Abstract
Physiological angiogenesis depends on the highly coordinated actions of multiple angiogenic regulators. CCN1 is a secreted cysteine-rich and integrin-binding matricellular protein required for proper cardiovascular development. However, our understanding of the cellular origins and activities of this molecule is incomplete. Here, we show that CCN1 is predominantly expressed in angiogenic endothelial cells (ECs) at the leading front of actively growing vessels in the mouse retina. Endothelial deletion of CCN1 in mice using a Cre-Lox system is associated with EC hyperplasia, loss of pericyte coverage and formation of dense retinal vascular networks lacking the normal hierarchical arrangement of arterioles, capillaries and venules. CCN1 is a product of an immediate-early gene that is transcriptionally induced in ECs in response to stimulation by vascular endothelial growth factor (VEGF). We found that CCN1 activity is integrated with VEGF receptor 2 (VEGF-R2) activation and downstream signaling pathways required for tubular network formation. CCN1-integrin binding increased the expression of and association between Src homology 2 domain-containing protein tyrosine phosphatase-1 (SHP-1) and VEGF-R2, which leads to rapid dephosphorylation of VEGF-R2 tyrosine, thus preventing EC hyperproliferation. Predictably, CCN1 further brings receptors/signaling molecules into proximity that are otherwise spatially separated. Furthermore, CCN1 induces integrin-dependent Notch activation in cultured ECs, and its targeted gene inactivation in vivo alters Notch-dependent vascular specification and remodeling, suggesting that functional levels of Notch signaling requires CCN1 activity. These data highlight novel functions of CCN1 as a naturally optimized molecule, fine-controlling key processes in physiological angiogenesis and safeguarding against aberrant angiogenic responses.
KEY WORDS: CCN1, Matricellular, Retinal angiogenesis, VEGF, Knockout mouse
Summary: Mice lacking endothelial CCN1 show severe defects in retinal vessel network formation and barrier integrity, associated with endothelial cell hyperproliferation.
INTRODUCTION
CCN1 (CYR61 – Mouse Genome Informatics) is a member of the Cysteine-rich protein 61/Connective tissue growth factor/Nephroblastoma-overexpressed (CCN) family of secreted matricellular proteins. CCN1 protein expression is crucial for successful cardiovascular development and embryonic viability (Mo and Lau, 2006). CCN1-null embryos are defective in vessel bifurcation at the chorionic plate, leading to a paucity of sprouting vessels that penetrate into the labyrinth and thus, an undervascularized placenta. In adults, the expression of CCN1 is associated with sites of angiogenesis and inflammation, such as in wound healing, arthritis, tumors and vessels damaged by angioplasty or atherosclerosis (Brigstock, 2003; Lee et al., 2007).
CCN1 is encoded by an inducible immediate-early gene, which is transcriptionally activated when responsive cells are stimulated by growth or mechanical factors (Chaqour, 2013; Huang et al., 2007). The encoded protein is organized into four conserved modular domains that contain binding sites for integrins, including ανβ3, ανβ5, ανβ1 and α6β1, heparan sulfate proteoglycans, growth factors and extracellular matrix (ECM) proteins (Holbourn et al., 2008; Jun and Lau, 2011). Through interactions with these molecules, CCN1 modulates cell adhesion, migration, proliferation, differentiation and even cell lineage commitment (Hasan et al., 2011; Yang et al., 2008). In the rabbit ischemic hind limb and rat cornea models, CCN1 promotes microvessel growth and improves collateral blood flow to an even larger extent than vascular endothelial growth factor (VEGF) (Hinkel et al., 2014; Rayssac et al., 2009). However, several key issues remain unresolved, including how this protein regulates vascular cell function and behavior in vivo.
VEGF is a potent, diffusible, endothelial-specific mitogen that is released in response to hypoxia, and, upon binding to the VEGF receptor 2 (VEGF-R2), expressed by the vascular endothelium, elicits angiogenesis (Bautch, 2012). Similarly, the pathologic transformations of the retinal vasculature seen in intraocular vascular disease are associated with increased expression of and signaling through VEGF-R2 (Lutty et al., 2006; Miller et al., 2013). Vascular growth and morphogenesis are initiated, at least in part, by VEGF-derived signals and ECM-integrin-cytoskeletal signaling, which promote simultaneous sprouting (i.e. cord formation) and luminogenesis of endothelial cell (EC)-lined tubes (Bautch, 2012). In both normal and pathological angiogenesis, sprouts are headed by migrating endothelial tip cells, which signal to their adjacent stalk ECs to downregulate their tip cell markers through Notch-mediated lateral inhibition (Siemerink et al., 2013). The VEGF/Notch pathways establish an adequate ratio between stalk and tip cell populations (Lobov et al., 2007). However, despite the prominent role of VEGF and Notch, their signaling mechanisms are context-dependent, suggesting that additional modulators and potential targets are yet to be identified (De Bock et al., 2013).
Here, we provide the first evidence that CCN1 regulates tissue-stage-specific functions during retinal vascular development. We uncovered unique sets of relevant genes targeted by CCN1 and identified a CCN1-based mechanism crucial for the coordinated regulation of retinal vascular network formation.
RESULTS
CCN1 expression is associated with angiogenic cell behavior
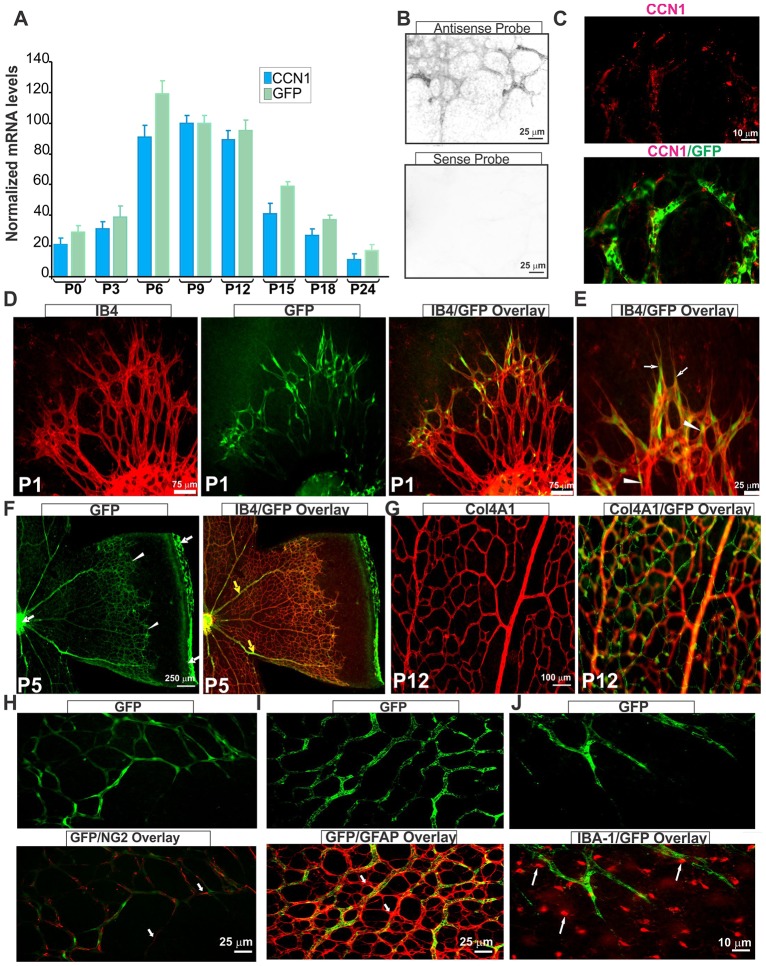
To determine the spatio-temporal pattern and site of action of CCN1 during retinal vessel development, we used a CCN1:GFP reporter transgenic mouse line in which the GFP reporter gene was placed downstream of a large CCN1 promoter segment. As shown in Fig. 1A, both endogenous CCN1 and GFP transcript levels increased progressively between P0 and P12 during the budding of superficial vessels and when the secondary deep layers of the retinal vasculature grow radially outward toward the inner nuclear and outer plexiform layers of the retina. CCN1 and GFP mRNA levels then progressively declined and became barely detectable at postnatal day (P) 24, when the retinal vasculature was completely formed. In situ hybridization showed that signal with an antisense CCN1 probe was associated with the vasculature (Fig. 1B). Immunostaining with an anti-CCN1 antibody showed that CCN1 localizes pericellularly (Fig. 1C), probably due to its strong heparin-binding activity, which is consistent with previous data (Latinkic et al., 2001). At P1, GFP localized in the leading tip cells that possess numerous filopodia but also in the trailing stalk ECs in IB4-stained retinas (Fig. 1D,E). At subsequent developmental stages (e.g. P5), CCN1:GFP expression became increasingly enriched in and restricted to the cells of the advancing vascular front (Fig. 1F). CCN1:GFP signal then became barely detectable in the completely formed vessels following pericyte recruitment and basement membrane formation, although the signal persists in large veins but not in the arteries. CCN1:GFP expression reappeared again when the capillary beds begin to grow out from the superficially formed capillaries in the plexus (Fig. 1G). NG2+ pericytes exhibited little GFP fluorescence, which was undistinguishable from endothelial GFP given their abluminal localization (Fig. 1H). Neither glial fibrillary acidic protein (GFAP)- nor IBA-1+ microglial cells appeared to express the CCN1:GFP transgene (Fig. 1I,J), suggesting that CCN1 expression is largely associated with the angiogenic phenotype of ECs.
