Abstract
Adult malignant brainstem gliomas (BSGs) are poorly characterized due to their relative rarity. We have examined histopathologically confirmed cases of adult malignant BSGs to better characterize the patient and tumor features and outcomes, including the natural history, presentation, imaging, molecular characteristics, prognostic factors, and appropriate treatments. A total of 34 patients were identified, consisting of 22 anaplastic astrocytomas (AAs) and 12 glioblastomas (GBMs). The overall median survival for all patients was 25.8 months, with patients having GBMs experiencing significantly worse survival (12.1 vs. 77.0 months, p = 0.0011). The majority of tumors revealed immunoreactivity for EGFR (93.3 %) and MGMT (64.7 %). Most tumors also exhibited chromosomal abnormalities affecting the loci of epidermal growth factor receptor (92.9 %), MET (100 %), PTEN (61.5 %), and 9p21 (80 %). AAs more commonly appeared diffusely enhancing (50.0 vs. 27.3 %) or diffusely nonenhancing (25.0 vs. 0.0 %), while GBMs were more likely to exhibit focal enhancement (54.6 vs. 10.0 %). Multivariate analysis revealed confirmed histopathology for GBM to significantly affect survival (HR 4.80; 95 % CI 1.86–12.4; p = 0.0012). In conclusion, adult malignant BSGs have an overall poor prognosis, with GBM tumors faring significantly worse than AAs. As AAs and GBMs have differing imaging characteristics, tissue diagnosis may be necessary to accurately determine patient prognosis and identify molecular characteristics which may aid in the treatment of these aggressive tumors.
Keywords: Adult, Brainstem glioma, Clinicopathological, Molecular, Treatment
Introduction
Brainstem gliomas (BSGs) are primarily found in the pediatric population and are most commonly low grade (WHO I or II), accounting for 10 % of all brain tumors in these patients [1]. These lesions peak in incidence near the first decade of life and have varying prognoses [2]. Overall survival for low grade BSGs in children is poor, with less than 30 % surviving after 5 years [3]. Diffuse and/or high grade BSGs typically have an even poorer prognosis (median survival less than 1 year) compared to those that are low grade, focal, or exophytic [4]. On the other hand, the low grade BSGs which occur near the floor of the 4th ventricle and at the cervicomedullary junction have a median survival of more than 5 years as they often have exophytic components that are amenable to resection [5, 6]. Currently, BSGs in children are primarily diagnosed by their characteristic appearance on magnetic resonance imaging (MRI) due to the high risks of biopsy.
Adult BSGs are less well-characterized due to their rarity, only accounting for approximately 2 % of all brain tumors in adults [7]. The few studies that have reported on these tumors have suggested an improved survival compared to those in children, with a median survival of 7 years and a 5-year survival rate of 58 % [8]. Unlike children, BSGs in adults tend to be more clinically and radiographically heterogenous. In this population, diffuse intrinsic low grade BSGs are the most frequent subtype and have a median survival of 7.3 years, compared to malignant BSGs which have a median survival of 11.2–17 months [8, 9].
To our knowledge there are currently no studies that have examined the natural history, presentation and imaging characteristics, prognostic factors, and appropriate treatments for adult BSGs. In this study, we have performed a retrospective study of 34 histopathologically confirmed cases of adult malignant BSGs to better characterize the features and outcomes of these challenging tumors.
Methods
This study was approved by the institutional review board. The medical records of adult patients (18+ years) with brainstem lesions who were treated from 1998 to 2011 at the Duke University Medical Center were reviewed. All patients underwent either stereotactic biopsy or surgical resection. The pathology was determined by a senior board-certified neuropathologist initially by using standard H&E stained sections supplemented with additional diagnostic immunohistochemistries (IHC), which included glial fibrillary acidic protein (GFAP), MGMT, Ki-67, and s100, as well as synaptophysin and neurofilament protein to evaluate evidence of brain invasion. Also studied by IHC in the more recent cases were vascular endothelial growth factor (VEGF), VEGF receptor 2 (KDR), carbonic anhydrase IX (CAIX), and isocitrate dehydrogenase 1. The histopathological diagnoses of anaplastic astrocytoma (AA; WHO grade III) or glioblastoma (GBM; WHO grade IV) were rendered based on World Health Organization criteria. Additionally, epidermal growth factor receptor (EGFR) and phosphatase and tensin homolog (PTEN) chromosomal loci were studied by fluorescence in situ hybridization (FISH).
Variables analyzed included demographic parameters, presenting signs and symptoms, the duration of symptoms, karnofsky performance status (KPS), tumor histology and location, imaging characteristics, procedure performed (biopsy or resection), and the use of adjuvant therapy. Imaging analysis was performed by a board-certified neuroradiologist. The primary location and secondary sites of involvement were classified, as was supratentorial extension (defined as either T2 signal abnormality or enhancement above the plane of the tentorial incisura) and multifocality. Primary tumor location was defined as the area in which the tumor mass was centered, with secondary sites representing surrounding areas with tumor infiltration. Multifocality represented the presence of multiple areas of enhancement in different brainstem regions. Lesions were characterized either as focal or diffuse, and T1 post-contrast appearance was defined as either enhancing or non-enhancing. Tumors were therefore classified into four categories (diffuse enhancing, diffuse nonenhancing, focal enhancing, and focal nonenhancing) as previously described [10]. Focal tumors were defined as those with well-defined margins and occupied <50 % of the axial diameter of the brainstem while diffuse tumors were those with poorly-defined margins and occupied more than 50 % of the brainstem axial diameter. Tumor volume was determined using the greatest dimensions in three planes on contrast-enhanced MRI.
