Abstract
Introduction
Vietnam has been largely reliant on international support in its HIV response. Over 2006-2010, a total of US$480 million was invested in its HIV programmes, more than 70% of which came from international sources. This study investigates the potential epidemiological impacts of these programmes and their cost-effectiveness.
Methods
We conducted a data synthesis of HIV programming, spending, epidemiological, and clinical outcomes. Counterfactual scenarios were defined based on assumed programme coverage and behaviours had the programmes not been implemented. An epidemiological model, calibrated to reflect the actual epidemiological trends, was used to estimate plausible ranges of programme impacts. The model was then used to estimate the costs per averted infection, death, and disability adjusted life-year (DALY).
Results
Based on observed prevalence reductions amongst most population groups, and plausible counterfactuals, modelling suggested that antiretroviral therapy (ART) and prevention programmes over 2006-2010 have averted an estimated 50,600 [95% uncertainty bound: 36,300–68,900] new infections and 42,600 [36,100–54,100] deaths, resulting in 401,600 [312,200–496,300] fewer DALYs across all population groups. HIV programmes in Vietnam have cost an estimated US$1,972 [1,447–2,747], US$2,344 [1,843–2,765], and US$248 [201–319] for each averted infection, death, and DALY, respectively.
Conclusions
Our evaluation suggests that HIV programmes in Vietnam have most likely had benefits that are cost-effective. ART and direct HIV prevention were the most cost-effective interventions in reducing HIV disease burden.
Introduction
Over the past 25 years, much has changed in Vietnam’s attempts to mitigate both the population and personal burden of HIV/AIDS. There was an absence of effective targeted prevention programmes across the country in the early response [1]. As a result, Vietnam was unable to curb the spread of HIV in the 1990s [2]. Later, Vietnam adopted community peer-based prevention interventions, with an ambitious target to alter the course of the increasing epidemic in many parts of the country [3, 4]. In particular, condom promotion programmes targeting female sex workers (FSWs) and their clients, and needle syringe exchange programmes (NSPs) targeting people who inject drugs (PWID), were rolled-out nationwide from 2002 and 2003, respectively. Since 2005, the national antiretroviral therapy (ART) programme has been rapidly scaled up according to World Health Organization (WHO) guidelines [5].
Like other low- and middle-income countries, international donors contribute the majority of funding for HIV programmes in Vietnam. Over 2008–2010, a total of US$363 million was invested in the HIV response [6]. About US$172 million was from the United States President's Emergency Plan for AIDS Relief (PEPFAR); US$15 million from the Global Fund, primarily supporting HIV care and treatment, and US$30 million from the United Kingdom Department for International Development (DFID) and the World Bank (WB) to support harm reduction interventions. In all, approximately 27.5% was from domestic resources and private sector, which primarily focus on mass communication, sexual health promotion programmes for youths, and surveillance. To date, several studies with limited scale have been conducted to examine the impacts of these programmes [7–10]. Although they provided important insights into some HIV programmes, their evaluations were of isolated programmes (e.g., methadone maintenance therapy (MMT) or ART only), without considering interactions with other interventions. At present, no national evidence is available to demonstrate both population health benefits of the entire response and value for the substantial investment in the HIV response in the Vietnamese context.
The objectives of this study were to investigate the possible population impacts and cost-effectiveness of the HIV programmes implemented in Vietnam over the period 2006–2010 using a retrospective modelling approach. We used a population-based model of HIV transmission, informed by the latest epidemiological and programming data at a national scale, to compare the differences in epidemiological outcomes between the status quo and assumed counterfactual scenarios representing what is assumed to have occurred programmatically and behaviourally in the absence of the programmes. This is then used to conduct to a cost-effectiveness analysis.
Methods
Data collation
We synthesised HIV epidemiological, programming, and costing data in Vietnam from two main sources. In Vietnam, the transmission patterns of HIV epidemics have been monitored annually by the national sentinel surveillance system [2, 3] and the periodic behavioural surveillance surveys [11] or integrated biological and behavioural surveillance [12, 13]. In addition to these national surveys, regional studies with smaller sample sizes were published in international peer-reviewed journals or as internal reports in in-country health organizations. These data were primarily collected through our ongoing collaboration with the Department for HIV Monitoring and Evaluation of the Vietnam Administration of HIV Control (VAAC). VAAC has provided data on population size estimates, HIV prevalence trends across population groups, and the number of HIV diagnoses and people living with HIV (PLHIV) on ART in the entire country over time. For independent studies in parallel with the sentinel surveillance, a systematic review of published English literature between 1990 and 2011 was conducted to collate data on HIV disease burden, HIV-related risk behaviours, and access to health promotion programmes in key affected populations (KAPs); further details are provided in S1 File.
Data on HIV spending in Vietnam during 2006–2010 were obtained through ongoing stakeholder collaborations with the authors. Comprehensive report in HIV financing—the National AIDS Spending Assessment [6]–was available for the period 2008–2010, but not earlier. To fill this data gap, we extracted relevant spending data from major national-level financial reports collected by our local data team and via comprehensive online searches for the period 2006–2007. The spending breakdown of total budgets to donors, programme areas and supporting costs was done by using proportional allocations available for the period 2008–2010 (Table B in S1 File). A prior study estimated that the costs of voluntary HIV counselling and testing (VCT) services were US$7.2 per client [14]. The healthcare costs for untreated HIV-positive people were within the range of US$105–178 per person-year, depending on levels of CD4+ T-cell counts; the annual costs for people receiving first-line and second-line ART were estimated to be US$344 and US$1,533, respectively (personal communication with Department of Planning and Finance, VAAC) [15].
