Abstract
Stray cats are a common feature roaming the streets and alleys of Kuwait; they could be a source of parasites, including trematodes, that affect humans. A survey was conducted to identify feline trematodes and throw the light on their public health significance in Kuwait. Out of 240 stray cats trapped from different localities of Kuwait from June 2011 to May 2012, 59 (24.6%) were found to be infected with 14 species of trematodes. The most common were trematodes of the genus Heterophyes, particularly H. heterophyes and H. dispar that were found in respectively 15.8% and 10.8% of the cats examined. Other trematodes recorded, with lower prevalences, were Heterophyes nocens (2.9%), Haplorchis taichui (3.8%), Stictodora sawakinensis (2.1%), Stellantchasmus falcatus (1.6%), Echinochasmus japonicus (1.6%), and Mesostephanus dottrensi (1.3%). Centrocestus cuspidatus, Galactosomum fregatae, Ascocotyle sp., Mesostephanus appendiculatus, Haplorchis yokogawai, and Pygidiopsis genata showed the lowest prevalence (0.4%) and intensity. The majority of the trematodes are recorded for the first time in Kuwait and even in the Gulf region. The study reveals that stray cats are good indicators of fish-borne trematodes in the environment. As all trematodes recovered are zoonotic, their significance to public health should be considred.
Keywords: Heterophyes heterophyes, Heterophyes dispar, trematode, heterophyid, zoonosis, stray cat, Kuwait
INTRODUCTION
In Kuwait, stray cats are a common feature roaming streets and alleys, living among houses, on farms, and near abattoirs and fish markets, and feeding on garbage, insects, and small animals as well as on meat and fish offal. Consequently, they may contract infection with parasites and pollute environment with them posing threat to humans and domestic animals.
Kuwait, a small country located in the desert geographical region with sparse vegetation, has a rich marine environment that includes a long 170 km shoreline, and open water of the Arabian Gulf where large quantities of fish and shrimp are caught. The fisheries sector in the country is important, and seafood is considered a significant source of protein for the people. According to the 2012 census, Kuwait produces 3,686,328 kg of fish including 30,482 kg from aquaculture, in addition it imports 8,803,178 kg of fish, mainly refregirated from Egypt, Iran, India, Pakistan, and Saudi Arabia as well as mainly frozen fish from Southeast Asia.
Although parasites of stray cats have been surveyed in many parts of the world [1-7] as well as in the Gulf region [8-10], little is known about the status of tremtode infections, particularly fish-borne zoonotic trematodes (FZTs), in Kuwait [11]. To assess the prevalence of FZTs and the role of cats as reservoir hosts for fish-borne parasites, a systematic study on parasites of stray cats in Kuwait was performed, with the identification of the recovered trematodes and the evaluation of their zoonotic importance.
MATERIALS AND METHODS
Cat sampling and study sites
During the period extended from June 2011 to May 2012, stray cats were trapped from various districts to cover the 6 administrative Governorates (Al-Asema, Hawalli, Farwanyiah, Mubarak Al-Kabeir, Al-Jahara, and Al-Ahmadi) of Kuwait (Fig. 1) using special live traps baited with fish. In order to determine the geographical distribution and demographics, the districts, from which cats were captured, were grouped into 2 main localities on the basis of the following criteria, e.g., the standard of living of inhabitants, density of population, level of municipal services, and accumulation of garbage. Locality 1 contained the districts which are inhabited by well-to do-Kuwaitis with good service levels and low population densities, while Locality 2 contains the districts which are inhabited by expatriates with low service levels and high population densities. Some districts of Locality 2, inhabited by laborers and fishermen, were characterized by the accumulation of garbage and large numbers of stary cats.
Fig. 1.
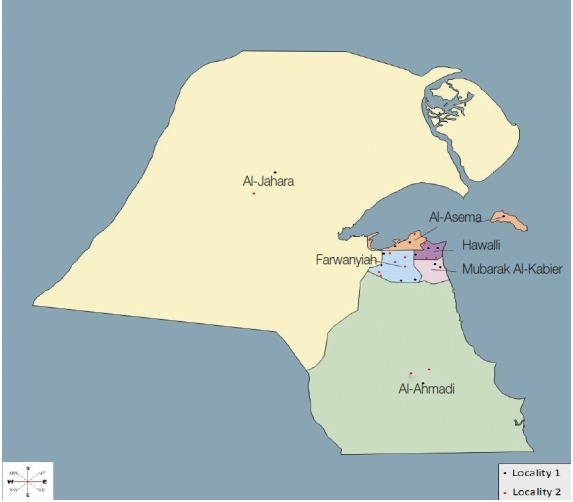
Surveyed areas of the 6 governorates of Kuwait, grouped into 2 main localities.