Statistical analysis
The data were summarized using medians for continuous variables, and counts and percentages for categorical variables. Differences in continuous variables were analyzed using the Mann–Whitney U test while categorical variables were evaluated using the Chi-squared test.
Survival was estimated using the Kaplan–Meier method with the log-rank test being used to evaluate the differences between survival curves. Univariate and stepwise multivariate analyses were performed to determine the effect of various patient, tumor, imaging, and treatment variables on overall survival. Values with p < 0.05 were considered statistically significant. All analyses were performed using SAS 9.3 (SAS Institute, Cary, NC, USA).
Results
Patient characteristics
A total of 34 adult patients with malignant BSGs were identified, consisting of 22 AA (64.7 %) and 12 GBM (35.3 %) cases. The median age of all patients was 42.5 years (range: 18–71 years), with patients having GBM tumors being older than those with anaplastic astrocytomas (AAs) (58 vs. 34 years, p = 0.063) (Table 1). While females accounted for 44.1 % of patients, significantly more females had AA tumors (59.1 vs. 16.7 %, p = 0.017). The majority of patients were caucasian (73.5 %).
Table 1.
Patient, tumor, treatment, and outcome characteristics of adult patients with malignant brainstem gliomas
| Characteristics | All Patients | Grade III | Grade IV | P value |
|---|---|---|---|---|
| Demographics | ||||
| Age, median | 42.5 | 34 | 58 | 0.063 |
| Gender, (female, %) | 44.1 | 59.1 | 16.7 | 0.017 |
| Race (%) | 0.054 | |||
| Caucasian | 73.5 | 86.4 | 50 | |
| Black | 23.5 | 13.6 | 41.7 | |
| Other | 2.9 | 0 | 8.3 | |
| Presentation | ||||
| DOS, median | 2.0 | 2.0 | 1.3 | 0.26 |
| KPS, median | 80.0 | 80.0 | 80.0 | 0.61 |
| No. of CN deficits, median | 3.0 | 3.0 | 2.5 | 0.75 |
| Ataxia/Gait disturbance (%) | 42.4 | 38.1 | 50.0 | – |
| Headache (%) | 27.3 | 33.3 | 16.7 | – |
| Nausea (%) | 18.2 | 23.8 | 8.3 | – |
| Facial weakness (%) | 24.2 | 9.5 | 50.0 | – |
| Facial numbness (%) | 15.2 | 14.3 | 16.7 | – |
| Emesis (%) | 15.2 | 19.0 | 8.3 | – |
| Diplopia (%) | 24.2 | 23.8 | 25.0 | – |
| Vertigo/dizziness (%) | 9.1 | 9.5 | 8.3 | – |
| Hearing loss (%) | 12.1 | 9.5 | 16.7 | – |
| Extremity weakness (%) | 18.2 | 9.5 | 33.3 | – |
| Blurry vision (%) | 12.1 | 9.5 | 16.7 | – |
| Dysarthria (%) | 9.1 | 4.8 | 16.7 | – |
| Paresthesia (%) | 6.1 | 4.8 | 8.3 | – |
| Tinnitus (%) | 3.0 | 0.0 | 8.3 | – |
| Tumor | ||||
| Primary location | 0.77 | |||
| Midbrain (%) | 15.2 | 13.6 | 18.2 | |
| Tectal plate (%) | 6.1 | 9.1 | 0 | |
| Pons (%) | 51.5 | 50.0 | 54.6 | |
| Medulla (%) | 27.3 | 27.3 | 27.3 | |
| Multifocal (%) | 35.5 | 40.0 | 27.3 | 0.48 |
| Supratentorial extension (%) | 35.5 | 30.0 | 45.5 | 0.39 |
| Tumor volume (cm3) | 5.4 | 5.4 | 6.8 | 0.89 |
| Imaging | ||||
| Category (%) | 0.028 | |||
| Diffuse enhancing | 41.9 | 50.0 | 27.3 | |
| Diffuse nonenhancing | 16.1 | 25.0 | 0.0 | |
| Focal enhancing | 25.8 | 10.0 | 54.6 | |
| Focal nonenhancing | 16.1 | 15.0 | 18.2 | |
| Enhancement (%) | 68.8 | 61.9 | 81.8 | |
| Treatment | ||||
| Resection (%) | 33.3 | 33.3 | 33.3 | 1.0 |
| Biopsy (%) | 66.7 | 66.7 | 66.7 | 1.0 |
| Radiation therapy (%) | 97.0 | 100 | 91.7 | 0.33 |
| Temozolomide (%) | 97.0 | 100 | 91.7 | 0.33 |
| Initial | 100 | 100 | 100 | – |
| Other chemotherapy (%) | 53.6 | 47.1 | 63.6 | 0.39 |
| Initial | 13.3 | 12.5 | 14.3 | – |
| On progression | 86.7 | 87.5 | 85.7 | – |
| Bevacizumab (%) | 33.3 | 29.4 | 40.0 | 0.57 |
| Initial | 22.2 | 20.0 | 25.0 | – |
| On progression | 77.8 | 80.0 | 75.0 | – |
| Irinotecan (%) | 25.9 | 23.5 | 30.0 | 0.71 |
| On progression | 100 | 100 | 100 | – |
| Outcome | ||||
| Progression-free survival | 6.7 | 6.1 | 6.7 | 0.22 |
| Overall survival | 25.8 | 77.0 | 12.1 | 0.0011 |
CN cranial nerve, DOS duration of symptoms, KPS Karnofsky performance status, No number
Clinical presentation
The median KPS at diagnosis was 80 (range: 50–90), with the median duration of symptoms prior to presentation being 2 months (range: 0–24 months) (Table 1). These variables did not differ significantly between AA and GBM tumors. Most patients presented with at least one cranial nerve palsy (88.2 %), with patients having a median of 3.0 cranial nerve deficits. The most common presenting symptoms were ataxia/gait disturbance (42.4 %), headache (27.3 %), diplopia (24.2 %), and facial weakness (24.2 %). Patients most uncommonly experienced dysarthria (9.1 %), vertigo/dizziness (9.1 %), parasthesias (6.1 %), and tinnitus (3.0 %).
