Abstract
Purpose
Carrying out the chemical composition and antiproliferative effects against cancer cells from different biological parts of Artemisia herba alba.
Methods
Essential oils were studied by gas chromatography coupled to mass spectrometry (GC–MS) and their antitumoral activity was tested against P815 mastocytoma and BSR kidney carcinoma cell lines; also, in order to evaluate the effect on normal human cells, oils were tested against peripheral blood mononuclear cells PBMCs.
Results
Essential oils from leaves and aerial parts (mixture of capitulum and leaves) were mainly composed by oxygenated sesquiterpenes 39.89% and 46.15% respectively; capitulum oil contained essentially monoterpenes (22.86%) and monocyclic monoterpenes (21.48%); esters constituted the major fraction (62.8%) of stem oil. Essential oils of different biological parts studied demonstrated a differential antiproliferative activity against P815 and BSR cancer cells; P815 cells are the most sensitive to the cytotoxic effect. Leaves and capitulum essential oils are more active than aerial parts. Interestingly, no cytotoxic effect of these essential oils was observed on peripheral blood mononuclear cells.
Conclusion
Our results showed that the chemical composition variability of essential oils depends on the nature of botanical parts of Artemisia herba alba. Furthermore, we have demonstrated that the differential cytotoxic effect depends not only on the essential oils concentration, but also on the target cells and the botanical parts of essential oils used.
Introduction
Artemisia herba-alba (Asteraceae) is a greenish-silver perennial dwarf shrub growing in arid and semi-arid climates. It occurs through the Mediterranean region in North Africa, Spain, deserts of Sinai Peninsula, the Middle East, Northwestern Himalayas, and in India. Artemisia herba alba Asso (desert wormwood, armoise blanche (Fr.), chich (Arab.) is a dwarf shrub with a rapid growth in dry and warm climates and in muddy areas [1].The various species are morphologically different from each other depending on geographical, environmental, and climatic conditions. The plant is green to light green, with strong, sturdy roots. The flowering time and harvesting is around May/June and continues until October in some areas [2]. This plant is used as a flavouring and as a medicine herb for the treatment of colds, coughing, intestinal disturbances and as an antidiabetic agent [3, 4]. Investigations on the medicinal properties of Artemisia herba alba extracts reported anti-diabetic, leishmanicidal, antibacterial, and antifungal properties [5, 6]. Over the last decades, studies on Artemisia herba alba were focused on its essential oils. Their composition through the world revealed a high level of polymorphism and led to the definition of several chemotypes. Because of their importance in the fragrance industry, numerous studies on Artemisia herba alba essential oils have been published [7, 8]. Two reviews are also available [9, 10].
In Spain, essential oil from Artemisia herba alba [8, 11] showed that monoterpene hydrocarbons and oxygenated monoterpenes are the most abundant skeletons, but large amounts of sesquiterpenes also were found in some populations. Camphor, 1,8-cineole, p-cymene and davanone were the major chemical compounds found. Two oil types were found for plants grown in Israel and Sinai those of cineole-thujane bornane type and the pinane type with monoterpene skeletons [8].
In Jordan, regular monoterpenes were predominant and the principal components were α- and β-thujones, classifying the Artemisia herba alba as being a thujone chemotype [12]. In Morocco, sixteen chemotypes were found, and twelve of them have monoterpenes as major components of essential oils. The remaining four chemotypes, have sesquiterpene skeletons as the major fraction. Investigations reported no correlation between chemotypes and geographic distribution [13].
In Algerian essential oil, monoterpenes were the major components, essentially camphor, α- and β-thujones, 1,8-cineole and chrysanthenyl derivatives [2, 14]. In Tunisian oil, oxygenated monoterpenes were found to be the major components of Artemisia herba alba oil extracted from aerial parts [15, 16].
This paper reports the chemical composition and the biological activity against cancer cell lines of the essential oils, obtained from different biological parts of Artemisia herba alba collected at Er-Rachidiya (Central- East region of Morocco).
Materials and Methods
Ethics statement
Artemisia herba alba Asso plant was harvested in Imilchil, Errachidia district central Eastern of Morocco (W 5°39′25.43″ - N 32°12′15.059″) in June 2007 (No specific permits were required for the described field studies or for the collection of plant material).The field studies did not involve endangered or protected species.
Human peripheral blood mononuclear cells (PBMCs): the blood samples were collected from the authors of this manuscript (Mounir Tilaoui,Hassan Ait Mouse; Abdeslam Jaafari and Abdelmajid Zyad) under medical surveillance. Approved by Sultan Moulay Slimane University committee, accreditation No 2008/01-2014. The authors receive written informed consent from the blood donors.
Plant material
Artemisia herba alba Asso plant was harvested in Imilchil, Errachidia district, central Eastern region of Morocco (W 5°39′ 25.43″ - N 32°12′ 15.059″) in June 2007 (No specific permits were required for the described field studies or for the collection of plant material). Whole aerial parts (mixture of capitulum and leaves) on isolated leaves, capitulum (flower head) or stems were shade-dried at room temperature with ventilation. The obtained dry matter was isolated separately from the plant, minced and immediately hydrodistilled to obtain essential oils.
Gas chromatography and mass spectroscopic analysis (GC-MS)
Analytical gas chromatography was carried out using a Trace GC ULTRA gas chromatography system fitted with a VB-5 (Methylpolysiloxane with 5% of phenyl) column (30 m x 0.25 mm, 0.25 μm film thickness). Carrier gas was helium at a flow rate of 1.4 mL/min. Column temperature was initially kept at 40°C for 2min, and then gradually increased to 300°C at a rate of 20°C/min. Samples (1μL, appropriately diluted in ethyl acetate) were injected at 220°C.
Mass spectrometry analysis was carried on a Polaris Q MS coupled to GC ULTRA with an ionic trap mass detector operating in the EI mode (70eV). Components identification was done by GC and GC-MS (according to fragmentation patterns) and by using NIST (National Institute of Standards and Technology) MS Search database.
Cell culture
The murine mastocytoma cell line (P815, ATCC: TIB64) and the kidney carcinoma cell lines of hamsters (BSR, ATCC: CCL10) were kindly donated by the laboratory of Dr. Michel Lepoivre, 841 Institute of Biochemistry, University of Paris XI, France. The cell lines were cultured in DMEM (Dulbecco’s modified Eagle’s medium) supplemented with 10% Heat-inactivated fetal calf serum (Gibco BRL, Cergy Pontoise, France), penicillin G- streptomycin (1%), and 0.2% sodium bicarbonate (Sigma) at 37°C in a humidified atmosphere containing 5% CO2.