Laboratory investigations
The captured cats were transferred to the laboratory where date of examination, site of trapping, gender and age, were recorded. The age of cats was assessed by dentition, body size, and maturation of genital organs. The cats were grouped by age into adults (more than 6 months) and juveniles (≤6 months). After anesthetizing cats using Rompun 2% (Bayer, Leverkusen, Germany) intramuscular (1.5 ml/10 kg), they were killed humanely by intracardial injection with T61 (Schering-Plough Intervet, Elkhorn, Nebraska, USA), 1-4 ml according to age and weight.
Organ examination for trematodes
Extra-intestinal organs: Bile ducts and air passages were opened, and liver and lung tissues were dissected using fine scissors in a petri dish containing normal saline. Then, all the contents were examined under a stereomicroscope for detection of trematodes.
Intestinal tract: The gastrointestinal tracts were removed from cats at necropsy. The intestines were separated and opened in containers containing normal saline; the mucous membrane of each organ was scraped between the blades of a forceps. The contents were then washed out over a 500 µm sieve with tap water. The residues were systematically searched under a stereomicroscope for trematodes, and any specimens found were transferred to 70% ethanol for preservation until identification.
Preparation of specimens for identification
The worms were counted. For identification, they were stained with lactophenol cotton blue (LPCB) using the method of Henedi and El-Azazy [12]. Some specimens were stained with alum carmine, cleared in xylene and clove oil, and mounted in Canada balsam.
Trematode identification
The recovered parasites were identified using the keys/textbooks of Soulsby [13] and Bray et al. [14,15]. In addition, helminth specimens were sent to John M. Kinsella, Helm-West Laboratory, Missoula, Montana, USA for confirmation, and voucher specimens were deposited in the US National Parasite Collection (accession no. 107983-107988). Measurement was done using a micrometer slide and a digital camera (Leica EC3, Wetzlar, Germany). All measurements are given in µm, as averages.
Statistical analysis
Data were analyzed and statistical comparisons were performed using Statistix® (copyright© 1985-2003, analytical software, version 8, Tallahassee, Florida, USA). Summary data for the parasite intensity are expressed as arithmetic means ± SEM (standard error of mean). The mean intensity of infection as defined by Bush et al. [16] is the average intensity of a particular parasite species among the infected members of a particular host species. The prevalence and confidence intervals (CI) were calculated as described by Sokal and Rohlf [17]. The prevalence is the proportion of infected hosts among all hosts examined. CI of prevalence indicates the accuracy of the estimation using CI 95% is advisable.
RESULTS
Overall infection rate and species of trematodes recovered
A total of 240 stray cats were examined, 117 from Locality 1 and 123 from Locality 2 (Table 1). Cats were captured around houses of fishermen, fish markets, and trash containers. A total of 59 (24.6%) cats were found to be infected with FZTs, and 23 (39.0%) being infected with more than 2 different species. Total 14 species of FZTs were recovered in the small intestine of cats. Among them, the great majority (11 spp.) were members of the family Heterophyidae, 2 species (Mesostephanus dottrensi and M. appendiculatus) were those of the Cyathocotylidae, and only 1 species, Echinochasmus japonicus, belonged to the Echinostomatidae. Three species of the genus Heterophyes (H. heterophyes, H. dispar, and H. nocens) were detected in 15.8%, 10.8%, and 2.9% of stray cats examined, and their average numbers were 141, 83, and 203 worms per cat infected, respectively. Trematodes including Centrocestus cuspidatus, Galactosomum fregatae, Ascocotyle sp., M. appendiculatus, H. yokogawai, and Pygidiopsis genata showed the lowest prevalence (0.4%) and intensity (Table 2).
Table 1.
Demographic data (site, gender, age, and season) of stray cats examined in Kuwait
| Locality | Season |
|||||||
|---|---|---|---|---|---|---|---|---|
| Wet |
Dry |
|||||||
| Male |
Female |
Male |
Female |
|||||
| Adult | Juvenile | Adult | Juvenile | Adult | Juvenile | Adult | Juvenile | |
| 1 | 14 | 9 | 19 | 7 | 21 | 13 | 21 | 13 |
| 2 | 20 | 9 | 41 | 10 | 10 | 10 | 14 | 9 |
| Total | 34 | 18 | 60 | 17 | 31 | 23 | 35 | 22 |
Table 2.
