Abstract
Chronic aerobic exercise has been shown to increase exercise efficiency, thus allowing less energy expenditure for a similar amount of work. The extent to which skeletal muscle mitochondria play a role in this is not fully understood, particularly in an elderly population. The purpose of this study was to determine the relationship of exercise efficiency with mitochondrial content and function. We hypothesized that the greater the mitochondrial content and/or function, the greater would be the efficiencies. Thirty-eight sedentary (S, n = 23, 10F/13M) or athletic (A, n = 15, 6F/9M) older adults (66.8 ± 0.8 years) participated in this cross sectional study. 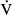 O2peak was measured with a cycle ergometer graded exercise protocol (GXT). Gross efficiency (GE, %) and net efficiency (NE, %) were estimated during a 1-h submaximal test (55%
O2peak was measured with a cycle ergometer graded exercise protocol (GXT). Gross efficiency (GE, %) and net efficiency (NE, %) were estimated during a 1-h submaximal test (55% 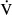 O2peak). Delta efficiency (DE, %) was calculated from the GXT. Mitochondrial function was measured as ATPmax (mmol/L/s) during a PCr recovery protocol with 31P-MR spectroscopy. Muscle biopsies were acquired for determination of mitochondrial volume density (MitoVd, %). Efficiencies were 17% (GE), 14% (NE), and 16% (DE) higher in A than S. MitoVD was 29% higher in A and ATPmax was 24% higher in A than in S. All efficiencies positively correlated with both ATPmax and MitoVd. Chronically trained older individuals had greater mitochondrial content and function, as well as greater exercise efficiencies. GE, NE, and DE were related to both mitochondrial content and function. This suggests a possible role of mitochondria in improving exercise efficiency in elderly athletic populations and allowing conservation of energy at moderate workloads.
O2peak). Delta efficiency (DE, %) was calculated from the GXT. Mitochondrial function was measured as ATPmax (mmol/L/s) during a PCr recovery protocol with 31P-MR spectroscopy. Muscle biopsies were acquired for determination of mitochondrial volume density (MitoVd, %). Efficiencies were 17% (GE), 14% (NE), and 16% (DE) higher in A than S. MitoVD was 29% higher in A and ATPmax was 24% higher in A than in S. All efficiencies positively correlated with both ATPmax and MitoVd. Chronically trained older individuals had greater mitochondrial content and function, as well as greater exercise efficiencies. GE, NE, and DE were related to both mitochondrial content and function. This suggests a possible role of mitochondria in improving exercise efficiency in elderly athletic populations and allowing conservation of energy at moderate workloads.
Keywords: Chronic exercise, delta efficiency, gross efficiency, net efficiency
Introduction
Elderly adults typically display low levels of exercise efficiency, which could hamper normal activities of daily living (Martin et al. 1992; Woo et al. 2006). High levels of efficiency are important since they allow one to expend less energy for a given amount of physical work. Skeletal muscle mitochondrial dysfunction is suggested to lead to metabolic perturbations, particularly in older adults (Conley et al. 2000; Menshikova et al. 2006). These perturbations lead to changes with cellular dynamics and are associated with loss of muscle quality as well as increases in oxidative stress (Peterson et al. 2012). More recently, Santanasto et al. (2014) also described this relationship between mitochondrial dysfunction and muscle ailments by showing that a lower mitochondrial function relates to greater fatigability in older adults. A sedentary lifestyle is touted as the primary culprit for decreases in mitochondrial function and content (Broskey et al. 2014). On the other hand, physical activity, in the form of structured aerobic exercise, can help ameliorate these conditions (Lanza et al. 2008; Conley et al. 2013a).
Exercise efficiency is broadly defined as the ratio of mechanical work rate over energy expenditure and is typically expressed by four different equations with the most common being either gross or delta efficiency (Ettema and Loras 2009). Historically, Gaesser and Brooks (1975) described the latter as being the most preferred and reliable measure at moderate and high power outputs. Comparisons of exercise efficiency between young active versus young sedentary adults show that those who are active have a higher efficiency than those who are sedentary (Mogensen et al. 2006). Young and old comparisons show that older adults have decreases in efficiency and/or expend more energy for walking (Martin et al. 1992; Malatesta et al. 2003; Mian et al. 2006; Ortega and Farley 2007; Conley et al. 2013b). We have previously shown that older obese sedentary adults improved exercise efficiency with 4 months of exercise alone or in combination with weight loss (Amati et al. 2008), however it is still uncertain how exercise efficiency differs between older adults who are habitually active versus age-matched sedentary counterparts.
Furthermore, the relationship between this difference in efficiency and the extent at which mitochondrial content and function contribute to these differences has only been partially explored. A couple of groups already showed in young adults that no relationship exists between exercise efficiency and mitochondrial content measured by citrate synthase activity (Mallory et al. 2002; Mogensen et al. 2006). Conley et al. (2013b) showed that reduced mitochondrial efficiency correlates with reduced delta efficiency; however, this was in a young and old cross-sectional comparison.
The purpose of this paper was to look at differences in exercise efficiency between older athletic adults and older sedentary adults matched for age. We hypothesized that athletic older adults will have higher exercise efficiencies as well as higher mitochondrial content and function. A secondary hypothesis was that this increased exercise efficiency related to increased mitochondrial content and function.