Tumor characteristics
Tumors were most commonly centered in the pons (51.5 %), followed by the medulla (27.3 %), midbrain (15.2 %), and tectal plate (6.1 %), with AA and GBM tumors having a similar location distribution (Table 1). The majority of tumors (72.7 %) were seen to extend into other brainstem regions. Most midbrain tumors extended into the tectal plate, while all tectal tumors extended into the midbrain. Pontine tumors most frequently remained confined to the pons (37.5 %), with the rest of these tumors extending into the midbrain (31.3 %) or medulla (31.3 %). Most medullary tumors extended into the pons (77.8 %), with the remaining being localized to the medulla. Multifocality and supratentorial extension were each present in 35.5 % of cases, and did not significantly differ between AA and GBM tumors (p = 0.48 and 0.39, respectively). Tumor volume was also similar between groups (5.4 vs. 6.8 cm3, p = 0.89).
Immunohistochemical and molecular profiles
Immunohistochemical examination revealed all tested tumors to express GFAP (n = 28). All cases revealed intracellular synaptophysin and neurofilament protein indicative of infiltrative tumors. Four of seven (57.1 %) tumors revealed macrophage infiltrates immunoreactive for HAM56. The mean Ki-67 proliferation index was 14 %, ranging from 1 to 70 %. AA tumors were seen to have a mean proliferation index of 16.1 % compared to 8.6 % for GBM tumors. Fourteen of 15 (93.3 %) revealed immunoreactivity for EGFR while MGMT expression was identified in 64.7 % (11 of 17) of cases. Most grade IV tumors (83.3 %) expressed MGMT while only 54.5 % of grade III tumors were seen to stain positive. Two of 16 (12.5 %) tumors tested expressed EGFR variant III (EGFRvIII). Also found to be expressed in select tumors included CAIX (42.9 %; 3/7), VEGF (75 %; 3/4), KDR (50 %; 2/4), platelet-derived growth factor receptor-A (PDGFR-A) (100 %; 3/3), PDGFR-B (100 %; 3/3), and hypoxia-inducible factor-2 alpha (HIF-2 alpha) (100 %; 1/1).
Fluorescence in situ hybridization analysis revealed most tumors to exhibit chromosomal numerical abnormalities (CNA) affecting the loci of EGFR (92.9 %; 13/14), MET (100 %; 4/4), PTEN (61.5 %; 8/13), and 9p21 (80 %; 4/5). The most common type of EGFR CNA observed was polysomy (84.6 %), followed by amplification (15.4 %). High EGFR copy numbers were also common, with a mean copy number of 6.3 (range: 3.1–22.3). MET gain mutations were seen in all tumors tested, with a mean copy number of 3.9 indicating CNA affecting the whole of chromosome 7. However, PTEN most commonly had monosomies (87.5 %), with few cases exhibiting polysomy (12.5 %). IDH mutations were seen in 40 % of tested cases (2 of 5), all of which were AAs.
Imaging features
Various patterns were identified on MRI, including diffuse enhancement (41.9 %), diffuse non-enhancement (16.1 %), focal enhancement (25.8 %), and focal non-enhancement (16.1 %). Overall pattern distribution differed significantly between AA and GBM tumors (p = 0.028). AAs more commonly appeared diffusely enhancing (50.0 vs. 27.3 %) and diffusely nonenhancing (25.0 vs. 0.0 %), while GBMs were more likely to appear focal enhancing (54.6 vs. 10.0 %). The focal nonenhancing pattern was seen in both AA and GBM tumors at similar rates (15.0 vs. 18.2 %). Overall enhancement upon gadolinium administration was seen in 68.8 % of tumors, with AA and GBM tumors enhancing at similar rates (61.9 vs. 81.8 %, p = 0.25) (Table 1).
Treatment
All patients underwent either stereotactic biopsy (66.7 %) or surgical resection (33.3 %), with no difference in procedure choice between AA and GBM tumors (p = 1.0). Procedure choice was also not seen to differ based on tumor location. Nearly all patients underwent radiotherapy (97.0 %), receiving from 5,580 to 6,300 cGy. Most patients also received concurrent temozolomide (97.0 %), ranging from 75 to 300 mg/m2 (58.8 % received 150–300 mg/m2 for days 1–5 every 28 days and 41.2 % received 75 mg/m2 daily). Other chemotherapeutics were also used in 53.6 % of patients upon tumor progression, most commonly consisting of irinotecan (37.0 %; 70–125 mg/m2), bevacizumab (33.3 %; 10–15 mg/kg), lomustine (14.8 %; 110 mg/m2) and etoposide (14.8 %; 50–100 mg/m2).
Outcome
Following stereotactic biopsy, no patients experienced postoperative complications. Of those who underwent surgical resection, 1 (9.1 %) patient had new neurological deficit while another experienced a cerebrospinal fluid leak. No significant change in overall performance status was seen postoperatively.
