Abstract
Tapeworms of the genus Spirometra are pseudophyllidean cestodes endemic in Korea. At present, it is unclear which Spirometra species are responsible for causing human infections, and little information is available on the epidemiological profiles of Spirometra species infecting humans in Korea. Between 1979 and 2009, a total of 50 spargana from human patients and 2 adult specimens obtained from experimentally infected carnivorous animals were analyzed according to genetic and taxonomic criteria and classified as Spirometra erinaceieuropaei or Spirometra decipiens depending on the morphology. Morphologically, S. erinaceieuropaei and S. decipiens are different in that the spirally coiled uterus in S. erinaceieuropaei has 5-7 complete coils, while in S. decipiens it has only 4.5 coils. In addition, there is a 9.3% (146/1,566) sequence different between S. erinaceieuropaei and S. decipiens in the cox1 gene. Partial cox1 sequences (390 bp) from 35 Korean isolates showed 99.4% (388/390) similarity with the reference sequence of S. erinaceieuropaei from Korea (G1724; GenBank KJ599680) and an additional 15 Korean isolates revealed 99.2% (387/390) similarity with the reference sequences of S. decipiens from Korea (G1657; GenBank KJ599679). Based on morphologic and molecular databases, the estimated population ratio of S. erinaceieuropaei to S. decipiens was 35: 15. Our results indicate that both S. erinaceieuropaei and S. decipiens found in Korea infect humans, with S. erinaceieuropaei being 2 times more prevalent than S. decipiens. This study is the first to report human sparganosis caused by S. decipiens in humans in Korea.
Keywords: Spirometra erinaceieuropaei, Spirometra decipiens, mitochondrial gene, molecular identification, morphology
INTRODUCTION
Tapeworms of the genus Spirometra are pseudophyllidean cestodes. The genus was established under the name Spirometra by Mueller [1] in 1937, although this name had been used earlier by Faust et al. [2] in 1929. There have been some controversies surrounding this genus because of its species classification; however, it has been recognized as independent and separate to Diphyllobothrium at the generic level. In 1952, Wardle and McLeod [3] recognized Spirometra mansoni Joyeux and Houdemer, 1927 and Spirometra erinacei Faust, Campbell and Kellogg, 1929 as independent species, with S. mansoni as the type species. In 1959, Yamaguti [4] classified S. mansoni and S. erinacei as the same species and put under the name S. erinaceieuropaei (hyphen has been omitted later by other authors). In 1972, Iwata [5] insisted that all species in the genus Spirometra are synonyms of S. erinacei Faust, Campbell and Kellogg, 1929. However, in 1974, Mueller [6] distinguished S. mansonoides distributed in North America from S. mansoni distributed in Asian region.
The species of the genus Spirometra, including S. erinaceieuropaei (S. erinacei), S. decipiens, S. mansoni, S. ranarum, and S. mansonoides are all intestinal parasites of canine and feline hosts [1-6]. The main characters of species differentiation are the spirally coiled uterus of S. erinaceieuropaei (5-7 coils) and S. decipiens (4−4½ coils) [2]. The life cycle of these parasites require 2 different intermediate hosts; the fresh water cyclops as the first intermediate host, and vertebrates such as amphibians and reptiles as the second intermediate hosts [6,7]. The procercoids develop in cyclops, and the plerocercoids (spargana) develop in frogs or snakes and cause sparganosis in humans.
Human sparganosis has been reported sporadically worldwide. The first case in Korea was reported under the name Sparganum mansoni [8]. Between 1924 and 1974, snakes and frogs were identified as the second intermediate host causing human sparganosis in 63 patients [9]. Between 1975 and 1989, an additional 56 cases of human sparganosis were reviewed [10]. Further studies identified adult worm infections with S. erinaceieuropaei (under the name S. erinacei) in 2 human patients [7]. The experimental complete life cycle was established in the laboratory [11].
However, the cases of human sparganosis have not been clearly identified in the aspect of what kind(s) of Spirometra species were involved in human infections. Accurate identification of Spirometra spp. in Korea had been difficult because of lack of morphologic and molecular characterizing features. Therefore, a reliable taxonomic criterion based on morphologic characteristics and molecular data is needed. The present study aimed to provide information for classification and identification of Spirometra spp. in Korea by focusing on morphologic differences as well as nucleotide sequencing of mitochondrial genes of S. erinaceieuropaei and S. decipiens.