Methods
Subjects
Older adults between the age of 60 and 80 years old in good general health, nonsmokers, and weight stable were recruited for this study. Volunteers were considered athletes or sedentary based on self-reported frequency of habitual levels of exercise. Those who are considered athletes engaged in three or more structured aerobic exercise sessions per week for more than 1 year. Individuals were defined as sedentary if they engaged in one or less structured exercise sessions per week. Volunteers were excluded if they took any medication known to affect muscle metabolism, such as corticosteroids or enhancers of insulin sensitivity. The research protocol was accepted by the ethics committee of the Canton of Vaud and all volunteers gave written informed consent.
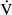 O2peak
O2peak
Peak oxygen consumption was determined as described previously (Broskey et al. 2014). Briefly, the graded exercise test was conducted on an electronically braked cycle ergometer (Lode B.V., Groningen, The Netherlands) combined with continuous ECG, heart rate, and blood pressure recordings. 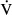 O2 was computed with indirect calorimetry (Metalyzer3B, Cortex GmbH, Leipzig, Germany). The exact protocol was the following with increments derived every 2 min: sedentary men started at 50 W and increased in increments of 25 W, male athletes started at 50 W and increased one increment of 50 W, increased again of 50 W, then the remainder were increments of 25 W, and all women started at 25 W and increased in increments of 25 W. Both groups performed the test until volitional exhaustion or if one of the American College of Sports Medicine established criteria for maximal testing had been reached (Walter et al. 2010).
O2 was computed with indirect calorimetry (Metalyzer3B, Cortex GmbH, Leipzig, Germany). The exact protocol was the following with increments derived every 2 min: sedentary men started at 50 W and increased in increments of 25 W, male athletes started at 50 W and increased one increment of 50 W, increased again of 50 W, then the remainder were increments of 25 W, and all women started at 25 W and increased in increments of 25 W. Both groups performed the test until volitional exhaustion or if one of the American College of Sports Medicine established criteria for maximal testing had been reached (Walter et al. 2010).
Submaximal bike test
Subjects were instructed to avoid strenuous exercise 2 days before the test. To ensure adequate glycogen stores, subjects were asked to eat their habitual diet ensuring that they eat at least 200 g of carbohydrates per day for the 3 days before the submaximal test as previously described (Amati et al. 2008). After a 12-h overnight fast, participants reported to the laboratory and biked for 1-h on the electronically braked cycle ergometer at the exact 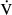 O2 corresponding to 55% of
O2 corresponding to 55% of 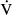 O2 peak. Subjects were instructed to pedal at a controlled cadence between 60 and 65 rpms due to this being reported previously as a stable cadence to calculate efficiency (Sidossis et al. 1992; Francescato et al. 1995). Oxygen consumption was recorded via indirect calorimetry for 5 min at four time points (15, 30, 45, and 60 min). This testing protocol was chosen to replicate what was published previously and was successful for outcomes of efficiency in sedentary and trained elderly individuals (Amati et al. 2008). Each volunteer was able to complete the 1-h test accordingly.
O2 peak. Subjects were instructed to pedal at a controlled cadence between 60 and 65 rpms due to this being reported previously as a stable cadence to calculate efficiency (Sidossis et al. 1992; Francescato et al. 1995). Oxygen consumption was recorded via indirect calorimetry for 5 min at four time points (15, 30, 45, and 60 min). This testing protocol was chosen to replicate what was published previously and was successful for outcomes of efficiency in sedentary and trained elderly individuals (Amati et al. 2008). Each volunteer was able to complete the 1-h test accordingly.
DXA
Lean body mass (LBM) was determined by dual energy X-ray absorptiometry (DiscoveryA; Hologic Inc, Bedford, MA).
Resting energy expenditure
On a separate day from the exercise testing, indirect calorimetry was used to determine resting energy expenditure (REE) using an open canopy system (Quark, Cosmed, Rome, Italy). After an overnight fast and stay at the Clinical Research Center at ∽6:30 A.M. subjects were placed under the canopy while resting in bed. They were told to close their eyes and try to sleep, refrain from fidgeting, and not to perform any type of activity (i.e., watching TV or reading). The duration was at least 30 min with the first 5 min of data discarded to assure steady state.
Muscle biopsies
Percutaneous muscle biopsies were obtained following the REE, in the fasted state, from the vastus lateralis under local anaesthesia (buffered lidocaïne) as previously described (Amati et al. 2011). Controlled conditions included no exercise for 48 h, a standardized dinner (consisting of 7 kcal/kg of body weight with 50% carbohydrates, 20% proteins, and 30% fats) followed by an overnight fast prior to the biopsy. After trimming of visible adipose tissue with a dissecting microscope (MZ6; Leica Microsystems, Wetzlar, Germany), one portion of the specimen (5 mg) was fixed in glutaraldehyde solution (EMS, Hatfield, PA) for transmission electron microscopy as described previously (Broskey et al. 2013).