Cytotoxicity assay
The starting inoculums of 2 x 105 P815 cells/mL and 4.8 x 104 BSR cells/mL were used in the exponential phase of growth. Cellular viability was determined by the MTT reduction assay using (3- [4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide, MTT) [17]. BSR and P815 cell lines were harvested from starting cultures at the exponential growth phase. After a phosphate buffer saline (PBS) wash, the harvested cells were poured in flat-bottomed 96-well microtiter plates containing 100 μL of complete medium per well. Then, the cells were treated with essential oils dissolved in Dimethyl sulfoxide (DMSO) with the appropriate concentrations (all essential oils studied here which are dissolved in DMSO, are soluble in culturing media). Control cells were treated with DMSO, at a final concentration 0, 5% (v/v). After 48-h incubation in a humidified atmosphere at 37°C, 5% CO2, 20 μL MTT solution (5 mg/mL PBS) was added. After 4-h incubation under the same conditions, the cleavage of MTT to formazan by metabolically active cells was quantified by scanning the plates at 540 and 630 nm using a MultisKan EX apparatus. The cell viability was calculated by the formula:
Where, OD: Optical Density, Controlled cells: DMSO + cells, OD of essential oils: treated cells.
Effect on human peripheral blood mononuclear cells (PBMCs)
This test was realised in order to evaluate the effect of the essential oils on human normal cells using the MTT colorimetric assay described above. The viability of PBMCs cells was evaluated by trypan bleu dye exclusion using Malassez Haemocytometer. Cell concentrations were determined as the cells were mixed with 0.2% trypan blue and counted microscopically with a Malasez haemocytometer using the following procedure: 10 μL of cell suspension mixed with 40 μl 0.2% trypan blue and 10 μL of the same mixture was transferred to Malassez haemocytometer. The percentage of cell viability was calculated via the formula: % cell viability = viable cells (unstained) / total number of cells x 100. Cell viability before the cytotoxicity experiment was above 98%.
To isolate the PBMCs, blood samples were collected from healthy donors in heparinized tubes. The blood sample was diluted with the same volume of PBS. After that, the diluted blood sample was carefully layered on Ficoll-hypaque (Amersham Pharmacia Biotech AB). The mixture was centrifuged under at 2000tr/mn for 30 mn. The undisturbed lymphocyte layer was carefully transferred out. The lymphocytes were washed twice with phosphate-buffered solution (PBS) and resuspended in DMEM media. Essential oils were added at the same concentrations able to induce a cytotoxic activity against tumor cells (P815 and BSR). The PBMCs were seeded at a density of (1 × 105 cells/mL) in 96 well plates.
Statistical analysis
All the experiments were performed in three replicate. Data are reported as the mean ± S.D. n = 3. The significance of differences was estimated using Student’s paired t-test. The difference was considered statistically significant when the P-value was less than 0.05. All statistical analyses were performed using STATISTICA software.
Results and Discussion
Chemical composition of the essential oil
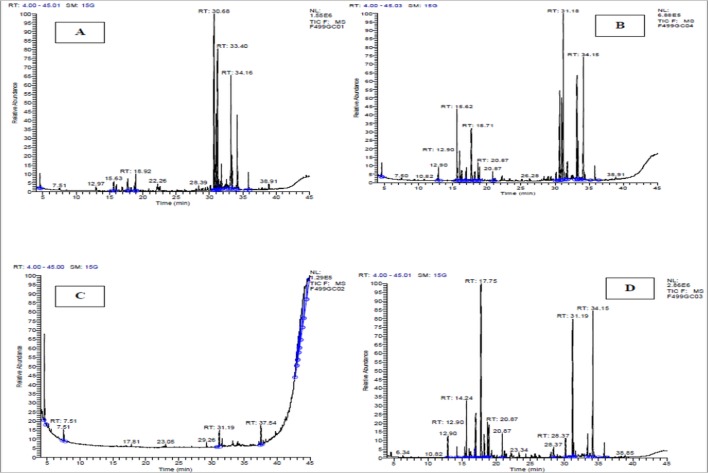
The chemical composition of the essential oil was investigated using both GC and GC-MS techniques (Fig 1). Table 1 lists the components identified in the essential oil of different parts of Artemisia herba alba (capitulum, leaves, aerial parts and stems). Table 2 shows that essential oil of leaves is composed mainly by oxygenated sesquiterpenes (39.89%), sesquiterpenes (29.04%) and esters (23.97%). Monoterpenes with thujone skeleton (1.24%) and monoterpenes with pinane skeleton (4.48%) are represented by low contents. These compounds are represented by variable concentrations in other parts of the plant studied: β- thujone (1.24%) in leaves and (6.14%) in aerial part, bisabolon oxide (17.55) in aerial parts and 10.27% in leaves. Nevertheless, (+) 2,3,6,7-tetramethyl-, 4,4aα,5,8,8aβ,9β,9aα,10,10aα-decahydroanthracen-9-ol and β-Guaiene are absent in other parts of the plant. In stems essential oil, esters constitute the major fraction with 62.8%. On the other side, essential oil of capitulum is relatively rich in terpenoids compared to other biological parts of the plant; oxygenated monoterpenes and monocyclic monoterpenes are the most abundant with 22.86% and 21.48% respectively. Other important compounds are represented by the sesquiterpenes (12.92%), the monoterpenes with thujane skeleton (10.91%), and the monoterpenes with skeleton pinane (9.24%), while monoterpenes with bornane skeleton were found with low percentage (3.31%).
Fig 1. Essential oils chromatogram of different biological parts from Artemisia herba alba.
A: Leaves, B: Capitulum, C: Stems, D: Aerial parts (Mixture of leaves and capitulum).
