Abstract
The use of nanoparticles (NPs) has become a significant area of research in Dentistry.
Objective
The aim of this study was to investigate the physical, antibacterial activity and bond strength properties of conventional base, core build and restorative of glass ionomer cement (GIC) compared to GIC supplemented with titanium dioxide (TiO2) nanopowder at 3% and 5% (w/w).
Material and Methods
Vickers microhardness was estimated with diamond indenter. Compressive and flexural strengths were analyzed in a universal testing machine. Specimens were bonded to enamel and dentine, and tested for shear bond strength in a universal testing machine. Specimens were incubated with S. mutans suspension for evaluating antibacterial activity. Surface analysis of restorative conventional and modified GIC was performed with SEM and EDS. The analyses were carried out with Kolmogorov-Smirnov, ANOVA (post-hoc), Tukey test, Kruskal-Wallis, and Mann Whitney.
Results
Conventional GIC and GIC modified with TiO2 nanopowder for the base/liner cement and core build showed no differences for mechanical, antibacterial, and shear bond properties (p>0.05). In contrast, the supplementation of TiO2 NPs to restorative GIC significantly improved Vickers microhardness (p<0.05), flexural and compressive strength (p<0.05), and antibacterial activity (p<0.001), without interfering with adhesion to enamel and dentin.
Conclusion
GIC supplemented with TiO2 NPs (FX-II) is a promising material for restoration because of its potential antibacterial activity and durable restoration to withstand the mastication force.
Keywords: Glass ionomer cements, TiO2 nanoparticles, Antibacterial activity, Physical properties, Shear bond strength
INTRODUCTION
Glass ionomer cement (GIC) possesses certain properties of adhesive 23 , biocompatibility 2 , and fluoride releasing 3 , which have led to worldwide use as luting, base, liners and restorative materials. However, the major disadvantages are fracture toughness, low wear-resistance and in the past high dissolution in a water sorption 23 resulting in a base, build or restoration failure leading to a growth of bacterial proliferation consequential in secondary caries or teeth fracture. The incorporation of hydroxyethyl-methacrylate (HEMA) or bisphenol-glycidyl-methacrylate (Bis-GMA) enhanced properties for compressive strength, hardness, higher modulus of elasticity, higher resistance to solubility and resistance to bacterial adhesion 14 . Significant perfections have been developed since the invention of GIC, numerous filler components have been added including; silver-amalgam particles 1 , spherical silica 26 , zirconia 12 , glass fiber 13 , hydroxyapatite 20 , bioactive glass particles as pre-reacted glass ionomer particles (PRG), giomer restorative material 15 . The incorporation of the filler particles above to GIC has significantly modified the mechanical properties of cements; however, fillers can interfere with metabolic activities for bacterial adhesion and inhibit the antibacterial activity of GIC 4 . In contrast, the use of nanoparticles (NPs) has become a significant area of research in Dentistry, the main use have been focused in increasing the mechanical properties and antibacterial effect; altering the hydrogen bonding, respiratory process, DNA unwinding, cell wall synthesis and division by making “pits” in the wall and increasing the permeability resulting in a bacterial death 11 . Recently, incorporation of hydroxyapatite and fluoroapatite nanobioceramics into conventional GIC improved their mechanical properties and bond strength to dentine 22 . Titanium dioxide (TiO2) as an inorganic additive has many promising properties as it is chemically stable, biocompatible and antibacterial 28 . NPs have been proposed as reinforcing fillers to dental resin composites and epoxy 30 . It has recently been reported that (i) the incorporation of TiO2 NPs to GIC at 3% and 5% (w/w) significantly enhanced the fracture toughness, compressive strength, flexural strength and hardness, and (ii) GIC supplemented with TiO2 NPs showed antibacterial activity against Streptococcus mutans without interference with fluoride release; nevertheless, (iii) the incorporation of 7% of TiO2 NPs compromised the mechanical properties and adhesion 6 . We recently reported that, for TiO2 nanoparticles in culture with human gingival fibroblast (HGF) 9 and oral squamous cell carcinoma cells (HSC-2) 7 , some particles were incorporated into the cells, exclusively in the vacuoles and showed no cytotoxic nor hormetic growth stimulation at lower concentrations. However, TiO2 NPs exert pro-inflammatory action by Interleukin-1β (IL-1β) and stimulated the secretion of prostaglandin E2 (PGE2), Cyclooxygenase (COX) 1 and 2, and induced drastic metabolic changes 10 to the culture medium by HGF cells and TiO2 NPs also induced PGE2 production, in synergy with IL-1β, the enhanced production of PGE2 was not simply due to LPS contamination 9 . Also, the incorporation of TiO2 NPs to GIC exhibits acceptable to moderate biocompatibility in culture with human oral normal cells [pulp cells (HPC), gingival fibroblast (HGF), periodontal ligament fibroblast (HPLF)] and human cancer cells [oral squamous cell carcinoma (OSCC): HSC-2, HSC-3, HSC-4 and gingival carcinoma (Ca9-22)] 8 .