Electron microscopy
Transmission electron microscopy was used to measure mitochondrial volume density (MitoVd) as a marker of mitochondrial content. A recent validation and detailed description of this stereological method has been described elsewhere (Broskey et al. 2013). Briefly, twenty micrographs of the intramyofibrillar region were taken per subject with a Philips CM100 transmission electron microscope (FEI, Eindhoven, The Netherlands) at an acceleration voltage of 80 kV and ×33,000 of magnification, with a pixel size of 2.11 nm and a horizontal field width of 8.3 μm, with a Megaview III SIS digital camera (Olympus Soft Imaging Solutions, Münster, Germany), using the software SIS iTEM Megaview III (Olympus Soft Imaging Solutions). To avoid sampling bias, all images were taken using the Multiple Image Alignment plugin (MIA) of the iTEM software. The MIA plugin is a panorama-image function that allows acquisition of a larger image of the sample than the one observed by an automatic displacement of the sample under the electron beam. The micrographs are composed of an alignment of four images in the x-axis per five images in the y-axis, creating a large single micrograph of 20 images without changing the magnification. A grid was superimposed on each micrograph with squares of 500 × 500 (0.25 μm2). Upon placing the grid, the number of points (defined as two intersecting grid lines) that touched the mitochondria, were tallied and divided by the total number of points on the grid. This process was repeated for the other 19 images and averaged together to receive the volume density percentage for each grid.
PCr recovery
The rate of postexercise phosphocreatine (PCr) recovery reflects the oxidative ATP synthesis rate and was shown to be correlated with in vitro measurements of oxidative capacity (McCully et al. 1993). Briefly, the exercise protocol consisted of dynamic knee extensions against a rubber band (supine position, 1 extension/s, different resistance levels adapted to each subject's strength). Default exercise duration was 28s and 31P MR spectra were obtained before, during, and for 9 min after the end of exercise from the quadriceps. The recovery of PCr was fitted to the following formula PCr (t) = PCr0 + ΔPCr (1 – e−k*t), with PCr0 as the PCr signal intensity at the beginning of recovery, and ΔPCr as the exercise-induced decrease of the PCr signal. The oxidative phosphorylation capacity (ATPmax) was computed as the product of the PCr recovery rate constant (k) and the resting PCr content obtained from the resting spectrum and assuming a constant ATP concentration of 8.2 mmol/L (Conley et al. 2000). This method is described in detail in a previous manuscript (Broskey et al. 2014).
Exercise efficiency computations
Gross efficiency (GE) was computed as the power output (watts converted in kcal/min) over exercise energy expenditure (EEE in kcal/min) during the 1-h submaximal bike test and expressed as a ratio (Eq. 1) as previously described (Amati et al. 2008). Mean values of work, 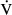 O2 and
O2 and 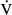 CO2 were averaged during the last 3 min at times 30, 45, and 60 min. The first 15 min of data were, if needed, used for workload adjustments to match exactly 55% of
CO2 were averaged during the last 3 min at times 30, 45, and 60 min. The first 15 min of data were, if needed, used for workload adjustments to match exactly 55% of 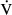 O2peak and thus were discarded. The reliability (test–retest) of this method and the absence of learning effect has been demonstrated in a previous manuscript (Amati et al. 2008).
O2peak and thus were discarded. The reliability (test–retest) of this method and the absence of learning effect has been demonstrated in a previous manuscript (Amati et al. 2008).
| 1 |
Net efficiency (NE) was measured from the submaximal test as power output (watts converted in kcal/min) over EEE minus REE (Eq. 2). It is important to note that due to research settings limitations, REE was measured on a different day as described above, and we did not measure REE in the seated position on the ergometer nor were any measurements of unloaded pedaling taken for the baseline subtraction for all volunteers.
| 2 |
Delta efficiency (DE) was measured in two ways. First, DE was obtained as the change in power output (delta watts converted in kcal/min) over the change in EEE (delta EEE) between two stages of the GXT (Eq. 3). Due to the fact that we witness some extremes, on one side 2 athletes did 6–8 stages, while on the other side of the spectrum one sedentary subject did only three stages; the two time points that were always present for all subjects and that were used for this computation were the peak and stage 1. Mean values of 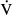 O2 and
O2 and 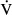 CO2 of the last 30 sec of these two stages were used for this computation. Secondly,
CO2 of the last 30 sec of these two stages were used for this computation. Secondly, 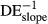 was also obtained by plotting a regression line for each subject with power output on the X axis and oxygen uptake on the Y axis. The inverse of the slope is DE (expressed in % by multiplying it *100) as described originally by Gaesser and Brooks (1975) and used in other publications (Sidossis et al. 1992; Marsh et al. 2000). Further, this second method was discussed recently by Reger et al. (2013), who stated the usefulness of reporting slopes and intercepts for delta efficiency values; with the former representing metabolic cost of biological processes as work rate increases and the latter being the metabolic cost of biological processes that remain stable at higher work rates. For all of our subjects, we took a minimum of three time points, which was previously reported as the minimum to produce a linear line for estimating DE from a slope (Poole et al. 1992).
was also obtained by plotting a regression line for each subject with power output on the X axis and oxygen uptake on the Y axis. The inverse of the slope is DE (expressed in % by multiplying it *100) as described originally by Gaesser and Brooks (1975) and used in other publications (Sidossis et al. 1992; Marsh et al. 2000). Further, this second method was discussed recently by Reger et al. (2013), who stated the usefulness of reporting slopes and intercepts for delta efficiency values; with the former representing metabolic cost of biological processes as work rate increases and the latter being the metabolic cost of biological processes that remain stable at higher work rates. For all of our subjects, we took a minimum of three time points, which was previously reported as the minimum to produce a linear line for estimating DE from a slope (Poole et al. 1992).
| 3 |
For all of these computations, EEE was calculated adapting the formula of Brouwer (Brouwer 1957) as previously described (Amati et al. 2008).
