Abstract
We have cloned a novel human mixed-lineage kinase gene, MLK4. Two alternatively spliced forms, MLK4α (580 aa) and MLK4β (1036 aa), have been identified and mapped to chromosomal band 1q42. MLK4 shows high amino acid homology to the kinase catalytic domain of MLK3 (72%), MLK1 (71%) and MLK2 (69%). Strong expression of MLK4 was detected in the human pancreas and kidneys. pCMV-MLK4β c-myc-tagged protein (human) was expressed in the cytoplasm and nucleus of transiently transfected COS-1 cells, while pCMV-MLK4α c-myc-tagged protein (human) was expressed in cytoplasm only. Both MLK4 isoforms reduced the colony formation ability of MCF7 cells by 85%–95% and almost totally suppressed cell proliferation in the CyQUANT cell proliferation assay. Human pCMV-MLK4β transgenic mice expressed the MLK4β in all tissues examined but no phenotypic abnormalities were observed. Thus, in this work, we present the cloning and sequencing of MLK4α and MLK4β for the first time; the data obtained suggest that MLK4 may function as a MAP kinase.
Keywords: MLK, protein kinase, gene cloning, tumor suppressor gene
Introduction
Protein kinases play an important role in many signaling pathways, and therefore have the potential to contribute to various diseases ranging from cancer and inflammation to diabetes and cardiovascular disorders.
Mixed-lineage kinases (MLKs) are serine/threonine protein kinases that regulate signalling by the c-Jun amino-terminal kinase (JNK) and p38 mitogen-activated protein kinase (MAPK) pathways. MAPK cascades exist in all eukaryotes and orchestrate diverse cellular activities, including mitosis, programmed cell death, motility and metabolism. Substrates for MAPKs include transcription factors, phospholipases, other protein kinases, cytoskeleton-associated proteins and membrane receptors.1
MAPK cascades typically consist of a MAPK, a MAPKK (MAP kinase kinase) and a MAPKKK (MAP kinase kinase kinase).2 Transmission of the signals is achieved by sequential phosphorylation and activation of the cascade components.3 At least three independent MAPK pathways have been identified in mammalian cells. They include the extracellular signal-regulated kinase (ERK) pathway, the c-Jun N-terminal kinase (JNK)/stress activated protein kinases (SAPK) pathway and the p38 pathway. The ERK pathway regulates cell proliferation, cell differentiation and developmental processes.4 The JNK/SAPK and p38 pathways are involved in cellular stress responses and apoptosis.5
The MLKs function as MAPKKKs. The MLKs are characterized by the unique catalytic domain that is a hybrid between those found in serine/threonine and tyrosine kinases.6 The MLKs can be divided into three subfamilies.1,7
The first subgroup, which includes MLK1–MLK4, is characterized by an amino-terminal SRC-homology (SH3) domain, followed sequentially by a kinase domain, a leucine-zipper region and a Cdc42/Rac-interactive binding (CRIB) motif that interacts with the RHO-family GTPase Rac and Cdc42. MLK1–MLK4 share 69%–72% sequence identity within their catalytic domains and approximately 64%–92% sequence identity with their other domains and motifs. The carboxyl termini of these proteins are proline-rich but have different sequences, indicating that these regions might serve different regulatory functions.
The second subfamily includes two genes, dual leucine zipper-bearing kinase (DLK) and leucine zipper bearing kinase (LZK).8,9
The third subgroup of MLKs consists of zipper sterile-α-motif kinases (ZAKs), ZAKα and ZAKβ.10,11
Some MLKs are restricted in their cell and tissue expression (like MLK1 and MLK2), whereas several others are widely produced (MLK3, ZAKα). The diverse regulatory domains that are present in the MLKs indicate that these proteins are probably regulated differently by different upstream stimuli and participate in selective interactions with other proteins that target MLKs to different subcellular localizations.1,12
The physiological roles of MLKs have not been defined and it seems that the initial picture of MLKs as comparatively selective regulators of the JNK group of MAPKs is no longer valid; in fact, MLKs may have a more general upstream role than was initially postulated.1,12
It was shown that MLKs might contribute to neurogenerative diseases, and they have been suggested as attractive therapeutic targets for the treatment of diseases such as Parkinson’s and Huntington’s.1,13,14 Still, MLK2, MLK3, DLK and LZK are the only MLKs that have been studied in detail.6,9,15 In this work, we present the cloning of MLK4α and MLK4β for the first time, and an analysis of their genomic structure, chromosomal localization, expression patterns and initial functional characterization.
Results
Cloning and nucleotide sequence analysis of MLK4α and MLK4β
The NotI linking clone NR5-DM9 (partial sequence 480 bp, GenBank accession No. AJ311799) showed 87% identity over 63 nucleotides with human MLK3 cDNA (GenBank accession No. NM_002419) and 83% identity over 87 nucleotides with human MLK1 cDNA (GenBank accession No. AF251442). BLASTN analysis revealed that this NotI linking clone was identical to part of the PAC clone RP5-862P8 (GenBank accession No. AL133380). Based on the similarity between the PAC clone RP5-862P8 and the human MLK3 gene, we designed the primers KINB and KINC and amplified a part of the human MLK4 gene (630–1734 bp) from Heart Marathon-Ready™ cDNA using polymerase chain reaction (PCR) (Fig. 1).
Figure 1.
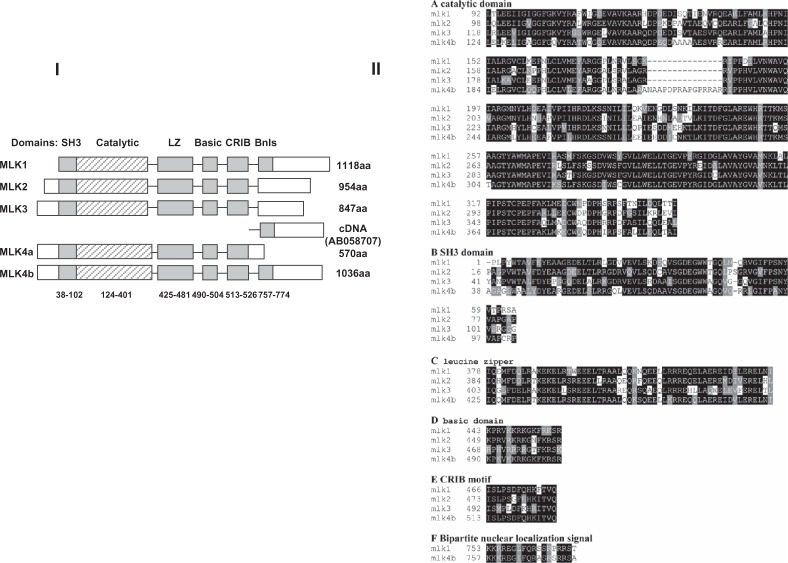
Nucleotide and amino acid sequences of MLK4α (A) and MLK4β (B). The following features are indicated: kinase catalytic domain (shaded), the SH3 domain (underlined), the leucine zipper domain (⋯), the basic domain (*), the CRIB motif (Image) and the bipartite nuclear localization signal (black box). MLK4α and MLK4β have an identical N-terminal region (1–558 aa) and share all domains, except the bipartite nuclear localization signal.