MATERIALS AND METHODS
Specimens and experimental infection to animals
A total of 50 human-infected plerocercoids (1979-2009) and 2 adult tapeworms recovered from experimentally infected cat and dog were analyzed (Table 1). The plerocercoids from humans were obtained from hospitals and researchers in different parts of the country. The procedures for obtaining 2 adult tapeworms were as follows: In April 2008, a plerocercoid of S. decipiens was collected from a snake (Rhabdophis tigrinus tigrinus) and used to infect a cat. In May 2009, a plerocercoid of S. erinaceieuropaei was collected from a 58-year-old man; a small part of the larva was cut for molecular studies and the remainder was used to infect a dog. After 8 weeks of infection, the cat and the dog were sacrificed and the adult tapeworms were recovered from the intestine. The adult worms were pressed and fixed in alcohol-formalin-acetic acid (AFA) for carmine staining. The vaginal opening, uterus, uterine pore, cirrus, genital pore, testes, and vitellaria of mature and gravid proglottids were observed, and longitudinal sections of gravid proglottids were stained with hematoxylin and eosin (H&E) to observe their relationships. The morphologic data were compared to those of Faust et al. [2]. Some of the gravid proglottids were preserved in a -70˚C deep freezer and in 70% ethanol for further use. Experimental animals were used following the ethical guidelines in commission of laboratory animals in Chungbuk National University, Cheongju, Korea (2008). Parasite materials used in this study were provided by the Parasite Resource Bank of Korea National Research Resource Center, Republic of Korea.
Table 1.
Spargana specimens analyzed in this study (1979-2009)
| Code | Locality | Sex/Age | Year | Molecular identification | Genbanka |
|---|---|---|---|---|---|
| G1249 | Cheongju (Chungbuk) | M/49 | 2001 | S. erinaceieuropaei | |
| G1254 | Goesan (Chungbuk) | M/60 | 1996 | ″ | |
| G1286 | Chungbuk | M/50 | 1997 | ″ | |
| G1295 | Cheongwon (Chungbuk) | F/78 | 1998 | ″ | |
| G1300 | Jeju | - | 2002 | S. decipiens | |
| G1301 | Jeju | - | 2002 | ″ | |
| G1302 | Jeju | - | 2002 | ″ | |
| G1326 | Sancheong (Gyoungnam) | M/64 | 2002 | S. erinaceieuropaei | |
| G1327 | Sancheong (Gyoungnam) | M/64 | 2002 | ″ | |
| G1328 | Sancheong (Gyoungnam) | M/64 | 2002 | S. decipiens | |
| G1343 | Seoul | - | 1979 | ″ | |
| G1344 | - | F/59 | - | S. erinaceieuropaei | |
| G1345 | Seoul | - | - | ″ | |
| G1346 | Seoul | - | - | ″ | |
| G1347 | Seoul | - | - | ″ | |
| G1348 | - | F/51 | 1987 | ″ | |
| G1353 | Seoul | F/66 | 2003 | ″ | |
| G1354 | Seoul | M/79 | 2003 | ″ | |
| G1356 | Cheongju (Chungbuk) | M/37 | 2000 | ″ | |
| G1357 | - | M/41 | 1999 | ″ | |
| G1393 | Seoul | F/42 | 2003 | ″ | |
| G1394 | Seoul | - | 2003 | ″ | |
| G1395 | Seoul | F/49 | 2003 | ″ | |
| G1396 | Seoul | - | 2003 | ″ | |
| G1398 | Cheonan (Chungnam) | F/44 | 2003 | ″ | |
| G1401 | Seoul | F/59 | 2003 | ″ | |
| G1402 | Cheonan (Chungnam) | F/49 | 2003 | ″ | |
| G1404 | Busan | - | 1990 | ″ | |
| G1405 | Busan | - | 1990 | ″ | |
| G1406 | Busan | F/23 | 1990 | S. decipiens | |
| G1407 | Busan | F/47 | 1994 | ″ | |
| G1409 | Busan | M/65 | 1995 | S. erinaceieuropaei | |
| G1410 | Busan | M/65 | 1995 | ″ | |
