.6 Muscle Lipid Comp: Increased, 2.3±1.3 mmol/kg in WL low GI+EX and 1.4±0.9 in WL high GI+EX. No group difference found. |
| Kelly et al. 2011[41] |
N= 28 Mean age: 66±1 yrs Gender: 83.3 % women BMI: 34.2±0.7 kg/m2 Health: insulin-resistant; sedentary |
Design: RCT Arms: WL low GI+EX (n=13) WL high GI+EX (n= 15) Duration: 3 mo |
Plasma and MNC TNF-α; IL-6; MCP-1; glucose; insulin; HbA1c; body composition via DXA |
Weight change: WL low GI+EX −6.5 kg; WL high GI+EX: −9.6; no significant difference between arms. Cardiometabolic Biomarkers: Significant reduction in fasting plasma glucose and insulin in both arms. Oral glucose tolerance was only reduced in the WL low GI+EX arm. Inflammatory Biomarkers: MNC and plasma TNF-α was reduced in the WL low GI+EX arm and increased in the WL high GI+EX arm. Plasma IL-6 was reduced in both arms with a significantly greater reduction in the WL low GI+EX arm. Body Comp: Fat mass and fat free mass were both significantly reduced in both arms, no difference between arms. |
| Santanasto et al. 2011[42] |
N= 36 Mean age: 70.3±5.9 yrs Gender: 83.3 % women BMI: 32.9±3.2 kg/m2 Health: Sedentary |
Design: RCT Arms: WL+EX ( n= 21) EX+Ed (n= 15) Duration: 6 mo |
Muscle lipid composition by CT of thigh; CT of abdomen; body composition by DXA; anthropometrics; SPPB; knee extensor strength, CHAMPS questionnaire. |
Weight change: −4.9±4.8 kg in WL+EX; −1.0±3.5 kg in EX+Ed Muscle Lipid Comp: Decreased in thigh of both groups, −18.1±17.5 cm in WL+EX and −5.4±9.9 cm in EX+Ed but group effect P<0.07. Body Comp: WL+EX decreased body fat, FFM, subcutaneous total visceral fat, SAT, VAT, and thigh fat, waist circumference and knee extensor strength and increased SPPB score. |
| Chomentowski et al. 2009[43] |
N= 29 Age: 67.2±4.2 Gender: 55.2% women BMI: 31.8±3.3 kg/m2 Health: impaired fasting glucose tolerance, impaired fasting glucose, or drug-naïve type 2 diabetes; sedentary |
Design: RCT Arms: WL+EX (n=18) WL (n=11) Duration: 4 mo |
Body composition by DXA; Thigh muscle cross-sectional area by CT-scan; skeletal muscle fiber size. |
Weight change: WL+EX: −9.2%±1.0%; WL: −9.1%±1.0% Body Comp: BW and total fat mass significantly decreased in both arms. WL (−4.3%±1.2%) lost significantly more lean mass compared to WL+EX (−1.1%±1.0%). CSA: Thigh muscle decreased in both arms. Muscle Fiber: Type 1 and II muscle fiber area significantly decreased in WL (−19.2%±7.9%; −16.6%±4.0%), but not in WL+EX (3.4%±7.5%; (−0.2%±6.5%) |
| Davidson et al. 2009[44] |
N= 117 Age: Women: 67.4±5.1 yrs; Men: 67.7±5.1 yrs Gender: 58.0% women BMI: Women: 30.5±2.0 kg/m2; Men: 30.4±2.7 kg/m2 Health: Abdominal obesity, Sedentary |
Design: RCT Arms: Control (n=28) Resistance EX (n= 36) Aerobic EX (n=37) Combined EX (n=35) Duration: 6 mo |
Chair stands; 2-minute step; 8-ft-up- and-go; seated arm curl; VO2 max; anthropometrics; body composition by MRI |
Weight change: Control; 0.28±0.37 kg; Resistance EX: −0.64±0.37 kg; Aerobic EX: −2.77±0.33 kg; Combined EX: −2.31±0.33 kg Function: Chair stands; 2-minute step; 8-ft-up-and-go; seated arm curl improved in all EX arms, with combined EX having greater improvements than Aerobic EX. VO2 increased in Aerobic EX and Combined EX. Body Comp: BW and total fat decreased in Aerobic and Combined EX compared to control and Resistance EX arms. Abdominal fat decreased in Aerobic EX and Combined EX compared to control only; FFM increased in Resistance EX ad Combined EX compared to control and Aerobic EX |
| Frimel et al. 2008[19] |
