Abstract
The Notch pathway has a fundamental role during cell-fate specification in the developing mammalian nervous system. During neocortical development, Notch signaling inhibits neuronal differentiation and maintains the neural stem/progenitor cell pool to permit successive waves of neurogenesis, which are followed by gliogenesis. In addition, recent evidence suggests that Notch signaling is not uniformly used among distinct proliferative neural cells types, with the canonical cascade functional in neural stem cells but attenuated in neurogenic progenitors. Although the role of Notch in neural development is increasingly well understood, it has recently become evident that Notch also has a role in brain tumor biology. Notch receptors are overexpressed in many different brain tumor types, and they may have an initiating role in some. Stem-like cells in brain tumors share many similarities with neural stem/progenitor cells and may require Notch for their survival and growth. Understanding the role of Notch signaling in neoplastic and non-neoplastic stem/progenitor populations will advance our understanding of basic principles regulating developmental and stem cell biology and may also lead to more effective therapies for brain tumors.
The Notch pathway regulates many different processes during mammalian development. Notch signaling has been particularly heavily studied in the developing nervous system, where Notch receptor activation inhibits neuronal differentiation and maintains the neural stem/progenitor cell pool. Notch signaling has also been found to promote glial character, and in light of findings that certain glial cells possess neural stem cell character, the ability of Notch to maintain progenitor character and to promote glial character is likely to be mechanistically related (Gaiano and Fishell 2002).
In addition to its role in development, Notch signaling has been implicated in many human cancers, including those in the brain. Because Notch pathway activation is well known for contributing to the maintenance of a proliferatively active cell state, the notion that aberrant Notch signaling could contribute to tumor formation is not surprising. The mechanisms by which Notch regulates neural stem cells in the developing nervous system are likely to be similar to those used during the regulation of putative brain tumor stem cells. As such, understanding parallels between both the signaling mechanisms and the cellular heterogeneity present in both settings (e.g., stem cell vs. transit-amplifying progenitor) will provide valuable insight.
In the first part of this chapter, we focus on Notch’s role in embryonic neural stem/progenitor cells in the developing mammalian neocortex. Ongoing work in the field has focused on understanding how the Notch signal transduction cascade is regulated and also on the nature of cell–cell interactions that mediate signaling. In the second part of this chapter, we address Notch signaling in brain tumor formation and growth and how this signaling pathway may regulate the maintenance and behavior of brain tumor stem cells. We also consider parallels between Notch function in embryonic neural stem/progenitor cells and in brain tumor stem cells.
NOTCH AND NEURAL PROGENITORS
Neocortical Development
The mammalian neocortex is a highly organized six-layered structure that develops at the anterior end of the neural tube. The first neural stem/progenitor cells (simply referred to as progenitors hereafter, unless otherwise noted) line the ventricles in a germinal area referred to as the ventricular zone (VZ). A second germinal area, termed the subventricular zone (SVZ), forms just beneath the VZ after the onset of neurogenesis. The VZ and SVZ both contain highly proliferative neural progenitor cells that undergo symmetric and asymmetric cell divisions to either maintain the proliferative pool or produce the neurons of the different cortical layers (Noctor et al. 2004). In the mouse, neurogenesis begins around embryonic day 10.5 (E10.5) and lasts until around E17.5, when gliogenesis begins in the SVZ (Molyneaux et al. 2007). The newborn neurons produced in the VZ or SVZ exit these areas by radial migration along the processes of radial glial cells to their final destination in the neocortical plate, where neuronal differentiation and circuit formation take place (Noctor et al. 2001, 2004).
At least three types of well-defined neural progenitors exist in the developing neocortex: neuroepithelial progenitors (NEPs), radial glial cells (RGCs), and intermediate progenitor cells (IPCs) (Pontious et al. 2007). NEPs span the neural tube from the ventricular (inner) surface to the pial (outer) surface and comprise the VZ before the onset of neurogenesis. NEPs initially divide symmetrically to expand the progenitor pool but undergo asymmetric divisions to yield the first neurons (Molyneaux et al. 2007). Early during neocortical development, many NEPs transform into RGCs, which also extend processes from the ventricular to pial surfaces, and in addition to a role as progenitors (see below), they serve as a migratory scaffold for newly generated neurons. RGCs differ from NEPs because they acquire aspects of astroglial character including expression of astrocyte-specific glutamate-aspartate transporter (GLAST) and brain lipid-binding protein (BLBP) (Götz and Barde 2005). Recent studies have shown that RGCs function as neuronal progenitors either by directly producing neurons (Noctor et al. 2001; Malatesta et al. 2003) or by producing IPCs, which give rise to neurons (Noctor et al. 2004). Time-lapse imaging in slice culture has shown that IPCs divide symmetrically to produce either two neurons or two IPCs (Noctor et al. 2004). IPCs are characterized by expression of Tbr2, Svet1, and Cux2 (Tarabykin et al. 2001; Nieto et al. 2004; Englund et al. 2005).
Several recent studies have suggested that a fourth neocortical progenitor cell type exists in addition to NEPs, RGCs, and IPCs. One study identified a short neural precursor (SNP) present during neurogenesis and located in the VZ (Gal et al. 2006). SNPs are morphologically distinct from RGCs in that their processes only span the VZ. In addition, SNPs are molecularly distinct from RGCs, with the former driving expression from the tubulin α1 (Tα1) promoter and the latter driving expression from the GLAST and BLBP promoters (Gal et al. 2006).
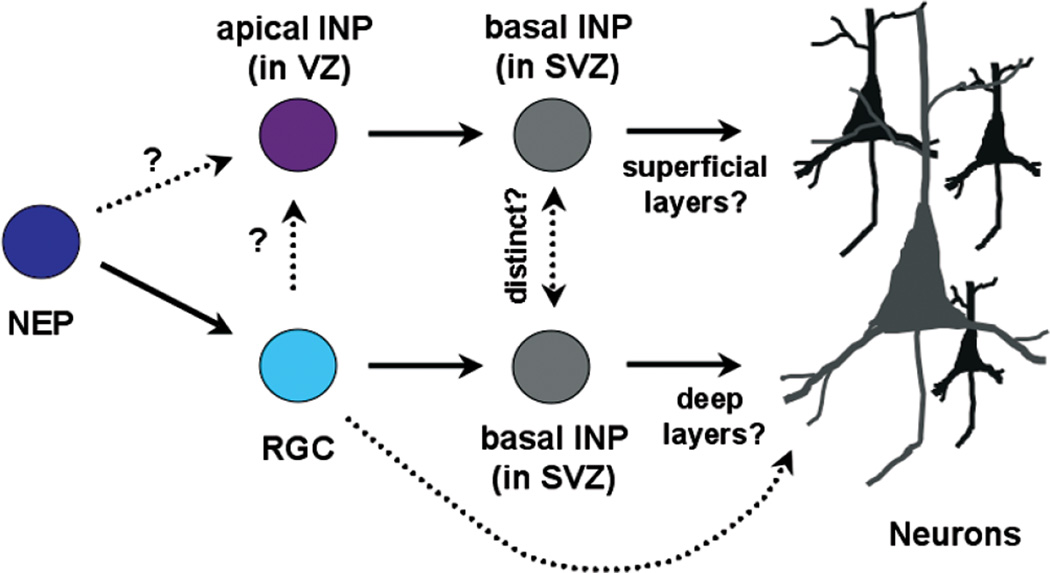
In support of the existence of two molecularly distinct VZ progenitor subtypes, we have recently shown that Notch signaling is not uniformly used in the VZ. Those cells that drive expression from the GLAST promoter (RGCs) are able to robustly activate the canonical Notch signaling cascade, whereas those VZ cells that drive expression from the Tα1 promoter (presumptive SNPs) have attenuated canonical Notch signaling (Mizutani et al. 2007; see below for further discussion). The relationship between the latter cell type, referred to as intermediate neural progenitor (INP), and the IPCs present primarily in the SVZ is unclear, although because both are neurogenic, it is reasonable to hypothesize that the former give rise in some part to the latter. If so, it may ultimately make more sense to call them both INPs but with designations that specify apical (aINPs or aIPCs in the VZ) or basal (bINPs or bIPCs in the SVZ) locations. We favor retention of the term “neural” to distinguish these cells from intermediate progenitors in other tissues. For a model of the lineage relationships among the four different neocortical progenitor subtypes described here (NEPs, RGCs, SNP/INPa, IPC/INPb), see Fig. 1.