Based in the previously reports, we expected that the supplementation of TiO2 NPs to GIC enhance its mechanical and antibacterial properties, the objective of this research is to investigate the physical properties (microhardness, flexural and compressive strength), the antibacterial activity and the bond strength of base, core build up and restorative GIC compared to GIC modified with TiO2 nanopowder at 3% and 5% (w/w).
MATERIAL AND METHODS
Powder of each GIC was blended with TiO2 nanopowder, anatase phase, particle size <25 nm (Sigma-Aldrich, St. Louis, MO, USA) at 3% and 5% (w/w). GIC powder and TiO2 NPs were mixed in a vortex for one minute.
Vickers microhardness test
GIC cylinders (9.5x1 mm) (n=5) were made in a Teflon mold according to ADA specification 27 after being prepared following the manufacturer´s instruction. The recommended powder/liquid (P/L) ratio of 2.6/1 g was mixed for cements. Cylinders were tested in ISO 9001:2008 certified diamond indenter (DongGuan Sinowon precision instruments, Nancheng, China) with 10 N and a dwell time of 10 s were employed for 10 indentations across the specimens of each group resulting in 50 indentations of each group. Since Vickers microhardness test is more sensitive to measurement errors than Knoop test and best for small rounded areas, we decided to use the method based on the ISO 9917-1:2007 16 .
Flexural and compressive strength
Twenty cylinder specimens were prepared as mentioned above. Cylinders were subjected to three points bending in a universal testing machine (AGS-X, Shimadzu, Kyoto, Japan) at cross speed of 1 mm/min (MPa). Flexural strength (MPa) was calculated using the following formula:
O´=3Pl/2bd2
where O´ is the flexural strength, P (N) is the load at fracture, l is the distance between the two supports (mm), b is the width of the specimen (mm), and d is the thickness (mm). On the other hand, compressive strength of specimens was performed by the universal testing machine at cross speed of 1 mm/min (MPa), and calculated using the following equation:
CS=2P/πdh
where CS is the compressive strength, P (N) is the load at fracture, d is the diameter of specimen (mm), and h is the thickness (mm). Flexural and compressive strength were determined according to ISO 9917-1:2007 16 and ISO 9917-2:2010 17 .
Shear bond strength to enamel and dentine
A total of 180 freshly extracted anterior bovine teeth were stored in 0.1 thymol solution. Teeth were randomly divided into the nine groups (n=20/group). Samples were fixed in acrylic resin (NicTone 62, MDC Dental, Guadalajara, Mexico) with a label bearing the number of each sample. A mounting jig was used to align each tooth’s labial surface. Standardized GIC blocks (4x4x1 mm) were preformed in a metal mold following the manufacturer’s instructions. Before adhering the block to the dental surfaces with fresh cement, the sample surfaces were finished with #400 waterproof abrasive paper (Fuji Star, Sankyo, Rikagaku, Okegawa, Japan). In the case of enamel bond strength, vestibular surface was sandblasted (Micro Cab, Danville, San Ramon, CA, USA) with 50 µm of aluminum dioxide (Danville, San Ramon, CA, USA) for one minute. Then, teeth underwent ultrasonic cleaning for one minute (Quantrex, Kearny, NJ, USA). Consequently, for testing the bond strength in dentin, the vestibular surfaces of the teeth were reduced approximately 1.5 mm with a high speed diamond bur (SS White Burs Inc, Lakewood, NJ, USA). At that point, dentinal surface was sandblasted and underwent ultrasonic cleaning as mentioned above. Immediately after direct bonding the GIC block with appropriate powder/liquid proportion, samples were stored in water at 37°C during 24 h. Shear bond strength to enamel and dentine was carried out in a universal testing machine at cross speed of 1 mm/min (MPa). Force was applied at the interface of the GIC block and dental surface.
Antibacterial activity
Suspension of approximately 10 5 Streptococcus mutans (S. mutans, ATCC 35668) was cultivated in brain heart infusion broth (Becton Dickinson, NJ, USA) for 18 hours. Bacteria solution was sub-cultivated in brain heart agar (Becton Dickinson, NJ, USA). Immediately, blocks (4x4x1 mm) of the different conventional GIC and GIC modified with TiO2 NPs at 3% and 5% (w/w) were set in direct contact over the agar containing the bacteria, after 24 hours of incubation at 37°C, inhibit halos were measured with electronic digital caliper (NSK, Tochigi, Japan). Three blocks were set on each 100 mm plate containing the brain heart agar. Experiment was performed in triplicate to obtain reproducible data.