Statistical procedures
Data are reported as mean ± SEM. Group differences were analyzed by independent T tests. Correlations were performed with Spearman ρ correlation coefficient. The significance level was set at 0.05. Statistical analyses were performed using JMP v.9 (SAS, Cary, NC).
Results
Fifteen athletes (A) and 23 sedentary (S) men and women participated in this study (Table1). Groups did not differ in gender proportions or age. S was significantly higher in weight, BMI, and percent body fat. 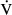 O2peak was greater in A than S when normalized by LBM.
O2peak was greater in A than S when normalized by LBM.
Table 1.
Subject characteristics.
| Athletes (n = 15) | Sedentary (n = 23) | |
|---|---|---|
| Gender, Male/Female | 9/6 | 13/10 |
| Age | 68.53 ± 1.19 | 65.74 ± 0.96 |
| Body Weight (kg) | 61.95 ± 3.64 | 85.08 ± 2.94* |
| Body Mass Index (kg/m2) | 21.91 ± 0.99 | 28.22 ± 0.80* |
| Percent Body Fat (%) | 20.01 ± 1.95 | 32.16 ± 1.58* |
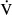 O2peak (L/min) O2peak (L/min) |
2.29 ± 0.15 | 2.11 ± 0.12 |
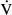 O2peak (mL/min/kg LBM) O2peak (mL/min/kg LBM) |
47.48 ± 1.75 | 37.93 ± 1.41* |
Values are mean ± SEM, LBM, lean body mass.
Significant difference between groups, P < 0.05.
For GE, we fixed the 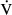 O2 during the last three stages of the submaximal bike test, the average
O2 during the last three stages of the submaximal bike test, the average 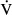 O2 was at 1.25 ± 0.07 L/min for the athletes and 1.23 ± 0.09 L/min with no statistical difference between groups (P = 0.92). These values, which corresponded to 55% of each subjects'
O2 was at 1.25 ± 0.07 L/min for the athletes and 1.23 ± 0.09 L/min with no statistical difference between groups (P = 0.92). These values, which corresponded to 55% of each subjects' 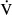 O2peak, produced the following power outputs for A (77.27 ± 6.08 W) and S (66.62 ± 5.02 W). There were no statistical differences between these power outputs (P = 0.19). The
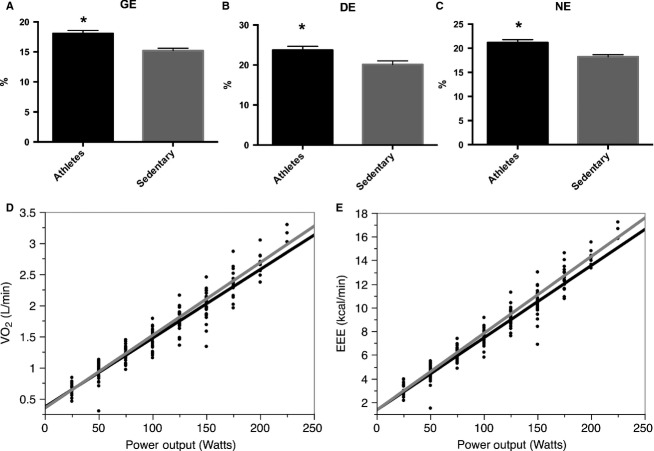
O2peak, produced the following power outputs for A (77.27 ± 6.08 W) and S (66.62 ± 5.02 W). There were no statistical differences between these power outputs (P = 0.19). The 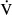 O2 corresponding to this wattage was checked to be below the ventilatory threshold; thus, removing any possibility of a slow component during this moderate intensity exercise test. As shown in Figure1, A had both a higher GE and DE than S (panel A and B). Mean regression lines for each group are presented in panel D where Mean
O2 corresponding to this wattage was checked to be below the ventilatory threshold; thus, removing any possibility of a slow component during this moderate intensity exercise test. As shown in Figure1, A had both a higher GE and DE than S (panel A and B). Mean regression lines for each group are presented in panel D where Mean 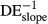 for A was 10.31 ± 0.44% and 9.97 ± 0.35% for S (P = 0.54). We also look at the slopes using EEE instead of
for A was 10.31 ± 0.44% and 9.97 ± 0.35% for S (P = 0.54). We also look at the slopes using EEE instead of 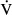 O2peak obtaining similarly nonsignificant results between A and S (panel E).
O2peak obtaining similarly nonsignificant results between A and S (panel E).
Figure 1.
Exercise efficiency differences between athletes and sedentary age-matched older adults. Exercise efficiency is expressed as either gross efficiency (GE, panel A), delta efficiency (DE, panel B), net efficiency (NE, panel C), the inverse of the slope obtained by regression lines of power output over oxygen uptake (VO2, panel D), or the slope between exercise energy expenditure (EEE) over power output (panel E). *P < 0.05.