Fig. 1.
CCN1 is expressed in endothelial cells in angiogenic vasculature. (A) Analysis of CCN1 and GFP transcript levels of CCN1:GFP reporter transgenic mice during postnatal development of the retinal vasculature. CCN1 and GFP mRNA levels as determined by qPCR were normalized to those of the acidic ribosomal phosphoprotein (ARBP) (n=5). (B) In situ hybridization of CCN1 sense and antisense probes in P4 flat-mounted mouse retinas, showing positive signal with the antisense probe in the retinal vasculature. (C) Immunohistochemical localization of the CCN1 protein in P4 flat-mounted retina of CCN1:GFP reporter mice. Images show CCN1 and merged CCN1/GFP signals. (D,E) Flat-mounted retinas stained with isolectin B4 (IB4; red). Images depict IB4, GFP and/or merged IB4/GFP staining. Arrows and arrowheads indicate CCN1:GFP reporter signals in endothelial tip and stalk cells, respectively. (F,G), Immunostaining of flat-mounted retinas of CCN1:GFP reporter mice at P5 and P12. Note the increasingly enriched and restricted GFP signal to the advancing vascular front (white arrowheads) and large veins (yellow arrows). GFP signal can also be seen in the remnants of hyaloid vessels found around the optic nerve and at the edge of retinal leaflets (white arrows). In G, note the absence of CCN1:GFP signal in the superficial capillaries in the plexus visualized with anti-α1 type IV collagen (Col4A1) antibodies and increased CCN1:GFP signal in deeper plexuses. (H-J) Flat-mounted CCN1:GFP mouse retinas stained with anti-NG2 (H), anti-GFAP (I) and anti-IBA-1 (J) antibodies, depicting pericyte, astrocyte and microglia localization, respectively (white arrows).
Defects in retinal vessel migration, density and morphology in EC-specific CCN1-deficient mice
To examine the function of endothelial CCN1, we generated mice with EC-specific deletion of CCN1 by combining the Cdh5(PAC)-CreERT2 allele with CCN1flox (supplementary material Fig. S1A,B). CCN1/Cyr61 gene deletion in ECs was induced by administering 4-hydroxytamoxifen (4HT) into newborn CCN1flox/flox, Cdh5(PAC)-CreERT2-CCN1flox/+ and Cdh5(PAC)-CreERT2-CCN1flox/flox (hereafter referred to as CCN1+/+, iΔEC+/− and iΔEC−/−, respectively). The allele was effectively recombined in retinal ECs as shown by crossing mice with Cdh5(PAC)-CreERT2 and Gt(ROSA)26Sortm1Sor alleles (supplementary material Fig. S1D). Western blot analysis of the CCN1 protein in retinal protein lysates showed remarkably reduced levels of CCN1 protein levels in iΔEC−/− mice compared with CCN1+/+ mice (supplementary material Fig. S1C). Retinas from 4HT- or sunflower oil-injected littermate Cdh5(PAC)-CreERT2 exhibited a vascular phenotype identical to CCN1flox/flox mouse retinas and were used as controls in all experiments (Fig. 2A,B).
Fig. 2.
Retinal vascular abnormalities following EC-specific inactivation of the CCN1 gene. (A,B) Representative immunofluorescence images of whole-mounts of retinas of CCN1+/+, Cdh5-Cre and iΔEC−/− mice upon induction of EC-specific CCN1 deletion from P1 to P3 and analysis at P4. Staining was performed with either IB4 (a, b, c) or anti-Col4A1 antibody (d), which also allows visualization of the retinal vasculature. (C-E) Analysis and quantification of vascular parameters of representative retinas from CCN1+/+ and iΔEC−/− mice at P4 using the AngioTool software. Fields of view at the sprouting vascular front of the retinal vascular networks from control and mutant mice included regions of capillary-sized vessels directly adjacent to radial arterioles. Graphical representation of the analysis of the vascular area, branching index, lacunarity and vascular progression are shown in C, D, E and F, respectively. **P<0.01, ***P<0.001 versus CCN1+/+ (n=4-5).
Tamoxifen-induced recombination in the retinal endothelium resulted in severe vascular defects, including formation of a dense immature network lacking a normal hierarchical organization of blood vessels (Fig. 2A-C). Enlargement and apparent coalescence of retinal vessels were evident, causing loss of specific features of vascular architecture in iΔEC−/− mice. A relatively high recombination efficiency (65±21%) was uniformly associated with these vascular defects, whereas lower efficiencies (<25%) resulted in a wild-type-like vascular phenotype. The iΔEC−/− mutant mouse retinas displayed a striking vascular dysmorphology, as all types of blood vessels were enlarged and anastomosed randomly with each other. The percentage of the area covered by vessels and the branching index were significantly increased after loss of CCN1, suggesting severe defects in vascular remodeling (Fig. 2D,E). Conversely, vessel lacunarity, the distribution of gaps/lacunae surrounding blood vessels (Gould et al., 2011), was significantly diminished, suggesting altered vessel morphogenesis and tubulogenesis. At P8, the progression of the vascular plexus to the retinal periphery was markedly delayed in iΔEC−/− mouse retinas compared with age-matched control retinas (Fig. 2F). However, retinal ECs of iΔEC−/− mice associate with GFAP+ astrocytes in a manner closely resembling wild-type retinas, as both showed filopodial alignment along astrocyte processes (supplementary material Fig. S2A). Fibronectin is produced largely by astrocytes and accumulates in the vascular front, forming a trail-like network that guide migration of the endothelial tip cells (Stenzel et al., 2011); and its expression was not significantly altered after endothelial loss of CCN1 (supplementary material Fig. S2B). However, fewer NG2+ pericytes were recruited to the vasculature of iΔEC−/− mice, providing little mural cell coverage over the enlarged vessels (supplementary material Fig. S3A). Electron microscopy further showed that, whereas the wild-type mouse capillaries were adequately sized (<10 mm of diameter), covered by a continuous basement membrane and mural cells, those of iΔEC−/− retinas vessels exhibited an altered morphology with no pericyte coverage and in some cases a collapsed lumen and local edema (supplementary material Fig. S3B). An overt extravasation of systemically injected fluorescein isothiocyanate (FITC)-albumin was observed in iΔEC−/− retinal vessels compared with the wild-type counterparts (supplementary material Fig. S4A,B). Thus, loss of CCN1 resulted in a breach of barrier function of retinal vessels.
Loss of CCN1 induces EC hyperproliferation by increasing VEGF-R2 activation
EC proliferation enables sprout growth in length and diameter and both specification and proliferation of ECs in emerging sprouts are coordinated by the VEGF/VEGF-R2 and Notch signaling pathways. Therefore, we first monitored cell growth within 300-400 μm of the leading edge of the growing plexus, using BrdU labeling. BrdU+ cells at the angiogenic front showed a fourfold increase of retinal EC proliferation in iΔEC−/− mouse retinas compared with that of CCN1+/+ mice (Fig. 3A,B). The IB4+ regions in whole-mount retinas were appreciably similar to those of type IV collagen immunoreactivity in both iΔEC−/− and control littermates, although empty sleeves of matrix deposits rich in collagen IV were reduced in iΔEC−/− compared with control littermates (Fig. 3C,D). Thus, loss of CCN1 promoted excessive incorporation of ECs into the wall of preformed tubes instead of new sprouts, suggesting dysregulated proliferation and remodeling.
Fig. 3.
Loss of CCN1 induces EC hyperproliferation. (A) Immunofluorescence images showing BrdU incorporation (green) together with IB4 staining (red) in the retina at P6 of CCN1+/+, iΔEC+/− and iΔEC−/− mice. (B) Quantitative analysis of EC proliferation at P6 as measured by counting BrdU+ nuclei. Equivalent areas of retinas from CCN1+/+, iΔEC+/− and iΔEC−/− mice were compared. Data are means±s.e.m. *P<0.05 versus CCN1+/+; **P<0.001 versus CCN1+/+ (n=5). (C) Whole-mount α1 type IV collagen (Col4A1, red) and isolectin B4 (IB4, green) staining of P5 retinas from CCN1+/+ control and iΔEC−/− mice. (D) Quantitation of empty collagen IV sleeves in iΔEC−/− and littermate control retinal vasculature. *P<0.05 versus CCN1+/+ (n=5).