| G1411 | Busan | F/55 | 2001 | ″ | |
| G1412 | Busan | M | 2003 | S. decipiens | |
| G1413 | Busan | M/71 | 2003 | ″ | |
| G1476 | Busan | M/71 | 2003 | S. erinaceieuropaei | |
| G1530 | Busan | M/72 | 2003 | ″ | |
| G1574 | Gangwon | F/68 | 2002 | S. decipiens | |
| G1576 | Gangwon | F/57 | 2005 | S. erinaceieuropaei | |
| G1578 | Jinju (Gyeongnam) | M/42 | 2005 | ″ | |
| G1622b | Chungbuk | S. decipiens | KJ599679 | ||
| G1625 | Seoul | F/52 | 2006 | ″ | |
| G1628 | Gangwon | F/57 | 2002 | ″ | |
| G1657 | Cheongju (Chungbuk) | M/59 | 2008 | ″ | |
| G1681 | Cheongju (Chungbuk) | F/46 | 2008 | S. erinaceieuropaei | |
| G1718 | Cheongju (Chungbuk) | F/42 | 1994 | ″ | |
| G1719 | Chungbuk | M/45 | 2008 | S. decipiens | |
| G1720 | Chungbuk | F/67 | 2009 | S. erinaceieuropaei | |
| G1721 | Jinju (Gyeongnam) | - | 2002 | S. decipiens | |
| G1724c | Cheongju (Chungbuk) | M/58 | 2009 | S. erinaceieuropaei | KJ599680 |
Accession numbers in GenBank, National Center for Biotechnology Information, USA.
The sparganum was obtained from a snake and a Spirometra decipiens adult was recovered after experimental infection of a cat.
The sparganum was obtained from a patient (molecular study was done cutting a small part of this worm) and a S. erinaceieuropaei adult was recovered from experimental infection of a dog.
The 2 adult tapeworms, code numbers G1724 (S. erinaceieuropaei) and G1622 (S. decipiens), were used as the reference criteria for morphologic and genetic observations using mitochondrial cox1 sequences.
DNA extraction, PCR, and nucleotide sequencing
A single proglottid was chopped into small pieces and the total genomic DNA was extracted using a DNeasy tissue Kit (Qiagen, Valencia, California, USA) according to the manufacturer’s instructions. Thirty-eight specimens were fixed in formalin fixative and the rest of them were preserved in a -70˚C deep freezer and in 70% ethanol. Genomic DNA of specimens preserved in formalin was extracted using the protocols of Jeon et al. [12]. The partial mitochondrial cox1 gene was amplified by PCR.
PCR were performed in a 50 μl reaction mixture with 0.01 μg/μl of genomic DNA, 10× PCR buffer (20 mM Mg+), 10 mM dNTP mixture, 10 pmoles of each primer, and 2.5 U/μl Taq DNA polymerase (High Fidelity PCR system, Roche, Mannheim, Germany). PCRs were performed in a GeneAmp PCR System 9700 (Applied Biosystems, Langen, Germany). The PCR primers used were p1f (5´-TGGTTTTTTGGACATCCTGAA-3´) and p1r (5´-ATCACATAATGAAAGTGAGCC-3´), which amplified a 440 bp product corresponding to the positions 707-1,146 bp of the cox1 gene. The second set of PCR primers, used for sequencing, was p1f1 (5´-GTGTTGATTTTGCCTGGGTTT-3´) and p1r1 (5´-TACAAACCAAGTATCATGTAA-3´), which yielded a 390 bp product corresponding to positions 732-1,122 bp of the cox1 gene. These were designed from S. erinaceieuropaei (KJ599680) and S. decipiens (KJ599679). The PCR reaction was performed as follows: 1 cycle of initial denaturation at 94˚C for 3 min, 35 cycles at 94˚C for 20 sec, 46˚C for 40 sec, 72˚C for 1 min, and incubation at 72˚C for 4 min. This resulted in 390 bp DNA fragments, which were isolated on a 1.0% agarose gel, excised under long-wave UV light, and extracted using a QIAquick PCR purification kit (Qiagen).