Figure 1.
Schematic depicting potential lineage relationships between different neural progenitor subtypes present during neocortical neurogenesis. Note that the apical INP (aINP) is likely to be the same as the short neural precursor (SNP) described in the text, whereas the basal INP (bINP) is the same as the intermediate progenitor cell (IPC) described in the text, which is located primarily in the SVZ.
Notch Signaling in Neural Progenitors
The Notch signaling pathway is evolutionarily conserved and has a fundamental role during animal development and in the adult, in particular by regulating cell-fate specification (Yoon and Gaiano 2005; Louvi and Artavanis-Tsakonas 2006). The function of Notch signaling was first characterized in the fly where heterozygous loss-of-function mutants displayed notching in the wing margin, and homozygous loss-of-function mutants contained supernumery neurons, in what was termed a “neurogenic” phenotype (Louvi and Artavanis-Tsakonas 2006). Since then, Notch function has been well documented in the developing nervous system, immune system, and the skin, muscle, and cardiovascular system, as well as in many other contexts.
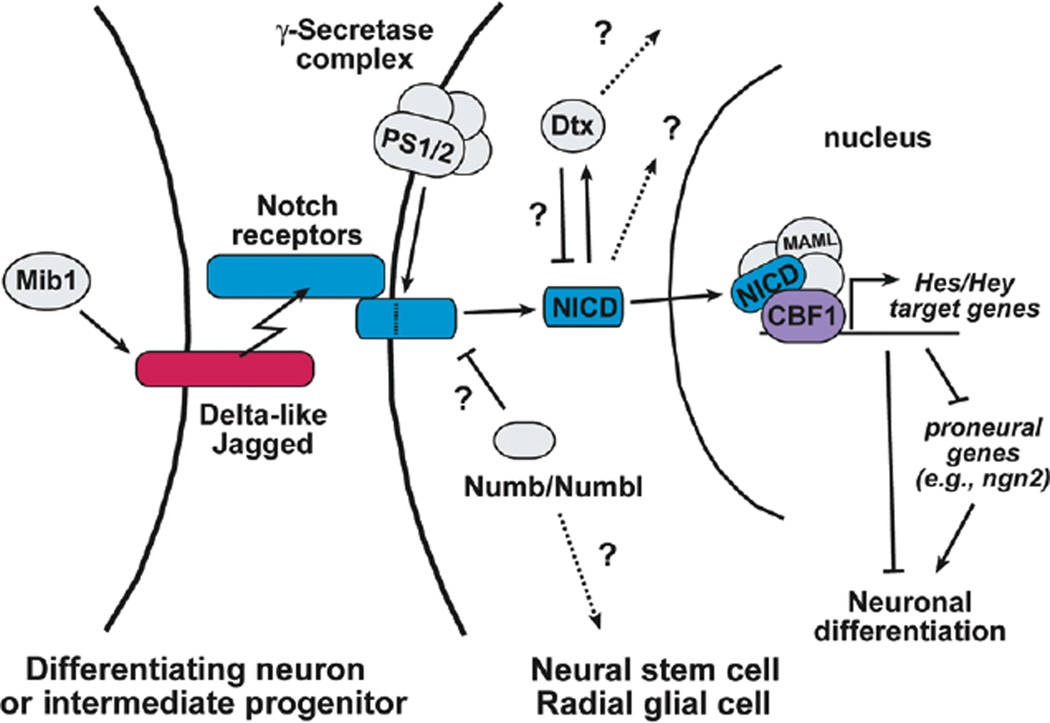
The Notch signal is mediated through contact between adjacent cells. In mammals, there are four Notch receptors (Notch1–4) and multiple ligands of the Delta-like (Dll) and Jagged (Jag) families, all of which are single-pass transmembrane proteins. Upon ligand binding, the Notch receptors are cleaved by the γ-secretase complex, releasing the Notch intracellular domain (NICD). NICD translocates into the nucleus and associates with CBF1 and Mastermind-like (MAML) (Wu et al. 2000) to create a transcriptional activator complex (see Fig. 2). In the developing nervous systems, that complex activates the expression of the Hes and Hey genes (Iso et al. 2003), which are basic helix-loop-helix (bHLH) transcriptional repressors that antagonize the function of the proneural genes such as the Neurogenins and thereby inhibit neuronal differentiation (Bertrand et al. 2002).
Figure 2.
The Notch signaling pathway depicted between two cells in the neocortical germinal zone. The ligand-expressing cell may be either a neuron or an intermediate progenitor, whereas the signal-receiving cell is inhibited from undergoing neuronal differentiation. (PS1/2) Presenilin 1 and 2, the catalytic subunits of the γ-secretase complex; (Mib1) mindbomb1; (Dtx) Deltex; (NICD) Notch intracellular domain; (MAML) Mastermind-like; (CBF1) C-promoter-binding factor 1.
The role of Notch signaling in the embryonic mammalian nervous system has been investigated using both loss- and gain-of-function approaches. Gain-of-function studies have revealed that Notch signaling inhibits neuronal and oligodendroglial differentiation while promoting progenitor and astroglial character (for review, see Gaiano and Fishell 2002). For example, in the mammalian neocortex, retroviral expression of activated forms of Notch1 or Notch3 in vivo promotes RGC identity embryonically and astrocyte fate postnatally (Gaiano et al. 2000; Dang et al. 2006b). Consistent with the gain-of-function findings, loss of Notch signaling results in an increase in neuronal differentiation and diminished progenitor maintenance (for review, see Yoon and Gaiano 2005; Yoon et al. 2008). Interestingly, although Notch signaling is required for neural progenitor maintenance, it does not appear to be necessary for the initial generation of neural stem cell/progenitors in vivo (Hitoshi et al. 2002).
Notch Signaling: Puzzles and Progress
Although the core components of the Notch signaling cascade are known, both the nature of the cell–cell contacts that activate that signaling and the molecular mechanisms that modulate it remain areas of active investigation. Most prominent among pathway elements with uncertain function are the Deltex, Numb, and Numblike proteins. For example, some studies provide evidence that Deltex proteins (of which there are four in mammals) positively regulate Notch signaling (Xu and Artavanis-Tsakonas 1990; Matsuno et al. 1995; Ramain et al. 2001). However, other studies have suggested that Deltex can inhibit Notch/CBF1-dependent signaling, while positively transmitting a Notch signal that is CBF1-independent (Yamamoto et al. 2001; Patten et al. 2006).
Another ongoing area of some confusion in the Notch field relates to the role of the modulators numb and numblike (numbl). Numb was first identified in Drosophila, where it was shown to be an inhibitor of Notch during neural development (Spana and Doe 1996). Numb protein is localized asymmetrically during cell division such that one of the daughter cells receives most of the Numb protein and will therefore have Notch signaling inhibited, whereas the other daughter will not. The role of Numb with respect to Notch in the mammalian nervous system has been far less clear (see Yoon and Gaiano 2005; Petersen et al. 2006). Several numb and numbl knockout studies have been reported, but they show contradictory findings, with some indicating that Numb and Numblike promote differentiation (Zilian et al. 2001; Li et al. 2003) and others showing that they promote maintenance of the progenitor pool (Zhong et al. 2000; Petersen et al. 2002, 2004). Other functions have recently been ascribed to Numb and Numblike, including regulation of cell adhesion (Kuo et al. 2006; Rasin et al. 2007; Zhou et al. 2007), but how such functions relate to Notch signaling, if at all, remains to be determined.