Model
We used the Optima model for the quantitative evaluation [16]. Details of this model are provided in S1 File. Briefly, Optima is a compartmental mathematical model of HIV that divided the overall 15–49 year old population in Vietnam into seven distinct risk populations as follows: males/females in the general population, direct/indirect FSWs, clients of sex workers, men who have sex with men (MSM), and PWID. Through a set of ordinary differential equations, the model tracks HIV transmission and the number of HIV-positive people and their rates of disease progression via four different CD4+ T-cell count categories (>500, 351–500, 200–350, and <200 cells/μL). The key model variable is the force-of-infection of HIV (λ), which is the probability with which uninfected individuals in each population become infected. It is expressed as λ = 1 −(1 − β)n, where β represents the transmission probability of HIV in each risk (sexual or injecting) event and n is the effective number of the events [17]. The model follows infected individuals through various stages of HIV infection and enables the calculation of disease burden in the populations at each of these stages. In this model, the Markov chain Monte Carlo algorithm was used to sample 1,000 sets of model parameters and then calibrate the model estimates to the observed epidemiological data [16]. A total of 40 calibrations with the best goodness-of-fit to the data were selected for computing summary statistics including median and 95% uncertainty bound. Further details are provided in S1 File.
Effectiveness and cost-effectiveness analysis
Five programmes were selected for this evaluation including: NSPs and MMT for PWID, condom promotion for FSWs/clients and for MSM, and ART. Efficacy of these interventions in reducing HIV transmission in the target populations (e.g. 95% (85–99%) for consistent and correct condom use [18] and 96% (90–98%) for people who are virally suppressed while on ART [19, 20]) were included in the Optima model. The evaluation was based on defined counterfactuals, that is, assumptions about what would have occurred had the programmes not been implemented at a national level. For the counterfactuals, we assumed that key behavioural or coverage indicators associated with each programme (Table G in S1 File) would remain constant at the level prior to programme commencement. This approach differed from previous cost-effectiveness analyses of HIV programmes in Vietnam [8–10], which were largely dependent on the probability of behavioural change associated with a specific intervention for a single population (e.g., MMT for PWID). Our model, calibrated to reflect what actually occurred across population groups across the entire country, was used to simulate the epidemic according to these changed parameter assumptions. The model outputs were then compared between these two scenarios in terms of the estimated number of new infections, deaths, and DALYs for each programme and across all programmes. Absolute DALYs with the model for all scenarios were quantified by using available disability weights (Table F in S1 File) [21, 22]. We additionally assessed programme cost-effectiveness by estimating the costs required for averting one infection, HIV-related death, or DALY. We used an annual discount rate of 3%.
Results
Overview of HIV expenditure during 2006–2010
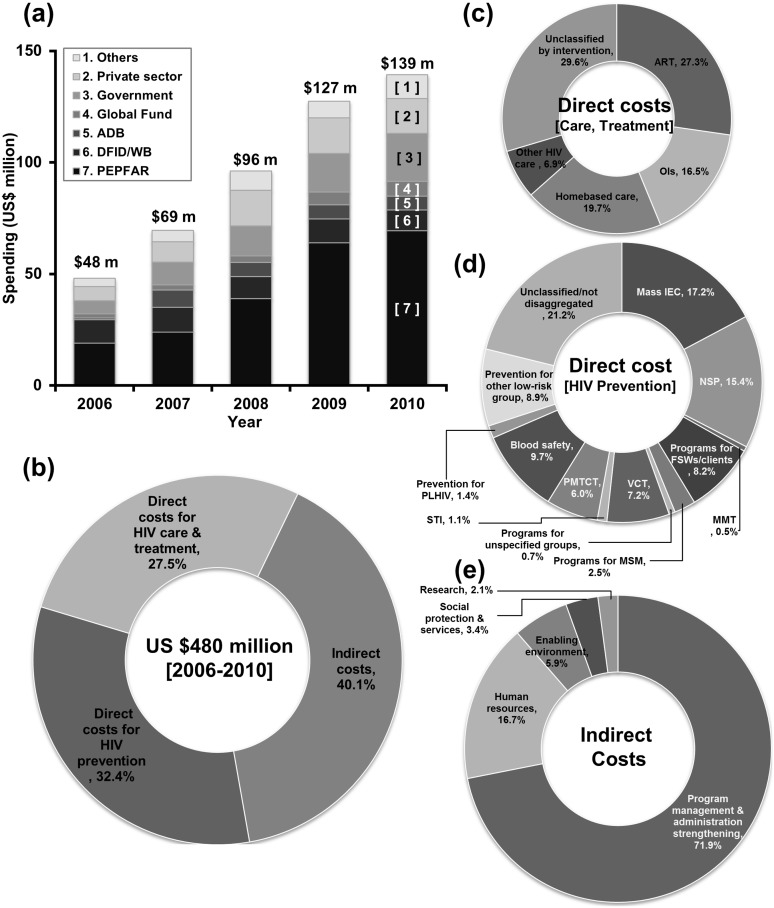
Overall, about US$480.3 million was spent on the HIV programmes in Vietnam during 2006–2010 with annual spending nearly tripling from US$48.0 million in 2006 to US$139.2 million in 2010 (Fig 1a). The domestic contribution to the total HIV spending was at a low level and international sources accounted for most of funding (72.7%). PEPFAR was the largest source of support for HIV/AIDS programmes in Vietnam (44.8%) and was followed by DFID/WB (10.8%). From 2006 through 2010, expenditures for prevention programmes totalled US$155.8 million, with a substantial amount, of US$23.9 million (15.4%), spent on NSPs, followed by US$12.8 million (8.2%) for condom promotion programmes for FSWs and their clients (Fig 1d). Over the same period, US$132.1 million was directly allocated to HIV care and treatment programmes, with US$36.0 million (27.3%) directly spent on ART (Fig 1c). The amount of funding spent on FSW/clients was only one-half of the funding allocated to PWID despite a far greater numbers of new infections. Only US$3.8 million (2.5%) of the total funding for HIV prevention programmes was allocated to MSM who contributed 27.2% of all new infections during 2006–2010 (Figs 1d and 3a).
Fig 1. Budget allocation by donors and HIV programmatic areas, 2006–2010.
(a) Overall HIV spending by donors. (b) Total funding by programmatic areas. (c) Direct cost for HIV care and treatment. (d) Direct cost for HIV preventions. (e) Indirect cost. ADB, Asian Development Bank; ART, antiretroviral therapy; DFID, the United Kingdom Department for International Development; FSW, female sex workers; IEC, information, education, and communication; MMT, methadone maintenance therapy; MSM, men who have sex with men; NSP, needle syringe exchange program; OIs, opportunistic infections; PEPFAR, the United States President's Emergency Plan for AIDS Relief; PLHIV, people living with HIV; PMTCT, preventing mother to child transmission of HIV; STI, sexually transmitted infections; VCT, voluntary HIV counselling and testing; WB, the World Bank.
