Abstract
Objectives
Oral lichen planus (OLP) is a chronic inflammatory oral mucosal disease. Cytokines play an important role in the pathogenesis and disease progression of OLP. Various reports have implicated cytokine gene polymorphisms in susceptibility to develop some immune mediated conditions including OLP. The purpose of this study was to investigate the association of tumor necrosis factor (TNF)-α, TNF-β and interleukin (IL)-10 gene polymorphisms with the OLP risk.
Material and Methods
Forty two unrelated patients with OLP and 211 healthy volunteers were genotyped for TNF-α (-308 G/A), TNF-β (+252A/G), IL-10 (-1082G/A), IL-10 (-819C/T), and IL-10 (-592C/A) polymorphisms.
Results
The frequencies of allele A and genotype GA of TNF-α (-308G/A) were significantly higher while allele G and GG genotypes were lower in OLP patients as compared to the controls (P<0.001). The frequency of GA genotype of TNF-β (+252A/G) was significantly higher in patients than in controls while the AA genotype was completely absent in OLP patients. These results indicated that allele A and genotype GA of TNF-α (-308G/A) as well as the GA genotype of TNF-β (+252A/G) polymorphisms are associated with OLP risk. The frequencies of alleles and genotypes of -1082G/A, -819C/T and -592C/A polymorphisms in IL-10 gene did not differ significantly between OLP patients and controls (P>0.05). However, haplotype ATA extracted from 1082G/A, -819C/T, -592C/A polymorphisms of IL-10 were more prevalent in OLP patients when compared to controls indicating its possible association with OLP susceptibility.
Conclusion
It is concluded that TNF-α (-308G/A), TNF-β (+252A/G) and IL-10 (-1082G/A, -819C/T and -592C/A) polymorphisms are associated with the susceptibility of OLP, thus giving additional support for the genetic basis of this disease.
Keywords: Oral lichen planus, Tumor necrosis factors, Interleukin-10, Genetic polymorphism
INTRODUCTION
Oral lichen planus (OLP) is a chronic inflammatory oral condition whose etiology is not fully elucidated. It is the prototype of oral lichenoid lesions characterized by T-cell mediated immune responses and abnormal epithelial keratinization cycles 6 . OLP manifests as white striations, white papules, white plaques, erythema, erosions, or blisters predominantly affecting the buccal mucosa, tongue and gingival. The OLP lesions may co-exist with cutaneous and genital lesions, or may be the only disease manifestations. The prevalence of OLP ranges between less than 1 and 3% of the general population 17 . The prevalence of OLP in Arab countries including Saudi Arabia has been reported ranging from 0.35 to 1.7% 2 .
The OLP lesions are consistently more persistent than the dermal lesions and have been reported to carry a risk of malignant transformation to oral squamous cell carcinoma of 1-2% (reported range of malignant transformation 0–12.5%) 10 . Clinically, OLP may be divided into three subtypes: reticular, erythematous (atrophic), and erosive 23 and more than one subtype of OLP may be present in an individual patient. The pathogenesis of OLP is very complex and involves possible antigen presentation by the oral keratinocytes that could be either of an exogenous or an endogenous origin 9 . This antigenic trigger is accompanied by a mixed inflammatory response comprising of mainly T-cells, macrophages, and mast cells, as well as the associated cytokines and cytotoxic molecules 9 , 10 . It is a disease associated with middle aged people and is more common among women 4 .
Cytokines play an important role in the pathogenesis and disease progression of OLP and a strong body of evidence suggests that OLP is a T-cell-mediated disease. The genetic factors that influence the immune function have been suggested to contribute to the OLP 6 , 12 . The gene polymorphisms of T-helper cell subtype Th1/Th2 cytokines, tumor necrosis factor (TNF)-α, and interleukin-10 (IL-10) have been reported to affect the susceptibility to, and the progression of OLP 3 , 14 . Recently Chauhan, et al. 8 (2013) suggested that proinflammatory cytokines are an important factor in understanding the disease burden of OLP and their comorbid factors. However, immunogenetic studies of OLP have given controversial results and the precise cause of OLP is still unclear.