Table 1. Essential oils chemical composition (%) of leaves, stems, capitulum and aerial parts (mixture of capitulum and leaves) of Artemisia herba alba.
| Leaves | Stems | Capitulum | Aerial parts | Formula | MW | |
|---|---|---|---|---|---|---|
| Monocyclic monoterpenes | ||||||
| Terpinen-4-ol | - | - | 0.89 | 2.43 | C10H16O | 152.23 |
| Piperitone | - | - | 1.01 | 2.69 | C10H16O | 152.23 |
| 3,5-Heptadienal, 2-ethylidene-6-methyl- | - | - | - | 1.74 | C10H14O | 150.21 |
| (+-)-2,3,6,7-tetramethyl-1,4,4aα,5,8,8aβ,9β,9aα,10,10aα-decahydroanthracen-9-ol | - | - | 19.58 | - | ||
| Monoterpenes with thujane skeleton | ||||||
| β-thujone | 1.24 | - | 7.00 | 6.14 | C10H16O | 152.23 |
| α- thujone | - | - | 2.91 | - | C10H16O | 152.23 |
| 3-thujanol | - | - | 1.07 | - | C10H16O | 152.23 |
| Cis-sabinol | - | - | - | 2.40 | C10H16O | 152.23 |
| Thujone | - | - | - | 1.01 | C10H16O | 152.23 |
| Trans-sabinene hydrate | - | - | - | 1.24 | C10H20O | 172.26 |
| Monoterpenes with bornane skelton | ||||||
| Camphor | - | - | 1.45 | 5.12 | C10H16O | 152.23 |
| Fenchol | - | - | 1.86 | 3.86 | C10H18O | 154.25 |
| Monoterpenes with pinane skelton | ||||||
| Verbenol | 2.16 | - | 5.99 | 21.83 | C10H16O | 152.24 |
| Myrtenol | 2.32 | - | 1.74 | 4.19 | C10H16O | 152.23 |
| Chrysanthenone | - | - | 1.51 | - | C10H14O | 150.22 |
| Sesquiterpenes | ||||||
| Widdrene | 9.95 | - | - | - | C15H24 | 204.35 |
| α-bergamotene | 3.31 | - | - | - | C15H24 | 204.35 |
| α-longipinene | - | - | 1.84 | - | C15H24 | 204.35 |
| β-Guaiene | - | - | 11.08 | - | C15H24 | 204.35 |
| α –bulnesene | 15.78 | - | - | - | C15H24 | 204.35 |
| Ester | ||||||
| Acetic acid, butyl ester | 1.49 | 10.50 | 1.19 | - | C6H12O2 | 116.15 |
| 2,5-Octadecadiynoic acid, methyl ester | 22.48 | 3.08 | 9.51 | - | C21H38O2 | 322.52 |
| 1-Butanol, 3-methyl-, acetate | - | 2.10 | - | - | C7H14O2 | 130.18 |
| [1,1'-Bicyclopropyl]-2-octanoic acid, 2'-hexyl-, methyl ester | - | 5.79 | - | - | C21H38O2 | 322.52 |
| Cyclopropaneoctanoic acid, 2-[[2-[(2thylcyclopropyl)methyl]cyclopropyl]methyl]-, methyl ester | - | 4.65 | - | - | C22H38O2 | 334.53 |
| Ethyl linoleate | - | 19.28 | - | - | C20H36O2 | 308.50 |
| Linoleic acid | - | 6.31 | - | - | C18H32O2 | 280.44 |
| 13,16-Octadecadiynoic acid, methyl ester | - | 11.09 | - | - | C21H38O2 | 322.52 |
| Oxygenated sesquiterpenoids | ||||||
| Caryophyllene oxide | 1.49 | - | - | 1.76 | C15H24O | 220.35 |
| Farnesene epoxide, E | 4.97 | - | 4.38 | 17.08 | C15H24O | 220.35 |
| Bisabolone oxide | 10.27 | - | 13.64 | 17.55 | C15H24O2 | 236.35 |
| trans-(Z)-α-bisabolene epoxide | - | 2.86 | - | - | C15H24O | 220.35 |
| 1,2–15,16-Diepoxyhexadecane | - | - | - | C20H40O2 | 312.53 | |
| Eucalyptol (1,8-Cineole) | 20.37 | 7.71 | 1.49 | 2.27 | C10H18O | 154.24 |
| Α-bisabolol oxide A | - | - | 2.20 | 2.26 | C15H26O2 | 238.36 |
| Bergamotol, Z-α-trans | 2.79 | - | 0.97 | 2.24 | C15H24O | 220.35 |
| α-Bisabolol oxide | - | - | - | 2.99 | C15H26O2 | 238.37 |
| Yield of essential oils (%) | 0,15 | 0,12 | 0,6 | 0,22 |
Table 2. Essential oils monoterpenes compounds of Artemisia herba alba (%).
| Leaves | Stems | Capitulum | Aerial parts | |
|---|---|---|---|---|
| Oxygenated sesquiterpenoids | 39,89 | 10,57 | 22,86 | 46,15 |
| Sesquiterpenes | 29,04 | 12.92 | ||
| Esters | 23,97 | 62,8 | ||
| Monoterpenes with pinane skelton | 4,48 | 9,24 | 26,02 | |
| Monoterpenes with thujane skeleton | 1,24 | 10,91 | 10.78 | |
| Monocyclic monoterpenes | 21,48 | 6.59 | ||
| Monoterpenes with bornane skelton | 3,31 | 8.98 | ||
| Fatty acid derivate | 17.22 |
The essential oil extracted from aerial parts (mixture of capitulum and leaves) is very rich in oxygenated sesquiterpenes (46.15%) and pinane skeleton (26.02%), other constituents are representative by monoterpene skeleton with thujane (10.78%), monoterpenes with bornane skeleton (8.98%) and monocyclic monoterpenes (6.59%). In Tunisian Artemisia herba alba essential oil, oxygenated monoterpenes and oxygenated sesquiterpenes represent the major fraction of the oil [18].
Thujone was reported as the major component in the essential oil from Tunisia (64%), whereas it was absent in Spain for all the essential oils studied [8, 11]. In this study, thujone concentration was 1.04%. In previous studies, Benjilali and Richard identified seven chemotypes with high concentration varying between 34% and 94% of α and β thujone in Artemisia herba alba essential oils collected from different Moroccan regions [19, 21]. Also, in Algeria, α thujone and β thujone were characterized as the major components in the studied oil [22,23]. In our investigation, β thujone concentration was 6.14% but α thujone was not detected in aerial parts oil. On the basis of the results obtained by Ouyahya et al [10] for three Moroccan artemisia oils (Artemisia negri, Artemisia mesatlantica and Artemisia herba alba) it appears that the percentage composition of α and β thujone vary according to the geographical growth site, season, environmental and climatic conditions.
It has been reported in almost all samples described in the literature that cineole is one of the most common constituents in Artemisia herba alba essential oil. It has been found as a major component in the essential oil from Spain (Eastern and central Spain) [8, 11], Israel [8], Egypt [24], and in other Moroccan locations [13]. Its concentration reaches high values (superior to 40%) in Spain and Israel [11, 8]. In Tunisia none or very low values were found for 1,8-cineole concentration [15,16], like essential oils from Algeria and Jordan [12, 14]. In this study 1,8-cineole concentration varied between 1.49% in aerial parts and 20.37% in leaves.