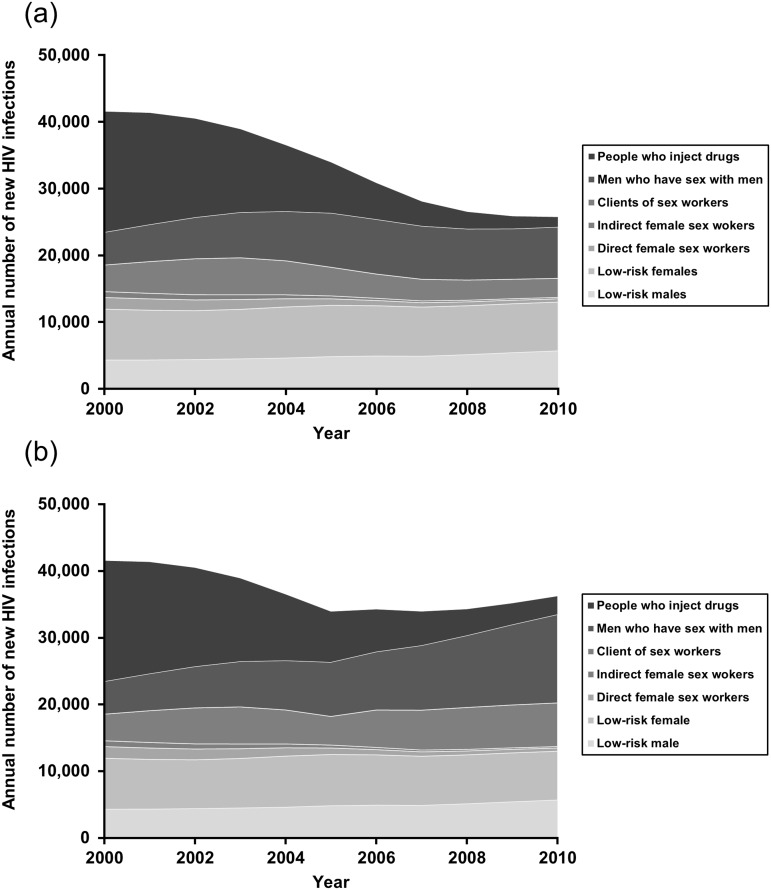
Fig 3. Projected median annual number of new HIV infections in Vietnam by population groups, 2000–2010.
(a) Status quo. (b) Counterfactual scenario (without programmes).
Projected plausible epidemiological impacts of HIV programmes
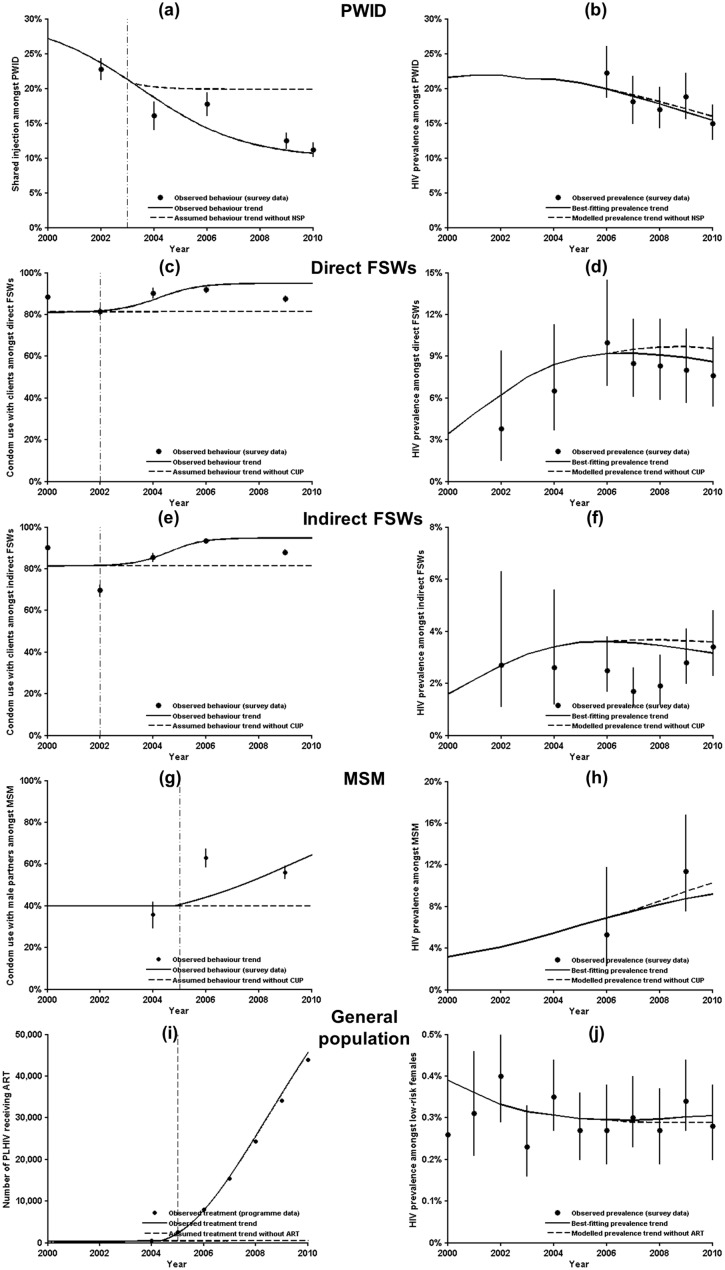
Behaviour trends indicated lower risk behaviours over time amongst PWID and different groups of FSWs (Fig 2a, 2c and 2e). Condom use by MSM was increasing, but remained at low levels over the study period (Fig 2g). Despite these changes, our model trajectories of HIV prevalence were within confidence intervals of observed data. However, prevalence trends decreased amongst PWID and FSWs associated with the reduction in risk behaviour. According to our assumed counterfactual scenarios of coverage and risk levels without the interventions, the prevention programmes reduced prevalence and brought a decline in prevalence amongst KAPs, particularly PWID and FSWs (Fig 2b, 2d and 2f), but not MSM (Fig 2h). A low but gradually increasing percentage of condom use amongst MSM under the status quo, given the relatively limited investment in HIV programmes targeting MSM, would contribute to the slowing down of HIV transmission in MSM but was impossible to reserve its rising trend. Of note, implementation of the ART programme had led to a slightly higher HIV prevalence in general population, as compared to the counterfactual scenario in which ART was absent (0.30% versus 0.29% in 2010, respectively, Fig 2j), due to its large population benefits in reducing the numbers of HIV-related deaths and incident infections (41,616 [95% uncertainty bound: 35,406–53,515] and 14,249 [10,426–21,23], respectively, Table 1).