TNF-α and TNF-β genes are located in tandem on chromosome 6 between the Class I and Class II cluster of the major histocompatibility complex (chromosome 6p21.1–6p21.3). TNF-α-308G/Apolymorphism (rs1800629) has been reported to be associated with several autoimmune/inflammatory diseases including OLP 3 , 8 . The genetic variation at position −308 of the TNF-α gene results in two allelic forms in which the presence of guanine (G) defines the common variant and the presence of adenine (A) defines the less common one. The A-allele of TNFα-308G/A polymorphism displays increased gene transcription as compared to the common allele G. It has been shown to produce levels of TNF-α transcription 6–7 fold greater 31 . TNF-β +252A/G polymorphism (rs909253) has been reported at position +252 within the first intron of the TNF-β gene, consisting of guanine (TNF-β +252G) on one allele and adenine (TNF-β +252Α) on the alternate allele 18 . The presence of G at this position defines the mutated allele known as TNF-β 1 (allele-1) which is a less frequent allele and is associated with higher TNF-α and TNF−β production 18 .
Interleukin-10 (IL-10) gene maps to the junction of 1q31-q32. It shifts the Th1/Th2 balance by down regulating the Th1 responses and by suppression of pro-inflammatory cytokines, such as TNF-α and interferon gamma (IFN γ) secretion 26 . Three promoter polymorphisms: -1082A/G (rs18000896), -819T/C (rs1800871) and -592A/C (rs1800872) are reportedly involved in the IL-10 transcription rate, thereby directly affecting its production level 19 . The -1082G, -819C and -592C alleles (GCC haplotype) have been associated with elevated levels of IL-10 production 15 while ACC and ATA haplotypes exhibit intermediate and low IL-10 gene transcription respectively 27 . In this study we examined the association of five single nucleotide polymorphisms (SNPs) of TNF-α, TNF-β, and IL-10 genes in the etiopathogenesis of OLP in Saudi patients.
MATERIAL AND METHODS
Study group
A total of 253 Saudi subjects visiting Prince Sultan Military Medical City (PSMMC), Riyadh, Saudi Arabia were recruited for this study. Forty two (16 male, 26 female) unrelated patients with oral lichen planus, ages ranging from 27 to 72 years, and 211 (140 male, 71 female) unrelated, healthy patients matched voluntary blood donors with ages ranging from 20 to 65 years from the same population were studied for polymorphisms in TNF-α, TNF-β and IL-10 genes. Patients with any other inflammatory/autoimmune diseases were excluded from the study. This study was approved by the research and ethical committee of PSMMC and written informed consent was obtained from each subject before recruitment.
OLP was diagnosed according to the clinical manifestations and histopathological criteria of the World Health Organization. All patients were diagnosed through a review of the patient’s history, physical examination and histological findings by an oral pathologist. A 4 mm punch biopsy of lesional tissue that extends into the submucosa was performed. Symptoms were bilateral, more or less symmetrical lesions, and a lace-like network of slightly raised gray-white lines (reticular pattern). Erosive, atrophic, bullous, and plaque-type lesions were only accepted as a subtype in the presence of reticular lesions elsewhere in the oral mucosa. Histopathological criterium were the presence of a well-defined band-like zone of cellular infiltration that was confined to the superficial part of the connective tissue, consisting mainly of lymphocytes, signs of “liquefaction degeneration” in the basal cell layer, and the absence of epithelial dysplasia 28 . No patient was suspected to have drug or restoration related lichenoid lesions, and no patient showed histologic signs of dysplasia. In cases where the clinical and histological findings were inconclusive, direct immunofluorescence (DIF) microscopy of perilesional mucosa was used to exclude autoimmune vesiculobullous disorders.
PCR amplification
Genomic DNA was extracted from the peripheral blood of OLP patients and controls using the QIA ampR DNA mini kit (QIAGEN Hilden, North Rhine-Westphalia, Germany). TNF-α, TNF-β and IL-10 genes were amplified using amplification refractory mutation systems (ARMS)-PCR methodology 21 to detect polymorphisms at positions -308 of TNF-α, +252 in intron1 of TNF-β and at loci -592, -819, and -1082 of IL-10 genes. PCR amplification was carried out using Ready to Go PCR Beads (GE Healthcare, Little Chalfont, Buckinghamshire, UK). Reactions consisted of 10 denaturation temperature cycles for 15 s at 94°C, annealing for 50 s at 65°C and extension for 40 s at 72°C. Then 25 denaturation cycles of 20 s at 94°C, annealing for 50 s at 59°C and then extension of 50 s at 72°C. Final extension was performed at 72°C for 7 min. A positive control was included in the PCR assay by amplification of the human growth hormone gene. The amplified products for the various samples were separated on the 1.5% agarose gel, stained with ethidium bromide and photographed.