The entire cox1 gene was amplified by PCR. Primers were designed from the complete sequence of the S. erinaceieuropaei and S. decipiens mitochondrial genome and used for amplifying the full cox1 gene. Primers spcox1f (5´-GTA TTG AAG GAA TTA GTT AGG TTA-3´) and spcox1r (5´-CAA CCC AAT TAA ATT AAG TTC CAC-3´) were used to amplify and sequence the cox1 gene. The PCR reaction was performed as follows: 1 cycle of initial denaturation at 94˚C for 3 min, 30 cycles at 94˚C for 1 min, 50˚C for 1 min, 72˚C for 2 min, and extension at 72˚C for 10 min. Cyclic sequencing was done from 4 fragments using a Big-Dye terminator sequencing kit (version 3.2, Applied Biosystems, Foster City, California, USA), and the reaction products were directly sequenced using a DNA sequencer (3739XL model, Applied Biosystems).
DNA sequence analyses
The sequences were assembled and aligned using Geneious 6.1.5 (Biomatter, Auckland, New Zealand) and identified by BLAST searches and comparisons with the sequences of S. erinaceieuropaei and S. decipiens, which had been deposited in GenBank database. The molecular identification of Spirometra tapeworm specimens was based on the similarity of the nucleotide sequences of the cox1 gene and the phylogenetic relationship with those of S. erinaceieuropaei (KJ599680) and S. decipiens (KJ599679). The phylogenetic analysis was conducted with PAUP 4.0 [13]. The phylogenetic trees were constructed using the maximum likelihood (ML), maximum parsimony (MP), and neighbor-joining (NJ) methods, using D. nihonkaiense as an outgroup.
RESULTS
Morphologic features of S. erinaceieuropaei and S. decipiens
The length of strobilae was 68 cm in S. erinaceieuropaei and 47 cm in S. decipiens. The scolex was spatulate, measuring 0.2 mm in diameter by 1.0 mm length in S. erinaceieuropaei and 0.5 mm diameter by 1.5 mm length in S. decipiens. The neck was immediately behind the scolex and measured 0.5 cm in S. erinaceieuropaei and 1.0 cm in S. decipiens segmentation. The genital primordium measured 2.0-2.5 cm in S. erinaceieuropaei and 2.5-3.0 cm in S. decipiens, and was located at the back of the scolex.
The mature proglottids with eggs were first visible almost 14 cm behind the scolex in both S. erinaceieuropaei and S. decipiens. The width and length of gravid proglottids was 6.8-10.8 mm by 1.1-2.0 mm in S. erinaceieuropaei, and 3.1-4.2 mm by 2.1-3.2 mm in S. decipiens (Table 2). The genital pore was situated ventrally on the midline in the anterior 1/5 of the segment in S. erinaceieuropaei and S. decipiens. The uterine pores were on the midline behind the anterior margin of the terminal ball, 80-100 µm in S. erinaceieuropaei and 80-110 µm in S. decipiens, respectively. The uterus consisted of 5-7 loops on each side in S. erinaceieuropaei and 4-4.5 loops in S. decipiens. The dumbbell-shaped ovary was connected to the uterus and situated near the posterior margin of the segment in both S. erinaceieuropaei and S. decipiens (Fig. 1A, B). Longitudinal sections of the cirrus sac showed an oval structure, 200-250 µm in length by 120-150 µm in width in S. erinaceieuropaei, and 200-220 µm by 150-170 µm in S. decipiens. The seminal vesicle was elliptical and measured 200-250 µm in length by 100-150 µm in width in S. erinaceieuropaei, and 240-280 µm by 100-150 µm in S. decipiens. The testes measured 70-80 µm in maximal diameter in both S. erinaceieuropaei and S. decipiens (Fig. 1C, D). The eggs measured 57-61 µm by 33-36 µm in S. erinaceieuropaei and 56-60 µm by 34-36 µm in S. decipiens.
Table 2.
Morphologic features of Spirometra erinaceieuropaei and S. decipiens
| Organs | Morphologic features |
S. erinaceieuropaei |
S. decipiens |
Others |
|---|---|---|---|---|
| Size (mm) | Size (mm) | |||
| Scolex | Spatulate | 0.2 | 0.5 | Diameter |
| 1 | 1.5 | Length | ||
| Gravid proglottids | Trapezoid | 3.1-4.2 | 6.8-10.8 | Width |
| 2.1-3.2 | 1.1-2.0 | Length | ||
| Uterus | Coiling | 5-7 | 4-4½ | Loops |
| Eggs | Operculate | 0.057-0.061 × 0.033-0.036 | 0.056-0.060 × 0.034-0.036 | Length width |
Fig. 1.