SEM and EDS analysis
Standardized GIC blocks (4x4x1 mm) of FX-II conventional, FX-II 3% (w/w) TiO2 NPs, and FX-II 5% (w/w) TiO2 NPs were prepared in the metallic mold and covered with microslide glass. Samples were gently polished and finished with #400, 1000 and 1500 waterproof abrasive paper (Fuji Star, Sankyo, Rikagaku, Okegawa, Japan). Subsequently, blocks were ultrasonically cleaned for five minutes in distilled water (Quantrex, Kearny, NJ, USA). All samples were adhered to aluminum stubs with conductive tape, coated with carbon and observed under SEM (PHILIPS XL-30, North Billerica, MA, USA) with secondary electrons at ×100, ×500, and ×3,000 magnification by 20 kV. Energy-dispersive X-ray (EDS) analysis was developed at the same time of SEM micrographs. An area of approximately 20×15 µm was selected for analysis; relative values were obtained after 300 s of measurement.
Statistical analysis
Mean values and standard deviations were estimated. Vickers microhardness data were subjected to Kolmogorov-Smirnov normality test and ANOVA (post-hoc) Tukey test. In order to examine compressive and flexural strength, shear bond strength to enamel and dentine, and antibacterial activity data were analyzed with non-parametric Kruskal-Wallis and multiple comparisons of Mann-Whitney, the analyses were carried out with SPSS 18.0 (SPSS Inc., Chicago, Ill, USA). A value of 0.05 was considered statistically significant.
RESULTS
Vickers microhardness
Vickers microhardness data indicated normality and ANOVA test showed statistical differences (p<.0001) between groups and post-hoc Tukey test results are enlisted in Table 1. It must be mentioned that, in all cases, the size of the indentations was larger than the filler particles, based on the size of fillers reported by the manufacturer. Data showed a significant increase in microhardness for the FX-II containing 3% and 5% (w/w) TiO2 NPs compared to the conventional cement. Nevertheless, core shade and base cement did not present increased microhardness values; actually, the inclusion of nanopowder at both concentrations decreased the microhardness.
Table 1. Mean (standard deviation) of Vickers microhardness (VHN) (n=50), flexural (O´) and compressive strength (Cs) and shear bond strength to enamel and dentin (n=20) of GIC and GIC incorporated with 3% and 5% (w/w) TiO2 nanopowder.
| Cement | GroupƟ | VHN | O´ | Cs | Enamel bond strength | Dentin bond strength |
|---|---|---|---|---|---|---|
| Core shade base cement (Gray) | Conventional GIC | 56.9±9.6a | 22.4±6.9a | 7.1±3.9a | 1.92±1.11a | 1.90±.92a |
| GIC-3% (w/w) TiO2 | 47.1±6.5b | 18.1±5.6a | 8.8±3.0ab | 1.30±.49a | 1±.40b | |
| GIC-5% (w/w) TiO2 | 57.6±7.1a | 21.2±6.8a | 9.6±2.5b | 2.61±1.52a | 1.40±.86b | |
| Base cement (Yellow) | Conventional GIC | 61.2±7.6a | 20.8±5.6a | 7±3.2a | 2.61±1.33a | .84±.28a |
| GIC-3% (w/w) TiO2 | 54.1±5.5b | 20.2±5.9a | 7.5±3.1b | 1.78±1.08b | .82±.20a | |
| GIC-5% (w/w) TiO2 | 58.4±5.2a | 18.3±4.3a | 5.4±2.4c | 1.78±.91b | .87±.21a | |
| FX-II Enhanced restoration (A2) | Conventional GIC | 54.3±9.0a | 15.1±2.9a | 5.6±2.3a | 1.89±1.39a | 1.32±.74a |
| GIC-3% (w/w) TiO2 | 64.2±3.3b | 20.2±4.1b | 7.3±1.6b | 1.96±1.47a | 1.50±.66a | |
| GIC-5% (w/w) TiO2 | 63.8±4.1b | 21.4±5.0c | 8.6±1.5c | 2.20±1.41a | .99±.46a |
* GIC: Glass ionomer cement.
Ɵ TiO2: Titanium dioxide nanopowder.
Mean values for each cement group with the same superscript letter (column) are not significantly different (p>0.05), while mean values with different letters are significantly different (p<0.05). Vickers microhardness was analyzed with ANOVA (post-hoc) Tukey test, while flexural and compressive strength, shear bond strength to enamel and dentin were analyzed by Mann Whitney test.