Statistical analysis
The frequencies of alleles and genotypes were calculated. Hardy-Weinberg equilibrium was determined using Hardy-Weinberg Equilibrium Calculator for 2 Alleles (http//www.had2know.com/academics/hardy-weinberg-equilibrium-calculator-2alleles.html). The Bayesian method of the PHASE program (version 2.1) was applied to reconstruct the haplotype (http://stephenslab.uchicago.edu/software.html). The differences in allele/genotype frequencies between patients and controls were analyzed by Fisher’s exact test using the CalcFisher software 13 . P values ≤0.05 were considered significant. The strength of the association of the disease with respect to a particular allele/genotype is expressed by odd ratio interpreted as relative risk (RR) following the Woolf’s method as out lined by Schallreuter, et al. 22 (1993). It was calculated only for those alleles/genotypes which were increased or decreased in OLP patients when compared to the control group. The RR was calculated for all the subjects using the formula given below:
 |
a = number of patients with expression of allele or genotype
b = number of patients without expression of allele or genotype
c = number of controls with expression of allele or genotype
d = number of controls without expression of allele or genotype.
Etiologic Fraction (EF) indicates the hypothetical genetic component of the disease. The values 0.0 - 0.99 are of significance. EF was calculated for a positive association only where RR>1 using the following formula 25 .
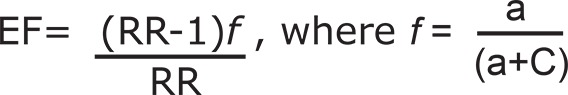 |
Preventive Fraction (PF) indicates the hypothetical protective effect of one specific allele/ genotype for the disease. PF was calculated for negative association only where RR<1 using the following formula 25 . Values <1.0 indicate the protective effect of the allele/ genotype against the manifestation of disease.
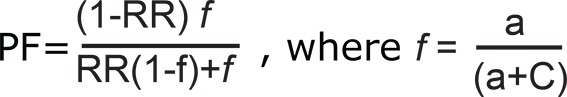 |
RESULTS
Among OLP patients the male to female ratio was 16:26 (1:1.6). There was no significant difference in clinical manifestation or prognosis comparing men to women in our study. Thirty-five patients (83.333%) had lesions on the buccal mucosa, three (7.14 %) each on the tongue and gingival while one patient (2.39%) had lesions on the palate. The majority of patients (85%) had white lichen. In our patients, reticular type OLP was the commonest (80.95%) followed by erosive (23.81%) and then atrophic types (4.76%).
The genotype and allele frequencies of TNF-α (-308G/A) and TNF-β (+252A/G) promoter polymorphisms are presented in Table 1. In both the lichen planus patients and control groups the genotype distributions were in Hardy-Weinberg equilibrium. The frequency of the heterozygous genotype GA was significantly higher in OLP patients than in the control (P<0.001) whereas the frequency of homozygous genotypes GG was significantly lower in OLP than controls (P<0.001). The frequency of allele-A was significantly higher in OLP patients than control subjects (P<0.001). On the other hand, allele-G was significantly lower in OLP patients when compared to the control (P<0.001). The difference in frequency of the AA genotype between the two groups was not statistically significant.
Table 1. Genotype and allele frequencies of TNF-α and TNF-β variants in oral lichen planus patients and matched controls.
| Genotype/ allele | OLP (N=42) | Control (N=200) | |||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | P-value | RR | EF†/ PF | |
| -308 G/A | |||||||
| GG | 5 | 11.9 | 110 | 55 | <0.001* | 0.11 | 0.257 |
| GA | 36 | 85.72 | 76 | 38 | <0.001* | 9.789 | 0.288† |
| AA | 1 | 2.38 | 14 | 7 | 0.479 | 0.324 | 0.121 |
| G allele | 46 | 54.76 | 296 | 74 | <0.001* | 0.425 | 0.153 |
| A allele | 38 | 45.24 | 104 | 26 | <0.001* | 2.351 | 0.153† |
| +252 A/G | |||||||
| GG | 4 | 9.52 | 28 | 14 | 0.616 | 0.647 | 0.063 |
| GA | 38 | 90.48 | 148 | 74 | 0.025* | 6.767 | 0.173† |
| AA | 0 | 0 | 24 | 12 | 0.010* | - | - |
| G allele | 46 | 54.76 | 204 | 51 | 0.55 | 1.163 | 0.026† |
| A allele | 38 | 45.24 | 196 | 49 | 0.55 | 0.859 | 0.026 |
N=number of subjects,*statistically significant, RR=relative risk, EF=etiologic fraction, PF=preventive fraction
The frequency of the GA genotype of TNF-β (+252A/G) promoter polymorphism was significantly higher in patients group than in the controls. Homozygous AA genotype was completely absent in OLP patients whereas it was present in 12% of the controls. The frequencies of alleles of TNF-β (+252A/G) polymorphism were not significantly different between OLP patients and healthy controls (Table 1). Albeit, the frequencies of allele-G were slightly higher in the OLP patients than they were in the control subjects.