Camphor was found at high concentrations in essential oil samples from Southern Tunisia [18] and Algeria (19.14%) [14]. In Morocco, a previous study showed that camphor is one of the most encountered components in Artemisia herba alba essential oil. Five chemotypes were defined as camphor type oils [20]. In our work, we found 5.12% of Camphor.
Chrysanthenone is a common compound in oils from Israel, Spain (28.2% -36.4%), Tunisia (17%) and Algeria (22.5% sample from Sidi Aissa) [2], and even from other Moroccan samples (77%). Here it was not detected in aerial parts from Artemisia herba alba; it was rather present as a minor concentration in capitulum (1.2%). Among the compounds identified in other Artemisia herba alba oils, and not detected in this study, the absence of davanon is worth noting; it has been found to be the major constituent in some populations of Artemisia herba alba from other regions of Morocco [21, 25] and as a minor constituent in samples from Israeli [8, 26] and the Sinai desert and surroundings [8, 27]. In Spain, its concentration reaches high values (superior to 39,1%) but it is absent in other essential oils samples [11].
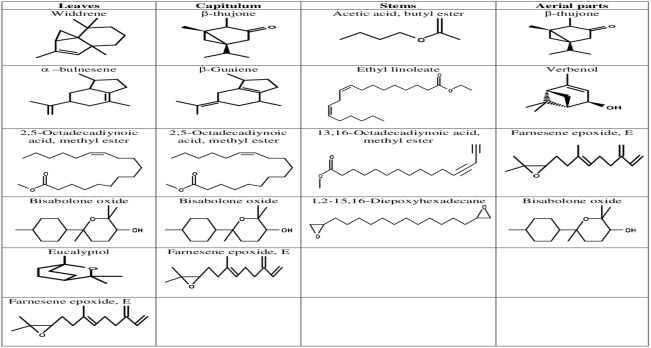
Our results show that essential oils from different botanical parts of Artemisia herba alba studied don’t contain camphor, thujone, and 1.8 cineole as the main components like other Moroccan [22], Algerian [2] and Israeli types of essential oils. Furthermore, our study identifies new molecules that have not been cited in other studies and the possible characterization of a new chemothypes (verbenol, trans α bisabolone, Farnesene epoxyde) of Artemisia herba alba essential oils (Fig 2). Trans α bisabolone and farnesene epoxyde, have been reported to exhibit potential antitumor activities [28, 29].
Fig 2. Structures of the most abundant compounds identified in different parts of Artemisia herba alba essential oils.
Through the study of the essential oils by GC-MS, we have been able to identify and quantify the composition of the essential oils of Artemisia herba alba, which varies in different parts of plants. These qualitative and quantitative differences in the chemical composition of essential oils might be attributed to several factors such as geographical factors (location), climatic effects of the plants, harvest season, nature of the soil, age of the plant parts (young or adult), the state of plant material used (dried or fresh), the part of the plant used, time of collection, the extraction process, genetic variability (chemotype), [30] etc.
Our results are in concordance with other reports that demonstrated how plant growth are affected by genetic and environmental factors, and how these factors contribute to differences in the chemical variation of essential oils of plants with different chemotypes [31, 32]. The chemical variation of essential oils of different chemotypes of Thymus species from different locations or growing in the same habitat have been attributed to the difference in environmental and genetic factors [31, 33, 34, 35]. Furthermore, ecological factors, climatic characteristics, particularly, light and temperature, have also been reported to influences the production of essential oils as well as other active agents in plants [32, 36, 37, 38, 39].
Anticancer activity of Artemisia herba alba essential oils
Chemotherapy is not giving significant benefits and it is often associated to systemic toxicity and drug resistance. Thus, searching for new therapeutic agents that are able to circumvent the chemotherapy side effects, remains a more efficient to reduce toxicity and increase their effectiveness against cancer. Therefore, we carried out the in vitro anticancer activity of essential oils obtained from different biological parts of Artemisia herba alba growth in Morocco. Artemisia herba alba is a frequent component of herbal mixtures used in the traditional medicine [40, 3, 4].
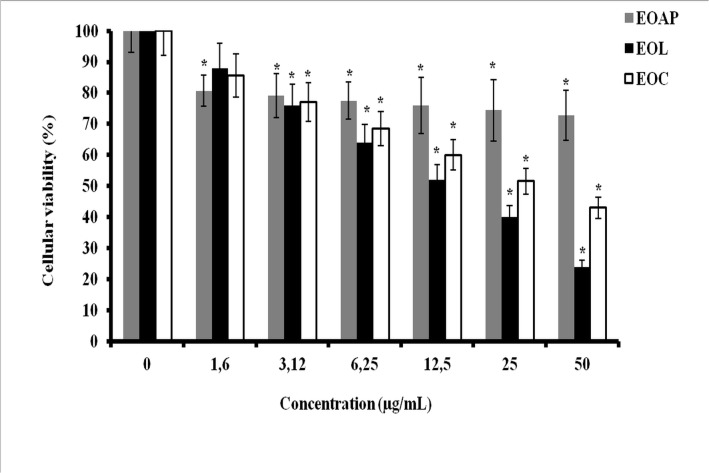
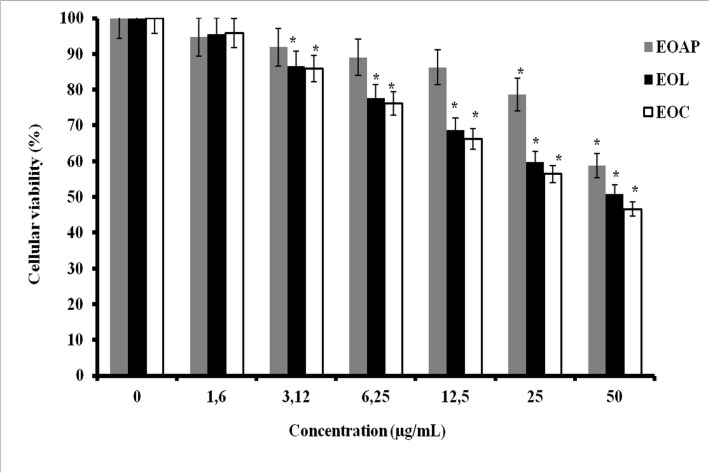
In vitro anti-tumor cytotoxicity assays of Artemisia herba alba oils were performed against P815 and BSR cell lines. These oils include: the essential oils of leaves, capitulum and aerial parts. The results obtained are reported in Figs 3 and 4; it is shown that all essential oils have an important dose dependant cytotoxic effect against P815 and BSR cell lines. However, in P815 cell line (Fig 3) the cytotoxicity decreased in the following order: essential oil of leaves > essential oil of capitulum > essential oil of aerial parts. The concentration leading to 50% cytotoxicity (IC50) was about 15μg/ mL (P<0.05) for leaves oil and 36μg/ml for capitulum essential oil, however; the essential oil of aerial parts did not show an important cytotoxicity and did not reach IC50 (P<0.05). In BSR cell line (Fig 4), the cytotoxicity induced by essential oils studied shows that capitulum oil (IC50 = 20μg/mL, P<0.05)) is slightly sensitive to leaves oil (IC50 = 26μg/mL, P<0.05) and more sensitive than aerial parts oils (IC50 = 50μg/mL) (P<0.05). The results suggest that the cytotoxicity effect can be attributed to the nature of chemical compound content in essential oils extracted from different botanical parts of Artemisia herba alba and on cancer cell lines target.