The 3′-RACE product of human MLK4α gene was obtained as described in the Materials and Methods section. It contained a poly(A) tail of 30 adenine nucleotides and a typical polyadenylation site (AATAAA) 18 nucleotides upstream from the poly(A) tail. The 5′-end of the MLK4α gene was cloned from the Heart Marathon-Ready cDNA. The cloned sequence revealed that the first ATG (Met) codon is situated at 262 nucleotides and this codon is situated within the sequence CCCATGG, which is consistent with the Kozak model.16
A BLASTN search of the EMBL and EST databases for MLK4α nucleotide sequences revealed a human EST (GenBank accession No. AW408639) representing a different spliced form of MLK4 at the 3′-end. Using the PAC clone RP5-862P8, the human MLK3 and sequences of several ESTs (Gen-Bank accession Nos. AL135711 and BE867187) that are situated downstream from the 3′UTR of MLK4α cDNA on the PAC clone, we designed the primers KIND (derived from the common part of MLK4α and AW408639) and KINF (derived from the AL135711 and BE867187 sequences). With these primers, a 3′ segment of human MLK4β was cloned from the Heart Marathon-Ready cDNA. MLK4β cDNA has four additional exons at the 3′-end and an open reading frame that is 466 aa longer than MLK4α.
The primers KIN5/KIN3a and KIN5/KIN3b were used to amplify complete coding sequences of spliced variants of MLK4α (84–2171 bp, 570 amino acids) and MLK4β (84–3476 bp, 1036 amino acids).
Sequence analysis revealed that MLK4α and MLK4β genes consist of 6 and 10 exons and span more than 46.3 kb and 56.2 kb of genomic DNA, respectively. The complete sequence of the inserts in the plasmids containing MLK4α (3910 bp; GenBank accession No. AJ311797) and MLK4β (4667 bp; GenBank accession No. AJ311798) is shown in Figure 1(A, B) and its different predicted domains are shown in Figure 2.
Figure 2.
Functional domains of MLK4. I) Composite structure of human MLKs, showing the relative positions of the Src-homology-3 (SH3), catalytic, leucine-zipper (LZ), basic, and Cdc42/Rac-interactive binding (CRIB) domains, and the Bipartite nuclear localization signal (Bnls). cDNA (AB058707) was cloned by Nagase et al.21 See text for discussion. II) Alignment of the deduced MLK4β sequences domains with those of MLK1, MLK2 and MLK3. A) Alignment of the kinase catalytic domains; B) Alignment of the SH3 domains; C) Alignment of the double leucine zipper domains; D) Alignment of the basic domains; E) Alignment of the CRIB motifs; F) Alignment of the bipartite nuclear localization signals. Black and gray boxes indicate identical and similar amino acid residues, respectively.
Expression Patterns of MLK4
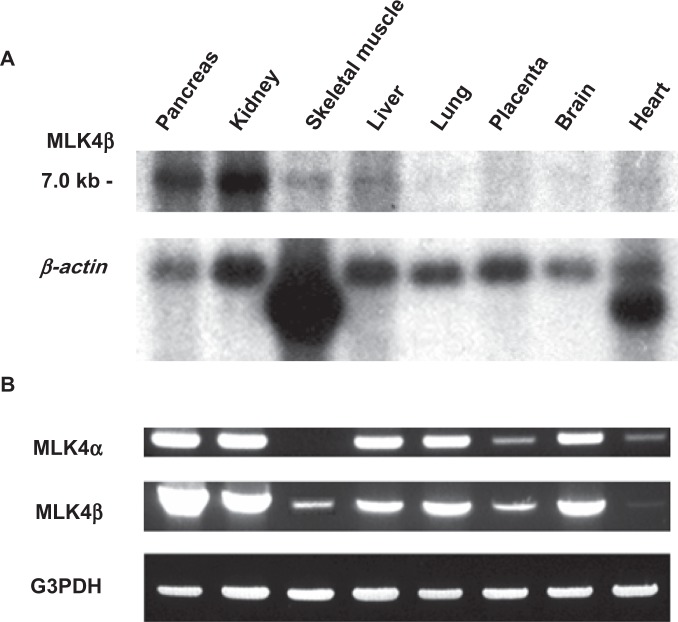
To determine the distribution of MLK4α and MLK4β mRNA within the tissues, a multiple human tissue Northern blot (Clontech, Palo Alto, CA, USA) was hybridized to the non-coding 3′-end of specific DNA fragments of MLK4α (1970–3910 bp) and MLK4β (2087–4667 bp). As shown in Figure 3A, one transcript of about 7 kb was observed for the MLK4β gene. This transcript was detected in the pancreas, kidney, skeletal muscle, liver and heart. Northern blot hybridization for MLK4α did not result in any specific signal (data not shown), indicating a low abundance of MLK4α mRNA variant.
Figure 3.
Expression analysis of MLK4α and MLK4β: A) Northern blot hybridization for MLK4β, B) PCR with multiple tissue cDNA panel for MLK4α and MLK4β.
To investigate the possibility that MLK4α still was expressed at low levels, we checked its expression by reverse transcription PCR (RT-PCR) using multiple tissue cDNA panels. Figure 3B shows that both MLK4α and MLK4β mRNAs were expressed in the pancreas, kidney, liver, lung and brain. Expression in the heart and placenta was detected at a lower level. Only MLK4β was expressed in skeletal muscle.
Chromosomal Localization of MLKα and MLK4β
Using fluorescent in situ hybridization (FISH) we assigned the NotI linking clone NR5-DM9 (insert size 5 kb) to chromosomal band 1q42 (Fig. 4). A number of diseases have been localized to this region, including a predisposition to prostate cancer17 and papillary renal cell carcinoma.18
Figure 4.