The results of SNPs for IL-10(-1082G/A), IL-10(-592C/A), IL-10(-819C/T), and corresponding alleles and genotypes are summarized in Table 2. The genotype distributions of IL-10 polymorphisms in patient and controls were in Hardy-Weinberg equilibrium. The frequencies of -1082GG and 1082GA genotypes were slightly higher while the frequency of -1082AA genotype was lower in OLP patients when compared to the control subjects, however, the differences were not statistically significant.
Table 2. Genotype and allele frequencies of IL-10 variants in oral lichen planus patients and matched controls.
| Genotype/ allele | OLP (N=42) | Control (N=186) | |||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | P-value | RR | EF*/PF | |
| -1082G/A | |||||||
| GG | 5 | 11.9 | 14 | 7.53 | 0.357 | 1.6 | 0.098* |
| GA | 33 | 78.5 | 140 | 75.27 | 0.841 | 1.2 | 0.032* |
| AA | 4 | 9.5 | 32 | 12.2 | 0.251 | 0.51 | 0.095 |
| G-allele | 43 | 51.2 | 168 | 45.16 | 0.334 | 1.3 | 0.047* |
| A-allele | 41 | 48.8 | 204 | 54.84 | 0.334 | 0.78 | 0.048 |
| -819C/T | |||||||
| CC | 12 | 28.5 | 88 | 41.71 | 0.122 | 0.559 | 0.086 |
| CT | 25 | 59.5 | 102 | 48.34 | 0.239 | 1.5 | 0.065* |
| TT | 5 | 11.9 | 21 | 9.95 | 0.78 | 1.2 | 0.032* |
| C-allele | 49 | 62 | 278 | 65.88 | 0.521 | 0.846 | 0.026 |
| T-allele | 30 | 38 | 144 | 34.12 | 0.521 | 1.182 | 0.172* |
| -592C/A | |||||||
| CC | 12 | 28.5 | 88 | 41.71 | 0.122 | 0.559 | 0.086 |
| CA | 25 | 59.5 | 102 | 48.34 | 0.239 | 1.5 | 0.065* |
| AA | 5 | 11.9 | 21 | 9.95 | 0.78 | 1.2 | 0.032* |
| C-allele | 49 | 62 | 278 | 65.88 | 0.521 | 0.846 | 0.026 |
| A-allele | 30 | 38 | 144 | 34.12 | 0.521 | 1.182 | 0.172* |
N=number of subjects,*statistically significant, RR=relative risk, EF=etiologic fraction, PF=preventive fraction
Similarly the frequencies of alleles and genotypes of IL-10 (-819C/T) and IL-10 (-592C/A) polymorphisms did not differ significantly between OLP patients and controls (P>0.05). Albeit, the frequencies of genotypes -819 CT, -819 TT, 592 CA and 592 AA were higher in OLP patients while -819 CC and -592 CC genotypes showed a reverse pattern with lower frequency in OLP patients when compared to the controls (Table 2). The frequencies of haplotypes extracted from 1082G/A, -819C/T, -592C/A polymorphisms of IL-10 are shown in Table 3. The haplotype ATA was significantly increased in OLP patients when compared to controls (P=0.008).
Table 3. Haplotype analysis of IL-10 -1082G/A, -819T/C and -592C/A polymorphisms in OLP and control groups.
| Haplotype | OLP (N=42) | Controls (N=186) | |
|---|---|---|---|
| N (Frequency) | N (Frequency) | P-value | |
| GCC | 7 (0.166) | 42 (0.225) | 0.533 |
| ACC | 13 (0.309) | 82 (0.440) | 0.165 |
| ATA | 20 (0.476) | 48 (0.258) | 0.008* |
| ACA | 2 (0.047) | 14 (0.075) | 0.742 |
N=number of subjects,*statistically significant
The frequencies of alleles and genotypes of all five polymorphisms with gender are summarized in Table 4. No statistically significant difference was found in the frequencies of alleles or genotypes of TNF-α, TNF-β and IL-10 gene polymorphisms between male and female OLP patients.