Although confusion persists with respect to certain aspects of the Notch cascade, progress has recently been made in other areas, including cell–cell signaling during neocortical development. As indicated above, Notch signaling occurs between a signal-sending cell (expressing Notch ligands) and a signal-receiving cell (expressing Notch receptors). Traditionally, it has been assumed that the signal-sending cells were newborn neurons and this remains likely to be true to some extent. However, recent work examining the role of the protein mindbomb-1 (mib1), an E3 ubiquitin ligase required for Notch ligand endocytosis and consequently receptor activation, suggests that IPCs also have a role in activating Notch receptors to maintain RGC character in the VZ (Yoon et al. 2008). Conditional inactivation of mib1 in the developing neocortex results in the loss of Notch activation, depletion of RGCs, and premature differentiation into IPCs and neurons. Of particular interest, this study provides strong evidence that ligands on one progenitor cell type (IPCs) can activate Notch receptors on another progenitor cell type (RGCs). In addition, this study details one of the best examples of Notch loss of function in the mammalian nervous system resulting in precocious neuronal differentiation at the expense of progenitor maintenance.
In another recent advance with respect to Notch signaling, real-time imaging has revealed that expression of the target gene Hes1 oscillates in embryonic neocortical progenitors in a manner similar to what has been shown before and during somitogenesis (Shimojo et al. 2008). As a direct consequence of Hes1 oscillations, the expression of Ngn2 and Dll1 also oscillate in neural progenitors but inversely with respect to Hes1. This work suggests that neocortical progenitors are maintained by a pulsatile inhibition of neurogenesis, but they are poised to be driven toward neurogenesis upon a sustained drop in Hes1 and the resulting sustained increase in Ngn2. Many questions remain to be addressed, including whether the timing of the Hes1 oscillations (2-hour periodicity) is important and how the decision to stabilize low (or potentially high) levels of Hes1 is achieved.
Differential Notch Signaling in Neural Progenitors
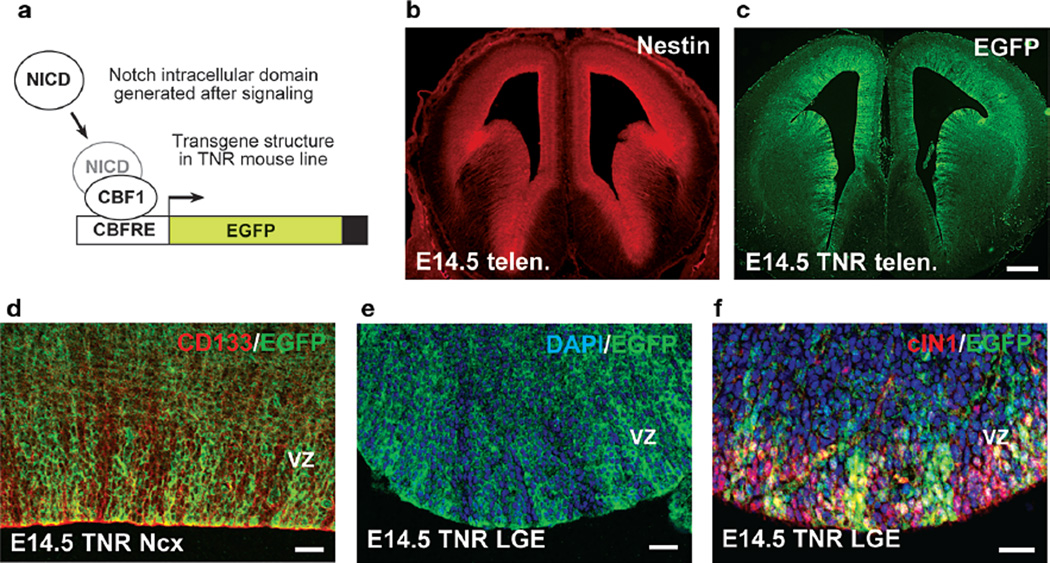
Our group has recently shown that differential Notch use in neural progenitors exists and likely contributes to the generation of progenitor heterogeneity, in particular in the VZ (Mizutani et al. 2007). This was first observed using a transgenic Notch reporter (TNR) line that expresses enhanced green fluorescent protein (EGFP) as a readout of CBF1-dependent Notch signaling. The transgene includes four CBF1-binding sites and the SV40 basal promoter, together referred to as the CBF1-responsive element (CBFRE), upstream of EGFP. Examination of either transgene expression or reporter expression from constructs delivered into the embryonic brain transiently revealed VZ progenitor heterogeneity (Fig. 3). As indicated above, those VZ cells that contained canonical Notch signaling (and thus were EGFP+) were found to be RGCs, which drove expression from the GLAST promoter. In addition, those VZ cells that exhibited attenuated canonical Notch signaling (and thus expressed little to no detectable EGFP) drove expression from the Tα1 promoter and were likely neurogenic progenitors (Fig. 4). As suggested above, here we will call such cells aINPs for apical (VZ) intermediate neural progenitors.
Figure 3.
Notch signaling heterogeneity exists in the embryonic forebrain. Strategy to detect CBF1 activation in the TNR line (a). Nestin (b) is expressed in the telencephalic (telen.) VZ, as is EGFP in TNR embryos (c). EGFPhi cells in the E14.5 neocortex (Ncx, d) and lateral ganglionic eminence (LGE, e) are interspersed with EGFPlo/neg cells. Double labeling reveals that cleaved (activated) Notch1 (red) is present in cells expressing high and low levels of EGFP (f). Bars: (b,c) 200 µm; (d–f) 25 µm. (Reprinted from Mizutani et al. 2007 [© Nature Publishing Group].).
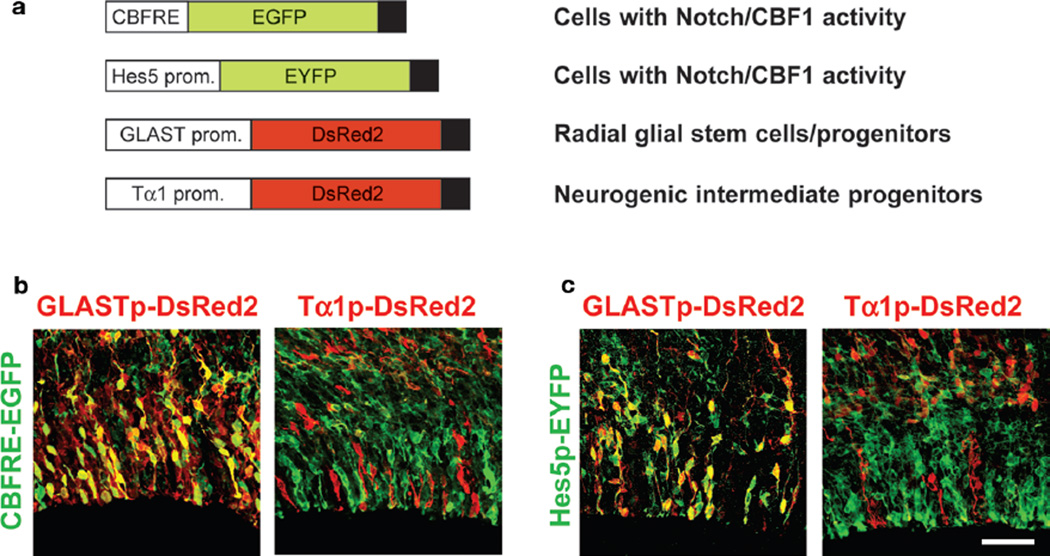
Figure 4.
In utero electroporation was used to deliver plasmids designed to identify cells with CBF1 activity (CBFRE-EGFP, Hes5p-EYFP), radial glial NSC (GLASTp-DsRed2), or INP character (Tα1p-DsRed2). (a) After E12.5 coelectroporations, VZ cells with CBF1 activity (green) at E15.5 are predominantly GLASTp-DsRed2+ and Tα1p-DsRed2− (b,c). Bar, 50 µm. (Modified from Mizutani et al. 2007 [© Nature Publishing Group].).
Additional in vivo analysis revealed that aINPs did not activate the CBFRE even when NICD1 expression driven in those cells could inhibit their differentiation (Mizutani et al. 2007). Furthermore, the clustered organization of aINPs raised the interesting possibility that those cells possess a heritable block to CBF1 activation. We performed additional analysis of RGCs and aINPs by using fluorescence-activated cell sorting (FACS) to isolate cells that were expressing the apical progenitor marker CD133 and were either EGFP+ or EGFP−. Consistent with the classification of those cells as RGCs and aINPs, respectively, the former were found to exhibit stem cell character in vitro and after in vivo transplantation, whereas the latter were biased primarily toward becoming neurons, but also oligodendrocytes in vivo.