Resting energy expenditure was higher in S than A with 0.97 ± 0.04 kcal/min versus 0.86 ± 0.03 kcal/min, respectively (P < 0.05). EEE was similar at 55% of 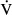 O2peak with 6.04 ± 0.38 kcal/min in the S and 6.21 ± 0.46 in the A (P > 0.05). This gave a NE higher in the A compared to the S (Fig.1, panel C). Although REE is to be taken with caution as it was performed on a different day that the submaximal exercise test, this does not take away the message of the sedentary being less efficient. This is the nature of the different efficiencies and the importance of presenting their various expressions.
O2peak with 6.04 ± 0.38 kcal/min in the S and 6.21 ± 0.46 in the A (P > 0.05). This gave a NE higher in the A compared to the S (Fig.1, panel C). Although REE is to be taken with caution as it was performed on a different day that the submaximal exercise test, this does not take away the message of the sedentary being less efficient. This is the nature of the different efficiencies and the importance of presenting their various expressions.
With regard to mitochondrial content and function (Fig.2), A had a higher MitoVd (panel A) and higher ATPmax (panel B) than S. Confirming our results of the previous study (Broskey et al. 2014), these two outcomes are positively related (ρ = 0.57, P = 0.0002, panel C).
Figure 2.
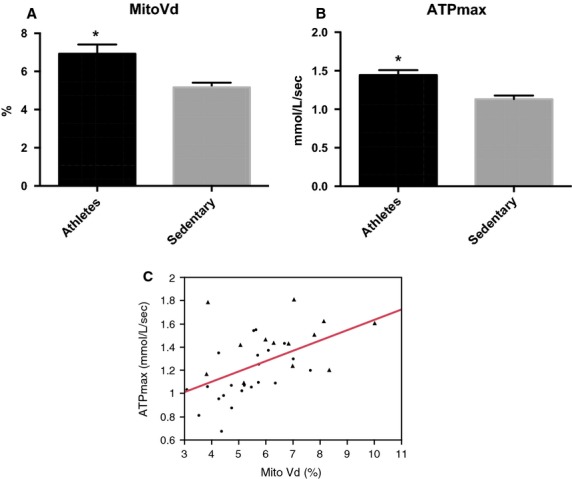
Mitochondrial content and function differences between athletes and sedentary age-matched older adults. Comparison between mitochondrial volume density (MitoVd, panel A) and ATPmax (panel B), relationship between MitoVd and ATPmax (panel C). *P < 0.05. Athletes (triangle) and sedentary (circle) groups.
Spearman correlations between the exercise efficiencies and mitochondrial measurements are presented in Table2. Strong correlations exist between both mitochondrial content (MitoVd) and function (ATPmax) with GE, NE, and DE. The plots of these correlations are shown in Figure3.
Table 2.
Spearman correlations between exercise and mitochondrial measurements.
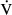 O2peak O2peak
|
GE | NE | DE | ATPmax | MitoVd | |
|---|---|---|---|---|---|---|
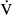 O2peak (mL/min/kg LBM) O2peak (mL/min/kg LBM) |
1.0 | 0.69** | 0.53* | −0.12 | 0.19 | 0.43* |
| GE (%) | 1.0 | 0.95** | 0.10 | 0.50* | 0.36* | |
| NE (%) | 1.0 | 0.13 | 0.42* | 0.44* | ||
| DE (%) | 1.0 | 0.44* | 0.41* | |||
| ATPmax [mmol/L/s] | 1.0 | 0.60** | ||||
| MitoVd (%) | 1.0 |
GE, gross efficiency; NE, net efficiency; DE, delta efficiency; ATPmax, maximal rate of ATP production; MitoVd, mitochondrial volume density.
P < 0.05
P < 0.0001.
Figure 3.
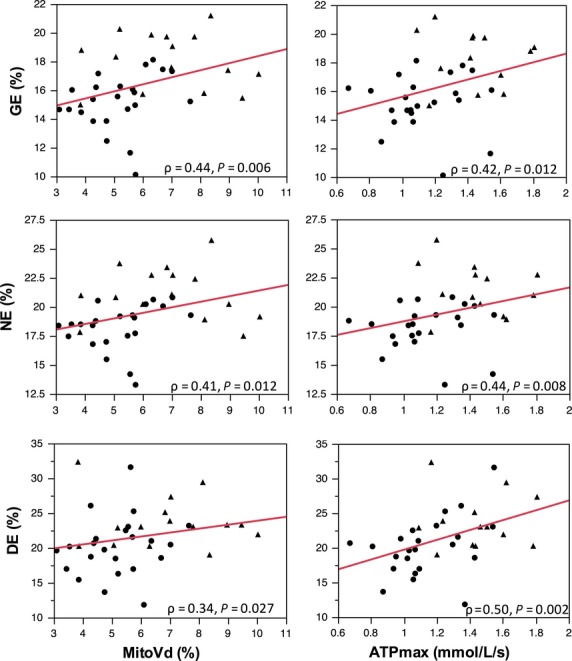
Relationship between exercise efficiencies (GE, gross efficiency; NE, net efficiency; DE, delta efficiency) and mitochondrial content (MitoVd) and function (ATPmax). Athletes (triangle) and sedentary (circle) groups. ρ = spearman rho correlation coefficient.