Table 4. Genotype and allele frequencies in male and female oral lichen planus patients.
| Genotype/ allele | Male (N=16) | Female (N=26) | |||
|---|---|---|---|---|---|
| N | % | N | % | P-value | |
| TNF-α (-308) | |||||
| GG | 2 | 12.5 | 3 | 11.54 | 1 |
| GA | 14 | 87.5 | 22 | 84.61 | 1 |
| AA | 0 | 0 | 1 | 3.85 | 0.99 |
| G-allele | 18 | 56.25 | 28 | 53.85 | 0.99 |
| A-allele | 14 | 43.75 | 24 | 46.15 | 0.99 |
| TNF-β (+525) | |||||
| GG | 1 | 6.25 | 3 | 11.54 | 1 |
| GA | 15 | 93.75 | 23 | 88.46 | 1 |
| AA | 0 | 0 | 0 | 0 | - |
| G-allele | 17 | 53.13 | 29 | 55.77 | 0.82 |
| A-allele | 15 | 46.87 | 23 | 44.23 | 0.82 |
| IL-10 (-1082) | |||||
| GG | 1 | 6.25 | 4 | 15.38 | 0.63 |
| GA | 15 | 93.75 | 18 | 69.23 | 0.12 |
| AA | 0 | 0 | 4 | 15.38 | 0.27 |
| G-allele | 17 | 53.13 | 26 | 50 | 0.82 |
| A-allele | 15 | 46.87 | 26 | 50 | 0.82 |
| IL-10 (-819) | |||||
| CC | 6 | 37.5 | 6 | 23.08 | 0.48 |
| CT | 8 | 50 | 17 | 65.38 | 0.35 |
| TT | 2 | 12.5 | 3 | 11.54 | 1 |
| C-allele | 20 | 62.5 | 29 | 55.77 | 0.65 |
| T-allele | 12 | 37.5 | 23 | 44.23 | 0.65 |
| IL-10 (-592) | |||||
| CC | 6 | 37.5 | 6 | 23.08 | 0.48 |
| CA | 8 | 50 | 17 | 65.38 | 0.35 |
| AA | 2 | 12.5 | 3 | 11.54 | 1 |
| C-allele | 20 | 62.5 | 29 | 55.77 | 0.65 |
| A-allele | 12 | 37.5 | 23 | 44.23 | 0.65 |
DISCUSSION
In this study 62% of the OLP patients were women. OLP has previously been reported to be more prevalent in females than males in American, British, Chinese, Iranian, Italian, Japanese and Spanish populations 4 , 5 , 20 . In our patients OLP was more frequent in the third to fourth decade of life and the median age of the patients group was 42 years. Reticular OLP was the most common (80.95%) with the buccal mucosa as the most affected site (83.33%). These results are compatible with previous studies from various ethnic groups 5 , 20 . There was no significant difference in the clinical manifestation or prognosis when comparing men to women however, in our study, these observations should be taken with caution as the study’s sample size is small.
Our results on TNF-α promoter polymorphism in Saudi OLP patients suggested that genotype GA-positive individuals at position -308 of TNF-α are at higher risk for OLP, whereas GG genotypes might be negatively associated with the disease. Data analysis further demonstrated that allele A (TNF-α 2 allele) increases the risk of OLP, whereas allele G (TNF-α 1 allele) reduces OLP risk. Similarly higher frequencies of TNF-α 308 A allele and -308 GA genotype have been reported in Italian 7 , Indian 8 , and Chinese patients with an OLP 3 , 12 . Predominance of the AA genotype was also reported in Thai 14 and Brazilian patients with OLP 32 . Further, genetic polymorphisms of cytokine-encoding genes are also known to predispose malignant diseases and have been associated with oral precancerous lesions (OPCLs). TNF-α (-308G/A), IL-6 and TGF-β polymorphisms have been associated with the development of OPCLs in Taiwanese patients 11 .
Allele A (TNF-α 2 allele) lies on the extended haplotype HLA-A1-B8-DR3-DQ2, which is associated with high TNF-α production. The allele A (TNF-α 2 allele) has been demonstrated to be a much stronger transcriptional activator than the common allele G (TNF-α 1 allele) 31 . Moreover, TNF-α (-308G/A) promoter polymorphism has a direct effect on TNF-α gene regulation (increased TNF-α expression) and may be responsible for the association of allele A with the high TNF-α phenotype and more severe diseases 31 . Further populations bearing a higher proportion of the allele A (TNF-α 2 allele) are reportedly predisposed to several metabolic, degenerative, inflammatory, and autoimmune diseases 1 .