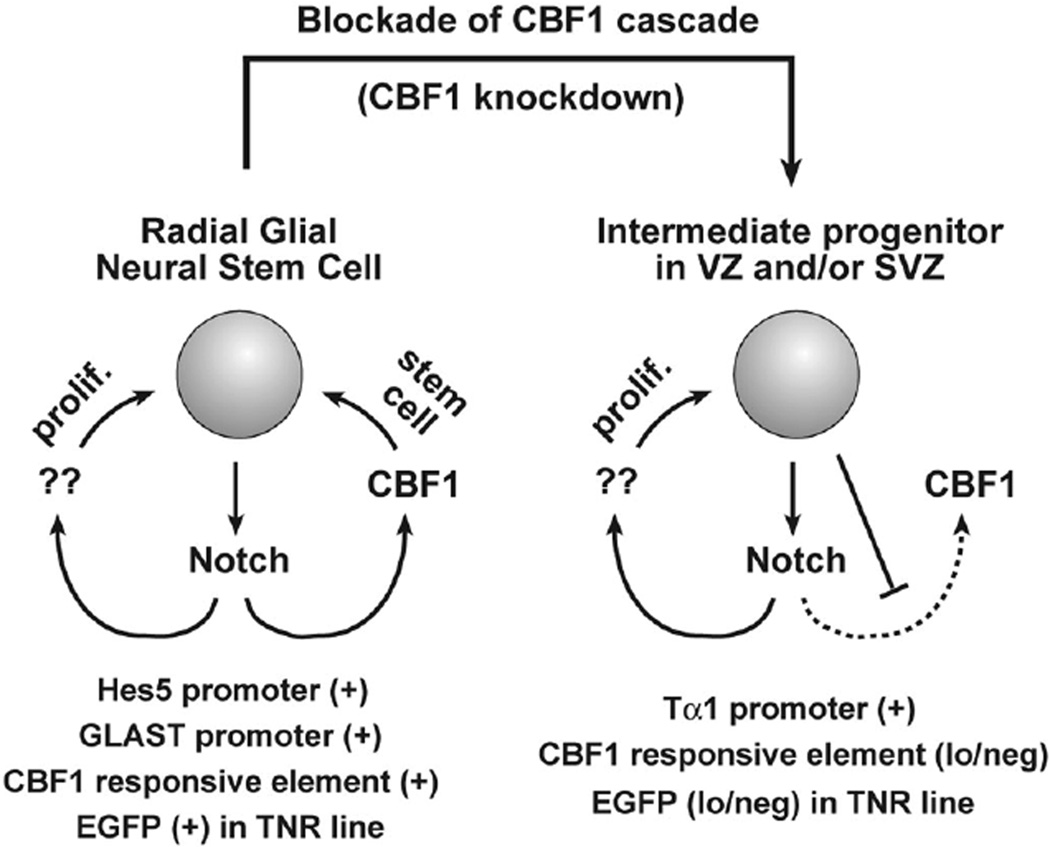
All told, our results indicate that the telencephalic VZ contains two progenitor subtypes that can be distinguished based on Notch/CBF1 signaling levels (Fig. 5). How Notch signaling in RGCs and aINPs is differentially regulated remains unknown, although it appears that aINPs possess a yet to be identified regulator of Notch signaling (a known or novel modulator) that can antagonize NICD signaling through CBF1 or might instead shunt NICD to noncanonical targets (CBF1-dependent or otherwise). Interestingly, we have shown that CBF1 knockdown, using short hairpin RNA (shRNA), promotes the conversion of RGCs to aINPs in vivo, suggesting that the differential Notch/CBF1 signaling observed between RGCs and aINPs is not just correlative, but has a causative role in the transition from one cell type to the other. Future studies will provide novel insight into how Notch signaling regulates different neural progenitor subtypes and into the general mechanisms of Notch pathway regulation.
Figure 5.
A model summarizing the molecular characteristics of radial glial neural stem cells (NSCs), and intermediate neural progenitors (INPs). Both cell types use Notch signaling to remain undifferentiated. However, the former utilize the canonical CBF1 signaling cascade, whereas the latter possess a heritable block to CBF1 signaling. (Reprinted from Mizutani et al. 2007 [© Nature Publishing Group].)
NOTCH IN BRAIN CANCER
Given the significant role of the Notch pathway in development, it is not surprising that it would also have an important role in oncogenesis. Notch has been known to be active in cancers for some time. In 1991, it was shown that a translocation in T-cell acute lymphoblastic leukemia (T-ALL) causes the expression of a truncated, constitutively active form of Notch1 (Ellisen et al. 1991). Subsequently, it has been demonstrated that Notch pathway dysregulation is quite common in a wide range of hematopoietic and epithelial cancers (Bolos et al. 2007; Aster et al. 2008). In most tumors, Notch acts as an oncogene. In some cases, however, it appears that Notch has a protective effect against the formation of tumors, as has been shown for Notch1 in the skin (Nicolas et al. 2003).
Notch dysregulation was first demonstrated in brain cancers rather recently, when it was found that cell lines derived from malignant human gliomas overexpress the Notch ligands Jag1 and Dll1 (Ignatova et al. 2002). It has subsequently been shown that Notch pathway alterations are present in a wide range of brain tumor types, including neoplasms resembling stem-like embryonic neural cells, various glial cell types, and meningothelial cells. Many of these tumors are extremely aggressive, with no known effective therapy, and the possibility of targeting such cancers using agents that block Notch signaling has generated considerable excitement (Dirks 2008; Fan and Eberhart 2008). As such, the fact that Notch may have an important role in stem-like brain tumor cells is of great clinical interest.
Astrocytomas
The gliomas, a heterogeneous group of tumors, are classified based on their morphological resemblance to glial cell types, and they include multiple grades of astrocytomas, oligodendrogliomas, and ependymomas (Louis et al. 2007). Accumulating evidence suggests that Notch pathway components are expressed in gliomas and have an important functional role in their formation and growth. The most malignant astrocytic tumors, known as glioblastomas, overexpress Notch receptors and ligands (Ignatova et al. 2002; Purow et al. 2005). Inhibition of the pathway via Notch1-, Jag1- and Dll1-directed small interfering RNA (siRNA) leads to decreased glioblastoma proliferation in vitro, increased apoptosis, and decreased tumorgenicity in vivo (Purow et al. 2005). Similarly, pathway inhibition with a γ-secretase inhibitor (which prevents the activating cleavage of Notch receptors, thereby preventing NICD translocation to the nucleus) represses growth of glioma cell lines (Kanamori et al. 2007). Conversely, overexpression of activated Notch1 in a human glioma cell line results in increased colony (neurosphere)-forming ability and cell growth rate (Zhang et al. 2008). In further support of an oncogenic role for Notch, it has been shown that small genomic deletions including the Notch2 locus correlate with better survival in human glioma patients (Boulay et al. 2007).
Expression profiles of Notch pathway members appear to correlate with tumor grade or clinical outcome in various ways. In one large study that included multiple sample sets, a microarray analysis of glioblastomas suggested that the tumors could be divided into three subtypes based on their gene expression profiles and that these subtypes correlated with age of onset and prognosis (Phillips et al. 2006). The subclass associated with the best prognosis was the “proneural” class, which was shown to have increased expression of genes associated with normal neurogenesis, as well as the Notch ligands Dll1 and Dll3, the Notch target Hey2, and the proneural gene Mash1 (Phillips et al. 2006). The other subclasses, associated with less favorably prognoses, did not have increased expression of Notch pathway components and instead showed a strong correlation with epidermal growth factor receptor (EGFR) activation.