Prevalence and mean intensity of trematodes in stray cats from Kuwait relative to age
| All cats (n = 240) |
Adult (n = 160) |
Young (n = 80) |
||||
|---|---|---|---|---|---|---|
| Positive (%) | Intensity ± SEM | Positive (%) | Intensity ± SEM | Positive (%) | Intensity ± SEM | |
| All trematodes | 24.6 | 154 ± 19 | 28.7 | 116 ± 17 | 16.2 | 288 ± 23 |
| Heterophyes heterophyes | 15.8 | 141 ± 17 | 28.3 | 85 ± 11 | 10.0 | 351 ± 24 |
| Heterophyes nocens | 2.9 | 203 ± 17 | 3.8 | 187 ± 18 | 1.3 | 301 ± 0 |
| Heterophyes dispar | 10.8 | 83 ± 12 | 13.8 | 70 ± 11 | 5.0 | 156 ± 14 |
| Haplorchis yokogawai | 0.4 | 8 ± 3 | 0.6 | 8 ± 3 | - | - |
| Haplorchis taichui | 3.8 | 4 ± 2 | 5.0 | 4 ± 2 | 1.3 | 2 ± 0 |
| Stellantchasmus falcatus | 1.6 | 5 ± 2 | 2.5 | 5 ± 2 | - | - |
| Centrocestus cuspidatus | 0.4 | 3 ± 0 | 0.6 | 3 ± 0 | - | - |
| Ascocotyle sp. | 0.4 | 1 ± 0 | - | - | 1.3 | 1 ± 0 |
| Pygidiopsis genata | 0.4 | 1 ± 0 | - | - | 1.3 | 1 ± 0 |
| Stictodora sawakinensis | 2.1 | 7 ± 3 | 3.1 | 7 ± 3 | - | - |
| Galactosomum fregatae | 0.4 | 2 ± 0 | 0.6 | 2 ± 0 | - | - |
| Mesostephanus. dottrensi | 1.3 | 4 ± 2 | 0.6 | 6 ± 0 | 2.5 | 3 ± 1 |
| Mesostephanus appendiculatus | 0.4 | 1 ± 0 | - | - | 1.3 | 1 ± 0 |
| Echinochasmus japonicus | 1.6 | 5 ± 2 | 2.5 | 5 ± 2 | - | - |
Brief description of trematodes recovered (unit: µm) Heterophyes heterophyes
Pear-shaped, 1,256×379 (n=10). Oral sucker terminal, 35×61. Pre-pharynx clear, 60; pharynx, 47×45. Esophagus, 200 long. Ventral sucker separated from genital sucker, large, well developed, located at middle of the body between 2 ceca, 146×169. Genital sucker about the same size of ventral sucker, 132×157, located posterio-lateral to ventral sucker, armed with incomplete crown of 70-80 spines (Fig. 2). Ovary small, compact, pretesticular, 65×69. Testes oval to round, horizontal, at caudal region, right, 118×98; left, 121×97. Vitelline glands restricted in 2 patches anterior to testes. Eggs small, yellow 25×15.
Fig. 2.
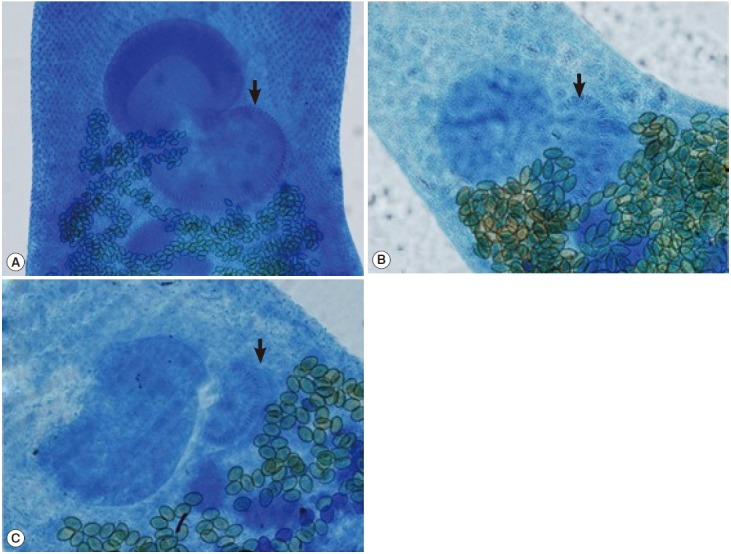
Heterophyes spp. ventro-genital region. The number of the rodlets on the genotyl (arrow) is about 77 in H. heterophyes (A), 52 in H. nocens (B), and 30 in H. dispar (C). ×100.