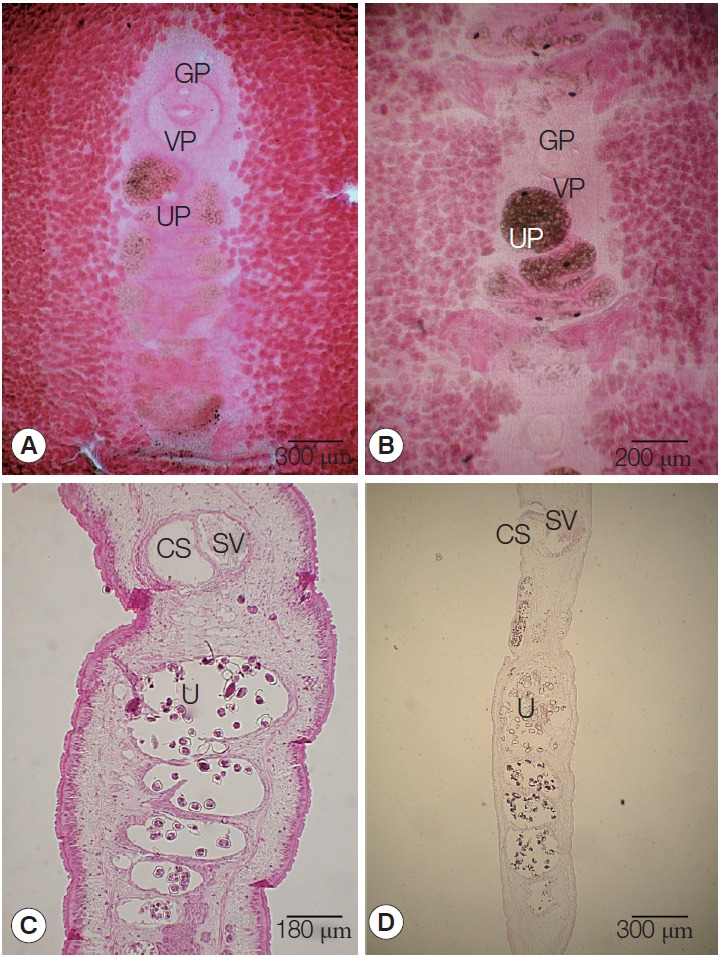
Gravid proglottids of Spirometra erinaceieuropaei and Spirometra decipiens. (A, B) Whole mounted specimens of proglottids showing the uterus and cirrus sac (×40), (C, D) Longitudinal section of a gravid proglottids showing the cirrus sac (CS), seminal vesicle (SV), and uterus (U) (H&E stain).
Sequence divergence of mitochondrial cox1 gene
The entire cox1 gene of both S. erinaceieuropaei and S. decipiens was 1,566 bp in length and showed 146 polymorphic sites. The sequence difference between S. erinaceieuropaei and S. decipiens was 9.3% (146/1,566) in the cox1 gene. Partial cox1 sequences (390 bp) from 35 Korean isolates showed 99.4% (388/390) similarity to references of the Korean S. erinaceieuropaei (G1724; GenBank KJ599680), while the remaining15 Korean isolates showed 99.2% (387/390) similarity with reference sequences of the Korean S. decipiens (G1657; GenBank KJ599679). The sequence difference in partial cox1 gene between S. erinaceieuropaei and S. decipiens was 9.2%. The ratio of S. erinaceieuropaei and S. decipiens according to molecular data was 35:15. The phylogenetic relationship of the 50 isolates derived by different analytical methods (MP, NJ, and ML) is seen in Fig. 2, with D. nihonkaiense basal to the S. erinaceieuropaei-S. decipiens clades.
Fig. 2.