Next, we determined the expression pattern of the different isoforms of VEGF and their receptors. VEGF-A, VEGF-B and VEGF-C mRNA levels were not significantly altered upon endothelial-specific CCN1 deletion, indicating that increased EC growth was not caused by increased tissue hypoxia or VEGF expression (Fig. 4A). The expression of VEGF-R1 and VEGF-R2 was not altered either, but VEGF-R3 levels were slightly but significantly reduced in iΔEC−/− retinas compared with control retinas (Fig. 4B). Immunostaining of whole-retinal mount for VEGF-R3 corroborated the changes observed at the mRNA levels (supplementary material Fig. S5A,B). Similarly, transcript levels of VEGF-R3 were significantly reduced in iΔEC−/− lung tissue, which is particularly enriched with endothelial cells (supplementary material Fig. S5C). Interestingly, hyperproliferation of ECs in iΔEC−/− retinas correlated well with a significant increase of VEGF-R2 tyrosine phosphorylation at the Y1175 (pY1175) site of its kinase domain, whereas phosphorylation at the Y1214 site was not significantly altered (Fig. 4C,D). Phosphorylation at Y1175 supports EC survival and proliferation by inducing Akt and mitogen-activated protein kinase (MAPK) p44/42 phosphorylation, whereas phosphorylation at the Y1214 site promotes p38 activation and cell migration (Olsson et al., 2006). Thus, loss of CCN1 induced differential activation of the VEGF-R2 kinase that controls EC proliferation and tubulogenesis.
Fig. 4.

Loss of CCN1 potentiates VEGF receptor activation. (A,B) Quantification by qPCR of endogenous levels of VEGF-A, VEGF-B and VEGF-C (A) and of VEGF-R1, VEGF-R2 and VEGF-R3 (B) mRNAs in CCN1+/+, iΔEC+/− and iΔEC−/− mouse retinas. Transcript levels in non-mutant CCN1+/+ were set to 100% to facilitate comparisons and analyses among the three groups of mice. *P<0.05 versus CCN1+/+ (n=5). (C,D) VEGF-R2 activation upon endothelial-specific CCN1 deletion in mice. Lysates from retinas were analyzed by immunoprecipitation with an anti-VEGF-R2 antibody followed by immunoblotting with either anti-pY1175 or anti-pY1214 antibody. VEGF-R2 protein band in the total protein input was visualized with anti-VEGF-R2 antibody. Densitometric quantification of the phosphorylated and non-phosphorylated forms of VEGF-R2 using ImageJ software is shown in D. **P<0.05 versus CCN1+/+ (n=3).
CCN1 induces Src-homology 2-domain-containing protein tyrosine phosphatase (SHP)-1 expression and association with VEGF-R2
We further examined whether and how CCN1 directly influences VEGF-induced EC proliferation. Stimulation of cultured ECs with VEGF induced a rapid but transient expression of CCN1, the protein levels of which peaked within 2 h after cell stimulation by VEGF and decreased progressively thereafter (Fig. 5A). Cell transfection with CCN1 siRNA effectively reduced CCN1 protein levels by 64-95%. Stimulation with VEGF significantly increased EC growth rate (Fig. 5B). Paradoxically, VEGF-induced cell growth was further increased upon inhibition of CCN1 expression by siRNA. Stimulation of the cells with CCN1 alone through adenoviral gene transfer increased cell proliferation (Fig. 5C). However, EC growth was significantly reduced upon adenovirus-mediated co-expression of the CCN1 and Vegf genes, suggesting that CCN1 expression potentiates the mitogenic activity of VEGF. Analyses of VEGF-R2 tyrosine kinase activity showed that phosphorylation at the Y1175 site of the kinase domain of VEGF-R2 was enhanced upon CCN1 suppression by siRNA in VEGF-stimulated cells, whereas phosphorylation at the Y1214 site was reduced, suggesting that CCN1 differentially affected VEGF-R2 downstream signaling (Fig. 5D).
Fig. 5.
CCN1-induced SHP-1 expression/activity modulates VEGF-induced EC proliferation. (A,B) ECs were exposed to VEGF for increasing periods of time (A, left panel) in the presence or absence of a scrambled siRNA (siR-Scrbl) or siR-CCN1 (A, right panel). Protein lysates were analyzed by western immunoblotting. The proliferation rate was determined using the CyQUANT direct cell proliferation assay. *P<0.001 versus control; **P<0.005 versus VEGF+siR-Scrbl (n=3). (C) Cells were transduced with adenoviral vector expressing either GFP (Ad-GFP), VEGF (Ad-VEGF) or CCN1 (Ad-CCN1), and the proliferation rate was determined following cell transduction with either Ad-GFP, Ad-VEGF or Ad-CCN1 in serum-free medium for 16 h. *P<0.001 versus Ad-GFP; **P<0.05 versus Ad-VEGF (n=4). (D) VEGF-R2 phosphorylation at the Y1175 and Y1214 sites in cells treated as described in A,B. Cell lysates were analyzed by immunoprecipitation with an anti-VEGFR-2 antibody to determine total VEGF-R2 protein input. (E) SHP-1 and SHP-2 mRNA levels were determined by qPCR in cells transduced with either Ad-GFP or Ad-CCN1 and incubated for 16 h in serum-free medium. *P<0.001 versus Ad-GFP (n=3). (F,G) SHP-1 protein expression (F) and activity (G) in cells transfected with siR-Scrbl or siR-CCN1 and left untreated (Ctrl) or treated with VEGF. (H) Cells were transduced with Ad-CCN1 and/or Ad-VEGF and protein extracts were analyzed by western immunoblotting (upper panel). Immunoprecipitation of protein extracts with anti-SHP-1, and further detection with antibodies against VEGF-R2 or CCN1 is shown (lower panel). (I) Effect of function-blocking antibodies against different integrin subunits on the formation of the complex including VEGF-R2, SHP-1 and CCN1. Cells were incubated in serum-free medium with integrin-blocking antibodies for up to 4 h. Cell lysates were analyzed as described in H. (J-L) SHP-1 protein levels as determined by western blotting (J), densitometric scanning (K) and activity (L) in retinal lysates of CCN1+/+, iΔEC+/− and iΔEC−/− mice. **P<0.01 versus CCN1+/+ (n=3).
VEGF-R2 phosphorylation is regulated by members of the Src-homology 2-domain-containing protein tyrosine phosphatase (SHP) family of phosphatases (Nakamura et al., 2008). Dephosphorylation of VEGF-R2 at Y1175 is mediated by SHP-1, but not by SHP-2, another phosphatase of the SHP family. We determined the effects of CCN1 on the expression pattern of SHP-1 and SHP-2 in ECs. Adenovirus-mediated overexpression of CCN1 induced a ninefold increase of SHP-1 mRNA levels but had no effects on those of SHP-2 (Fig. 5E). Stimulation of cells with VEGF did not significantly affect SHP-1 levels. However, siRNA-mediated suppression of CCN1 markedly reduced SHP-1 protein levels in VEGF-stimulated cells (Fig. 5F). SHP-1 activity, which is regulated by both tyrosine and serine phosphorylation (Bhattacharya et al., 2008), was measured by immunoprecipitation and phosphatase assay. As shown in Fig. 5G, SHP-1 activity was significantly reduced upon suppression of CCN1 in VEGF-stimulated cells. The association of SHP-1 with VEGF-R2 was further determined by immunoprecipitation and western blot analysis in cells stimulated with CCN1 and/or VEGF. As shown in Fig. 5H, SHP-1 co-precipitated by both VEGF-R2 and CCN1 in cells co-expressing both CCN1 and VEGF. VEGF alone did not induce a detectable association of SHP-1 with VEGF-R2, suggesting that CCN1 acts, at least in part, by increasing the expression, activity and recruitment of SHP-1 to the VEGF-R2. In addition, neutralizing antibodies against β1 integrin abolished SHP-1 recruitment to the VEGF-R2 and the formation of the complex with CCN1, suggesting that SHP-1 activity is dependent on CCN1 signaling through β1 integrin binding.
We further determined the effects of EC-specific deletion of CCN1 on SHP-1 protein levels and activity in the retina. As shown in Fig. 5I-K, loss of CCN1 function was associated with 60% and 40% reduction of SHP-1 protein levels and activity, respectively, as compared with control CCN1+/+ mouse retinas. In transverse sections of retinas from CCN1+/+ mice, SHP-1 colocalized with EC endomucin, a typical endothelial marker, as well as in ganglion cell and outer nuclear layers (supplementary material Fig. S6; and see Lyons et al., 2006). Immunostaining with antibody against P-SHP-1, the active Y536 phosphorylated form of SHP-1, was predominantly associated with ECs, whereas little or no immunostaining was found in other cell types in control mouse retinas. P-SHP-1 immunostaining was either weak or undetectable in retinas from iΔEC−/− mice, further confirming that loss of CCN1 reduced SHP-1 expression in the endothelium. In agreement with previous reports (Pei et al., 1994), SHP-1 was catalytically inactive in the outer nuclear and ganglion cell layers as a consequence of Src homology 2 domain-mediated auto-inhibition. Thus, SHP-1 acts as a downstream target of CCN1 in the endothelium during retinal vascular development.