N= 30 Age: WL: 70.3±4.8 yrs; WL+EX: 68.7±4.3 yrs Gender: 60% women BMI: WL: 36.9±4.9kg/m2; WL+EX:36.7±5.1kg/m2 Health: Mild to moderate frailty; sedentary |
Design: RCT Arms: WL (n=15) WL+EX (n=15) Duration: 6 mo |
1-RM; body composition by DXA; anthropometrics |
Weight change: WL: −10.7±4.5 kg, 10.6±4.6%; WL±EX: −9.7±4.0 kg, 100±3.9% Function: WL+EX increased in upper and lower extremity strength (1-RM). Body Comp: WL and WL+EX decreased BW and FM; WL+EX lost less FFM (1.8±1.5 vs. 5.4±3.7 kg), and upper (0.1±0.2 vs. 0.2±0.2 kg) and lower (0.9±0.8 vs. 2.0±0.9 kg) extremity FFM compared to WL arm. |
| Lambert et al. 2008[45] |
N= 16 Age: 69±1 yrs Gender: EX: 50% women; WL: 50% women BMI: 38±2 kg/m2 Health: Frail; difficulty or need for assistance in two IADLS of one ADL |
Design: RCT Arms: EX WL Duration: 12 weeks |
Skeletal muscle mRNAs for toll-like receptor-4; mechano-growth factor, TNF-α; IL-6; HsCRP; body composition via DXA |
Weight change: WL: 7.1%; EX: 0% Inflammation/ROS Biomarkers: EX decreased TLR-4 mRNA; IL-6 mRNA; TNF-α mRNA; EX increased MGF mRNA |
| Solomon et al. 2008[46] |
N= 23 Mean age: 66 ± 1 yrs Gender: Both BMI: 33.2±1.4 kg/m2 Health: Impaired glucose tolerance |
Design: RCT Arms: EX (n=12) WL+EX (n=11) Duration: 12 wks |
Muscle lipid composition by histochemistry of muscle biopsy; body composition by hydrostatic weighing; anthropometrics. |
Weight change: EX: −3.3 kg; WL+EX: −7.9 kg Muscle Lipid Comp: Decreased −25.9±12.4% in EX and −34.3±17.6% in WL+EX, no group difference found. Body Comp: No change in FFM. WL+EX had greater decreases in body and fat mass. |
| Villeral et al. 2008[47] |
N= 27 Mean age: 70 ± 5 yrs Gender: Both BMI: 39±5 kg/m2 Health: Sedentary; mild to moderate frailty defined by physical performance score, peak VO2, and IADLS and ADL |
Design: RCT Arms: WL+EX (n=17) Control (n=10 Duration: 1 year |
BW, BMD, BMC, bone metabolism markers, and strength assessed by 1-RM. |
Weight change: WL+EX: −10.1±2.0%; Control: +1.2±1.3% BMD: Decreased greater in WL+EX arm compared to control at total hip (−2.4±2.5% vs. 0.1±2.1%), trochanter (−3.3±3.1% vs. 0.2±3.3%), and intertrochanter (−2.7±3.0% 0.3±2.7%). BMC: Decreased greater in WL+EX compared to control arm at total hip (−2.4±4.7% vs. 0.9±2.0%), trochanter (−4.1±7.0% vs. 1.4±6.1%), and intertrochanter (−2.4±5.7% vs. 0.6±2.0%). Bone markers: Greater Increased in WL+EX arm compared to control in C-terminal telopeptide (101±79% vs. 12±35%) and osteocalcin (66±61% vs. −5±15%). Hormones: Decreased greater in WL+EX compared to control arm in serum leptin (−30±25% vs. 2±12%) and estradiol (−14±21% vs.. 0/1±14%) Strength: Significant improvements in upper and lower body compared to control/ |
| Miller et al. 2006[48] |
N= 87 Age: Control: 69.3±0.9 yrs; WL+EX : 69.7±0.9 yrs Gender: WS: 60.5% women; WL+EX : 63.6% BMI: Control : 34.3±3.9 kg/m2; WL: 34.9±4.9 kg/m2 Health: Symptomatic knee OA; difficulty with 1 or more: lifting and carrying groceries, walking one-quarter mile, getting in and out of a chair, or going up and down stairs |
Design: RCT Arms: Control (n=43) WL+EX (n=44) Duration: 6 mo |
WOMAC; 6-minute walk distance test; stair climb test; body composition by DXA; anthropometrics |
Weight change: Control: −0.1±0.7 kg; WL+EX: −8.3 ±0.7 kg Function: Compared to control WL+EX had improvements in WOMAC score in WL+EX; walking distance; faster stair climb in WL+EX. Body Comp: BW, WC, FM, and FFM decreased in WL+EX compared to control. |
| Villareal et al. 2006[49] |
N= 27 Age: Control: 71.1±5.1 yrs; WL+EX : 69.4±4.6 yrs Gender: Both BMI: Control: 39.0±5.0 kg/m2; WL+EX: 38.5±5.3 kg/m2 Health: Mild to moderate frailty |