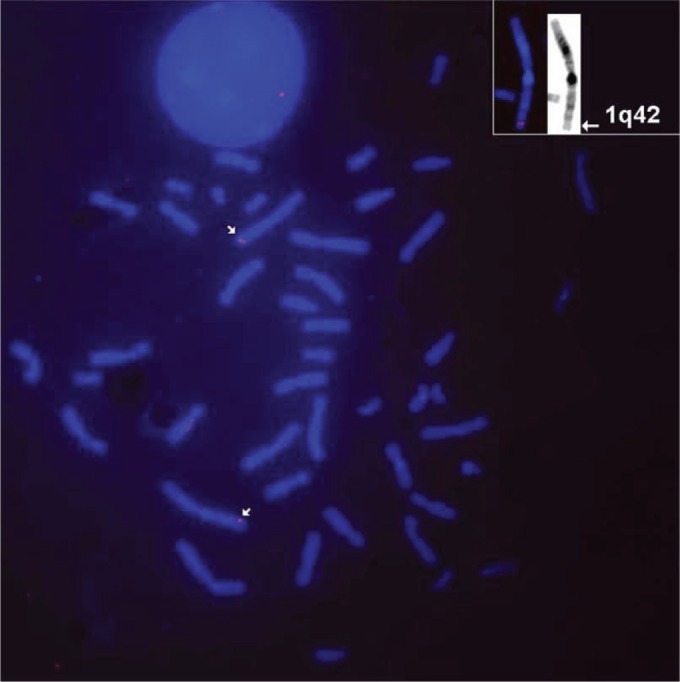
FISH-based assignment of MLK4 to human chromosome band 1q42.
Subcellular Localization of MLKα and MLK4β
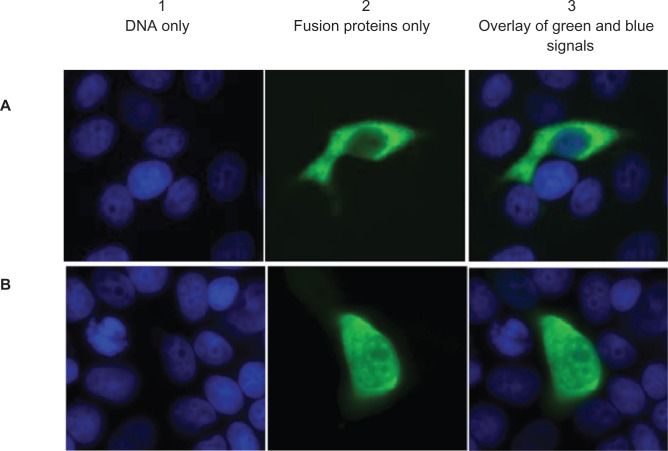
According to the sequence data, a bipartite nuclear localization signal is present only in the longer MLK4β. To study the localization of both MLK4 variants in the cell, MLK4α and MLK4β were cloned into a pCMV-Tag3 vector (pCMV-MLK4α and pCMV-MLK4β) for the c-myc-tagged expression of both proteins. COS-1 cells were transfected with either pCMV-MLK4α or pCMV-MLK4β and immunostained with α-myc antibody 9E10 (Fig. 5A, B). The data thus obtained showed that pCMV-MLK4β fusion protein was localised in the cytoplasm and nucleus of transiently transfected COS-1 cells, while pCMV-MLK4α was present in the cytoplasm only.
Figure 5.
Cellular localization of A) MLK4α and B) MLK4β c-myc fusion proteins. COS-1 cells were transfected with pCMV-MLK4α and pCMV-MLK4β constructs, and the c-myc-tag was stained with mouse α-c-myc monoclonal antibody (green). DNA is stained blue. (A1 and B1) DNA only; (A2 and B2) fusion proteins only; (A3 and B3) overlay of green and blue signals.
Functional Analyses of MLKα and MLK4β in Vitro
To study the possible effect of MLK4 expression on a cell’s viability and proliferation, colony formation tests and cell proliferation rate analyses were performed for MLK4-transfected cells. Human breast cancer cells (MCF7) were transfected with either pCMV-MLK4α or pCMV-MLK4β constructs and used for the experiments. To evaluate the viability of the transfected cells, they were seeded on 10-cm cell culture dishes at the same cell density; the number of colonies growing on each plate was counted after two weeks in the presence of G418 (Fig. 6A). Both MLK4 isoforms reduced colony formation ability by 85% for MLK4α and 95% for MLK4β compared to cells transfected with the empty vector.
Figure 6.
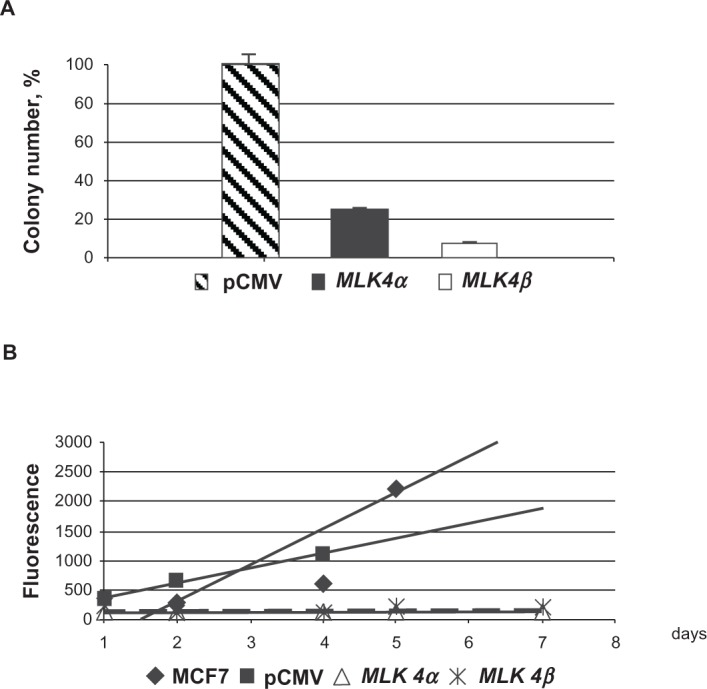
Colony formation and cell proliferation assays with pCMV-MLK4α and pCMV-MLK4β. A) Colony formation. The number of G418-resistant colonies in MCF7 cells transfected with empty pCMV-Tag3 vector was set at 100%. B) The CyQUANT NF Cell Proliferation assay was used to measure the cell proliferation rate. Two stably transfected clones for each construct (pCMV-MLK4α and pCMV-MLK4β; see Methods) for were used in the experiment, which was done in triplicate. Original MCF7 cells and MCF7 cells stably transfected with pCMV-Tag3A plasmid were used as controls.