Flexural and compressive strength
The supplementation of 3% and 5% (w/w) TiO2 NPs into FX-II enhanced flexural strength (p<0.05) and compressive strength (p<0.0001), compared to the conventional cement. The minimal supplementation at 3% improved the properties of definitive restoration cement. Core shade build up cement improved only compressive strength when 5% (w/w) (p<0.05) TiO2 NPs were incorporated, compared to the conventional GIC. Base cement did not (p<0.05) show better properties with the addition of TiO2 NPs compared to conventional GIC. The results are summarized in Table 1.
Shear bond strength
Data for shear bond strength (MPa) to enamel and dentine showed no statistical differences between the conventional GIC and that modified with TiO2 NPs (neither at 3% nor at 5%). There was a slight but insignificant increase in the shear bond strength to enamel in the case of the core shade with 5% (w/w) TiO2 NPs, and FX-II with 3% and 5% (w/w) TiO2 NPs (Table 1).
Antibacterial activity
Bacterial growth activity (Table 2) was reduced on direct contact to FX-II conventional, FX-II 3% and 5% (w/w) TiO2 NPs. Inhibit halos values (n=18) obtained corresponded to 0.92±.22 mm, 2.11±0.82 mm, and 1.53±0.79 mm, respectively. When the antibacterial activity of FX-II 3% and 5% (w/w) TiO2 NPs was compared with conventional FX-II, significant differences were observed (p<0.001) in both groups, and no difference was observed between FX-II 3% and 5% (w/w) TiO2 NPs. The minimum supplementation of 3% or 5% (w/w) TiO2 NPs to GIC showed higher antibacterial activity against S. mutans than conventional FX-II. Nevertheless, core shade and base cement conventional GIC with or without modification with TiO2 nanopowder showed no antibacterial properties in any specimens.
Table 2. Antibacterial activity of GIC and GIC incorporated with 3% and 5% (w/w) TiO2 nanopowder against Streptococcus mutans (ATCC 35668).
| Cement | GroupƟ | n | Inhibit halos (mm) |
|---|---|---|---|
| Core shade base cement (Gray) | Conventional GIC* | 18 | None |
| GIC-3% (w/w) TiO2 | 18 | None | |
| GIC-5% (w/w) TiO2 | 18 | None | |
| Base cement (Yellow) | Conventional GIC | 18 | None |
| GIC-3% (w/w) TiO2 | 18 | None | |
| GIC-5% (w/w) TiO2 | 18 | None | |
| FX-II Enhanced restoration (A2) | Conventional GIC | 18 | 0.92±0.22a |
| GIC-3% (w/w) TiO2 | 18 | 2.11±0.82b | |
| GIC-5% (w/w) TiO2 | 18 | 1.53±0.79b |
* GIC: Glass ionomer cement.
Ɵ TiO2: Titanium dioxide nanopowder.
Mean values for each cement group with the same superscript letter (column) are not significantly different (p>0.05), while mean values with different letters are significantly different (p<0.001) (Mann-Whitney test).
SEM and EDS analysis
Representative SEM micrographs are shown in Figure 1. Topographically, there are no apparent differences in the finish surfaces for FX-II, FX-II 3% and 5% (w/w) TiO2 NPs. In Figure 1C, hybrid particles are observed, microparticles uniformly lay between (matrix) macroparticles, and such particles seem to be grouped of TiO2 NPs due to their angular and semispherical shape confirmed by the 1D micrograph. The composition of conventional GIC FX-II, FX-II 3% and 5% (w/w) TiO2 NPs are shown in Table 3. Based on EDS data, all materials showed dominant portions of carbon and oxygen. Titanium was detected in FX-II containing 3% and 5% (w/w) TiO2 NPs, while the concentration of oxygen increased and strontium decreased, by incorporating the TiO2 NPs.
Figure 1. Blocks (4x4x1 mm) of (a) conventional FX-II, (b) FX-II 3% (w/w) TiO2, and (c) FX-II 5% (w/w) TiO2. Samples were gently polished and finished with #400, #1,000, and #1,500 waterproof abrasive paper and ultrasonically cleaned. Topographically, there are no differences between specimens. Nevertheless, hybrid particles are observed, microparticles (1c, black circle and arrow) are uniformly lay between (matrix) macroparticles, and such particles seem to be grouped of TiO2 nanoparticles due to their angular and semispherical shape confirmed by the 1d micrograph and EDS of this area, the zone exhibits higher concentration of titanium (a%=0.36%).