Heterophyes nocens
Oval to elongate body, 1,157×390 (n=10). Oral sucker terminal, 44×50. Pre-pharynx short, 64 long; followed by small pharynx, 37×37. Esophagus, 157 long. Ventral sucker large, 150×170. Crescent genital sucker posterio-lateral to ventral sucker carries 50-62 spines (Fig. 2), 110×120. Seminal vesicle bilobed, between genital sucker and ovary. Ovary globular, 62×81. Testes horizontal at posterior part of the body, right, 95×77; left, 98×81. Eggs numerous, 25×15.
Heterophyes dispar
Elongate to pear-shaped body, 943×318 (n=10). Oral sucker terminal to subterminal, small, 54×67. pre-pharynx, 64 long; pharynx, 48×45. Esophagus, 159 long. Ventral sucker large, 126×150. Genital sucker small, armed with 15-35 spines (Fig. 2), at the lower left side of ventral sucker, 81×57. Ovary round, 73×64. Testes obligue or opposite, right, 104×73; left, 115×70. Seminal receptacles at lower right side of the ovary. Eggs numerous, 25×10.
Stictodora sawakinensis
Body elongate, pyriform 870×208 (n=10). Oral sucker round, subterminal, 52×52. Pre-pharynx, 56 long; Pharynx, 40×41. Esophagus, 46 long. Ventral sucker and gonotyl enclosed in ventrogenital sac. Ventrogenital sac looks like egg plant, divided in 2 portions, smaller 46×55 armed with 6-10 longitudinal rows of spines which trifid or single tipped, vary in number (14-28), large portion, saccular, 99×65. Seminal vesicle, bilobed or tripartite, posterior to ventrogenital sac. Testes obliquely, oval to round in shape located at second third of the body. Upper testis, 95×50; lower, 49×52. Ovary between testes, 39×50. Vitellaria post-testicular extending outside cecal field. Eggs oval, dark yellow, 20×10.
Ascocotyle (Phagicola) longa
Body pyriform, 889×265, (n=1). A small prolongation, 10, is seen at the top of oral sucker. Oral sucker, 51×51, surrounded by single crown of 16 well-developed spines. At the back of oral sucker, dorsal to prephrynx, extends a tapered muscular extensible prolongation. Pre-pharynx, 204 long. Pharynx muscular, well developed, 80×50, esophagus short, 20. Ventral sucker spherical, 80×80. Testes equal, opposite in position, 80×50. Ovary lies posterior ventral sucker, obscured by numerous eggs. Vitelline glands follicular, in latero-posterior region. Eggs, 50×30, fill area between ventral sucker and testes.
Haplorchis yokogawai
Oval to pyriform body, 662×295 (n=10). Oral sucker terminal, 45×53. Pre-pharynx short, 21 long. Pharynx oval to spherical, 29×34. Esophagus, 120 long. Ventral sucker small, 66×82 with apex composing of large ventral lobe armed with numerous tiny spines and larger pair of sclerites in addition to 3 smaller lobes armed with tiny spines (reduced cavity, armed with small spines). Gonotyl absent. Ventrogenital sac unarmed, 142×119. Ovary round, 47×49 posterior to ventral sucker at left side. Testis single, 79×71 at posterior part of the body. Eggs numerous, uncountable, distributed around and posterior to ventral sucker, 20×10.
Haplorchis taichui
Body elongated, 562×197 (n=4). Oral sucker terminal, 32×50. Pre-pharynx, 10 long. Pharynx, 27×30. Esophagus, 85 long. Ventral sucker median, 60×55 possesses reduced cavity, armed with 1 or 2 ventral crescent shaped groups of 12-16 sclerites. Seminal vesicle between ventral sucker and ovary. Ovary globular, 53×53. Testis single, fan shaped, lobulated, at posterior part of the body, 46×36. Vitteline glands at posterior part of the body, masked with large amount of yellow eggs which are 20×10.
Stellantchasmus falcatus
Small pyriform body, 528×180 (n=10). Oral sucker terminal, 30×39. Pre-pharynx short, 26. Pharynx globular, 23×29. Esophagus, 106 long. Ventral sucker semicircle, 30×31, slightly deviated to right side between 2 caeca, armed with minute spines encircling mouth and forming 2 denses. Seminal vesicle, elongated sac-like, situated at the opposite side of ventral sucker, 66×24. Ovary submedian, lies between ventral sucker and right testis, 40×40. Testes horizontal, right, 65×41; left, 64×44.Vitellaria follicular. Eggs, 20×10.
Centrocestus cuspidatus
Body very small 468×186, with unique conical flask shape, with a spiny neck, (n=3). Oral sucker sub-terminal, 32×57, with dorsal lip armed with 2 circles of alternating spines. Inner circle contains 9-21 spines, outer contains 10-30 spines. Pre-pharynx, 41 long. Pharynx, 30×40. Esophagus short, 59 long. Ventral sucker, 40×60. Seminal vesicle bipartite. Ovary, 60×90. Testes opposite, right, 70×30; left, 80×90. Eggs large, 35×22, few in number (5-11).