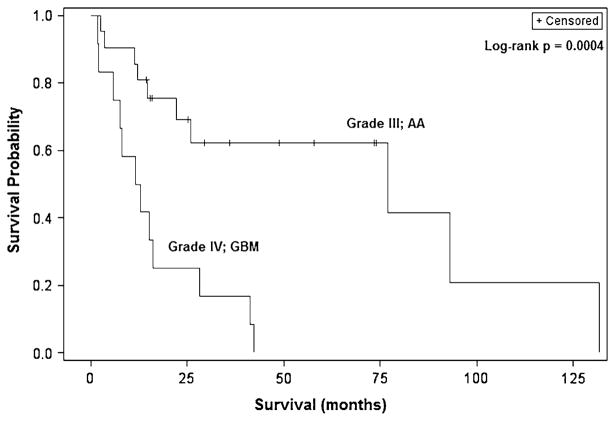
The overall median survival for all patients was 25.8 months, with patients having GBM tumors experiencing significantly worse survival than those with AA tumors (12.1 vs. 77.0 months, p = 0.0011) (Tables 1 and 2; Fig. 1). Progression-free survival was similar in both groups. Univariate analysis revealed age greater than 40 years (HR 3.59;95 %CI 1.37–9.44; p = 0.0096), midbrain location (HR 3.25; 95 % CI 1.01–10.4; p = 0.048), and grade IV pathology (HR 4.05; 1.64–10.0; p = 0.0025) to be significant predictors of worse survival. Duration of symptoms for 2 months or greater was the only variable associated with improved survival (HR 0.39; 95 % CI 0.16–0.96; p = 0.039).
Table 2.
Univariate and log-rank analysis of various patient, tumor, imaging and treatment characteristics
| Characteristic | HR (95 % CI) | P value | Survival (months) | P value |
|---|---|---|---|---|
| Patient characteristics | ||||
| Age, years | ||||
| < 40 | 1.00 | N/A | 77.0 | |
| ≥40 | 3.59 (1.37–9.44) | 0.0096 | 14.5 | 0.0058 |
| Gender | ||||
| Male | 1.00 | N/A | 16.2 | |
| Female | 0.81 (0.34–1.90) | 0.62 | 25.8 | 0.62 |
| Race | ||||
| Non-caucasian | 1.00 | N/A | 12.8 | |
| Caucasian | 0.49 (0.20–1.21) | 0.12 | 42.1 | 0.11 |
| Presentation characteristics | ||||
| DOS, months | ||||
| < 2 | 1.00 | N/A | 12.0 | |
| ≥2 | 0.39 (0.16–0.96) | 0.039 | 42.1 | 0.033 |
| KPS | ||||
| ≥80 | 1.00 | N/A | 41.3 | |
| < 80 | 1.92 (0.79–4.63) | 0.15 | 12.0 | 0.14 |
| No. of CN deficits | ||||
| < 3 | 1.00 | N/A | 25.8 | |
| ≥3 | 1.39 (0.59–3.27) | 0.45 | 28.1 | 0.45 |
| Tumor characteristics | ||||
| Location | ||||
| Pons | 1.00 | N/A | 42.1 | |
| Tectal plate | 2.89 (0.58–14.4) | 0.20 | 18.9 | 0.18 |
| Midbrain | 3.25 (1.01–10.4) | 0.048 | 5.9 | 0.039 |
| Medulla | 1.41 (0.49–4.08) | 0.53 | 41.3 | 0.53 |
| Unifocal | 1.00 | N/A | 28.1 | |
| Multifocal | 1.32 (0.51–3.41) | 0.57 | 16.2 | 0.57 |
| Local disease | 1.00 | N/A | 41.3 | |
| Supratentorial extension | 1.34 (0.54–3.37) | 0.52 | 16.2 | 0.52 |
| Tumor volume | 1.00 (0.98–1.03) | 0.94 | N/A | – |
| Grade III | 1.00 | N/A | 77.0 | |
| Grade IV | 4.05 (1.64–10.0) | 0.0025 | 12.1 | 0.0011 |
| Imaging characteristics | ||||
| Category (%) | ||||
| Diffuse enhancing | 1.00 | N/A | 28.1 | |
| Diffuse nonenhancing | 0.54 (0.11–2.63) | 0.44 | 77.0 | 0.44 |
| Focal enhancing | 2.06 (0.71–5.92) | 0.18 | 13.6 | 0.17 |
| Focal nonenhancing | 1.35 (0.34–5.30) | 0.67 | 41.3 | 0.67 |
| Non-enhancing | 1.00 | N/A | 41.3 | |
| Enhancing | 1.36 (0.49–3.78) | 0.56 | 16.2 | 0.55 |
| Treatment characteristics | ||||
| Biopsy | 1.00 | N/A | 22.0 | |
| Resection | 0.64 (0.25–1.68) | 0.37 | 42.1 | 0.36 |
| No other chemotherapy | 1.00 | N/A | Not reached | |
| Other chemotherapy | 2.10 (0.73–6.06) | 0.17 | 16.2 | 0.16 |
| No bevacizumab | 1.00 | N/A | 28.1 | |
| Bevacizumab | 1.12 (0.38–3.28) | 0.84 | 16.2 | 0.84 |
| No Irinotecan | 1.00 | N/A | 28.1 | |
| Irinotecan | 1.19 (0.40–3.48) | 0.76 | 22.0 | 0.76 |
CN cranial nerve, DOS duration of symptoms, KPS Karnofsky performance status, NA not applicable, No number
Fig. 1.