At first glance, the above findings may seem to contradict the notion that Notch has an oncogenic role in gliomas. However, these data are in agreement with other recent observations on Notch component expression in gliomas of varying grades. For example, proportionately fewer glioblastomas overexpress NICD1 as compared with other less malignant astrocytic and oligodendroglial tumors (Cheung et al. 2006). Additionally, it has been shown that Mash1, which is inhibited by the Notch pathway, is up-regulated in some types of high-grade gliomas, possibly due to a decrease in Notch pathway activity (Somasundaram et al. 2005). These data suggest that Notch pathway activation may be important for low-grade gliomas but that the pathway is turned off in a portion of higher-grade tumors. Alternatively, some high-grade tumors may form independent of Notch pathway overexpression, whereas low-grade gliomas may be more commonly initiated via a Notch-dependent mechanism.
Other studies have yielded at least partially contradictory results with respect to the role of Notch in astrocytomas. In contrast to the decreased expression of active Notch1 reported by some researchers in more malignant astrocytomas, at least one study reported a positive correlation between increased levels of the Notch pathway target Hey1 and increased tumor grade (Hulleman et al. 2008). Interactions of Notch with other oncogenic pathways are complicated and are likely to contribute to seemingly contradictory findings with respect to the role of Notch in particular. For example, one recent study suggested that EGFR is regulated by Notch1 via a p53-dependent mechanism (Purow et al. 2008), complicating the picture painted by Phillips and colleagues. All told, it is clear that there are many gaps in our understanding of the role Notch has signaling in gliomas, although progress is certainly being made.
Ependymomas
The Notch pathway also affects other types of glial brain tumors, including ependymomas, in which Notch pathway expression patterns have been used to infer the cell of origin. Taylor et al. (2005) have found that increased expression of the Notch pathway ligand Jag1 is present in supratentorial ependymomas but not in those arising in the posterior fossa or spinal cord. Because this pattern correlates with that seen in non-neoplastic radial glial cells, the authors have suggested that these act as cells of origin for supratentorial ependymomas, with radial glia identified using other markers serving similar roles in the more caudal neuroaxis. We have also detected high-level expression of Notch ligands, receptors, and targets in human ependymoma samples, and it appears that Notch receptors cluster at the tips of the distinctive tumor processes that rosette around blood vessels (C. Eberhart, unpubl.).
Medulloblastomas
Medulloblastomas are malignant embryonal brain tumors that arise in the cerebellum, usually in children. They are thought to develop from committed progenitor cells in the external germinal layer (EGL) or from stem/progenitor cells along the ventricles (Eberhart 2007). Notch2 activity is present in the developing cerebellar EGL and has a role in maintaining this proliferative pool of progenitors, whereas Notch1 is expressed in differentiating cells (Solecki et al. 2001). Interestingly, we have observed that Notch2 promotes the growth of medulloblastoma cell lines, whereas Notch1 does not have this effect and in fact inhibits growth (Fan et al. 2004). These findings suggest that the two Notch receptors recapitulate their roles in normal cerebellar development in precursor-derived cerebellar tumors. Other groups have also shown that Notch pathway components, specifically Notch2, Jag1, and Hes1, show increased expression in mouse medulloblastoma models (Hallahan et al. 2004; Dakubo et al. 2006). It has also been observed that knockdown of Notch pathway activity in Sonic hedgehog-initiated tumors leads to increased cell death (Hallahan et al. 2004), indicating that the Notch pathway has an important role in medulloblastoma tumor maintenance.
Other Brain Tumors
Two final low-grade brain tumor types in which Notch has been implicated are meningioma and choroid plexus papilloma (CPP). In meningioma cell lines, introduction of activated Notch1 or Notch2 causes tetraploidy, and it has been speculated that Notch has a role in meningioma initiation (Baia et al. 2008). We have demonstrated more directly a tumor-initiating role for Notch3 in murine tumors. We found that constitutively active Notch3 (NICD3) is capable of causing CPP formation in mice when retrovirally expressed during neural development (Dang et al. 2006a). Strikingly, Notch1 does not have the same effect when injected at the same times during development, indicating that the various Notch receptors are not functionally equivalent with respect to tumor initiation in the mouse brain (Gaiano et al. 2000). We also found that Notch receptors and targets were expressed in CPPs from human patients (Dang et al. 2006a).
Brain Tumor Stem Cells
The idea that brain tumors contain a small population of stem-like cells capable of self-renewal, indefinite division, and “differentiation” into all of the cell types found in a given tumor is gaining acceptance in the field (Reya et al. 2001; Zhou and Zhang 2008). However, the identifying characteristics of cancer stem cells remain unclear. Relatively recently, it was shown that brain tumors contain subpopulations of cells that proliferate through numerous passages under neural stem cell culture conditions, give rise to a heterogeneous population of cells, and form xenografts recapitulating the phenotype of the tumors from which they were derived (Hemmati et al. 2003; Galli et al. 2004; Singh et al. 2004). Tumor-derived neurosphere colonies containing such stem-like cells have been derived from human glioblastomas (Galli et al. 2004), medulloblastomas, pilocytic astrocytomas, gangliogliomas (Singh et al. 2003), and ependymomas (Taylor et al. 2005).
The identification of robust molecular markers of cancer stem cells has been a significant challenge. One group used CD133, a cell surface marker previously shown to be present in neural progenitors (Uchida et al. 2000), to enrich for stem-like brain tumor cells. However, it has also been shown that not all brain tumors contain CD133+ stem-like cells, and that CD133− cells can initiate xenografts from these tumors (Beier et al. 2007). In addition to CD133, stem-like brain tumor cells have also been prospectively identified on the basis of their expression of ABC-type transporters and resulting ability to efflux Hoechst dye 33342 faster than more differentiated cells in the population (Patrawala et al. 2005). This “side population” (SP, as defined by FACS) has also been demonstrated to be enriched for neural progenitors in fetal and adult brain (Murayama et al. 2002). In brain cancer, it was shown that the SP is enriched for cells able to form more clones in a neurospheres assay, suggesting that it coincides with cancer stem cells in brain tumors (Patrawala et al. 2005).
The intermediate filament Nestin can also serve as a marker of undifferentiated or poorly differentiated cells in brain tumors. Nestin was first found to be expressed in NEPs in the developing central nervous system (Lendahl et al. 1990) and is now known to be expressed in both stem cells and more differentiated neural progenitor cells (Wiese et al. 2004). Interestingly, it has been reported that Notch signaling is able to drive nestin gene transcription, and coexpression of the two proteins has been shown in Kras-induced tumors (Shih and Holland 2006). These data further support the idea that Notch may have a role in regulating cell fate and stem cell maintenance in brain tumors.
The role of Notch in maintaining neural progenitors has been well established, yet our understanding of the role it has in stem-like tumor cells is much less advanced. We have shown that inhibition of the Notch pathway by a γ-secretase inhibitor leads to an up to fivefold decrease in CD133+ cells and a complete loss of side population within medulloblastoma cell lines (Fan et al. 2006). We have also demonstrated that the growth of these cell lines was dramatically decreased by the Notch blockade and that the treated cell lines were unable to form colonies in soft agar or xenografts in athymic mice (Fan et al. 2006). These results suggest that the stem-cell-like population in these tumors may have been eliminated as a consequence of the Notch blockade.
CONCLUSIONS
The Notch signaling pathway has not only a vital role in the developing mammalian nervous system, but also an important role in oncogenesis. In neural development, molecular interactions among progenitors and/or more differentiated cells activate Notch receptors to maintain a neural stem/progenitor state and inhibit neuronal differentiation. Notch signaling has also been implicated in many cancers, including brain cancer, where pathway components are aberrantly expressed. The pathway appears in most cases to promote brain tumor formation and growth, but in some contexts, Notch signaling may suppress tumors or help to define a subset of patients with less aggressive disease.