To study the influence of the MLK4 gene on MCF7 cell proliferation, we measured the DNA accumulation rate in cell clones stably transfected with MLK4α-and MLK4β (see Methods) using the CyQUANT NF Cell Proliferation Assay (Fig. 6B). The CyQUANT NF Cell Proliferation Assay is based on measuring cellular DNA content via fluorescent dye binding (see Methods). The assay is designed to produce an analytical linear response from at least 100–20,000 cells per well in most cell lines. Almost no cell proliferation was shown both for MLK4α- and MLK4β-transfected cells compared to the control MCF7 cells transfected with the vector only. The strong inhibition of the viability and cell proliferation by MLK4 suggests the possible involvement of MLK4 in programmed cell death/apoptosis, as was shown for MLK1, MLK2 and DLK.1,19
Transgenic MLK4 Mice
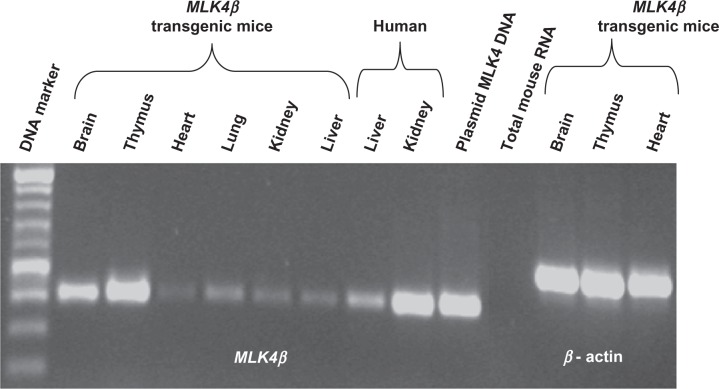
To study the effect of MLK4 expression at the organism level, MLK4β-transgenic mice were produced (see Methods). We chose MLK4β because almost all coding information in MLK4α (except for 12 amino acids in the C-terminal end) is present in MLK4β. The aim of this experiment was to see rapid, strong changes in transgenic mice. Fifty-six offspring were genotyped by PCR using human MLK4 primers and seven MLK4-positive mice were found. A different human MLK4 expression level was shown for the different mouse tissues, ranging from the highest expression being found in the thymus to weaker expression in the heart, kidney and liver (Fig. 7). Surprisingly, no phenotypical abnormalities were observed for the human MLK4-transgenic mice compared to the non-transgenic mice after 12 months’ observation.
Figure 7.
RT-PCR analysis of MLK4β expression in the different transgenic mouse tissues. M—DNA marker; 1–6—transgenic mouse tissues (1 = brain, 2 = thymus, 3 = heart, 4 = lung, 5 = kidney, 6 = liver); 7—human liver; 8—human kidney; 9—plasmid MLK4 DNA (positive control), 10—total mouse RNA (negative control), 11–13—actin expression control for the transgenic mouse tissues (11 = brain, 12 = thymus, 13 = heart).
Discussion
In this work, we present cloning and sequencing of MLK4α (3910 bp, GenBank accession No. AJ311797) and MLK4β (4667 bp, GenBank accession No. AJ311798) for the first time.20 Using FISH, we assigned the MLK4 to chromosomal band 1q42. Two MLK4 isoforms have different 3′-sequences and an identical 5′-region (1–1936 bp). The 5′ UTR of 261 bp contains an in-frame stop codon at position 118. The open reading frames of the MLK4α and MLK4β genes consist of 1710 and 3108 bp, respectively. They code for 570 aa and 1036 aa proteins. The 3′UTR length is 1938 bp for MLK4α cDNA and 1297 bp for MLK4β cDNA. A Poly(A) tail was identified for the MLK4α gene only. Independently, a cDNA clone (KIAA1804) containing 476 aa from the 3′-end of the MLK4β was cloned by Nagase et al.21 (GenBank accession No. AB 058707). This clone missed 560 aa of MLK4β from the 5′-end and thus lost all functional domains except bipartite nuclear localization signal (Fig. 2 and see below). We ran a comparison between the RefSeq entry NM_034535 and MLK4β, and found nine differences. These differences are likely to be polymorphisms because they are present both in MLK4α and −4β.
MLK4α and MLK4β cDNAs encode amino acid polypeptides with a calculated molecular mass of 62.9 kDa and 113.8 kDa, respectively. Both of them have an identical N-terminal region (1–558 aa), which contains the SRC homology (SH3) domain (38–102 aa), the kinase catalytic domain (124–401 aa), the leucine zipper domain (425–481 aa), the basic domain (490–504 aa) and the Cdc42/Rac interactive binding (CRIB) motif (513–526 aa) (Fig. 2). A search for significant matches against the PROSITE library of protein profiles was done via the ISREC server (http://www.isrec.isb-sib.ch/software/PFSCAN_form.html).
The MLK4 catalytic domain is a hybrid between those in the serine/threonine and tyrosine protein kinases with 69%–72% homology to MLK3 (GenBank accession No. NP_002410), MLK1 (GenBank accession No. AAG44591) and MLK2 (GenBank accession No. Q02779); see Figure 2.
The SRC homology 3 (SH3) domain of MLK4 is similar to that of MLK3 (68% identity), MLK1 (66% identity) and MLK2 (64% identity) (Fig. 2). It is generally assumed that the SH3 domain is involved in specific protein–protein interactions and recognizes proline-rich sequences containing the core PxxP.22
The leucine zipper domain of MLK4 (425–481 aa) displays 84% identity to MLK1, 73% identity to MLK2 and 66% identity to MLK3 (Fig. 2). The domain is able to promote homodimerization and binding to other proteins with similar domains.
The basic domain of MLK4 (490–504 aa) is located C-terminally to the leucine zipper domain, in contrast to location of basic domain in the transcription factors.23 The basic domain of MLK4 is similar to that of MLK1 and MLK2 with 80% identity but has only 53% identity to MLK3 (Fig. 2).
The CRIB motif is highly similar to that of MLK1 (92% identity), MLK2 (85% identity) and MLK3 (71% identity) (Fig. 2). It was shown that MLK2 and MLK3 interact with Rac and Cdc42 in a GTP-dependent manner.24 The CRIB motif of MLK3 is required for its activation.25
In addition, MLK4β has a bipartite nuclear localization signal (757–774 aa). The nuclear localization signal of the MLK4α protein, however, was not found (Fig. 2). Within the MLK1, MLK2 and MLK3 genes, the bipartite nuclear localization signal was found in MLK1 only.