Galactosomum fregatae
Body elongated, rounded at both extremities, with slight constriction behind ventrogenital complex, covered with spines and scattered pigment granules 2,877×475 (n=2). Oral sucker subterminal, large; 90×100. Pre-pharynx, 325 long. Pharynx, 100×90. Esophagus very short, 87 long. Ventrogenital complex at the beginning of second third of body, it's composed of ventral genital sac that’s connected to lobulated seminal vesicle by prostatic ejaculatory duct. Ventral genital sac looks like a rose, contains ventral sucker and genital atrium. Ventral sucker, 143×155 with 2 separate groups of spines, first group at right with large spines arranged alternatively in horse shoe manner, while second group of spines opposite to first one and composed of 2 sets of spines, first set tiny, arranged in crescent order which is connected to another set of small spines arranged in small patch. Genital atrium, 124×166. Seminal vesicle strongly developed lying opposite to acetabulum, 430×160, composed of 2 chambered separated by a distinct constriction, upper oval to round while lower elongated, sac like. Testes diagonal, upper, 100×170; lower, 170×120. Ovary round, post-acetabulam, 120×110. Seminal receptacle large, 120×90 post ovarian. Eggs numerous, 20×10.
Pygidiopsis genata
Body looks like conical flask, with maximum width at the ovary level, 550×240 (n=1). Oral sucker large, terminal, without appendage, 40×50. Pre-pharynx, 36. Pharynx muscular, 30×40. Ceca reach ovary and turn somewhat dorsomedially at terminal portion. Ventral sucker median, round, globular, 60×40. Genital sucker crescent-like, lobulated, spineless, attached to ventral sucker, 60×30. Seminal vesicle bipartite, post acetabulum, 50×28. Ovary round, post to seminal vesicle, 50×40. Seminal receptacles attached to ovary, 40×48. Testes oval, opposite, right, 30×60; left, 40×40. Eggs scattered on area posterior to ventral sucker and anterior to testes, 20×10.
Mesostephanus dottrensi
Pyriform in shape, with small dorsoterminal appendage at end of body, 1,000×398 (n=6). Oral sucker, 47×57. Pre-pharynx absent; pharynx, 41×49. Esophagus, 62 long. Intestinal ceca reaching close to posterior extremity. Between 2 ceca; 2 columns, each of 6 small dense patches. Acetabulum, 47×52. A hold fast organ occupies the region behind ventral sucker. Two large testes, subspherical or oval, tandem, at posterior half of the body. Upper, 102×100; lower, 107×100. Ovary pyramidal, between testes, 140×140. Cirrus sac long, well developed, 270×65; genital pore terminal. Vitellaria well-developed; in horse-shoe manner, composed of large follicles confined around gonads, but not confluent posteriorly. Vaginal sphincter ellipsoidal. Coffee bean-shaped eggs large; yellowish in color, few in number (1-3 eggs) 74×51.
Mesostephanus appendiculatus
Body, 680×410 (n=1). Oral sucker, 54 long. Pre-pharynx very short. Pharynx, 50×36; esophagus length 25. Ventral sucker, 60×60. Tribocytic organ well developed. Ovary intertesticular, 60×50. Testes oblique, in posterior part of the body, right, 116×70; left, 118×75. Cirrus sac and cirrus well developed. Vitellaria follicular arranged in semi circle at posterior half of the body. Eggs, 107×60.
Echinochasmus japonicus
Body ovoid or plump, 1,200×39 (n=6). Anterior part of the body armed with collar of 24 spines (12 on each side) alternating in position. Head crown, 75×90. Oral sucker spherical, 40×50. Pre-pharynx between 2 sets of spines, 51 long. Pharynx, 70×110. Esophagus, 113 long. Caecum blind, reaching end of posterior extremity of trematode. Ventral sucker large, 150×110. Ovary posterior to ventral sucker, 130×160. Cirrus sac large, elongated to oval, between the intestinal bifurcation and ventral sucker. Seminal receptacle large, sac-like between ovary and upper testis. Testes tandem, posterior at one-third body, upper, transversely oval, 110×120; lower, rounded, 160×180. Ootype obvious between ovary and upper testis. Vitelline glands extend on from acetabular level to posterior body extremity. Eggs few (1-2) and measure 90×50.