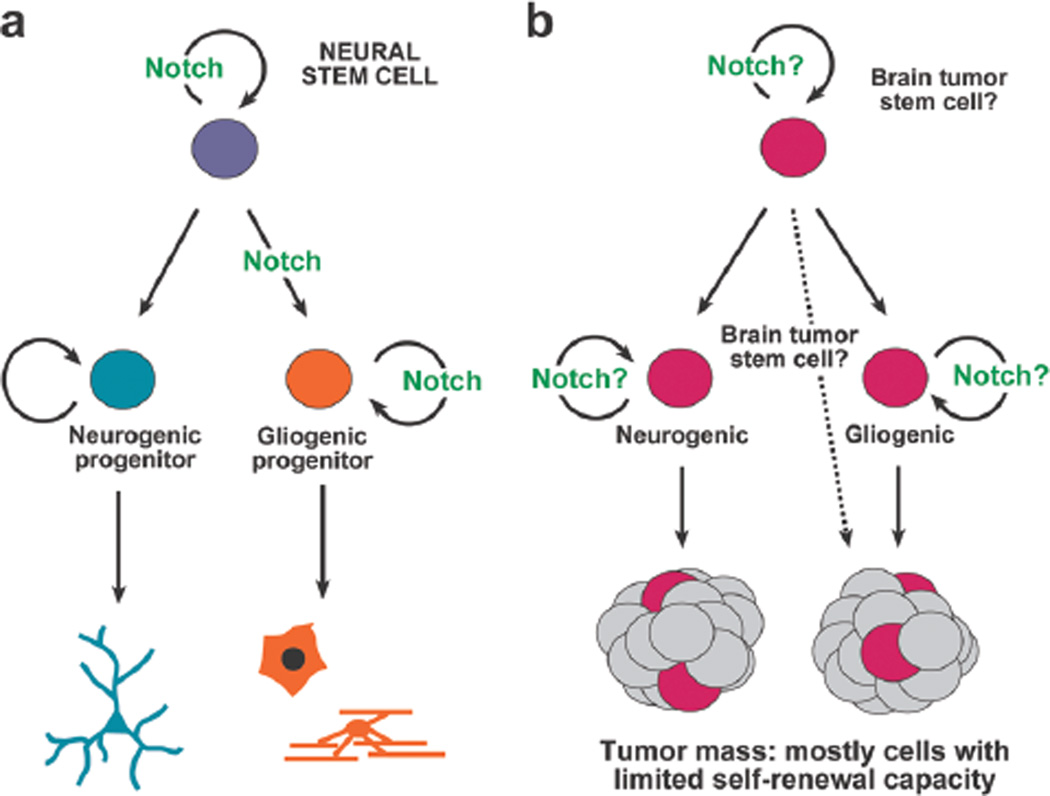
Although the data we have reviewed summarize much of what is known about Notch in neural progenitors during development, and Notch in brain tumors, it is currently not clear how to connect these two aspects of Notch signaling directly. It is tempting to speculate, based on the cancer stem cell model, that Notch signaling contributes to brain cancer formation by regulating brain tumor stem cells in a manner akin to how the pathway normally regulates neural progenitors (see Fig. 6). Aberrant Notch signaling could maintain an undifferentiated and proliferative active state that would contribute to tumor formation and growth. Furthermore, our recent findings that (1) canonical Notch signaling is used in embryonic neural stem cells (i.e., RGCs) but not in more restricted neurogenic progenitors (INPs) (Mizutani et al. 2007) and (2) Notch inhibition preferentially ablates stem-like tumor cells while sparing other proliferating cell types (Fan et al. 2006) suggest that understanding the heterogeneous nature of neural stem/progenitor cells in normal and neoplastic contexts is likely to provide insight directly relevant to the eventual eradication of brain cancer.
Figure 6.
Schematic representation depicting the idea that there may be parallels between embryonic neural progenitor subtypes and brain tumor stem cells and that Notch signaling, well known to have a role in regulating the former (a), may also have an aberrant role in regulating the latter (b).
REFERENCES
- Aster JC, Pear WS, Blacklow SC. Notch signaling in leukemia. Annu. Rev. Pathol. 2008;3:587–613. doi: 10.1146/annurev.pathmechdis.3.121806.154300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baia G, Stifani S, Kimura ET, McDermott MW, Pieper RO, Lal A. Notch activation is associated with tetraploidy and enhanced chromosomal instability in meningiomas. Neoplasia. 2008;10:604–612. doi: 10.1593/neo.08356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier D, Han P, Proescholdt M, Lohmeier A, Wischhusen J, Oefner PJ, Aigner L, Brawanski A, Bogdahn U, Beier CP. CD133+ and CD133− GBM-derived CaSCs show differential growth characteristics and molecular profiles. Cancer Res. 2007;67:4010–4015. doi: 10.1158/0008-5472.CAN-06-4180. [DOI] [PubMed] [Google Scholar]
- Bertrand N, Castro DS, Guillemot F. Proneural genes and the specification of neural cell types. Nat. Rev. Neurosci. 2002;3:517–530. doi: 10.1038/nrn874. [DOI] [PubMed] [Google Scholar]
- Bolos V, Grego-Bessa J, Pompa JL. Notch signaling in development and cancer. Endocr. Rev. 2007;28:339–363. doi: 10.1210/er.2006-0046. [DOI] [PubMed] [Google Scholar]
- Boulay JL, Miserez AR, Zweifel C, Sivasankaran B, Kana V, Ghaffari A, Luyken C, Sabel M, Zerrouqi A, Wasner M, Van Meirs E, Tolnay M, Reifenberger G, Merlo A. Loss of NOTCH2 positively predicts survival in subgroups of human glial brain tumors. PLoS One. 2007;2:e576. doi: 10.1371/journal.pone.0000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung HC, Corley LJ, Fuller GN, McCutcheon IE, Cote GJ. Polypyramidine tract binding protein and Notch1 are independently re-expressed in glioma. Mod. Pathol. 2006;19:1034–1041. doi: 10.1038/modpathol.3800635. [DOI] [PubMed] [Google Scholar]
- Dakubo GD, Mazerole CJ, Wallace VA. Expression of Notch and Wnt pathway components and activation of Notch signaling in medulloblastomas from heterozygous patched mice. J. Neuro-Oncol. 2006;79:221–227. doi: 10.1007/s11060-006-9132-2. [DOI] [PubMed] [Google Scholar]
- Dang L, Fan X, Chaudhry A, Wang M, Gaiano N, Eberhart CG. Notch3 signaling initiates choroid plexus tumor formation. Oncogene. 2006a;25:487–491. doi: 10.1038/sj.onc.1209074. [DOI] [PubMed] [Google Scholar]
- Dang L, Yoon K, Wang M, Gaiano N. Notch3 signaling promotes radial glial/progenitor character in the mammalian telencephlaon. Dev. Neurosci. 2006b;28:58–69. doi: 10.1159/000090753. [DOI] [PubMed] [Google Scholar]
- Dirks PB. Brain tumor stem cells: Bringing order to the chaos of brain cancer. J. Clin. Oncol. 2008;26:2916–2924. doi: 10.1200/JCO.2008.17.6792. [DOI] [PubMed] [Google Scholar]
- Eberhart CE. In search of the medulloblast: Neural stem cells and embryonal brain tumors. Neurosurg. Clin. N. Am. 2007;18:59–69. doi: 10.1016/j.nec.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Ellisen LW, Bird J, West DC, Soreng AL, Reynolds TC, Smith SD, Sklar J. TAN-1, the human homolog of the Drosophila Notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell. 1991;66:649–661. doi: 10.1016/0092-8674(91)90111-b. [DOI] [PubMed] [Google Scholar]
- Englund C, Fink A, Lau C, Pham D, Daza RA, Bulfone A, Kowalczyk T, Hevner RF. Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J. Neurosci. 2005;25:247–251. doi: 10.1523/JNEUROSCI.2899-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Eberhart CG. Medulloblastoma stem cells. J. Clin. Oncol. 2008;26:2821–2827. doi: 10.1200/JCO.2007.15.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Mikolaenko I, Elassan I, Ni XZ, Wang Y, Ball D, Brat DJ, Perry A, Eberhart CG. Notch1 and Notch2 have opposite effects on embryonal brain tumor growth. Cancer Res. 2004;64:7787–7793. doi: 10.1158/0008-5472.CAN-04-1446. [DOI] [PubMed] [Google Scholar]
- Fan X, Matsui W, Khaki L, Stearns D, Chun J, Li YM, Eberhart CG. Notch pathway inhibition depletes stem-like cells and blocks engraftment in embryonal brain tumors. Cancer Res. 2006;66:7445–7452. doi: 10.1158/0008-5472.CAN-06-0858. [DOI] [PubMed] [Google Scholar]
- Gaiano N, Fishell G. The role of Notch in promoting glial and neural stem cell fates. Annu. Rev. Neurosci. 2002;25:471–490. doi: 10.1146/annurev.neuro.25.030702.130823. [DOI] [PubMed] [Google Scholar]
- Gaiano N, Nye JS, Fishell G. Radial glial identity is promoted by Notch1 signaling in the murine forebrain. Neuron. 2000;26:395–404. doi: 10.1016/s0896-6273(00)81172-1. [DOI] [PubMed] [Google Scholar]