Fig 2. Observed HIV prevalence trends amongst key affected populations and low-risk females under the status quo versus projected prevalence trends in the absence of targeted programmes.
Risk behaviours or interventions (left panels) and HIV prevalences (right panels) for each population group. HIV prevalence amongst pregnant women (as the low-risk female population) was assumed to be representative for the prevalence amongst the general population. Curves presented in the five figures in the left column were obtained from a generalised 4-parameter logistic function, whereas curves in the five right-hand figures were model trajectories. Solid curves represent median of 40 best-fitting trends under the status quo. Dashed curves represent assumed behaviour or modelled prevalence trends in the absence of programmes (i.e. the counterfactual). Black dots with solid vertical lines represent observed survey data with 95% confidence intervals. The vertical dash line indicates the initial year of programme implementation.
Table 1. Estimated effectiveness and cost-effectiveness of past HIV/AIDS programmes in Vietnam, 2006–2010.
| Programme cost | Estimated infections averted (95% UB) | Estimated deaths averted (95% UB) | Estimated DALYs averted (95% UB) | Programme cost per infection averted (95% UB) | Programme cost per death averted (95% UB) | Programme cost per DALY averted (95% UB) | |
|---|---|---|---|---|---|---|---|
| Needle and syringe programme | $23,935,822 | 5,212 (2,761, 7,522) | 338 (160, 517) | 16,306 (8,533, 24,253) | $4,592 (3,182, 8,669) | $70,816 (46,298, 149,599) | $1,493 (987, 2,805) |
| Methadone maintenance therapy programme | $853,296 | Nil | Nil | Nil | Nil | Nil | Nil |
| Condom promotion programme for FSWs | $12,769,281 | 12,035 (8,467, 20,240) | 1,022 (748, 1,421) | 42,231 (30,817, 64,150) | $1,061 (631, 1,508) | $12,494 (8,986, 17,071) | $302 (199, 414) |
| Condom promotion programme for MSM | $3,838,991 | 10,652 (3,080, 13,797) | 678 (250, 810) | 37,190 (10,874, 45,509) | $360 (278, 1,246) | $5,662 (4,739, 15,356) | $103 (84, 353) |
| Antiretroviral therapy programme (including PMTCT) | $45,392,265 | 14,249 (10,426, 21,023) | 41,616 (35,406, 53,515) | 276,401 (221,365, 349,243) | $3,186 (2,159, 4,354) | $1,091 (848, 1,282) | $164 (130, 205) |
| All programmes a | $99,747,684 | 50,570 (36,314, 68,921) | 42,557 (36,070, 54,129) | 401,550 (312,207, 496,278) | $1,972 (1,447, 2,747) | $2,344 (1,843, 2,765) | $248 (201, 319) |
DALY, disability adjusted life-year; FSW, female sex workers; MSM, men who have sex with men; PMTCT, prevention of mother-to-child transmission of HIV; UB, uncertainty bound of model simulations.
aThis included expenditure on NSP, MMT programme, condom use promotion programmes for FSWs and MSM, sexually transmitted infections programme, voluntary HIV counselling and testing programme, and ART.
Our modelling indicates that Vietnam’s HIV investments between 2006 and 2010 have averted a total of 50,570 [36,314–68,921] infections and 42,557 [36,070–54,129] deaths, corresponding to a 33.5% and 35.2% reduction of population numbers of new infections and deaths, respectively (Table 1). Furthermore, we estimated 27,180 [20,770–53,390] new infections occurring in Vietnam in 2010, showing a 35.4% reduction from 42,102 [36,811–53,826] cases in 2001 (Fig 3a). Whereas there has been a considerable reduction in new infections amongst both PWID and FSWs/clients over 2006–2010, approximately 8,000 MSM were newly infected with HIV every year since 2005. Among the five targeted programmes, the most effective intervention in reducing new infections was estimated to be the ART programme (14,249 [10,426–21,23], 9.5% of all new infections). It was followed by condom promotion programmes for FSWs/clients (12,035 [8,467–20,240], 7.8%), MSM (10,652 [3,080–13,797], 7.1%), and NSPs (5,212 [2,761–7,522], 3.5%). The prevention programmes were estimated to have led to a total of 401,550 [312,207–496,278] fewer DALYs over 2006–2010 (Table 1).
Projected cost-effectiveness of HIV programmes
With a total amount of US$480.3 million spent during 2006–2010, each infection and HIV-related death averted cost an estimated US$9,498 [6,969–13,227] and US$11,287 [8,874–13,317], respectively. Yet these costs were about five times lower when just direct investment on HIV prevention and ART programme costs were considered (US$1,972 [1,447–2,747] and US$2,344 [1,843–2,765], respectively). Each DALY averted was estimated to cost approximately US$1,196 [968–1,538] when the entire HIV investment was included and only US$248 [201–319] when only direct programme costs were included (Table 1).
Discussion
Vietnam has had a declining HIV epidemic trend between 2006 and 2010 [23]. ART, NSPs and condom promotion programmes for FSW/clients and MSM are likely to have been largely responsible for bringing about the decline. In this study, we found that there is not convincing evidence of population impact. Nevertheless, our model-based estimates suggest that HIV programmes have averted approximately 50,600 infections, 42,600 deaths, and 401,600 DALYs over 2006–2010, corresponding to costs of US$1,972, US$2,344, and US$248 per infection, death, and DALY averted, respectively. According to most willingness-to-pay thresholds [24], these ratios are likely to be deemed highly cost-effective.