Discussion
Exercise efficiency is higher in young individuals that are endurance trained than those who live a sedentary lifestyle (Mogensen et al. 2006). Furthermore, efficiency is lower in older adults than younger individuals (Martin et al. 1992; Malatesta et al. 2003; Mian et al. 2006; Ortega and Farley 2007; Conley et al. 2013b). Here we show that exercise efficiency is also higher in older athletes than those who are sedentary and matched for age. In addition, we show that GE, NE, and DE correlate with both mitochondrial content expressed as mitochondrial volume density and mitochondrial function expressed as in vivo maximal ATP production.
Our finding that exercise efficiency is greater in those who engage in physical activity is consistent with previous findings in younger subjects (Boning et al. 1984; Mogensen et al. 2006). In our cohort, the athletes expended less energy for the same relative power output. Therefore, they were more efficient, with higher percentages than the sedentary group of all efficiency measurements. Thus, our data shows that athletes have a higher efficiency in an elderly age-matched population similar to younger athletes versus younger sedentary counterparts (Mogensen et al. 2006). Further, the efficiency values found in our older athletes are in the range with the theoretical values published in 1969 by Whipp and Wasserman (1969). Using a cohort of 8 young males (mean age 25 ± 2.3), the authors concluded that since the energy release for phosphorylative coupling is around 60% and the energy required for contractile coupling being around 53%, the product of these two for muscular efficiency should give a maximal value of approximately 32%.
Part of our observations may be explained by fiber type content. We have shown previously that older athletes have significantly more type I muscle fibers than age-matched sedentary individuals (Amati et al. 2011). It has been shown that individuals with more type 1 fibers display higher levels of cycling efficiency (Coyle et al. 1992). On the contrary, differences in efficiencies between groups cannot be explained by the athletes being better at cycling since most of our athletes engaged in running rather than cycling endurance activities. In further support of this claim, researchers have reported in the past no differences in exercise efficiency between well-trained cyclists and recreational cyclists (Boning et al. 1984; Nickleberry and Brooks 1996). It could also be thought that athletes would have more of a preferred biking cadence than the sedentary group, which could affect efficiency. However, this is not the case in our cohort since we controlled the pedaling cadence for both groups during the test (mean cadence for A 67 ± 1 vs. S 65 ± 1, P > 0.05). The fact that 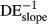 and other outcomes from the regression analyses of the GXT were not significantly different between our cohorts may be explained by the limitations of such computations in a population made of extreme characteristics. Practically, as some of the sedentary women reached
and other outcomes from the regression analyses of the GXT were not significantly different between our cohorts may be explained by the limitations of such computations in a population made of extreme characteristics. Practically, as some of the sedentary women reached 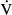 O2peak after three stages, we first computed all slopes in all subjects with all time points and repeated the procedure with only submaximal time points, reaching similar results. We believe that the variability of these results reflects the reality of the population between 60 and 80 years old. Thus, in this broad population, we believe that ‘static’ computations of efficiency (GE and NE), such as using 1 h of submaximal exercise with clear evidence of steady state, are more accurate than ‘dynamic’ measures taking into account each step of the GXT where the number of stages is highly variable.
O2peak after three stages, we first computed all slopes in all subjects with all time points and repeated the procedure with only submaximal time points, reaching similar results. We believe that the variability of these results reflects the reality of the population between 60 and 80 years old. Thus, in this broad population, we believe that ‘static’ computations of efficiency (GE and NE), such as using 1 h of submaximal exercise with clear evidence of steady state, are more accurate than ‘dynamic’ measures taking into account each step of the GXT where the number of stages is highly variable.
In addition, the athletes group had both a higher mitochondrial content and function, which correlated positively with all efficiencies. Therefore, although causality cannot be proved in this study, the higher efficiency in the athletes group could be due to both a higher mitochondrial content and function. Two studies in the past showed no relationship with citrate synthase activity (typically used a marker of mitochondrial content/function) and exercise efficiency (Mallory et al. 2002; Mogensen et al. 2006). Citrate synthase measurements have a downside in that it only represents one enzyme of the TCA cycle. It is difficult to actually decipher how much it portrays mitochondrial content and/or function. Instead in our manuscript, we have separate measures for mitochondrial content and function and show a relationship with all types of exercise efficiency.
In regard to age, several cross-sectional studies have shown that older adults expend more energy than younger counterparts of the same activity levels (Martin et al. 1992; Malatesta et al. 2003; Mian et al. 2006; Ortega and Farley 2007). More recently, Conley et al. (2013b) showed that a reduction in delta efficiency in older adults, compared to younger, is due to a reduction in mitochondrial efficiency. In that manuscript, Conley normalizes ATPmax by mitochondrial content and defines this as “mitochondrial efficiency”. Although our objective was not to compare different age groups, but to compare athletes versus sedentary older subjects, we explored our data using Conley's approach. Upon normalizing ATPmax with mitochondrial volume, our values of mitochondrial efficiency were similar to the elderly group in Conley's paper (approximately 0.2 for both studies). However, in our cohort, we see no differences between athletes and sedentary groups in regard to mitochondrial efficiency using this normalization and no relationship with any measure of exercise efficiency. These differences in mitochondrial efficiency between the two studies could be explained by the different exercise protocols used for the measurement of delta efficiency. In their paper, they used a ramp protocol with workloads increasing on a fixed rate of 10, 12 or 14 W/min, based on self-reported fitness and body mass.