Endothelial-specific deletion of CCN1 altered Rho GTPase activation and MAPK signaling cascades
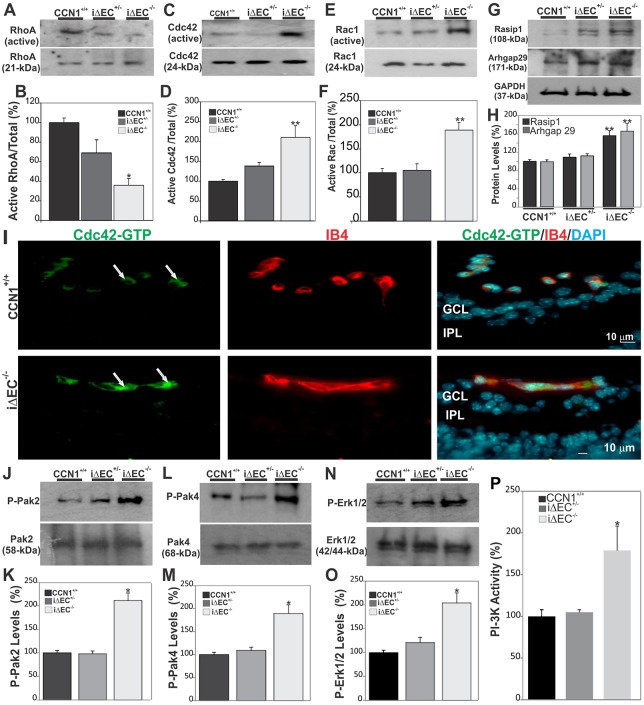
VEGF signaling regulates EC polarization and Rho GTPase-dependent non-muscle myosin-induced cell shape changes required for vessel luminization (Strilić et al., 2009). Thus, we determined whether enlargement and apparent coalescence of retinal vessels in CCN1 mutant retinas was concomitant with changes in the activation state of monomeric G-proteins Cdc42/Rac1/RhoA and their upstream and downstream signaling regulators. As shown in Fig. 6A,B, RhoA GTPase was weakly activated in control retinas, and its activated form was further reduced in iΔEC−/− mutant retinas. This is consistent with the notion that RhoA activity must be suppressed during vascular lumen formation, allowing for reduced internal cell contractility and for ECs to flatten and for lumen morphogenesis to occur (Koh et al., 2009). Conversely, CCN1 deletion resulted in a stronger activation of Cdc42 and Rac1 in iΔEC−/− mouse retinas (Fig. 6C-F). Cdc42 and Rac1 induce the formation of tube networks and prevent tube collapse by blocking the activation of RhoA and its downstream target ROCK (Davis et al., 2011). Immunostaining of transverse sections of retinas from control and iΔEC−/− mutant mice showed that the active Cdc42-GTP was intensely localized within endothelial tubes of CCN1 mutant mice (Fig. 6I). Meanwhile, Rho GTPases are directly regulated by the adaptor protein ras-interacting protein 1 (Rasip1), involved in cell spreading, and its interacting partner Rho GTPase-activating protein 29 (Arhgap29). In iΔEC−/− mouse retinas, the levels of Rasip1 and Arhgap29 were markedly increased (Fig. 6G,H). Rasip1, which is a specific endothelial marker, is an immediate specific target of VEGF-R2 signaling, as Rasip1 expression has been shown to be undetectable in VEGF-R2 null embryos lacking ECs (Xu et al., 2011). Rasip1 reduces the activity of Rho GTPases in part by recruiting Arhgap29 (Xu et al., 2009). Furthermore, the active phosphorylated forms of key GTPase effector kinases, such as pPak4 (activated by Cdc42), pPak2 (activated by Rac1 and Cdc42) and P-Erk1/2, were increased in iΔEC−/− retinas compared with those of CCN1+/+ mice (Fig. 6J-O). Similarly, the activity of phosphoinositide 3-kinase (PI3K), which signals to Akt1 by phosphorylating multiple angiogenic substrates (Lee et al., 2014), was significantly increased in iΔEC−/− mouse retinas (Fig. 6P). Thus, hyperactivation of the luminization signaling cascade downstream of VEGF-R2 in the absence of CCN1 induced aberrant tubulogenesis of the vasculature (supplementary material Fig. S8).
Fig. 6.
Loss of CCN1 potentiates VEGF-R2 downstream signaling through Rho GTAse and MAPK activation during retinal vessel formation. (A-F) Rho A, Cdc42 and Rac activation status as determined by GTPase assay in retinal extracts from wild-type, iΔEC+/− and iΔEC−/− mice. Protein band signals were normalized to total input of each GTPase (B,D,F). **P<0.01 versus CCN1+/+ (n=3). (G,H) Expression of signaling kinases (Rasip1 and Arhgap29) upstream of Cdc42/Rac1 GTPases. The same blots were stripped and washed before subsequent incubation with antibody against the indicated proteins. Experiments were performed on at least three different retinal lysate preparations with similar results. **P<0.05 versus CCN1+/+. (I) Effects of CCN1 deletion on the activation of Cdc42. Transverse sections of P6 retinas were labeled with either Cdc42-GTP-specific antibody or IB4. Note that the active Cdc42-GTP was largely localized within the vasculature of both control CCN1+/+ and iΔEC−/− mice (arrows). GCL, ganglion cell layer; IPL, inner plexiform layer. (J-O) Phosphorylation status of signaling kinases (Pak2, Pak4 and Erk1/2) downstream of Cdc42/Rac1 GTPases. Phosphorylated protein levels were normalized to those of the corresponding non-phosphorylated protein signal. *P<0.01 versus CCN1+/+. (P) PI3-K activity in retinal protein lysates as determined by PI3-K activity ELISA assay. *P<0.001 versus CCN1+/+ (n=4).
CCN1 regulates Dll4/Notch signaling
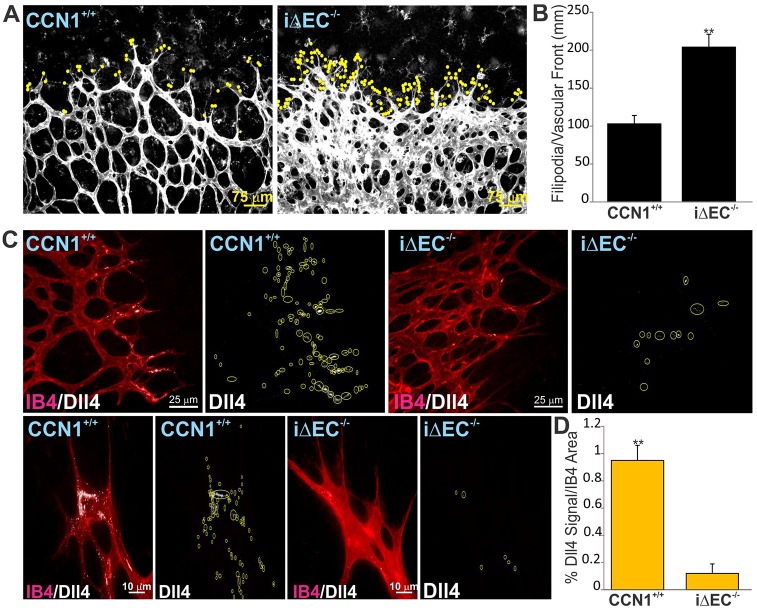
VEGF signaling also regulates phenotypical plasticity of ECs through inter-endothelial signaling via Dll4 and Notch (Erber et al., 2006). Endothelial deletion of CCN1 resulted in a 2.1-fold increase in the number of endothelial tip cells located at the retinal vascular edge compared with wild-type control retinas (Fig. 7A,B). Dll4 immunostaining was localized in tip cells but was also visible in adjacent stalk cells at the advancing front of the vascular edge, defined as the distal-most 20% of the vascular plexus (Fig. 7C). Dll4 protein distribution showed a typical sparse localization within the cytoplasm and along the edge of tip and stalk cells of retinal vessels. By contrast, Dll4 signal was conspicuously reduced or absent in tip and stalk cells at the edge of the growing plexus of iΔEC−/− mice, where retinal vessels coalesced into large flat sinuses (Fig. 6C,D). These changes were recapitulated in lung tissue, as both the expression of Dll4 and the Notch target genes hairy and enhancer of split 1 (Hes1), hairy and enhancer of split related-2 (Herp2/Hey1) and Ephb4 significantly decreased in the tissue from iΔEC−/− versus CCN+/+ mice (supplementary material Fig. S5D). Thus, reduction of Dll4 expression and hyperproliferation of ECs in iΔEC−/− mice are consistent with reduced Notch 1 signaling upon endothelial inactivation of the CCN1 gene. In cultured ECs, CCN1 suppression by siRNA significantly reduced VEGF-induced Dll4 expression (supplementary material Fig. S7A). Concordantly, cultured ECs plated on a CCN1-coated surface showed a strong Dll4 upregulation at both the mRNA and protein levels, whereas no significant Dll4 increase was found upon cell adhesion to either type IV collagen or fibronectin (supplementary material Fig. S7B-D). Similarly, Notch activity as determined using the RBP-jk luciferase Notch-reporter was significantly increased (4.3-fold) in cells stimulated with Ad-CCN1 compared with those stimulated with the control Ad-GFP (supplementary material Fig. S7E,F). Under these same conditions, the endogenous expression of the Dll4 and Notch targets, Hes1 and Hey1 genes, was upregulated in Ad-CCN1 versus Ad-GFP-expressing cells. Cell treatment with function-blocking antibodies against integrin subunits showed that Notch reporter activity was significantly reduced in the presence of anti-β1, anti-β3 and anti-αvβ3 neutralizing antibodies, indicating that CCN1 binding to several integrin subtypes induced Notch pathway activation (supplementary material Fig. S7F). Thus, the establishment of a properly patterned vascular tree is, at least in part, dependent on CCN1-integrin signaling during sprouting angiogenesis in the retina.