Condom promotion programmes for FSWs/clients have demonstrated plausible moderate population impacts as about a quarter of the averted new infections during 2006–2010 were attributed to this programme. Despite being cost-effective, its cost-effectiveness ratios are much higher than countries with similar national FSW programmes (in India: US$104 and US$11 for each infection and DALY averted) [25]. The difference may be partly explained by the shorter duration of our evaluation (5-year versus 20-year time horizon). This ratio may become similar to those studies for other settings [26] if the currently large amount of indirect costs associated with programme administration and management could be reduced.
NSPs have had a major and noticeable impact on the HIV epidemic in Vietnam, which adds to a growing body of global evidence of the effectiveness of the NSPs in reducing HIV transmission amongst PWID [27]. The finding is strongly supported by the low level of receptive sharing of syringes recently reported amongst Vietnamese PWID (13% in the past month in 2009) [13], which is already comparable to those in high-income settings (e.g., Australia, 16% in 2009 [28]; Canada, 15% in 2006 [29]). Ongoing funding for NSPs is essential to curb the HIV epidemic in Vietnam, despite the estimated costs per infection averted by Vietnam’s NSPs found to be considerably higher than that of local NSPs in other low- and middle-income countries with a rising burden of HIV amongst PWID such as Odessa, Ukraine (US$97) [30], Dhaka, Bangladesh (US$110) [31], Yunnan, China (US$138–198) [32], and Svetlogorsk, Belarus (US$359) [33]. The higher cost per infection of Vietnamese NSPs may be explained by the large-scale implementation of the programmes that is often associated with higher cost requirements and an apparent decline in new HIV infections amongst Vietnamese PWID over the study period. Unlike NSPs, which have been long implemented in areas of Vietnam, the low-level coverage of MMT since its initial roll out in 2008, estimated to be 5.6% by 2012 [34], has limited our attempts to evaluate its population health effects. Future studies are required to better define the effects of this programme for PWID in Vietnam. Despite this, continued scale-up of MMT is likely to improve the overall impact and cost-effectiveness of the HIV response in Vietnam [35]. MMT also leads to other social and public health gains [36, 37].
Our projection of a rapidly expanding HIV epidemic amongst MSM suggests a shift in the modes of HIV transmission in Vietnam. It is possible that the limited investment in HIV prevention programmes targeting MSM over the past decade in this country has substantially fuelled homosexual HIV transmission. Shifting prevention resources to this population, which is known to be at a much higher risk of HIV infection globally [38], is urgently needed to curb the epidemic. Specifically, promotion of condom use by MSM is a priority strategy to prevent the sexual transmission of HIV to their serodiscordant same-sex partners, as well as to limit the spread of HIV into the wider community through unprotected sexual activities with their female partners [13]. Overlapping sexual and injecting behaviours are also common amongst Vietnamese MSM [39], which makes it important to coordinate condom and harm reduction programmes for drug-using MSM to minimise the cross-transmission of HIV.
Cohen and colleagues [19] showed substantial prevention benefits of providing early ART in reducing heterosexual transmission of HIV in serodiscordant couples, from which the treatment as prevention (TasP) strategy has been recommended for many settings. Despite the potentially large preventive benefits of Vietnam’s ART programmes and the low cost per infection of the public-health ART approach used in Vietnamese ART programme [5, 15, 40], as observed in this study, it is important to emphasize that there are several key barriers for a successful roll-out of TasP in Vietnam. The first is the constrained government budget for HIV interventions, the weakness of the government-funded HIV healthcare system, and a shortage of health professionals. These difficulties make the TasP strategy difficult to roll out and scale up at the national level in the immediate future. Alternatively, a narrower scope of early and immediate initiation of ART regardless of CD4+ T-cell count level for specific HIV-positive subgroups, as per recent recommendations of the WHO [41, 42], may be a feasible option for Vietnam. Low access to HIV treatment amongst Vietnamese PLHIV is another hurdle. Despite a significant effort since 2005 to rapidly scale up HIV testing and ART for PLHIV, by 2012, amongst the 197,300 people diagnosed with HIV in Vietnam, fewer than half were linked to HIV care and only a third were on ART [43]. Thus a critical area of need in Vietnam is an improved continuum of HIV care and treatment through timely diagnosis, referral to HIV care, and early ART initiation for eligible PLHIV.
With a heavy reliance on international donors for the HIV response and a foreseeable reduction in funding in the near future, a scale-up of domestic revenue to maintain effective responses to the epidemic in Vietnam is required. The limited resources mean every dollar for HIV responses needs to be spent effectively. A recent study on population-level cost-effectiveness of the large community-based Avahan programme in south India strongly indicated that KAP-focused interventions are highly cost-effective [44]. Our findings recommend a shift of available resources away from the general populations and towards the KAPs in Vietnam, which is consistent with a recent systematic review of HIV funding landscape in other Asian settings [45]. NSPs and condom programmes amongst MSM and FSW/clients should be specifically prioritised.
Our study has several limitations. Empirical data on the actual effects of an intervention for KAPs on changing their high-risk behaviours and incidence rates between the intervention and control groups have been lacking in Vietnam. Therefore, we could not perform an evaluation of evidence-based interventions in this study with a well-defined control group (or counterfactual). Instead, this evaluation depends on assumptions of conditions remaining unchanged had the interventions not taken place. It is possible that risk behaviours may have decreased without investment in the specific interventions. Furthermore, behavioural and surveillance data were taken from a series of cross-sectional surveys amongst KAPs, which may not be completely comparable over time and thus contribute in part to wide uncertainty bounds of the findings. Several other model parameters used in our analysis also have large uncertainties. For example, the HPTN052 clinical trial demonstrated a 96% reduction in rates of sexual transmission amongst serodiscordant heterosexual couples in nine African countries [19], whereas no difference or a smaller reduction (26%) in HIV risks were found from other observational studies amongst Ugandan and Chinese serodiscordant couples [46, 47]. More importantly, preventive effects of ART on homosexual and injection sharing transmission of HIV are still unknown and assumed in this study to be identical to that of heterosexual transmission. In a national evaluation, Nguyen et al. [48] further showed a low CD4+ cell count among people initiating therapy for HIV, indicating a long lag time from HIV infection to HIV diagnosis and care among Vietnamese people living with HIV. This may suggest that the observed decline in diagnosis over the study period of 2006–2010 may be contributed in part by the effects of the HIV programmes implemented in the earlier period (i.e., 2000–2005). However, our study was hindered by incomplete costing data for the period 2000–2005, which has limited our further investigation of the relationship between HIV spending with key behavioural indicators. Spending data were not stratified by provincial/regional levels, so the cost-effectiveness of programmes in specific Vietnamese provinces/regions could not be assessed in this analysis. Thus, while we used the best available evidence to conduct this study, the extreme paucity of empirical data and the resultant reliance on informed assumptions renders it impossible to make definitive claims about the effectiveness of the programmes considered.