Effect of demographic factors on parasitism
Older cats had a significantly higher infection rate, 46 adult cats had a significantly higher intensity and infection rate with trematodes than juveniles did (P=0.03). In addition, trematodes showed significantly higher prevalence and intensity in cats which were trapped in Locality 2 than in Locality 1 (P=0.03). These data were very much influenced by prevalences of Heterophyes spp., which constituted the majority of the trematode community. Heterophyes spp. were influenced by age and site but not by season and gender; significantly higher (P=0.00) prevalences were observed in adults and in Locality 2 when compared to juveniles and Locality 1, respectively (Table 3). The prevalence of trematode infection was higher (29.5%) in the wet season than in the dry season (18.9%), while the intensity of infection was higher in the dry season (mean=279.1 burdens) than in the wet season (mean=84.9 burdens). However, these differences were not statistically significant (P>0.05).
Table 3.
Prevalence and 95% confidence intervals (C.I.) of parasites in cats (240) of different age groups, sexes, and seasons
| Sex |
Age |
Season |
Locality |
Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Adult | Juvenile | Wet | Dry | 1 | 2 | |||
| Prevalence | 21.7 | 26.9 | 28.7* | 16.2 | 29.5 | 18.9 | 13.71 | 34.9a | 24.6 | |
| Range | Low | 14.27 | 19.57 | 8.94 | 21.87 | 21.8 | 12.1 | 8.0 | 26.5 | 19.3 |
| High | 30.76 | 35.21 | 26.19 | 36.43 | 38.1 | 27.5 | 21.3 | 44.1 | 30.5 | |
Statistically significant (P<0.05).
DISCUSSION
All trematodes recorded in this study are transmitted by eating raw or undercooked fish, and most of them have been reported to cause health problems in humans [18,19]. The role of stray cats as reservoir hosts of fish-borne trematodes is highlighted in this study; however, their role in sustaining transmission of them is questionable. Little is known about the epidemiology of fish-borne trematodiasis in Kuwait.
It is known that cats and other fish-eating mammals and birds act as final hosts for fish-borne trematodes. However, few studies have been done on the role of reservoir hosts, including cats, in the epidemiology of FZTs; in some endemic areas in Southeast Asian countries, it has been suggested that a minor role is played [18,19]. However, in Vietnam, cats, dogs, and pigs have been found to play major roles in the epidemiology of FZTs in aquaculture [20].
In the present study, although it is evident that cats were aquired infection with trematodes by eating raw fish, it is not possible to know the source of infection, i.e., via local or imported fish. This point needs more investigation. Locally, no systematic studies have been conducted to investigate infection of fish with metacercariae. However, on 2 occasions, Stictodora fuscata and S. tridactyla were reported after experimental feeding of kittens with metacercaria-infected fish species, respectively, Liza macrolepis (mullet) and Aphanius dispar (killfish), caught from Kuwait Bay [21,22]. On other hand, metacercariae of fish-born trematodes have been detected in fish in some countries nearby Kuwait [23-27].
Kuwait imports fish from many countries where FZTs are endemic, e.g., Egypt [25], Iran [28], India [29,30], and Southeast Asia [18,19]. It is worth mentioning that metacercariae in fish tissues can survive and be infective for 10-18 days at -12˚C and for 3-7 days at -20˚C [31]. Importation of fish from a wide variety of geographical and ecological regions can explain the broad spectrum of FZTs diversity and recording a large number of species in this study.
Heterophyes spp., particularly H. heterophyes, were the most prevalent trematodes and the most abundant helminth parasites in the present study. In previous studies in Kuwait, H. heterophyes was the only trematode recorded from dogs [32] and cats [11]. In addition, it has been recorded from humans and animals in other Gulf countries, e.g., Iran [28], United Arab Emirates [9], and Iraq [33]. Seo [34] and Chai et al. [35] reported H. heterophyes infections in Korean workers who returned from Saudi Arabia, Bahrain, and Kuwait. This could indicate that heterophyiasis is endemic in the Gulf region.
The significantly higher prevalence observed in adult cats, is believed to be caused by longer exposure and accumulation of parasites [36]. This could be also related to the competition for food, where adults have a greater capacity to obtain fish offal at garbage containers near fish markets in comparison to younger cats. Higher prevalence and intensity rates of trematodes were observed in cats captured in Locality 2 where expatriate laborers clean fish by themselves and throw the offal in garbage to be accessed by stray cats. Another reason for the higher prevalence rates at Locality 2 could be that the inhabitants prefer the consumption of imported fish, which could have higher numbers of FZTs.
The great majority of FZTs are recorded for the first time in Kuwait, and even in the Gulf region. This study proved that stray cats are good indicators of FZTs in the environment of Kuwait. The paper draws the attention of food safety services, fishery officials, marketing agencies, and public and veterinary authorities in Kuwait to the risk, which may be posed by consumption of raw or under cooked seafood.