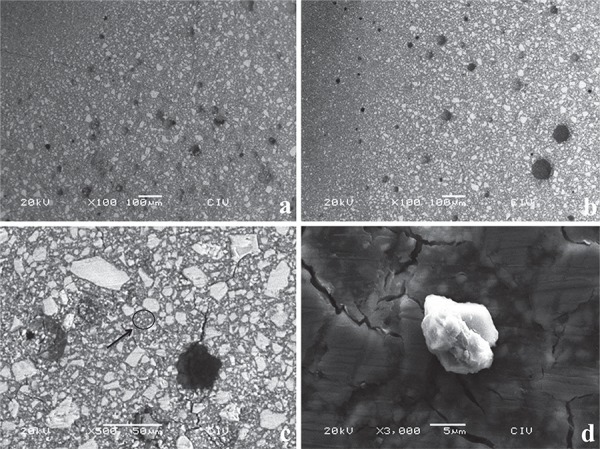
Table 3. Energy-dispersive X-ray (EDS) analysis of conventional FX-II, FX-II with 3% and 5% (w/w) TiO2 nanopowder. Values represent atomic percentage (a%).
| Element | FX-II | FX-II-3% (w/w) TiO2 | FX-II-5% (w/w) TiO2 |
|---|---|---|---|
| C | 78.1 | 59.6 | 60.3 |
| O | 11.2 | 30.56 | 30.63 |
| F | 2.4 | 5.72 | 5.7 |
| Al | 3.3 | 1.68 | 1.33 |
| Si | 2.93 | 1.34 | 1.08 |
| P | 0.85 | 0.37 | 0.28 |
| S | 0.006 | 0.001 | 0 |
| Ti | 0 | 0.11 | 0.17 |
| Sr | 1.16 | 0.57 | 0.44 |
| Total | 100% | 100% | 100% |
FX-II: Enhanced restorative cement
TiO2: Titanium dioxide nanopowder
DISCUSSION
Flexural and compressive strength
Compressive and flexural tests are used in Dentistry for laboratory simulation of the stress that may result from forces applied clinically to a restorative, base/liner or core build material 24 . Most mastication forces are compressive in nature, but exact critical value is unknown 28 . Therefore, it is important to investigate whether compressive force contributes to fracture failure during mastication process. The minimum value necessary to resist the masticatory forces in the posterior teeth would be 125 MPa, while 100 MPa for primary dentition 29 . Flexural forces are generated under clinical situations, and the dental materials need to withstand the repeated flexing, bending, and twisting forces. Microhardness test is a parameter frequently used to evaluate the material surfaces resistance to plastic deformation by penetration 28 . The powder/liquid ratio of GIC has an influence on the mechanical properties and bond strength 31 .
Improvement in flexural strength of the GIC FX-II was significantly higher at concentrations of 3% and 5% (w/w) TiO2 NPs than conventional. Therefore, Ketac-Molar (3M ESPE, Seefeld, Germany) and Fuji IX (GC Corporation, Tokyo, Japan) have showed higher values of flexural strength than the cements in this study when performed specimens of 25x2x2 mm. The reported flexural strength values of the cements above represent 33.3, 34.5 MPa, respectively 19 . Supplementation of 3% and 5% (w/w) TiO2 NPs to FX-II have results similar to those reported by Elsaka, et al. 6 (2011) when restorative GIC (Kavitan Plus, SpofaDental, Czech Republic) was modified with TiO2 NPs.
On the other hand, the test procedures for compressive strength are not complicated. Although the compression specimen has a convenient cylindrical geometry, perfection of the ends (which is essential to produce uniform contact between the specimen and the testing device) is difficult to achieve. Compressive strengths for GIC FX-II containing 3% and 5% (w/w) TiO2 NPs were higher than that of conventional GIC. Compressive strengths of different conventional GIC such as Ketac Molar (3M ESPE), Fuji IX (GC), and Ketac-fil plus (3M ESPE) (146.28 to 152.41 MPa) were higher than that of GIC studied here with or without supplementation of TiO2 NPs; the difference in values can be explained by the size of specimens (4 mm diameter and 6 mm high) 19 . Flexural and compressive strength improvement of FX-II containing 3% and 5% (w/w) TiO2 NPs can be attributed to the small sizes of the TiO2 particles supplemented into the glass powder and the presence of the NPs can occupy the empty spaces between the larger GIC glass particles and act as additional bonding sites for the polyacrylic polymer; this means that the base cement did not incorporate particles because of the small size particles and greater surface of TiO2 NPs compared to those of the glass.