Conclusions
Our model-based estimates suggest that HIV programmes in Vietnam have most likely been cost-effective in reducing the HIV disease burden; however, with counterfactual projections overlapping with intervals around observed data, the evidence for effectiveness is only moderate. With a highly concentrated epidemic in Vietnam, it is imperative to shift HIV resources from programmes for the general population towards programmes for the KAPs for greatest population benefits.
Supporting Information
Flow chart of article selection (Figure A). HIV prevalence among various populations in Vietnam, 2000–2011 (Figure B). Number of reported HIV diagnosis in Vietnam, 2000–2011 (Figure C). Number of PWID registered methadone maintenance therapy (Figure D). Number of people receiving ART in Vietnam, 2006–2010 (Figure E). Model schematic for HIV infection progression (Figure F). Calibrated input parameters by Optima (Figure G). Projected prevalence levels are shown by population group (Figure H). Summary of literature among key affected populations in Vietnam (Table A). Estimated HIV spending in Vietnam, 2006–2010 (Table B). Population interactions (Table C). Mathematical modelling inputs (Table D). Healthcare costs of HIV infected people in 2009 (Table E). Disability-weights for cost effectiveness calculations (Table F). Selected behaviours affected by HIV prevention programmes (Table G).
(DOCX)
Acknowledgments
This work could not have been accomplished without the assistance of colleagues in Vietnam. We thank Nguyen Thi Mai at the World Bank Office in Vietnam; Nguyen Phuong Thuy at Hanoi School of Public Health for their contributions and support. The authors wish to thank Louisa Wright for assistance with English language editing and proofreading. The Kirby Institute is funded by the Australian Government, Department of Health and Ageing and is affiliated with the UNSW Australia. The findings and conclusions in this report do not necessarily represent the position of the Vietnamese Ministry of Health, of the authors’ institutions, and of the Vietnamese or Australian Governments.
Data Availability
A full description of the mathematical model and parameter values are provided in the Supplementary Materials.
Funding Statement
This research was supported by the World Bank under the terms of the contract number: 8004397. QDP is supported by an AusAID PhD scholarship. DPW’s work was funded in part by the Australian National Health and Medical Research Council. The Kirby Institute is funded by the Australian Government, Department of Health and Ageing and is affiliated with the UNSW Australia.
References
- 1. Quan VM, Chung A, Abdul-Quader AS. The feasibility of a syringe-needle-exchange program in Vietnam. Subst Use Misuse. 1998;33(5):1055–67. [DOI] [PubMed] [Google Scholar]
- 2. Quan VM, Chung A, Long HT, Dondero TJ. HIV in Vietnam: the evolving epidemic and the prevention response, 1996 through 1999. J Acquir Immune Defic Syndr. 2000;25(4):360–9. [DOI] [PubMed] [Google Scholar]
- 3. Hien NT, Long NT, Huan TQ. HIV/AIDS epidemics in Vietnam: evolution and responses. AIDS Educ Prev. 2004;16(Suppl A):137–54. [DOI] [PubMed] [Google Scholar]
- 4. Vuong T, Ali R, Baldwin S, Mills S. Drug policy in Vietnam: a decade of change? Int J Drug Policy. 2012;23(4):319–26. 10.1016/j.drugpo.2011.11.005 [DOI] [PubMed] [Google Scholar]
- 5. Srikantiah P, Ghidinelli M, Bachani D, Chasombat S, Daoni E, Mustikawati DE, et al. Scale-up of national antiretroviral therapy programs: progress and challenges in the Asia Pacific region. AIDS. 2010;24:S62–S71. [DOI] [PubMed] [Google Scholar]
- 6. The Joint United Nations Programme on HIV/AIDS (UNAIDS). Vietnam national AIDS spending assessment, 2008–2010. Hanoi, Vietnam: UNAIDS; 2012. Available at: http://www.aidsdatahub.org/vietnam-national-aids-spending-assessment-2008-2010-unaids-2012. Accessed 25 July 2012. 2012. [Google Scholar]
- 7. Kato M, Granich R, Bui DD, Tran HV, Nadol P, Jacka D, et al. The potential impact of expanding antiretroviral therapy and combination prevention in Vietnam: towards elimination of HIV transmission. J Acquir Immune Defic Syndr. 2013;63(5):e142–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tran BX, Ohinmaa A, Duong AT, Nguyen LT, Vu PX, Mills S, et al. The cost-effectiveness and budget impact of Vietnam's methadone maintenance treatment programme in HIV prevention and treatment among injection drug users. Glob Public Health. 2012;7(10):1080–94. 10.1080/17441692.2012.736259 [DOI] [PubMed] [Google Scholar]
- 9. Tran BX, Ohinmaa A, Duong AT, Nguyen LT, Vu PX, Mills S, et al. Cost-effectiveness of integrating methadone maintenance and antiretroviral treatment for HIV-positive drug users in Vietnam's injection-driven HIV epidemics. Drug Alcohol Depend. 2012;125(3):260–6. 10.1016/j.drugalcdep.2012.02.021 [DOI] [PubMed] [Google Scholar]
- 10. Tran DA, Wilson DP, Shakeshaft A, Ngo AD, Reyes J, Doran C, et al. Cost-effectiveness of antiretroviral therapy expansion strategies in Vietnam. AIDS Patient Care STDS. 2014;28(7):365–71. 10.1089/apc.2014.0016 [DOI] [PubMed] [Google Scholar]