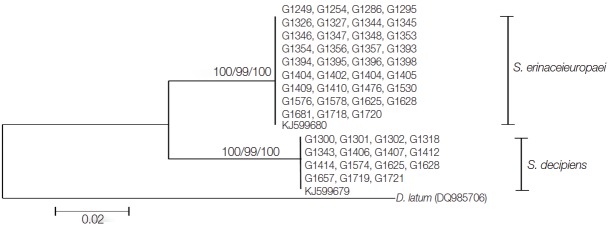
A phylogenetic tree of spirometrid tapeworms based on partial cox1 sequences inferred from neighbor-joining analysis. Numbers on branches indicate the bootstrap supporting values based on 1,000 replicates and bootstrap values by the maximum parsimony, maximum likelihood, and neighbor-joining methods are shown from left to right. The partial cox1 sequences of S. erinaceieuropaei and S. decipiens were divided into 2 clades, which differed by 29-32 nucleotides from each other. There were 390 bp corresponding to positions 732-1,122 bp of the cox1 gene.
DISCUSSION
Diesing in 1854 used the terms sparganum, dubium, ligula, and plerocercoides for the larval spirometriid worms and assigned the name Dubium erinacei-europaei (Rudolphi, 1819) for the larval tapeworm encysted in the intercostal muscles of a European hedgehog (Erinaceus europaeus) [2,3]. In 1929, Faust et al. [2] reviewed spirometriid species (under the name Diphyllobothrium) with morphologic and biological studies; they reported S. houghtoni, S. erinacei, and S. okumurai. Later, the spirometrid (Spirometra) species were described to include S. decipiens Gedoelst, 1911, S. reptans Meggitt, 1924, S. ranarum Meggitt, 1925, S. mansoni Joyeux and Houdemer, 1927, S. felis Southwell, 1928, S. houghtoni, S. erinacei, and S. okumurai Faust, Campbell and Kellogg, 1929, S. serpentis Yamaguti, 1935, and S. mansonoides Mueller, 1935 [3]. The name S. erinacei was later substituted by S. erinacei-europaei by Yamaguti [4]. However, the name S. erinacei has been used repeatedly by various workers in Asia and Australia [7,14,15] and even at present by many authors. In the meantime, Schmidt [16] used the name Diphyllobothrium erinaceieuropaei in 1986 (it should be Spirometra erinaceieuropaei), and articles using Spirometra erinaceieuropaei for this tapeworm began to appear from 1997 in PubMed Database (National Library of Medicine, Washington DC, USA).
In this study, the morphologic characteristics of Spirometra species were observed as in the above studies. Morphologic features of S. erinaceieuropaei and S. decipiens are different in that the spirally coiled uterus of S. erinaceieuropaei consists of 5-7 complete turns, while that of S. decipiens consist of only 4-4.5 coils [2]. This is the major differential features between the 2 species and our specimens completely fit to the criteria of those uterine morphology with some other features; the uterine pore lies in the midline, a little distance behind the anterior margin of the uterine terminal ball in S. erinaceieuropaei, while in S. decipiens it is a conspicuous sphincter with a ventral position under the bulge of the terminal uterine coil. The vaginal pore is a broad crescent slit behind the male genital pore in S. erinaceieuropaei, while in S. decipiens it is crescent shaped and elliptical. The cirrus is strongly muscular and elongated in shape in S. decipiens, while in S. erinaceieuropaei it is smaller than in other related species. The morphologic characteristics of the male and female reproductive organs of S. mansoni overlap those of S. decipiens in terms of the number of rotations of the spirally coiled uterus, the shape of the terminal uterus, the vaginal opening, and the cirrus.
The taxonomic status of spirometrid tapeworms has been unsettled. It would be useful to determine which Spirometra species are present in Korea causing human sparganosis. The most common form of transmission to humans is by drinking water contaminated with procercoid larvae. In addition, transmission can also occur by eating raw or undercooked flesh of second intermediate hosts such as snakes and frogs, which is why the disease is more prevalent in adult males, who are the main consumers of these infection sources [9]. A previous study reported that snakes are an important source of human sparganosis; the cases had eaten raw snakes in about 50% of all cases in Korea [9]. However, another study revealed an epidemiological discrepancy between the eating habit and the ratio of Spirometra species distribution (unpublished observation). The mitochondrial genotype of sparganum infecting snakes has been shown to be different from that of human-infecting sparganum (unpublished observation). Thus, additional studies are strongly needed to identify the correct intermediate hosts and the infection route. Natural infection with S. decipiens has been mostly observed in carnivorous animals such as cats and dogs. However, according to our data, no natural hosts and adult tapeworms of S. erinaceieuropaei were available; therefore, the infection route of S. erinaceieuropaei for humans still remains unclear.