The presence of this bipartite nuclear localization signal is the most likely explanation for the difference in the subcellular localization of the two MLK4 proteins: pCMV-MLK4β fusion protein was expressed in the cytoplasm and nucleus of transiently transfected COS-1 cells, while pCMV-MLK4α was expressed in cytoplasm only. As it was mentioned in the Introduction, different localization of the other MLK family members was reported: a cytoskeletal localization for MLK224 and nuclear localization for ZAKα/MLTKα.26 It is likely that the two MLK4 variants, which have different localizations, could have different functions.
Despite a high level of domain homology at the N-terminal region, the C-terminal regions of the MLKs are divergent.11 The C-terminal region of MLK4β is rich in proline, which is similar to MLK1, MLK2 and MLK3. This proline-rich region contains several general consensus motifs (PxxP) for SH3 binding sequences22 and may be involved in the protein–protein interaction with the SH327 and WW28 domains of other proteins (WW domains bind proline-rich proteins containing the PPXY or PPLP core motifs), or with its own SH3 domain.
MLK4 displays a high level of amino acid identity and a very similar domain structure to MLK1, MLK2 and MLK3. It has been shown that all tested MLKs (MLK2, MLK3, DLK and LZK) function as MAPK-KKs.1 The data therefore suggest that MLK4 may also function as a MAPKKK.
Our experiments clearly indicate that both forms of MLK4 inhibit viability and cell growth in vitro (colony formation experiments and CyQUANT NF proliferation assay).
On the other hand, it was shown that wild-type MLK3 overproduction induces transformation of NIH3T3 fibroblasts, whereas catalytically inactive MLK3 does not.29 ZAKα/MLTKα was also reported to function as an oncogene-inducing neoplastic cell transformation.26 Another report indicated that MLK3 inhibits Rac-mediated cellular transformation.30
The controversial results of the possible involvement of MLK4 and other MLKs in cell proliferation suggest that a more detailed functional study of the MLK family will be necessary.
Mutational analysis of the MLK4 kinase domain in 147 colorectal cancers revealed a rather high frequency (6.8%) of somatic mutations.31 In this study, our MLK4 sequence was used (GenBank database, accession No. AJ311798). However, Shao et al. 2007 did not find any mutations in the MLK4 kinase domain in 46 colorectal samples isolated from Japanese patients and only two of 24 cell lines in the study harbored the mutation R470C in the MLK4 gene.32 No mutations in MLK4 were found in gastric and hepatocellular carcinomas from Korean patients either, suggesting that the MLK4 kinase domain mutations are rare and may not contribute to the development of these carcinomas.33
The relevance of these observations is unclear and currently, no evidence suggests that any MLK family member functions as an oncogene in humans. MLK4β-transgenic mice were produced but, surprisingly, no obvious phenotypical abnormalities were observed compared to the non-transgenic mice after 12 months’ observation. This result is in disagreement with the strong effect of MLK4 in vitro. It is interesting that MLK3−/− knockout mice were also viable and healthy.34 These results indicate that we are in the initial stages of understanding the physiological role of MLK4 and other MLKs, and further work is necessary to clarify the functional significance of MLK4α and MLK4β proteins.
Conclusions
In summary, in this work, we present the cloning and sequencing of MLK4α and MLK4β for the first time. Using FISH, we assigned the MLK4 gene to chromosomal band 1q42. A number of diseases have been localized to this region, see for example.35,36 pCMV-MLK4β fusion protein was localized in the cytoplasm and nucleus of transiently transfected COS-1 cells, while pCMV-MLK4α was present in cytoplasm only. Both forms of MLK4 strongly inhibited viability and MCF7 cell proliferation in vitro. These data showed that MLK4 genes could be important not only for basic science but could also be promising avenues of cancer diagnostics and therapeutics.
Materials and Methods
NotI linking library construction
The construction and sequencing of NotI linking libraries has been described previously.37–40 In brief, genomic DNA of the CBMI-Ral-Sto cell line was completely digested with BamHI and self-ligated with T4 ligase at a low concentration. To eliminate any remaining linear molecules, the sticky ends were partially filled-in with Klenow enzyme in the presence of dATP and dGTP.41. Subsequently, DNA was digested with NotI and ligated to λSK17 and λSK22. To convert the library into plasmid form, 2 μg of λDNA was digested with SalI, self-ligated with T4 ligase and transformed into E. coli XL1-Blue MRF cells (Stratagene, La Jolla, CA, USA). The restriction enzymes, Klenow enzyme and T4 ligase used in this work have been manufactured by Roche, (Mannheim, Germany). All molecular biology and microbiology procedures were performed according to standard methods.
Molecular Cloning of Human MLK4
MLK4 gene fragment (630–1734 bp) from Heart Marathon-Ready cDNA (Clontech, Palo Alto, CA, USA) has been obtained by PCR, using the primers KINB and KINC (for primer sequences, see Table 1) and according to the manufacturer’s instructions.
Table 1.
Primer sequences.
| Primers | Sequences |
|---|---|
| KINB | 5′GCTGGAGCTGAAGGAGCTCATCG3′ |
| KINC | 5′GGGCTTCTCCTGGTTTAGCTGGAA3′ |
| KIND | 5′CAAGCTCATGAAAGAATGCTGGCAACAAG3′ |
| KINE | 5′CAGTTGACTGCTATTGAGGGGGCAGTGAT3′ |
| KINF | 5′GCAAATTTACCAAGCTCTCGGGTAGCCATT3′ |
| KINA | 5′CAGGGCCTGGGCACGACCATG3′ |
| KING | 5′CGAACTCCAGCACCAGGCAGAGGT3′ |
| KIN5 | 5′GGCCGAGGCTGGACCCTTT3′ |
| KIN3a | 5′GGCCAGGTCGCATCACCAA3′ |
| KIN3b | 5′CACAGCTTTTGAAAACACAGCAGCATGAAA3′ |
| KINI | 5′GGATAAGACCTCTCTCCGATGGCAACAGTC3′ |
| G3PDHF | 5′GGGCGCCTGGTCACAA3′ |
| G3PDHR | 5′AACATGGGGGCATCAGCAGA3′ |
| KINRTF | 5′CACAGTCTTTCGACAAGAAGAATTTG3′ |
| KINRTR | 5′AGACTATTCGTAGGAGCCATCTCAATG3′ |
| ActinHS-Fov | 5′GAGCAAGAGAGGCATCCTCACC3′ |
| ActinHS-Rev | 5′GGAAGGAAGGCTGGAAGAGTGC3′ |
PCR product was cut and extracted from agarose gel using the JETquick gel extraction kit (Genomed GmbH, Bad Oeynhausen, Germany) and cloned using the TOPO TA cloning kit for sequencing (Invitrogen BV, Groningen, Netherlands). Plasmid DNA was isolated with the GFX™ micro plasmid prep kit (AmershamPharmaciaBiotech, Uppsala, Sweden). Sequencing was done using an ABI 310 Sequencer with the ABI PrismR BigDye™ terminator cycle sequencing ready reaction kit (Perkin Elmer, Foster City, CA, USA) as previously described.40
3′-RACE was performed with Heart Marathon-Ready cDNA using two gene-specific primers combined with two adaptor primers, AP1 and AP2. PCRs were done according to the manufacturer’s protocol with the AdvantageR 2 PCR enzyme system (Clontech). For 3′-RACE of MLK4α, the KIND and KINE primers were used.