- 11. National AIDS Standing Bureau, Family Health International, United States Agency for International Development. HIV/AIDS behavioral surveillance survey Vietnam 2000. Hanoi, Vietnam; 2001. Available at: http://www.aidsdatahub.org/behavioral-surveillance-survey-vietnam-2000-bss-round-1-results-national-aids-standing-bureau-fhi-usaid-et-al-2000. Accessed 25 July 2012. [Google Scholar]
- 12. Ministry of Health. Results from the HIV/STI integrated biological and behavioral surveillance (IBBS) in Vietnam, 2005–2006. Ha Noi, Vietnam: Ministry of Health; 2006. Available at: http://www.inthealth.ku.dk/reach/resources/surveillance.pdf/. Accessed 15 April 2014. [Google Scholar]
- 13. Ministry of Health. Results from the HIV/STI intergrated biological and behavioral surveillance (IBBS) in Vietnam round II, 2009. Hanoi, Vietnam: Ministry of Health; 2011. Available at: http://aidsdatahub.org/dmdocuments/Vietnam_IBBS_Round_II_2009.pdf. Accessed 22 September 2011. [Google Scholar]
- 14. Family Health International. Cost analyses of selected USAID-supported HIV interventions managed by FHI 360 in Vietnam: outreach, HIV testing and counseling (HTC), and anti-retroviral treatment (ART) 2009–2010. Hanoi, Vietnam, 2012: FHI 360/Urban Care. [Google Scholar]
- 15. Duong AT, Kato M, Bales S, Do NT, Minh Nguyen TT, Thanh Cao TT, et al. Costing analysis of national HIV treatment and care program in Vietnam. J Acquir Immune Defic Syndr. 2014;65(1):e1–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kerr CC, Stuart RM, Gray RT, Shattock A, Fraser N, Benedikt C, et al. Optima: a model for HIV epidemic analysis, program prioritization, and resource optimization. J Acquir Immune Defic Syndr.2015;69(3):365–76. [DOI] [PubMed] [Google Scholar]
- 17. RØTtingen J-A, Garnett GP. The epidemiological and control implications of HIV transmission probabilities within partnerships. Sex Transm Dis. 2002;29(12):818–27. [DOI] [PubMed] [Google Scholar]
- 18. Patel P, Borkowf CB, Brooks JT, Lasry A, Lansky A, Mermin J. Estimating per-act HIV transmission risk: A systematic review. AIDS. 2014;28(10):1509–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. 10.1056/NEJMoa1105243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wilson DP, Law MG, Grulich AE, Cooper DA, Kaldor JM. Relation between HIV viral load and infectiousness: a model-based analysis. Lancet. 2008;372(9635):314–20. 10.1016/S0140-6736(08)61115-0 [DOI] [PubMed] [Google Scholar]
- 21. Salomon JA, Vos T, Hogan DR, Gagnon M, Naghavi M, Mokdad A, et al. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2129–43. 10.1016/S0140-6736(12)61680-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stouthard MEA, Essink-Bot M-L, Bonsel GJ, on behalf of the DDWG. Disability weights for diseases: a modified protocol and results for a Western European region. Eur J Public Health. 2000;10(1):24–30. [Google Scholar]
- 23.The Joint United Nations Programme on HIV/AIDS (UNAIDS). HIV in Asia and the Pacific UNAIDS report 2013. Bangkok, Thailand: UNAIDS's Regional Support Team for Asia and the Pacific; 2013. Available at: http://www.aidsdatahub.org/HIV-in-Asia-and-the-Pacific-UNAIDS-Report-2013. Accessed 20 June 2014.
- 24. Shillcutt S, Walker D, Goodman C, Mills A. Cost effectiveness in low- and middle-income countries. Pharmacoeconomics. 2009;27(11):903–17. 10.2165/10899580-000000000-00000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Prinja S, Bahuguna P, Rudra S, Gupta I, Kaur M, Mehendale SM, et al. Cost effectiveness of targeted HIV prevention interventions for female sex workers in India. Sex Transm Infect. 2011;87(4):354–61. 10.1136/sti.2010.047829 [DOI] [PubMed] [Google Scholar]
- 26. Hogan DR, Baltussen R, Hayashi C, Lauer JA, Salomon JA. Achieving the millennium development goals for health: cost effectiveness analysis of strategies to combat HIV/AIDS in developing countries. BMJ. 2005;331(7530):1431–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aspinall EJ, Nambiar D, Goldberg DJ, Hickman M, Weir A, Van Velzen E, et al. Are needle and syringe programmes associated with a reduction in HIV transmission among people who inject drugs: a systematic review and meta-analysis. Int J Epidemiol. 2013;43(1):235–48. 10.1093/ije/dyt243 [DOI] [PubMed] [Google Scholar]
- 28.Iversen J, Chow S, Maher L. Australian needle and syringe program survey: national data report, 2009–2013. The Kirby Institute, UNSW Australia, 2014. Available at: http://www.kirby.unsw.edu.au/surveillance/Australian-NSP-Survey-Data-Reports. Accessed 30 June 2014.
- 29.Public Health Agency of Canada. I-Track: enhanced surveillance of risk behaviours among injecting drug users in Canada. Phase I Report, August 2006. Surveillance and Risk Assessment Division, Centre for Infectious Disease Prevention and Control, Public Heath Agency of Canada, 2006. Available at: www.phac-aspc.gc.ca/i-track/sr-re-1/index-eng.php. Accessed 30 June 2014.