Fig 3. Cytotoxic effect of Artemisia herba alba essential oils on P815 cell line.
P815 cells treated with different concentrations of essential oils from aerial parts (EOAP), leaves (EOL) and capitulum (EOC). Bars show the mean percentage ± SD. *P<0.05.
Fig 4. Cytotoxic effect of Artemisia herba alba essential oils on BSR cell line.
BSR cells treated with different concentrations of essential oils from aerial parts (EOAP), leaves (EOL) and capitulum (EOC) of Artemisia herba alba for 48h. Bar graph shows the mean percentage ± SD. *P < 0.05.
On basis of literature data it is possible to hypothesize that the cytotoxic effects exhibited by essential oils, under these experimental conditions, could be related to an overall action of the compounds present in oils, especially to sesquiterpene compounds [41, 42]. The essential oil of leaves, which presents a higher concentration of sesquiterpenes (68.93%) and particularly oxygenated sesquiterpenes (39.89%), has been shown to be more active than other essential oils extracted from Artemisia herba alba. In fact, the anticancer activities of sesquiterpenes have been reported in the literature. It has been found that α-humulene is active against A-549, DLD-1 and LNCaP cell lines [43, 44, 45]. Also, caryophyllene exhibited antiproliferative activity against K562 cell [46]. Therefore, our results on tumor cell lines suggest that the anticancer activity of the essential oils from different biological parts of Artemisia herba alba may be related to active sesquiterpenes associated to the synergism of other natural products present in the essential oil composition.
The difference in cytotoxic activity of essential oils extracted from different biological parts, indicate that cancer cell lines studied differ with respect to their sensitivity to the substances contained in the essential oils of Artemisia herba alba and the molecular characteristics of the cells as well. A study of cytotoxicity induced by different essential oils in Saccharomyces cerevisiae shows that these cells are more sensitive to Artemisia herba Alba, this cytotoxicity is accompanied by the induction of cytoplasmic mutation, indicating mitochondrial damage and impairment of oxidative metabolism [47]. In our study, although the essential oils of some parts of the plant not shown a cytotoxic effect against the PBMCs (EOC, and EOL), some of them demonstrate a cytotoxic effect on PBMCs (EOAP). On the other hand, the microscopic comparison of the viable cancer cells and the PBMCs treated with 50μg/mL by the essential oils from leaves and capitulum (EOC and EOL), shown after 24h lysis of tumor cell lines (70%) using the bleu trypan exclusion method. Whereas, the PBMCs don’t demonstrate the bleu coloration reflecting their viability, this observation was not shown after treatment with the EOAP which demonstrate a lysis effect toward the PBMCs (Fig 5). Moreover, Tilaoui et al, (2011) reported the cytotoxic activity of aerial part essential oil of Artemisia herba alba on CEM cancer cell lines with IC50 = 6μg/mL [40]. The cytotoxicity of Artemisia herba alba essential oils could be related to the production of reactive oxygen species (ROS). It is reported that exposure to essential oils strongly affects the cell wall and membranes and damages mitochondria. This may lead to mitochondrial dysfunction and to a radical burst of reactive oxygen species that triggers gene induction and apoptotic cell death [48, 49]. In fact, mitochondrial dysfunction is known to increase intracellular concentrations of DNA-damaging species such as superoxide and peroxide ions linked to apoptotic death [50, 51].
Fig 5. Effect of Artemisia herba alba essential oils against the PBMC.
PBMC were prepared from human normal donors by Ficoll-hypaque density centrifugation. Cells were incubated in 96-well microtiter plates in the presence of different essential oils (essential oil of leaves: EOL, essential oils of capitulum: EOC and essential oils of aerial parts: EOAP) at different concentrations (0, 4 μg/ml–50μg/ml). After 48 h incubation, viability was determined using MTT assay as described in materials and methods. Bar graph shows the mean percentage ± SD. *P < 0.05.
Earlier studies have shown that plants are known to display variation in the concentration of the bioactive phytochemicals depending on intrinsic factors like the age of the plant, and on extrinsic factors like the geographical climate, circadian rhythm, the nature of the soil, and the season. The cytotoxic activity of essential oils might be due to the synergic effects of all the terpenes in the essential oil, or perhaps there are some other active compounds responsible for the antiproliferative activity of the essential oil, which deserves attention in any future study [52, 53]. However, it must be kept in mind that essential oils are complex mixtures of numerous molecules, and one might wonder if their biological effects are the result of synergism among all molecules or reflect only the activities of the main molecules present at the highest concentrations according to the gas chromatographic analysis. Generally, the major components are found to reflect quite well the biophysical and biological features of the essential oils from which they were isolated, with the amplitudes of their effects being dependent on their concentration when they are tested on their own or in essential oils [54]. Also, the differential effects observed between cancer cell lines could be related on the target of these cells, as P815 is a suspension cell line and BSR is an adherent cell lines. Our result is in accordance with Kumura et al, (2004), who demonstrate that inhibition of cell proliferation by Methotrexate is different in suspension (FM3A, 2B4 and THP-1) and adherent (NIH3T3 and V79) cells [55].