To amplify the 3′-end of MLK4β the KIND and KINF primers were applied.
To obtain sequences from the 5′-end of MLK4, including the first ATG (Met), we used the KINA and KING primers.
To obtain gene fragments of MLK4α cDNA (84–2171 bp, as in AJ311797) and MLK4β cDNA (84–3476 bp, as in AJ311798), which contain a complete open reading frame, the following primers were used: KIN5 and KIN3a for MLK4α, and KIN5 and KIN3b for MLK4β.
Expression Patterns and Chromosomal Localization of MLK4 Gene
Northern blot hybridization with human multiple tissue #7760-1 Northern blot (Clontech) and PCR with human multiple tissue cDNA panels (Clontech) were used to determine the expression patterns of MLK4α and MLK4β. Human multiple tissue Northern blot was consecutively hybridized at 42 °C for 16 hours with three [32P] labeled probes: i) the 3′-end of MLK4α cDNA (1970–3910 bp), ii) the 3′-end of MLK4β cDNA (2087–4667 bp) and iii) β-actin cDNA as previously described.42
PCR with multiple tissue cDNA panels (Clontech) was done with primers KIND and KIN3a for MLK4α, KINF and KINI for MLK4β, and G3PDHF and G3PDHR for G3PDH. To amplify the gene fragments of MLK4α, MLK4β and G3PDH, 40, 30 and 20 cycles of amplification were performed, respectively. PCR was carried out with 30 sec denaturation at 95 °C, 20 sec annealing at 60 °C and 1 min extension at 72 °C. Initial denaturation was done for 4 min at 95 °C.
The FISH of the NotI linking clone NR5-DM9 (insert size 5 kb) (GenBank accession No. AJ324357) with normal metaphase chromosomes was done as described previously.43 Sixty metaphase spreads with specific signals have been analyzed.
Subcellular Localization of the MLK4 Gene by Immunostaining
For cellular localization of the corresponding proteins, we cloned MLK4 gene into the pCMV-Tag3 vector (GenBank accession No. AF072997) to express c- myc-MLK4 fusion proteins. The full-length MLK4 coding sequences from pCR4-TOPO clones (for MLK4α, the plasmid was digested by NcoI and EcoRI, and for MLK4β, by NcoI and SpeI) were blunted by Klenow enzyme (Invitrogen BV, Groningen, Netherlands) and reinserted into a pCMV-Tag3A digested by EcoRV.
COS-1 cells (http://phage.atcc.orghttp://phage.atcc.org) were cultured in IMDM with 10% fetal bovine serum (Gibco, Paisley, UK) and transfected with either pCMV-MLK4α or pCMV-MLK4β constructs using Lipofectamine Plus Reagent (Invitrogen) according to the manufacturer’s protocol. The immunostaining was done as described.44 We used mouse monoclonal antibody 9E10 (Abcam, Cambridge, UK) against c-myc-tag, fused with MLK4α or MLK4β and the FITC-conjugated swine anti-mouse secondary antibody F0205 (Dako, Glostrup, Denmark). Hoechst 33258 (Sigma-Aldrich, St. Louis, USA) was used for DNA counterstaining at a concentration of 0.4 μg/mL.
Colony Formation Assay
MCF7 breast cancer cells were also cultured in IMDM. Transient transfection with either pCMV-MLK4α, pCMV-MLK4β or original pCMV-Tag3A vector was done using Lipofectamine Plus Reagent. Transfected cells were stripped 24–48 h after transfection with trypsin/EDTA (Sigma) and plated on 100 mm cell culture dishes at 500–1000 cells per plate. After selection with 500 μg/mL G418 (Cayla, Toulouse, France) for two weeks, Giemsa-stained colonies were photographed and counted.
Cell Proliferation Assay
To obtain MCF7 cells stably transfected with pCMV-MLK4α or pCMV-MLK4β (transfection was performed as described above), selection with 500 μg/mL G418 was done for 10 weeks due to very slow cell growth. Two PCR positive clones for each construct were used to measure the cell proliferation rate using the CyQUANT NF Cell Proliferation Assay (Invitrogen) according to the manufacturer’s protocol. Briefly, cells were plated at a density of 100–500 cells per well in a 96-well plate (8–12 identical wells in total). Parental MCF7 cells stably transfected with the pCMV-Tag3A vector were used as a control. The number of cells per well was counted every 24 hours: growth medium was removed, 80 μL of green-fluorescent CyQUANT GR dye (which exhibits strong fluorescence enhancement when bound to cellular nucleic acid) was added to the well and incubated for 30 min at 37 °C. The fluorescence intensity of each sample was measured using a fluorescence microplate reader with excitation at 485 nm and emission detection at 530 nm (SpectraMax Gemini, Molecular Devices, Sunnyvale, USA). Plotted data points represent averages of triplicate samples, and the plotted line is a linear regression fit of all data points. The assay is designed to produce a linear analytical response from at least 100–20,000 cells per well in most cell lines.
Transgenic Mice
The pCMV-MLK4β construct(4 ng/μL) was linearized with BstBI and microinjected into the male pronucleus of eggs from B6CBA F1 crosses of common house mice (Mus musculus L.). The eggs were transferred into the pseudopregnant D1 recipient strain. The injection buffer contained 10 mM Tris-HCl, pH 7.5 and 0.1 mM EDTA. A total of 136 eggs were transferred, 56 offspring were genotyped by PCR and seven of them were found to be positive. The transgenic founders were crossed with CD1 mice and F1 progeny were used for the analysis of MLK4β expression in different mouse tissues. Both PCR and RT-PCR were carried out with KINRTF and KINRTR primers for 25 cycles with 25 sec denaturation at 95 °C, 20 sec annealing at 60 °C and 1 min extension at 72 °C; β-actin was used as a housekeeping control (primers ActinHS-Fov and ActinHS_Rev). Animal experiments were approved by North Stockholm Ethical Committee (decision 150/08) on 29 May of 2008.