Kaplan–Meier overall survival curves for adult patients with malignant BSGs showing significantly worse survival for those with GBM tumors compared to AAs
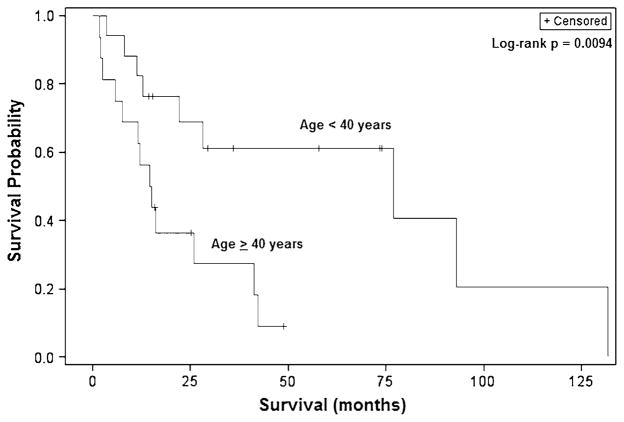
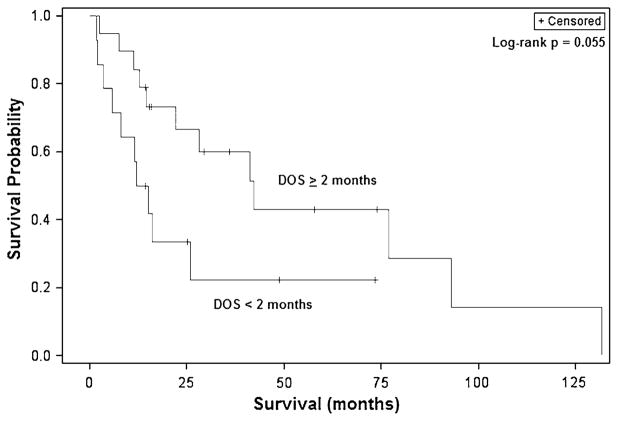
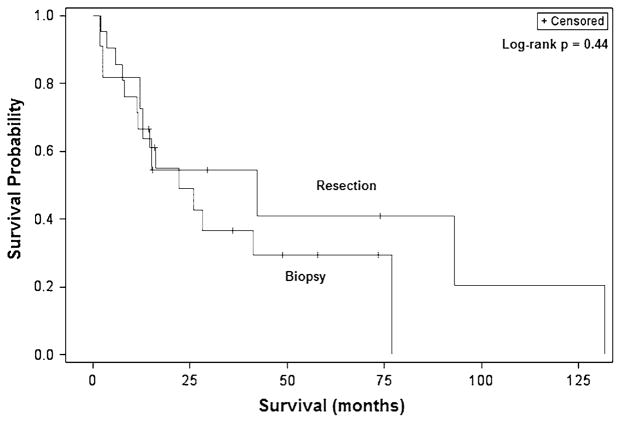
Similarly, log-rank analysis demonstrated patients 40 years and older to have a significantly worse median survival than younger patients (14.5 vs. 77.0 months, p = 0.0058) (Fig. 2). Patients with tumors in the midbrain also had a poorer survival than those with tumors in the pons (5.9 vs. 42.1 months, p = 0.039). Patients experiencing symptoms for two or more months experienced improved survival and had a median survival of 42.1 months compared to 12.0 months for those presenting more acutely (p = 0.033) (Fig. 3). Notably, while surgical resection nearly doubled survival compared to biopsy alone (42.1 vs. 22.0 months), this difference was not statistically significant (p = 0.36) (Fig. 4). Stratification by tumor location revealed a slight benefit with resection for midbrain and pontine tumors though this also was not significant.
Fig. 2.
Kaplan–Meier overall survival curves for adult patients with malignant BSGs demonstrating significantly worse survival for patients aged 40 years and older
Fig. 3.
Kaplan–Meier overall survival curves for adult patients with malignant BSGs showing worse survival for those with presenting with duration of symptoms less than 2 months
Fig. 4.
Kaplan–Meier overall survival curves for adult patients with malignant BSGs demonstrating resection to not confer any survival benefit compared to biopsy alone
Multivariate stepwise analysis revealed the duration of symptoms, KPS, and GBM pathology to affect survival. As seen in the univariate analysis, duration of symptoms 2 months and greater was associated with improved survival (HR 0.33; 95 % CI 0.12–0.88; p = 0.026). KPS less than 80 (HR 2.85; 95 % CI 1.11–7.34; p = 0.030) and GBM pathology (HR 4.80; 95 % CI 1.86–12.4; p = 0.0012) were seen to be significant independent predictors of survival.
Discussion
Brainstem gliomas in adults are an uncommon, genetically and radiographically heterogeneous group of tumors. In the few retrospective studies of adult BSGs in the literature, there has been little discussion of histologically proven high grade tumors due to the small number of overall cases. In the current study, we have evaluated to our knowledge the largest collection of adult malignant BSGs to help better define the associated patient, tumor, imaging, and outcome characteristics.