Vickers microhardness
The GIC FX-II enhanced restoration containing 3% and 5% (w/w) TiO2 NPs exhibit significantly higher Vickers microhardness compared to conventional GIC, while GIC with 5% (w/w) TiO2 NPs for base and core build showed no statistical differences in relation to conventional cement. The 3% (w/w) TiO2 NPs rather decreased the Vickers microhardness; the supplementation of TiO2 NPs to FX-II powder possibly is related to the fewer glass particles on the surface of GIC, which result in greater amount of acid to react with the NPs. Different studies have focused on determining the hardness of conventional and modified GIC. Thus, conventional GIC as Ketac-fil (3M, ESPE), Fuji IX (GC), and Ionofil Molar (VOCO, Cuxhaven, Germany) have values of 90, 69.7, and 57.4 VHN, respectively 18 . Conventional FX-II enhanced restoration showed 54.3 VHN, lower values than the other GICs. On the other hand, the supplementation of 3% and 5% (w/w) TiO2 NPs to conventional FX-II showed higher values of 64.2 and 63.8 VHN, respectively. Meanwhile, microhardness values of metal reinforced cements like Fuji IX GP (GC) (from 54.44 to 61.77 VHN) 21 showed lower microhardness than FX-II supplemented with TiO2 NPs; thus, Kavitan Plus restorative (SpofaDental) containing 3% (w/w) TiO2 NPs represents 48.34 VHN 6 . Microhardness values of RMGIC such as Photac Fil (3M, ESPE), Vitremer (3M, St. Paul, MN, USA), and Fuji II LC (GC) showed values of 46.2, 51.4, 69.2 VHN 21 , respectively.
Shear bond strength
The chemical adhesion of GIC to enamel and dentin is achieved by reaction of phosphate ions in the dental tissue with carboxylate groups in the polyacrylic acid. Several factors can influence the bond strength, one of which is the type of dental substrate. Theoretical considerations and results of experiments show that enamel is much more susceptible to adhesion than dentin 21 . Enamel has a surface that is essentially homogeneous, dense, and mainly composed of hydroxyapatite, which possesses high surface energy. Dentin has a heterogeneous surface, containing dental tubules that contain odonto-plastic processes, consists of approximately 30% volume organic matter, and consequently has low surface energy 19 . The enamel bond strength of different GIC modified with 3% and 5% (w/w) TiO2 NPs studied here showed similar values in relation to conventional cements except for Core shade containing 5% (w/w) TiO2 NPs, which showed significantly higher values when bonding to enamel surface. Data reported here have similar or lower values of enamel shear bond strength than different studies carried out with conventional GIC such as Ketac-fil plus (3M ESPE), Ketac-Molar (3M ESPE), and Fuji IX (GC). These cements have reported values as follows: 4.9, 5.31, and 5 MPa when debonding 3 mm in diameter of GIC adhered to enamel surface 5 , 25 . These low values were observed due to the sensitivity of GIC to moisture during setting. In our study, the comparison of scores recorded among the conventional GIC and GIC supplemented with 3% and 5% (w/w) TiO2 NPs demonstrated that there is no difference between groups of cements, except for the core shade cement, which conventionally has higher adherence to dentinal surface than GIC modified with TiO2 NPs. Results can be explained by the incorporation of TiO2 NPs to powder of GIC, which does not interfere with the shear bond strength to dentin. In addition, some studies recorded shear bond strength values of 2.05, 308, and 3.79 MPa, respectively, for GIC Ketac-fil plus (3M ESPE), Ketac-Molar (3M ESPE), and Fuji IX (GC) 5 , 25 . Therefore, when both enamel and dentinal surfaces were sandblasted, the values of shear bond strength increase twice when debonding GIC specimens.
Consequently, GIC containing 3% and 5% (w/w) TiO2 NPs seem to be much more susceptible to dissolution in contact to water than conventional cement; it can be explained by the low ionic attraction between filler particles and TiO2 NPs and the heterogeneous distribution of NPs into the filler particles when mixed at the recommended powder/liquid ratio.
Antibacterial activity
On the other hand, the minimum supplementation of 3% or 5% (w/w) TiO2 NPs to the FX-II showed better antibacterial activity against S. mutans (ATCC 35668) than conventional FX-II. Similar antibacterial activity results are obtained for specimens of restorative GIC Kavitan Plus (SpofaDental) added with 3%, 5%, and 7% (w/w) TiO2 nanopowder on direct contact to S. mutans (ATCC 27351) reported by Elsaka, et al. 6 (2011). The base cement and core shade cement showed no antibacterial activity, possibly explained by the agglomeration of TiO2 NPs forming a conjugated particle that was not perfectly incorporated between the filler particles and matrixes of GIC as well as that particle attraction was positioned near the center of the cement without reactive surfaces in direct contact to bacteria, leading to ineffective bacterial growth inhibition. The antibacterial mechanism suggested that TiO2 NPs to produced reactive oxygen species (ROS), specifically, hydroxyl free radicals and peroxide, as previously reported 27 .
SEM and EDS analysis
Due to the unique properties detected in the FX-II supplemented with TiO2 NPs at 3% and 5% (w/w), SEM observation and EDS analysis were performed to identify the topographical aspect and chemical interaction and composition of supplemented GIC; however, it is necessary to investigate the chemical interaction between TiO2 NPs and GIC composition by specific analyses, such as transmission electron microscopy (TEM) and sophisticated spectroscopies. Findings of EDS analysis showed as follows: between higher TiO2 amounts, lesser carbon composition and higher quantity of oxygen. On the other hand, the fluor composition when TiO2 NPs is added to conventional FX-II GIC powder at 3% and 5% increases, probably, due to the suitable interaction of glass particles and NPs showed better antibacterial effect 28 .