Acknowledgments
This work was supported by research grants from the Swedish Cancer Society, the Swedish Research Council, the Swedish Foundation for International Cooperation in Research and Higher Education (STINT), the Swedish Institute, the Karolinska Institute and the Russian Foundation for Basic Research. EG was recipient of fellowship from the Concern Foundation/Los Angeles and the Cancer Research Institute/New York.
Footnotes
Disclosure
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers of this paper report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material.
References
- 1.Gallo KA, Johnson GL. Mixed-lineage kinase control of JNK and p38 MAPK pathways. Nat Rev Mol Cell Biol. 2002;3:663–72. doi: 10.1038/nrm906. [DOI] [PubMed] [Google Scholar]
- 2.Schaeffer HJ, Weber MJ. Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol Cell Biol. 1999;19:2435–44. doi: 10.1128/mcb.19.4.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yasuda J, Whitmarsh AJ, Cavanagh J, Sharma M, Davis RJ. The JIP group of mitogen-activated protein kinase scaffold proteins. Mol Cell Biol. 1999;19:7245–4. doi: 10.1128/mcb.19.10.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Treisman R. Regulation of transcription by MAP kinase cascades. Curr Opin Cell Biol. 1996;8:205–15. doi: 10.1016/s0955-0674(96)80067-6. [DOI] [PubMed] [Google Scholar]
- 5.Robinson MJ, Cobb MH. Mitogen-activated protein kinase pathways. Curr Opin Cell Biol. 1997;9:180–6. doi: 10.1016/s0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- 6.Dorow DS, Devereux L, Dietzsch E, Kretser TDe. Identification of a new family of human epithelial protein kinases containing two leucine/isoleucine-zipper domains. Eur J Biochem. 1993;213:701–10. doi: 10.1111/j.1432-1033.1993.tb17810.x. [DOI] [PubMed] [Google Scholar]
- 7.Handley ME, Rasaiyaah J, Barnett J, et al. Expression and function of mixed lineage kinases in dendritic cells. Int Immunol. 2007;19:923–33. doi: 10.1093/intimm/dxm050. [DOI] [PubMed] [Google Scholar]
- 8.Holzman LB, Merritt SE, Fan G. Identification, molecular cloning, and characterization of dual leucine zipper bearing kinase. A novel serine/threonine protein kinase that defines a second subfamily of mixed lineage kinases. J Biol Chem. 1994;269:30808–17. [PubMed] [Google Scholar]
- 9.Sakuma H, Ikeda A, Oka S, Kozutsumi Y, Zanetta JP, Kawasaki T. Molecular cloning and functional expression of a cDNA encoding a new member of mixed lineage protein kinase from human brain. J Biol Chem. 1997;272:28622–9. doi: 10.1074/jbc.272.45.28622. [DOI] [PubMed] [Google Scholar]
- 10.Liu TC, Huang CJ, Chu YC, et al. Cloning and expression of ZAK, a mixed lineage kinase-like protein containing a leucine-zipper and a sterile-alpha motif. Biochem Biophys Res Commun. 2000;274:811–6. doi: 10.1006/bbrc.2000.3236. [DOI] [PubMed] [Google Scholar]
- 11.Gotoh I, Adachi M, Nishida E. Identification and characterization of a novel MAP kinase kinase kinase, MLTK. J Biol Chem. 2001;276:4276–86. doi: 10.1074/jbc.M008595200. [DOI] [PubMed] [Google Scholar]
- 12.Handley ME, Rasaiyaah J, Chain BM, Katz DR. Mixed lineage kinases (MLKs): a role in dendritic cells, inflammation and immunity? Int J Exp Pathol. 2007;88:111–26. doi: 10.1111/j.1365-2613.2007.00531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang LH, Besirli CG, Johnson EM. Mixed-lineage kinases: a target for the prevention of neurodegeneration. Annu Rev Pharmacol Toxicol. 2004;44:451–74. doi: 10.1146/annurev.pharmtox.44.101802.121840. [DOI] [PubMed] [Google Scholar]
- 14.Silva RM, Kuan CY, Rakic P, Burke RE. Mixed lineage kinase-c-jun N- terminal kinase signaling pathway: a new therapeutic target in Parkinson’s disease. Movement Disorders. 2007;20:653–64. doi: 10.1002/mds.20390. [DOI] [PubMed] [Google Scholar]
- 15.Ing YL, Leung IW, Heng HH, Tsui LC, Lassam NJ. MLK-3: identification of a widely-expressed protein kinase bearing an SH3 domain and a leucine zipper-basic region domain. Oncogene. 1994;9:1745–50. [PubMed] [Google Scholar]
- 16.Kozak M. An analysis of 5′-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987;15:8125–48. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berthon P, Valeri A, Cohen-Akenine A, et al. Predisposing gene for early-onset prostate cancer, localized on chromosome 1q42.2-43. Am J Hum Genet. 1998;62:1416–24. doi: 10.1086/301879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Launonen V, Vierimaa O, Kiuru M, et al. Inherited susceptibility to uterine leiomyomas and renal cell cancer. Proc Natl Acad Sci U S A. 2001;98:3387–92. doi: 10.1073/pnas.051633798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu Z, Maroney AC, Dobrzanski P, Kukekov NV, Greene LA. The MLK family mediates c-Jun N-terminal kinase activation in neuronal apoptosis. Mol Cell Biol. 2001;21:4713–24. doi: 10.1128/MCB.21.14.4713-4724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kvasha SM, Protopopov AI, Zabarovsky ER, Rynditch AV, Kashuba VI. Isolation, expression analysis and chromosomal mapping of a novel human kinase gene MLK4. Biopolymers and Cell. 2001;17:302–7. [Google Scholar]
- 21.Nagase T, Nakayama M, Nakajima D, Kikuno R, Ohara O. Prediction of the coding sequences of unidentified human genes. XX. The complete sequences of 100 new cDNA clones from brain which code for large proteins in vitro. DNA Res. 2001;8:85–95. doi: 10.1093/dnares/8.2.85. [DOI] [PubMed] [Google Scholar]