In the present study, we first report human sparganosis caused by S. decipiens, which was identified from spargana collected between 1979 and 2010 in Korea. Spirometra infection was identified as S. erinaceieuropaei in 35 cases and S. decipiens in 15 cases. This was done through sequence analysis of the mitochondrial cox1 gene and by morphologic characteristics of the adult worms. Our results indicated that S. erinaceieuropaei and S. decipiens are human-infecting Spirometra tapeworms in Korea, with S. erinaceieuropaei infections accounting for 2/3 of all Spirometra infections.
Acknowledgments
One author (Hyeong-Kyu Jeon) was supported by the Research Fellow Grant for young scientist from National Research Foundation of Korea (NRF-2012R1A1A2042993). This work was supported by a research grant from Chungbuk National University in 2014.
Footnotes
We have no conflict of interest related to this work.
REFERENCES
- 1.Mueller JF. A repartition of the genus Diphyllobothrium. J Parasitol. 1937;23:308–310. [Google Scholar]
- 2.Faust EC, Campbell HE, Kellogg CR. Morphological and biological studies on the species of Diphyllobothrium in China. Am J Hyg. 1929;9:560–583. [Google Scholar]
- 3.Wardle RA, McLeod JA. The Zoology of Tapeworms. Minneapolis, USA: University of Minnesota Press; 1952. pp. 559–615. [Google Scholar]
- 4.Yamaguti S. Systema Helminthum. Vol. II. The cestodes of vertebrates. New York, USA: Interscience Publishers; 1959. pp. 358–361. [Google Scholar]
- 5.Iwata S. Experimental and morphological studies of Manson's tapeworm, Diphyllobothrium erinacei, Rudolphi. Special reference with its scientific name and relationship with Sparganum proliferum, Ijima. Progr Med Parasitol Jpn. 1972;4:536–590. [Google Scholar]
- 6.Mueller JF. The biology of Spirometra. J Parasitol. 1974;60:3–14. [PubMed] [Google Scholar]
- 7.Lee SH, We SJ, Sohn WM, Hong ST, Chai JY. Experimental life history of Spirometra erinacei. Korean J Parasitol. 1990;28:161–173. doi: 10.3347/kjp.1990.28.3.161. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi H. On the animal parasites in Chosen (Korea). Second report. Acta Med Keijo. 1928;11:109–124. [Google Scholar]
- 9.Cho SY, Bae JH, Seo BS. Some aspects of human sparganosis in Korea. Korean J Parasitol. 1975;13:60–77. doi: 10.3347/kjp.1975.13.1.60. [DOI] [PubMed] [Google Scholar]
- 10.Min DY. Cestode infections in Korea. Korean J Parasitol. 1990;28(suppl):123–44. doi: 10.3347/kjp.1990.28.suppl.123. [DOI] [PubMed] [Google Scholar]
- 11.Lee SH, Chai JY, Seo BS, Cho SY. Two cases of human infection by adult of Spirometra erinacei. Korean J Parasitol. 1984;22:71–77. doi: 10.3347/kjp.1984.22.1.66. [DOI] [PubMed] [Google Scholar]
- 12.Jeon HK, Kim KH, Eom KS. Molecular identification of Taenia specimens after long-term preservation in formalin. Parasitol Int. 2011;60:203–205. doi: 10.1016/j.parint.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Swofford DL. Paup*: phylogenetic analysis using parsimony (*and other methods). Version 4. Sunderland, Massachusetts, USA: Sinauer Associates; 2003. [Google Scholar]
- 14.Bearup AJ. Observations on the life cycle of Diphyllobothrium (Spirometra) erinacei in Australia (Cestoda: Diphyllobothriidae) Aust J Sci. 1948;10:183. [PubMed] [Google Scholar]
- 15.Yamane Y, Okada N, Takihara M. On a case of long term migration of Spirometra erinacei larva in the breast of a woman. Yonago Acta Med. 1975;19:207–213. [PubMed] [Google Scholar]
- 16.Schmidt GD. CRC Handbook of Tapeworm Identification. 2nd Printing. Boca Raton, Florida, USA: CRC Press, Inc; 1986. pp. 93–95. [Google Scholar]