Fig. 7.
Endothelial-specific deletion of CCN1 impairs Dll4/Notch Signaling. (A) Typical vascular fronts of flat-mounted IB4-stained retinas from control CCN1+/+ and iΔEC−/− mouse pups. IB4 staining was overexposed to visualize the filopodia (scored with yellow dots) at the vascular front. (B) Quantification of filopodia-rich endothelial tip cells in the retina of iΔEC−/− mice and their control counterparts. Values represent means±s.d. Tip cells were counted in four equivalent areas of retinas of six control and six iΔEC−/− mouse retinas. *P<0.05 versus CCN1+/+. (C) Typical pattern of Dll4 protein localization in tip and stalk cells in the retina of P4 control and mutant iΔEC−/− mice. Whole-retinal-mount immunostaining for IB4 (in red) and Dll4 (white) in retinas of P4 control and mutant iΔEC−/− mice. Yellow circles highlight the Dll4 staining. (D) Quantification of the percentage of Dll4+ signal normalized to IB4+ area shown in C.
DISCUSSION
The ECM is composed of various bioactive molecules with potential functions in the onset and/or termination of angiogenesis. Studies aimed at unraveling the effects of specific ECM proteins on the behavior of ECs and the shaping of the vascular network could conceivably identify new therapeutic targets in the ECM and substantially improve existing tools for clinical use in angiogenesis-driven diseases, including the preponderant neovascular diseases of the retina (Yan and Chaqour, 2013). Here, we show that CCN1 is largely expressed by retinal angiogenic ECs at the forefront of the growing vascular network during sprouting angiogenesis. Specific deletion of CCN1 in ECs induced endothelial hyperplasia and the formation of a vascular network with immature characteristics, as retinal vessels failed to form correctly and to remodel from an initial primitive plexus into their typical hierarchical organization. In addition, the mouse model of EC-specific CCN1 deletion revealed that not only does CCN1 regulate the dynamics of endothelial cell phenotypical plasticity but also fine-tunes via integrin-binding canonical VEGF action. Previously, Mo et al. have shown that ubiquitous CCN1 gene deletion in mice led to embryonic lethality as a result of severe vascular defects and abnormal heart development (Mo and Lau, 2006). This conventional knockout of CCN1, which largely focused on analyzing the vascular phenotype of the aorta, revealed spatially disorganized, some apoptotic, ECs, and pericytes that were loosely associated with a disintegrated basal lamina (Mo et al., 2002). The dissimilarities of the vascular phenotypes of EC-specific and ubiquitous CCN1 deletion might reflect both the uniqueness of the mechanisms underlying postnatal sprouting angiogenesis in the retina and the cell type- and context-specific activities of CCN1, which depend on the availability of interacting partners in different vascular beds. Of note, the severe phenotype in conventional CCN1 mutant mice made it challenging to distinguish a direct effect of CCN1 gene deletion from a secondary effect (e.g. placental insufficiency). Conversely, EC-specific loss of CCN1 in mice, unambiguously demonstrated that CCN1 is a crucial component of the EC secretome with autocrine and paracrine activities.
CCN1 deletion selectively enhanced VEGF-R2 phosphotyrosine activity at Y1175, which supports Akt and MAPK p44/42 phosphorylation (supplementary material Fig. S8) by, at least in part, decreasing the gene expression and protein activity of the phosphatase SHP-1. Interestingly, Hayashi et al. have shown that in mouse embryoid bodies and zebra fish, VEGF-R2 phosphorylation was regulated by the vascular endothelial-phosphotyrosine phosphatase (VE-PTP), which dephosphorylates VEGF-R2 on Y1175 (Hayashi et al., 2013). Whether VE-PTP similarly affects VEGF-R2 phosphorylation during sprouting angiogenesis in the retina is unknown. Nonetheless, the same study also showed that VE-PTP activity was localized to VEGF-R2 of endothelial junctions, as its silencing preferentially increased the junctional localization of active VEGF-R2. As SHP-1 has not, thus far, been shown to be confined to specific cellular localization, the action of both phosphatases on VEGF-R2 are probably required for a complete and efficient control of VEGF-R2 activity. Moreover, our data showed that the formation of the VEGF-R-SHP-1 complex was not constitutive but rather required CCN1 interaction with integrin β1. Of the 22 currently recognized integrin heterodimers, at least seven (αvβ3, αvβ5, αvβ1, α1β1, α2β1, α3β1 and α5β1) are expressed by ECs and have been implicated in vascular morphogenesis (Hynes and Bader, 1997). In particular, β1 integrin was shown to be a negative regulator of proliferation (Yamamoto et al., 2015), and its loss led to disruption of arterial EC polarity and lumen formation, vessel-branching abnormalities and formation of hemorrhaging vessels (Zovein et al., 2010), mimicking, at least in part, the endothelial CCN1 deletion phenotype. CCN1 signaling involves either interaction with a single integrin type or the concerted binding to several integrins and/or growth factors and growth factor receptors. CCN1 co-immunoprecipitation with VEGF-R2 and SHP-1 indicated that CCN1 is part of a larger signaling complex, including membrane receptors as well as extracellular and intracellular components. Interestingly, Guillon-Munos et al. have previously demonstrated that CCN1 physically interacts with VEGF (Guillon-Munos et al., 2011). Whether such interaction occurs in vivo and regulates VEGF bioavailability and VEGF-R2 signaling is still unknown. A scenario which also merits further investigations is the molecular basis for CCN1-dependent regulation of SHP-1 activity, which largely resides within the C-terminus of SHP-1 and includes (i) phosphorylation of serines and tyrosines by kinases, such as Lyn, Lck and Src (Frank et al., 2004); (ii) membrane lipid interaction and lipid raft localization; and (iii) proline-rich domain interaction motifs for recruitment of SH3 domain-containing proteins (Kawakami et al., 2012).
Another important result of our study design is that CCN1 expression affected Dll4/Notch signaling, which regulates endothelial tip and stalk phenotypes. Endothelial loss of CCN1 reduced Dll4 expression and produced striking morphological changes, similar to those reported for Dll4/Notch inhibition (Cristofaro et al., 2013). Thus far, hypoxia-driven VEGF-VEGF-R2 signaling has been widely reported as a major inducer of the expression of Dll4 in tip cells (Sainson and Harris, 2006). Our studies further showed that endothelial loss of CCN1 in vivo or interference with VEGF-induced CCN1 expression in vitro significantly reduced Dll4 expression in ECs. In addition, loss of CCN1 was accompanied by a simultaneous reduction of VEGF-R3, which, as suggested by other studies, activates Notch in a Dll4-independent non-canonical manner (Tammela et al., 2011). Thus, integrin-dependent action of CCN1 on Dll4 expression and Notch signaling might reflect/mediate not only VEGF-R2- but also VEGF-R3-dependent regulation of Notch signaling. Several studies have previously used mouse genetics to investigate the regulation of Dll4/Notch-dependent cell-cell signaling by VEGF-R2 and VEGF-R3. Benedito et al. showed that Dll4 protein expression in retinal tip cells is only weakly modulated by VEGF-R2 signaling (Benedito et al., 2012). Reversibly, Notch inhibition had no major impact on VEGF-R2 expression, and perturbed endothelial sprouting and proliferation even in the absence of VEGF-R2. The same study emphasized a rather important role of VEGF-R3 in the regulation of sprouting of ECs with low Notch signaling activity. Another study by Zarkada et al. demonstrated that genetic ablation of VEGF-R2 alone or in combination with VEGF-R3 prevented the increase of vascular density induced by Notch activation, suggesting that VEGF-R2 but not VEGF-R3 is required for the hypersprouting that occurred in the absence of Notch activation (Zarkada et al., 2015). In the same study, VEGF-R2 was required independently of VEGF-R3 for endothelial Dll4 upregulation and angiogenic sprouting, and for VEGF-R3 function in angiogenesis. Discrepancies among these observations reported by different groups of investigators might largely be due to the different approaches used to examine the functions of individual VEGF receptors and the dose-dependent effects of receptor functions. Of particular interest, increased Dll4 was observed in VEGF-R2-deficient mouse endothelium after Notch inhibition, suggesting that one or more additional factor(s) is or are involved (Zarkada et al., 2015). This is consistent with the independent action of CCN1 on the activation of Dll4/Notch signaling. However, a separate study by Stenzel et al. has also identified laminin, alpha 4 (Lama4) as a regulator of tip cell specification (Stenzel et al., 2011). Thus, the action of CCN1 and Lama4 might be coordinated both temporally and spatially to modulate Dll4 expression and Notch 1 activity during the highly dynamic process of inter-endothelial cell communication.