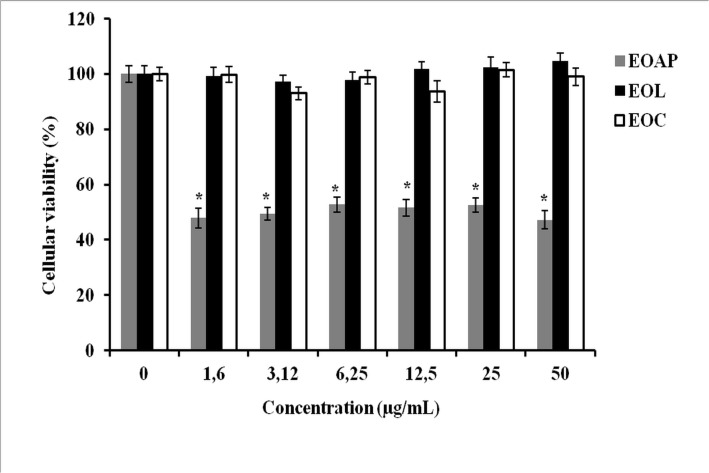
Effect of Artemisia herba alba essential oils against PBMCs
The majority of clinically approved anticancer drugs are characterized by a narrow therapeutic window that results mainly from a high systemic toxicity of the drugs in combination with an evident lack of tumor selectivity [56]. In this connection, we tested our essential oils against the human peripheral blood mononuclear cells (PBMC) in order to determine their effects against normal cells. The results obtained are represented in Fig 5. It is depicted in this figure that the essential oil of aerial parts causes around 50% of cytotoxicity into PBMC. However, the essential oils of leaves and capitulum were shown at a concentration as being able to induce a cytotoxic activity against tumor cells (P815 and BSR); no cytotoxicity effect was observed (Fig 6) but, instead of that, leaves and capitulum oils produced a slight proliferative effect on normal PBMC with 105% and 102% of viability (P<0.05) after 48 h of treatment for leaves and capitulum respectively. (Fig 5). The differential cytotoxic effect toward PBMCs and cancer cells (Fig 6) could be explained by the difference of the metabolism of each cell line type. Also, it might be due to the differential expression of the genes and consequently the modulation of the activity of essential oils. Similar observations in vivo are also known, where it has been seen that a plant extract exhibits antiperoxidative and pro-oxidative effect for cardiac and liver tissues respectively [57].
Fig 6. Effect of essential oils from different organs plant of Artemisia herba alba against normal cells (PBMCS) and cancer cell lines (P815, BSR).
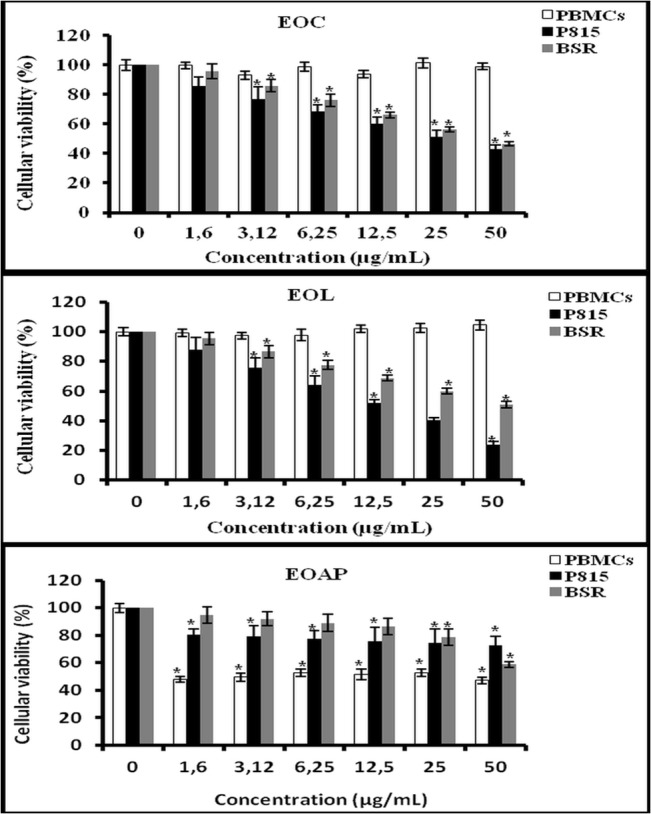
EOC: essential oils of capitulum; EOL: essential oils of leaves; EOAP: essential oils of aerial parts (mixture of leaves and capitulum). Bar graph shows the mean percentage ± SD. *P < 0.05.
Conclusion
In Summary, the present work confirms the very important chemical variability in Artemisia herba alba essential oils. As far as we could investigate, such study can be considered as the deep detailed report on the chemical composition and the in vitro anticancer activity of the essential oils extracted from stems, leaves, capitulum and mixture of capitulum and leaves of Artemisia herba alba. Furthermore, this study identifies new molecules that have not been cited in other studies and the possible characterization of new chemotypes of Artemisia herba alba essential oils. The antitumor activity of the essential oils from different biological parts of Artemisia herba alba has been determined comprehensively against two cancer cells lines P815 and BSR. Overall, P815 cell lines are more sensitive than BSR cell lines to essential oils effect; our results suggest that leaves and capitulum essential oils are more cytotoxic than aerial parts studied. However, no cytotoxic effect of these essential oils was observed on the human normal cells, apart from a slight proliferative effect. It seems likely that Artemisia herba alba could play an important role in combating cancer. Clearly, determining the bioactive compound(s) in Artemisia herba alba essential oils that inhibit cancer cell lines proliferation will need further investigation, to clarify the mechanism of action and their potential use.
Acknowledgments
The authors would like to thank Prof. Khalid Chaouch (Department of English, Faculty of Literature and Human Sciences) for reviewing the manuscript.
Data Availability
All relevant data are within the paper.
Funding Statement
The work was funded by a grant from the CNRST (PROTARSIII, D61/07), Rabat, Morocco.
References
- 1. Belhattab R, Amor L, Barroso JG, Pedro LG, Cristina Figueiredo A (2014) Essential oil from Artemisia herba-alba Asso grown wild in Algeria: Variability assessment and comparison with an updated literature survey. Arab J Chem 7: 243–251. [Google Scholar]
- 2. Vernin G, Merad O, Vernin GMF, Zamkotsian RM, Parkanyi C (1995) GC-MS analysis of Artemisia herba alba Asso essential oils from Algeria. Dev Food Sci 37: 147–205. [Google Scholar]
- 3. Bailey C, Danin A (1981) Bedouin plant utilization in Sinai and the Negev. Econ Bot 35: 145–162. [Google Scholar]
- 4. Jouad H, Haloui M, Rhiouani H, El Hilaly J, Eddouks M (2001) Ethnobotanical survey of medicinal plants used for the treatment of diabetes, cardiac and renal diseases in the North centre region of Morocco (Fez–Boulemane). J Ethnopharmacol 77: 175–182. [DOI] [PubMed] [Google Scholar]
- 5. Yashphe J, Segal R, Breuer A, Erdreich-Naftali G (1979) Antibacterial activity of Artemisia herba-alba. J Pharm Sci 68: 924–925. [DOI] [PubMed] [Google Scholar]
- 6.Hatimi S, Boudouma M, Bichichi M, Chaib N, Idrissi NG (2000) Evaluation in vitro of antileishmanien activity of Artemisia herba-alba Asso. Franco-African meeting of pediatrics. pp. 57–70.