Design: RCT Arms: Control (n=10) WL+EX (n=17) Duration: 6 mo |
PPT; VO2 max; FSQ score; 1-RM; knee extensor and flexor strength; dynamic balance (obstacle course); static balance (single limb leg stance time); gait speed; SF-36; body composition by DXA; anthropometrics |
Weight change: Control: 0.7±2.7 kg; WL+EX: −8.2 ±5.7 kg Function: WL+EX inc; VO2; FSQ score; knee extension and flexor strength; gait speed; physical function (SF-36); role limitations (SF-36); bodily pain (SF-36); vitality (SF-36), and change in health (SF-36). WL+EX improved one leg limb stand and obstacle course time. Body Comp: BW and FM decreased in WL+EX. No difference between FFM loss in control and WL+EX |
| Messier et al. 2004[50] | N= 252 Age: healthy lifestyle: 69±0.1 yrs; WL: 68±0.7 yrs; EX: 69±0.8 yrs; WL+EX: 69±0.8 yrs Gender: Control: 68% women; WL: 72% women; EX: 74% women; WL+EX: 74% women BMI: Control: 34.2±0.6 kg/m2; WL: 34.5±0.6 kg/m2; EX: 34.2±0.6 kg/m2; WL+EX: 34.0±0.7 kg/m2 Health: Knee pain, radiographic evidence of knee OA, sedentary, self-reported physical disability |
Design: RCT Arms: Control (n=78) WL (n=82) EX (n=80) WL+EX (n=76) Duration: 18 mo |
WOMAC; 6-minute walk; timed stair-climb; Body composition by DXA; anthropopmetircs |
Weight change: Control: 1.2% WL: 4.5%; EX: 3.7%; WL+EX: 5.7% Function: WL+EX decreased in WOMAC score compared to control; WL+EX and WL decreased in WOMAC score compared to baseline scores. 6-minute walk distance increased in WL+EX and EX compared to control and stair-climb time decreased in WL+EX. Body Comp: WL+EX and WL decreased in BW compared to control. |
| Nicklas et al. 2004[51] |
N= 252 Age: Control: 69±0.1 yrs; WL: 68±0.7 yrs; EX: 69±0.8 yrs; WL+EX: 69±0.8 yrs Gender: Control: 68% women; WL: 72% women; EX: 74% women; WL+EX: 74% women BMI: Control: 34.2±0.6 kg/m2; Diet only: 34.5±0.6 kg/m2; EX only: 34.2±0.6 kg/m2; WL+EX: 34.0±0.7 kg/m2 Health: Knee pain, radiographic evidence of knee OA, sedentary, self-reported physical disability |
Design: RCT Arms: Control WL EX WL+EX Duration: 18 mo |
IL-6; TNF-α; IL-6sR; sTNFR1; sTNFR2; CRP |
Weight change: Control: 2.3% WL: 12.8%; EX: 4.1%; WL+EX: 8.2% Inflammation/ROS Biomarkers: WL decreased C-reactive protein, IL-6, and sTNFR1. Decreases in sTNFR1 correlated with decreases in BW. |
ADL = activities of daily living; Anthropometrics = body weight, height, BMI, waist circumference; BW = body weight; BEI = bioelectric impedance; BMC = bone mineral content; BMD = bone mineral density; CHAMPS = Community Health Activities Model Program for Seniors physical activity questionnaire. CT = computed tomography. CRP = C-reactive protein; DXA = duel energy X-ray absorptiometry; EX = exercise intervention; FM = fat mass; FFM = fat free mass; FSQ = functional status questionnaire; GI = glycemin index; 3H-MRS = proton magnetic resonance spectroscopy; HsCRP = high sensitivity C-reactive protein; IADL = Instrumental activities of daily living; IL-6= Interleukin-6; IL-6sR = soluble IL-6 receptor; MGF = mechano-growth factor; OA = osteoarthritis; PPT = physical performance test; RCT = ransomized control trial; ROM = range of motion; SF-36 = short form health survey; SPPB = short physical performance battery; sTNFR1 =s oluble TNF-α receptor 1; sTNFR2 = soluble TNF-α receptor 2; TLR-4 = toll-like receptor-4; VO2 max = cardiorespiratory fitness; WC = waist circumference; WL = weight loss intervention; WL+EX = weight loss and exercise intervention; WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index;1-RM = 1 repetition maximum
Complications and Concerns