- 22.Kaneko T, Li L, Li SS. The SH3 domain—a family of versatile peptide- and protein-recognition module. Front Biosci. 2008;13:4938–52. doi: 10.2741/3053. [DOI] [PubMed] [Google Scholar]
- 23.Landschulz WH, Johnson PF, McKnight SL. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988;240:1759–64. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- 24.Nagata K, Puls A, Futter C, et al. The MAP kinase MLK2 co-localizes with activated JNK along microtubules and associates with kinesin superfamily motor KIF3. EMBO J. 1998;17:149–58. doi: 10.1093/emboj/17.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bock BC, Vacratsis PO, Qamirani E, Gallo KA. Cdc42-induced activation of the mixed-lineage kinase SPRK in vivo. Requirement of the Cdc42/Rac interactive binding motif and changes in phosphorylation. J Biol Chem. 2000;275:14231–41. doi: 10.1074/jbc.275.19.14231. [DOI] [PubMed] [Google Scholar]
- 26.Cho YY, Bode AM, Mizuno H, Choi BY, Choi HS, Dong Z. A novel role for mixed-lineage kinase-like mitogen-activated protein triple kinase alpha in neoplastic cell transformation and tumor development. Cancer Res. 2004;64:3855–64. doi: 10.1158/0008-5472.CAN-04-0201. [DOI] [PubMed] [Google Scholar]
- 27.Ren R, Mayer BJ, Cicchetti P, Baltimore D. Identification of a ten-amino acid proline-rich SH3 binding site. Science. 1993;259:1157–61. doi: 10.1126/science.8438166. [DOI] [PubMed] [Google Scholar]
- 28.Ermekova KS, Zambrano N, Linn H, et al. The WW domain of neural protein FE65 interacts with proline-rich motifs in Mena, the mammalian homolog of Drosophilaenabled. J Biol Chem. 1997;272:32869–77. doi: 10.1074/jbc.272.52.32869. [DOI] [PubMed] [Google Scholar]
- 29.Hartkamp J, Troppmair J, Rapp UR. The JNK/SAPK activator mixed lineage kinase 3 (MLK3) transforms NIH 3T3 cells in a MEK-dependent fashion. Cancer Res. 1999;59:2195–202. [PubMed] [Google Scholar]
- 30.Lambert JM, Karnoub AE, Graves LM, Campbell SL, Der CJ. Role of MLK3-mediated activation of p70 S6 kinase in Rac1 transformation. J Biol Chem. 2002;277:4770–7. doi: 10.1074/jbc.M109379200. [DOI] [PubMed] [Google Scholar]
- 31.Bardelli A, Parsons DW, Silliman N, et al. Mutational analysis of the tyrosine kinome in colorectal cancers. Science. 2003;300:949. doi: 10.1126/science.1082596. [DOI] [PubMed] [Google Scholar]
- 32.Shao RX, Kato N, Lin LJ, et al. Absence of tyrosine kinase mutations in Japanese colorectal cancer patients. Oncogene. 2007;26:2133–5. doi: 10.1038/sj.onc.1210007. [DOI] [PubMed] [Google Scholar]
- 33.Soung YH, Lee JW, Kim SY, et al. Kinase domain mutation of MLK4 gene is uncommon in gastric and hepatocellular carcinomas. Dig Liver Dis. 2006;38:283. doi: 10.1016/j.dld.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 34.Brancho D, Ventura JJ, Jaeschke A, Doran B, Flavell RA, Davis RJ. Role of MLK3 in the regulation of mitogen-activated protein kinase signaling cascades. Mol Cell Biol. 2005;25:3670–81. doi: 10.1128/MCB.25.9.3670-3681.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berthon P, Valeri A, Cohen-Akenine A, et al. Predisposing gene for early-onset prostate cancer, localized on chromosome 1q42.2-43. Am J Hum Genet. 1998;62:1416–24. doi: 10.1086/301879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Launonen V, Vierimaa O, Kiuru M, et al. Inherited susceptibility to uterine leiomyomas and renal cell cancer. Proc Natl Acad Sci U S A. 2001;98:3387–92. doi: 10.1073/pnas.051633798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zabarovsky ER, Boldog F, Erlandsson R, et al. New strategy for mapping the human genome based on a novel procedure for construction of jumping libraries. Genomics. 1991;11:1030–9. doi: 10.1016/0888-7543(91)90029-e. [DOI] [PubMed] [Google Scholar]
- 38.Zabarovsky ER, Allikmets R, Kholodnyuk I, et al. Construction of representative NotI linking libraries specific for the total human genome and for human chromosome 3. Genomics. 1994;20:312–6. doi: 10.1006/geno.1994.1175. [DOI] [PubMed] [Google Scholar]
- 39.Wang JY, Zabarovsky ER, Talmadge C, et al. Somatic cell hybrid panel and NotI linking clones for physical mapping of the human chromosome 3. Genomics. 1994;20:105–13. doi: 10.1006/geno.1994.1133. [DOI] [PubMed] [Google Scholar]
- 40.Kutsenko AS, Gizatullin RZ, Al-Amin AN, et al. NotI flanking sequences: a tool for gene discovery and verification of the human genome. Nucleic Acids Res. 2002;30:3163–70. doi: 10.1093/nar/gkf428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zabarovsky ER, Allikmets RL. An improved technique for the efficient construction of gene libraries by partial filling-in of cohesive ends. Gene. 1986;42:119–23. doi: 10.1016/0378-1119(86)90158-7. [DOI] [PubMed] [Google Scholar]
- 42.Kashuba VI, Protopopov AI, Kvasha SM, et al. hUNC93B1: a novel human gene representing a new gene family and encoding an unc-93-like protein. Gene. 2002;283:209–17. doi: 10.1016/s0378-1119(01)00856-3. [DOI] [PubMed] [Google Scholar]
- 43.Protopopov AI, Gizatullin RZ, Vorobieva NV, et al. Human chromosome 3: high-resolution fluorescence in situ hybridization mapping of 40 unique NotI linking clones homologous to genes and cDNAs. Chromosome Res. 1996;4:443–7. doi: 10.1007/BF02265051. [DOI] [PubMed] [Google Scholar]
- 44.Pokrovskaja K, Mattsson K, Kashuba E, Klein G, Szekely L. Proteasome inhibitor induces nucleolar translocation of Epstein-Barr virus-encoded EBNA-5. J Gen Virol. 2001;82:345–8. doi: 10.1099/0022-1317-82-2-345. [DOI] [PubMed] [Google Scholar]