- 7. Khafagy SM, Gharbo SA, Sarg TM (1971) Phytochemical investigation of Artemisia herba alba. Planta Med 20: 90–96. [DOI] [PubMed] [Google Scholar]
- 8. Feuerstein I, Danin A, Segal R (1988) Constitution of the essential oil from an Artemisia herba-alba population of Spain. Phytochemistry 27: 433–434. [Google Scholar]
- 9.Sandra P, Bicchi C (1987) Capillary gas chromatography in essential oil analysis. Available: http://agris.fao.org/agris-search/search.do?recordID=US201300603328.
- 10. Ouyahya A, Negre R, Viano J, Lozano YF, Gaydou EM (1990) Essential oils from Moroccan Artemisia negrei, A. mesatlantica and A. herba alba. Lebensm-Wiss Technol 23: 528–530. [Google Scholar]
- 11. Salido S, Valenzuela LR, Altarejos J, Nogueras M, Sánchez A, Cano E (2004) Composition and infraspecific variability of Artemisia herba-alba from southern Spain. Biochem Syst Ecol 32: 265–277. [Google Scholar]
- 12. Hudaib MM, Aburjai TA (2006) Composition of the essential oil from Artemisia herba-alba grown in Jordan. J Essent Oil Res 18: 301–304. [Google Scholar]
- 13.Lamiri A, Belanger A, Berrada M, Zrira S, Benjilali B (1997) Chemical polymorphism of Artemisia herba-alba Asso from Morocco. Rabat Maroc: 69–79.
- 14. Dob T, Benabdelkader T (2006) Chemical composition of the essential oil of Artemisia herba-alba Asso grown in Algeria. J Essent Oil Res 18: 685–690. [Google Scholar]
- 15. Akrout A (2004) Essential oil study of some pastoral plants from Matmata (south Tunisia). Cah Options Med 62: 289–292. [Google Scholar]
- 16. Aicha N, Ines S, Mohamed BS, Ines B, Soumaya K, Kamel G, et al. (2008) Chemical composition, mutagenic and antimutagenic activities of essential oils from (Tunisian) Artemisia campestris and Artemisia herba-alba. J Essent Oil Res 20: 471–477. [Google Scholar]
- 17. Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65: 55–63. [DOI] [PubMed] [Google Scholar]
- 18. Mohsen H, Ali F (2009) Essential oil composition of Artemisia herba-alba from southern Tunisia. Molecules 14: 1585–1594. 10.3390/molecules14041585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benjilali B, Richard H, Liddle P (1985) Chémotypes d’Armoise blanche du Maroc Artemisia herba-alba. Federagrario Torino. 131–151 p.
- 20. Benjilali B, Richard H (1980) Etude de quelques peuplements d’Armoise blanche du Maroc Artemisia herba-alba. Riv Ital EPPOS 62: 69–74. [Google Scholar]
- 21. Benjilali B, Sarris J, Richard H (1982) Nouveaux chémotypes d’Artemisia herba-alba. Sci Aliment 2: 515–527. [Google Scholar]
- 22. Boutekedjiret C, Charchari S, Belabbes R, Bessiere JM (1992) Contribution à l’étude de la composition chimique de l’huile essentielle d’Artemisia herba-alba Asso. Riv Ital EPPOS 3: 39–42. [Google Scholar]
- 23. Fleisher Z, Fleisher A, Nachbar RB (2002) Chemovariation of Artemisia herba alba Asso. Aromatic Plants of the Holy Land and the Sinai. Part XVI. J Essent Oil Res 14: 156–160. [Google Scholar]
- 24. Soliman MM (2007) Phytochemical and toxicological studies of Artemisia L.(Compositae) essential oil against some insect pests. Arch Phytopathol Plant Prot 40: 128–138. [Google Scholar]
- 25. Lawrence BM (1988) Armoise oil. Natural flavour and fragrance materials. Perfum Flavorist Ed Essent Oils 1991: 52–54. [Google Scholar]
- 26. Segal R, Feuerstein I, Danin A (1987) Chemotypes of Artemisia herba-alba in Israel based on their sesquiterpene lactone and essential oil constitution. Biochem Syst Ecol 15: 411–416. [Google Scholar]
- 27. El-Sayed AM, Seida AA (1990) Comparative study of the major constituents of the essential oils of wild and cultivated Egyptian Artemisia herba-alba with those of plants produced abroad. Bull Fac Pharm 28: 57–58. [Google Scholar]
- 28. Wiseman DA, Werner SR, Crowel PL (2007) Cell cycle arrest by the isoprenoids perillyl alcohol, geraniol, and farnesol is mediated by p21Cip1 and p27Kip1 in human pancreatic adenocarcinoma cells. J Pharmacol Exp Ther 320: 1163–1170. [DOI] [PubMed] [Google Scholar]
- 29. Isaac O (1979) Pharmacological investigations with compounds of chamomile on the pharmacology of (-)-alpha-bisabolol and bisabolol oxides. Planta Med 35:118–124. [DOI] [PubMed] [Google Scholar]
- 30. Bandoni A, (2000) Los Recursos Vegetales Aromáticos en Latinoamérica Editorial de laUniversidad Nacional de La Plata; Argentina, p. 417 [Google Scholar]
- 31. Loziene K, Venskutonis PR (2005) Influence of environmental and genetic factors on the stability of essential oil composition of Thymus pulegioides. Biochem Syst Ecol 33: 517–525. [Google Scholar]
- 32. Clark RJ, Menary RC (1980) Environmental effects on peppermint (Mentha piperita L.). II. Effects of temperature on photosynthesis, photorespiration and dark respiration in peppermint with reference to oil composition. Funct Plant Biol 7: 693–697. [Google Scholar]
- 33. Jaafari A, Mouse HA, M’Bark LA, Tilaoui M, Elhansali M, Lepoivre M, et al. (2009) Differential antitumor effect of essential oils and their major components of Thymus broussonettii: relationship to cell cycle and apoptosis induction. Herba Pol 55: 36–50. [Google Scholar]
- 34. Jaafari A, Mouse HA, Rakib EM, Tilaoui M, Benbakhta C, Boulli A, et al. (2007) Chemical composition and antitumor activity of different wild varieties of Moroccan thyme. Rev Bras Farmacogn 17: 477–491. [Google Scholar]