Among the limitations of study, further in-depth antibacterial activity tests are necessary to be performed in future research to obtain reliable results using not only S. mutans but also aerobic, anaerobic and facultative bacterial. Further research is necessary to understand the fluor releasing from the GIC modified with TiO2 nanopowder.
CONCLUSIONS
GIC supplemented with TiO2 NPs is a promising dental material to be used as enhanced restoration due to its potential antibacterial properties and use in high-tension restoration considering the force of mastication.
REFERENCES
- 1.Bala O, Arisu HD, Yikilgan I, Arslan S, Gullu A. Evaluation of surface roughness and hardness of different glass ionomer cements. Eur J Dent. 2012;6:79–86. [PMC free article] [PubMed] [Google Scholar]
- 2.Brentegani LG, Bombonato KF, Carvalho TL. Histological evaluation of the biocompatibility of a glass-ionomer cement in rat alveolus. Biomaterials. 1997;18:137–140. doi: 10.1016/s0142-9612(96)00110-x. [DOI] [PubMed] [Google Scholar]
- 3.Coffey JP, Robertello FJ, Lynde TA, King P. Fluoride release of glass ionomer-based luting cements in vitro. J Prosthet Dent. 1999;82:172–176. doi: 10.1016/s0022-3913(99)70152-6. [DOI] [PubMed] [Google Scholar]
- 4.Dhull KS, Nandlal B. Comparative evaluation of fluoride release from PGR-composites and compomer on application of topical fluoride: an in vitro study. J Indian Soc Pedod Prevent Dent. 2009;27:27–32. doi: 10.4103/0970-4388.50813. [DOI] [PubMed] [Google Scholar]
- 5.El-Askary FS, Nassif MS, Fawzy AS. Shear bond strength of glass-ionomer adhesive to dentin: effect to smear layer thickness and different dentin conditioners. J Adhes Dent. 2008;10:471–479. [PubMed] [Google Scholar]
- 6.Elsaka SE, Hamouda IM, Swain MV. Titanium dioxide nanoparticles addition to a conventional glass-ionomer restorative: influence on physical and bacterial properties. J Dent. 2011;39:589–598. doi: 10.1016/j.jdent.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Contreras R, Scougall-Vilchis RJ, Contreras-Bulnes R, Ando Y, Kanda Y, Hibino Y, et al. Effect of TiO2 nanoparticles on cytotoxic action of chemotherapeutic drugs against a human oral squamous cell carcinoma cell line. In Vivo. 2014;28:209–215. [PubMed] [Google Scholar]
- 8.Garcia-Contreras R, Scougall-Vilchis RJ, Contreras-Bulnes R, Kanda Y, Nakajima H, Sakagami H. Effect of TiO2 nano glass ionomer cements against normal and cancer oral cells. In Vivo. 2014;28:895–907. [PubMed] [Google Scholar]
- 9.Garcia-Contreras R, Scougall-Vilchis RJ, Contreras-Bulnes R, Kanda Y, Nakajima H, Sakagami H. Induction of prostaglandin E2 production by TiO2 nanoparticles in human gingival fibroblast. In Vivo. 2014;28:217–222. [PubMed] [Google Scholar]
- 10.Garcia-Contreras R, Susigmoto M, Umemura N, Kaneko M, Hatakeyama Y, Soga T, et al. Alteration of metabolomic profiles by titanium dioxide nanoparticles in human gingivitis model. Biomaterials. 2015;57:33–40. doi: 10.1016/j.biomaterials.2015.03.059. [DOI] [PubMed] [Google Scholar]
- 11.García-Contreras R, Argueta-Figueroa, Mejía-Rubalcava C, Jiménez Martínez R, Cuevas-Guajardo S, Sánchez-Reyna PA, et al. Perspectives for the use of silver nanoparticles in dental practice. Int Dent J. 2011;61:297–301. doi: 10.1111/j.1875-595X.2011.00072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu YW, Yap AU, Cheang P, Koh YL, Khor KA. Development of zirconia-glass ionomer cement composites. J Non Cryst Solids. 2005;351:508–514. [Google Scholar]
- 13.Hammouda IMN. Addition of glass fibers to conventional glass ionomer and composite resin restorative materials. Int J Mat Sci. 2007;2:123–136. [Google Scholar]
- 14.Hibino Y, Kuramochi K, Harashima A, Honda M, Yamazaki A, Nagasawa Y, et al. Correlation between the strength of glass ionomer cements and their bond strength to bovine teeth. Dent Mater J. 2004;23:656–660. doi: 10.4012/dmj.23.656. [DOI] [PubMed] [Google Scholar]