The results of this study also show that the GA genotype of TNF-β (+252 A/G) was positively associated with OLP risk, whereas genotype AA might be protective. This report is the first to show an association between TNF-β (+252 A/G) polymorphism and OLP. This +252 A/G polymorphism on intron 1 of TNF-β is in linkage disequilibrium with a missense variant (Thr 26 Asn) in exon3 18 . It is also closely linked to TNF-α, which is in linkage disequilibrium with HLA-A, B8, and DR3 30 and has been reported to define a high TNF-α expressing haplotype in addition to a modifying expression of TNF-β itself 18 . TNF-β (+252A/G) polymorphism has also been associated with numerous autoimmune diseases 1 . High serum and salivary levels of proinflammatory cytokine and TNF have been detected in OLP patients 24 . Thalidomide, a known TNF suppressant has been used to successfully treat OLP showing the implication of high TNF-α expressing genotypes of TNF-α and -β polymorphisms in the pathogenesis of OLP 29 .
Our results show no significant differences in the frequencies of alleles and genotypes of IL-10 (1082G/A), IL-10 (-592C/A), IL-10 (-819C/T) polymorphisms between OLP patients and healthy Saudi controls. However, haplotype ATA from 1082G/A, -819C/T, -592C/A of IL-10 had significant association with OLP in Saudi patients. Two other studies dealing with the IL-10 gene polymorphisms in OLP populations could also not find any difference in the frequencies of IL-10 alleles and genotypes between the patients with OLP and healthy controls 3 , 32 . However,the -1082A/-819T/-592A (ATA) haplotype of the IL-10 polymorphisms, which correlated with a lower serum level of IL-10, has a significant association with OLP in a Chinese cohort with Han ethnicity 3 . Thus, this study together with the study from China supports that the low producer ATA haplotype of IL-10 polymorphisms is linked to the risk of OLP.
Our results also indicated that the frequencies of alleles and genotypes of all the five polymorphisms studied are independent of gender (Table 4). However, these results should be taken with caution as the number of males and females is too low for any significant conclusion.
Besides TNF-α and IL-10, a number of inflammation and immune regulation-related cytokines, including interferon-gamma (IFN-γ), IL-4, and IL-8, have been suggested to have an important role in the pathogenesis of OLP. Abnormal expression patterns of various inflammation-related cytokines, including IL-10, and TNF-α, in lesions, saliva, serum and peripheral blood mononuclear cells from patients with OLP has also been reported, which may reflect the immune dysregulation status of cytokines and emergence as central players in the immunopathogenesis of OLP 16 . Recently Carrozzo 7 (2014) suggested that the pro inflammatory milieu associated with degranulated mast cells might play a role in helping T cells breach the epidermal basement membrane in OLP lesions.
CONCLUSION
The polymorphisms in TNF-α (-308), TNF-β (+252) and IL-10 (-1082, -819 and -592) gene are associated significantly with the risk of OLP susceptibility. However, further studies are required that use a larger sample size to confirm this association.
ACKNOWLEDGEMENTS
The authors thank S. Sadaf Rizvi and Mohammad Al-Asmari for their help with laboratory work.