- 35. Robles C, Garzino S (2000) Infraspecific variability in the essential oil composition of Cistus monspeliensis leaves. Phytochemistry 53: 71–75. [DOI] [PubMed] [Google Scholar]
- 36. Burbott AJ, Loomis WD (1967) Effects of light and temperature on the monoterpenes of peppermint. Plant Physiol 42: 20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Staroscik JA, Wilson AA (1982) Seasonal and regional variation in the quantitative composition of cold-pressed lemon oil from California and Arizona. J Agric Food Chem 30: 835–837. [Google Scholar]
- 38. Razafimamonjison G, Jahiel M, Duclos T, Ramanoelina P, Fawbush F, Danthu P (2014) Bud, leaf and stem essential oil composition of Syzygium aromaticum from Madagascar, Indonesia and Zanzibar. Int J Basic App Sci 3: 224–233. [Google Scholar]
- 39. Marzoug BHN, Romdhane M, Lebrihi A, Mathieu F, Couderc F, Abderraba M, et al. (2011) Eucalyptus oleosa essential oils: chemical composition and antimicrobial and antioxidant activities of the oils from different plant parts (Stems, Leaves, Flowers and Fruits). Molecules 16: 1695–1709 10.3390/molecules16021695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tilaoui M, Mouse HA, Jaafari A, Aboufatima R, Chait A, Zyad A (2011) Chemical composition and antiproliferative activity of essential oil from aerial parts of a medicinal herb Artemisia herba-alba. Rev Bras Farmacogn 21: 781–785. [Google Scholar]
- 41. Sharma A, Bajpai VK, Shukla S (2013) Sesquiterpenes and Cytotoxicity Natural Products. Springer; pp. 3515–3550. Available: http://link.springer.com/10.1007/978-3-642-22144-6_152. Accessed 18 June 2014. [Google Scholar]
- 42. Tilaoui M, Mouse HA, Jaafari A, Zyad A (2014) Differential Effect of Artemisinin Against Cancer Cell Lines. Nat Prod Bioprospecting 4: 189–196. 10.1007/s13659-014-0024-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Legault J, Pichette A (2007) Potentiating effect of β-caryophyllene on anticancer activity of α-humulene, isocaryophyllene and paclitaxel. J Pharm Pharmacol 59: 1643–1647. [DOI] [PubMed] [Google Scholar]
- 44. Sylvestre M, Pichette A, Longtin A, Nagau F, Legault J (2006) Essential oil analysis and anticancer activity of leaf essential oil of Croton flavens L. from Guadeloupe. J Ethnopharmacol 103: 99–102. [DOI] [PubMed] [Google Scholar]
- 45. Loizzo MR, Tundis R, Menichini F, Saab AM, Statti GA, Menichini F (2007) Cytotoxic activity of essential oils from Labiatae and Lauraceae families against in vitro human tumor models. Anticancer Res 27: 3293–3299. [PubMed] [Google Scholar]
- 46. Lampronti I, Saab AM, Gambari R (2006) Antiproliferative activity of essential oils derived from plants belonging to the Magnoliophyta division. Int J Oncol 29: 989–996. [PubMed] [Google Scholar]
- 47. Bakkali F, Averbeck S, Averbeck D, Zhiri A, Idaomar M (2005) Cytotoxicity and gene induction by some essential oils in the yeast Saccharomyces cerevisiae. Mutat Res Toxicol Environ Mutagen 585: 1–13. [DOI] [PubMed] [Google Scholar]
- 48. Ferguson LR, Von Borstel RC (1992) Induction of the cytoplasmic “petite”mutation by chemical and physical agents in Saccharomyces cerevisiae. Mutat Res Mol Mech Mutagen 265: 103–148. [DOI] [PubMed] [Google Scholar]
- 49. Krems B, Charizanis C, Entian K-D (1995) Mutants of Saccharomyces cerevisiae sensitive to oxidative and osmotic stress. Curr Genet 27: 427–434. [DOI] [PubMed] [Google Scholar]
- 50. Ehlers RA, Hernandez A, Bloemendal LS, Ethridge RT, Farrow B, Evers BM (1999) Mitochondrial DNA damage and altered membrane potential (ΔΨ) in pancreatic acinar cells induced by reactive oxygen species. Surgery 126: 148–155. [PubMed] [Google Scholar]
- 51. Weiss CN, Ito K (2014) DNA damage response, redox status and hematopoiesis. Blood Cells Mol Dis 52: 12–18. 10.1016/j.bcmd.2013.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jaafari A, Tilaoui M, Mouse HA, M’bark LA, Aboufatima R, Chait A, et al. (2012) Comparative study of the antitumor effect of natural monoterpenes: relationship to cell cycle analysis. Rev Bras Farmacogn 22: 534–540. [Google Scholar]
- 53. Buhagiar JA, Podesta MT, Wilson AP, Micallef MJ, Ali S (1998) The induction of apoptosis in human melanoma, breast and ovarian cancer cell lines using an essential oil extract from the conifer Tetraclinis articulata. Anticancer Res 19: 5435–5443. [PubMed] [Google Scholar]
- 54. Ipek E, Zeytinoglu H, Okay S, Tuylu B A, Kurkcuoglu M, Baser HCK (2005) Genotoxicity and antigenotoxicity of Origanum oil and carvacrol evaluated by Ames almonella/microsomal test. Food Chemistry 93: 551–556. [Google Scholar]
- 55. Kimura E, Nishimura K, Sakata K, Oga S, Kashiwagi K, Igarashi K (2004) Methotrexate differentially affects growth of suspension and adherent cells. Int J Biochem Cell Biol. 36:814–25. [DOI] [PubMed] [Google Scholar]
- 56. Kratz F, Müller IA, Ryppa C, Warnecke A (2008) Prodrug strategies in anticancer chemotherapy. ChemMedChem 3: 20–53. [DOI] [PubMed] [Google Scholar]
- 57. Parmar HS, Panda S, Jatwa R, Kar A (2006) Cardio-protective role of Terminalia arjuna bark extract is possibly mediated through alterations in thyroid hormones. Pharmazie 61: 793–795. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.