The best solution for halting cyclic and progressive functional and metabolic deterioration in those obese older adults with complicated health concerns awaits further study before evidence-based recommendations can be made. For individuals who are able, the use of physical activity, specifically, resistance exercise, appears to be the best approach to protect muscle and bone while undergoing intentional weight loss. Therefore, we strongly advocate for the combined approach of a weight reduction diet plus a program of exercise. However, in the case of the obese, frail, older adult who is unable to achieve a level of physical activity sufficient to provide this protection, the best approach in terms of long term health impact is unknown.[22] Future studies of generous protein intakes as a means of preserving lean muscle mass during weight reduction may yield guidance on this issue.[21 23–25]
The risk of developing serious chronic health conditions increases with age. Unfortunately, these conditions sometimes lead to pronounced conditions of wasting, also known as cachexia.[26] In these cases, the “reverse epidemiology” of obesity or “obesity paradox” is often observed, meaning that those with a high BMI survive longer than those with a lower body weight. This phenomenon has been confirmed in the case of cancer cachexia[27], end stage renal disease[28], and chronic heart failure[29], as well as a number of other conditions common in old age. The increased survival attributed to obesity in these situations is thought to be due to the availability of larger body stores of both energy (fat) and lean mass, as well as a better nutritional state overall.[30] Taking into consideration the points made in this section, we underscore that an individualized approach to obesity, with careful consideration of health and quality of life priorities, should be taken in the following situations:
Disability that precludes physical exercise
Osteoporosis
Muscle wasting
Moderate to advanced dementia
An ultimately terminal condition
Disease states that might progress to a state of wasting/cachexia (e.g., advanced renal disease, heart failure, COPD, certain cancers)
Summary
Biological heterogeneity is a hallmark of aging and approaches to managing obesity in late life must take into account this heterogeneity. Interventions for restoring optimal body mass need to consider medical history and future health trajectories so that recommendations can be tailored to the needs of the individual. The combination of exercise (particularly resistance training) with a gradual weight reduction diet is the best means to protect lean muscle mass and bone mineral density during weight loss. This approach is recommended for adults under 80 years of age who are experiencing metabolic and/or functional problems as a result of a BMI ≥30 and who are physically able to exercise. For obese adults who are 80 years of age or older and/or who have potential contraindications for weight reduction, a regimen of healthy diet plus individualized exercise geared towards weight maintenance and enhanced muscle mass is recommended. Further study is needed to identify the diet and exercise strategies most suitable for overweight older adults, namely the optimal approaches for preventing the development of obesity and slowing the progression of obesity-related chronic health conditions in this age group. Determination of the optimal body mass and composition for older adults based on the age and health status of the individual continues to be a very active area of study in the geriatrics field.