- 15.Ikemura K, Tay FR, Endo T, Phashley DH. A review of chemical-approach and ultramorphological studies in the development of fluoride-releasing dental adhesives comprising new pre-reacted glass ionomer (PGR) fillers. Dent Mater J. 2008;27:315–339. doi: 10.4012/dmj.27.315. [DOI] [PubMed] [Google Scholar]
- 16.International Organization for Standardization . ISO 9917:2007: Dentistry-water-based cements-part 1: powder/liquid acid-base cements. Geneva: International Organization for Standardization; 2007. [Google Scholar]
- 17.International Organization for Standardization . ISO 9917-2:2010: Water-based cements - Part 2: Resin-modified cements. Geneva: International Organization for Standardization; 2010. [Google Scholar]
- 18.Khouw-Liu VH, Anstice HM, Pearson GJ. An in vitro investigation of a poly (vinyl phosphonic acid) based cement with four conventional glass-ionomer cement Part 2: Maturation in relation to surface hardness. J Dent. 1999;27:359–365. doi: 10.1016/s0300-5712(98)00062-1. [DOI] [PubMed] [Google Scholar]
- 19.Lohbauer U. Dental glass ionomer cements as permanent filling material? Properties, limitations and future trends. Materials. 2010;3:76–96. [Google Scholar]
- 20.Lucas ME, Arita K, Nishino M. Toughness, bonding and fluoride-release properties of hydroxyapatite-added glass ionomer cement. Biomaterials. 2003;24:3787–3794. doi: 10.1016/s0142-9612(03)00260-6. [DOI] [PubMed] [Google Scholar]
- 21.Magni E, Ferrari M, Hickel R, Ilie N. Evaluation of the mechanical properties of dental adhesives and glass-ionomer cements. Clin Oral Invest. 2010;14:79–87. doi: 10.1007/s00784-009-0259-3. [DOI] [PubMed] [Google Scholar]
- 22.Moshaverinia A, Ansari S, Moshaverinia M, Roohpour N, Darr JA, Rehman I. Effect of incorporation of hidroxyapatite and fluoroapatite nanobioceramics into conventional glass ionomer cements (GIC) Acta Biomater. 2008;4:432–440. doi: 10.1016/j.actbio.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 23.Pereira LC, Nunes MC, Dibb RG, Powers JM, Roulet JF, Navarro MF. Mechanical properties and bond strength of glass-ionomer cements. J Adhes Dent. 2002;4:73–80. [PubMed] [Google Scholar]
- 24.Peutzfeldt A. Restorative materials for the direct technique. In: Adhesion: the silent revolution in dentistry. Chicago: Quintessence Publishing; 2000. pp. p. 61–p. 80. [Google Scholar]
- 25.Souza-Zaroni WC, Nhani VT, Ciccone-Nogueira JC, Chinalatti MA, Palma-Dibb RG, Corona SA. Shear bond strength of glass-ionomer cements to air-abraded dentin. J Adhes Dent. 2006;8:233–237. [PubMed] [Google Scholar]
- 26.Tjandrawinata R, Irie M, Susuki K. Effect of 10wt% spherical silica filler addition on the various properties of conventional and resin-modified glass-ionomer cements. Acta Odntol Scand. 2005;63:371–375. doi: 10.1080/00016350500206819. [DOI] [PubMed] [Google Scholar]
- 27.Wang H, Tang B, Li X, Ma Y. Antibacterial properties and corrosion resistance of nitrogen-doped TiO2 coatings on stainless steel. J Mat Sci Technol. 2011;27:309–316. [Google Scholar]
- 28.Wang L, D´Alpino PH, Lopes LG, Pereira JC. Mechanical properties of dental restorative material: relative contribution of laboratory test. J Appl Oral Sci. 2003;11:162–167. doi: 10.1590/s1678-77572003000300002. [DOI] [PubMed] [Google Scholar]
- 29.Williams JA, Billington RW. Increase in compressive strength of glass ionomer restorative materials with respect to time: a guide to their suitability for use in posterior primary dentition. J Oral Rehab. 1989;16:475–479. doi: 10.1111/j.1365-2842.1989.tb01368.x. [DOI] [PubMed] [Google Scholar]
- 30.Xia Y, Zhang F, Xie H, Gu N. Nanoparticle-reinforced resin-based dental composites. J Dent. 2008;36:450–455. doi: 10.1016/j.jdent.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 31.Xie D, Brantley WA, Culbertson BM, Wang G. Mechanical properties and microstructures of glass-ionomer cements. Dent Mater. 2000;16:129–138. doi: 10.1016/s0109-5641(99)00093-7. [DOI] [PubMed] [Google Scholar]