Outcome and prognostic factors
In this study, the overall median survival for all patients was 25.8 months. We have demonstrated age greater than 40 years, duration of symptoms, midbrain location, and grade IV pathology to result in significantly worse median survival on univariate analysis. However, KPS <80 and GBM pathology were seen to be poor independent predictors of survival while duration of symptoms >2 months was a favorable independent predictor for survival. Though age is a strong prognostic factor in supratentorial GBM cases, adjustment for grade IV pathology significantly attenuated the effect of older age, suggesting that the effect of older age in the univariate analysis was due to the higher incidence of GBM tumors in these patients [11–14]. Therefore, while previous studies have grouped grade III and IV tumors together using the general term “malignant BSGs”, our data supports significantly different prognosis between the two groups. Also as seen in supratentorial malignant gliomas, low preoperative KPS was a significant predictor of worse survival. In contrast, duration of symptoms 2 months and greater, which did not significantly different between grades, was seen to result in improved survival. Previous studies have also supported increased survival of those with a longer history of symptoms, suggesting that these patients have less aggressive tumors [9].
Imaging and tumor characteristics
Adult BSGs may have a heterogenous appearance on MRI and, therefore, it cannot be reliably used to predict tumor grade. A study by Dellaretti et al. [10] demonstrated MRI findings to vary for malignant BSGs, with these lesions occurring in 21.9 % of diffuse non-enhancing, 58.1 % of diffuse enhancing, and 30.4 % of focal enhancing lesions. No high grade tumors were found in lesions appearing focal and non-enhancing. This study has similarly demonstrated malignant BSG tumors to have varying appearances. Interestingly, the imaging patterns significantly differed between AA and GBM tumors, potentially aiding the preoperative identification of more aggressive malignant BSGs. GBM tumors were more likely to appear focal enhancing (54.6 %), with none of these tumors appearing diffusely non-enhancing. In contrast, AA tumors more commonly appeared diffusely enhancing (50.0 %) or diffusely non-enhancing (25.0 %). Additional studies are needed to characterize these tumors using diffusion and perfusion imaging so that further unique imaging features can be ascertained for preoperative identification.
This study is to our knowledge the first to describe the molecular characteristics of adult malignant BSGs. MGMT has been the subject of debate for its role in conferring a survival advantage when treated with temozolomide due to enhanced sensitivity to alkylating agents. MGMT promoter methylation, leading to gene silencing, has been demonstrated in 45–80 % of supratentorial malignant gliomas [15–17]. While MGMT protein expression was only absent in 35.3 % of patients, malignant BSGs likely have a similar molecular profile as supratentorial malignant gliomas and may therefore be equally responsive to temozolomide therapy. Interestingly, the vast majority of grade IV BSGs exhibited MGMT expression, potentially indicating these tumors to be more resistant to temozolomide compared to AA tumors. The EGFR locus is also commonly altered in malignant gliomas, with polysomy being found in nearly 100 % of high grade astrocytomas and amplifications occurring in 10 % of grade III and 45 % of grade IV tumors [18, 19]. Malignant BSGs are similarly affected, with 92.9 % of tested tumors having chromosomal abnormalities. Nonetheless, the small sample size of tumors genetically tested in this study precludes definitive conclusions and further studies are needed to better characterize malignant BSGs. Furthermore, the reliability of IHC to characterize MGMT status is limited due to the poor correlation between MGMT protein expression and promoter methylation. Additional studies using polymerase chain reaction to genetically analyze malignant BSGs are therefore needed.
Diagnosis and treatment
In the pediatric population, MRI is typically used for the diagnosis and classification of BSGs, with treatment often administered without pathological confirmation. However in adults, BSGs may have a more heterogeneous appearance, raising the concern for other types of lesions. Therefore, radiological features are not sufficient for the diagnosis and classification of these tumors in adults. As 31.2 % of malignant BSGs may appear as focally or diffusely non-enhancing lesions, there is considerable risk in classifying non-enhancing lesions as low grade tumors. Therefore tissue diagnosis is indispensable for making the correct diagnosis, determining prognosis and guiding treatment.
Many studies have evaluated the safety and efficiency of biopsy. A study involving stereotactic brainstem biopsies in 46 adults only demonstrated one case of perioperative morbidity, with no permanent morbidity or mortality in any patients [20]. However other studies have demonstrated significant complications following biopsy, occurring in up to 10 % of cases [21]. In a study by Dellaretti et al. [10] in which 96 brainstem biopsies were performed, there was one procedure-related death, with nine patients experiencing a slight deterioration in symptoms. In our study, we did not observe any morbidity or mortality following biopsy. Additionally, biopsy is frequently able to achieve pathological diagnosis, with typical diagnosis rates>90 % [10, 22, 23].
Currently the role of resection in the treatment of BSGs is controversial. Resection in this highly eloquent region can involve significant morbidity and mortality. However attempts at surgical resection have demonstrated improved survival in cases involving focal tumors. A study by Kestle et al. [24] involved resection of brainstem juvenile pilocytic astrocytoma in 28 patients, with gross total resection (GTR) or resection with linear enhancement (RLE) achieved in 12 of 25 patients. Six of 28 patients experienced long-term new neurological deficits following resection. However, survival was increased following greater resection, with 10-year progression free survival being 74 % following GTR or RLE, but only 19 % in the presence of solid residual tumor. Another study by Teo et al. [25] evaluated 34 patients undergoing endoscope-assisted microsurgery for focal BSGs and reported GTR in 35 %, near total resection in 56 %, and subtotal in 9 % of patients. As most patients with malignant BSGs received STR and died before the first postoperative year, the authors questioned the justification for resection of these tumors. Teo et al. also addressed the morbidity associated with the resection of these lesions, with postoperative status improving in 65 % of patients and worsening in 15 %. The reported rates of worsened neurological outcome following surgery in other studies range from 15 to 31 % [24–26]. In this study, only one (9.1 %) patient who underwent resection experienced a permanent neurological deficit.