Key Points.
Obesity is common in older adults, contributing to significant morbidity and reducing functional independence and quality of life.
Older adults with a BMI <23 kg/m2 are advised to emphasize a diet of high energy and nutrient density and participate in exercise (resistance) training in order to achieve a gradual increase in body mass, especially muscle mass.
Older adults who are overweight (BMI 25 to 29.9 kg/m2) are advised to remain weight stable, emphasize a diet of high nutrient density, and actively participate in exercise training that enhances aerobic endurance and improves muscle mass.
For adults aged 60 to 79 years who are obese (BMI ≥30 kg/m2) and experiencing metabolic and/or functional deficits, a weight loss therapy that minimizes muscle and bone losses can be beneficial. Ideally, this therapy should be a combination of calorie reduction and exercise training that includes resistance exercise.
In the case of obese adults who are older than 79 years, and/or those who have serious chronic illness or disabilities, a weight maintenance approach is best advised, given the scarcity of information about the benefit/risk of weight reduction in these situations.
Acknowledgments
The authors acknowledge the support of the National Institutes of Health via training grant support for KPS (T32 AG000029) and the Claude D. Pepper Older Americans Independence Center (P30 AG028716).
References
- 1.Mathus-Vliegen EM. Obesity and the elderly. Journal of clinical gastroenterology. 2012;46(7):533–44. doi: 10.1097/MCG.0b013e31825692ce. [DOI] [PubMed] [Google Scholar]
- 2.Porter Starr KN, McDonald SR, Bales CW. Obesity and Physical Frailty in Older Adults: A Scoping Review of Lifestyle Intervention Trials. J Am Med Dir Assoc. 2014;15(4):240–50. doi: 10.1016/j.jamda.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dey DK, Lissner L. Obesity in 70-year-old Subjects as a Risk Factor for 15-year Coronary Heart Disease Incidence. Obes Res. 2003;11(7):817–27. doi: 10.1038/oby.2003.113. [DOI] [PubMed] [Google Scholar]
- 4.Fagot-Campagna A, Bourdel-Marchasson I, Simon D. Burden of Diabetes in an Aging Population: Prevalence, Incidence, Mortality, Characteristics and Quality of Care. Diabetes Metab. 2005;31(Spec No 2):5S35–5S52. doi: 10.1016/s1262-3636(05)73650-8. MDOI-DM-12-2005-31-HS2-1262-3636-101019-200509654. [DOI] [PubMed] [Google Scholar]
- 5.Vincent HK, Vincent KR, Lamb KM. Obesity and mobility disability in the older adult. Obes Rev. 2010;11(8):568–79. doi: 10.1111/j.1467-789X.2009.00703.x. OBR703 [pii] [DOI] [PubMed] [Google Scholar]
- 6.Jensen GL, Hsiao PY. Obesity in older adults: relationship to functional limitation. Curr Opin Clin Nutr Metab Care. 2010;13(1):46–51. doi: 10.1097/MCO.0b013e32833309cf. [DOI] [PubMed] [Google Scholar]
- 7.Naugle KM, Higgins TJ, Manini TM. Obesity and use of compensatory strategies to perform common daily activities in pre-clinically disabled older adults. Arch Gerontol Geriatr. 2012;54(2):e134–8. doi: 10.1016/j.archger.2011.10.017. Epub 2011 Dec 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rolland Y, Lauwers-Cances V, Cristini C, et al. Difficulties with Physical Function Associated with Obesity, Sarcopenia, and Sarcopenic-obesity in Community-dwelling Elderly Women: the EPIDOS (EPIDemiologie de l’OSteoporose) Study. Am J Clin Nutr. 2009;89(6):1895–900. doi: 10.3945/ajcn.2008.26950. Epub 2009 Apr 15. [DOI] [PubMed] [Google Scholar]
- 9.Fakhouri TH, Ogden CL, Carroll MD, et al. NCHS data brief, no 106. National Center for Health Statistics; Hyattsville, MD: 2012. Prevalence of obesity among older adults in the United States, 2007–2010. [PubMed] [Google Scholar]