Taken together, these studies suggest that CCN1-EC cross-talk controls, directly via integrin signaling and indirectly by fine tuning VEGF signaling, the phenotypical plasticity of ECs and vessel morphogenesis. This information could be useful for the design of therapeutic approaches to treat neovascular diseases of the eye.
MATERIALS AND METHODS
Mice
GENSAT Tg(Cyr61-EGFP)HA63Gsat (015210-UCD) mice, referred to herein as CCN1:GFP mice carrying enhanced green fluorescent protein (GFP) under the control of the CCN1 promoter, were developed under the NINDS-funded GENSAT BAC transgenic project (Gong et al., 2003) and obtained from the Mutant Mouse Regional Resource Center. CCN1:GFP mice were initially generated and maintained in an FVB/N-Swiss Webster background and later backcrossed for >10 times in the C57BL/6J genetic background. CCN1flox/flox mice (Kim et al., 2013) and Cdh5 (PAC)-CreERT2 (Sorensen et al., 2009) and ROSA26 Cre reporter (JAX Lab) (Soriano, 1999) transgenic lines have been described previously. Mice were handled and housed according to the approved Institutional Animal Care and Use Committee (IACUC) protocol 11-10251 of SUNY Downstate Medical Center, NY, USA.
Generation of the CCN1 conditional allele
Mice with endothelial-specific deletion of CCN1 were generated by cross-breeding CCN1flox/flox with Cdh5 (PAC)-CreERT2 mice to produce CCN1flox/+ CDH5-Cre−/− and CCN1flox/+ CDH5-Cre+/−. The latter were further crossed among each other or with CCN1flox/flox to produce CCN1flox/+CDH5-Cre+/−, CCN1flox/+CDH5-Cre−/− or CCN1flox/floxCDH5-Cre+/−mice. A solution of 4-hydroxytamoxifen (4HT) was dissolved in ethanol at 10 mg/ml, and then four volumes of sunflower seed oil were added. Samples of 4HT were thawed and diluted in sunflower seed oil prior to intraperitoneal injection of 100 µl to mouse pups. Lactating mothers were simultaneously given a single daily injection of 4HT (2 mg) to increase recombination efficiency (Weber et al., 2009). Genotyping was determined by qPCR to identify mice with floxed alleles, hemizygous floxed allele and Cre allele (iΔEC+/−), and homozygous floxed alleles and one Cre allele (iΔEC−/−) (supplementary material Fig. S1A). Recombination levels in iΔEC−/− mice as compared with CCN1+/+ were determined as described previously (Kim et al., 2013).
RNA in situ hybridization
Eyes were fixed in 4% paraformaldehyde (PFA) for 2 min and dissected in phosphate-buffered saline (PBS). Retinas were flattened and fixed in 100% ice-cold methanol for 16 h. Retinas were further fixed in 4% PFA for 10 min and washed in PBS before digestion for 10 min in proteinase K (80 µg/ml). In situ hybridization was performed using denatured CCN1 RNA probes as previously described (O'Brien and Lau, 1992).
Bromo-2-deoxyuridine (BrdU) incorporation assay
BrdU was administered at 10 mg/kg intraperitoneally at P5. For BrdU labeling, retinas were digested with proteinase K (10 µg/ml), fixed in 4% PFA, treated with DNase I (0.1 U/ml) for 2 h at 37°C and incubated with anti-BrdU antibody (BD Pharmingen). ECs were visualized by staining with isolectin B4 (Vector Laboratories, RL-1102; 1:250 dilution) or anti-endomucin antibody (eBioscience, 14-4852; 1:250 dilution) and BrdU was detected using directly conjugated mouse anti-BrdU Alexa 488 (Molecular Probes, A21202; 1:500 dilution).
Quantification of retinal vessel density, migration and lacunarity
Fields of views at the sprouting vascular front of the retinal vascular networks from control and mutant mice were captured using a 40× objective lens and included regions of capillary-sized vessels directly adjacent to radial arterioles (Chang et al., 2007). Vascular parameters were measured using the AngioTool software (Zudaire et al., 2011). For each quantification, at least four fluorescent images/retina were taken from 4-5 mice. The data are presented as means±s.e.m. The statistical significance of differences among mean values was determined by one-way ANOVA, and two-tailed t-test statistical analysis was performed with a P-value <0.05.
RNA isolation and quantitative analysis of mRNA
Quantitative analysis of mRNAs by qPCR was performed using TaqMan technology on ABI 7000 sequence detection system (Applied Biosystems) as previously described (Chintala et al., 2012). The primers used are further described in the supplementary material methods.
Immunofluorescence and immunoblotting
Immunohistochemical analyses of retinal tissue and protein analyses by western imunoblotting were performed as previously described (Chintala et al., 2012). For further details, see the supplementary material methods.
Cell culture infection, transfection and luciferase reporter assay
In vitro studies were performed with retinal ECs (CellPro Labs) and maintained in culture according to the manufacturer's instructions. Cells at 80% confluence were transduced with adenoviral vectors and/or transfected with scrambled siRNA, CCN1 siRNAs (Qiagen) or luciferase reporter plasmids [e.g. CBF-1/RBP-JK-driven promoter plasmid (Promega) and the pCMV-Cluc2 vector (New England Biolabs) containing the Cypridina luciferase gene as a control]. Cells were incubated in low-glucose Dulbecco's Modified Eagle Medium without serum as previously described (Lee et al., 2007; Liu et al., 2008) and processed for analyses as described in the text. The proliferation rate of ECs was determined using the CyQUANT Direct Cell Proliferation Assay (Invitrogen). Cell adhesion on individual extracellular matrix substrates was performed as previously described (Estrach et al., 2011; Liu et al., 2008).
Immunoprecipitation
Total proteins (∼10 mg) of cultured cell lysates were transferred to tubes with antibody-bound protein G beads and rocked gently at 4°C overnight. Non-specific bound proteins were removed with five washes with 1× PBS containing 1% NP-40. Immunoprecipitation products were extracted from the protein G beads using Laemmli sample buffer and were further analyzed by western immunoblotting.
Rho GTPase activation pull-down, PI-3 kinase and SHP-1 activity assays
The activation of the Rho GTPases, RhoA, Cdc42 and Rac was determined as previously described (Han et al., 2003), using Activation Assay Biochem Kits (Cytoskeleton). SHP-1 phosphatase activity in cell and tissue lysates was determined by immunoprecipitation of SHP-1, followed by a colorimetric assay using the SensoLyte pNPP protein phosphatase assay kit (AnaSpec). PI-3 kinase activity was determined using PI3 kinase activity ELISA assay (Echelon Biosciences). For further details, see the supplementary material methods.
Statistical analyses
Statistical analyses were performed using the Prism software for Windows Version 4 from GraphPad, as previously described (Choi et al., 2013; Liu et al., 2008).
Supplementary Material
Acknowledgements
The authors would like to thank Sangmi Lee and Jinok Choi for their assistance and helpful discussion. We are also indebted to the Cancer Research Technology Center (UK) for sharing the CDh5(Pac)-Cre ERT2 mice.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
H.C. performed in vivo deletion of CCN1 and vascular phenotype analyses. I.K. and L.Y. performed the in vitro studies, including cell infection and transfection and data analyses. L.L. prepared and tested CCN1-floxed constructs and generated mice with the floxed CCN1 gene. M.G. contributed to vascular phenotype analyses and interpretation. B.C. designed the entire project and experimental approaches and wrote the paper.