In summary, older adults who are regularly participating in structured physical activity have higher exercise efficiency than age-matched sedentary counterparts. This is also true for both mitochondrial content and function. Elderly who are more athletic can exercise more efficiently and utilize a lesser amount of energy for a given power output; thus, conserving energy stores and exerting less effort. The higher exercise efficiency in the athletes group can be explained by a higher amount of mitochondria occupying their skeletal muscle volume as well as a faster ability to regenerate ATP. Therefore, continuous exercise at an older age can possibly ameliorate the conditions of disease states attributed to decreases in mitochondrial function by increasing mitochondrial content, function, and whole-body efficiency.
Acknowledgments
We appreciate the cooperation of our research volunteers, our research coordinator Nadina Becirovic, and the nursing staff, Christiane Pellet and Francoise Secretan, from the Clinical Research Center of the University Hospital of the University of Lausanne (CHUV). Ours thanks to Cyril Besson, Chantal Daucourt, and Anaelle Jullierat for their assistance in the exercise laboratory, Karin Zwygart at the MR research center and the DXA technicians. Parts of this work have been accepted and presented at the annual scientific meeting of the American College of Sports Medicine.
Conflict of Interest
The authors declare no conflict of interest. The results of the present study do not constitute endorsement by APS.
References
- Amati F, Dube JJ, Shay C. Goodpaster BH. Separate and combined effects of exercise training and weight loss on exercise efficiency and substrate oxidation. J. Appl. Physiol. (1985) 2008;105:825–831. doi: 10.1152/japplphysiol.90384.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amati F, Dube JJ, Alvarez-Carnero E, Edreira MM, Chomentowski P, Coen PM, et al. Skeletal muscle triglycerides, diacylglycerols, and ceramides in insulin resistance: another paradox in endurance-trained athletes? Diabetes. 2011;60:2588–2597. doi: 10.2337/db10-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boning D, Gonen Y. Maassen N. Relationship between work load, pedal frequency, and physical fitness. Int. J. Sports Med. 1984;5:92–97. doi: 10.1055/s-2008-1025887. [DOI] [PubMed] [Google Scholar]
- Broskey NT, Daraspe J, Humbel BM. Amati F. Skeletal muscle mitochondrial and lipid droplet content assessed with standardized grid sizes for stereology. J. Appl. Physiol. (1985) 2013;115:765–770. doi: 10.1152/japplphysiol.00063.2013. [DOI] [PubMed] [Google Scholar]
- Broskey NT, Greggio C, Boss A, Boutant M, Dwyer A, Schlueter L, et al. Skeletal muscle mitochondria in the elderly: effects of physical fitness and exercise training. J. Clin. Endocrinol. Metab. 2014;99:1852–1861. doi: 10.1210/jc.2013-3983. [DOI] [PubMed] [Google Scholar]
- Brouwer E. On simple formulae for calculating the heat expenditure and the quantities of carbohydrate and fat oxidized in metabolism of men and animals, from gaseous exchange (Oxygen intake and carbonic acid output) and urine-N. Acta Physiol. et Pharmacol. Neerl. 1957;6:795–802. [PubMed] [Google Scholar]
- Conley KE, Jubrias SA. Esselman PC. Oxidative capacity and ageing in human muscle. J. Physiol. 2000;526(Pt 1):203–210. doi: 10.1111/j.1469-7793.2000.t01-1-00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley KE, Amara CE, Bajpeyi S, Costford SR, Murray K, Jubrias SA, et al. Higher mitochondrial respiration and uncoupling with reduced electron transport chain content in vivo in muscle of sedentary versus active subjects. J. Clin. Endocrinol. Metab. 2013a;98:129–136. doi: 10.1210/jc.2012-2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley KE, Jubrias SA, Cress ME. Esselman P. Exercise efficiency is reduced by mitochondrial uncoupling in the elderly. Exp. Physiol. 2013b;98:768–777. doi: 10.1113/expphysiol.2012.067314. [DOI] [PubMed] [Google Scholar]
- Coyle EF, Sidossis LS, Horowitz JF. Beltz JD. Cycling efficiency is related to the percentage of type I muscle fibers. Med. Sci. Sports Exerc. 1992;24:782–788. [PubMed] [Google Scholar]
- Ettema G. Loras HW. Efficiency in cycling: a review. Eur. J. Appl. Physiol. 2009;106:1–14. doi: 10.1007/s00421-009-1008-7. [DOI] [PubMed] [Google Scholar]
- Francescato MP, Girardis M. di Prampero PE. Oxygen cost of internal work during cycling. Eur. J. Appl. Physiol. 1995;72:51–57. doi: 10.1007/BF00964114. [DOI] [PubMed] [Google Scholar]
- Gaesser GA. Brooks GA. Muscular efficiency during steady-rate exercise: effects of speed and work rate. J. Appl. Physiol. 1975;38:1132–1139. doi: 10.1152/jappl.1975.38.6.1132. [DOI] [PubMed] [Google Scholar]