REFERENCES
- 1.Al-Harthi F, Zouman A, Arfin M, Tariq M, Al-Asmari A. Tumor necrosis factor-α and -β genetic polymorphisms as a risk factor in Saudi patients with vitiligo. Genet Mol Res. 2013;12:2196–2204. doi: 10.4238/2013.July.8.1. [DOI] [PubMed] [Google Scholar]
- 2.Al-Nasser L, El-Metwally A. Oral lichen planus in Arab countries: a review. J Oral Pathol Med. 2014;43:723–727. doi: 10.1111/jop.12136. [DOI] [PubMed] [Google Scholar]
- 3.Bai J, Jiang L, Lin M, Zeng X, Wang Z, Chen Q. Association of polymorphisms in the tumor necrosis factor-alpha and interleukin-10 genes with oral lichen planus: a study in a Chinese cohort with Han ethnicity. J Interferon Cytokine Res. 2009;29:381–388. doi: 10.1089/jir.2008.0089. [DOI] [PubMed] [Google Scholar]
- 4.Bermejo-Fenoll A, Sánchez-Siles M, López-Jornet P, Camacho-Alonso F, Salazar-Sánchez NJ. A retrospective clinicopathological study of 550 patients with oral lichen planus in south-eastern Spain. Oral Pathol Med. 2010;39:491–496. doi: 10.1111/j.1600-0714.2010.00894.x. [DOI] [PubMed] [Google Scholar]
- 5.Carbone M, Arduino PG, Carrozzo M, Gandolfo S, Argiolas MR, Bertolusso G, et al. Course of oral lichen planus: a retrospective study of 808 northern Italy patients. Oral Dis. 2009;15:235–243. doi: 10.1111/j.1601-0825.2009.01516.x. [DOI] [PubMed] [Google Scholar]
- 6.Carrozzo M. Understanding the pathobiology of oral lichen planus. Curr Oral Health Rep. 2014;1:173–179. [Google Scholar]
- 7.Carrozzo M, Uboldi de Capei M, Dametto E, Fasano ME, Paolo Arduino P, Broccoletti R, et al. Tumor necrosis factor-alpha and interferon-gamma polymorphisms contribute to susceptibility to oral lichen planus. J Invest Dermatol. 2004;122:87–94. doi: 10.1046/j.0022-202X.2003.22108.x. [DOI] [PubMed] [Google Scholar]
- 8.Chauhan I, Beena VT, Srinivas L, Sathyan S, Banerjee M. Association of cytokine gene polymorphisms with oral lichen planus in Malayalam-speaking ethnicity from South India (Kerala) J Interferon Cytokine Res. 2013;33:420–427. doi: 10.1089/jir.2012.0115. [DOI] [PubMed] [Google Scholar]
- 9.Farhi D, Dupin N. Pathophysiology, etiologic factors, and clinical management of oral lichen planus, part I: facts and controversies. Clin Dermatol. 2010;28:100–108. doi: 10.1016/j.clindermatol.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez-Moles MA, Scully C, Gil-Montoya JA. Oral lichen planus: controversies surrounding malignant transformation. Oral Dis. 2008;14:229–243. doi: 10.1111/j.1601-0825.2008.01441.x. [DOI] [PubMed] [Google Scholar]
- 11.Hsu HJ, Yang YH, Shieh TY, Chen CH, Kao YH, Yang CF, et al. Role of cytokine gene (interferon-γ, transforming growth factor-β1, tumor necrosis factor-α, interleukin-6, and interleukin-10) polymorphisms in the risk of oral precancerous lesions in Taiwanese. Kaohsiung J Med Sci. 2014;30:551–558. doi: 10.1016/j.kjms.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Jin X, Wang J, Zhu L, Wang L, Dan X, Zeng X, et al. Association between -308 G/A polymorphism in TNF-α gene and lichen planus: a meta-analysis. J Dermatol Sci. 2012;68:127–134. doi: 10.1016/j.jdermsci.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Khan HA. A visual basic software for computing Fisher’s exact probability. J Stat Softw. 2003;8:1–7. [Google Scholar]
- 14.Kimkong I, Hirankarn N, Nakkuntod J, Kitkumthorn N. Tumour necrosis factor-alpha gene polymorphisms and susceptibility to oral lichen planus. Oral Dis. 2011;17:206–209. doi: 10.1111/j.1601-0825.2010.01722.x. [DOI] [PubMed] [Google Scholar]
- 15.Koss K, Satsangi J, Fanning GC, Welsh KI, Jewell DP. Cytokine (TNF alpha, LT alpha and IL-10) polymorphisms in inflammatory bowel diseases and normal controls: differential effects on production and allele frequencies. Genes Immun. 2000;1:185–190. doi: 10.1038/sj.gene.6363657. [DOI] [PubMed] [Google Scholar]
- 16.Lu R, Zhang J, Sun W, Du G, Zhou G. Inflammation-related cytokines in oral lichen planus: an overview. J Oral Pathol Med. 2015;44:1–14. doi: 10.1111/jop.12142. [DOI] [PubMed] [Google Scholar]