Funding
This work was supported by grants from the National Eye Institute of the National Institutes of Health [EY022091 to B.C.] and Research to Prevent Blindness. Deposited in PMC for release after 12 months.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.121913/-/DC1
References
- Bautch V. L. (2012). VEGF-directed blood vessel patterning: from cells to organism. Cold Spring Harb. Perspect. Med. 2, a006452 10.1101/cshperspect.a006452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedito R., Rocha S. F., Woeste M., Zamykal M., Radtke F., Casanovas O., Duarte A., Pytowski B. and Adams R. H. (2012). Notch-dependent VEGFR3 upregulation allows angiogenesis without VEGF–VEGFR2 signalling. Nature 484, 110-114. 10.1038/nature10908 [DOI] [PubMed] [Google Scholar]
- Bhattacharya R., Kwon J., Wang E., Mukherjee P. and Mukhopadhyay D. (2008). Src homology 2 (SH2) domain containing protein tyrosine phosphatase-1 (SHP-1) dephosphorylates VEGF Receptor-2 and attenuates endothelial DNA synthesis, but not migration. J Mol. Signal. 3, 8 10.1186/1750-2187-3-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigstock D. R. (2003). The CCN family: a new stimulus package. J. Endocrinol. 178, 169-175. 10.1677/joe.0.1780169 [DOI] [PubMed] [Google Scholar]
- Chang K.-H., Chan-Ling T., McFarland E. L., Afzal A., Pan H., Baxter L. C., Shaw L. C., Caballero S., Sengupta N., Calzi S. L. et al. (2007). IGF binding protein-3 regulates hematopoietic stem cell and endothelial precursor cell function during vascular development. Proc. Natl. Acad. Sci. USA 104, 10595-10600. 10.1073/pnas.0702072104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaqour B. (2013). Molecular control of vascular development by the matricellular proteins (CCN1/Cyr61) and (CCN2/CTGF). Trends Dev. Biol. 7, 59-72. [PMC free article] [PubMed] [Google Scholar]
- Chintala H., Liu H., Parmar R., Kamalska M., Kim Y. J., Lovett D., Grant M. B. and Chaqour B. (2012). Connective tissue growth factor regulates retinal neovascularization through p53 protein-dependent transactivation of the matrix metalloproteinase (MMP)-2 gene. J. Biol. Chem. 287, 40570-40585. 10.1074/jbc.M112.386565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J., Lin A., Shrier E., Lau L. F., Grant M. B. and Chaqour B. (2013). Degradome products of the matricellular protein CCN1 as modulators of pathological angiogenesis in the retina. J. Biol. Chem. 288, 23075-23089. 10.1074/jbc.M113.475418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristofaro B., Shi Y., Faria M., Suchting S., Leroyer A. S., Trindade A., Duarte A., Zovein A. C., Iruela-Arispe M. L., Nih L. R. et al. (2013). Dll4-Notch signaling determines the formation of native arterial collateral networks and arterial function in mouse ischemia models. Development 140, 1720-1729. 10.1242/dev.092304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis G. E., Stratman A. N., Sacharidou A. and Koh W. (2011). Molecular basis for endothelial lumen formation and tubulogenesis during vasculogenesis and angiogenic sprouting. Int. Rev. Cell Mol. Biol 288, 101-165. 10.1016/B978-0-12-386041-5.00003-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bock B. K., Georgiadou M. and Carmeliet P. (2013). Role of endothelial cell metabolism in vessel sprouting. Cell Metab. 18, 634-647. 10.1016/j.cmet.2013.08.001 [DOI] [PubMed] [Google Scholar]
- Erber R., Eichelsbacher U., Powajbo V., Korn T., Djonov V., Lin J., Hammes H.-P., Grobholz R., Ullrich A. and Vajkoczy P. (2006). EphB4 controls blood vascular morphogenesis during postnatal angiogenesis. EMBO J. 25, 628-641. 10.1038/sj.emboj.7600949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrach S., Cailleteau L., Franco C. A., Gerhardt H., Stefani C., Lemichez E., Gagnoux-Palacios L., Meneguzzi G. and Mettouchi A. (2011). Laminin-binding integrins induce Dll4 expression and Notch signaling in endothelial cells. Circ. Res. 109, 172-182. 10.1161/CIRCRESAHA.111.240622 [DOI] [PubMed] [Google Scholar]
- Frank C., Burkhardt C., Imhof D., Ringel J., Zschörnig O., Wieligmann K., Zacharias M. and Bohmer F.-D. (2004). Effective dephosphorylation of Src substrates by SHP-1. J. Biol. Chem. 279, 11375-11383. 10.1074/jbc.M309096200 [DOI] [PubMed] [Google Scholar]
- Gong S., Zheng C., Doughty M. L., Losos K., Didkovsky N., Schambra U. B., Nowak N. J., Joyner A., Leblanc G., Hatten M. E. et al. (2003). A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature 425, 917-925. 10.1038/nature02033 [DOI] [PubMed] [Google Scholar]
- Gould D. J., Vadakkan T. J., Poché R. A. and Dickinson M. E. (2011). Multifractal and lacunarity analysis of microvascular morphology and remodeling. Microcirculation 18, 136-151. 10.1111/j.1549-8719.2010.00075.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillon-Munos A., Oikonomopoulou K., Michel N., Smith C. R., Petit-Court A., Canepa S., Reverdiau P., Heuze-Vourc'h N., Diamandis E. P. and Courty Y. (2011). Kallikrein-related peptidase 12 hydrolyzes matricellular proteins of the CCN family and modifies interactions of CCN1 and CCN5 with growth factors. J. Biol. Chem. 286, 25505-25518. 10.1074/jbc.M110.213231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J.-S., Macarak E., Rosenbloom J., Chung K. C. and Chaqour B. (2003). Regulation of Cyr61/CCN1 gene expression through RhoA GTPase and p38MAPK signaling pathways. Eur. J. Biochem. 270, 3408-3421. 10.1046/j.1432-1033.2003.03723.x [DOI] [PubMed] [Google Scholar]
- Hasan A., Pokeza N., Shaw L., Lee H.-S., Lazzaro D., Chintala H., Rosenbaum D., Grant M. B. and Chaqour B. (2011). The matricellular protein cysteine-rich protein 61 (CCN1/Cyr61) enhances physiological adaptation of retinal vessels and reduces pathological neovascularization associated with ischemic retinopathy. J. Biol. Chem. 286, 9542-9554. 10.1074/jbc.M110.198689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M., Majumdar A., Li X., Adler J., Sun Z., Vertuani S., Hellberg C., Mellberg S., Koch S., Dimberg A. et al. (2013). VE-PTP regulates VEGFR2 activity in stalk cells to establish endothelial cell polarity and lumen formation. Nat. Commun. 4, 1672 10.1038/ncomms2683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkel R., Trenkwalder T., Petersen B., Husada W., Gesenhues F., Lee S., Hannappel E., Bock-Marquette I., Theisen D., Leitner L. et al. (2014). MRTF-A controls vessel growth and maturation by increasing the expression of CCN1 and CCN2. Nat. Commun. 5, 3970 10.1038/ncomms4970 [DOI] [PubMed] [Google Scholar]
- Holbourn K. P., Acharya K. R. and Perbal B. (2008). The CCN family of proteins: structure–function relationships. Trends Biochem. Sci. 33, 461-473. 10.1016/j.tibs.2008.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B.-L., Dornbach L. M. and Lyons K. M. (2007). The 5′ untranslated regions (UTRs) of CCN1, CCN2, and CCN4 exhibit cryptic promoter activity. J. Cell Commun. Signal. 1, 17-32. 10.1007/s12079-007-0003-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. and Bader B. L. (1997). Targeted mutations in integrins and their ligands: their implications for vascular biology. Thromb. Haemost. 78, 83-87. [PubMed] [Google Scholar]
- Jun J.-I. and Lau L. F. (2011). Taking aim at the extracellular matrix: CCN proteins as emerging therapeutic targets. Nat. Rev. Drug Discov. 10, 945-963. 10.1038/nrd3599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami T., Xiao W., Yasudo H. and Kawakami Y. (2012). Regulation of proliferation, survival, differentiation, and activation by the Signaling Platform for SHP-1 phosphatase. Adv. Biol. Regul. 52, 7-15. 10.1016/j.advenzreg.2011.09.003 [DOI] [PubMed] [Google Scholar]
- Kim K.-H., Chen C.-C., Monzon R. I. and Lau L. F. (2013). Matricellular protein CCN1 promotes regression of liver fibrosis through induction of cellular senescence in hepatic myofibroblasts. Mol. Cell. Biol. 33, 2078-2090. 10.1128/MCB.00049-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh W., Sachidanandam K., Stratman A. N., Sacharidou A., Mayo A. M., Murphy E. A., Cheresh D. A. and Davis G. E. (2009). Formation of endothelial lumens requires a coordinated PKCepsilon-, Src-, Pak- and Raf-kinase-dependent signaling cascade downstream of Cdc42 activation. J. Cell Sci. 122, 1812-1822. 10.1242/jcs.045799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latinkic B. V., Mo F. E., Greenspan J. A., Copeland N. G., Gilbert D. J., Jenkins N. A., Ross S. R. and Lau L. F. (2001). Promoter function of the angiogenic inducer Cyr61gene in transgenic mice: tissue specificity, inducibility during wound healing, and role of the serum response element. Endocrinology 142, 2549-2557. 10.1210/en.142.6.2549 [DOI] [PubMed] [Google Scholar]