Approximately a third of patients (33.3 %) underwent surgical resection, with surgery being performed for select situations. Resection was considered for tumors that had an exophytic component and could be resected without entering the brainstem parenchyma, tumors suspected of other pathology and could not easily be biopsied, and tumors that resembled lesions unsafe for stereotactic biopsy, e.g. vascular lesions. Patients who underwent resection had a median survival nearly two times longer than those who underwent biopsy alone though this was not significant. While this may be due to the benefit of tumor debulking, the increased survival seen may also be due to favorable clinical and molecular tumor characteristics in the resection group. Due to the limited sample size of this study, larger multicenter studies are needed to provide more conclusive evidence for the use of surgical resection.
Following histological diagnosis, adjuvant therapy primarily consists of fractionated radiotherapy or stereotactic radiosurgery for small focal tumors, followed by chemotherapy. Temozolomide is most commonly used as a first line agent due to the significant survival benefit seen in supratentorial malignant gliomas [27]. However, due to the low incidence of MGMT gene silencing in malignant BSGs, other therapies may prove more efficacious. Evaluation of the effect of bevacizumab and irinotecan did not reveal improvements in survival, potentially indicating malignant BSGs to be more treatment resistant than comparable supratentorial tumors. As a result, prospective studies are greatly needed to evaluate novel agents for the treatment of malignant BSGs in pediatric and adult patients.
Conclusions
Adult malignant BSGs have an overall poor prognosis, with grade IV tumors faring significantly worse than grade III tumors. GBM proven pathology and KPS <80 were independent prognostic factors for worse survival, with duration of symptoms 2 months or greater being a favorable prognostic factor. AA and GBM tumors have varying imaging characteristics with GBM tumors most commonly appearing focally enhancing, while AA tumors tended to appear more diffuse. These tumors less frequently have MGMT gene silencing than supratentorial malignant gliomas, but commonly have EGFR gene aberrations. Treatment involves aggressive chemoradiotherapy, and may involve resection in select cases. If not amenable to resection, tissue diagnosis may be necessary, allowing for the determination of prognosis and molecular characteristics which may aid in the treatment of these aggressive tumors.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Ethical Standards The data collection and analyses performed in this study comply with the current laws of the United States.
Contributor Information
Ranjith Babu, Division of Neurosurgery, Department of Surgery, Duke University Medical Center, 2624, Durham, NC 27710, USA.
Peter G. Kranz, Department of Radiology, Duke University Medical Center, Durham, NC 27710, USA
Vijay Agarwal, Email: vjagarwal@gmail.com, Division of Neurosurgery, Department of Surgery, Duke University Medical Center, 2624, Durham, NC 27710, USA.
Roger E. McLendon, Department of Pathology, Duke University Medical Center, Durham, NC 27710, USA
Steven Thomas, Department of Biostatistics and Bioinformatics, Duke University School of Medicine, Durham, NC 27710, USA.
Allan H. Friedman, Division of Neurosurgery, Department of Surgery, Duke University Medical Center, 2624, Durham, NC 27710, USA
Darell D. Bigner, Preston Robert Tisch Brain Tumor Center, Duke University Medical Center, Durham, NC 27710, USA
Cory Adamson, Division of Neurosurgery, Department of Surgery, Duke University Medical Center, 2624, Durham, NC 27710, USA. Neurosurgery Section, Durham VA Medical Center, Durham, NC, USA.
References
- 1.Farwell JR, Dohrmann GJ, Flannery JT. Central nervous system tumors in children. Cancer. 1977;40:3123–3132. doi: 10.1002/1097-0142(197712)40:6<3123::aid-cncr2820400656>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 2.Tokuriki Y, Handa H, Yamashita J, Okumura T, Paine JT. Brainstem glioma: an analysis of 85 cases. Acta Neurochir (Wien) 1986;79:67–73. doi: 10.1007/BF01407447. [DOI] [PubMed] [Google Scholar]
- 3.Littman P, Jarrett P, Bilaniuk LT, Rorke LB, Zimmerman RA, Bruce DA, Carabell SC, Schut L. Pediatric brain stem gliomas. Cancer. 1980;45:2787–2792. doi: 10.1002/1097-0142(19800601)45:11<2787::aid-cncr2820451113>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 4.Farmer JP, Montes JL, Freeman CR, Meagher-Villemure K, Bond MC, O’Gorman AM. Brainstem Gliomas. A 10-year institutional review. Pediatr Neurosurg. 2001;34:206–214. doi: 10.1159/000056021. [DOI] [PubMed] [Google Scholar]
- 5.Bricolo A, Turazzi S, Cristofori L, Talacchi A. Direct surgery for brainstem tumours. Acta Neurochir Suppl (Wien) 1991;53:148–158. doi: 10.1007/978-3-7091-9183-5_25. [DOI] [PubMed] [Google Scholar]
- 6.Constantini S, Epstein F. Surgical indication and technical considerations in the management of benign brain stem gliomas. J Neurooncol. 1996;28:193–205. doi: 10.1007/BF00250199. [DOI] [PubMed] [Google Scholar]