- Gal JS, Morozov YM, Ayoub AE, Chatterjee M, Rakic P, Haydar TF. Molecular and morphological heterogeneity of neural precursors in the mouse neocortical proliferative zone. J. Neurosci. 2006;26:1045–1056. doi: 10.1523/JNEUROSCI.4499-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F, Vescovi A. Isolation and characterization of tumorgenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- Götz M, Barde Y. Radial glial cells defined and major intermediates between embryonic stem cells and CNS neurons. Neuron. 2005;46:369–372. doi: 10.1016/j.neuron.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Hallahan AR, Pritchard JI, Hansen S, Benson M, Stoeck J, Hatton BA, Russell TL, Ellenbogen RG, Bernstein ID, Beachy PA, Olson J. The SmoA1 mouse model reveals that Notch signaling is critical for the growth and survival of sonic hedgehog-induced medulloblastomas. Cancer Res. 2004;64:7794–7800. doi: 10.1158/0008-5472.CAN-04-1813. [DOI] [PubMed] [Google Scholar]
- Hemmati HD, Nakano I, Lazareff JA, Masterman-Smith M, Geschwind DH, Bronner-Fraser M, Kornblum HI. Cancerous stem cells can arise from pediatric brain tumors. Proc. Natl. Acad. Sci. 2003;100:15178–15183. doi: 10.1073/pnas.2036535100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitoshi S, Alexson T, Tropepe V, Donoviel D, Elia AJ, Nye JS, Conlon RA, Mak TW, Berstein A, van der Kooy D. Notch pathway molecules are essential for the maintenance, but not the generation, of mammalian neural stem cells. Genes Dev. 2002;16:846–858. doi: 10.1101/gad.975202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulleman E, Quarto M, Vernell R, Masserdotti G, Colli E, Kros JM, Levi D, Gaetani P, Tunici P, Finocchiaro G, Rodriguez y Baena R, Capra M, Helin K. A role for the transcription factor HEY1 in glioblastoma. J. Cell. Mol. Med. 2008;13:136–146. doi: 10.1111/j.1582-4934.2008.00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatova TN, Kukekov VG, Laywell ED, Suslov ON, Vrionis FD, Steindler DA. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia. 2002;39:193–206. doi: 10.1002/glia.10094. [DOI] [PubMed] [Google Scholar]
- Iso T, Kedes L, Hamamori Y. HES and HERP families: Multiple effectors of the Notch signaling pathway. J. Cell. Physiol. 2003;194:237–255. doi: 10.1002/jcp.10208. [DOI] [PubMed] [Google Scholar]
- Kanamori M, Kawaguchi T, Nigro JM, Feuerstein BG, Berger MS, Miele L, Pieper RO. Contribution of Notch signaling activation to human glioblastoma multiforme. J. Neurosurg. 2007;106:417–427. doi: 10.3171/jns.2007.106.3.417. [DOI] [PubMed] [Google Scholar]
- Kuo CT, Mirzadeh Z, Soriano-Navarro M, Rasin M, Wang D, Shen J, Sestan N, Garcia-Verdugo J, Alvarez-Buylla A, Jan LY, Jan YN. Postnatal deletion of Numb/Numblike reveals repair and remodeling capacity in the subventricular neurogenic niche. Cell. 2006;127:1253–1264. doi: 10.1016/j.cell.2006.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- Li HS, Wang D, Shen Q, Schonemann MD, Gorski JA, Jones KR, Temple S, Jan LY, Jan YN. Inactivation of Numb and Numblike in embryonic dorsal forebrain impairs neurogenesis and disrupts cortical morphogenesis. Neuron. 2003;40:1105–1118. doi: 10.1016/s0896-6273(03)00755-4. [DOI] [PubMed] [Google Scholar]
- Louis DN, Ohgaki H, Wiestler OD, Cavenee WA. WHO classification of tumours of the central nervous system. Lyon, France: IARC Press; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louvi A, Artavanis-Tsakonas S. Notch signaling in vertebrate neural development. Nat. Rev. Neurosci. 2006;7:93–102. doi: 10.1038/nrn1847. [DOI] [PubMed] [Google Scholar]
- Malatesta P, Hack MA, Hartfuss E, Kettenmann H, Klinkert W, Kirchhoff F, Götz M. Neuronal or glial progeny: Regional differences in radial glia fate. Neuron. 2003;37:751–764. doi: 10.1016/s0896-6273(03)00116-8. [DOI] [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, Menezes JR, Macklis JD. Neuronal subtype specification in the cerebral cortex. Nat. Rev. Neurosci. 2007;8:427–437. doi: 10.1038/nrn2151. [DOI] [PubMed] [Google Scholar]
- Matsuno K, Diederich RJ, Go MJ, Blaumueller CM, Artavanis-Tsakonas S. Deltex acts as a positive regulator of Notch signaling through interactions with the Notch ankyrin repeats. Development. 1995;121:2633–2644. doi: 10.1242/dev.121.8.2633. [DOI] [PubMed] [Google Scholar]
- Mizutani K, Yoon K, Dang L, Tokunaga A, Gaiano N. Differential Notch signaling distinguishes neural stem cells from intermediate progenitors. Nature. 2007;449:351–355. doi: 10.1038/nature06090. [DOI] [PubMed] [Google Scholar]
- Murayama A, Matsuzaki Y, Kawaguchi A, Shimazaki T, Okano H. Flow cytometric analysis of neural stem cells in the developing and adult mouse brain. J. Neurosci. Res. 2002;15:837–847. doi: 10.1002/jnr.10339. [DOI] [PubMed] [Google Scholar]
- Nicolas M, Wolfer A, Raj K, Kummer J, Mill P, van Noort M, Hui C, Levers H, Dotto GP, Radtke F. Notch1 functions as a tumor suppressor in mouse skin. Nat. Genet. 2003;33:416–421. doi: 10.1038/ng1099. [DOI] [PubMed] [Google Scholar]
- Nieto M, Monuki ES, Tang H, Imitola J, Haubst N, Khoury SJ, Cunningham J, Gotz M, Walsh CA. Expression of Cux-1 and Cux-2 in the subventricular zone and upper layers II–IV of the cerebral cortex. J. Comp. Neurol. 2004;479:168–180. doi: 10.1002/cne.20322. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Martinez-Cerdeno V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat. Neurosci. 2004;7:136–144. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- Patrawala L, Calhoun T, Schneider-Broussard R, Zhou J, Claypool K, Tang DG. Side population is enriched in tumorigenic, stem-like cancer cells, whereas ABCG2+ and ABCG2− cancer cells are similarly tumorigenic. Cancer Res. 2005;65:6207–6219. doi: 10.1158/0008-5472.CAN-05-0592. [DOI] [PubMed] [Google Scholar]
- Patten BA, Sardi SP, Koirala S, Nakafuku M, Corfas G. Notch1 signaling regulates radial glia differentiation through multiple transcriptional mechanisms. J. Neurosci. 2006;26:3102–3108. doi: 10.1523/JNEUROSCI.4829-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen PH, Zou K, Hwang JK, Jan YN, Zhong W. Progenitor cell maintenance requires numb and numblike during mouse neurogenesis. Nature. 2002;419:929–934. doi: 10.1038/nature01124. [DOI] [PubMed] [Google Scholar]
- Petersen PH, Zou K, Krauss S, Zhong W. Continuing role for mouse Numb and Numbl in maintaining progenitor cells during cortical neurogenesis. Nat. Neurosci. 2004;7:803–811. doi: 10.1038/nn1289. [DOI] [PubMed] [Google Scholar]
- Petersen PH, Tang H, Zou K, Zhong W. The enigma of the Numb-Notch relationship during mammalian embryogenesis. Dev. Neurosci. 2006;28:156–168. doi: 10.1159/000090761. [DOI] [PubMed] [Google Scholar]
- Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD, Misra A, Nigro JM, Colman H, Soroceanu L, Williams PM, Modrusan Z, Feuerstein BG, Aldape K. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9:157–173. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Pontious A, Kowalczyk T, Englund C, Hevner RF. Role of intermediate progenitor cells in cerebral cortex development. Dev. Neurosci. 2007;30:24–32. doi: 10.1159/000109848. [DOI] [PubMed] [Google Scholar]
- Purow BW, Haque RM, Noel MW, Su Q, Burdick MJ, Lee J, Sundaresan T, Pastorino S, Park JK, Mikolaenko I, Maric D, Eberhart CG, Fine HA. Expression of Notch-1 and its ligands, Delta-like-1 and Jagged-1, is critical for glioma cell survival and proliferation. Cancer Res. 2005;65:2353–2363. doi: 10.1158/0008-5472.CAN-04-1890. [DOI] [PubMed] [Google Scholar]
- Purow BW, Sundaresan TK, Burdick MJ, Kefas BA, Comeau LD, Hawkinson MP, Su Q, Kotliarov Y, Lee J, Zhang W, Fine HA. Notch-1 regulates transcription of the epidermal growth factor receptor through p53. Carcinogenesis. 2008;29:918–925. doi: 10.1093/carcin/bgn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramain P, Khechumian K, Seugnet L, Arbogast N, Ackermann C, Heitzler P. Novel Notch alleles reveal a Deltex-dependent pathway repressing neural fate. Curr. Biol. 2001;11:1729–1738. doi: 10.1016/s0960-9822(01)00562-0. [DOI] [PubMed] [Google Scholar]
- Rasin MR, Gazula VR, Breunig JJ, Kwan KY, Johnson MB, Liu-Chen S, Li HS, Jan LY, Jan YN, Rakic P, Sestan N. Numb and Numbl are required for maintenance of cadherin-based adhesion and polarity of neural progenitors. Nat. Neurosci. 2007;10:819–827. doi: 10.1038/nn1924. [DOI] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- Shih AH, Holland EC. Notch signaling enhances nestin expression in gliomas. Neoplasia. 2006;12:1072–1082. doi: 10.1593/neo.06526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimojo H, Ohtsuka T, Kageyama R. Oscillations in Notch signaling regulate maintenance of neural progenitors. Neuron. 2008;58:52–64. doi: 10.1016/j.neuron.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumor initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- Solecki DJ, Liu XL, Tomoda T, Fang Y, Hatten ME. Activated Notch2 signaling inhibits differentiation of cerebellar granule neuron precursors by maintaining proliferation. Neuron. 2001;31:557–568. doi: 10.1016/s0896-6273(01)00395-6. [DOI] [PubMed] [Google Scholar]
- Somasundaram K, Reddy SP, Vinnakota K, Britto R, Subbarayan M, Nambiar S, Hebar A, Samuel C, Shetty M, Sreepathi HK, Santosh V, Hedge AS, Hedge S, Kondaiah P, Rao MR. Upregulation of ASCL1 and inhibition of Notch signaling pathway characterize progressive astrocytoma. Oncogene. 2005;24:7073–7083. doi: 10.1038/sj.onc.1208865. [DOI] [PubMed] [Google Scholar]
- Spana EP, Doe CQ. Numb antagonizes Notch signaling to specify sibling neuron cell fates. Neuron. 1996;17:21–26. doi: 10.1016/s0896-6273(00)80277-9. [DOI] [PubMed] [Google Scholar]
- Tarabykin V, Stoykova A, Usman N, Gruss P. Cortical upper layer neurons derive from the subventricular zone as indicated by Svet1 gene. Development. 2001;128:1983–1993. doi: 10.1242/dev.128.11.1983. [DOI] [PubMed] [Google Scholar]
- Taylor MD, Poppleton H, Fuller C, Su X, Liu Y, Jensen P, Magdaleno S, Dalton J, Calabrese C, Board J, Macdonald T, Rutka J, Guha A, Gajjar A, Curran T, Gilbertson RJ. Radial glia cells are candidate stem cells of ependymoma. Cancer Cell. 2005;8:323–335. doi: 10.1016/j.ccr.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, Tsukamoto AS, Gage FH, Weissman IL. Direct isolation of human central nervous system stem cells. Proc. Natl. Acad. Sci. 2000;97:14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese C, Rolletschek A, Kania G, Blyszczuk P, Tarasov KV, Tarasova Y, Wersto RP, Boheler KR, Wobus AM. Nestin expression—A property of multi-lineage progenitor cells? Cell. Mol. Life Sci. 2004;61:2510–2522. doi: 10.1007/s00018-004-4144-6. [DOI] [PubMed] [Google Scholar]
- Wu L, Aster JC, Blacklow SC, Lake R, Artavania-Tsakonas S, Griffin JD. MAML1, a human homologue of Drosophila Mastermind, is a transcriptional coactivator for NOTCH receptors. Nat. Genet. 2000;26:484–489. doi: 10.1038/82644. [DOI] [PubMed] [Google Scholar]
- Xu T, Artavanis-Tsakonas S. deltex, a locus interacting with the neurogenic genes, Notch, Delta and mastermind in Drosophila melanogaster. Genetics. 1990;126:665–677. doi: 10.1093/genetics/126.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N, Yamamoto S, Inagaki F, Kawaichi M, Fukamizu A, Kishi N. Role of Deltex-1 as a transcriptional regulator downstream of the Notch receptor. J. Biol. Chem. 2001;276:45031–45040. doi: 10.1074/jbc.M105245200. [DOI] [PubMed] [Google Scholar]
- Yoon K, Gaiano N. Notch signaling the mammalian nervous system: Insights from mouse mutants. Nat. Neurosci. 2005;8:709–715. doi: 10.1038/nn1475. [DOI] [PubMed] [Google Scholar]
- Yoon K, Koo B, Im S, Jeong H, Ghim J, Kwon M, Moon J, Miyata T, Kong Y. Mind bomb 1-expressing intermediate progenitors generate Notch signaling to maintain radial glial cells. Neuron. 2008;58:519–531. doi: 10.1016/j.neuron.2008.03.018. [DOI] [PubMed] [Google Scholar]
- Zhang XP, Zheng G, Zou L, Liu HL, Hou LH, Zhou P, Yin DD, Zheng QJ, Liang L, Zhang SZ, et al. Notch activation promotes cell proliferation and the formation of neural stem cell-like colonies in human glioma cells. Mol. Cell. Biochem. 2008;307:101–108. doi: 10.1007/s11010-007-9589-0. [DOI] [PubMed] [Google Scholar]
- Zhong W, Jiang M-M, Schonemann MD, Meneses JJ, Pedersen RA, Jan LY, Jan YN. Mouse numb is an essential gene involved in cortical neurogenesis. Proc. Natl. Acad. Sci. 2000;97:6844–6849. doi: 10.1073/pnas.97.12.6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Zhang Y. Cancer stem cells. Cell Cycle. 2008;7:1360–1370. doi: 10.4161/cc.7.10.5953. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Atkins JB, Rompani SB, Bancescu DL, Petersen PH, Tang H, Zou K, Stewart SB, Zhong W. The mammalian Golgi regulates numb signaling in asymmetric cell division by releasingACBD3 during mitosis. Cell. 2007;129:163–178. doi: 10.1016/j.cell.2007.02.037. [DOI] [PubMed] [Google Scholar]
- Zilian O, Saner C, Hagedorn L, Lee H-Y, Säuberli E, Suter U, Sommer L, Aguet M. Multiple roles of mouse Numb in tuning developmental cell fates. Curr. Biol. 2001;11:494–501. doi: 10.1016/s0960-9822(01)00149-x. [DOI] [PubMed] [Google Scholar]