- 30. Vickerman P, Kumaranayake L, Balakireva O, Guinness L, Artyukh O, Semikop T, et al. The cost-effectiveness of expanding harm reduction activities for injecting drug users in Odessa, Ukraine. Sex Transm Dis. 2006;33(10):S89–S102. [DOI] [PubMed] [Google Scholar]
- 31. Guinness L, Vickerman P, Quayyum Z, Foss A, Watts C, Rodericks A, et al. The cost-effectiveness of consistent and early intervention of harm reduction for injecting drug users in Bangladesh. Addiction. 2010;105(2):319–28. 10.1111/j.1360-0443.2009.02755.x [DOI] [PubMed] [Google Scholar]
- 32. Zhang L, Yap L, Xun Z, Wu Z, Wilson D. Needle and syringe programs in Yunnan, China yield health and financial return. BMC Public Health. 2011;11(1):250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kumaranayake L, Vickerman P, Walker D, Samoshkin S, Romantzov V, Emelyanova Z, et al. The cost-effectiveness of HIV preventive measures among injecting drug users in Svetlogorsk, Belarus. Addiction. 2004;99(12):1565–76. [DOI] [PubMed] [Google Scholar]
- 34. Nguyen TTM, Nguyen LT, Pham MD, Vu HH, Mulvey KP. Methadone maintenance therapy in Vietnam: an overview and scaling-up plan. Adv Prev Med. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wammes JJG, Siregar AY, Hidayat T, Raya RP, van Crevel R, van der Ven AJ, et al. Cost-effectiveness of methadone maintenance therapy as HIV prevention in an Indonesian high-prevalence setting: a mathematical modeling study. Int J Drug Policy. 2012;23(5):358–64. 10.1016/j.drugpo.2012.06.009 [DOI] [PubMed] [Google Scholar]
- 36. Marsch LA. The efficacy of methadone maintenance interventions in reducing illicit opiate use, HIV risk behavior and criminality: a meta-analysis. Addiction. 1998;93(4):515–32. [DOI] [PubMed] [Google Scholar]
- 37. Van Den Berg C, Smit C, Van Brussel G, Coutinho R, Prins M. Full participation in harm reduction programmes is associated with decreased risk for human immunodeficiency virus and hepatitis C virus: evidence from the Amsterdam Cohort Studies among drug users. Addiction. 2007;102(9):1454–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Beyrer C, Baral SD, van Griensven F, Goodreau SM, Chariyalertsak S, Wirtz AL, et al. Global epidemiology of HIV infection in men who have sex with men. Lancet. 2012;380(9839):367–77. 10.1016/S0140-6736(12)60821-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pham QD, Nguyen TV, Hoang CQ, Cao V, Khuu NV, Phan HTT, et al. Prevalence of HIV/STIs and associated factors among men who have sex with men in An Giang, Vietnam. Sex Transm Dis. 2012;39(10):799–806. [DOI] [PubMed] [Google Scholar]
- 40. Gilks CF, Crowley S, Ekpini R, Gove S, Perriens J, Souteyrand Y, et al. The WHO public-health approach to antiretroviral treatment against HIV in resource-limited settings. Lancet. 2006;368(9534):505–10. [DOI] [PubMed] [Google Scholar]
- 41. Easterbrook PJ, Irvine CJ, Vitoria M, Shaffer N, Muhe LM, Negussie EK, et al. Developing the 2013 WHO consolidated antiretroviral guidelines. AIDS. 2014;28:S93–S100. [DOI] [PubMed] [Google Scholar]
- 42. Eaton JW, Menzies NA, Stover J, Cambiano V, Chindelevitch L, Cori A, et al. Health benefits, costs, and cost-effectiveness of earlier eligibility for adult antiretroviral therapy and expanded treatment coverage: a combined analysis of 12 mathematical models. Lancet Glob Health. 2014;2(1):e23–e34. 10.1016/S2214-109X(13)70172-4 [DOI] [PubMed] [Google Scholar]
- 43.Family Health International. Improving the HIV cascade of services in Vietnam. ICAAP, Bangkok, Thailand, November 2013: Family Health International. Available at: http://www.slideshare.net/scfhi/improving-the-hiv-cascade-of-services-in-vietnam. Accessed 20 July 2014.
- 44. Vassall A, Pickles M, Chandrashekar S, Boily M-C, Shetty G, Guinness L, et al. Cost-effectiveness of HIV prevention for high-risk groups at scale: an economic evaluation of the Avahan programme in south India. Lancet Glob Health. 2014;2(9):e531–e40. 10.1016/S2214-109X(14)70277-3 [DOI] [PubMed] [Google Scholar]
- 45. Craig AP, Thein HH, Zhang L, Gray RT, Henderson K, Wilson D, et al. Spending of HIV resources in Asia and Eastern Europe: systematic review reveals the need to shift funding allocations towards priority populations. J Int AIDS Soc. 2014;17(1):188822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jia Z, Mao Y, Zhang F, Ruan Y, Ma Y, Li J, et al. Antiretroviral therapy to prevent HIV transmission in serodiscordant couples in China (2003–11): a national observational cohort study. Lancet. 2013;382(9899):1195–203. 10.1016/S0140-6736(12)61898-4 [DOI] [PubMed] [Google Scholar]
- 47.Birungi J, Wang H, Ngolobe MH, Muldoon K, Khanakwa S, King R, et al. Lack of effectiveness of antiretroviral therapy (ART) as an HIV prevention tool for serodiscordant couples in a rural ART program without viral load monitoring in Uganda [abstract no. TUAC0103]. Presented at: 19th International AIDS Conference, Washington, USA, July 2012. Available from: http://www.iasociety.org/Abstracts/A200745964.aspx. Accessed 13 August 2014.
- 48. Nguyen DB, Do NT, Shiraishi RW, Le YN, Tran QH, Huu Nguyen H, et al. Outcomes of antiretroviral therapy in Vietnam: Results from a national evaluation. PLoS One. 2013;8(2):e55750 10.1371/journal.pone.0055750 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flow chart of article selection (Figure A). HIV prevalence among various populations in Vietnam, 2000–2011 (Figure B). Number of reported HIV diagnosis in Vietnam, 2000–2011 (Figure C). Number of PWID registered methadone maintenance therapy (Figure D). Number of people receiving ART in Vietnam, 2006–2010 (Figure E). Model schematic for HIV infection progression (Figure F). Calibrated input parameters by Optima (Figure G). Projected prevalence levels are shown by population group (Figure H). Summary of literature among key affected populations in Vietnam (Table A). Estimated HIV spending in Vietnam, 2006–2010 (Table B). Population interactions (Table C). Mathematical modelling inputs (Table D). Healthcare costs of HIV infected people in 2009 (Table E). Disability-weights for cost effectiveness calculations (Table F). Selected behaviours affected by HIV prevention programmes (Table G).
(DOCX)
Data Availability Statement
A full description of the mathematical model and parameter values are provided in the Supplementary Materials.