- 7.White HH. Brain stem tumors occurring in adults. Neurology. 1963;13:292–300. doi: 10.1212/wnl.13.4.292. [DOI] [PubMed] [Google Scholar]
- 8.Kesari S, Kim RS, Markos V, Drappatz J, Wen PY, Pruitt AA. Prognostic factors in adult brainstem gliomas: a multicenter, retrospective analysis of 101 cases. J Neurooncol. 2008;88:175–183. doi: 10.1007/s11060-008-9545-1. [DOI] [PubMed] [Google Scholar]
- 9.Guillamo JS, Monjour A, Taillandier L, Devaux B, Varlet P, Haie-Meder C, Defer GL, Maison P, Mazeron JJ, Cornu P, Delattre JY. Brainstem gliomas in adults: prognostic factors and classification. Brain. 2001;124:2528–2539. doi: 10.1093/brain/124.12.2528. [DOI] [PubMed] [Google Scholar]
- 10.Dellaretti M, Touzet G, Reyns N, Dubois F, Gusmao S, Pereira JL, Blond S. Correlation between magnetic resonance imaging findings and histological diagnosis of intrinsic brainstem lesions in adults. Neuro Oncol. 2012;14:381–385. doi: 10.1093/neuonc/nor215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Filippini G, Falcone C, Boiardi A, Broggi G, Bruzzone MG, Caldiroli D, Farina R, Farinotti M, Fariselli L, Finocchiaro G, Giombini S, Pollo B, Savoiardo M, Solero CL, Valsecchi MG Brain Cancer Register of the Fondazione IINCB . Prognostic factors for survival in 676 consecutive patients with newly diagnosed primary glioblastoma. Neuro Oncol. 2008;10:79–87. doi: 10.1215/15228517-2007-038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, Lang FF, McCutcheon IE, Hassenbusch SJ, Holland E, Hess K, Michael C, Miller D, Sawaya R. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95:190–198. doi: 10.3171/jns.2001.95.2.0190. [DOI] [PubMed] [Google Scholar]
- 13.Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011;115:3–8. doi: 10.3171/2011.2.JNS10998. [DOI] [PubMed] [Google Scholar]
- 14.Buckner JC. Factors influencing survival in high-grade gliomas. Semin Oncol. 2003;30:10–14. doi: 10.1053/j.seminoncol.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 15.Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, Bromberg JE, Hau P, Mirimanoff RO, Cairncross JG, Janzer RC, Stupp R. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 16.van den Bent MJ, Dubbink HJ, Sanson M, van der Lee-Haarloo CR, Hegi M, Jeuken JW, Ibdaih A, Brandes AA, Taphoorn MJ, Frenay M, Lacombe D, Gorlia T, Dinjens WN, Kros JM. MGMT promoter methylation is prognostic but not predictive for outcome to adjuvant PCV chemotherapy in anaplastic oligodendroglial tumors: a report from EORTC brain tumor group study 26951. J Clin Oncol. 2009;27:5881–5886. doi: 10.1200/JCO.2009.24.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wick W, Hartmann C, Engel C, Stoffels M, Felsberg J, Stockhammer F, Sabel MC, Koeppen S, Ketter R, Meyermann R, Rapp M, Meisner C, Kortmann RD, Pietsch T, Wiestler OD, Ernemann U, Bamberg M, Reifenberger G, von Deimling A, Weller M. NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J Clin Oncol. 2009;27:5874–5880. doi: 10.1200/JCO.2009.23.6497. [DOI] [PubMed] [Google Scholar]
- 18.Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu L, Backlund LM, Nilsson BR, Grander D, Ichimura K, Goike HM, Collins VP. Clinical significance of EGFR amplification and the aberrant EGFRvIII transcript in conventionally treated astrocytic gliomas. J Mol Med (Berl) 2005;83:917–926. doi: 10.1007/s00109-005-0700-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rachinger W, Grau S, Holtmannspotter M, Herms J, Tonn JC, Kreth FW. Serial stereotactic biopsy of brainstem lesions in adults improves diagnostic accuracy compared with MRI only. J Neurol Neurosurg Psychiatry. 2009;80:1134–1139. doi: 10.1136/jnnp.2009.174250. [DOI] [PubMed] [Google Scholar]
- 21.Guillamo JS, Doz F, Delattre JY. Brain stem gliomas. Curr Opin Neurol. 2001;14:711–715. doi: 10.1097/00019052-200112000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Kondziolka D, Lunsford LD. Results and expectations with image-integrated brainstem stereotactic biopsy. Surg Neurol. 1995;43:558–562. doi: 10.1016/0090-3019(95)00009-7. [DOI] [PubMed] [Google Scholar]
- 23.Roujeau T, Machado G, Garnett MR, Miquel C, Puget S, Geoerger B, Grill J, Boddaert N, Di Rocco F, Zerah M, Sainte-Rose C. Stereotactic biopsy of diffuse pontine lesions in children. J Neurosurg. 2007;107:1–4. doi: 10.3171/PED-07/07/001. [DOI] [PubMed] [Google Scholar]
- 24.Kestle J, Townsend JJ, Brockmeyer DL, Walker ML. Juvenile pilocytic astrocytoma of the brainstem in children. J Neurosurg. 2004;101:1–6. doi: 10.3171/ped.2004.101.2.0001. [DOI] [PubMed] [Google Scholar]
- 25.Teo C, Siu TL. Radical resection of focal brainstem gliomas: is it worth doing? Childs Nerv Syst. 2008;24:1307–1314. doi: 10.1007/s00381-008-0647-z. [DOI] [PubMed] [Google Scholar]
- 26.Weiner HL, Freed D, Woo HH, Rezai AR, Kim R, Epstein FJ. Intra-axial tumors of the cervicomedullary junction: surgical results and long-term outcome. Pediatr Neurosurg. 1997;27:12–18. doi: 10.1159/000121219. [DOI] [PubMed] [Google Scholar]
- 27.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO European Organisation for R, Treatment of Cancer Brain T, Radiotherapy G, National Cancer Institute of Canada Clinical Trials G. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]