- Lee H.-Y., Chung J.-W., Youn S.-W., Kim J.-Y., Park K.-W., Koo B.-K., Oh B.-H., Park Y.-B., Chaqour B., Walsh K. et al. (2007). Forkhead transcription factor FOXO3a is a negative regulator of angiogenic immediate early gene CYR61, leading to inhibition of vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ. Res. 100, 372-380. 10.1161/01.RES.0000257945.97958.77 [DOI] [PubMed] [Google Scholar]
- Lee M. Y., Luciano A. K., Ackah E., Rodriguez-Vita J., Bancroft T. A., Eichmann A., Simons M., Kyriakides T. R., Morales-Ruiz M. and Sessa W. C. (2014). Endothelial Akt1 mediates angiogenesis by phosphorylating multiple angiogenic substrates. Proc. Natl. Acad. Sci. USA 111, 12865-12870. 10.1073/pnas.1408472111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Yang R., Tinner B., Choudhry A., Schutze N. and Chaqour B. (2008). Cysteine-rich protein 61 and connective tissue growth factor induce deadhesion and anoikis of retinal pericytes. Endocrinology 149, 1666-1677. 10.1210/en.2007-1415 [DOI] [PubMed] [Google Scholar]
- Lobov I. B., Renard R. A., Papadopoulos N., Gale N. W., Thurston G., Yancopoulos G. D. and Wiegand S. J. (2007). Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc. Natl. Acad. Sci. USA 104, 3219-3224. 10.1073/pnas.0611206104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutty G. A., Chan-Ling T., Phelps D. L., Adamis A. P., Berns K. I., Chan C. K., Cole C. H., D'Amore P. A., Das A., Deng W. T. et al. (2006). Proceedings of the Third International Symposium on Retinopathy of Prematurity: an update on ROP from the lab to the nursery (November 2003, Anaheim, California). Mol. Vis. 12, 532-580. [PubMed] [Google Scholar]
- Lyons B. L., Smith R. S., Hurd R. E., Hawes N. L., Burzenski L. M., Nusinowitz S., Hasham M. G., Chang B. and Shultz L. D. (2006). Deficiency of SHP-1 protein-tyrosine phosphatase in “viable motheaten” mice results in retinal degeneration. Invest Ophthalmol. Vis. Sci. 47, 1201-1209. 10.1167/iovs.05-1161 [DOI] [PubMed] [Google Scholar]
- Miller J. W., Le Couter J., Strauss E. C. and Ferrara N. (2013). Vascular endothelial growth factor a in intraocular vascular disease. Ophthalmology 120, 106-114. 10.1016/j.ophtha.2012.07.038 [DOI] [PubMed] [Google Scholar]
- Mo F.-E. and Lau L. F. (2006). The matricellular protein CCN1 is essential for cardiac development. Circ. Res. 99, 961-969. 10.1161/01.RES.0000248426.35019.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo F.-E., Muntean A. G., Chen C.-C., Stolz D. B., Watkins S. C. and Lau L. F. (2002). CYR61 (CCN1) is essential for placental development and vascular integrity. Mol. Cell. Biol. 22, 8709-8720. 10.1128/MCB.22.24.8709-8720.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Patrushev N., Inomata H., Mehta D., Urao N., Kim H. W., Razvi M., Kini V., Mahadev K., Goldstein B. J. et al. (2008). Role of protein tyrosine phosphatase 1B in vascular endothelial growth factor signaling and cell-cell adhesions in endothelial cells. Circ. Res. 102, 1182-1191. 10.1161/CIRCRESAHA.107.167080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien T. P. and Lau L. F. (1992). Expression of the growth factor-inducible immediate early gene cyr61 correlates with chondrogenesis during mouse embryonic development. Cell Growth Differ. 3, 645-654. [PubMed] [Google Scholar]
- Olsson A.-K., Dimberg A., Kreuger J. and Claesson-Welsh L. (2006). VEGF receptor signalling – in control of vascular function. Nat. Rev. Mol. Cell Biol. 7, 359-371. 10.1038/nrm1911 [DOI] [PubMed] [Google Scholar]
- Pei D., Lorenz U., Klingmuller U., Neel B. G. and Walsh C. T. (1994). Intramolecular regulation of protein tyrosine phosphatase SH-PTP1: a new function for Src homology 2 domains. Biochemistry 33, 15483-15493. 10.1021/bi00255a030 [DOI] [PubMed] [Google Scholar]
- Rayssac A., Neveu C., Pucelle M., Van den Berghe L., Prado-Lourenco L., Arnal J.-F., Chaufour X. and Prats A.-C. (2009). IRES-based vector coexpressing FGF2 and Cyr61 provides synergistic and safe therapeutics of lower limb ischemia. Mol. Ther. 17, 2010-2019. 10.1038/mt.2009.211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainson R. C. A. and Harris A. L. (2006). Hypoxia-regulated differentiation: let's step it up a Notch. Trends Mol. Med. 12, 141-143. 10.1016/j.molmed.2006.02.001 [DOI] [PubMed] [Google Scholar]
- Siemerink M. J., Klaassen I., Van Noorden C. J. F. and Schlingemann R. O. (2013). Endothelial tip cells in ocular angiogenesis: potential target for anti-angiogenesis therapy. J. Histochem. Cytochem. 61, 101-115. 10.1369/0022155412467635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen I., Adams R. H. and Gossler A. (2009). DLL1-mediated Notch activation regulates endothelial identity in mouse fetal arteries. Blood 113, 5680-5688. 10.1182/blood-2008-08-174508 [DOI] [PubMed] [Google Scholar]
- Soriano P. (1999). Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 21, 70-71. 10.1038/5007 [DOI] [PubMed] [Google Scholar]
- Stenzel D., Franco C. A., Estrach S., Mettouchi A., Sauvaget D., Rosewell I., Schertel A., Armer H., Domogatskaya A., Rodin S. et al. (2011). Endothelial basement membrane limits tip cell formation by inducing Dll4/Notch signalling in vivo. EMBO Rep. 12, 1135-1143. 10.1038/embor.2011.194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strilić B., Kučera T., Eglinger J., Hughes M. R., McNagny K. M., Tsukita S., Dejana E., Ferrara N. and Lammert E. (2009). The molecular basis of vascular lumen formation in the developing mouse aorta. Dev. Cell 17, 505-515. 10.1016/j.devcel.2009.08.011 [DOI] [PubMed] [Google Scholar]
- Tammela T., Zarkada G., Nurmi H., Jakobsson L., Heinolainen K., Tvorogov D., Zheng W., Franco C. A., Murtomäki A., Aranda E. et al. (2011). VEGFR-3 controls tip to stalk conversion at vessel fusion sites by reinforcing Notch signalling. Nat. Cell Biol. 13, 1202-1213. 10.1038/ncb2331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber T., Böhm G., Hermann E., Schütz G., Schönig K. and Bartsch D. (2009). Inducible gene manipulations in serotonergic neurons. Front. Mol. Neurosci. 2, 24 10.3389/neuro.02.024.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K., Chong D. C., Rankin S. A., Zorn A. M. and Cleaver O. (2009). Rasip1 is required for endothelial cell motility, angiogenesis and vessel formation. Dev. Biol. 329, 269-279. 10.1016/j.ydbio.2009.02.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K., Sacharidou A., Fu S., Chong D. C., Skaug B., Chen Z. J., Davis G. E. and Cleaver O. (2011). Blood vessel tubulogenesis requires Rasip1 regulation of GTPase signaling. Dev. Cell 20, 526-539. 10.1016/j.devcel.2011.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H., Ehling M., Kato K., Kanai K., van Lessen M., Frye M., Zeuschner D., Nakayama M., Vestweber D. and Adams R. H. (2015). Integrin beta1 controls VE-cadherin localization and blood vessel stability. Nat. Commun. 6, 6429 10.1038/ncomms7429 [DOI] [PubMed] [Google Scholar]
- Yan L. and Chaqour B. (2013). Cysteine-rich protein 61 (CCN1) and connective tissue growth factor (CCN2) at the crosshairs of ocular neovascular and fibrovascular disease therapy. J. Cell Commun. Signal. 7, 253-263. 10.1007/s12079-013-0206-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R., Amir J., Liu H. and Chaqour B. (2008). Mechanical strain activates a program of genes functionally involved in paracrine signaling of angiogenesis. Physiol. Genomics 36, 1-14. 10.1152/physiolgenomics.90291.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarkada G., Heinolainen K., Makinen T., Kubota Y. and Alitalo K. (2015). VEGFR3 does not sustain retinal angiogenesis without VEGFR2. Proc. Natl. Acad. Sci. USA 112, 761-766. 10.1073/pnas.1423278112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zovein A. C., Luque A., Turlo K. A., Hofmann J. J., Yee K. M., Becker M. S., Fassler R., Mellman I., Lane T. F. and Iruela-Arispe M. L. (2010). Beta1 integrin establishes endothelial cell polarity and arteriolar lumen formation via a Par3-dependent mechanism. Dev. Cell 18, 39-51. 10.1016/j.devcel.2009.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zudaire E., Gambardella L., Kurcz C. and Vermeren S. (2011). A computational tool for quantitative analysis of vascular networks. PLoS. ONE 6, e27385 10.1371/journal.pone.0027385 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.