- 10.Winter JE, MacInnis RJ, Wattanapenpaiboon N, et al. BMI and all-cause mortality in older adults: a meta-analysis. Am J Clin Nutr. 2014;99(4):875–90. doi: 10.3945/ajcn.113.068122. [DOI] [PubMed] [Google Scholar]
- 11.Flegal KM, Kit BK, Graubard BI. Overweight, obesity, and all-cause mortality--reply. JAMA. 2013;309(16):1681–2. doi: 10.1001/jama.2013.3101. [DOI] [PubMed] [Google Scholar]
- 12.Johnson MA, Bales CW. Is there a best body mass index for older adults? Moving closer to evidence-based recommendations regarding “overweight,” health, and mortality. Journal of nutrition in gerontology and geriatrics. 2014;33(1):1–9. doi: 10.1080/21551197.2014.875817. [DOI] [PubMed] [Google Scholar]
- 13.Waters DL, Ward AL, Villareal DT. Weight loss in obese adults 65years and older: a review of the controversy. Exp Gerontol. 2013;48(10):1054–61. doi: 10.1016/j.exger.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Decaria JE, Sharp C, Petrella RJ. Scoping review report: obesity in older adults. Int J Obes (Lond) 2012;36(9):1141–50. doi: 10.1038/ijo.2012.29. [DOI] [PubMed] [Google Scholar]
- 15.Darmon P. Intentional weight loss in older adults: useful or wasting disease generating strategy? Curr Opin Clin Nutr Metab Care. 2013 doi: 10.1097/MCO.0b013e32835f503f. [DOI] [PubMed] [Google Scholar]
- 16.Weinheimer EM, Sands LP, Campbell WW. A Systematic Review of the Separate and Combined Effects of Energy Restriction and Exercise on Fat-free Mass in Middle-aged and Older Adults: Implications for Sarcopenic Obesity. Nutr Rev. 2010;68(7):375–88. doi: 10.1111/j.1753-4887.2010.00298.x. [DOI] [PubMed] [Google Scholar]
- 17.Villareal DT, Chode S, Parimi N, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011;364(13):1218–29. doi: 10.1056/NEJMoa1008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anton SD, Manini TM, Milsom VA, et al. Effects of a weight loss plus exercise program on physical function in overweight, older women: a randomized controlled trial. Clin Interv Aging. 2011;6:141–9. doi: 10.2147/cia.s17001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frimel TN, Sinacore DR, Villareal DT. Exercise attenuates the weight-loss-induced reduction in muscle mass in frail obese older adults. Med Sci Sports Exerc. 2008;40(7):1213–9. doi: 10.1249/MSS.0b013e31816a85ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Villareal DT, Smith GI, Sinacore DR, et al. Regular multicomponent exercise increases physical fitness and muscle protein anabolism in frail, obese, older adults. Obesity (Silver Spring) 2011;19(2):312–8. doi: 10.1038/oby.2010.110. Epub 2010 May 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mathus-Vliegen EMH, Basdevant A, Finer N, et al. Prevalence, Pathophysiology, Health Consequences and Treatment Options of Obesity in the Elderly: A Guideline. Obesity facts. 2012;5(3):460–83. doi: 10.1159/000341193. [DOI] [PubMed] [Google Scholar]
- 22.Miller SL, Wolfe RR. The Danger of Weight Loss in the Elderly. J Nutr Health Aging. 2008;12(7):487–91. doi: 10.1007/BF02982710. [DOI] [PubMed] [Google Scholar]
- 23.McDonald SR, Porter Starr KN, Mauceri L, et al. Meal-based enhancement of protein quality and quantity during weight loss in obese older adults with mobility limitations: Rationale and design for the MEASUR-UP trial. Contemp Clin Trials. 2015;40:112–23. doi: 10.1016/j.cct.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paddon-Jones D, Leidy H. Dietary protein and muscle in older persons. Curr Opin Clin Nutr Metab Care. 2014;17(1):5–11. doi: 10.1097/mco.0000000000000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verreijen AM, Verlaan S, Engberink MF, et al. A high whey protein-, leucine-, and vitamin D-enriched supplement preserves muscle mass during intentional weight loss in obese older adults: a double-blind randomized controlled trial. Am J Clin Nutr. 2015;101(2):279–86. doi: 10.3945/ajcn.114.090290. [DOI] [PubMed] [Google Scholar]