- Lanza IR, Short DK, Short KR, Raghavakaimal S, Basu R, Joyner MJ, et al. Endurance exercise as a countermeasure for aging. Diabetes. 2008;57:2933–2942. doi: 10.2337/db08-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malatesta D, Simar D, Dauvilliers Y, Candau R, Borrani F, Prefaut C, et al. Energy cost of walking and gait instability in healthy 65- and 80-yr-olds. J. Appl. Physiol. (1985) 2003;95:2248–2256. doi: 10.1152/japplphysiol.01106.2002. [DOI] [PubMed] [Google Scholar]
- Mallory LA, Scheuermann BW, Hoelting BD, Weiss ML, McAllister RM. Barstow TJ. Influence of peak VO2 and muscle fiber type on the efficiency of moderate exercise. Med. Sci. Sports Exerc. 2002;34:1279–1287. doi: 10.1097/00005768-200208000-00008. [DOI] [PubMed] [Google Scholar]
- Marsh AP, Martin PE. Foley KO. Effect of cadence, cycling experience, and aerobic power on delta efficiency during cycling. Med. Sci. Sports Exerc. 2000;32:1630–1634. doi: 10.1097/00005768-200009000-00017. [DOI] [PubMed] [Google Scholar]
- Martin PE, Rothstein DE. Larish DD. Effects of age and physical activity status on the speed-aerobic demand relationship of walking. J. Appl. Physiol. (1985) 1992;73:200–206. doi: 10.1152/jappl.1992.73.1.200. [DOI] [PubMed] [Google Scholar]
- McCully KK, Fielding RA, Evans WJ, Posner JS, Jr, Leigh JD. Relationships between in vivo and in vitro measurements of metabolism in young and old human calf muscles. J. Appl. Physiol. (1985) 1993;75:813–819. doi: 10.1152/jappl.1993.75.2.813. [DOI] [PubMed] [Google Scholar]
- Menshikova EV, Ritov VB, Fairfull L, Ferrell RE, Kelley DE. Goodpaster BH. Effects of exercise on mitochondrial content and function in aging human skeletal muscle. J. Gerontol. A Biol. Sci. Med. Sci. 2006;61:534–540. doi: 10.1093/gerona/61.6.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mian OS, Thom JM, Ardigo LP, Narici MV. Minetti AE. Metabolic cost, mechanical work, and efficiency during walking in young and older men. Acta Physiol. (Oxf) 2006;186:127–139. doi: 10.1111/j.1748-1716.2006.01522.x. [DOI] [PubMed] [Google Scholar]
- Mogensen M, Bagger M, Pedersen PK, Fernstrom M. Sahlin K. Cycling efficiency in humans is related to low UCP3 content and to type I fibres but not to mitochondrial efficiency. J. Physiol. 2006;571:669–681. doi: 10.1113/jphysiol.2005.101691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickleberry BL., Jr Brooks GA. No effect of cycling experience on leg cycle ergometer efficiency. Med. Sci. Sports Exerc. 1996;28:1396–1401. doi: 10.1097/00005768-199611000-00008. [DOI] [PubMed] [Google Scholar]
- Ortega JD. Farley CT. Individual limb work does not explain the greater metabolic cost of walking in elderly adults. J. Appl. Physiol. (1985) 2007;102:2266–2273. doi: 10.1152/japplphysiol.00583.2006. [DOI] [PubMed] [Google Scholar]
- Peterson CM, Johannsen DL. Ravussin E. Skeletal muscle mitochondria and aging: a review. J. Aging Res. 2012;2012:194821. doi: 10.1155/2012/194821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole DC, Gaesser GA, Hogan MC, Knight DR. Wagner PD. Pulmonary and leg VO2 during submaximal exercise: implications for muscular efficiency. J. Appl. Physiol. (1985) 1992;72:805–810. doi: 10.1152/jappl.1992.72.2.805. [DOI] [PubMed] [Google Scholar]
- Reger M, Peterman JE, Kram R. Byrnes WC. Exercise efficiency of low power output cycling. Scand. J. Med. Sci. Sports. 2013;23:713–721. doi: 10.1111/j.1600-0838.2012.01448.x. [DOI] [PubMed] [Google Scholar]
- Santanasto AJ, Glynn NW, Jubrias SA, Conley KE, Boudreau RM, Amati F, et al. Skeletal muscle mitochondrial function and fatigability in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2014 doi: 10.1093/gerona/glu134. doi: 10.1093/gerona/glu134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidossis LS, Horowitz JF. Coyle EF. Load and velocity of contraction influence gross and delta mechanical efficiency. Int. J. Sports Med. 1992;13:407–411. doi: 10.1055/s-2007-1021289. [DOI] [PubMed] [Google Scholar]
- Walter R, Thompson NFG. Pescatello LS. ACSM's guidelines for exercise testing and prescription. Philadelphia: Lippincott Williams & Wilkins; 2010. [Google Scholar]
- Whipp BJ. Wasserman K. Efficiency of muscular work. J. Appl. Physiol. 1969;26:644–648. doi: 10.1152/jappl.1969.26.5.644. [DOI] [PubMed] [Google Scholar]
- Woo JS, Derleth C, Stratton JR. Levy WC. The influence of age, gender, and training on exercise efficiency. J. Am. Coll. Cardiol. 2006;47:1049–1057. doi: 10.1016/j.jacc.2005.09.066. [DOI] [PubMed] [Google Scholar]