Acknowledgments
The authors are grateful to the Kuwait Foundation for Advancement of Sciences for financial support as well as to John M. Kinsella, Helm-West Laboratory, Missoula, Montana, USA, for his kind help in verifying the identification of trematodes.
Footnotes
We have no conflict of interest related to this work.
REFERENCES
- 1.McGlade TR, Robertson ID, Elliot AD, Read C, Thompson RCA. Gastrointestinal parasites of domestic cats in Perth, Western Australia. Vet Parasitol. 2003;117:251–262. doi: 10.1016/j.vetpar.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 2.Tolbert MK. Prevalence of Dirofilaria immitis and gastrointestinal helminths in cats euthanized at animal control agencies in northwest Georgia. Vet Parasitol. 2004;119:319–326. doi: 10.1016/j.vetpar.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 3.Labarthe N, Serrao ML, Ferreira AMR, Almeida NKO, Guerrero J. A survey of gastrointestinal helminths in cats of the metropolitan region of Rio de Janeiro, Brazil. Vet Parasitol. 2004;123:133–139. doi: 10.1016/j.vetpar.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Sohn WM, Chai JY. Infection status with helminthes in feral cats purchased from a market in Busan, Republic of Korea. Korean J Parasitol. 2005;43:93–100. doi: 10.3347/kjp.2005.43.3.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borthakur SK, Mukharjee SK. Gastrointestinal helminthes in stray cats (Felis catus) from Aizawl, Mizoram, India. Southeast Asian J Trop Med Public Health. 2011;42:255–258. [PubMed] [Google Scholar]
- 6.Khalafalla RE. A survey study on gastrointestinal parasites of stray cats in Northern region in Nile Delta, Egypt. PLoS One. 2011;6: doi: 10.1371/journal.pone.0020283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chai JY, Bahk YY, Sohn WM. Trematodes recovered in the small intestine of stray cats in the Republic of Korea. Korean J Parasitol. 2013;51:99–106. doi: 10.3347/kjp.2013.51.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Changizi E, Mobedi I, Salimi-Bejestani MR, Razaei-Doust A. Gastrointestinal helminthic parasites in stray cats (Felis catus) from North of Iran. Iranian J Parasitol. 2007;2:25–29. [Google Scholar]
- 9.Schuster RK, Thomas K, Sivakumar S, O' Donovan D. The parasite fauna of stray domestic cats (Felis catus) in Dubai, United Arab Emirates. Parasitol Res. 2009;105:125–134. doi: 10.1007/s00436-009-1372-6. [DOI] [PubMed] [Google Scholar]
- 10.Abu-Madi MA, Behnke JM, Prabhaker KS, Al-Ibrahim R, Lewis JW. Intestinal helminthes of feral cat populations from urban and suburban districts of Qatar. Vet Parasitol. 2010;168:284–292. doi: 10.1016/j.vetpar.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 11.Abdul-Salam J, Baker K. Prevalence of intestinal helminths in stray cats in Kuwait. Pakistan Vet J. 1990;10:17–21. [Google Scholar]
- 12.Henedi AAM, El-Azazy OME. A simple technique for staining of platyhelminthes with the lactophenol cotton blue stain. J Egypt Soc Parasitol. 2013;43:419–423. doi: 10.12816/0006398. [DOI] [PubMed] [Google Scholar]
- 13.Soulsby EJL. Helminths, Arthropods and Protozoa of Domesticated animals. 7th ed. London, UK: Bailliere Tindall; 1982. pp. 8–87. [Google Scholar]
- 14.Bray RA, Gibson DI, Jones A. Keys to the Trematoda. Vol. 1. London, UK: CAB International and Natural History Museum; 2002. pp. 1–521. [Google Scholar]
- 15.Bray RA, Gibson DI, Jones A. Keys to the Trematoda. Vol. 3. London, UK: CAB International and Natural History Museum; 2008. pp. 1–824. [Google Scholar]
- 16.Bush AO, Lafferty KO, Lotz JM, Shostak AW. Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol. 1997;83:575–583. [PubMed] [Google Scholar]
- 17.Sokal RR, Rohlf FJ. Biometry: the principles and practice of statistics in biological research. 4th ed. New York, USA: WH Fereeman and Co; 1994. pp. 1–937. [Google Scholar]