- 26.Evans WJ, Morley JE, Argiles J, et al. Cachexia: a new definition. Clin Nutr. 2008;27(6):793–9. doi: 10.1016/j.clnu.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez MC, Pastore CA, Orlandi SP, et al. Obesity paradox in cancer: new insights provided by body composition. Am J Clin Nutr. 2014;99(5):999–1005. doi: 10.3945/ajcn.113.071399. [DOI] [PubMed] [Google Scholar]
- 28.Park J, Ahmadi SF, Streja E, et al. Obesity paradox in end-stage kidney disease patients. Progress in cardiovascular diseases. 2014;56(4):415–25. doi: 10.1016/j.pcad.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clark AL, Fonarow GC, Horwich TB. Obesity and the obesity paradox in heart failure. Progress in cardiovascular diseases. 2014;56(4):409–14. doi: 10.1016/j.pcad.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 30.Soeters PB, Sobotka L. The pathophysiology underlying the obesity paradox. Nutrition. 2012;28(6):613–5. doi: 10.1016/j.nut.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 31.Beavers KM, Gordon MM, Easter L, et al. Effect of Protein Source during Weight Loss on Body Composition, Cardiometabolic Risk and Physical Performance in Abdominally Obese, Older Adults: A Pilot Feeding Study. J Nutr Health Aging. 2015;19(1):87–95. doi: 10.1007/s12603-015-0438-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beavers KM, Beavers DP, Nesbit BA, et al. Effect of an 18-month physical activity and weight loss intervention on body composition in overweight and obese older adults. Obesity (Silver Spring) 2014;22(2):325–31. doi: 10.1002/oby.20607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beavers KM, Ambrosius WT, Nicklas BJ, et al. Independent and combined effects of physical activity and weight loss on inflammatory biomarkers in overweight and obese older adults. J Am Geriatr Soc. 2013;61(7):1089–94. doi: 10.1111/jgs.12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rejeski WJ, Brubaker PH, Goff DC, Jr, et al. Translating weight loss and physical activity programs into the community to preserve mobility in older, obese adults in poor cardiovascular health. Arch Intern Med. 2011;171(10):880–6. doi: 10.1001/archinternmed.2010.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beavers DP, Beavers KM, Loeser RF, et al. The independent and combined effects of intensive weight loss and exercise training on bone mineral density in overweight and obese older adults with osteoarthritis. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2014;22(6):726–33. doi: 10.1016/j.joca.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Solomon TP, Haus JM, Cook MA, et al. A Low-Glycemic Diet Lifestyle Intervention Improves Fat Utilization during Exercise in Older Obese Humans. Obesity (Silver Spring) 2013 doi: 10.1002/oby.20411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Armamento-Villareal R, Sadler C, Napoli N, et al. Weight loss in obese older adults increases serum sclerostin and impairs hip geometry but both are prevented by exercise training. J Bone Miner Res. 2012;27(5):1215–21. doi: 10.1002/jbmr.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shah K, Armamento-Villareal R, Parimi N, et al. Exercise training in obese older adults prevents increase in bone turnover and attenuates decrease in hip bone mineral density induced by weight loss despite decline in bone-active hormones. J Bone Miner Res. 2011;26(12):2851–9. doi: 10.1002/jbmr.