- 17.McCartan BE, Healy CM. The reported prevalence of oral lichen planus: a review and critique. J Oral Pathol Med. 2008;37:447–453. doi: 10.1111/j.1600-0714.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 18.Messer G, Spengler U, Jung MC, Honold G, Blömer K, Pape GR, et al. Polymorphic structure of the tumor necrosis factor (TNF) locus: an NcoI polymorphism in the first intron of the human TNF-beta gene correlates with a variant amino acid in position 26 and a reduced level of TNF-beta production. J Exp Med. 1991;173:209–219. doi: 10.1084/jem.173.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mormann M, Rieth H, Hua TD, Assohou C, Roupelieva M, Hu SL, et al. Mosaics of gene variations in the interleukin-10 gene promoter affect interleukin-10 production depending on the stimulation used. Genes Immun. 2004;5:246–255. doi: 10.1038/sj.gene.6364073. [DOI] [PubMed] [Google Scholar]
- 20.Pakfetrat A, Javadzadeh-Bolouri A, Basir-Shabestari S, Falaki F. Oral lichen planus: a retrospective study of 420 Iranian patients. Med Oral Patol Oral Cir Bucal. 2009;1:E315–E318. [PubMed] [Google Scholar]
- 21.Perrey C, Turner SJ, Pravica V, Howell WM, Hutchinson IV. ARMS-PCR methodologies to determine IL-10, TNF-alpha, TNF-beta and TGF-beta 1 gene polymorphisms. Transpl Immunol. 1999;7:127–128. doi: 10.1016/s0966-3274(99)80030-6. [DOI] [PubMed] [Google Scholar]
- 22.Schallreuter KU, Levenig C, Kühnl P, Löliger C, Hohl-Tehari M, Berger J. Histocompatibility antigens in vitiligo: Hamburg study on 102 patients from northern Germany. Dermatology. 1993;187:186–192. doi: 10.1159/000247240. [DOI] [PubMed] [Google Scholar]
- 23.Schlosser BJ. Lichen planus and lichenoid reactions of the oral mucosa. Dermatol Ther. 2010;23:251–267. doi: 10.1111/j.1529-8019.2010.01322.x. [DOI] [PubMed] [Google Scholar]
- 24.Sklavounou-Andrikopoulou A, Chrysomali E, Iakovou M, Garinis GA, Karameris A. Elevated serum levels of the apoptosis related molecules TNF-alpha, Fas/Apo-1 and Bcl-2 in oral lichen planus. J Oral Pathol Med. 2004;33:386–390. doi: 10.1111/j.1600-0714.2004.00221.x. [DOI] [PubMed] [Google Scholar]
- 25.Svejgaard A, Platz P, Ryder LP. HLA and disease 1982 - a survey. Immunol Rev. 1983;70:193–218. doi: 10.1111/j.1600-065x.1983.tb00715.x. [DOI] [PubMed] [Google Scholar]
- 26.Thio CL. Host genetic factors and antiviral immune responses to hepatitis C virus. Clin Liver Dis. 2008;12:713–726. doi: 10.1016/j.cld.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turner DM, Williams DM, Sankaran D, Lazarus M, Sinnott PJ, Hutchinson IV. An investigation of polymorphism in the interleukin-10 gene promoter. Eur J Immunogenet. 1997;24:1–8. doi: 10.1111/j.1365-2370.1997.tb00001.x. [DOI] [PubMed] [Google Scholar]
- 28.Van der Meij EH, Mast H, van der Waal I. The possible premalignant character of oral lichen planus and oral lichenoid lesions: a prospective five-year follow-up study of 192 patients. Oral Oncol. 2007;43:742–748. doi: 10.1016/j.oraloncology.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 29.Wang Z, Yao H, Cui B, Ning G, Tang GY. Genetic linkage analysis of oral lichen planus in a Chinese family. Genet Mol Res. 2011;10:1427–1433. doi: 10.4238/vol10-3gmr1137. [DOI] [PubMed] [Google Scholar]
- 30.Wilson AG, Vries N, Pociot F, di Giovine FS, van der Putte LBA, Duff GW. An allelic polymorphism within the human tumor necrosis factor alpha promoter region is strongly associated with HLA A1, B8, and DR3 alleles. J Exp Med. 1993;177:557–560. doi: 10.1084/jem.177.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilson AG, Symons JA, McDowell TL, McDevitt HO, Duff GW. Effects of a polymorphism in the human tumor necrosis factor alpha promoter on transcriptional activation. Proc Natl Acad Sci USA. 1997;94:3195–3199. doi: 10.1073/pnas.94.7.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xavier GM, Sá AR, Guimarães AL, Silva TA, Gomez RS. Investigation of functional gene polymorphisms interleukin-1beta, interleukin-6, interleukin-10 and tumor necrosis factor in individuals with oral lichen planus. J Oral Pathol Med. 2007;36:476–481. doi: 10.1111/j.1600-0714.2007.00560.x. [DOI] [PubMed] [Google Scholar]