- 18.Chai JY, Murrell KD, Lymbery AJ. Fish-borne parasitic zoonoses: status and issues. Int J Parasitol. 2005;35:1233–1254. doi: 10.1016/j.ijpara.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 19.Chai JY, Lee SH. Food-borne intestinal trematode infections in the Republic of Korea. Parasitol Int. 2002;51:129–154. doi: 10.1016/s1383-5769(02)00008-9. [DOI] [PubMed] [Google Scholar]
- 20.Lan-Anh NT, Phuong NT, Murrell KD, Johansen MV, Dalsgaard A, Thu LT, Kim-Chi TT, Thamsborg SM. Animal reservoir hosts and fish-borne zoonotic trematode infections on fish farms, Vietnam. Emerg Infect Dis. 2009;15:540–546. doi: 10.3201/eid1504.081147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdul-Salam J, Nair SBH, Ashkanani H. Surface ultrastructure of Stictodora fuscatum (Trematoda: Heterophyidae) from Kuwait Bay. Parasitol Día. 2000;24:55–59. doi: 10.1016/s1383-5769(00)00027-1. (online) [DOI] [PubMed] [Google Scholar]
- 22.Abdul-Salam J, Sreelatha BS, Ashkanani H. Surface ultrastructure of Stictodora tridactyla (Trematoda: Heterophyidae) from Kuwait Bay. Parasitol Int. 2000;49:1–7. doi: 10.1016/s1383-5769(00)00027-1. [DOI] [PubMed] [Google Scholar]
- 23.Farahnak A, Massoud J. Medically important metacercariae (larva trematodes) in Khuzestan fishes, Iran. Acta Med Iranica. 1999;37:59–62. [Google Scholar]
- 24.Ali AH. Pathological effects of helminths partasitic on some local fishes. M. Sc. thesis. College of Agriculture, University of Basrah; Iraq: 2001. pp. 1–174. (in Arabic) [Google Scholar]
- 25.El-Seify MA, Desouky ARY, Bazh EK. Epidemiological studies on some fish-borne parasites. Egypt Vet Med Soc Parasitol J. 2005;2:570–592. [Google Scholar]
- 26.Kardousha MM. Helminth parasite larvae collected from Arabian Gulf fish. IV. Description of 4 larvae including two metacercariae, one didymozoid and one acanthocephalan. Arab Gulf J Sci Res. 2005;23:23–27. [Google Scholar]
- 27.Karimian E, Ghorbani R, Hajimoradlou A. First occurrence and intensity of Posthodiplostomum cuticola (Nordmann, 1832) (Digenea; Diplostomatidae) metacercariae in monkey goby (Neogobius pallasi berg, 1916) in the Zarringol stream, Golestan Province, Iran. Global Veterinaria. 2013;10:505–510. [Google Scholar]
- 28.Massoud J, Jalali H, Reza M. Studies on trematodes of the family Heterophyidae (Odhner, 1914) in Iran. 1. Preliminary epidemiological surveys in man and carnivores in Khuzestan. J Helminthol. 1981;55:255–260. doi: 10.1017/s0022149x00027851. [DOI] [PubMed] [Google Scholar]
- 29.Rajavelu G, Raja EE. On helminth parasites in domestic cat in Madras. Cheiron. 1988;17:11–14. [Google Scholar]
- 30.Jacob L, Pillaik M. Incidence of parasitic infection in dogs in Thrissur, Kerala. J Vet Anim Sci. 1991;22:149–150. [Google Scholar]
- 31.Fan PC. Viability of metacercariae of Clonorchis sinensis in frozen or salted fresh water fish. Int J Parasitol. 1988;28:603–605. doi: 10.1016/s0020-7519(97)00215-4. [DOI] [PubMed] [Google Scholar]
- 32.Abdul-Salam J. Intestinal helminth parasites of stray dogs in Kuwait. Arab Gulf J Sci Res. 1986;4:659–663. [Google Scholar]
- 33.Mohammad KM. The parasitic fauna and the food habits of the wild jungle cat Felis chausfuraxde Winton, 1898, in Iraq. Bull Iraq Nat Hist Mus. 2008;10:65–78. [Google Scholar]
- 34.Seo BS. Biology and clinical aspects of Heterophyidae. Human Sci. 1979;3:784–791. (in Korean) [Google Scholar]
- 35.Chai JY, Seo BS, Lee SH, Hong SJ, Sohn WM. Human infections by Heterophyes heterophyes and H. dispar imported from Saudi Arabia. Korean J Parasitol. 1986;24:82–88. doi: 10.3347/kjp.1986.24.1.82. [DOI] [PubMed] [Google Scholar]
- 36.Dung DT, De NV, Waikagul J, Dalsgaard A, Chai JY, Sohn WM, Murrell D. Fishborne zoonotic intestinal trematodes, Vietnam. Emerg Infect Dis. 2007;13:1828–1833. doi: 10.3201/eid1312.070554. [DOI] [PMC free article] [PubMed] [Google Scholar]