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dube JJ, Amati F, Toledo FG, et al. Effects of weight loss and exercise on insulin resistance, and intramyocellular triacylglycerol, diacylglycerol and ceramide. Diabetologia. 2011;54(5):1147–56. doi: 10.1007/s00125-011-2065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haus JM, Solomon TP, Lu L, et al. Intramyocellular lipid content and insulin sensitivity are increased following a short-term low-glycemic index diet and exercise intervention. Am J Physiol Endocrinol Metab. 2011;301(3):E511–6. doi: 10.1152/ajpendo.00221.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelly KR, Haus JM, Solomon TP, et al. A low-glycemic index diet and exercise intervention reduces TNF(alpha) in isolated mononuclear cells of older, obese adults. J Nutr. 2011;141(6):1089–94. doi: 10.3945/jn.111.139964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Santanasto AJ, Glynn NW, Newman MA, et al. Impact of weight loss on physical function with changes in strength, muscle mass, and muscle fat infiltration in overweight to moderately obese older adults: a randomized clinical trial. J Obes. 2011 doi: 10.1155/2011/516576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chomentowski P, Dube JJ, Amati F, et al. Moderate exercise attenuates the loss of skeletal muscle mass that occurs with intentional caloric restriction-induced weight loss in older, overweight to obese adults. J Gerontol A Biol Sci Med Sci. 2009;64(5):575–80. doi: 10.1093/gerona/glp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davidson LE, Hudson R, Kilpatrick K, et al. Effects of exercise modality on insulin resistance and functional limitation in older adults: a randomized controlled trial. Arch Intern Med. 2009;169(2):122–31. doi: 10.1001/archinternmed.2008.558. [DOI] [PubMed] [Google Scholar]
- 45.Lambert CP, Wright NR, Finck BN, et al. Exercise but not diet-induced weight loss decreases skeletal muscle inflammatory gene expression in frail obese elderly persons. Journal of Applied Physiology. 2008;105(2):473–78. doi: 10.1152/japplphysiol.00006.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Solomon TP, Sistrun SN, Krishnan RK, et al. Exercise and diet enhance fat oxidation and reduce insulin resistance in older obese adults. J Appl Physiol. 2008;104(5):1313–9. doi: 10.1152/japplphysiol.00890.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Villareal DT, Shah K, Banks MR, et al. Effect of weight loss and exercise therapy on bone metabolism and mass in obese older adults: a one-year randomized controlled trial. J Clin Endocrinol Metab. 2008;93(6):2181–7. doi: 10.1210/jc.2007-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller GD, Nicklas BJ, Davis C, et al. Intensive weight loss program improves physical function in older obese adults with knee osteoarthritis. Obesity (Silver Spring) 2006;14(7):1219–30. doi: 10.1038/oby.2006.139. [DOI] [PubMed] [Google Scholar]
- 49.Villareal DT, Banks M, Sinacore DR, et al. Effect of weight loss and exercise on frailty in obese older adults. Arch Intern Med. 2006;166(8):860–6. doi: 10.1001/archinte.166.8.860. [DOI] [PubMed] [Google Scholar]
- 50.Messier SP, Loeser RF, Miller GD, et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion Trial. Arthritis Rheum. 2004;50(5):1501–10. doi: 10.1002/art.20256. [DOI] [PubMed] [Google Scholar]
- 51.Nicklas BJ, Ambrosius W, Messier SP, et al. Diet-induced weight loss, exercise, and chronic inflammation in older, obese adults: a randomized controlled clinical trial. The American Journal of Clinical Nutrition. 2004;79(4):544–51. doi: 10.1093/ajcn/79.4.544. [DOI] [PubMed] [Google Scholar]